Abstract
Atrial fibrillation (AF) and Atrial Tachyarrhythmias (AT) are the most common clinical arrhythmias and their worst issue is a well-recognized correlation with ischemic stroke. High incidence of “subclinical” AF/ATs has been demonstrated in several trials (TRENDS, ASSERT, CRYSTAL AF, EMBRACE) in patients with both cardiac implantable electronic devices (CIEDS) and external loop recorders. Moreover, a relationship between device-detected AF/ATs and stroke risk has been observed in the same studies. However, while the net clinical benefit of the antithrombotic treatment is well established in patients with “clinical” atrial fibrillation, there may be a lower benefit in patients with device-detected arrhythmias. Subclinical AF/ATs may be considered as a marker of stroke risk rather than the proximate cause and their burden may be used in combination with CHA2DS2-VASC and HAS-BLED scores to identify high-risk population who deserves anticoagulation.
Today the remote monitoring associated with the CIEDs is effective in the early detecting of AF/ATs by avoiding delays in the therapy evaluation, as demonstrated by several trials (TRUST, CONNECT, COMPAS). However clinical evidence for stroke risk reduction by remote monitoring is still awaited; the recent trial IMPACT failed to demonstrate that the handling of the anticoagulation therapy guided by device-detected ATs and remote monitoring improves the patients’ outcome.
The challenges for clinicians are to deal with the huge data entry, to define new organizational models, to improve device patient management and to continuously update AF guidelines in according to the great amount of data offered by the new technology.
Keywords: Atrial Fibrillation, Atrial Tachyarrhytmias, Cryptogenic Stroke, Remote Monitoring, Cardiac Implantable Electronic Devices
Introduction
Atrial fibrillation (AF) and Atrial Tachyarrhythmias (AT) are the most common clinical arrhythmias.[1] They have been associated with compromised hemodynamics, heart rate irregularity, uncontrolled ventricular rate and lower exercise capacity.[2] However their worst issue is a well-recognized correlation with ischemic stroke.[3,4] These adverse events due to AF or ATs are common and frequently devastating. AF is known to increase the risk of stroke up to 5-fold and the risk of mortality up to 2-fold; 15% of all strokes are caused by AF.[5] Anticoagulant therapy can reduce the risk of stroke by 60-70%.[4,6] Therefore the focus has to be moved on the detection of subclinical AF episodes and, possibly, on their correct quantification, especially in patient at high thromboembolic risk. However, this task results difficult due to the often paroxysmal and asymptomatic nature of these arrhythmias.[7] AF/ATs may go undetected with the use of traditional monitoring techniques ([Table 1]) and the patients often do not report any symptoms. AF can be asymptomatic in up to 30-40% of cases.[2,4] Consequently many patients with subclinical AF/ATs may suffer ischemic strokes which are defined as “cryptogenic”: it is known that embolic risk in AF is independent of symptoms.[8] Subclinical atrial arrhythmias may be unmasked only with a more aggressive monitoring technique. Recently, an high incidence of subclinical AF and ATs has been demonstrated thanks to the cardiac implantable electronic devices (CIEDs).[9,10] Pacemakers (PMs) and implantable cardioverter defibrillators (ICDs) should be seen not only as therapeutic devices but also as diagnostic tools which can prevent serious adverse events, especially thromboembolic ones. In addition implantable subcutaneous cardiac monitor (ICM) can be used to allow continuous monitoring over extended periods of time. This may lead to a more patient-centered approach: the anticoagulant therapy can be adjusted for each individual by considering both the presence and the duration of specific arrhythmic episodes as well as clinical risk scores.
Table 1. Methods for cardiac rhythm monitoring.
| PRO | CONS | |
|---|---|---|
| NON INVASIVE | ||
| Hospital Telemetry | Accurate, also for asymptomatic events | Only hospitalized patients |
| Holter ECG | Easy to use, continuous recording, also for asymptomatic events | Short monitoring |
| Event Recorder | Longer monitoring Rhythm-symptoms correlation | Not for asymptomatic events Requires patient’s trigger |
| INVASIVE | ||
| Implantable Loop Recorder | Long periods of monitoring Remote monitoring Asymptomatic events | Expensive and invasive False negative and positive Does not offer therapy |
| Pacemakers and Defibrillators | Asymptomatic events Also therapeutic | Only if therapeutic indication Expensive and invasive Complications |
Relation Between Subclinical Device-Detected Atrial Tachyarrhythmias and Cryptogenic Stroke
Cryptogenic strokes account for about 20% of all ischemic strokes.[2] Patients with cryptogenic strokes are usually treated with antiplatelet therapy, but they have an high recurrence rate of cerebral ischemic events. Moreover, the etiology of a subsequent stroke episode can be different from the first: for example 10-15% of patients with a first atherothrombotic event suffer a recurrent cardioembolic stroke (mainly caused by AF).[8] This background underlies the importance of a comprehensive approach involving the screening for subclinical AF/ATs since they are a possible cause of “idiopathic” cerebral ischemic events.
