Abstract
To overcome limitations of minimally invasive surgical ablation as a standalone procedure in eliminating atrial fibrillation (AF), hybrid approaches incorporating adjunctive endovascular catheter ablation have been proposed in recent years. The endovascular component targets residual conduction gaps and identifies additional electrophysiological targets with the goal of minimizing recurrent atrial arrhythmia. We performed a systematic review of published studies of hybrid AF ablation, analyzing 432 pooled patients (19% paroxysmal, 29% persistent, 52% long-standing persistent) treated using three different approaches: A. bilateral thoracoscopy with bipolar radiofrequency (RF) clamp-based approach; B. right thoracoscopic suction monopolar RF catheter-based approach; and C. subxiphoid posterior pericardioscopic (“convergent”) approach. Freedom from recurrence off antiarrhythmic medications at 12 months was seen in 88.1% [133/151] for A, 73.4% [47/64] for B, and 59.3% [80/135] for C, with no significant difference between paroxysmal (76.9%) and persistent/long-standing persistent AF (73.4%). Death and major surgical complications were reported in 8.5% with A, 0% with B and 8.6% with C. A critical appraisal of hybrid ablation is presented, drawing from experiences and insights published over the years on catheter ablation of AF, with a discussion of the rationale underlying hybrid ablation, its strengths and limitations, where it may have a unique role in clinical management of patients with AF, which questions remain unanswered and areas for further investigation.
Keywords: Atrial Fibrillation, Hybrid Ablation, Surgical Ablation, Rotors, Maze
Introduction
The traditional invasive cut-and-sew maze procedure, pioneered by Dr. Cox[1,2] and with subsequent revisions culminating in the Cox-maze III, has had a durably high success rate in treating atrial fibrillation (AF), with 80-90% of patients having maintained sinus rhythm without antiarrhythmic medications on long-term clinical follow-up.[3,4] Since the simplification of this procedure using surgical catheter ablation technologies as in the Cox-maze IV,[5] most maze procedures in the USA are facilitated with surgical catheter ablation.[6] Subsequently, minimally-invasive surgical approaches utilizing endoscopically delivered epicardial ablation were developed, parallel to developments in percutaneous catheter-based ablation strategies and technologies,[7-14] obviating the need for cardiopulmonary bypass and curtailing postoperative recovery, whilst maintaining a vantage for adjunctive interventions such as epicardial autonomic modulation and left atrial appendage (LAA) excision.[15-21] These developments were accompanied by increasing number of patients having standalone surgery for AF, constituting 8% of all surgical AF ablations registered in the Society of Thoracic Surgeons Adult Cardiac Surgery Database between 2005 and 2010.[22] However, whereas published outcomes from ablation-facilitated open surgical Cox-maze IV and the traditional cut-and-sew Cox-maze III have been comparable,[4,23-26] patients treated with minimally-invasive epicardial surgical ablation have fared less well, in particular those with non-paroxysmal AF.[27,28] On pooled analysis of published results, 75% of paroxysmal, 67% of persistent and only 43% of long-standing persistent AF patients are free of arrhythmia recurrence off antiarrhythmic medications.[27] A recent systematic review estimated a 10-20% higher rate of recurrent atrial arrhythmias after minimally-invasive surgery as compared to open ablation-based surgery, and although there are no adequately controlled trials or registry data comparing the two approaches directly, this likely underestimates the difference as most open surgical AF ablations in published series have been in patients with long-standing persistent AF (56%, with 8% paroxysmal) whilst most minimally-invasive epicardial ablations were in those with paroxysmal AF (59%, with 8% long-standing persistent).[28]
Although the mechanisms underlying this observed difference in outcomes are not fully ascertained, electrophysiological observations during minimally invasive epicardial ablations or at the time of catheter ablation of recurrent atrial arrhythmias following such procedures[29-33] suggest that these paradoxically inferior results often reflect the demonstrated limitations of current ablation tools in creating transmural lesions sets when applied endoscopically on the epicardium of the beating heart.[34-36] Up to 40% of patients undergoing minimally invasive AF surgery have been reported to develop recurrent atrial flutter, with 50% of isolated pulmonary veins having reconnected at time of repeat ablation,30 which is significantly higher than rates of 5 to 10% reported following endocardial surgical ablation,[37-39] and is responsive to further catheter ablation.[20] By slowing conduction yet failing to impart conduction block, such lesions are not only ineffective at preventing fibrillatory wave propagation[40] and AF from recurring[41-43] but also establish the tissue substrate required for reentrant atrial tachycardia.[44-54] With multiple, incomplete lines, reentrant atrial tachycardia circuits become complex and increasingly challenging to subsequently map and ablate.[29]
To overcome these limitations of minimally invasive surgical ablation as a standalone procedure in abolishing AF, hybrid ablation was developed, incorporating an adjunctive percutaneous catheter procedure to bridge conduction gaps in the anatomically-based surgical ablation lines as well as additional targets determined electrophysiologically.[55-59] This paired utility of surgical and catheter based approaches has been advocated as providing the combined advantage of both, whilst allowing each to overcome the limitation of the other,[55,57,59] Ensuring conduction block at the time of surgery significantly reduces recurrence rates; of 93 patients undergoing either open chest or minimally invasive surgical AF ablation, maintenance of AF off antiarrhythmic medications at 12 months was 87% with confirmation of conduction block vs 48% without.[32]
In this review, we critically appraise the published experience on hybrid ablation, placing it within the context of the experiences and insights attained over the years from catheter ablation of AF. In doing so, we provide a perspective on the rationale underlying hybrid ablation, its strengths and limitations, where it may have a unique role in clinical management of patients with AF, which questions remain unanswered and areas for further investigation.
Systematic Review of Published Literature on Hybrid Ablation of Atrial Fibrillation
Peer-reviewed publications reporting on 5 or more clinical cases undergoing adjunctive minimally invasive epicardial ablation and percutaneous endovascular catheter ablation were identified using Pubmed.gov (US National Library of Medicine, National Institute of Health) using the search term “atrial fibrillation AND (hybrid OR convergent) AND (ablation OR surgery)” (last updated 3/4/2015). This was supplemented by searches on Google Scholar and review of individual studies’ references. We identified 11 unique studies reporting on a total of 432 patients who have undergone standalone hybrid ablation at 11 centers (7 European, 3 North American, 1 Asian) and pooled available demographic, procedural and outcome data of 432 ([Tables 1-3]).[33,56,57,59-66] We included 2 publications where we felt that duplication of case ascertainment was minimal on account of a limited data overlap in recruitment of a patient subset[62,65] and excluded 5 publications with probably significant patient overlap selecting in preference those publications which were more recent with greater patient numbers.[55,67-70] A further 4 studies (231 patients) were not included as they were published in journals not indexed by the US National Library of Medicine.[71-74]
Table 1. Systematic Review of Hybrid Ablation Studies – Patient Demographics.
**Excludes 7 patients reported by Lee et al56 as results pooled with 18 patients undergoing non-hybrid minimally invasive AF surgery; $Additional 8 paroxysmal AF patients not included on account of excluding one study (8 paroxysmal AF and 28 long standing persistent AF)70 with significant overlap in reported patients with Bisleri et al.60; ¥University of Ljubljana Medical Center, Ljubljana, Slovenia; Silesian Center For Heart Diseases, Zabrze, Poland; Herz-und Gefaesszentrum, Bad Bevensen, Germany; L’Institut Mutualiste Montsouris, Paris, France PAF – Paroxysmal AF, PrAF – persistent AF, LSPrAF – long-standing persistent AF; NA - not available; LVEF - left ventricular ejection fraction; RF - radiofrequency
| First Author | No. patients$ | Publication Year | Institution(s) | Procedural Approach | Energy source | Age (mean±SD, years) | PAF | PrAF | LSPrAF | Lone AF only |
|---|---|---|---|---|---|---|---|---|---|---|
| Mahapatra[57] | 15 | 2011 | University of Virginia, Charlottesville, USA | Thoracoscopic Clamp-based | RF | 59.5±2.4 | 0 | 9 | 6 | No |
| Lee[56] | 7 | 2011 | Northwestern Memorial Hospital, Northwestern University, Chicago, USA | Thoracoscopic Clamp-based | RF | NA | NA | NA | NA | No |
| La Meir[59] | 19 | 2012 | University Hospital Maastricht, Maastricht, The Netherlands | Thoracoscopic Encircling Catheter | RF | 60.8±8.6 | 5 | 4 | 10 | No |
| Pison[33] | 26 | 2012 | University Hospital Maastricht, Maastricht, The Netherlands | Thoracoscopic Clamp-based | RF±cryoballoon* | 57.2±8.6 | 15 | 0 | 1 | No |
| Bisleri[60] | 45 | 2013 | University of Brescia Medical School, Brescia, Italy | Thoracoscopic Encircling Catheter | RF | 62.3±9.8 | 0 | 0 | 45 | Yes |
| Gehi[61] | 101 | 2013 | FirstHealth Arrhythmia Center, Pinehurst and University of North Carolina at Chapel Hill, Chapel Hill, USA | Subxiphoid Pericardioscopic (Convergent) | RF | 62.9±9.6 | 17 | 37 | 47 | No |
| La Meir[62] | 35 | 2013 | University Hospital Maastricht, Maastricht, The Netherlands | Thoracoscopic Clamp-based | RF | 57.1±9.5 | 16 | 8 | 11 | No |
| Kurfirst[63] | 30 | 2014 | Hospital Ceske Budejovic, Ceske Budejovice, Czech Republic | Thoracoscopic Clamp-based | RF | 61±8 | 0 | 0 | 30 | No |
| Lee[64] | 10 | 2014 | Samsung Medical Center, Seoul, South Korea | Thoracoscopic Clamp-based | RF | 56.1±7.6 | 1 | 0 | 9 | No |
| Pison[65] | 78 | 2014 | University Hospital Maastricht, Maastricht, The Netherlands | Thoracoscopic Clamp-based | RF | 60.5±7.5 | 29 | 34 | 15 | Yes |
| Gersak[66] | 73 | 2014 | Multicenter¥ (Slovenia, Poland, Germany, France) | Subxiphoid Pericardioscopic (Convergent) | RF | 56.3±10.5 | 0 | 22 | 51 | No |
| TOTAL** | 432 | mean 60.0 | 83 | 124 | 225 |
Table 3. Systematic Review of Hybrid Ablation Studies – Outcomes.
83 Journal of Atrial Fibrillation Featured Review Featured Review *Excludes readmissions for delayed staged procedures; **Excludes 7 patients reported by Lee et al56 as results pooled with 18 patients undergoing non-hybrid minimally invasive AF surgery; $Data for all minimally invasive patients including 7 with hybrid and 18 with non-hybrid surgical ablation, with 12 month follow-up; #1 sudden death, 1 death from atrioesophageal fistula PAF – Paroxysmal AF, PrAF – persistent AF, LSPrAF – long-standing persistent AF; NA - not available
| First Author | No. patients | AF type: PAF/PrAF/LSPrAF | Procedure time (min) | Hospital stay (days)* | Death (N) | Death or Major Complication (N) | Acute non-fatal thromboembolism (N) | Major Surgical Complications (non-embolic, non fatal) (N) | No. with minimum 12-month FU | Confirmation of AF recurrence | 12-month AAD-free SR maintanance – All (N) | 12-month AAD free FU for PAF (N) | 12-month AAD free FU for PrAF/LSPrAF |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thoracoscopic Clamp-based Approach | 88.1% | 78.3% | 88.2% | ||||||||||
| Mahapatra[57] | 15 | 0/9/6 | Both components: mean 450±20 | median 5.0 (IQR 5.0–5.5) | 0 | 0 | 0 | None | 14 | 7 day event monitor, AF >30 secs | 13/14 | - | 13/14 |
| Lee[56] | 7 | NA | NA | median 5 (IQR 4-6)* | 0 | 1 | 0 | 1 prolonged ventilation postop* | NA | Continuous ECG monitoring (duration and criteria not stated) or pacemaker/ICD monitoring | (12/23)$ | NA | NA |
| Pison[33] | 26 | 15/10/1 | Both components: mean 280±84 | mean 7±2 | 0 | 2 | 0 | 1 pleural effusion requiring drainage, 1 surgical incision pain prolonging hospital stay | 24 | 7-day Holter monitoring, AF/AT >30 secs | 20/24 | 11/15 | 10/11 |
| La Meir[62] | 35 | 16/8/11 | Both components: median 268 (IQR 186–477) | median 3.4 (IQR 2.6–4.1) | 0 | 0 | 0 | None | 35 | 7 day Holter, AF/AT >30 secs | 32/35 | 14/16 | 16/19 |
| Kurfirst[63] | 30 | 0/0/30 | Surgical component: mean 210±30 | mean 4.5±3 | 0 | 5 | 0 | 2 left pulmonary artery bleeding requiring sternotomy, 2 phrenic nerve palsies persistent at 12 months, 1 tamponade postop day 24 with overanticoagulation | 0 | 7 day Holter, AF/AT >30 secs | (27/30 at mean 208±29 days) | - | (27/30 at mean 208±29 days) |
| Lee[64] | 10 | 1/0/9 | Surgical component: mean 221 | median 12 | 0 | 3 | 1 stroke | 1 reexpansion pulmonary edema and pneumonia, 1 pericardial effusion | 0 | Holter (duration not specified) AF/AT >30 secs | (10/10 at median 7.6 months [range 6.7 - 11.6]). | NA | NA |
| Pison[65] | 78 | 29/34/15 | NA | median 6 (IQR 5.5-8) | 0 | 6 | 0 | 1 reoperation for bleeding, 1 bleeding not requiring reoperation, 2 pneumonia, 2 complete heart block requiring pacemaker implantation | 78 | 7 day Holter, AF/AT >30 secs | 68/78 | 22/29 (76%) | 43/49 |
| Thoracoscopic Encircling Catheter - based Approach | 73.4% | 60.0% | 74.6% | ||||||||||
| La Meir[59] | 19 | 5/4/10 | Both components: median 216 (IQR 132–391) | median 3.6 (IQR 2.7–4.3) | 0 | 0 | 0 | None | 19 | 7 day Holter, AF/AT >30 secs | 7/19 | 3/5 | 4/14 |
| Bisleri[60] | 45 | 0/0/45 | Epicardial component: mean 85±9 | mean 3.9±1.4 | 0 | 0 | 0 | None | 45 | ILR, AF>5 min or overall AF burden >0.5% | 40/45 | - | 40/45 |
| Subxiphoid Convergent Approach | 59.3% | - | 51.5% | ||||||||||
| Gehi[61] | 101 | 17/37/47 | NA | mean 4.4 | 2# | 7 | 1 TIA | 2 retroperitoneal bleeding, 2 tamponade | 69 | 24 hour Holter, AF/AT >30 secs | 46/69 | NA | NA |
| Gersak[66] | 73 | 0/22/51 | Surgical component: 112±38 | NA | 0 | 8 | 1 stroke | 2 bleeding requiring sternotomy, 2 bleeding not requiring reoperation, 1 tamponade, 1 pericardial effusion, 1 pleural effusion | 66 | ILR (n=48/73) or Holter (24 hours or 7 days), criteria not stated | 34/66 | - | 34/66 |
| TOTAL** | 432 | 83/124/225 | 2 | 32 | 3 | 27 | 350 | 260 | 50 | 160 | |||
| % of TOTAL | 0.5% | 7.4% | 0.7% | 6.3% | 81.0% | 74.3% | 76.9% | 73.4% | |||||
The 432 pooled patients had a mean age of 60 years (range, 56 to 63 years), mean CHADS2 scores (reported in 5 studies, n=250, 58%) 1 to 1.6, mean left atrial diameter (reported in all studies) 4.3 to 5.2 cm, mean left ventricular ejection fraction (reported in 11 studies, n=368, 85%) 47 to 62%, and mean AF duration (reported in all studies) 2.8 to 7.0 years ([Table 1]). AF was categorized in accordance with guidelines[75] as paroxysmal (individual episodes lasting ≤7 days) in 19% (83/432), persistent (continuous AF >7 days) in 29% (124/432) and long-standing persistent (continuous AF > 12 months) in 52% (225/432). A history of prior AF ablation was present in 35% (112/319 with reported data). Four studies (n=163, 38%) included only persistent and/or long-standing persistent AF patients. No study reported inclusion of patients with valvular AF (i.e. associated with rheumatic mitral stenosis, prosthetic or bioprosthetic valve, or mitral valve repair[75]) or prior cardiac surgery, although these data were not specifically reported in 5 [n=245, 57%] and 4 [n=210, 49%] studies respectively, whilst 2 studies [n=123, 28%] included only lone AF patients.
