Abstract
Sarcoidosis is a systemic granulomatous disease that affects the myocardium. Although ventricular arrhythmias are well known manifestations of cardiac involvement, there is increasing evidence that a significant proportion of patients with cardiac sarcoidosis (CS) also have atrial arrhythmias, atrial fibrillation being the most frequent. The incidence and mechanism of atrial fibrillation in CS is not precisely known. The management of atrial fibrillation in patients with CS is currently done according to the general guidelines for management of atrial fibrillation. Evidence is emerging regarding the additional role of immunosuppression for the treatment of atrial arrhythmias in CS. This paper reviews the incidence, possible mechanisms and treatment strategies of atrial fibrillation in patients with CS.
Keywords: Atrial Fibrillation, Outcomes, Arrhythmia Control, Cardiac Sarcoidosis, Sarcoidosis
Incidence and Diagnosis
Sarcoidosis is a granulomatous disease that affects predominantly the lungs of young adults aged 25-45 years and to a lesser extent other organs, including the heart. The annual incidence of sarcoidosis in the United States has been estimated to be 10.9 per 100,000 in whites and 35.5 per 100,000 in African Americans.[1] The prevalence of CS varies significantly depending on the population studied and methodology used for detection. In a series of 40 patients with systemic sarcoidosis, 17 out of 31 asymptomatic patients (54.8%) had subclinical cardiac MRI abnormalities,[2] while in another series from the Netherlands, only 3 of 82 (3.7%) asymptomatic patients with pulmonary sarcoidosis had CS.[3]
Therefore the screening and diagnosis of CS represent a challenge for the clinician. A definite diagnosis of CS is made through the presence of non-caseating granulomas on histological examination of myocardial tissue with no alternative cause (negative organismal stains). A myocardial biopsy is not often performed. For most cases the diagnosis of CS is “probable” using the expert consensus criteria which consist of the pesence of a histological diagnosis of extracardiac sarcoidosis in addition to one or more of the following: steroid +/- immunosuppressant-responsive cardiomyopathy or heart block, unexplained reduced LVEF (<40%), unexplained sustained (spontaneous or induced) VT, Mobitz type II 2nd degree or 3rd degree heart block, or a pattern consistent with cardiac sarcoidosis on cardiac PET, cardiovascular magnetic resonance (CMR) or gallium uptake study.[4] The three major manifestations of CS are
conduction abnormalities
ventricular arrhythmias
heart failure, yet supraventricular arrhythmias are frequently seen in patients with CS.
The exact incidence of atrial fibrillation in CS is difficult to know, as a proportion is undetected due to lack of symptoms. Cain et al., in a recent study, examined 192 consecutive patients who underwent CMR. Atrial arrhythmias were documented more frequently than ventricular arrhythmias in patients with sarcoidosis with cardiac involvement and were 3 times more prevalent than in patients with sarcoidosis without cardiac involvement.[5] In a series of 100 patients with confirmed CS reported by Viles-Gonzalez et al., the prevalence of supraventricular arrhythmias was 32%.[6] Atrial fibrillation was the most common arrhythmia (18%), followed by atrial tachycardia (7%), atrial flutter (5%), and other supraventricular tachycardias (2%).[6] In study reported by Betensky at al., of 45 patients with CS and an implantable cardioverter-defibrillator (ICD), 6 patients (13.3%) received inappropriate ICD therapies mostly representing atrial fibrillation.[7] However, it is likely that not all episodes of atrial fibrillation led to defibrillator shocks, so the true prevalence may be higher.
Mechanism
The cause of atrial fibrillation in patients with CS is likely due to granulomatous involvement of the atrium leading to inflammation and scarring, and raised end-diastolic pressures from sarcoid involvement of the lung and left ventricle. In a landmark study examining the clinical and autopsy findings of 113 hearts with CS, granulomas were detected in the left ventricle in 97% and in the atria in 22% of patients with symptomatic CS. Of note, 15 out of 89 patients (17%) in this study had atrial arrhythmias.[8]
While it is plausible that granulomas in the left atrium lead to scarring and the development of atrial fibrillation, there are other factors that contribute to the development of atrial arrhythmia. Left atrial enlargement (LAE) secondary to LV dysfunction is believed to be a contributing factor. In data from our center reported by Viles-Gonzalez et al, the incidence of supraventricular arrhythmias among patients with and without LAE was 267.8 and 38.3 per 1,000 person-years, respectively (RR, 6.99; 95% CI, 3.31-14.77).[6] Among the variables studied in a multivariate analysis, LAE was the only variable associated with atrial arrhythmias. Age, race, gender, systolic and diastolic ventricular dysfunction, presence of pulmonary sarcoidosis, right atrial enlargement, mitral valve disease, systemic and pulmonary hypertension or use of steroids/immunosuppressant were not significant. In a recent report using speckle tracking echocardiography, left and right atrial reservoir functions were significantly lower in CS patients compared to controls.[9] This report suggests that atrial mechanical function is impaired early in patients with CS before left ventricular dysfunction is seen on conventional echocardiography. The role of inflammation in the genesis of AF in these patients has been supported by a number of case series, in which glucocorticoids significiantly reduced patients’ arrhythmic burden.[10] Data from our laboratory has suggested that pulmonary vein triggers may also play a causal role:4 out of 5 (80%) CS patients with AF who underwent pulmonary vein isolation were free from recurrent AF at a median follow up of 18 months.[11]
Treatment
As discussed above CS is thought to cause atrial arrhythmias through two primary mechanisms:
sarcoid infiltration of the lungs and ventricles, leading to elevated end-diastolic pressure and atrial remodeling, and
direct granulomatous infiltration of the atria themselves, resulting in inflammation and fibrosis.[12,13]
Treatment of elevated filling pressures centers on the use of cardiac-specific drugs for the management of systolic and diastolic dysfunction, and anti-arrhythmic drugs for control of symptomatic arrhythmia. While no single anti-arrhythmic drug strategy is favored, it is recommended that class IC agents generally be avoided due to the high incidence of myocardial scar in this population.[4] Management of myocardial sarcoid infiltration has largely employed the use of immunosuppressive therapy, though such treatment has never been assessed in a clinical trial format. As a result, choice of immunosuppressive agent, dose and duration remains widely variable in the treatment of CS.
Immunosuppression
In the Delphi Study, which sought to establish an expert consensus on the diagnosis and treatment of CS, it was found that clinical management varies widely among physicians. More than three-quarters, however, agreed that immunomodulatory therapy should be instituted on the basis of ventricular dysfunction or arrhythmia, or a positive FDG-PET scan. The majority indicated that they would treat CS in the presence of conduction abnormalities or a positive MRI. Prednisone was widely the treatment of choice, though there was no particular agreement on strength or duration of therapy.[14]
Data on the use of corticosteroids for the treatment of CS has centered predominantly on the amelioration of ventricular arrhythmia, conduction block, and LV dysfunction; its use for treatment of atrial arrhythmia is largely extrapolated from such studies.[15-19] A systematic review by Sadek, et al., identified 10 retrospective studies examining the utility of steroids in CS.[20] All studies included had involved at least three patients with at minimum three months of follow up. Of those patients presenting with CS-related conduction disease, 47% showed improvement in conduction after initiation of steroids. None of the 16 patients with conduction disorders who did not received steroids improved.[15,17,19,21-23] Four studies have examined the effect of steroids on LV dysfunction, noting preservation or improvement in patients with normal or mild-to-moderate LV dysfunction, respectively.[15,17,19,24] Those subjects with severe LV dysfunction failed to derive any benefit, perhaps a reflection of the degree of disease progression. Data on the use of corticosteroids in ventricular arrhythmia has been similarly encouraging, though its use in both studies was confounded by the concomitant administration of anti-arrhythmic drugs.[19,25]
Dose and duration of corticosteroid therapy remains poorly established. Most studies have recommended initiation of prednisone at 1 mg/kg per day. However, retrospective review of CS patients managed on high dose (>30 mg/day) versus low dose (<30 mg/day) prednisone showed no difference in outcome, raising the possibility that CS flares can be effectively managed on lower doses of corticosteroids, thus mitigating the many side-effects of intensive steroid therapy.[18] Most experts at this time favor an early period of intensive prednisone therapy (approximately 0.5 mg/kg), followed by slow titration to a minimum suppressive dose (typically 5-10 mg/day).[14] This dose is maintained indefinitely, as there are several worrisome reports of sudden death following complete cessation of steroid therapy.[26]
Steroid-sparing immunosuppressants, including methotrexate, hydroxychloroquine, cyclophosphamide, and azathioprine, have been employed with varying degrees of success.[27,28] Though their use in pulmonary sarcoidosis has become well established, data regarding their efficacy in the treatment of CS remains scarce. They are typically employed when steroid therapy becomes limited by side effects, or as an adjunct to low-dose steroid therapy.
Studies directly examining the role of corticosteroids in CS-related atrial arrhythmia are limited to case reports and small series.[10,29-31] In each case, steroids either eliminated or significantly reduced the burden of atrial arrhythmia in patients refractory to anti-arrhythmic therapy and, in one case, catheter ablation. It may stand to reason that reduction of myocardial inflammation and improvement in left ventricular function would have a moderating role on arrhythmic burden in the atrium in much the same way it does in the ventricle, though this hypothesis requires further validation in larger studies.
Catheter Ablation
Research on the role of catheter ablation in the treatment of CS has focused primarily on ventricular tachycardia, in which the majority of arrhythmia is caused by macro-reentry around areas of granulomatous scar. Studies have consistently shown reasonably high procedural non-inducibility with somewhat more measured long-term success rates.[32-34]
While more limited, data regarding the treatment of atrial arrhythmia using catheter ablation has also shown some promise. Abnormal automaticity, triggered activity, and macro-reentry have all been described in non-AF atrial arrhythmia.[11,35] In our group’s published experience, 9 patients with CS underwent catheter ablation—2 for paroxysmal AF, 3 for persistent AF, 1 for cavotricuspid isthmus-dependent flutter, 2 for atypical flutters, and 1 for both CTI-dependent flutter and paroxysmal AF. Mean follow up was 1.8 ± 1.9 years. In the patients with paroxysmal AF, programmed stimulation with and without isoproterenol infusion failed to identify atrial triggers. Both underwent circumferential pulmonary vein isolation (PVI), and remained free from recurrence at follow up. In the patients with persistent AF, bipolar voltage mapping revealed a small area of low voltage in the septal area in one patient and diffuse, extensive left atrial scar in another ([Figure 1]). Both underwent PVI and complex fractionated atrial electrogram (CFAE) ablation. The remaining patient with persistent AF had minimal atrial scar and had PVI alone. One patient had subsequent recurrence and was started on anti-arrhythmic drug therapy with good response. Microreentrant circuits were seen originating from the septum and left atrial anterior wall in the patients with atypical flutters and were successfully ablated.[11]
Figure 1. Postero-anterior and anterior-posterior views of electro-anatomical voltage map of the left atrium (CARTO) in a 45 year old patient with paroxysmal atrial fibrillation prior to ablation. Left atrial appendage is not included. There is extensive atrial scar represented by areas of low voltage. Purple areas had voltage above 0.5 V rest of the left atrium had voltage <0.5V, red areas <0.05 V and thus represent atrial scar.
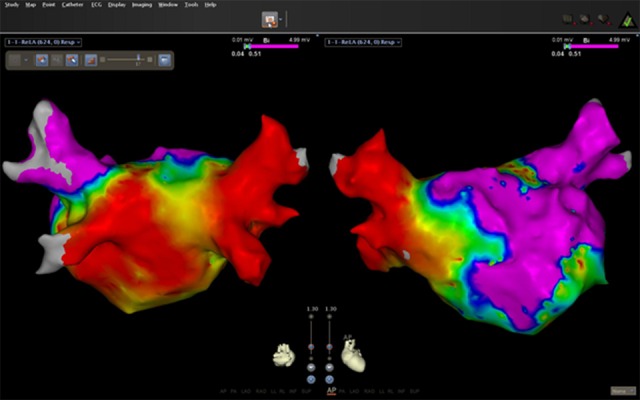
The success of PVI in those patients with AF suggests a causal role of the pulmonary veins despite the diffuse nature of CS. However, not all patients underwent electro-anatomical mapping to define the extent of left and right atrial scar; further study of atrial scar burden in these patients would help better define the nature of atrial arrhythmia in CS patients.
Anticoagulation
Patients with sarcoidosis may be at increased risk of venous thromboembolism, suggesting a hypercoagulable state.[36] It remains unknown whether CS patients with AF are at increased risk of LA thrombosis beyond traditional risk factors. Further, the efficacy of novel oral anti-coagulants (NOACs) in CS patients with AF has not been studied. At present the use of anti-coagulation, including NOACS, for CS patients with AF is suggested on the basis of the CHA2DS2-VASC score as is done for non-valvular AF, in keeping with current guidelines.[4]
Treatment Recommendations
Given the prevalence of atrial fibrillation in the general population, the diagnosis of CS cannot be made on the basis of the presence of atrial arrhythmias alone. Further, identifying the presence of inflammation or scar within the atria is limited using current imaging modalities, though progress in this area is ongoing.[37] The routine use of immunosuppression for the treatment of atrial arrhythmias as the sole manifestation of cardiac sarcoidosis is therefore not recommended. Rather, we recommend that a trial of immunosuppression may be considered in the presence of a confirmed or probable diagnosis of CS as defined by the 2014 HRS Consensus Statement.[4]
At this time, data on catheter ablation in CS remains too limited to recommend a distinct ablation strategy in these patients. It is encouraginPatients with sarcoidosis g, however, that routine PVI offered high rates of freedom from recurrent arrhythmia at medium-term follow up. It is thus our recommendation that catheter ablation employing PVI (with or without the addition of ancillary therapies) be offered to patients with symptomatic AF despite medical therapy.
Disclosures
None.
References
- 1.Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am. J. Respir. Crit. Care Med. 1999 Aug;160 (2):736–55. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 2.Vignaux Olivier, Dhote Robin, Duboc Denis, Blanche Philippe, Devaux Jean-Yves, Weber Simon, Legmann P. Detection of myocardial involvement in patients with sarcoidosis applying T2-weighted, contrast-enhanced, and cine magnetic resonance imaging: initial results of a prospective study. J Comput Assist Tomogr. 2002 Nov 20;26 (5):762–7. doi: 10.1097/00004728-200209000-00017. [DOI] [PubMed] [Google Scholar]
- 3.Smedema Jan-Peter, Snoep Gabriel, van Kroonenburgh Marinus P G, van Geuns Robert-Jan, Dassen Willem R M, Gorgels Anton P, Crijns Harry J G M. Cardiac involvement in patients with pulmonary sarcoidosis assessed at two university medical centers in the Netherlands. Chest. 2005 Jul;128 (1):30–5. doi: 10.1378/chest.128.1.30. [DOI] [PubMed] [Google Scholar]
- 4.Birnie David H, Sauer William H, Bogun Frank, Cooper Joshua M, Culver Daniel A, Duvernoy Claire S, Judson Marc A, Kron Jordana, Mehta Davendra, Cosedis Nielsen Jens, Patel Amit R, Ohe Tohru, Raatikainen Pekka, Soejima Kyoko. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014 Jul;11 (7):1305–23. doi: 10.1016/j.hrthm.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 5.Cain Matthew A, Metzl Mark D, Patel Amit R, Addetia Karima, Spencer Kirk T, Sweiss Nadera J, Beshai John F. Cardiac sarcoidosis detected by late gadolinium enhancement and prevalence of atrial arrhythmias. Am. J. Cardiol. 2014 May 1;113 (9):1556–60. doi: 10.1016/j.amjcard.2014.01.434. [DOI] [PubMed] [Google Scholar]
- 6.Viles-Gonzalez Juan F, Pastori Luciano, Fischer Avi, Wisnivesky Juan P, Goldman Martin G, Mehta Davendra. Supraventricular arrhythmias in patients with cardiac sarcoidosis prevalence, predictors, and clinical implications. Chest. 2013 Apr;143 (4):1085–90. doi: 10.1378/chest.11-3214. [DOI] [PubMed] [Google Scholar]
- 7.Betensky Brian P, Tschabrunn Cory M, Zado Erica S, Goldberg Lee R, Marchlinski Francis E, Garcia Fermin C, Cooper Joshua M. Long-term follow-up of patients with cardiac sarcoidosis and implantable cardioverter-defibrillators. Heart Rhythm. 2012 Jun;9 (6):884–91. doi: 10.1016/j.hrthm.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Fleming H A. Sarcoidosis of the heart. Am. J. Med. 1978 May;64 (5):915–6. doi: 10.1016/0002-9343(78)90550-8. [DOI] [PubMed] [Google Scholar]
- 9.Tigen Kursat, Sunbul Murat, Karaahmet Tansu, Tasar Onur, Dundar Cihan, Yalcinsoy Murat, Takir Mumtaz, Akkaya Esen. Early Detection of Bi-ventricular and Atrial Mechanical Dysfunction Using Two-Dimensional Speckle Tracking Echocardiography in Patients with Sarcoidosis. Lung. 2015 Oct;193 (5):669–75. doi: 10.1007/s00408-015-9748-0. [DOI] [PubMed] [Google Scholar]
- 10.Srivatsa Uma N, Rogers Jason. Sarcoidosis and atrial fibrillation: a rare association and interlink with inflammation. Indian Pacing Electrophysiol J. 2012 Nov;12 (6):290–1. doi: 10.1016/s0972-6292(16)30569-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willner Jonathan M, Viles-Gonzalez Juan F, Coffey James O, Morgenthau Adam S, Mehta Davendra. Catheter ablation of atrial arrhythmias in cardiac sarcoidosis. J. Cardiovasc. Electrophysiol. 2014 Sep;25 (9):958–63. doi: 10.1111/jce.12424. [DOI] [PubMed] [Google Scholar]
- 12.Roberts W C, McAllister H A, Ferrans V J. Sarcoidosis of the heart. A clinicopathologic study of 35 necropsy patients (group 1) and review of 78 previously described necropsy patients (group 11). Am. J. Med. 1977 Jul;63 (1):86–108. doi: 10.1016/0002-9343(77)90121-8. [DOI] [PubMed] [Google Scholar]
- 13.Tavora Fabio, Cresswell Nathaniel, Li Ling, Ripple Mary, Solomon Carol, Burke Allen. Comparison of necropsy findings in patients with sarcoidosis dying suddenly from cardiac sarcoidosis versus dying suddenly from other causes. Am. J. Cardiol. 2009 Aug 15;104 (4):571–7. doi: 10.1016/j.amjcard.2009.03.068. [DOI] [PubMed] [Google Scholar]
- 14.Hamzeh Nabeel Y, Wamboldt Frederick S, Weinberger Howard D. Management of cardiac sarcoidosis in the United States: a Delphi study. Chest. 2012 Jan;141 (1):154–62. doi: 10.1378/chest.11-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banba Kimikazu, Kusano Kengo Fukushima, Nakamura Kazufumi, Morita Hiroshi, Ogawa Aiko, Ohtsuka Fuyo, Ogo Keiko Ohta, Nishii Nobuhiro, Watanabe Atsuyuki, Nagase Satoshi, Sakuragi Satoru, Ohe Tohru. Relationship between arrhythmogenesis and disease activity in cardiac sarcoidosis. Heart Rhythm. 2007 Oct;4 (10):1292–9. doi: 10.1016/j.hrthm.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Chiu Chiung-Zuan, Nakatani Satoshi, Zhang Guican, Tachibana Teruo, Ohmori Fumio, Yamagishi Masakazu, Kitakaze Masafumi, Tomoike Hitonobu, Miyatake Kunio. Prevention of left ventricular remodeling by long-term corticosteroid therapy in patients with cardiac sarcoidosis. Am. J. Cardiol. 2005 Jan 1;95 (1):143–6. doi: 10.1016/j.amjcard.2004.08.083. [DOI] [PubMed] [Google Scholar]
- 17.Kato Yasuchika, Morimoto Shin-ichiro, Uemura Akihisa, Hiramitsu Shinya, Ito Teruo, Hishida Hitoshi. Efficacy of corticosteroids in sarcoidosis presenting with atrioventricular block. Sarcoidosis Vasc Diffuse Lung Dis. 2003 Jun;20 (2):133–7. [PubMed] [Google Scholar]
- 18.Yazaki Y, Isobe M, Hiroe M, Morimoto S, Hiramitsu S, Nakano T, Izumi T, Sekiguchi M. Prognostic determinants of long-term survival in Japanese patients with cardiac sarcoidosis treated with prednisone. Am. J. Cardiol. 2001 Nov 1;88 (9):1006–10. doi: 10.1016/s0002-9149(01)01978-6. [DOI] [PubMed] [Google Scholar]
- 19.Yodogawa Kenji, Seino Yoshihiko, Ohara Toshihiko, Takayama Hideo, Katoh Takao, Mizuno Kyoichi. Effect of corticosteroid therapy on ventricular arrhythmias in patients with cardiac sarcoidosis. Ann Noninvasive Electrocardiol. 2011 Apr;16 (2):140–7. doi: 10.1111/j.1542-474X.2011.00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sadek Mouhannad M, Yung Derek, Birnie David H, Beanlands Rob S, Nery Pablo B. Corticosteroid therapy for cardiac sarcoidosis: a systematic review. Can J Cardiol. 2013 Sep;29 (9):1034–41. doi: 10.1016/j.cjca.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Chapelon-Abric Catherine, de Zuttere Dominique, Duhaut Pierre, Veyssier Pierre, Wechsler Bertrand, Huong Du Le Thi, de Gennes Christian, Papo Thomas, Blétry Olivier, Godeau Pierre, Piette Jean-Charles. Cardiac sarcoidosis: a retrospective study of 41 cases. Medicine (Baltimore) 2004 Nov;83 (6):315–34. doi: 10.1097/01.md.0000145367.17934.75. [DOI] [PubMed] [Google Scholar]
- 22.Kandolin Riina, Lehtonen Jukka, Kupari Markku. Cardiac sarcoidosis and giant cell myocarditis as causes of atrioventricular block in young and middle-aged adults. Circ Arrhythm Electrophysiol. 2011 Jun;4 (3):303–9. doi: 10.1161/CIRCEP.110.959254. [DOI] [PubMed] [Google Scholar]
- 23.Okamoto H, Mizuno K, Ohtoshi E. Cutaneous sarcoidosis with cardiac involvement. Eur J Dermatol. 1999 Sep;9 (6):466–9. [PubMed] [Google Scholar]
- 24.Kudoh Hirokazu, Fujiwara Sei, Shiotani Hideyuki, Kawai Hiroya, Hirata Kenichi. Myocardial washout of 99mTc-tetrofosmin and response to steroid therapy in patients with cardiac sarcoidosis. Ann Nucl Med. 2010 Jun;24 (5):379–85. doi: 10.1007/s12149-010-0376-8. [DOI] [PubMed] [Google Scholar]
- 25.Futamatsu Hideki, Suzuki Jun-ichi, Adachi Susumu, Okada Hiroyuki, Otomo Kenichiro, Ohara Takahiro, Hashimoto Yuji, Kakuta Tsunekazu, Iesaka Yoshito, Yamaguchi Hiroaki, Sakurada Harumizu, Sato Akira, Obayashi Tohru, Niwa Akihiro, Hirao Kenzo, Isobe Mitsuaki. Utility of gallium-67 scintigraphy for evaluation of cardiac sarcoidosis with ventricular tachycardia. Int J Cardiovasc Imaging. 2006 Jun 10;22 (3-4):443–8. doi: 10.1007/s10554-005-9043-x. [DOI] [PubMed] [Google Scholar]
- 26.Sekiguchi M, Yazaki Y, Isobe M, Hiroe M. Cardiac sarcoidosis: diagnostic, prognostic, and therapeutic considerations. Cardiovasc Drugs Ther. 1996 Nov;10 (5):495–510. doi: 10.1007/BF00050989. [DOI] [PubMed] [Google Scholar]
- 27.Demeter S L. Myocardial sarcoidosis unresponsive to steroids. Treatment with cyclophosphamide. Chest. 1988 Jul;94 (1):202–3. doi: 10.1378/chest.94.1.202. [DOI] [PubMed] [Google Scholar]
- 28.Vorselaars Adriane D M, Cremers Johanna P, Grutters Jan C, Drent Marjolein. Cytotoxic agents in sarcoidosis: which one should we choose? Curr Opin Pulm Med. 2014 Sep;20 (5):479–87. doi: 10.1097/MCP.0000000000000078. [DOI] [PubMed] [Google Scholar]
- 29.Namboodiri Narayanan, Stiles Martin K, Young Glenn D, Sanders Prashanthan. Electrophysiological features of atrial flutter in cardiac sarcoidosis: a report of two cases. Indian Pacing Electrophysiol J. 2012 Nov;12 (6):284–9. doi: 10.1016/s0972-6292(16)30568-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Golwala Harsh, Dernaika Tarek. Atrial fibrillation as the initial clinical manifestation of cardiac sarcoidosis: a case report and review of the literature. J Cardiovasc Med (Hagerstown) 2015 Jan;16 Suppl 2 ():S104–12. doi: 10.2459/JCM.0b013e328343b589. [DOI] [PubMed] [Google Scholar]
- 31.Mohsen Amr. The anti-arrhythmic effects of prednisone in patients with sarcoidosis. Acta Cardiol. 2011 Dec;66 (6):803–5. doi: 10.1080/ac.66.6.2136967. [DOI] [PubMed] [Google Scholar]
- 32.Dechering Dirk G, Kochhäuser Simon, Wasmer Kristina, Zellerhoff Stephan, Pott Christian, Köbe Julia, Spieker Tilmann, Piers Sebastiaan R D, Bittner Alex, Mönnig Gerold, Breithardt Günter, Wichter Thomas, Zeppenfeld Katja, Eckardt Lars. Electrophysiological characteristics of ventricular tachyarrhythmias in cardiac sarcoidosis versus arrhythmogenic right ventricular cardiomyopathy. Heart Rhythm. 2013 Feb;10 (2):158–64. doi: 10.1016/j.hrthm.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 33.Jefic Dane, Joel Binjou, Good Eric, Morady Fred, Rosman Howard, Knight Bradley, Bogun Frank. Role of radiofrequency catheter ablation of ventricular tachycardia in cardiac sarcoidosis: report from a multicenter registry. Heart Rhythm. 2009 Feb;6 (2):189–95. doi: 10.1016/j.hrthm.2008.10.039. [DOI] [PubMed] [Google Scholar]
- 34.Koplan Bruce A, Soejima Kyoko, Baughman Kenneth, Epstein Laurence M, Stevenson William G. Refractory ventricular tachycardia secondary to cardiac sarcoid: electrophysiologic characteristics, mapping, and ablation. Heart Rhythm. 2006 Aug;3 (8):924–9. doi: 10.1016/j.hrthm.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 35.Zipes MM SJ, Katz JT. Atrial arrhythmias are common and arise from diverse mechanisms in patients with cardiac sarcoidosis. Heart rhythm: the official journal of the Heart Rhythm Society. 2013;10:0–0. [Google Scholar]
- 36.Swigris Jeffrey J, Olson Amy L, Huie Tristan J, Fernandez-Perez Evans R, Solomon Joshua J, Sprunger David, Brown Kevin K. Increased risk of pulmonary embolism among US decedents with sarcoidosis from 1988 to 2007. Chest. 2011 Nov;140 (5):1261–6. doi: 10.1378/chest.11-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldberger Jeffrey J, Arora Rishi, Green David, Greenland Philip, Lee Daniel C, Lloyd-Jones Donald M, Markl Michael, Ng Jason, Shah Sanjiv J. Evaluating the Atrial Myopathy Underlying Atrial Fibrillation: Identifying the Arrhythmogenic and Thrombogenic Substrate. Circulation. 2015 Jul 28;132 (4):278–91. doi: 10.1161/CIRCULATIONAHA.115.016795. [DOI] [PMC free article] [PubMed] [Google Scholar]