Recent studies have observed the relationship between device-detected AF/ATs and stroke risk. The TRENDS[9] trial was a prospective, multicenter observational study that enrolled 2486 patients after CIED implantation (pacemakers or defibrillators with an implanted atrial lead), all aged >65 years and with >1 risk factor for stroke (mean CHADS2 was 2.2). Patients with and without prior AF were also included. Device-detected AF/AT was defined as any Atrial High Rate Episode (AHRE) >175 bpm lasting at least 20 seconds, further refined by device-specific algorithms. Subclinical AHREs were diagnosed in 45% of 1988 patients without a documented history of prior AF. A daily AF/AT burden >5.5 hours (defined “high burden”) appeared to double the thromboembolic risk in the following 30 days with an annualized thromboembolic event rate of 2.4%. The risk remained increased even after the adjustment for other risk factors. The rate of thromboembolic events observed in “high burden” AF/AT group of TRENDS was, anyway, far below from the 4-4.5% annual rate expected from AF patients with average CHADS2 score ≥2.[2,3,4] The annualized thromboembolic event rate was 1.1% for either subsets with “zero” or “low” AF/AT burden. However the difference in hazard ratio (HR) between “low” and “high” burden AHRE groups was not statistically different.
The ASSERT trial[10] was a prospective, multicenter, observational study designed to evaluate if subclinical episodes of AHREs can be associated with an increased risk of ischemic stroke, in patients without previous evidence of AF. 2580 patients with an implanted pacemaker (n=2451) or defibrillator (n=129), and an implanted atrial lead, were enrolled and monitored for 3 months to detect subclinical atrial tachyarrhythmias and for a mean of 2.5 years for the primary outcome of ischemic stroke or systemic embolism. There was a substantial incidence of subclinical (asymptomatic) AF/ATs. These arrhythmias were detected in 10.1% of patients within the first 3 months after implantation and at least once in 34.7% of the patients during a mean follow-up period of 2.5 years. The second major finding of the study was that subclinical ATs were independently associated with an increase, by a factor of 2.5, in the risk of ischemic stroke or systemic embolism and this risk was independent of other risk factors. The annualized thromboembolic event rate has been found equal to 2.1% in the subgroup with CHADS2 score >2 (similar to TRENDS). The risk of ischemic stroke or systemic embolism associated with ATs before 3 months was 13%, which is similar to the one associated with clinical atrial fibrillation reported by previous studies. The study also suggested that the risk of stroke was higher when episodes of subclinical ATs were of longer duration (AHRE >190 bpm lasting >6 minutes), but the study was underpowered for this analysis. Anyway the incremental stroke risk has been observed for longer and more numerous subclinical episodes. Subclinical AHREs have been reported 8 times more common than clinical (symptomatic) AF.
Data from TRENDS and ASSERT are also supported by several smaller prospective trials which evaluated the relationship between AHREs and embolic events in patients with PMs and ICDs. Capucci et al.[11] found that in 725 patients with dual-chamber PMs AHRE lasting>5 minutes did not significantly increase embolic risk, whereas episodes>24 hours did (odds ratio 3.1). Botto et al.[12] analyzed embolic risk by combining duration and burden of AHREs with CHADS2 score. 568 patients with a dual-chamber pacemaker were followed for the first year after implantation and stratified by using a combination of AHRE burden and CHADS2 score. Separate populations with different stroke risk emerged: in patients with CHADS2 score >1 and cumulative AHRE>24 hours and in those with CHADS2 score ≥2 and AHRE>5 minutes the annualized thromboembolic event rate was found as high as 5%.
Subclinical atrial tachyarrhythmias can also be detected by using implantable subcutaneous cardiac monitors (ICMs) or external loop recorders. The CRYSTAL AF study[13] was a prospective, multicenter, international, randomized study to determine the incidence of AF among patients randomized to ICM vs standard monitoring. Eligible patients (n=441) were older than 40 years and had a stroke within the last 90 days defined as cryptogenic after to have undergone 12-lead ECG, 24-hour ECG monitoring, transesophageal echocardiography (TEE), computed tomographic angiography or magnetic resonance angiography of the head and neck to rule out an arterial source, and screening for hypercoagulable states in patients younger than 55 years. Standard monitoring was left to the discretion of the attending physician and therefore represents daily practice. AF was detected at a rate of 8.9%, 12.4%, and 30% in the ICM arm and 1.4%, 2%, and 3% in the standard monitoring arm at 6, 12 and 36 months, respectively. At 12 months, the median time from randomization to AF detection in the ICM arm was 84 days, and 79% of these episodes were asymptomatic. More than 92% of patients in the ICM arm with AF detected at 12 months had a day with >6 minutes of AF, a threshold found in the ASSERT study to confer an increased risk of subsequent ischemic stroke. At 36 months, AF was detected at a rate of 30% among ICM patients compared to 3 % among control patients (HR 8.8, P<0001). Oral anticoagulant therapy (OAC) was prescribed for 96.6 % of ICM patients in whom AF was detected, by suggesting that physicians found the amount of AF detected clinically relevant. This study demonstrates that long-term continuous monitoring with an ICM is significantly more effective than standard arrhythmia monitoring for the identification of subclinical AF in patients who suffered a cryptogenic stroke.
Similar findings could be demonstrated by using an external loop recorder. The recent EMBRACE study[14] randomly assigned 572 patients (age ≥ 55 years) with cryptogenic stroke to 30-day event triggered external loop recorder vs conventional 24-hour Holter monitoring. Unlike CRYSTAL AF, TEE or intracranial vascular imaging was not required as part of the stroke workup. The primary end-point (detection of AF ≥ 30 seconds within 90 days) was met in 16.1% and 3.2% of patients in the event recorder and control arms, respectively. The secondary end-point (detection of AF ≥ 2.5 minutes within 90 days) was met in 9.9% and 2.5% of patients in the event recorder and control arms, respectively. OAC was prescribed in 18.6% of patients in the event recorder arm vs 11.1% of patients in the control arm, presumably because of the higher rates of AF detection. Compliance with the protocol in the intervention arm was reasonably high at 82% completing ≥3 weeks of monitoring, which may not be easily replicated in clinical practice. This study demonstrated that 30-day event-triggered recorder was significantly more effective than conventional 24-hour Holter monitoring for identification of AF in patients who suffered a cryptogenic stroke. Prolonged monitoring nearly doubled the proportion of patients who subsequently received anticoagulant therapy for secondary prevention of stroke. At 90-days follow up 87% of patients with AF episodes in the study group were receiving OAC. This finding is a clinically meaningful change in treatment that has the potential to avoid recurrent strokes. The common practice of relying on 24 to 48 hours monitoring for AF after either a stroke or a TIA of undetermined cause is insufficient and should be considered only as an initial screening.