The published experience encompasses 3 different surgical approaches, each utilizing unique radiofrequency ablation tools ([Table 2], [Figure 1]): bilateral thoracoscopy with circumferential and linear lesions (sometimes referred to as LAMP [La Meir, Ailawadi, Mahapatra, Pison] hybrid ablation) created using bipolar radiofrequency clamps and ablation pens (Atricure, West Chester, OH) respectively (6 studies, n=194, 45%); right-sided thoracoscopy with simultaneous isolation of pulmonary veins and posterior left atrium using a suction monopolar radiofrequency catheter (Estech Cobra Adhere XL, Atricure, West Chester, OH) designed to deliver an encircling linear lesion (2 studies, n=64, 15%); and subxiphoid posterior pericardioscopy (through laparoscopic incision of the central diaphragmatic tendon) with linear ablation using a vacuum irrigated unipolar radiofrequency device (Numeris Guided Coagulation System with VisiTrax, nContact Surgical, Inc, Morrisville, NC, USA) to isolate or debulk the posterior left atrium and partially isolate the pulmonary veins (2 studies, n=174, 40%, referred to as the convergent procedure). Pulmonary vein isolation (PVI) was a common end-point in all studies. One clamp-based study[33] included 5 patients with severe COPD who underwent only right thoracoscopic radiofrequency epicardial ablation with adjunctive endovascular left-sided pulmonary vein cryoablation to avoid bilateral pneumothoraces.
Table 2. Systematic Review of Hybrid Ablation Studies – Ablation Details.
**Excludes 7 patients reported by Lee et al56 as results pooled with 18 patients undergoing non-hybrid minimally invasive AF surgery; §Selected patients only; *Persistent and/or long-standing persistent AF patients only; #Dissection of ganglion fat pads prior to box lesion application and HFS confirmation of all except right inferior ganglion; ₸Immediate staged in 2 centers, delayed at >2 weeks in 1 center, 50% immediate and 50% delayed >2 months in 1 center; ǁElectroanatomical mapping was not utilized in Slovenia; ŦSites of epicardial ablation identified with fluoroscopic visualization of ablation device in situ. N/A - not applicable - performed as part of box lesion; CS - coronary sinus; CFAE: complex fractionated atrial electrograms
| First Author | No. patients | Ablation Technology | Epicardial Atrial Ablation | Intraprocedural Confirmation of Conduction Block: Epicardial | Ganglion Ablation (identification method) | LOM ablation | LAA Intervention | Endocardial Ablation: Staging | Endocardial Ablation | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Electroanatomical Mapping | Confirming Conduction Block | Findings | Intervention | Reinduction | Triggers | Flutters | CFAE | |||||||||
| Thoracoscopic Clamp-based Approach (n=194, 45%)** | ||||||||||||||||
| Mahapatra[57] | 15 | Bipolar clamp, bipolar linear RF pen (Atricure, West Chester, OH) | Bilateral PVI, roof line, anterior line, mitral isthmus line, CS ablation, SVC isolation | PVI: entrance ± exit; linear: none | Yes (HFS) | Yes | Excision (staple) | Delayed staged 4.3±1.3 days | Yes | PV bidirectional block, SVC isolation, bidirectional block across linear lesions, AF inducibility (isoproterenol) | Gaps in 0 PVI and SVC lesions, 27% (4/15) of roof lines and 27% (4/15) of mitral isthmus lines | Consolidation of surgical ablation lines, CTI line, CS ablation, mitral isthmus line and CFAE ablation if AF inducible, ablation of inducible flutters ablated | Isoproterenol | No | Yes | Yes |
| Lee[56] | 7 | Bipolar RF clamp, monopolar RF pen (Medtronic, Minneapolis, MN) | Bilateral PVI | PVI: entrance ± exit; linear: bidirectional | Yes (HFS) | No | Excision (staple) | Delayed staged >3 months if recurrent AF/flutter | Yes (presumed) | PV bidirectional block, criteria for block across lines not stated | PV reconnections in all 7 patients (mean 2 per patient) at 6±3 months, all with confirmed entrance and/or exit block at time of surgery | Consolidation of surgical ablation lines, roof and mitral isthmus line, CTI line if typical flutter | Unknown | Yes | Yes | No |
| Pison[33] | 26 | Bipolar clamp, bipolar linear RF pen (Atricure, West Chester, OH) | Bilateral PVI, box lesion§, roof line§, mitral isthmus line§, CS ablation, intercaval line§, SVC isolation. Stepwise based on electrophysiological endpoint of non-inducibility of atrial arrhythmia. | PVI: entrance + exit; linear: bidirectional | No | No | Excision (staple)§ | Immediate staged | NoŦ | PV bidirectional block, bidirectional block across linear lesions | Gaps in 0 PVI lesions, 23% (5/22) of box and 100% (3/3) of mitral isthmus lines | Consolidation of surgical ablation lines, CTI line if typical flutter (n=3) | Rapid atrial pacing and isoproterenol | No | Yes | No |
| La Meir[62] | 35 | Bipolar clamp, bipolar linear RF pen (Atricure, West Chester, OH) | Bilateral PVI, box lesion, mitral isthmus line*, CS ablation, intercaval line*, SVC isolation* | PVI: entrance + exit; linear: bidirectional | Yes (HFS) | No | Exclusion (staple or clip)§ | Immediate staged | NoŦ | PV bidirectional block, bidirectional block across linear lesions, AF inducibility (rapid atrial pacing) | Gaps in linear ablation lesions in 14% (5/35) | Consolidation of surgical ablation lines, additional linear ablation if AF inducible, CTI line if typical flutter (n=3) | Rapid atrial pacing and isoproterenol | No | No | No |
| Kurfirst[63] | 30 | Bipolar clamp, bipolar linear RF pen (Atricure, West Chester, OH) | Bilateral PVI, box lesion, additional roof line | PVI: entrance + exit; linear: bidirectional | Yes (HFS) | Yes (ligation + ablation) | Exclusion (clips)§ | Delayed staged 3 months | Yes | PV entrance block, unidirectional block across linear lesions, AF inducibility (rapid atrial pacing) | Gaps in PVI lesions in 87% of right PV, 77% of left PV, 67% of roof lines, and 40% of inferior lines | Consolidation of surgical ablation lines, CTI line and mitral isthmus line, ablation of inducible flutters | Rapid atrial pacing | No | Yes | No |
| Lee[64] | 10 | Bipolar clamp, bipolar linear RF pen (Atricure, West Chester, OH) | Bilateral PVI, inferior line | No | Yes (HFS) | Yes | Excision (staple) | Delayed staged 4 days in 8/10 | Yes | PV isolation (not detailed) | Gaps in PVI lesions in 12.5% (1/8) | Consolidation of surgical ablation lines, CTI line in 6/8 | No | No | No | No |
| Pison[65] | 78 | Bipolar clamp, bipolar linear RF pen (Atricure, West Chester, OH) | Bilateral PVI, box lesion, mitral isthmus line§, CS ablation, intercaval line§, SVC isolation§ | PVI: entrance + exit; linear: bidirectional | Yes (unknown)§ | No | Exclusion (staple or clip)§ | Immediate staged | NoŦ | PV bidirectional block, bidirectional block across linear lesions, AF inducibility (rapid atrial pacing) | Gaps in 0 PVI lesions and 36% (28/78) of box lesions | Consolidation of surgical ablation lines, CTI line if typical flutter (n=11), mitral isthmus line completion if perimitral flutter (n=10) | Rapid atrial pacing and isoproterenol | No | No | No |
| Thoracoscopic Encircling Catheter-based Approach (n=64, 15%)** | ||||||||||||||||
| La Meir[59] | 19 | Encircling suction monopolar RF catheter (Estech Cobra Adhere XL, Atricure, West Chester, OH) | Encircling lesion set - bilateral PVI and box lesion | No | (as part of box lesion)# | No | No | Immediate staged | NoŦ | Entrance and/or exit block across linear lesions | Gaps in 89% (17/19) of PVI lesions and 100% (19/19) box lesions | Consolidation of surgical ablation lines, mitral isthmus line in persistent AF patients (n=3), CTI line if typical flutter seen (n=3) | No | No | No | No |
| Bisleri[60] | 45 | Encircling suction monopolar RF catheter (Estech Cobra Adhere XL, Atricure, West Chester, OH) | Encircling lesion set - bilateral PVI and box lesion | PVI: entrance ± exit; linear: unidirectional | No | No | No | Delayed staged 30–45 days | Yes | PV bidirectional block, bidirectional block across linear lesions, AF inducibility (rapid atrial pacing) | PV reconnections (7%, 3/45) representing 3/4 with only exit block and 0/41 with bidirectional block immediately after surgical ablation | Consolidation of surgical ablation lines, CFAE if AF inducible (n=20), CTI line if typical flutter (n=11) | Rapid atrial pacing | No | Yes | Yes |
| Subxiphoid Convergent Approach (n=174, 40%)** | ||||||||||||||||
| Gehi[61] | 101 | Vacuum irrigated unipolar RF device (Numeris Guided Coagulation System with VisiTrax, nContact Surgical, Inc, Morrisville, NC, USA) | Non-encircling bilateral antral lesions, box lesion, additional roof line, mitral isthmus line*, CS ablation | PVI: none; linear: unidirectional | No | Yes (undissected) | No | Immediate staged | Yes | PV bidirectional block, unidirectional block across linear lesions, AF inducibility (rapid atrial pacing) | Gaps in PVI lesions in 4% (4/101) | Consolidation of surgical ablation lines, additional roof line (n=90), CS ablation (n=73), mitral isthmus line (n=84), CTI line (n=99), CFAE ablation (n=29). Stepwise based on electrophysiological endpoints of termination to sinus rhythm with no re-inducibility of AF or termination to atrial flutter/tachycardia | Rapid atrial pacing | No | No | Yes |
| Gersak[66] | 73 | Vacuum irrigated unipolar RF device (Numeris Guided Coagulation System with VisiTrax, nContact Surgical, Inc, Morrisville, NC, USA) | Non-encircling bilateral antral lesions, box lesion | No | No | Yes (undissected) | No | Mixed₸ | Mixedǁ | Entrance and/or exit block across linear lesions and PV | Gaps in all PVI at sites of pericardial reflections - superior margins bilaterally and inferior margin of right inferior pulmonary vein antrum. | Consolidation of surgical ablation lines | No | No | No | No |
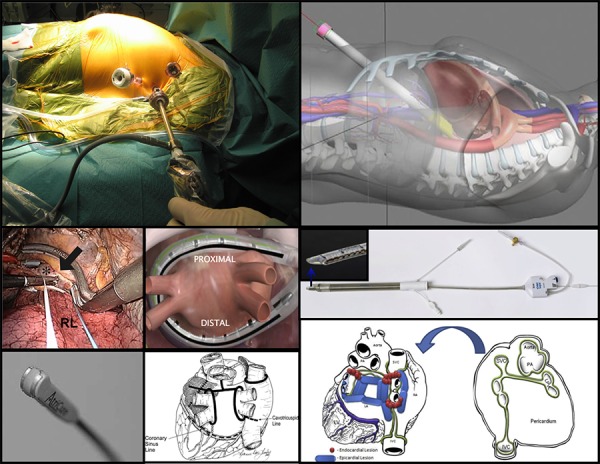
Figure 1. Hybrid ablation: access, tools and lesions sets. Top and middle left: Left thoracoscopic access as part of bilateral clamp-based approach; lesion (large arrow) created by bipolar radiofrequency clamp (Atricure, West Chester, OH) at right pulmonary vein antrum (asterix). RL – right lung. From Pison, J Am Coll Cardiol 2012;60(1):54-61, with permission. Bottom left: Bipolar pen used for linear ablations in bilateral clamp-based approach. From Sakamoto, J Thorac Cardiovasc Surg 2008;136(5):1295–1301, with permission. Bottom middle: Lesion set in bilateral clamp-based approach, from Mahapatra, Ann Thoracic Surg, Volume 91 , Issue 6 , 1890 – 1898, with permission. Central: Suction monopolar radiofrequency catheter (Estech Cobra Adhere XL, Atricure, West Chester, OH) positioned over the posterior left atrium as used in the right-sided thoracoscopic approach. From Muneretto, Innovations (Phila) 2012;7(4):254-8), with permission. Top right: Transabdominal, transdiaphragmatic access used in convergent approach. From Gehi, Heart Rhythm 2013;10(1):22-8, with permission. Middle and bottom right: Vacuum irrigated unipolar radiofrequency device (Numeris Guided Coagulation System with VisiTrax, nContact Surgical, Inc, Morrisville, NC) used in convergent approach; epicardial (blue) and endocardial (red) lesion set in convergent approach. From Gersack, J Thorac Cardiovasc Surg 2014;147(4):1411-6, with permission.
In addition to the differences in epicardial lesions created by these very different strategies and tools, timing of the endovascular catheter component also varied widely, from being performed immediately after surgery in 5 studies (“immediate-staged”, n=259, 60%) or after a delay ranging from 4 days to 3 months in 4 studies (“delayed-staged, n=100, 23%), with 1 multicenter study (n=73, 17%) reporting an immediate-staged procedure in 2 centers, delayed at >2 weeks in 1 center, and 50:50 split between immediate and delayed at >2 months in 1 center ([Table 2]). The endovascular component itself varied significantly between studies, such as whether electroanatomical mapping was utilized, choice of linear ablation lesions and which patients these were performed in, whether physiological targets such as triggers and complex fractionated atrial electrograms (CFAE) were targeted, and selection of end-points including intraprocedural confirmation of conduction block and re-induction protocols. There was variation in ganglion identification and ablation, ligament of Marshall ablation, and LAA ligation or excision ([Table 2]). There was diversity in approaches to peri-procedural antiarrhythmic and anticoagulant management.
Such diversity in approach is not surprising given the relative infancy of minimally-invasive surgical AF ablation and the novel ablation tools used,[27,76] as well as lack of consensus within the ablation community itself on optimal strategies for persistent and long-standing persistent AF.[77] An appreciation of electrophysiological principles underlying AF mechanisms and ablation approaches is central to understanding the role of these various approaches as therapeutic strategies for AF and their relative shortcomings.
Electrophysiological Perspective 1 – Heterogeneity of Atrial Fibrillation Mechanisms and Implications for Tailored Therapy
The pathogenesis of AF is often multifactorial. Applying the same therapy as a panacea may risk overtreating some and not addressing underlying mechanisms in others. Whilst the success of the maze procedure is consistent with the principle that a critical mass of atrial tissue is required to sustain fibrillatory conduction,[116-118] the cumulative experience from catheter ablation suggests both initiating triggers and arrhythmogenic substrate need to be addressed,[119,120] particularly in patients with persistent AF,[48,79,80,82-89,91,92,94,121-129] and underlying risk factors are identified and treated.[104,105]
Electrophysiological Perspective 2 – Insights from Catheter Ablation of Paroxysmal and Persistent Atrial Fibrillation
A major breakthrough in catheter ablation of AF was the finding that myocardial extensions into the pulmonary veins, previously recognized anatomically[132] and present in almost all human hearts ([Figure 2]),[133] are a dominant and progressive source of AF triggers amenable to ablation.[134,135] The mechanistic cogency of this approach was supported by studies demonstrating that isolating arrhythmogenic veins acutely terminates AF and prevents AF reinduction[140] even when tachycardia persists within the vein,[140,144-146] and also reduces arrhythmia recurrence compared to focal ablation[147] likely by addressing recurrent triggers from elsewhere in the pulmonary veins[148] or inter-connected veins.[149,150] Once it was realized that atrial myocardium adjacent to the pulmonary ostia, i.e. in the pulmonary vein antra, is a critical source of triggers leading to recurrence after ostial PVI,[151] in keeping with its histological and electrical homogeneity with the venous myocardial sleeves,[152] the approach was further modified to incorporate these areas (antral PVI or wide-area circumferential ablation [WACA]) with a further reduction in arrhythmia recurrence than with ostial PVI.[153-157] In addition to isolating venous[146,158-160] and antral arrhythmogenic foci,[151,161,164] proposed mechanisms have included disrupting pulmonary ostial anchors for AF drivers (discussed below)[128,165,166] and interrupting neuronal connections.[81,167,168] A number of randomized clinical trials have demonstrated the efficacy of this approach as a therapeutic strategy for paroxysmal AF,[7,09,10,169,174] and of consecutive patients undergoing radiofrequency PVI, approximately 75% and 90% are rendered arrhythmia-free off antiarrhythmic therapy after 1 and 2 ablation procedures, respectively,[99] and at 10 years, 75% of patients are arrhythmia free, although 40% require repeat ablation and there have been evolving approaches and technologies over this time.[100,175] In addition to symptomatic benefit and quality of life improvement, there is a significantly lower rate of progression to persistent AF (0.5-0.6% per year)[100,176,177] compared with pharmacological therapy (8.6% per year).[178]
Figure 2. Thoracic venous sources of atrial fibrillation triggers. Top: pulmonary vein and vena cavae. Arrows and asterexis identify myocardium in venous structures adjoining the left and right atria. Bottom: Diagram depicting location of the ligament (or vein) of Marshall, the remnant of the left-sided superior vena cava, which has varying degrees of patency in adult life. From Desimone, J Cardiovasc Electrophysiol 2012;23(12):1304-9, with permission. LAA – left atrial appendage; LIPV/RIPV/RSPV – left/right inferior/superior pulmonary vein; IVC/SVC – inferior/superior vena cava.
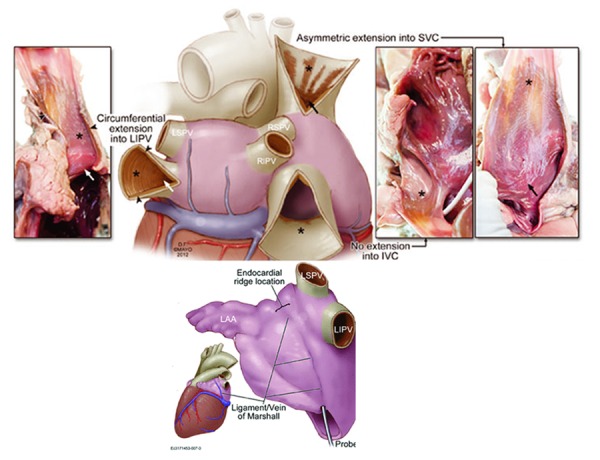
The catheter ablation experience allowed for additional insights into the pathophysiological significance of non-pulmonary thoracic venous myocardium. Atrial myocardium extending into the superior vena cava, seen in 80% of human hearts ([Figure 2]),[133] has similar electrophysiological characteristics to pulmonary venous extensions and can be a source of AF triggers.[179,180] It is also present in the coronary sinus, ligament of Marshall and, least commonly, azygous vein (6%).[133] Myocardium within the coronary sinus may be a source of rapid repetitive electrical activity during AF and electrically disconnecting it from the left atrium reduces sustained AF induction, suggesting a role in perpetuating AF.[181] The ligament of Marshall ([Figure 2]) is an epicardial remnant of the left superior vena cava, which maintains 3-French probe patency in 70% of hearts and consistently overlies the endocardial left lateral ridge between the pulmonary veins and left atrial appendage.[133] It contains both autonomic nerves and muscle fibres, the density of which varies along its length,[182] implicated in triggering or sustaining AF[183] and providing electrical continuity between the coronary sinus and pulmonary veins.[184]
Through experience with ablation, there has also been improved understanding on mechanisms and management of post-ablation atrial arrhythmia. Although early arrhythmia recurrence (<3 months) is a powerful predictor for long-term recurrence,[185-190] a significant proportion (30%) may go on to be arrhythmia free in the long-term without repeat ablation,[185,187] and early restoration of sinus rhythm during this period reduces long-term recurrence rates,[192] although additional antiarrhythmic use does not add incremental value to cardioversion.[192-195] For late recurrence (>3 months), repeat ablation is superior, as demonstrated by one randomized trial of paroxysmal AF patients with recurrence after antral PVI, with reduced AF burden, symptoms and progression to persistent AF (4 vs 23% at 36 months) compared to antiarrhythmic therapy.[196] This is mechanistically consistent with studies demonstrating that recurrence after PVI for paroxysmal AF is usually related to reconnected pulmonary vein conduction,[43,47,146,158,177,197-201] likely due to reversible ablation injury with absent circumferential scar on MRI,[202-205] or the presence of extrapulmonary ectopic AF triggers.[151,158,162] Extrapulmonary triggers are noted spontaneously in about 10% of patients undergoing AF ablation, being several fold more frequent with increasing AF burden and duration (3% of paroxysmal, 8% of persistent and 19% of long-standing persistent patients),[99] and in up to 45% following induction with pacing or isoproterenol infusion, most commonly arising from the posterior left atrial wall (20-40%), left atrial appendage (30%), or superior vena cava (30-40%), with a minority from the left atrial roof (8%), ligament of Marshall (8%), crista terminalis (5-10%), coronary sinus (1-10%), and interatrial septum (1-5%).[151,163,206-210] It is unknown whether routinely targeting these regions improves outcomes when they are not evident sources of triggers during ablation. For instance, routinely isolating the SVC as a dominant site of extrapulmonary venous triggers has been advocated,[180] whilst randomized trials testing this approach have reported conflicting results.[211-213]
To summarize, in keeping with the predominant trigger-based mechanism underlying paroxysmal AF, catheter ablation studies have demonstrated that an anatomically guided approach to PVI leads to sustained improvement off antiarrhythmic drugs in the majority of patients and offers a definite end point of electrical isolation of the pulmonary vein. Although the extent of atrial fibrosis correlates weakly with clinical phenotype,[78] with some paroxysmal AF patients having high atrial fibrosis burden, systematic substrate modification offers no incremental benefit,[214-222] even with late recurrent paroxysmal AF requiring re-ablation,[223] and may even predispose to macroreentrant atrial tachycardias.[216,224]
This contrasts with insights gained from catheter ablation of persistent and long-standing persistent AF, where dominant fibrillating frequency shifts away from the pulmonary veins to other left or even right atrial sites[144,161] with PVI offering limited success on its own[225-228] and additional substrate modification becoming necessary to improve ablation outcomes,[8,131,157,229,230] all in keeping with the underlying transition from a predominant trigger-based paradigm to one with increasing influence from extrapulmonary drivers and atrial substrate.[128,209,231] However, there is mechanistic overlap, such that in persistent AF there is evidence of ongoing dynamic interplay between pulmonary vein triggering and atrial activation[144] and PVI may acutely reduce left atrial dominant activation frequency,[232] partially organize AF without necessarily affecting dominant frequency,[233] or acutely terminate AF.[121,144,227] Accordingly, atrial substrate modification alone without PVI results in inferior outcomes.[228,234,235] Nonetheless, atrial substrate, no matter how defined, is a strong predictor of recurrence after catheter ablation of persistent AF[78,201,236-238] and outcomes have been better with more extensive atrial substrate modification.[124,230] To what extent this reflects current uncertainty over how best to identify pathogenic versus bystander substrate and appropriate end-points for ablation are areas of ongoing investigation.[131]
Electrophysiological Perspective 3 – Successful Substrate Modification and Ablation End Points in Persistent Atrial Fibrillation
An intriguing finding from the PRAGUE-12 randomized trial is that restoration of sinus rhythm was significantly higher with increasingly persistent AF comparing maze to no maze in patients undergoing cardiac surgery (paroxysmal 62 vs. 58%, persistent 72 vs. 50% and long-standing persistent AF 53 vs. 14%, P < 0.001).[239] The ability to observe in real time the effects of progressive substrate modification on atrial activation locally, remotely and globally whilst correlating this with structural, functional, and outcome data have allowed for novel insights into mechanism underlying arrhythmia and ablation efficacy in persistent and long-standing persistent AF. Improved catheter ablation outcomes have been reported when additional substrate modification is incorporated in an individualized, stepwise fashion to either reduce AF dominant frequency (>11%)[240] or terminate AF,[129,238] with adjunctive ablation of spontaneous and inducible atrial tachycardias.[127,241,242] With this approach, long-term freedom from AF off antiarrhythmic medication has been reported in 65-75% of patients,[129,238,240,242] and though 15-30% of patients with long-standing AF may require 3 or more procedures, recurrences are more likely to be from reentrant atrial tachycardia rather than recurrent AF,[129,238] albeit the benefits of AF termination decline with longer AF duration (>3 years in one study).[243] There is currently no consensus on what constitutes optimal substrate modification in persistent AF, with reported benefit from an anatomical approach through creating point-by-point ablation lines (linear ablation) following the success of the surgical maze procedure;[46,51,229,232,244-246] isolating the posterior left atrium in this manner,[247,248] ablating sites with complex fractionated atrial electrograms (CFAE) during fibrillatory conduction as a strategy to modify electrical substrate;[217,219,226,234,249-251] identifying and ablating focal drivers;[109,113,123,126,128,252,253] and ablating autonomic ganglia as AF modulators.[82,84,86,88,91,92,254]
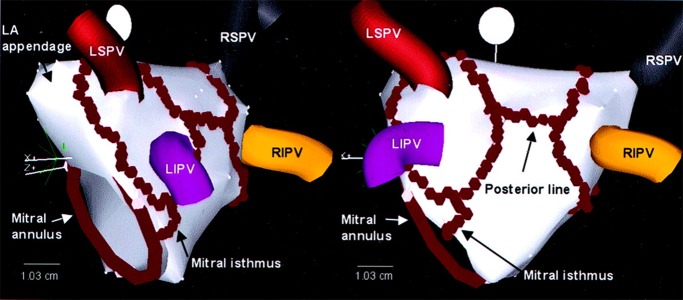
The most extensively investigated linear ablation lesions during catheter ablation are the roof line, which connects the superior pulmonary veins (to mitigate risk of atrio-esophageal fistula from ablating over the esophagus posteriorly), and mitral isthmus line connecting the left inferior pulmonary venous antrum to the mitral annulus ([Figure 3]).[48,153,232] When such ablation is successful, it results in dominant activation frequency reduction and cycle length prolongation, which may progress with further ablation to AF termination, often to a macroreentrant atrial tachycardia circuiting around the left atrial roof, mitral isthmus, or cavotricuspid isthmus.[48,232] Of those still in AF on completion of antral PVI, 60% are left non-inducible for AF after one linear lesion and 95% after two.[121] Analyzing frequency-domain transformed activation signaling during linear ablation reveals that acute reductions in dominant activation frequency do not occur until completion of conduction block and coincide with disappearance of discreet lower frequency sources, characteristics of which were consistent with reentrant drivers, an effect not seen after PVI or CFAE ablation in this study.[232] Linear ablation may therefore work by disrupting localized reentrant drivers. Results from whole atrial phase and organization index mapping also suggest this.[128,166,255] In addition, linear ablation may reduce subsequent macro-reentrant atrial tachycardia by creating lines of conduction block across critical anatomical isthmi, yet predispose to it when conduction block is not achieved.[51,54] The major limitation of linear ablation is therefore difficulty in achieving sustained conduction block,[50] particularly at the mitral isthmus ([Figure 4]) where 40-70% of patients require additional epicardial ablation from within the coronary sinus and 10-35% are left without conduction block,[256-258] especially in areas with anatomical irregularity (such as with a pouch), or interpositioned coronary vessels presumably causing a heat sink.[257-259] Even if successful conduction block is achieved at index procedure, late recovery of mitral isthmus conduction is seen in 75% of instances (much higher than after cavotricuspid isthmus block [25%]) and predisposes to perimitral flutter.[260] The incidence of perimitral flutter is higher after routine mitral isthmus ablation and requires further reablation to achieve lasting freedom from recurrence.[53]
Figure 3. Ablation lines created during left atrial ablation represented on a 3D electroanatomical mapping system (CartoTM). Individual ablation lesions are represented by red tags. From Oral, Circulation. 2003;108(19):2355-2360, with permission.
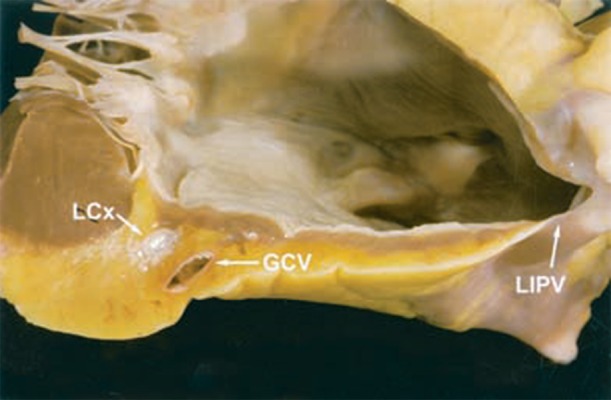
Figure 4. Human heart demonstrating cross-section of the mitral isthmus. The thickness of the atrial myocardium, epicardial fat and associated vasculature are demonstrated. From Becker, J Cardiovasc Electrophysiol 2004;15(7):809-812, with permission. LCx – left circumflex artery; GCV – great cardiac vein; LIPV – left inferior pulmonary vein.
As with antral PVI, gaps in conduction block in linear lesions resulting in reentrant atrial tachycardias is the most common cause for arrhythmia recurrence,[45,46,50,52-245] and may explain why the recently published STAR AF II randomized trial,[261] in which linear ablation resulted in acute conduction block in only 74% of patients and intraprocedural AF termination in 22%, failed to demonstrate benefit of linear ablation over antral PVI, and also why a recent meta-analysis demonstrated a significant reduction of AF recurrence with linear ablation but not with more extensive linear lesion sets.[235] In addition, although patients may manifest with perimitral flutter following persistent AF ablation, they still stand to benefit from confirmation of PVI and ablation of extra-pulmonary triggers, which may by itself result in more patients having reduced arrhythmia recurrence than if the mitral isthmus alone is ablated and blocked, indicating that triggering may underlie some macroreentry post-ablation flutters.[252] As for routinely creating linear lesions in the right atrium, cavotricuspid isthmus ablation during AF ablation does not reduce long-term arrhythmia recurrence[262,263] unless typical atrial flutter is demonstrated either clinically or during the ablation procedure.[264,265] These principles also extend to effective surgical lesion sets.[29] Limiting lesion sets also reduces the likelihood of complications, as illustrated by a recent meta-analysis comparing left atrial and biatrial maze which reported equivalent long-term success but with increased pacemaker implantation with biatrial maze.[266]
In a prior study CFAE ablation was reported to significantly improve ablation outcomes as an adjunct to PVI, but as standalone therapy the results were inferior to PVI (1 year arrhythmia-free rates 74 vs. 48 vs. 29% after one procedure in the STAR AF trial).[234] In about 20% of patients, AF terminates whilst ablating a CFAE[267,268] and an acute decrease in dominant frequency with CFAE ablation predicts reduced recurrence of AF.[269] Certain CFAEs are more likely to terminate AF or reduce its dominant activation frequency than others, such as those with greater duration of continuous activity or complexity,[270-273] whilst other CFAEs may represent bystander activation as suggested by studies demonstrating a reduction in CFAE number and location immediately following PVI or linear ablation.[268,271,274] Judicious selection of target sites is therefore needed, whilst identifying appropriate CFAE targets based on grading complexity is somewhat subjective and can be erroneously overestimated at overlapping structures including the interatrial septum. This may explain why, when guided by automated software protocols, CFAE ablation was reported to offer no additional benefit to antral PVI by the STAR AF II trial[261] and, as reported by another randomized trial, have inferior outcome to linear ablation despite more extensive lesions with greater cardiac enzyme release.[275] Further evidence of the importance of strategic CFAE ablation comes from a trial demonstrating no added benefit when right atrial CFAE ablation is routinely added to left atrial CFAE ablation.[114] The three published randomized studies directly comparing linear with CFAE ablation have reported either inferior performance of CFAE ablation,[275] as noted above, or equivalence of the two procedures,[261,276] though with CFAE ablation resulting in higher rates of intra-procedural AF termination261 and recurrences which are less likely fibrillation and more likely atrial tachycardia.[276]
The observation that terminating or slowing AF by ablation at discreet sites modifies atrial substrate in a manner which renders the atrium significantly less likely to fibrillate suggests that the influence of even advanced substrate surrogates in determining recurrence can be overcome by focal ablation.[128,166,245,255,267-273] Whole atrial activation, phase, frequency and organizational index mapping has identified the presence of rotor-like activity and focal high-frequency sources driving AF.[111-113,123,126,128,166,277-283] Ablation of these domains acutely terminates AF,[128,255] though with longer durations of AF these drivers become more numerous and acute termination is less frequent (75% and 15% of persistent and long-lasting AF terminated, respectively, >6 months cutoff).[128] Though their location varies between individuals,[123,128,255,282] they are predominantly left atrial (70%), with the rest being right atrial (30%),[128,255] and demonstrate relative spatiotemporal stability with most meandering over 5-10 cm2 areas around the pulmonary antra, antrally-associated septum and appendage, or left inferior wall/coronary sinus,[128] consistent with earlier data identifying these as sites where ablation is most likely to terminate AF.[245] What determines the observed relationship to these critical areas is unknown[284] and likely involves interplay between individual variation in atrial and pulmonary ostial geometry,[285] fiber orientation and anisotropy,[286-288] fibrosis,[289-291] regional variation in autonomic innervation and tissue response to autonomic input,[83,85,86,292] and geometrically governed variability in exposure to mechanical stress and pressure.[293-295] By imaging fibrosis using MRI, the likelihood of recurrent arrhythmia after ablation has been correlated to the extent of fibrosis left unablated, indicating that substrate modification works best when it targets such fibrotic areas, likely by converting proarrhythmic tissue with heterogenous fibrosis to homogenous inert scar.[230] A stepwise ablation approach also effectively reduces intrinsic scar burden,[296] indicating that both electrophysiologically and anatomically defined targets are likely co-localized. An approach tailored to targeting low voltage (<0.5mV) sites during sinus rhythm as areas representing fibrosis was recently shown to be feasible and associated with comparable outcomes to patients without any atrial scar.[297]
These data provide a mechanistic link between substrate, clinical characterization of AF and the observed responses to ablation. By identifying individual-specific mechanistic targets, providing novel insights into the role of atrial structural changes,[286-288,291,297,298] better understanding why the currently established substrate modification strategies of linear and CFAE ablation sometimes demonstrate greater effect[128,232,271,280,299,30] than at other times,[261,274,298,301-303] these approaches raise the possibility that successful persistent AF ablation need not necessarily depend on standardized, extensive atrial compartmentalization or debulking by providing novel patient-specific ablation end-points,[126,128,230,281,297,304] though this remains an area of ongoing investigation.
Electrophysiological Perspective 4 – Creating Effective Ablation Lesions: Endocardial vs. Epicardial Approaches
As with the endocardial approach, demonstrating effective conduction block across linear lesions is key to successful epicardial AF ablation.[29,32] With viable myocardial strands as thin as 1 mm allowing for electrical propagation,[40] creating contiguous transmural lesions is the goal with both approaches and dependent on choice of ablation energy,[305] electrode and catheter design, interplay of biophysical response characteristics of targeted tissue and its related anatomy,[306,307] including variation in atrial wall thickness regionally (thickest at the roof, mitral isthmus and left lateral ridge[308]) and between individuals (posterior wall 0.9 to 7.4 mm in one study),[309] atrial and pulmonary venous morphology[257,310,311] and associated vasculature.[257] The published hybrid ablation experience has thus far exclusively incorporated radiofrequency ablation ([Table 2]), which relies on current flow alternating at radiofrequency causing resistive tissue heating and depends fundamentally on electrode-tissue contact.[312,313] Additional variables affecting lesion depth include power and duration of ablation,[314] electrode size and orientation,[315-317] electrode tip cooling to prevent surface char and coagulum allowing for deeper lesions,[318,319] electrical tissue impedance,[314] and tissue heat sinks.[35,306,307,320] Monitoring electrode contact force, temperature rise, impedance fall, and electrogram diminution allows real-time monitoring of lesion evolution surrogates and safe ablation,[313] though none of these parameters obviate the need for confirming conduction block.[29]
Despite these measures, and documentation of block acutely, conduction may recover and whether this is from tissue regeneration or incomplete ablation and reversible injury is unknown.[284] The latter is supported by data demonstrating that common locations of late recovery after endovascular catheter ablation are sites with known difficulty in maintaining catheter tissue contact (close to the pulmonary veins, left lateral ridge, mitral isthmus, accessory pulmonary veins or a common left pulmonary vein ostium);[50,311,321-323] electroanatomical correlation of gaps with measured tissue contact force;[322,323] echocardiography[324] and contrast MRI showing evidence of reversible injury with edema which resolves on serial scans[205] with recovery over days to weeks, and incomplete scarring at ablation sites with conduction recovery;[203,204] and histological evidence of non-transmural lesions in pulmonary veins that reconnect late after PVI.[325] In addition, outcomes are better when areas with reversible injury are re-ablated once identified after a 60-90 minute wait period[326] and/or adenosine,[326-328] ablating to unexcitability as a superior tissue endpoint to conduction block,[329] measuring and maintaining tissue contact force to deliver more effective lesions,[322,330-332] and visually identifying gaps after electroanatomically tagging ablation lesions.[333,334]
The specific additional challenges with an epicardial approach include constraints from mediastinal anatomy and pericardial reflections[335] in accessing individual-specific arrhythmogenic triggers and substrate; the increased proximity and risk of collateral injury to great vessels, coronary arteries, lungs, mediastinum, esophagus, liver, diaphragmatic vessels, and phrenic nerves;[335] how well suited catheter design is for navigation, maintaining tissue contact over the smooth epicardial convexity of the beating heart and safe delivery of ablation energy; how well matched the selected ablation energy is for the local tissue environment and characteristics;[305,336] how to overcome endolumenal and intramural arterial heat sinks,[35,306,307,320] the presence of epicardial coronary vessels at key linear lesion sites (mitral and cavotricuspid isthmus), and presence of epicardial fat which limits energy delivery to underlying myocardium[305,306,337] as well as independently influencing AF pathophysiology and recurrence after endocardial ablation.[338]
To overcome these challenges, minimally invasive surgical approaches have incorporated versatile access and catheter designs to facilitate controlled tissue manipulation and ablation. The bipolar radiofrequency clamp (Atricure, West Chester, OH) which is utilized for PVI with the bilateral thoracoscopic hybrid approach has favorable preclinical results,[36,339] though a variable number of repeat applications (at least 3 and often more in clinical studies[29,31]) are required to achieve block and reconnection gaps are increasingly prevalent over time from none when tested immediately[33,65] to 12.5% after 4 days[64] and 87% of right and 77% of left pulmonary veins at 3 months.[63] Bipolar sources have been reported to create better lesions than monopolar sources with epicardial ablation.[340] Additional linear lesions created with the bipolar radiofrequency pen (Atricure, West Chester, OH), which again has good preclinical efficacy data,[34,341] resulted in reconnection gaps in 14-36% of box lesions at immediate staged endovascular testing,[62,65] 27% of roof and mitral isthmus lines at 4.3±1.3 days,57 and 63% of roof and 40% of inferior lines at 3 months[63] even when bidirectional block was confirmed during epicardial ablation. To overcome limitations in mitral isthmus linear ablation from attempts to avoid coronary arterial injury,[58] some investigators connected the left fibrous trigone to the mitral annulus.[29] However, whether this has similar efficacy and propensity to disrupt focal drivers or reentrant flutters is unknown. Similarly, ablation lines across Bachmann bundle are hard to establish due to atrial thickness and results in inter-atrial dyssynchrony.[63] The bilateral thoracoscopic approach additionally allows visualized access to the epicardial ganglia, ligament of Marshall, and left atrial appendage, can protect or maneuver away from critical anatomical structures, but requires collapsing the lung and opening the pericardium on each side.
The right-sided thoracoscopic approach and convergent (posterior pericardioscopic) approaches utilize specially designed linear ablation monopolar RF catheters incorporating suction to increase tissue contact and optimize catheter stability (Estech Cobra Adhere XL, Atricure, West Chester, OH, and Numeris Guided Coagulation System with VisiTrax, nContact Surgical, Inc, Morrisville, NC, USA, respectively), allowing for less invasive access than the bilateral thoracoscopic approach. Both of these catheters performed less well than the bipolar clamp in preclinical studies.[34] However, with the encircling suction catheter (Estech Cobra Adhere XL), when applications were repeated until entrance and/or exit block in conduction to/from the posterior left atrium, block was maintained after 1 month at endovascular in all with bidirectional block and 25% with unidirectional block at epicardial ablation.[60] In contrast, when block is not tested for during epicardial application, almost all had conduction gaps during immediate-staged endovascular study.[59] The ablation line also abolished standardized ganglionic responses except at the right inferior ganglion, which was located outside the box lesion.[59] A limitation with the unilateral right-sided approach is lack of access to the left atrial appendage. The convergent approach avoids thoracoscopy altogether, utilizing laparoscopy to guide subdiaphragmatic posterior pericardioscopy.[55,68] Space constraints limits placement of additional catheters for simultaneous electrophysiological monitoring and, although inferior and posterior left atrial surfaces are well visualized, the superior and anterior lesions need to be made without direct visualization and rely on knowing catheter angulation and orientation. Pericardial reflections lead to discontinuous lesions at both superior and right inferior pulmonary veins, necessitating routine endocardial touch-up lesions at these sites, whilst access to the ligament of Marshall, appendage and ganglia is limited and the esophagus is left more vulnerable than with the bilateral thoracoscopic approach.[55,68] Although the other hybrid approaches have isolated the posterior left atrium, the convergent approach has focused on debulking this region. There are no data comparing the two approaches directly.
When electroanatomical mapping was not utilized to register lesions during epicardial ablation, techniques to correlate with the endovascular component during immediate staging involved leaving the epicardial ablation catheter in situ to correlate fluoroscopically or gently prodding epicardially at the ablation line whilst correlating endocardial catheter position with intravascular ultrasound.
Electrophysiological Perspective 5 – Cardiac Autonomic Ganglia as Targets during Atrial Fibrillation Ablation
An autonomic etiology for AF was first recognized with description of vagally-induced AF in 1978.[342] Pulmonary vein isolation has reduced efficacy in treating paroxysmal vagotonic AF,[80] whereas atrial vagal denervation can abolish it.[88] The importance of autonomic influences on AF is also evident from a reduction in AF recurrence following antral PVI when vagal responses (bradycardia, atrioventricular block, hypotension) are fortuitously elicited during radiofrequency ablation and abolished,[81] the increase in late AF recurrence in those with high serum titres of autoantibodies against the beta-1 adrenoceptor and M2 muscarinic receptor at the time of cryoballoon PVI,[96] and in the post cardiac transplantation population, whose denervated recipient hearts are relatively resistant to AF with 70% lower AF incidence than matched patients undergoing cardiac surgery with left atrial maze.[90]
The atria are richly innervated by autonomic nerves[343] and between 700-1500 epicardial ganglionated autonomic neuronal plexi are associated with the heart, though numbers decline by up to 50% with age, with complex circuits involving both parasympathetic and sympathetic components.[344] The atrial ganglia are primarily clustered at the superior right atrium, superior left atrium, posterior right atrium, posteromedial left atrium, and the inferolateral aspect of the posterior left atrium.[344] Following the demonstration in dogs that ganglionic stimulation induces calcium-mediated pulmonary vein triggers[345] and enhances trigger-induction of AF, with the opposite effects with ganglionic block,292 studies began to focus on modifying local autonomic atrial input by targeting epicardial ganglia to improve outcomes of catheter AF ablation[82] and surgical maze.[346] After validation of the technique in dog experiments, eliciting bradycardic responses and atrioventricular block at sites of high frequency atrial burst pacing has been used to map ganglionic cluster sites from the endocardium[82] with descriptions of five common left atrial sites (superior left, inferior left, ligament of Marshall, anterior right, inferior right).[86] Although some ganglionic sites may not elicit such a response yet still exert modulatory influence,[84] whilst surgical approaches are able to directly visualize these ganglia, elimination of the high frequency stimulation response may serve as a useful ablation end-point. Such ganglionic responses were shown to be present in 86% of 216 patients after antral PVI and predicted arrhythmia recurrence in patients with paroxysmal AF (51 vs 8% at >6 months) but not persistent AF (40 vs 39%), even though a higher proportion of persistent AF patients had positive ganglionic responses.[94] Ganglia may also co-localize with CFAE although the mechanism is not fully explained.[83,85,86]
There is currently no consensus on whether to routinely perform ganglion ablation during catheter ablation of AF.[347] A meta-analysis of six randomized trials (342 patients) concluded that ganglion ablation improves the results of catheter PVI or surgical maze in reducing freedom from AF recurrence, but as standalone therapy the outcome is inferior to PVI,[348] with similar results in a recent trial of 242 patients with paroxysmal AF (at 2 years, freedom from recurrence 74 vs 56 vs 46%, respectively).[92] In persistent AF, even though retained ganglionic responses after antral PVI may not predict AF recurrence,[94] trials of ganglionic ablation during both catheter ablation[91] and surgical maze[346] have reported positively on efficacy. Long-term outcomes are unknown, with dog data demonstrating the reappearance of ganglionic responses with time,[349] presumably due to axonal regrowth, whilst isolated ganglionic ablation (i.e. without PVI) may be paradoxically proarrhythmic.[350]
Electrophysiological Perspective 6 – Stroke Prevention and Left Atrial Appendage Closure
Stroke mechanisms in AF are complex and demonstrating a role for fibrillation independent to atrial myopathy and vascular disease is challenging as both not only predispose to AF but also to stroke risk with AF.[351] There are at present no randomized data demonstrating that AF ablation, whether surgical or catheter-based, reduces stroke risk. Although Cox et al reported a low incidence of stroke (0.7%) after the cut-and-sew maze procedure in 265 patients followed for 11.5 years,[352] the majority had low stroke risk at baseline and the study was non-randomized. The PRAGUE-12 trial randomized 224 patients undergoing cardiac surgery to concomitant maze or no maze, and reported 1-year stroke rates of 2.7 vs. 4.3% (p=0.319).[239] Overly aggressive atrial compartmentalization and debulking may paradoxically increase stroke risk by rendering the atrium without contractile activity despite restoration of sinus rhythm, mitigating any benefit.[353] In a large, unselected catheter ablation cohort, 2% developed stroke after 1,347 patient-years follow-up, with no significant difference when sinus rhythm was maintained, though the study was probably underpowered as most patients had low baseline stroke risk.[175] A study of 4,212 patients who underwent AF ablation reported reduced risk of stroke, death and dementia compared to 16,848 age-gender matched controls with AF but no ablation, with similar rates to 16,848 age-gender matched controls without AF.[354] The results of the CABANA trial (clinicaltrials.gov/ NCT00911508), which aims to study the effects of catheter ablation on mortality, stroke and bleeding as compared to drug therapy, are awaited.
Recent advances in cardiac imaging have allowed an appreciation that morphological complexity of the LAA significantly influences thromboembolic risk, supporting a structural approach to thromboprophylaxis.[355] The efficacy of this approach was demonstrated by the WATCHMAN trial of percutaneous LAA occlusion.[356] A meta-analysis of 7 studies including 3,653 patients undergoing appendage closure (n = 1716) versus not (n = 1937) at the time of cardiac surgery reported a significantly reduced stroke incidence with closure (0.95 vs 1.9% at 30 days, 1.4 vs 4.1% at last follow-up) and reduced mortality (1.9 vs 5%).[357] Surgical approaches, however, have been limited by incomplete closure, with surgical amputation and oversewing yielding highest maintained closure rates.[351] Results of the LAAOS III trial (clinicaltrials.gov/ NCT01561651), which plans to randomize 4,700 patients undergoing cardiac surgery to LAA occlusion or no occlusion, are awaited. A number of minimally invasive approaches have been developed, using either suture or clip exclusion or staple excision, though none have yet been proven to reduce stroke.[351] In addition, LAA exclusion may not be regarded as a panacea for stroke reduction in AF, as vascular mechanisms may coexist and thrombi are more likely to be extra-appendicular in valvular AF patients.[358] There are also hemodynamic sequelae which are in keeping with loss of its compliance and atrial booster function.[359,360] In addition to modulating stroke risk, appendage ligation or excision results may serve as a form of substrate modification[361] by reducing atrial mass[118] and eliminating appendage triggers and drivers,[362,363] whereas electrical isolation without mechanical closure may paradoxically increase stroke risk through appendage blood stagnation.[364]
Electrophysiological Perspective 7 – Hemodynamic Impact of Atrial Fibrillation Ablation
In the majority of individuals, restoration of sinus rhythm with ablation leads to significant improvement in left ventricular function, effects that extend beyond rate control,[11,365,366] even when baseline ejection fraction is normal,[367] with improvements also reported after surgical maze.[368] Restoration and maintenance of sinus rhythm results in reverse atrial remodeling[8,369,370] and similar effects are seen after surgical maze,[371] although with time these effects can subsequently reverse[372] and it is unclear whether this is due to the maze procedure itself or persistent risk factors leading to progressive atrial myopathy. Without the expected benefits of reverse atrial remodeling from immediate restoration of sinus rhythm, such as those with paroxysmal AF and low arrhythmia burden, a reduction in atrial contractile function is seen after maze, more so with more extensive ablation (new left atrial dysfunction 8.5% after PVI compared to 30% after additional linear ablation).[373] Surgical maze may result in reduced atrial compliance which can cause severe, symptomatic pulmonary hypertension (“stiff left atrial syndrome”).[374] This has also been reported after catheter ablation in 1.4% of 1,380 patients, predisposing factors being smaller pre-procedural left atrial size (≤45mm), preexisting left atrial hypertension, increased baseline left atrial fibrosis, diabetes mellitus and obstructive sleep apnea.[375]
Electrophysiological Perspective 8 – Critical Appraisal of the Role of Hybrid Ablation in Improving Outcomes from Atrial Fibrillation Ablation
Outcome from Hybrid Ablation of Atrial Fibrillation: Results of Systematic Review
Published success rates from hybrid ablation ([Table 3]), defined as maintained sinus rhythm off antiarrhythmic medications at 12 months, are 74.3% overall (data available for 81% [350/432]), 76.9% for paroxysmal (data available for 76% [65/83]) and 73.4% for persistent / long-standing persistent AF patients (data available for 62% [218/349]). Methods used to detect recurrence varied from a 24-hour Holter monitoring at prespecified follow-up intervals to continuous ECG monitoring using implantable loop recorders or pacemakers and defibrillators ([Table 3]). Success rates differed significantly among the three approaches ([Table 3]), with highest rates reported with the bilateral thoracoscopic clamp-based approach (88.1% [133/151]), intermediate with the unilateral thoracoscopic suction encircling catheter-based approach (73.4% [47/64]) and lowest with the convergent approach (59.3% [80/135]), p<0.001). The difference in the proportion of patients with long-standing persistent AF (37% [72/205]), 86% [55/64] and 56% [98/174] respectively, p<0.001) may partly account for some of this difference in outcome ([Table 3]). However, when data were available, success rates in patients with persistent and long-standing persistent AF were similar to overall success rates (88.2% [82/93], 74.6% [44/59] and 51.5% [34/66] respectively, p<0.001). There was limited separately reported 12-month data for paroxysmal AF patients (78.2% [47/60], 60.0% [3/5], no data for convergent procedure, p=0.325).
Major complications ([Table 3]) were death (n=2, both with convergent approach), thromboembolic (n=3, of which 2 were with convergent approach, none fatal) and non-thromboembolic complications (n=27), consisting of 10 thoracic or retroperitoneal bleeds with or without rescue sternotomy, 6 tamponade/pericardial effusion, 2 complete heart block requiring pacemaker implantation, 2 phrenic nerve palsy, 2 pleural effusion, 4 respiratory complications and 1 with incisional pain delaying hospital discharge. Rate of death or non-fatal major complications were 7.4% overall, 8.5% with the bilateral thoracoscopic clamp-based approach, 0% with the thoracoscopic suction encircling catheter-based approach and 8.6% with the convergent approach ([Table 3]). Average length of hospital stay, when reported, was between 3.6 and 7 days (average for the three different approaches 5.6 vs. 3.8 vs. 4.4 days respectively, [Table 3]).
Hybrid Ablation vs. Sequential Catheter Ablation
For endovascular catheter ablation of paroxysmal AF, 18-month freedom from arrhythmia recurrence and antiarrhythmics was reported in 75% of 9,590 patients in a worldwide survey of 182 centers from 24 countries treated between 2004-6.[376] Results at 5 years are 47-78% after the first procedure[99,176,177] and 75-92% after repeat procedures.[99,100,176,177] In most cases, recurrence is due to recovered conduction at a prior ablation site.[99,177] A 12- to 24-month success rate of 73-92% has been reported from contemporary catheter ablation techniques to prevent late reconnection of pulmonary veins, which include using a force-sensing catheter (SmartTouch, Biosense Webster, Inc., Diamond Bar, CA) to ensure adequate contact force during ablation, a second generation cryoballoon catheter designed for better contact and surface temperature distribution (Arctic Front Advance, Medtronic, Minneapolis, MN), using failure to capture as an ablation endpoint, and incorporating a wait period and adenosine to identify reversible injury and unmask latent conduction.[326,329,331,377-379] It is unknown whether these strategies will be subject to similar rates of late attrition in success seen with earlier approaches.[177]
With persistent and long-standing persistent AF, endovascular catheter ablation yield sinus rhythm maintenance off antiarrhythmic medication in 35-77% at 12 months after a single procedure[122,129,238,240,242] and 64-79% at 18-24 months after repeat procedures.[129,242,243,376] Patients with persistent and long-standing persistent AF have a higher rate of late attrition than with paroxysmal AF,[380] with reported 5 year success rates of 45-81%.[99,129,381,382]
Complications of endovascular catheter AF ablation were seen in 6.3% of an estimated 93,801 procedures performed between 2000-10 in the National Inpatient Sample database.[383] Complications were cardiac in 2.5%, including 1.5% pericardial and 0.3% requiring rescue cardiac surgery, respiratory in 1.3%, postoperative hemorrhage in 3.4%, vascular complications in 1.5%, and neurological (thromboembolic) in 1%. In-hospital mortality was 0.5%. In the California State Inpatient Database, complications after first AF ablation between 2005-8 were seen in 5% of 4,156 patients, most commonly vascular.[384] The world-wide survey reported major complications in 4.5% of 20,825 catheter ablation procedures on 16,309 patients between 2003-6.[376] Complication rates were low in other large cohorts with patients undergoing multiple procedures (3.3% of 1,404 patients, 20% had repeat ablation;99 5.2% of 1,220 patients, 27% had repeat ablation176). Rates of pulmonary vein stenosis are 0.3-1.3% and atrio-esophageal fistula are 0-0.04%.[99,176,376] Studies reporting on the second generation cryoballoon ablation have reported higher rates of right phrenic nerve palsy (3.5-5.6%) than catheter radiofrequency ablation.[378,379] Cost-effectiveness analyses of endovascular catheter ablation in various developed countries’ healthcare models have demonstrated reasonable cost-effectiveness in patients who have paroxysmal AF, with improved quality of life and avoidance of future health care costs,[385,386] including when utilized as a first-line approach in younger patients,[387] although are sensitive to AF recurrence rates and impact on stroke risk.[388-393]
The FAST trial compared bilateral thoracoscopic epicardial ablation to endovascular catheter ablation, randomizing 124 patients with drug-refractory non-valvular AF (67% paroxysmal, 33% persistent, CHADS2 of 0 or 1 in 90%) to either surgical PVI using a bipolar radiofrequency clamp with intraoperative confirmation of block, LAA staple excision, ganglionated plexi ablation, ligament of Marshall transection and optional additional linear ablation (31%), or endovascular antral radiofrequency PVI with optional additional linear ablation (50%).[394] More patients in the surgical group were free from recurrent atrial arrhythmia off antiarrhythmics at 12 months (overall: 66 vs 37%, p=0.002; paroxysmal AF: 69 vs 35%, p=0.005; persistent AF: 56 vs 36%, p=0.341). It is unclear whether the difference in success was due to more durable lesions or the more diverse ablation targets with surgical ablation, but the results should be interpreted in light of the higher proportion with persistent AF in the endovascular catheter ablation group (41 vs 21%) and lower success rate of catheter ablation in comparison to the published contemporary data above, particularly with paroxysmal AF. There were more frequent procedural complications (23 vs 3.2%) and fewer thromboembolic events at 12 months (0/61 vs 2/63), with surgical ablation preventing an arrhythmia recurrence for every 3.4 and causing an additional complication for every 5.1 procedures.[394] The procedural complication rate was higher than that of the published hybrid ablation literature summarized above (7.4%).
To compare hybrid ablation with repeat catheter ablation, Mahapatra et al[57] matched their hybrid ablation group of 15 persistent and long-standing persistent AF patients to a control group of 30 long-standing persistent AF patients undergoing repeat catheter ablation, matching for left atrial size, duration and type of AF, lack of prior cardiac surgery, left ventricular ejection fraction and use of antiarrhythmic medications. The hybrid group had bilateral thoracoscopic clamp-based approach with delayed staged endovascular ablation at 4.3±1.3 days, multiple linear ablation sets, ganglion ablation, ligament of Marshall ablation, LAA excision, coronary sinus ablation and CFAE ablation. The catheter ablation group all had antral isolation, roof line, cavotricuspid isthmus line and optional mitral isthmus line (17 cases), coronary sinus ablation (9 cases), SVC isolation (11 cases) and CFAE ablation (12 cases). After a mean follow-up of 21 months, 87% (13/15) of hybrid ablation and 53% (16/30) of catheter-alone patients were free of atrial arrhythmia off antiarrhythmics. Repeat ablation was performed in 0/15 hybrid ablation and 3/30 catheter-alone patients.
In summary, the fundamental principle underlying hybrid ablation in assessing the eletrophysiological effects of lesions and ensuring that targets are ablated to specific endpoints to improve outcomes is incontrovertible. However, there is limited evidence supporting the concept that a multidimensional intervention targeting all possible arrhythmia mechanisms for all patients in the same sitting will result in superior results, even when these lesions are reinforced from both epicardial and endocardial sides to maximize the chances of sustained conduction block. Hybrid ablation procedures are associated with increased complications and longer post-procedural hospital stay, whilst cost-effectiveness studies have been limited by the lack of long-term outcome data.[395] Studies directly comparing hybrid with endovascular catheter ablation have had small numbers, differences in patient characteristics and ablation targets, variable periprocedural antiarrhythmic and anticoagulant management, different methods for identifying recurrence arrhythmia, and have not incorporated recent advances in endovascular ablation practice offering more durable lesions or better identifying individual-specific mechanistic targets, an understanding of which has implications on long-term tailored approaches. Outcome data from contemporary endovascular catheter approaches suggest similar success rates to hybrid ablation, both for paroxysmal and persistent patient groups, particular when repeat catheter ablations are accounted for. When considering the hybrid approach, matching intervention to mechanism is key for identifying targets, ablation endpoints, and the specific advantage over the endovascular approach for the patient at hand, balanced against the increased procedural complexity, more complications some of which are life-threatening, and longer hospital stay.
Hybrid Ablation vs. Cut-and-Sew Maze
Since the first description of the ablation-assisted open surgical maze yielding similar short-term outcomes to the traditional cut-and-sew technique, 5 others have reported that the traditional approach yields superior long-term outcomes, with hazard ratio for recurrent arrhythmia of 0.40 up to 5 years and 0.23 beyond 5 years.[396] In a meta-analysis of 16 randomized trials, the cut-and-sew approach was associated with higher sinus rhythm prevalence and lower stroke rates outcome compared to ablation-assisted approaches.[397] Lee et al compared 25 hybrid ablation patients (bilateral thoracoscopic clamp-based) to 38 cut-and-sew maze patients and reported 1 year freedom from AF and antiarrhythmic medication in 52% and 87.5%, respectively (p=0.004), even though the cut-and-sew group had more with long-standing persistent AF (40 vs 16%).[56] However, in their hybrid group, only 7 patients followed through with the endovascular catheter ablation component. There were more frequent complications in the cut-and-sew group (18 vs 4%). A systematic review comparing minimally invasive endocardial Cox-maze, minimally invasive epicardial ablation and hybrid ablation reported operative mortality at 0%, 0.5% and 0.9%; perioperative permanent pacemaker implantation in 3.5%, 2.7% and 1.5%, rescue median sternotomy in 0%, 2.4% and 2.5%, reoperation for bleeding in 1.0%, 1.5% and 2.2%, mean length of stay of 5.4, 6.0 and 4.6 days, and 12-month maintenance of sinus rhythm off antiarrhythmics in 87%, 72% and 71%, respectively.[28]
Immediate vs. Delayed Staged Hybrid Ablation
There is limited published data allowing direct comparison of immediate to delayed staging of the endovascular component of hybrid ablation. Immediate staging requires a laboratory hosting both surgical and electrophysiological setups, careful management of intraprocedural anticoagulation with transeptal and endoscopic accesses, and the available time and resources to complete both interventions in the same sitting. Delayed staging, where endovascular testing and ablation has been performed days to months after minimally invasive epicardial ablation, allows healing of the surgical wounds and time for reversible injury from ablation to abate and reconnections to establish, and has been shown to increase the likelihood of discovering PV reconnection during endocardial mapping versus a same day procedure (48% vs 14%).[398] It can be performed in separate surgical and electrophysiological laboratory setups. Patients may prefer a procedure where both components are completed in the same sitting or during the same hospitalization.
Future Directions
Atrial fibrillation ablation offers considerable benefits to patients towards symptom control and quality of life improvement.[75] The multiplicity and progressive nature of AF mechanisms and recovery of conduction may account for progressive attrition in maintaining sinus rhythm with long-term follow-up.[129,380] The pioneering developments that have led to the various hybrid ablation approaches are an opportunity in properly selected patients without having to recourse to open heart surgery. The choice of procedural approach utilized for hybrid ablation is important, given the difference in possible lesion sets, success rates, complications, adjunctive LAA closure and access to adjunctive ablation targets. However, randomized trial data are lacking and long-term efficacy is unproven. The PRHACA (Prospective, Randomized Comparison of Hybrid Ablation vs. Catheter Ablation) trial (clinicaltrials.gov/ NCT02344394) is an investigator sponsored trial which is currently recruiting patients with persistent AF to test the nContact system (convergent approach). The CONVERGE (Epi/Endo Ablation For Treatment of Persistent Atrial Fibrillation) trial (clinicaltrials.gov/NCT01984346) is an industry sponsored trial which is also recruiting persistent AF patients to test the nContact system. The SCALAF trial (clinicaltrials.gov/NCT00703157), which aims to compare efficacy of minimally invasive surgical and catheter-based PVI, is underway. Another active trial (clinicaltrials.gov/NCT02392338) is comparing hybrid ablation with minimally invasive thoracoscopic ablation alone in persistent AF.
A promise of progress comes from an improved understanding of how anatomical substrate relates to electrophysiological observations, better catheters and mapping technologies, novel energy sources, adequate management of recurrences, and identifying and treating underlying clinical risk factors. Whether hybrid ablation will add to this remains to be seen. In the absence of robust efficacy data, and given the increased risk of complications, associated morbidity and length of hospital stay as compared to catheter ablation, caution should be exercised in adopting this approach universally. The present data suggest that, perhaps, with appropriate patient selection, accurate identification of patient-specific mechanisms and targets, and selection of optimal access to maximize anatomical vantage to these targets, this novel approach may have a role in specific situations. It may be better suited as a concomitant procedure during cardiac surgery for other reasons, for example patients requiring thrombectomy or LAA closure. Alternatively, it may help translate novel therapeutic pathways to practice, such as controlled delivery of genetic vectors influencing arrhythmia mechanisms[399-401] or humeral mediators governing response to injury.[402-404]
Disclosures
None.
References
- 1.Cox J L, Boineau J P, Schuessler R B, Ferguson T B, Cain M E, Lindsay B D, Corr P B, Kater K M, Lappas D G. Successful surgical treatment of atrial fibrillation. Review and clinical update. JAMA. 1991 Oct 9;266 (14):1976–80. [PubMed] [Google Scholar]
- 2.Cox J L, Schuessler R B, D'Agostino H J, Stone C M, Chang B C, Cain M E, Corr P B, Boineau J P. The surgical treatment of atrial fibrillation. III. Development of a definitive surgical procedure. J. Thorac. Cardiovasc. Surg. 1991 Apr;101 (4):569–83. [PubMed] [Google Scholar]
- 3.Gaynor Sydney L, Schuessler Richard B, Bailey Marci S, Ishii Yosuke, Boineau John P, Gleva Marye J, Cox James L, Damiano Ralph J. Surgical treatment of atrial fibrillation: predictors of late recurrence. J. Thorac. Cardiovasc. Surg. 2005 Jan;129 (1):104–11. doi: 10.1016/j.jtcvs.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 4.Weimar Timo, Schena Stefano, Bailey Marci S, Maniar Hersh S, Schuessler Richard B, Cox James L, Damiano Ralph J. The cox-maze procedure for lone atrial fibrillation: a single-center experience over 2 decades. Circ Arrhythm Electrophysiol. 2012 Feb;5 (1):8–14. doi: 10.1161/CIRCEP.111.963819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaynor Sydney L, Diodato Michael D, Prasad Sunil M, Ishii Yosuke, Schuessler Richard B, Bailey Marci S, Damiano Nicholas R, Bloch Jeffrey B, Moon Marc R, Damiano Ralph J. A prospective, single-center clinical trial of a modified Cox maze procedure with bipolar radiofrequency ablation. J. Thorac. Cardiovasc. Surg. 2004 Oct;128 (4):535–42. doi: 10.1016/j.jtcvs.2004.02.044. [DOI] [PubMed] [Google Scholar]
- 6.Gammie James S, Haddad Michel, Milford-Beland Sarah, Welke Karl F, Ferguson T Bruce, O'Brien Sean M, Griffith Bartley P, Peterson Eric D. Atrial fibrillation correction surgery: lessons from the Society of Thoracic Surgeons National Cardiac Database. Ann. Thorac. Surg. 2008 Mar;85 (3):909–14. doi: 10.1016/j.athoracsur.2007.10.097. [DOI] [PubMed] [Google Scholar]
- 7.Wazni Oussama M, Marrouche Nassir F, Martin David O, Verma Atul, Bhargava Mandeep, Saliba Walid, Bash Dianna, Schweikert Robert, Brachmann Johannes, Gunther Jens, Gutleben Klaus, Pisano Ennio, Potenza Dominico, Fanelli Raffaele, Raviele Antonio, Themistoclakis Sakis, Rossillo Antonio, Bonso Aldo, Natale Andrea. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA. 2005 Jun 1;293 (21):2634–40. doi: 10.1001/jama.293.21.2634. [DOI] [PubMed] [Google Scholar]
- 8.Oral Hakan, Pappone Carlo, Chugh Aman, Good Eric, Bogun Frank, Pelosi Frank, Bates Eric R, Lehmann Michael H, Vicedomini Gabriele, Augello Giuseppe, Agricola Eustachio, Sala Simone, Santinelli Vincenzo, Morady Fred. Circumferential pulmonary-vein ablation for chronic atrial fibrillation. N. Engl. J. Med. 2006 Mar 2;354 (9):934–41. doi: 10.1056/NEJMoa050955. [DOI] [PubMed] [Google Scholar]
- 9.Jaïs Pierre, Cauchemez Bruno, Macle Laurent, Daoud Emile, Khairy Paul, Subbiah Rajesh, Hocini Mélèze, Extramiana Fabrice, Sacher Fréderic, Bordachar Pierre, Klein George, Weerasooriya Rukshen, Clémenty Jacques, Haïssaguerre Michel. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation. 2008 Dec 9;118 (24):2498–505. doi: 10.1161/CIRCULATIONAHA.108.772582. [DOI] [PubMed] [Google Scholar]
- 10.Cosedis Nielsen Jens, Johannessen Arne, Raatikainen Pekka, Hindricks Gerhard, Walfridsson Håkan, Kongstad Ole, Pehrson Steen, Englund Anders, Hartikainen Juha, Mortensen Leif Spange, Hansen Peter Steen. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N. Engl. J. Med. 2012 Oct 25;367 (17):1587–95. doi: 10.1056/NEJMoa1113566. [DOI] [PubMed] [Google Scholar]
- 11.Jones David G, Haldar Shouvik K, Hussain Wajid, Sharma Rakesh, Francis Darrel P, Rahman-Haley Shelley L, McDonagh Theresa A, Underwood S Richard, Markides Vias, Wong Tom. A randomized trial to assess catheter ablation versus rate control in the management of persistent atrial fibrillation in heart failure. J. Am. Coll. Cardiol. 2013 May 7;61 (18):1894–903. doi: 10.1016/j.jacc.2013.01.069. [DOI] [PubMed] [Google Scholar]
- 12.Morillo Carlos A, Verma Atul, Connolly Stuart J, Kuck Karl H, Nair Girish M, Champagne Jean, Sterns Laurence D, Beresh Heather, Healey Jeffrey S, Natale Andrea. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of paroxysmal atrial fibrillation (RAAFT-2): a randomized trial. JAMA. 2014 Feb 19;311 (7):692–700. doi: 10.1001/jama.2014.467. [DOI] [PubMed] [Google Scholar]
- 13.Hunter Ross J, Berriman Thomas J, Diab Ihab, Kamdar Ravindu, Richmond Laura, Baker Victoria, Goromonzi Farai, Sawhney Vinit, Duncan Edward, Page Stephen P, Ullah Waqas, Unsworth Beth, Mayet Jamil, Dhinoja Mehul, Earley Mark J, Sporton Simon, Schilling Richard J. A randomized controlled trial of catheter ablation versus medical treatment of atrial fibrillation in heart failure (the CAMTAF trial). Circ Arrhythm Electrophysiol. 2014 Feb;7 (1):31–8. doi: 10.1161/CIRCEP.113.000806. [DOI] [PubMed] [Google Scholar]
- 14.Pak Hui-Nam, Hwang Chun, Lim Hong Euy, Kim Jin Seok, Kim Young-Hoon. Hybrid epicardial and endocardial ablation of persistent or permanent atrial fibrillation: a new approach for difficult cases. J. Cardiovasc. Electrophysiol. 2007 Sep;18 (9):917–23. doi: 10.1111/j.1540-8167.2007.00882.x. [DOI] [PubMed] [Google Scholar]
- 15.Kottkamp Hans, Hindricks Gerhard, Autschbach Rüdiger, Krauss Beate, Strasser Bernhard, Schirdewahn Petra, Fabricius Alexander, Schuler Gerhard, Mohr Friedrich-Wilhelm. Specific linear left atrial lesions in atrial fibrillation: intraoperative radiofrequency ablation using minimally invasive surgical techniques. J. Am. Coll. Cardiol. 2002 Aug 7;40 (3):475–80. doi: 10.1016/s0735-1097(02)01993-9. [DOI] [PubMed] [Google Scholar]
- 16.Salenger Rawn, Lahey Stephen J, Saltman Adam E. The completely endoscopic treatment of atrial fibrillation: report on the first 14 patients with early results. Heart Surg Forum. 2004;7 (6):E555–8. doi: 10.1532/HSF98.20041111. [DOI] [PubMed] [Google Scholar]
- 17.Wolf Randall K, Schneeberger E William, Osterday Robert, Miller Doug, Merrill Walter, Flege John B, Gillinov A Marc. Video-assisted bilateral pulmonary vein isolation and left atrial appendage exclusion for atrial fibrillation. J. Thorac. Cardiovasc. Surg. 2005 Sep;130 (3):797–802. doi: 10.1016/j.jtcvs.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 18.Beyer Erik, Lee Richard, Lam Buu-Khanh. Point: Minimally invasive bipolar radiofrequency ablation of lone atrial fibrillation: early multicenter results. J. Thorac. Cardiovasc. Surg. 2009 Mar;137 (3):521–6. doi: 10.1016/j.jtcvs.2008.11.031. [DOI] [PubMed] [Google Scholar]
- 19.Edgerton James R, Jackman Warren M, Mack Michael J. A new epicardial lesion set for minimal access left atrial maze: the Dallas lesion set. Ann. Thorac. Surg. 2009 Nov;88 (5):1655–7. doi: 10.1016/j.athoracsur.2009.05.046. [DOI] [PubMed] [Google Scholar]
- 20.Han Frederick T, Kasirajan Vigneshwar, Kowalski Marcin, Kiser Robert, Wolfe Luke, Kalahasty Gautham, Shepard Richard K, Wood Mark A, Ellenbogen Kenneth A. Results of a minimally invasive surgical pulmonary vein isolation and ganglionic plexi ablation for atrial fibrillation: single-center experience with 12-month follow-up. Circ Arrhythm Electrophysiol. 2009 Aug;2 (4):370–7. doi: 10.1161/CIRCEP.109.854828. [DOI] [PubMed] [Google Scholar]
- 21.Kiser AC, Cockfield W. Paracardioscopic ex-maze procedure for atrial fibrillation. Multimedia manual of cardiothoracic surgery. MMCTS / European Association for Cardio-Thoracic Surgery. 2010;0:0–0. doi: 10.1510/mmcts.2008.003863. [DOI] [PubMed] [Google Scholar]
- 22.Ad Niv, Suri Rakesh M, Gammie James S, Sheng Shubin, O'Brien Sean M, Henry Linda. Surgical ablation of atrial fibrillation trends and outcomes in North America. J. Thorac. Cardiovasc. Surg. 2012 Nov;144 (5):1051–60. doi: 10.1016/j.jtcvs.2012.07.065. [DOI] [PubMed] [Google Scholar]
- 23.Khargi Krishna, Hutten Barbara A, Lemke Bernd, Deneke Thomas. Surgical treatment of atrial fibrillation; a systematic review. Eur J Cardiothorac Surg. 2005 Feb;27 (2):258–65. doi: 10.1016/j.ejcts.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Moten Simon C M, Rodriguez Evelio, Cook Richard C, Nifong L Wiley, Chitwood W Randolph. New ablation techniques for atrial fibrillation and the minimally invasive cryo-maze procedure in patients with lone atrial fibrillation. Heart Lung Circ. 2007;16 Suppl 3 ():S88–93. doi: 10.1016/j.hlc.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Damiano Ralph J, Schwartz Forrest H, Bailey Marci S, Maniar Hersh S, Munfakh Nabil A, Moon Marc R, Schuessler Richard B. The Cox maze IV procedure: predictors of late recurrence. J. Thorac. Cardiovasc. Surg. 2011 Jan;141 (1):113–21. doi: 10.1016/j.jtcvs.2010.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ad Niv, Henry Linda, Friehling Ted, Wish Marc, Holmes Sari D. Minimally invasive stand-alone Cox-maze procedure for patients with nonparoxysmal atrial fibrillation. Ann. Thorac. Surg. 2013 Sep;96 (3):792–8. doi: 10.1016/j.athoracsur.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 27.Krul Sébastien P J, Driessen Antoine H G, Zwinderman Aeilko H, van Boven Wim J, Wilde Arthur A M, de Bakker Jacques M T, de Groot Joris R. Navigating the mini-maze: systematic review of the first results and progress of minimally-invasive surgery in the treatment of atrial fibrillation. Int. J. Cardiol. 2013 Jun 5;166 (1):132–40. doi: 10.1016/j.ijcard.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 28.Je HG, Shuman DJ, Ad N. A systematic review of minimally invasive surgical treatment for atrial fibrillation: a comparison of the Cox-Maze procedure, beatingheart epicardial ablation, and the hybrid procedure on safety and efficacydagger. European journal of cardio-thoracic surgery. official journal of the European Association for Cardio-thoracic Surgery. 2015;0:0–0. doi: 10.1093/ejcts/ezu536. [DOI] [PubMed] [Google Scholar]
- 29.Lockwood Deborah, Nakagawa Hiroshi, Peyton Marvin D, Edgerton James R, Scherlag Benjamin J, Sivaram Chittur A, Po Sunny S, Beckman Karen J, Abedin Moeen, Jackman Warren M. Linear left atrial lesions in minimally invasive surgical ablation of persistent atrial fibrillation: techniques for assessing conduction block across surgical lesions. Heart Rhythm. 2009 Dec;6 (12 Suppl):S50–63. doi: 10.1016/j.hrthm.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 30.Kron Jordana, Kasirajan Vigneshwar, Wood Mark A, Kowalski Marcin, Han Frederick T, Ellenbogen Kenneth A. Management of recurrent atrial arrhythmias after minimally invasive surgical pulmonary vein isolation and ganglionic plexi ablation for atrial fibrillation. Heart Rhythm. 2010 Apr;7 (4):445–51. doi: 10.1016/j.hrthm.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 31.Krul Sébastien P J, Driessen Antoine H G, van Boven Wim J, Linnenbank André C, Geuzebroek Guillaume S C, Jackman Warren M, Wilde Arthur A M, de Bakker Jacques M T, de Groot Joris R. Thoracoscopic video-assisted pulmonary vein antrum isolation, ganglionated plexus ablation, and periprocedural confirmation of ablation lesions: first results of a hybrid surgical-electrophysiological approach for atrial fibrillation. Circ Arrhythm Electrophysiol. 2011 Jun;4 (3):262–70. doi: 10.1161/CIRCEP.111.961862. [DOI] [PubMed] [Google Scholar]
- 32.Gersak Borut, Kiser Andy C, Bartus Krzysztof, Sadowski Jerzy, Harringer Wolfgang, Knaut Michael, Wimmer-Greinecker Gerhard, Pernat Andrej. Importance of evaluating conduction block in radiofrequency ablation for atrial fibrillation. Eur J Cardiothorac Surg. 2012 Jan;41 (1):113–8. doi: 10.1016/j.ejcts.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pison Laurent, La Meir Mark, van Opstal Jurren, Blaauw Yuri, Maessen Jos, Crijns Harry J. Hybrid thoracoscopic surgical and transvenous catheter ablation of atrial fibrillation. J. Am. Coll. Cardiol. 2012 Jul 3;60 (1):54–61. doi: 10.1016/j.jacc.2011.12.055. [DOI] [PubMed] [Google Scholar]
- 34.Schuessler Richard B, Lee Anson M, Melby Spencer J, Voeller Rochus K, Gaynor Sydney L, Sakamoto Shun-Ichiro, Damiano Ralph J. Animal studies of epicardial atrial ablation. Heart Rhythm. 2009 Dec;6 (12 Suppl):S41–5. doi: 10.1016/j.hrthm.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melby Spencer J, Zierer Andreas, Kaiser Scott P, Schuessler Richard B, Damiano Ralph J. Epicardial microwave ablation on the beating heart for atrial fibrillation: the dependency of lesion depth on cardiac output. J. Thorac. Cardiovasc. Surg. 2006 Aug;132 (2):355–60. doi: 10.1016/j.jtcvs.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 36.Melby Spencer J, Gaynor Sydney L, Lubahn Jordon G, Lee Anson M, Rahgozar Paymon, Caruthers Shelton D, Williams Todd A, Schuessler Richard B, Damiano Ralph J. Efficacy and safety of right and left atrial ablations on the beating heart with irrigated bipolar radiofrequency energy: a long-term animal study. J. Thorac. Cardiovasc. Surg. 2006 Oct;132 (4):853–60. doi: 10.1016/j.jtcvs.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 37.Usui Akihiko, Inden Yasuya, Mizutani Shinichi, Takagi Yasushi, Akita Toshiaki, Ueda Yuichi. Repetitive atrial flutter as a complication of the left-sided simple maze procedure. Ann. Thorac. Surg. 2002 May;73 (5):1457–9. doi: 10.1016/s0003-4975(02)03506-3. [DOI] [PubMed] [Google Scholar]
- 38.Kobza Richard, Kottkamp Hans, Dorszewski Anja, Tanner Hildegard, Piorkowski Christopher, Schirdewahn Petra, Gerds-Li Jin-Hong, Hindricks Gerhard. Stable secondary arrhythmias late after intraoperative radiofrequency ablation of atrial fibrillation: incidence, mechanism, and treatment. J. Cardiovasc. Electrophysiol. 2004 Nov;15 (11):1246–9. doi: 10.1046/j.1540-8167.2004.04356.x. [DOI] [PubMed] [Google Scholar]
- 39.Deneke Thomas, Khargi Krishna, Grewe Peter H, Calcum Bernd, Laczkovics Axel, Keyhan-Falsafi Ali, Mügge Andreas, Lawo Thomas, Lemke Bernd. Catheter ablation of regular atrial arrhythmia following surgical treatment of permanent atrial fibrillation. J. Cardiovasc. Electrophysiol. 2006 Jan;17 (1):18–24. doi: 10.1111/j.1540-8167.2005.00265.x. [DOI] [PubMed] [Google Scholar]
- 40.Melby Spencer J, Lee Anson M, Zierer Andreas, Kaiser Scott P, Livhits Masha J, Boineau John P, Schuessler Richard B, Damiano Ralph J. Atrial fibrillation propagates through gaps in ablation lines: implications for ablative treatment of atrial fibrillation. Heart Rhythm. 2008 Sep;5 (9):1296–301. doi: 10.1016/j.hrthm.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bertaglia Emanuele, Stabile Giuseppe, Senatore Gaetano, Pratola Claudio, Verlato Roberto, Lowe Martin, Raatikainen Pekka, Lamberti Filippo, Turco Pietro. Documentation of pulmonary vein isolation improves long term efficacy of persistent atrial fibrillation catheter ablation. Int. J. Cardiol. 2014 Feb 1;171 (2):174–8. doi: 10.1016/j.ijcard.2013.11.055. [DOI] [PubMed] [Google Scholar]
- 42.Ouyang Feifan, Ernst Sabine, Chun Julian, Bänsch Dietmar, Li Yigang, Schaumann Anselm, Mavrakis Hercules, Liu Xingpeng, Deger Florian T, Schmidt Boris, Xue Yumei, Cao Jiang, Hennig Detlef, Huang He, Kuck Karl-Heinz, Antz Matthias. Electrophysiological findings during ablation of persistent atrial fibrillation with electroanatomic mapping and double Lasso catheter technique. Circulation. 2005 Nov 15;112 (20):3038–48. doi: 10.1161/CIRCULATIONAHA.105.561183. [DOI] [PubMed] [Google Scholar]
- 43.Willems Stephan, Steven Daniel, Servatius Helge, Hoffmann Boris A, Drewitz Imke, Müllerleile Kai, Aydin Muhammet Ali, Wegscheider Karl, Salukhe Tushar V, Meinertz Thomas, Rostock Thomas. Persistence of pulmonary vein isolation after robotic remote-navigated ablation for atrial fibrillation and its relation to clinical outcome. J. Cardiovasc. Electrophysiol. 2010 Oct;21 (10):1079–84. doi: 10.1111/j.1540-8167.2010.01773.x. [DOI] [PubMed] [Google Scholar]
- 44.Ouyang Feifan, Ernst Sabine, Vogtmann Thomas, Goya Masahiko, Volkmer Marius, Schaumann Anselm, Bänsch Dietmar, Antz Matthias, Kuck Karl-Heinz. Characterization of reentrant circuits in left atrial macroreentrant tachycardia: critical isthmus block can prevent atrial tachycardia recurrence. Circulation. 2002 Apr 23;105 (16):1934–42. doi: 10.1161/01.cir.0000015077.12680.2e. [DOI] [PubMed] [Google Scholar]
- 45.Ernst Sabine, Ouyang Feifan, Löber Felix, Antz Matthias, Kuck Karl-Heinz. Catheter-induced linear lesions in the left atrium in patients with atrial fibrillation: an electroanatomic study. J. Am. Coll. Cardiol. 2003 Oct 1;42 (7):1271–82. doi: 10.1016/s0735-1097(03)00940-9. [DOI] [PubMed] [Google Scholar]
- 46.Pappone Carlo, Manguso Francesco, Vicedomini Gabriele, Gugliotta Filippo, Santinelli Ornella, Ferro Amedeo, Gulletta Simone, Sala Simone, Sora Nicoleta, Paglino Gabriele, Augello Giuseppe, Agricola Eustachio, Zangrillo Alberto, Alfieri Ottavio, Santinelli Vincenzo. Prevention of iatrogenic atrial tachycardia after ablation of atrial fibrillation: a prospective randomized study comparing circumferential pulmonary vein ablation with a modified approach. Circulation. 2004 Nov 9;110 (19):3036–42. doi: 10.1161/01.CIR.0000147186.83715.95. [DOI] [PubMed] [Google Scholar]
- 47.Gerstenfeld Edward P, Callans David J, Dixit Sanjay, Russo Andrea M, Nayak Hemal, Lin David, Pulliam Ward, Siddique Sultan, Marchlinski Francis E. Mechanisms of organized left atrial tachycardias occurring after pulmonary vein isolation. Circulation. 2004 Sep 14;110 (11):1351–7. doi: 10.1161/01.CIR.0000141369.50476.D3. [DOI] [PubMed] [Google Scholar]
- 48.Haïssaguerre Michel, Hocini Mélèze, Sanders Prashanthan, Sacher Frederic, Rotter Martin, Takahashi Yoshihide, Rostock Thomas, Hsu Li-Fern, Bordachar Pierre, Reuter Sylvain, Roudaut Raymond, Clémenty Jacques, Jaïs Pierre. Catheter ablation of long-lasting persistent atrial fibrillation: clinical outcome and mechanisms of subsequent arrhythmias. J. Cardiovasc. Electrophysiol. 2005 Nov;16 (11):1138–47. doi: 10.1111/j.1540-8167.2005.00308.x. [DOI] [PubMed] [Google Scholar]
- 49.Hocini Mélèze, Sanders Prashanthan, Jaïs Pierre, Hsu Li-Fern, Weerasoriya Rukshen, Scavée Christophe, Takahashi Yoshihide, Rotter Martin, Raybaud Florence, Macle Laurent, Clémenty Jacques, Haïssaguerre Michel. Prevalence of pulmonary vein disconnection after anatomical ablation for atrial fibrillation: consequences of wide atrial encircling of the pulmonary veins. Eur. Heart J. 2005 Apr;26 (7):696–704. doi: 10.1093/eurheartj/ehi096. [DOI] [PubMed] [Google Scholar]
- 50.Rostock Thomas, O'Neill Mark D, Sanders Prashanthan, Rotter Martin, Jaïs Pierre, Hocini Mélèze, Takahashi Yoshihide, Sacher Fréderic, Jönsson Anders, Hsu Li-Fern, Clémenty Jacques, Haïssaguerre Michel. Characterization of conduction recovery across left atrial linear lesions in patients with paroxysmal and persistent atrial fibrillation. J. Cardiovasc. Electrophysiol. 2006 Oct;17 (10):1106–11. doi: 10.1111/j.1540-8167.2006.00585.x. [DOI] [PubMed] [Google Scholar]
- 51.Knecht Sébastien, Hocini Mélèze, Wright Matthew, Lellouche Nicolas, O'Neill Mark D, Matsuo Seiichiro, Nault Isabelle, Chauhan Vijay S, Makati Kevin J, Bevilacqua Michela, Lim Kang-Teng, Sacher Frederic, Deplagne Antoine, Derval Nicolas, Bordachar Pierre, Jaïs Pierre, Clémenty Jacques, Haïssaguerre Michel. Left atrial linear lesions are required for successful treatment of persistent atrial fibrillation. Eur. Heart J. 2008 Oct;29 (19):2359–66. doi: 10.1093/eurheartj/ehn302. [DOI] [PubMed] [Google Scholar]
- 52.Chae Sanders, Oral Hakan, Good Eric, Dey Sujoya, Wimmer Alan, Crawford Thomas, Wells Darryl, Sarrazin Jean-Francois, Chalfoun Nagib, Kuhne Michael, Fortino Jackie, Huether Elizabeth, Lemerand Tammy, Pelosi Frank, Bogun Frank, Morady Fred, Chugh Aman. Atrial tachycardia after circumferential pulmonary vein ablation of atrial fibrillation: mechanistic insights, results of catheter ablation, and risk factors for recurrence. J. Am. Coll. Cardiol. 2007 Oct 30;50 (18):1781–7. doi: 10.1016/j.jacc.2007.07.044. [DOI] [PubMed] [Google Scholar]
- 53.Matsuo Seiichiro, Wright Matthew, Knecht Sébastien, Nault Isabelle, Lellouche Nicolas, Lim Kang-Teng, Arantes Leonardo, O'Neill Mark D, Hocini Mélèze, Jaïs Pierre, Haïssaguerre Michel. Peri-mitral atrial flutter in patients with atrial fibrillation ablation. Heart Rhythm. 2010 Jan;7 (1):2–8. doi: 10.1016/j.hrthm.2009.09.067. [DOI] [PubMed] [Google Scholar]
- 54.Yokokawa Miki, Latchamsetty Rakesh, Ghanbari Hamid, Belardi Diego, Makkar Akash, Roberts Brett, Saint-Phard Wouter, Sinno Mohamad, Carrigan Thomas, Kennedy Robert, Suwanagool Arisara, Good Eric, Crawford Thomas, Jongnarangsin Krit, Pelosi Frank, Bogun Frank, Oral Hakan, Morady Fred, Chugh Aman. Characteristics of atrial tachycardia due to small vs large reentrant circuits after ablation of persistent atrial fibrillation. Heart Rhythm. 2013 Apr;10 (4):469–76. doi: 10.1016/j.hrthm.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 55.Kiser Andy C, Landers Mark, Horton Rodney, Hume Andrew, Natale Andrea, Gersak Borut. The convergent procedure: a multidisciplinary atrial fibrillation treatment. Heart Surg Forum. 2010 Oct;13 (5):E317–21. doi: 10.1532/HSF98.20091112. [DOI] [PubMed] [Google Scholar]
- 56.Lee Richard, McCarthy Patrick M, Passman Rod S, Kruse Jane, Malaisrie S Chris, McGee Edwin C, Lapin Brittany, Jacobson Jason T, Goldberger Jeffrey, Knight Bradley P. Surgical treatment for isolated atrial fibrillation: minimally invasive vs. classic cut and sew maze. Innovations (Phila) 2011 Nov;6 (6):373–7. doi: 10.1097/IMI.0b013e318248f3f4. [DOI] [PubMed] [Google Scholar]
- 57.Mahapatra Srijoy, LaPar Damien J, Kamath Sandeep, Payne Jason, Bilchick Kenneth C, Mangrum James M, Ailawadi Gorav. Initial experience of sequential surgical epicardial-catheter endocardial ablation for persistent and long-standing persistent atrial fibrillation with long-term follow-up. Ann. Thorac. Surg. 2011 Jun;91 (6):1890–8. doi: 10.1016/j.athoracsur.2011.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pison Laurent, Dagres Nikolaos, Lewalter Thorsten, Proclemer Alessandro, Marinskis Germanas, Blomström-Lundqvist Carina. Surgical and hybrid atrial fibrillation ablation procedures. Europace. 2012 Jul;14 (7):939–41. doi: 10.1093/europace/eus207. [DOI] [PubMed] [Google Scholar]
- 59.La Meir Mark, Gelsomino Sandro, Lorusso Roberto, Lucà Fabiana, Pison Laurant, Parise Orlando, Wellens Francis, Gensini Gian Franco, Maessen Jos. The hybrid approach for the surgical treatment of lone atrial fibrillation: one-year results employing a monopolar radiofrequency source. J Cardiothorac Surg. 2012;7 () doi: 10.1186/1749-8090-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bisleri Gianluigi, Rosati Fabrizio, Bontempi Luca, Curnis Antonio, Muneretto Claudio. Hybrid approach for the treatment of long-standing persistent atrial fibrillation: electrophysiological findings and clinical results. Eur J Cardiothorac Surg. 2013 Nov;44 (5):919–23. doi: 10.1093/ejcts/ezt115. [DOI] [PubMed] [Google Scholar]
- 61.Gehi Anil K, Mounsey J Paul, Pursell Irion, Landers Mark, Boyce Ker, Chung Eugene H, Schwartz Jennifer, Walker T Jennifer, Guise Kimberly, Kiser Andy C. Hybrid epicardial-endocardial ablation using a pericardioscopic technique for the treatment of atrial fibrillation. