Abstract
Syncope and atrial fibrillation are both common entities and frequently occur together in an acute clinical scenario. Treatment of each in this presentation requires acquiring a good history and understanding the presentation of the patient. In this manuscript, there are 5 case studies that demonstrate common misperceptions when attempting to treat syncope when it presents with the arrhythmia atrial fibrillation. Rarely, does atrial fibrillation cause syncope. However, when a patient presents in atrial fibrillation, it becomes the focus of therapy rather than trying to define the etiology of the syncopal episode. It may be that well thought out algorithms to treat atrial fibrillation in an acute setting are replacing deductive thinking particularly when it comes to diagnosing the cause of a syncopal spell.
Keywords: Syncope, Atrial Fibrillation
Introduction
Both syncope and atrial fibrillation are common disease entities, frequent contributors to the economic footprint of the healthcare system, both leading to frequent admissions and to significant morbidity. Both are often associated with other syndromes such as sinus node dysfunction and stroke. Both are multifactorial in etiology. As most electrophysiologist, and indeed most healthcare workers in fact, are aware: they can both be challenging to treat.
Both Are Common Clinical Entities
Syncope
Syncope is defined as a transient loss of consciousness, with unresponsiveness, loss of postural tone and spontaneous recovery. By definition, with spontaneous termination, specific attempts at resuscitation are not required. It is thought to occur in 40% of the population. It is a symptom frequently seen in 12%-48% of young healthy adults, most of whom do not seek evaluation. Nearly 60% with a syncopal episode who seek attention and have some evaluation will not have a recurrence. But it is a disease that affects all ages: the elderly in long-term residence care may have a 6% annual incidence of syncope. Falls occur in 20% of those over age 65 years and 10% of falls in this population are caused by syncope. Approximately, 1% to 3% of emergency department/ urgent care visits are related to syncope. Nearly, 35% of these individuals with an emergency department diagnosis of syncope are admitted. In 2000, there were over 200,000 hospital admissions with a diagnosis of syncope. The estimated cost was 2.5 billion dollars. In those hospitalized with an unclear etiology for their syncopal spell, the actual diagnosis remains a mystery in the vast majority (~ 90%).
Atrial Fibrillation
This is the commonness cardiac tachycardia. In the space of 30 years it has gone from a topic of clinical nuisance to now a disorder of major clinical importance. In the early 1990’s, the prevalence of atrial fibrillation was at the 1%-2% level of adults in the United States. Twenty years later, the prevalence ranges from 1% to 6% but is generally quoted to be in the 5% neighborhood. This is best explained by the overall increase in life expectancy in this country. The average female will live to be 81 years old and the average male will reach 76 years old. The prevalence of atrial fibrillation is both age and sex related. At age equivalents, more men have atrial fibrillation than women. Those individuals below age 50 years have an incidence that is less than 1% but it is greater than 8% in those over age 80 years. The incidence increases significantly after age 65 years where the incidence shifts from 1% to the 2% - 4% range. The trend has been for the total number of individuals with atrial fibrillation to increase in accordance with the aging population increase. The number of individuals with atrial fibrillation in 1980 was 1.3 million and in 1995 a little over 2 million. Naccarelli et al.[2] estimated that in 2005, 3.03 million individuals in the United States had atrial fibrillation and the projected prevalence may be 7.56 million by 2050.
Both Can Precede The Other
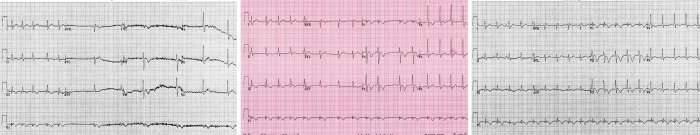
Case study#1: PK, a 35 years old male bartender was brought to the emergency department (ED) for recurrent syncope. He had worked the night before and following an episode of syncope, went home early from the bar. When he awoke the next morning, he had symptoms of dizziness, fatigue, and shortness of breath. Later that day he had another syncopal event and was brought to the hospital. Pertinent medical history included the fact that he had fractured ribs from a motor vehicle accident 3 weeks earlier and more recent 3 days of nausea and vomiting. His medicines on presentation included nuprin for pain and LIPO 6 (for weight loss). In the ED, he was found to be in atrial fibrillation with a ventricular response rate of 100 bpm and a systolic blood pressure less than 100 systolic. His respiratory rate was 18/ minute, temperature 100.4°F (38°C) and weight of 180 lbs. (81 kg). On physical examination, the jugular venous pressure was elevated and the pulse irregularly irregular. His lungs were clear and there were no additional cardiovascular findings. Although he was awake and oriented, he appeared anxious and agitated. Given his hemodynamic compromise, direct current cardioversion was performed of atrial fibrillation with two unsuccessful attempts. Cardiology was consulted and a new ECG ([Figure 1]) was performed as the patient has another witnessed episode of syncope. An urgent chest CT was performed but unfortunately the patient succumbed from what was eventually found to be massive bilateral pulmonary emboli in the ED.
Figure 1: This is ECG is recorded during syncope. Initially, there is atrial fibrillation, average rate 125 bpm. There is sudden ventricular rate slowing. There is an S1-Q3-T3 pattern and inverted T waves in the anterior precordial leads, which are findings consistent with pulmonary embolism.
Syncope
This case illustrates several points: atrial fibrillation rarely causes syncope and does not usually cause hypotension when ventricular rates are normal. One must consider the possibility that another another factor was involved. The patient had recurrent syncope with alternating tachycardia (atrial fibrillation) followed by profound sinus bradycardia suggesting some autonomic nervous system input. This was a young health individual on an NSAID plus a sympathomimetic weight loss supplement and was dehydrated from nausea and vomiting. He presented with shortness of breath, periodic hypotension with bradycardia alternating with tachycardia and an ECG with an S1-Q3-T3 pattern. The syncope in this instance was due to pulmonary emboli, which had lead to the initial presentation of atrial fibrillation.
Table 1. Clinical Presentation and Diagnosis.
| Onset | Slow | Fast | Fast |
|---|---|---|---|
| Recovery: | Slow | Slow | Fast |
| Metabolic | Cardiac | ||
| Hyperventilation | Structural: stenosis, | ||
| Intoxication | obstruction. | ||
| Hyperglycemia | Arrhythmia | ||
| Neurocardiogenic | |||
| Neurological | |||
| Cerebrovascular accidents | |||
| Seizures |
A thorough analysis of the history is mandatory and it is still integral to determining the correct diagnosis. The history in case #1 indicates that syncope had a “fast onset” and “rapid offset”. Most often this would point to a mechanism that is either cardiac, autonomic reflex, autonomic failure or postural in origin and not cerebrovascular in nature. This is not related to intoxication, hyperventilation or hypoglycemia since the onset of repeated syncopal spells is not one of slow onset and slow recovery.[3]
Atrial Fibrillation
In case #1, the aggressive approach to treating atrial fibrillation with direct current cardioversion in the emergency department reflects the use of rigid algorithmic logic (that can occasionally be seen in the ED). A patient presents with atrial fibrillation and syncope and is found to be hypotensive. The algorithm would dictate using direct current cardioversion to treat the atrial fibrillation when a patient presents with hypotension, pulmonary edema, symptomatic angina pectoris or acute myocardial infarction. However, algorithms do not take into account that the atrial fibrillation is not directly responsible for the hypotension but is just an innocent bystander. In the ECG shown, atrial fibrillation spontaneously alternates with sinus bradycardia during the episode of syncope, the mechanism of which seems related to an autonomic nervous system factor. We must assume that the initiating factor is adrenalin related to hypotension or possibly a neurocardiogenic vagal triggered mechanism. In this case, direct current cardioversion is inappropriate at this time in the ED.
Both Can Be Induced By An Increase In Vagal Tone
Case Study #2
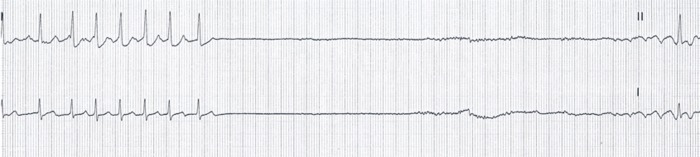
DK, a healthy 74 years old female is standing in her kitchen when she developed the sudden onset of nausea, followed by syncope. She presented to the emergency department (ED) in normal sinus rhythm. The physical examination and baseline laboratory evaluation were normal. In the ED, she had more nausea and a repeat syncopal episode. Telemetry monitoring showed sinus bradycardia followed by PR interval prolongation, complete AV block, asystole, then atrial fibrillation. ([Figure 2]) Atrial fibrillation persisted initially with a slow junctional escape rhythm then as the ventricular response increased there was spontaneous recovery.[4] Tilt table testing replicated syncope and atrial fibrillation. There is both vagal induced syncope and vagal induced atrial fibrillation.[4]
Figure 2: With the onset of vasovagal syncope, this continuous recording shows profound sinus slowing then complete AV block for 31 seconds followed by vagal induced atrial fibrillation with a slow ventricular response.
Syncope
In classic vasovagal syncope, patients may have a prodromal picture consisting of a flushed feeling, diaphoresis, nausea, abdominal pain or discomfort, lightheadedness and pallor.5 The elderly may not have this classical prodrome or it may be greatly abbreviated.[5] The classic triggers include prolonged standing, hot temperatures, emotional stress (like fear), venipuncture, pain, dehydration and systemic illness. It can also occur while seated and may be precipitated by alcohol.[5] The prevalence of vasovagal syncope ranges from 8-37% and all of reflex-mediated syncope ranges from 9-49% of all reflex-mediated syncope.[1] On average, a neurocardiogenic mechanism will be the cause of syncope about 18% of the time while some form of organic heart disease accounts for 4% and cardiac arrhythmia another 14%.1 Vasovagal syncope may be the explanation in as many as 31% of those over the age 65 years. With the advent of Tilt Table assessment of syncope, the incidence of unexplained syncope has been reduced by ½ to 17-18%.[1]
Using head up tilt testing, Brignole et al.[6] described three patterns of vasovagal syncope. The classic pattern is a (1) reflex decrease in blood pressure and heart rate, (2) a cardio-inhibitory (bradycardic) variety and (3) a dysautonomic response. Elderly patients tend to display the dysautonomic response with a slow decline in blood pressure with head up tilt. In the elderly, passive tilt table testing is positive in 32%-36% as compared to a younger population, in whom passive tilt table testing is positive in 60%-70%. The addition of nitroglycerin as a provocation agent increases the number of positive outcomes to 60%-70% in the elderly. This agent is safer than isoproterenol infusion is this population. Isoproterenol can be dangerous in those with ischemic heart disease, obstructive hypertrophic cardiomyopathy, aortic stenosis and accelerated hypertension. Head-up tilt table testing is very useful in elderly patients in helping to confirm one’s clinical impression of vasovagal or dysautonomic syncope.
Atrial Fibrillation
Coumel first recognized bradycardia-induced atrial fibrillation and ascribed this to vagal stimulation.[7-8] Attuel demonstrated that atrial refractory periods in patients with vagal induced atrial fibrillation shorten with slowing of the heart rate. This paradoxical finding linked with an increase in atrial dispersion of refractoriness is the mechanism for vagal induction of atrial fibrillation.[10] The patient population of vagal induced atrial fibrillation favors men over women (ratio 4:1) usually between the ages of 25 and 60 years. Despite recurrent bouts of atrial fibrillation over several years, there seems little progression to permanent fibrillation.
Episodes last from a few minutes to a few hours and tend to occur mostly at night or at rest. There is an association with gastrointestinal symptoms of nausea and indigestion as well as with alcohol ingestion.[11] Episodes can often trigger frequent urination. Digoxin, beta-blockers and calcium antagonist, (verapamil, diltiazem) are ineffective. Exercise and faster heart rates are salutary. It is generally thought that atrial fibrillation requires a critical mass of atrial tissue coupled with shortened atrial action potentials and increased dispersion of atrial refractoriness. Vagal mediated atrial fibrillation is usually in the setting of structurally normal atria. Therefore, the major factor in vagal facilitated atrial fibrillation is related to the degree of refractory period dispersion created by vagal shortening of atrial action potentials.[11] In case #2, this recently published example (4) of vagal induced atrial fibrillation is linked to vasovagal syncope. In this instance, tilt table testing was able to replicate both entities.
Both are Associated with Sinus Node Dysfunction
Case Study #3
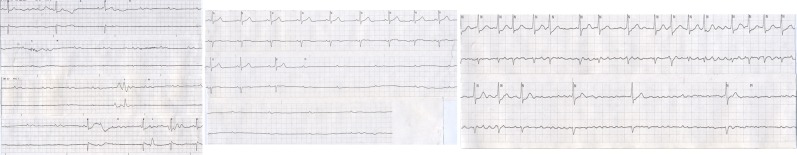
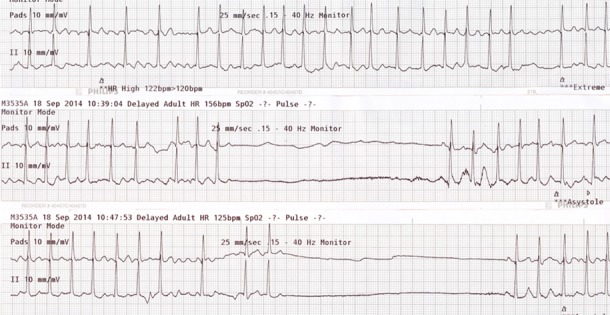
HV was a 74 years old female with a history of paroxysmal atrial fibrillation and recurrent syncope over a 4 year period. She had previously been on anticoagulation but this had been discontinued when she had a meningioma removed and had never been reinitiated. She continued to have infrequent palpitations. Recurrent atrial fibrillation had been documented on ECG at least once about one year prior. She described three distinct syncopal episodes, occurring 9 months, 3 months and one week prior to the current episode that had brought her to the ED. On the day of admission, she awakened with an upper respiratory infection. Her granddaughter noticed that she had seizure like activity and was unresponsive. She awoke spontaneously and appeared neurologically intact. Shortly there after, she had more seizure like activity that once again spontaneously recovered. Emergency medical personnel were called and she was found to be in atrial fibrillation with a rapid ventricular rate at 160 bpm on arrival. During transport, she was given a single dose of intravenous diltiazem. Syncope recurred in route to the ED. ([Figure 3]) She also had several further episodes of syncope and near-syncope in the ED. ([Figure 4]) Tachycardia-bradycardia syndrome was documented and a permanent dual chamber pacemaker was implanted.
Figure 3: During transport, the patient is given an intravenous injection of diltiazem. This recording during transport confirms the diagnosis of tachycardia-bradycardia syndrome with atrial fibrillation suddenly terminating and resulting in a 7.2 second pause followed by resumption of atrial fibrillation.
Figure 4: There are 3 separate recordings in the emergency department. Top: There is atrial fibrillation with an average rate at 140 bpm. The patient is awake an alert. Middle: There is atrial fibrillation that terminates with a resultant 4-second pause and near syncope. There is a single sinus beat then the return of atrial fibrillation with a rapid ventricular response. Bottom: There is another symptomatic episode when atrial fibrillation terminates with a resultant 4.4-second pause followed by a single sinus beat then recurrent atrial fibrillation and a rapid ventricular response.
Syncope
In older individuals, syncope when associated with paroxysmal atrial fibrillation should suggest the presence of underlying sinus node dysfunction. Tachycardia-bradycardia syndrome is the most common presentation of sick sinus syndrome. Syncope occurs as tachycardia terminates with protracted sinus pauses. Failure of the sinus node to overcome overdrive suppression by atrial fibrillation causes sinus arrest. Reduced sinus node automaticity is the major problem. These patients have suppression of their escape or subsidiary pacemakers by the overdrive suppression of atrial fibrillation. Subsidiary pacemaker failure is enhanced by medications such as digitalis, beta-blockers and the calcium antagonist diltiazem or verapamil. In case #3, the patient unfortunately had suffered recurrent episodes of syncope which were not recognized until she presented with seizure-like activity. The week prior, she had presented with a fall and resultant head trauma. Her superficial wound was cleaned and closed with stitches and she was discharged from the ED. Given that atrial fibrillation had been documented on previous ECG recordings in the electronic medical record in this case, the connection between recurrent syncope and atrial fibrillation should have suggested a clinical diagnosis of tachycardia-bradycardia syndrome prior to the more severe presentation of seizure activity from sinus arrest. Permanent pacing is recommended in patients with recurrent syncope in the setting of tachycardia-bradycardia syndrome to prevent symptoms.[12]
Atrial Fibrillation
Case #3 is a classic version with documented paroxysmal atrial fibrillation alternating with sinus bradycardia. It depicts how the algorithm to treat one particular entity, the tachycardia fails to take into account the second entity, the bradycardia. The intravenous diltiazem provided in the ambulance to treat her rapid ventricular rates accentuated the frequency and duration of sinus pauses and the recurrent seizure like activity that accompanied these pauses in the ED. Sometimes the approach that “the best medicine is less medicine” applies. The treatment of atrial fibrillation in this syndrome requires permanent pacing in conjunction with either antiarrhythmic agents or rate control drugs aimed at the AV junction. Atrial pacing alone generally does not help deter the recurrence of episodes of atrial fibrillation. However, ventricular pacing alone is recognized to cause an increase in the number of episodes of atrial fibrillation. Atrial fibrillation in those with sick sinus syndrome is mostly related to the extent of atrial scaring and fibrosis. Atrial fibrosis may be diffusely distributed with predominance in the areas of the sinus and AV nodes. Other influences, such as the degree of AV valvular regurgitation and the underlying imbalance between the parasympathetic and sympathetic limbs of the autonomic nervous system may also carry impact. Importantly, in the tachycardia-bradycardia syndrome, prognosis and mortality is linked to underlying cardiac pathology and function as well as systemic pathology. The arrhythmia is generally not the direct determinant of mortality given the currently available treatment modalities.[12]
Both Can Be Challenging
Case Study #4
DZ was a 57 years old male with palpitations and a remote history of syncope. He also had a prior history of nephritis with stage 3 renal insufficiency and persistent hyponatremia. He complained of persistent palpitations occurring generally at night and unrelated to exercise or emotion. An ECG on presentation documented atrial fibrillation with a rapid rate. ([Figure 5]) He was placed on flecainide 100 mg orally twice daily with resolution of atrial fibrillation and the associated palpitations. A follow up ECG showed sinus rhythm but on further review, also demonstrated type I Brugada pattern. ([Figure 6]) He has converted from atrial fibrillation to sinus rhythm on this agent. He has no symptoms of syncope or palpitations on flecainide even with the Brugada pattern ECG. Flecainide was discontinued and without any antiarrhythmic agent, he developed symptomatic atrial fibrillation again. He was hospitalized and quinidine gluconate 324 mg was started orally every 8 hours. On quinidine, he converts to sinus rhythm and the ECG was normal.
Figure 5: There is atrial fibrillation with an average ventricular rate at 90 bpm. The QRS complexes are narrow with a normal axis at +35 degrees. There is 1 mm J-point elevation with concave ST segments in V1 and V2 suggestive of a type 3 Brugada pattern.
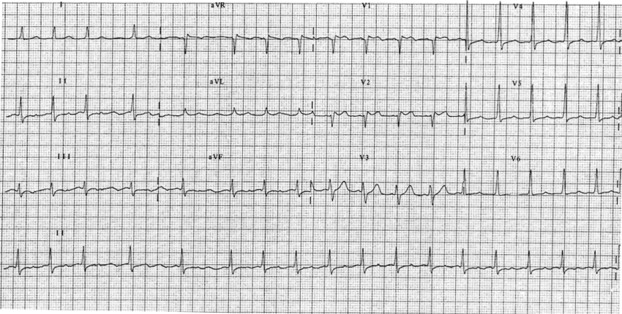
Figure 6: This ECG is recorded on Flecainide 100 mg orally bid. There is sinus rhythm with a type I Brugada pattern showing V1 and V2 ST elevation with inverted T waves.
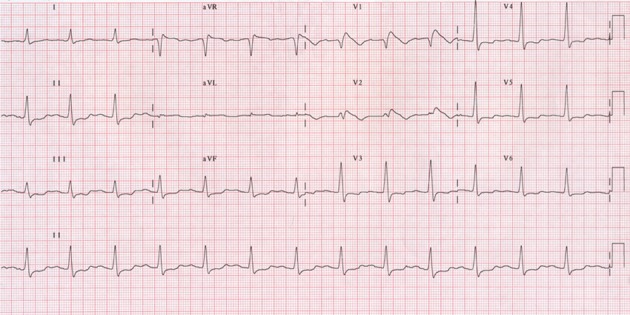
Syncope
This patient had syncope but this occurred in the setting of atrial fibrillation and was orthostatic related. In this patient, the type I Brugada pattern ECG was provoked by the class IC antiarrhythmic agent flecainide. He remained asymptomatic in sinus rhythm on flecainide. With discontinuation of flecainide, his ECG normalized but atrial fibrillation recurred. The class IA antiarrhythmic agent quinidine gluconate was successful at restoring sinus rhythm. This agent has both sodium channel and potassium Ito channel blocking activity. Inhibition of the potassium Ito channel helped to reverse the Brugada ECG pattern. Syncope in the setting of an ECG documenting Brugada pattern is an indication for placement of an ICD. However, drug or fever induced Brugada pattern ECG has a more benign prognosis and generally does not warrant further testing or ICD placement.
Atrial Fibrillation
Atrial fibrillation is a commonly seen arrhythmia in patients with Brugada syndrome. The traditional antiarrhythmic agents used to treat atrial fibrillation may be contraindicated in this setting. Specifically, the class IA agents (procainamide, disopyramide) and IC agents (flecainide, propafenone) should be avoided as well as amiodarone, the class III antiarrhythmic. Quinidine may be useful at treating both atrial fibrillation and the ventricular arrhythmias seen in patients with Brugada syndrome. Quinidine has a salutary effect on the ventricular arrhythmias and Brugada ECG pattern with the inhibition of the potassium Ito channel. The complexity of trying to treat atrial fibrillation with Brugada syndrome is not just challenging but may in itself be potentially lethal.
Both Can Be Potentially Lethal
Case Study #5
SW was a 35 years old male who suddenly develops palpitations at home followed by syncope. He is brought to the hospital by emergency services with tachycardia and hypotension. The ECG on admission revealed an irregular wide QRS complex tachycardia at a rate 215 bpm. Adenosine was given intravenously without any adverse effects but the rhythm does not change. The patient was seen by our cardiology consult service and following sedation had direct current cardioversion performed with a single 200 joules synchronized biphasic shock. The ECG ([Figures 7],[8]) was diagnostic of atrial fibrillation with pre-excitation and Wolff-Parkinson-White syndrome. The patient subsequently underwent electrophysiologic testing and successful catheter ablation of a left lateral accessory pathway.
Figure 7: This ECG is diagnostic of Wolff-Parkinson-White syndrome. It shows a rapid irregularly, irregularly wide QRS tachycardia at a ventricular rate of 220 bpm and is atrial fibrillation. There is antegrade conduction over the left lateral accessory pathway (RBBB configuration, right axis) with varying QRS widths.
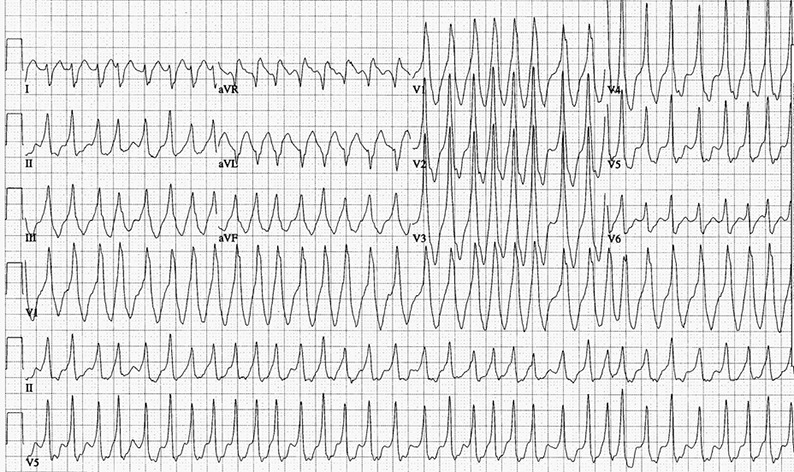
Figure 8: There is sinus rhythm after direct current cardioversion with a short PR interval and delta wave in most leads diagnostic of Wolff-Parkinson-White syndrome.
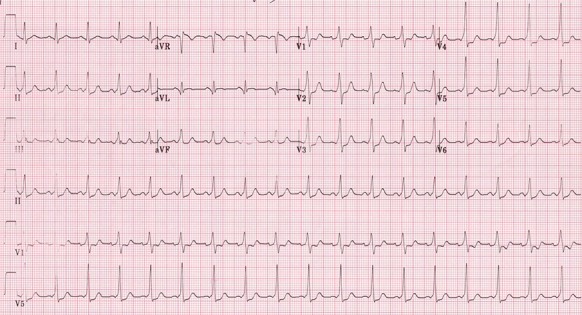
Syncope
As mentioned previously, atrial fibrillation rarely causes syncope. The notable exception is in the patient with Wolff-Parkinson-White (WPW) syndrome and a rapidly conducting accessory pathway. In this setting, syncope is seen when ventricular rates are exceedingly rapid. Syncope or near syncope with WPW are an indication for electrophysiology testing and catheter ablation to eliminate the accessory pathway.
Atrial Fibrillation
Atrial fibrillation may be seen in as high as a third of WPW patients. It is frequently associated with and triggered by the rapid rates of atrioventricular reentrant tachycardia. Wolff-Parkinson-White is a critical diagnosis in a young individual presenting with the ECG showing an irregular, rapid wide QRS complex tachycardia with varying degrees of QRS width. Patients resuscitated from cardiac arrest with atrial fibrillation and WPW rarely present with de novo ventricular fibrillation. Nearly all have mildly symptomatic episodes of tachycardia if not an overt presentation. Those at risk of sudden death have accessory pathways with very short antegrade effective refractory periods and the shortest R-R interval of less than 250 milliseconds. Agents that block conduction over the AV node and facilitate conduction over the accessory pathway are potentially lethal. Avoid the use of digoxin, diltiazem, verapamil and adenosine. Patients with atrial fibrillation and WPW should be referred to an electrophysiologist for evaluation and catheter ablation of the accessory pathway.
Both are the Focus of Algorithmic Therapy
In the five cases presented above, all are examples of syncope coupled with atrial fibrillation that when initially seen in the emergency department presented diagnostic and therapeutic dilemmas. All presented with or developed an arrhythmia, atrial fibrillation in the ED which tended to cause confusion in the approach to management. In 4 cases, the diagnosis responsible for syncope were: acute pulmonary embolism (case 1), vasovagal syncope (case 2), tachycardia bradycardia syndrome (case 3) and WPW and atrial fibrillation (case 5). In case 4, the syncope was remote from the ED presentation in atrial fibrillation. In all, the approach involved focusing on atrial fibrillation and rate control rather than focusing on making a diagnosis for syncope. This is an unfortunate common emergency department approach to the problem of syncope, which tends to focus on treatment of the rhythm instead.
The Concept of a Syncope Unit
There are several well thought out and constructed algorithms for risk stratification of syncope. ([Table 2])[13-22] These are designed to aid in identifying individuals with syncope at high risk for cardiovascular events, life threatening arrhythmias and short-term risk of mortality.[21,22] Most emphasize the presence of structural heart disease, coronary disease in particular prior infarction and left ventricular dysfunction with heart failure. The ECG criteria that may point to an arrhythmic mechanism for syncope include the presence of bundle branch block, bifascicular block, intraventricular conduction delay, bradycardia (<50 bpm), pre-excitation, prolonged QT interval, Brugada pattern and epsilon wave of arrhythmogenic right ventricular cardiomyopathy. The ROSE (“ Risk Stratification of Syncope in the Emergency Department) study included historical parameters, examination findings, biochemical, biomarkers and hematologic variables.[14] This study does not focus strictly on cardiac risk but other serious medical co-morbidities. All of these risk stratification studies are aimed at selecting the ED patient to be admitted for evaluation. ([Table 3])[21] The ROSE Study may be the best at defining the individual patient that merits hospitalization. The ROSE rule recommends admission for any one positive factor. Their mnemonic encompasses these risk factors, “BRACES” which includes: 1. BNP ≥ 300 pg/ml; 2. Bradycardia ≤50 in or pre ED; 3.Rectal examination positive for occult blood; 4.Anemia – hemoglobin ≤ 90 g/l; 5. Chest pain with syncope; 6. ECG with Q waves; 7. Saturation ≤ 94% on room air.[14]
Table 2. Syncope: Risk Stratification Studies.
| European Society of Cardiology (ESC) | 2004 (ref. 15) |
|---|---|
| Evaluation in the ED Study (SEEDS) | 2004 (ref. 16) |
| Osservatorio Epidemiologico sula Sincope | |
| Nel Lazio (OESIL) | 2003 (ref. 17) |
| Short Term Prognosis of Syncope (STePS) | 2008 (ref. 18) |
| San Francisco Syncope Rule (SFSR) | 2004 (ref. 19) |
| Evaluation of Guidelines in Syncope Study | |
| (EGSYS) | 2008 (ref. 20) |
Table 3. High Risk Syncope.
| Cardiac Diagnosis: |
|---|
| Cardiac Diagnosis: |
| Clinical: Syncope with exercise, Family history of SCD, |
| Previous MI. |
| Structural disease: low EF, Hypertrophic CM, Dilated CM |
| ECG: LBBB; RBBB+ LAFB; IVCD; LQT; Brugada; WPW; |
| Bradycardia < 50 bpm; Epsilon wave of ARVC/D |
| Rose Criteria: “BRACES”: |
| Central Nervous System Event: |
| TIA; CVA (with focal neurological signs); New seizures |
The diagnosis of an episode of syncope seen in the ED is made from history, physical examination, ECG or routine laboratory test in 50%. A diagnosis is established in another 21% of those sent to a “Syncope Unit” with still an unexplained diagnosis. Most specialized “Syncope Units” use a combination of algorithms that employ both (1) etiology and (2) mechanism.[22] The guidelines suggested by the European Society of Cardiology seem most practical.[23] Tilt table testing is useful for atypical presentations of neurocardiogenic syncope. Electrophysiologic testing is recommended for suspected cases of arrhythmogenic syncope. Exercise stress testing may be beneficial in those with a history of exercise related syncope. Short term monitoring with a Holter or loop recorder is employed universally. The real break through in diagnosing difficult cases has been the general adaptation of implantable loop recorders. When used in unexplained syncope, 32% have a diagnosis in 18 months and nearly 50% in 24 months.[22] There is nothing magical about the specialized “Syncope Unit”. All employ useful diagnostic algorithms and appropriate useful test but more importantly, they all have individuals that do not rely on a test or the algorithm for diagnosis.
In summary, syncope is very common. The diagnosis is identified from the history and physical in about 45%-50% of cases.1,[22] The number of patients with unexplained syncope still remains high, at around 17 %. Neurocardiogenic syncope accounts for nearly a third of all cases. The high-risk subgroups with a cardiac diagnosis are those with structural disease and arrhythmias that average about 4% and 14% respectively. This particular group has a 33% one-year mortality rate.[1] Most patients have a benign cause and a very low mortality risk.[22] There are adequate risk stratification protocols for recommending who should be hospitalized from the ED.[21-23] Our five cases presented here would indicate that there is a clinical disconnect when it comes to diagnosis of the etiology of syncope. The algorithm-designed approaches to the diagnosis of syncope and atrial fibrillation are intended to bridge this disconnect. Instead, use of a rigid algorithm approach may tend to camouflage the real clinical issues. Occasionally, treatment of symptoms can cloud the diagnosis of the true underlying disease. Unfortunately, it seems apparent that algorithms have replaced critical deductive thinking.
References
- 1.Wishwa N. An Overview of the evaluation and management of syncope. In Grubb BP, Olshansky B, (eds), Syncope: Mechanisms and Management, Futura Publishing Company, Inc . Armonk, NY. 1998;0:1–13. [Google Scholar]
- 2.Naccarelli Gerald V, Varker Helen, Lin Jay, Schulman Kathy L. Increasing prevalence of atrial fibrillation and flutter in the United States. Am. J. Cardiol. 2009 Dec 1;104 (11):1534–9. doi: 10.1016/j.amjcard.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 3.Luck JC. Syncope: Presentations, perspectives and nuances. J Cardiac Arrhythmias. 1997;2:1–3. [Google Scholar]
- 4.Sriram Chenni S, Naccarelli Gerald V, Luck Jerry C. An atypical case of vagally mediated atrial fibrillation in an elderly woman: electrocardiographic caveats to diagnosis. J Electrocardiol. 2014 Jul 26;47 (5):734–7. doi: 10.1016/j.jelectrocard.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Tan Maw Pin, Parry Steve W. Vasovagal syncope in the older patient. J. Am. Coll. Cardiol. 2008 Feb 12;51 (6):599–606. doi: 10.1016/j.jacc.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 6.Brignole M, Menozzi C, Del Rosso A, Costa S, Gaggioli G, Bottoni N, Bartoli P, Sutton R. New classification of haemodynamics of vasovagal syncope: beyond the VASIS classification. Analysis of the pre-syncopal phase of the tilt test without and with nitroglycerin challenge. Vasovagal Syncope International Study. Europace. 2000 Jan;2 (1):66–76. doi: 10.1053/eupc.1999.0064. [DOI] [PubMed] [Google Scholar]
- 7.Coumel P, Attuel P, Lavallée J, Flammang D, Leclercq J F, Slama R. [The atrial arrhythmia syndrome of vagal origin]. Arch Mal Coeur Vaiss. 1978 Jun;71 (6):645–56. [PubMed] [Google Scholar]
- 8.Coumel P. Paroxysmal atrial fibrillation: a disorder of autonomic tone? Eur. Heart J. 1994 Apr;15 Suppl A ():9–16. doi: 10.1093/eurheartj/15.suppl_a.9. [DOI] [PubMed] [Google Scholar]
- 9.Attuel P, Childers R, Cauchemez B, Poveda J, Mugica J, Coumel P. Failure in the rate adaptation of the atrial refractory period: its relationship to vulnerability. Int. J. Cardiol. 1982;2 (2):179–97. doi: 10.1016/0167-5273(82)90032-8. [DOI] [PubMed] [Google Scholar]
- 10.Le Heuzey J-Y, Boutjdir M, Gagey S, Lavergne T, Guise T. Cellular aspects of atrial vulnerability. In Atteul P, Coumel P, Jansen MJ, (eds): The Atrium in Health and Disease. . Mount Kisco, NY, Futura Publishing Company, Inc. 1989;pp:81–94. [Google Scholar]
- 11.Coumel P, Jain RC. Neurogenic and humoral influences of the autonomic nervous system in the determination of paroxysmal atrial fibrillation. In Atteul P, Coumel P, Jansen MJ, (eds): The Atrium in Health and Disease. . Mount Kisco, NY. Futura Publishing Company, Inc. 1989;0:213–232. [Google Scholar]
- 12.Kaplan B. The tachycardia-bradycardia syndrome. In Leon Resnekov, (ed): The Medical Clinics of North America. W. B. Saunders Company. Philadelphia . 1976;0:81–99. doi: 10.1016/s0025-7125(16)31921-6. [DOI] [PubMed] [Google Scholar]
- 13.Benditt David G, Can Ilknur. Initial evaluation of "syncope and collapse" the need for a risk stratification consensus. J. Am. Coll. Cardiol. 2010 Feb 23;55 (8):722–4. doi: 10.1016/j.jacc.2009.09.050. [DOI] [PubMed] [Google Scholar]
- 14.Reed Matthew J, Newby David E, Coull Andrew J, Prescott Robin J, Jacques Keith G, Gray Alasdair J. The ROSE (risk stratification of syncope in the emergency department) study. J. Am. Coll. Cardiol. 2010 Feb 23;55 (8):713–21. doi: 10.1016/j.jacc.2009.09.049. [DOI] [PubMed] [Google Scholar]
- 15.Brignole Michele, Alboni Paolo, Benditt David G, Bergfeldt Lennart, Blanc Jean-Jacques, Bloch Thomsen Paul Erik, van Dijk J Gert, Fitzpatrick Adam, Hohnloser Stefan, Janousek Jean, Kapoor Wishwa, Kenny Rose Anne, Kulakowski Piotr, Masotti Giulio, Moya Angel, Raviele Antonio, Sutton Richard, Theodorakis George, Ungar Andrea, Wieling Wouter. Guidelines on management (diagnosis and treatment) of syncope--update 2004. Europace. 2004 Nov;6 (6):467–537. doi: 10.1016/j.eupc.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Shen Win K, Decker Wyatt W, Smars Peter A, Goyal Deepi G, Walker Ann E, Hodge David O, Trusty Jane M, Brekke Karen M, Jahangir Arshad, Brady Peter A, Munger Thomas M, Gersh Bernard J, Hammill Stephen C, Frye Robert L. Syncope Evaluation in the Emergency Department Study (SEEDS): a multidisciplinary approach to syncope management. Circulation. 2004 Dec 14;110 (24):3636–45. doi: 10.1161/01.CIR.0000149236.92822.07. [DOI] [PubMed] [Google Scholar]
- 17.Colivicchi Furio, Ammirati Fabrizio, Melina Domenico, Guido Vincenzo, Imperoli Giuseppe, Santini Massimo. Development and prospective validation of a risk stratification system for patients with syncope in the emergency department: the OESIL risk score. Eur. Heart J. 2003 May;24 (9):811–9. doi: 10.1016/s0195-668x(02)00827-8. [DOI] [PubMed] [Google Scholar]
- 18.Costantino Giorgio, Perego Francesca, Dipaola Franca, Borella Marta, Galli Andrea, Cantoni Giulia, Dell'Orto Simonetta, Dassi Simonetta, Filardo Nicola, Duca Pier Giorgio, Montano Nicola, Furlan Raffaello. Short- and long-term prognosis of syncope, risk factors, and role of hospital admission: results from the STePS (Short-Term Prognosis of Syncope) study. J. Am. Coll. Cardiol. 2008 Jan 22;51 (3):276–83. doi: 10.1016/j.jacc.2007.08.059. [DOI] [PubMed] [Google Scholar]
- 19.Quinn James V, Stiell Ian G, McDermott Daniel A, Sellers Karen L, Kohn Michael A, Wells George A. Derivation of the San Francisco Syncope Rule to predict patients with short-term serious outcomes. Ann Emerg Med. 2004 Feb;43 (2):224–32. doi: 10.1016/s0196-0644(03)00823-0. [DOI] [PubMed] [Google Scholar]
- 20.Del Rosso A, Ungar A, Maggi R, Giada F, Petix N R, De Santo T, Menozzi C, Brignole M. Clinical predictors of cardiac syncope at initial evaluation in patients referred urgently to a general hospital: the EGSYS score. Heart. 2008 Dec;94 (12):1620–6. doi: 10.1136/hrt.2008.143123. [DOI] [PubMed] [Google Scholar]
- 21.Benditt David G, Nguyen John T. Syncope: therapeutic approaches. J. Am. Coll. Cardiol. 2009 May 12;53 (19):1741–51. doi: 10.1016/j.jacc.2008.12.065. [DOI] [PubMed] [Google Scholar]
- 22.Brignole Michele, Hamdan Mohamed H. New concepts in the assessment of syncope. J. Am. Coll. Cardiol. 2012 May 1;59 (18):1583–91. doi: 10.1016/j.jacc.2011.11.056. [DOI] [PubMed] [Google Scholar]
- 23.Moya Angel, Sutton Richard, Ammirati Fabrizio, Blanc Jean-Jacques, Brignole Michele, Dahm Johannes B, Deharo Jean-Claude, Gajek Jacek, Gjesdal Knut, Krahn Andrew, Massin Martial, Pepi Mauro, Pezawas Thomas, Ruiz Granell Ricardo, Sarasin Francois, Ungar Andrea, van Dijk J Gert, Walma Edmond P, Wieling Wouter. Guidelines for the diagnosis and management of syncope (version 2009). Eur. Heart J. 2009 Nov;30 (21):2631–71. doi: 10.1093/eurheartj/ehp298. [DOI] [PMC free article] [PubMed] [Google Scholar]