Oral Anticoagulation for Device-Detected Atrial Tachyarrhythmias
The benefits of OAC in patients with AF/AT are clear, leading to a substantial reduction of not only stroke risk, but also of stroke severity and mortality.[8] Underused of OAC in AF patients is a well described phenomenon with multiple causes and multifaceted aspects in a general AF population. The increasing prevalence of patients with CIEDs in combination with AHREs and their associated increased risk of stroke/embolism pose new clinical challenges to clinicians.[9,10,11,12] At present the management of patients with device-detected AF/AT remains controversial, and uncertainties exist about the duration of the longest episode, the cumulative duration and the individual stroke risk.[8] To date the only prospective randomized trial to address this question was the IMPACT study,[15] which was stopped early and was unable to demonstrate that daily remote monitoring for ATs with a predefined plan for anticoagulation is superior to a conventional strategy for identification of patients deserving OAC.[16] The incidence of AF/AT detected by PMs or ICDs can reach 50% but only <25% of these patients are treated with OAC.[8] On the other side, and inexplicably, when AF is detected with an ICM or an external loop recorder (like in CRYSTAL and EMBRACE) many more patients are anticoagulated.[13,14] There are several potential explanations for this trend. First of all, although Guidelines recognize the role of cardiac devices in detecting AHREs (a surrogate for AF/AT), there is no specific recommendation regarding their use for diagnosis and management in these patients.[1,6] Few evidences exist about a critical threshold for duration/number of AHRE burden, even if many short episodes could result in the same AF burden as single long-lasting episode.[8] Moreover while the net clinical benefit of antithrombotic treatment is well established in patients with “clinical” atrial fibrillation, there may be a lower benefit in patients with device-detected AF/AT: in patients with CHADS2 score >2, the annualized thromboembolic event rate associated with subclinical AHREs was 2.4% in TRENDS and 2.1% in ASSERT,[9,10] far below from the 4-4.5% annual rate expected in “clinical” AF patients with similar risk profile. So patients with device-detected AHREs (although having a higher risk compared to patients without AHREs) appear to be at lower risk for stroke compared to a “general” AF population: as a result the net clinical benefit of OAC may be reduced.[17] Another issue to consider is the lack of a temporal relationship between subclinical AF and stroke in studies of patients with CIEDs: in ASSERT study 73% of patients with thromboembolism did not show a temporal relationship between AHRE and embolic events.[8,10] Given the lack of temporal association between device-detected ATs and stroke, a clinician could consider AF only as a marker of stroke risk rather than the proximate cause; so monitoring atrial activity with a CIED could promote a “wait-and-see” approach.
Even if the overall stroke rate in patients with AHREs appears to be less than that in clinically recognized AF, it is crucial to identify a certain high-risk population who deserve anticoagulation, provided that embolic risk exceeds the risk of serious bleeding. By combining AF/AT burden with CHADS2 or CHA2DS2-VASC score and HAS-BLED score we can individualize OAC for appropriate patients at high risk for stroke.[12] It has been suggested that with a CHADS2 or CHA2DS2-VASC score of 1-2 the anticoagulation could be appropriate if a single AHRE episode exceeds 24 hours; with a score >2 the anticoagulation could be started for AHRE lasting > 6 minutes (the higher is the score the shorter is the AHRE duration threshold to start OAC).[12,17]
CIEDS and ATs Detection: Potentials, Technical Issues, Pitfalls, Clinical Implications
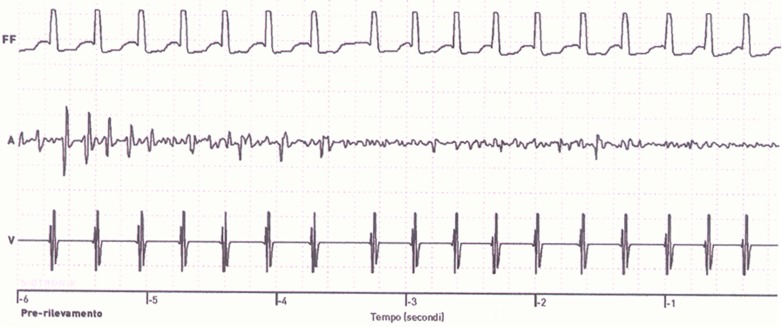
CIEDs are sensitive and specific for diagnosis of AF/ATs. The presence of an implanted atrial lead allows a continuous monitoring of atrial activity and the recording of the episodes in which the sensed atrial rate exceeds a predefined cutoff or deviates from a running average.[17] However, when a CIED is used for the detection of atrial arrhythmias all cardiac rhythm recordings must be adjudicated and reviewed by a qualified clinician to verify their diagnostic accuracy.[8] There are some factors that limit the diagnostic performance of a CIED: oversensing, undersensing, far-field sensing, cross talk, interference, inappropriate programmed detection criteria, differences between manufacturers in diagnostic algorithms for AHRE detection.[18,19] Moreover the ATs detection rate and the duration of the post-ventricular atrial blanking interval can influence the number of automatic mode-switching episodes.[8] The storing of electrograms (EGM) in the device memory improved the diagnostic accuracy by allowing to detect and document appropriate versus inappropriate sensing or detections. Several studies showed that the data retrieved from diagnostic counters may sometimes be misleading and, although AHRE are used as a surrogate for AF, the data must be interpreted with caution.[8,18,19] For example in the ASSERT study 17% of AHREs (>190 bpm lasting >6 minutes) were found to be false positives because of atrial oversensing, runs of premature atrial complexes, far-field R wave detection, repetitive non-re-entrant ventriculoatrial synchrony (RNRVAS). RNRVAS, in particular, was the single most common cause of false positive detection in the ASSERT study; it is triggered by a retrograde ventriculoatrial conduction with functional atrial undersensing and results from retrograde atrial activation during the post-ventricular atrial refractory period and functional atrial non-capture due to stimulation during the absolute refractory period, with the potential to trigger inappropriate mode switching ([Fig. 1]).[8] On the other hand false AF negatives have been observed when episodes were very brief or atrial sensing was unreliable.[8,17,18,19]
Figure 1. Episode of inappropriate mode switch due to repetitive nonreentrant ventriculoatrial synchrony (RNRVAS). See text for detailed description of the phenomenon.
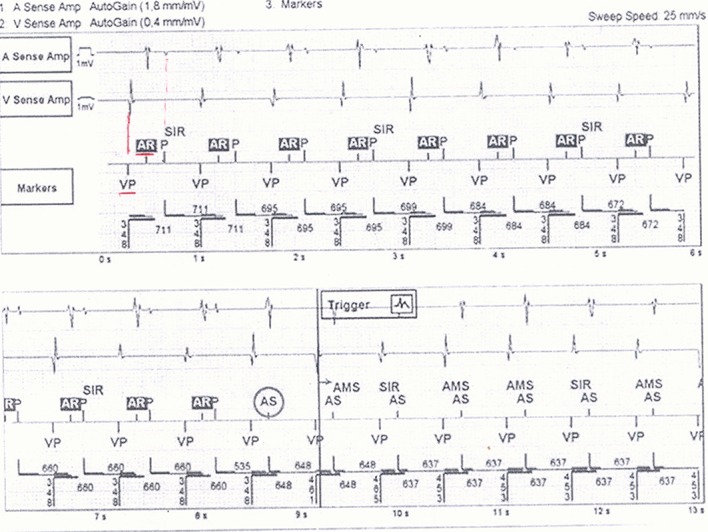
The need to insert an atrial electrode to detect ATs carries in itself a significant associated risk for complications and higher costs[20] which could be solved by using an atrial floating VDD lead if there is no need of atrial pacing. However, an acknowledged issue with VDD system is the dissatisfaction and the instability of the atrial sensing amplitude over time.[21] This may easily cause underestimation of atrial arrhythmic burden and the loss of synchronized ventricular pacing. Recently a single-lead ICD with atrial sensor (DX ICD Biotronik SE and Co. KG, Berlin, Germany), has been developed in order to maintain the crucial atrial information without implanting an atrial lead. The DX ICD system relies on an atrial floating dipole with enhanced sensing capabilities and has demonstrated to provide reliable atrial sensing in the medium to long term ([Fig. 2]).[22]
Figure 2. Example of atrial fibrillation detected in a single-lead defibrillator with atrial floating sensing dipole.
Regarding ICMs, three new devices with dedicated AF algorithms are on the market (Medtronic Reveal XT, model 9529, Medtronic Inc., Minneapolis, Minnesota; SJM Confirm ICM model DM2102, St Jude Medical Inc., Sunnyvale California and Biotronik BioMonitor, Biotronik SE and Co. KG, Berlin). The Medtronic Reveal XT reported to have an overall accuracy of 98.5% in AF detection.[23] These monitors usually detect AF by analyzing the irregularity and the incoherence of successive R-R intervals with high sensitivity and good specificity.[24] However also ICM are affected by to false AF detection due to oversensing or missed AF due to undersensing: so clinical evaluation of recorded episode is always fundamental.
Early Detection Of Atrial Arrhythmias With Daily Remote Monitoring
It is now clear that diagnostics of last-generation devices allow us to have a detailed and complete monitoring of the atrial arrhythmic episodes. These data become meaningful if they are early available for the physician to prevent arrhythmia-related severe adverse events.[25] Without remote monitoring any information is available only during in-hospital follow-up, usually scheduled every 6 or 12 months. This represents a great limitation, mainly for asymptomatic patients and for those with mild symptoms. The main potential advantage of daily remote control application in AF management is represented by early detection and early reaction to the arrhythmia occurrence.[26] A pilot Italian single-centre study involving 166 patients (73% pacemakers; Biotronik Home Monitoring [HM] system) demonstrated that 20% of patients had alerts for AF. The median reaction time to AF was reduced of 148 days compared with scheduled follow-up.[27] The HM-guided unscheduled follow-ups led to clinically significant reactions to AF such as antiarrhythmic drug therapy introduction or modification (48%), anticoagulation starting (45%), or external cardioversion (21%).
In the TRUST trial, (ICDs; Biotronik HM system), AF detection was 34.5 days earlier with remote monitoring vs standard follow-up (5.5 vs 40 days).[28]
In the CONNECT trial (ICDs; Medtronic CareLink system) the interval between an AF event longer than 12 h and the clinical reaction was eight times shorter with remote monitoring when compared with standard follow-up (3 vs 24 days).[29]
In the COMPAS trial (PMs; Biotronik HM system), although the study was not powered to make these comparisons, significant differences were observed between the two study groups (remote monitoring vs standard in-hospital follow-up) in the rates of hospitalizations for the management of atrial arrhythmias and strokes.[30] Several follow-ups prompted by remote monitoring, which enabled the early detection and management of atrial arrhythmias in the active group, may have prevented the development of more serious adverse events.
The potential benefit of remote continuous monitoring on 2-year incidence of stroke was modeled by running repeated Monte Carlo simulations based on a real population of 166 patients prospectively followed daily.[31] The results suggested that daily monitoring may reduce the 2-year stroke risk by 9 to 18% with an absolute reduction of 0.2 to 0.6%, compared with conventional inter-visit intervals of 6–12 months. Although this result was derived from a clinical experience performed using a particular paradigm for remote control (Biotronik HM system), it may apply to any remote monitoring system, provided that this is based on wireless automatic daily transmissions with immediate (within 24 hours) notification of AF episodes.
However, the clinical evidence for stroke risk reduction by remote monitoring is still awaited. As outlined before, the prospective randomized IMPACT trial[15] was stopped early. The study hypothesis was that daily remote monitoring for ATs with a predefined plan for anticoagulation would have been proved superior to a conventional strategy for the identification of patients deserving OAC. 2718 patients, with a dual-chamber or biventricular ICD, were enrolled and randomized 1:1 to either office visits or remote monitoring for AHRE detection (>200 bpm for 36 of 48 beats). When ATs were detected an anticoagulation prespecified protocol was started in the intervention group on the basis of CHADS2 score. Discontinuation of OAC was contemplated for patients without ATs recurrences over time and with low CHADS2 scores. Previous stroke, transient ischemic attack, systemic embolism and clinically documented atrial arrhythmias were Exclusion criteria. No significant differences existed in baseline demographics between the 2 groups. The incidence of ATs was similar for the 2 groups (33% control, 36% interventional group). The adjudication of device-based atrial EGM verified 60.5% of events as AF, 30% as atrial flutter, 9.5% as false positive episodes (with no significant differences between the 2 groups). After 5 years follow-up the Data Monitoring Committee recommended the trial termination, because of the failure to demonstrate any significant differences in the outcome between the two groups. No statistically significant difference was found in the primary outcome, which was a composite of ischemic stroke, systemic embolism, major bleeding and all-cause mortality. However, in the interventional group the OAC was started earlier (3 vs 54 days; p<0.001) by indicating that remote monitoring facilitated earlier reactions for ATs.[16] No clear explanations for these results are available; the compliance with the OAC in the interventional group was suboptimal, but the overall primary event rate was low nevertheless. Moreover, like in the previous studies[8,10] no temporal relationship was found between device-detected ATs and thromboembolic events. The investigators concluded that the beginning and the discontinuation of the anticoagulation therapy based on the presence of device-detected ATs available from the home monitoring did not improve clinical outcome in this specific population.[8]
The first experiences of AF home monitoring with ICMs have been carried out showing promising results.[32,33] A single center pilot study involving 186 patients suffering of AF and implanted with ICM equipped with daily remote monitoring (Biotronik HM system) demonstrated that 26% of the patients had a clinical interventions triggered by remote transmissions with a mean follow up of 6 months. All the clinical interventions were performed within 24 hours after the remote alert. The main frequent reaction was a therapy change.[33]
The Organization Model for Remote Monitoring of AF Patients
The HomeGuide Registry (Biotronik HM system) is a large registry which investigated the impact of remote monitoring of CIEDs on the patient management in daily practice.[34] The main result of the study showed that, by applying a structured organizational model, remote monitoring of CIEDs may be effectively introduced in standard clinical practice combining high effectiveness in clinical and device-related cardiovascular events detection, with a very low manpower and resource consumption. This is crucial for AF management, where early reaction from the remote notification is fundamental. The HomeGuide model is essentially based on a cooperative interaction between the roles of an expert reference nurse and a responsible physician, with an agreed list of respective tasks and responsibilities. Home Monitoring transmissions were reviewed by the nurse within two working days. In the case of critical alerts, such as AF episode detected, the responsible physician was contacted for the clinical decision.
The HomeGuide Registry showed that the applied organizational model may lead to an overall manpower for remote follow-ups which is less than one hour/month every 100 patients.[35] Such result was obtained in centers with different activity volumes recruited in different regions of Italy, by underlining the success of the applied workflow model and the used technology.
Conclusions
Patients with CIEDs represent a special population with multiple comorbidities predisposing to atrial arrhythmias, especially AF, which are often paroxysmal, intermittent and asymptomatic. “Subclinical” atrial tachyarrythmias are associated with a significant increase in the risk of stroke and systemic embolism and may be unmasked only with more aggressive monitoring techniques. Patients with dual-chamber pacemakers and implantable cardioverter defibrillators, as far as with implantable cardiac monitor, represent a unique opportunity to screen for and unmask silent AF episodes. Until further studies will be carried out, anticoagulation therapy should be individualized according to stroke risk scores in combination with the burden of AF/AT detected by the device. At the meantime, the recently designed ARTESIA study will evaluate if the treatment with Apixaban, compared to aspirin, could reduce the risk of ischemic stroke and systemic thromboembolism in pacemaker patients with subclinical AF ad additional risk factors for stroke.[35]
All these data become meaningful if they are early available and today this is possible thanks to the daily remote monitoring of the devices. The challenges for clinicians are to deal with the huge data entry, to define new organizational models, to improve device patient management and to continuously update AF guidelines according to the great amount of data offered by new technologies. Future AF guidelines should consider this peculiar scenario and, hopefully, make more specific recommendations, in particular regarding anticoagulation therapy.
Disclosures
None.
References
- 1.January Craig T, Wann L Samuel, Alpert Joseph S, Calkins Hugh, Cigarroa Joaquin E, Cleveland Joseph C, Conti Jamie B, Ellinor Patrick T, Ezekowitz Michael D, Field Michael E, Murray Katherine T, Sacco Ralph L, Stevenson William G, Tchou Patrick J, Tracy Cynthia M, Yancy Clyde W. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2014 Dec 2;64 (21):e1–76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 2.Chugh S S, Blackshear J L, Shen W K, Hammill S C, Gersh B J. Epidemiology and natural history of atrial fibrillation: clinical implications. J. Am. Coll. Cardiol. 2001 Feb;37 (2):371–8. doi: 10.1016/s0735-1097(00)01107-4. [DOI] [PubMed] [Google Scholar]
- 3.Independent predictors of stroke in patients with atrial fibrillation: a systematic review. Neurology. 2007 Aug 7;69 (6):546–54. doi: 10.1212/01.wnl.0000267275.68538.8d. [DOI] [PubMed] [Google Scholar]
- 4.Jauch Edward C, Saver Jeffrey L, Adams Harold P, Bruno Askiel, Connors J J Buddy, Demaerschalk Bart M, Khatri Pooja, McMullan Paul W, Qureshi Adnan I, Rosenfield Kenneth, Scott Phillip A, Summers Debbie R, Wang David Z, Wintermark Max, Yonas Howard. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013 Mar;44 (3):870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 5.Hart Robert G, Pearce Lesly A, Aguilar Maria I. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann. Intern. Med. 2007 Jun 19;146 (12):857–67. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 6.Camm A John, Lip Gregory Y H, De Caterina Raffaele, Savelieva Irene, Atar Dan, Hohnloser Stefan H, Hindricks Gerhard, Kirchhof Paulus. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation--developed with the special contribution of the European Heart Rhythm Association. Europace. 2012 Oct;14 (10):1385–413. doi: 10.1093/europace/eus305. [DOI] [PubMed] [Google Scholar]
- 7.Flaker Greg C, Belew Kathy, Beckman Karen, Vidaillet Humberto, Kron Jack, Safford Robert, Mickel Mary, Barrell Patrick. Asymptomatic atrial fibrillation: demographic features and prognostic information from the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study. Am. Heart J. 2005 Apr;149 (4):657–63. doi: 10.1016/j.ahj.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 8.Chen-Scarabelli Carol, Scarabelli Tiziano M, Ellenbogen Kenneth A, Halperin Jonathan L. Device-detected atrial fibrillation: what to do with asymptomatic patients? J. Am. Coll. Cardiol. 2015 Jan 27;65 (3):281–94. doi: 10.1016/j.jacc.2014.10.045. [DOI] [PubMed] [Google Scholar]
- 9.Glotzer Taya V, Daoud Emile G, Wyse D George, Singer Daniel E, Ezekowitz Michael D, Hilker Christopher, Miller Clayton, Qi Dongfeng, Ziegler Paul D. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ Arrhythm Electrophysiol. 2009 Oct;2 (5):474–80. doi: 10.1161/CIRCEP.109.849638. [DOI] [PubMed] [Google Scholar]
- 10.Healey Jeff S, Connolly Stuart J, Gold Michael R, Israel Carsten W, Van Gelder Isabelle C, Capucci Alessandro, Lau C P, Fain Eric, Yang Sean, Bailleul Christophe, Morillo Carlos A, Carlson Mark, Themeles Ellison, Kaufman Elizabeth S, Hohnloser Stefan H. Subclinical atrial fibrillation and the risk of stroke. N. Engl. J. Med. 2012 Jan 12;366 (2):120–9. doi: 10.1056/NEJMoa1105575. [DOI] [PubMed] [Google Scholar]
- 11.Capucci Alessandro, Santini Massimo, Padeletti Luigi, Gulizia Michele, Botto GianLuca, Boriani Giuseppe, Ricci Renato, Favale Stefano, Zolezzi Francesco, Di Belardino Natale, Molon Giulio, Drago Fabrizio, Villani Giovanni Q, Mazzini Elena, Vimercati Marco, Grammatico Andrea. Monitored atrial fibrillation duration predicts arterial embolic events in patients suffering from bradycardia and atrial fibrillation implanted with antitachycardia pacemakers. J. Am. Coll. Cardiol. 2005 Nov 15;46 (10):1913–20. doi: 10.1016/j.jacc.2005.07.044. [DOI] [PubMed] [Google Scholar]
- 12.Botto Giovanni L, Padeletti Luigi, Santini Massimo, Capucci Alessandro, Gulizia Michele, Zolezzi Francesco, Favale Stefano, Molon Giulio, Ricci Renato, Biffi Mauro, Russo Giovanni, Vimercati Marco, Corbucci Giorgio, Boriani Giuseppe. Presence and duration of atrial fibrillation detected by continuous monitoring: crucial implications for the risk of thromboembolic events. J. Cardiovasc. Electrophysiol. 2009 Mar;20 (3):241–8. doi: 10.1111/j.1540-8167.2008.01320.x. [DOI] [PubMed] [Google Scholar]
- 13.Sanna Tommaso, Diener Hans-Christoph, Passman Rod S, Di Lazzaro Vincenzo, Bernstein Richard A, Morillo Carlos A, Rymer Marilyn Mollman, Thijs Vincent, Rogers Tyson, Beckers Frank, Lindborg Kate, Brachmann Johannes. Cryptogenic stroke and underlying atrial fibrillation. N. Engl. J. Med. 2014 Jun 26;370 (26):2478–86. doi: 10.1056/NEJMoa1313600. [DOI] [PubMed] [Google Scholar]
- 14.Gladstone David J, Spring Melanie, Dorian Paul, Panzov Val, Thorpe Kevin E, Hall Judith, Vaid Haris, O'Donnell Martin, Laupacis Andreas, Côté Robert, Sharma Mukul, Blakely John A, Shuaib Ashfaq, Hachinski Vladimir, Coutts Shelagh B, Sahlas Demetrios J, Teal Phil, Yip Samuel, Spence J David, Buck Brian, Verreault Steve, Casaubon Leanne K, Penn Andrew, Selchen Daniel, Jin Albert, Howse David, Mehdiratta Manu, Boyle Karl, Aviv Richard, Kapral Moira K, Mamdani Muhammad. Atrial fibrillation in patients with cryptogenic stroke. N. Engl. J. Med. 2014 Jun 26;370 (26):2467–77. doi: 10.1056/NEJMoa1311376. [DOI] [PubMed] [Google Scholar]
- 15.Ip John, Waldo Albert L, Lip Gregory Y H, Rothwell Peter M, Martin David T, Bersohn Malcolm M, Choucair Wassim K, Akar Joseph G, Wathen Mark S, Rohani Pooyan, Halperin Jonathan L. Multicenter randomized study of anticoagulation guided by remote rhythm monitoring in patients with implantable cardioverter-defibrillator and CRT-D devices: Rationale, design, and clinical characteristics of the initially enrolled cohort The IMPACT study. Am. Heart J. 2009 Sep;158 (3):364–370.e1. doi: 10.1016/j.ahj.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Martin David T, Bersohn Malcolm M, Waldo Albert L, Wathen Mark S, Choucair Wassim K, Lip Gregory Y H, Ip John, Holcomb Richard, Akar Joseph G, Halperin Jonathan L. Randomized trial of atrial arrhythmia monitoring to guide anticoagulation in patients with implanted defibrillator and cardiac resynchronization devices. Eur. Heart J. 2015 Jul 7;36 (26):1660–8. doi: 10.1093/eurheartj/ehv115. [DOI] [PubMed] [Google Scholar]
- 17.DeCicco Anthony E, Finkel Jonathan B, Greenspon Arnold J, Frisch Daniel R. Clinical significance of atrial fibrillation detected by cardiac implantable electronic devices. Heart Rhythm. 2014 Apr;11 (4):719–24. doi: 10.1016/j.hrthm.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Benezet-Mazuecos Juan, Rubio José Manuel, Farré Jerónimo. Atrial high rate episodes in patients with dual-chamber cardiac implantable electronic devices: unmasking silent atrial fibrillation. Pacing Clin Electrophysiol. 2014 Aug;37 (8):1080–6. doi: 10.1111/pace.12428. [DOI] [PubMed] [Google Scholar]
- 19.Pollak W M, Simmons J D, Interian A, Atapattu S A, Castellanos A, Myerburg R J, Mitrani R D. Clinical utility of intraatrial pacemaker stored electrograms to diagnose atrial fibrillation and flutter. Pacing Clin Electrophysiol. 2001 Apr;24 (4 Pt 1):424–9. doi: 10.1046/j.1460-9592.2001.00424.x. [DOI] [PubMed] [Google Scholar]
- 20.Dewland Thomas A, Pellegrini Cara N, Wang Yongfei, Marcus Gregory M, Keung Edmund, Varosy Paul D. Dual-chamber implantable cardioverter-defibrillator selection is associated with increased complication rates and mortality among patients enrolled in the NCDR implantable cardioverter-defibrillator registry. J. Am. Coll. Cardiol. 2011 Aug 30;58 (10):1007–13. doi: 10.1016/j.jacc.2011.04.039. [DOI] [PubMed] [Google Scholar]
- 21.Sun Z H, Stjernvall J, Laine P, Toivonen L. Extensive variation in the signal amplitude of the atrial floating VDD pacing electrode. Pacing Clin Electrophysiol. 1998 Sep;21 (9):1760–5. doi: 10.1111/j.1540-8159.1998.tb00276.x. [DOI] [PubMed] [Google Scholar]
- 22.Iori Matteo, Giacopelli Daniele, Quartieri Fabio, Bottoni Nicola, Manari Antonio. Implantable cardioverter defibrillator system with floating atrial sensing dipole: a single-center experience. Pacing Clin Electrophysiol. 2014 Oct;37 (10):1265–73. doi: 10.1111/pace.12421. [DOI] [PubMed] [Google Scholar]
- 23.Hindricks Gerhard, Pokushalov Evgueny, Urban Lubos, Taborsky Milos, Kuck Karl-Heinz, Lebedev Dmitry, Rieger Guido, Pürerfellner Helmut. Performance of a new leadless implantable cardiac monitor in detecting and quantifying atrial fibrillation: Results of the XPECT trial. Circ Arrhythm Electrophysiol. 2010 Apr;3 (2):141–7. doi: 10.1161/CIRCEP.109.877852. [DOI] [PubMed] [Google Scholar]
- 24.Eitel Charlotte, Husser Daniela, Hindricks Gerhard, Frühauf Manuela, Hilbert Sebastian, Arya Arash, Gaspar Thomas, Wetzel Ulrike, Bollmann Andreas, Piorkowski Christopher. Performance of an implantable automatic atrial fibrillation detection device: impact of software adjustments and relevance of manual episode analysis. Europace. 2011 Apr;13 (4):480–5. doi: 10.1093/europace/euq511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varma Niraj, Stambler Bruce, Chun Sung. Detection of atrial fibrillation by implanted devices with wireless data transmission capability. Pacing Clin Electrophysiol. 2005 Jan;28 Suppl 1 ():S133–6. doi: 10.1111/j.1540-8159.2005.00083.x. [DOI] [PubMed] [Google Scholar]
- 26.Ricci Renato Pietro. Disease management: atrial fibrillation and home monitoring. Europace. 2013 Jun;15 Suppl 1 ():i35–i39. doi: 10.1093/europace/eut114. [DOI] [PubMed] [Google Scholar]
- 27.Ricci Renato Pietro, Morichelli Loredana, Santini Massimo. Remote control of implanted devices through Home Monitoring technology improves detection and clinical management of atrial fibrillation. Europace. 2009 Jan;11 (1):54–61. doi: 10.1093/europace/eun303. [DOI] [PubMed] [Google Scholar]
- 28.Varma Niraj, Epstein Andrew E, Irimpen Anand, Schweikert Robert, Love Charles. Efficacy and safety of automatic remote monitoring for implantable cardioverter-defibrillator follow-up: the Lumos-T Safely Reduces Routine Office Device Follow-up (TRUST) trial. Circulation. 2010 Jul 27;122 (4):325–32. doi: 10.1161/CIRCULATIONAHA.110.937409. [DOI] [PubMed] [Google Scholar]
- 29.Crossley George H, Boyle Andrew, Vitense Holly, Chang Yanping, Mead R Hardwin. The CONNECT (Clinical Evaluation of Remote Notification to Reduce Time to Clinical Decision) trial: the value of wireless remote monitoring with automatic clinician alerts. J. Am. Coll. Cardiol. 2011 Mar 8;57 (10):1181–9. doi: 10.1016/j.jacc.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 30.Mabo Philippe, Victor Frédéric, Bazin Patrick, Ahres Saïd, Babuty Dominique, Da Costa Antoine, Binet Didier, Daubert Jean-Claude. A randomized trial of long-term remote monitoring of pacemaker recipients (the COMPAS trial). Eur. Heart J. 2012 May;33 (9):1105–11. doi: 10.1093/eurheartj/ehr419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ricci Renato P, Morichelli Loredana, Gargaro Alessio, Laudadio Maria T, Santini Massimo. Home monitoring in patients with implantable cardiac devices: is there a potential reduction of stroke risk? Results from a computer model tested through monte carlo simulations. J. Cardiovasc. Electrophysiol. 2009 Nov;20 (11):1244–51. doi: 10.1111/j.1540-8167.2009.01543.x. [DOI] [PubMed] [Google Scholar]
- 32.Arrocha Alberto, Klein George J, Benditt David G, Sutton Richard, Krahn Andrew D. Remote electrocardiographic monitoring with a wireless implantable loop recorder: minimizing the data review burden. Pacing Clin Electrophysiol. 2010 Nov;33 (11):1347–52. doi: 10.1111/j.1540-8159.2010.02857.x. [DOI] [PubMed] [Google Scholar]
- 33.Petretta A, Giacopelli D, Pappone A, Grassini D, Cuko A, Saviano M, Vitale R, Ionescu B, Giannelli L, Ciaccio C, Baldi M, Vicedomini G, Pappone C. Incidence and timing of clinical interventions triggered by implantable leadless device remote transmissions. J Interv Card Electrophysiol. 2014;39(Suppl 1):S93–0. [Google Scholar]
- 34.Ricci Renato Pietro, Morichelli Loredana, D'Onofrio Antonio, Calò Leonardo, Vaccari Diego, Zanotto Gabriele, Curnis Antonio, Buja Gianfranco, Rovai Nicola, Gargaro Alessio. Effectiveness of remote monitoring of CIEDs in detection and treatment of clinical and device-related cardiovascular events in daily practice: the HomeGuide Registry. Europace. 2013 Jul;15 (7):970–7. doi: 10.1093/europace/eus440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ricci Renato Pietro, Morichelli Loredana, D'Onofrio Antonio, Calò Leonardo, Vaccari Diego, Zanotto Gabriele, Curnis Antonio, Buja Gianfranco, Rovai Nicola, Gargaro Alessio. Manpower and outpatient clinic workload for remote monitoring of patients with cardiac implantable electronic devices: data from the HomeGuide Registry. J. Cardiovasc. Electrophysiol. 2014 Nov;25 (11):1216–23. doi: 10.1111/jce.12482. [DOI] [PubMed] [Google Scholar]
- 36.A Comparison of Apixaban Versus Aspirin for Preventing Stroke in Patients with Pacemakers (ARTESIA). ClinicalTrial.gov. Identifier: NCT01938248. . Available at http://clinicaltrial.gov/ct2/show/ NCT01938248?=Artesia&rank=1 [Google Scholar]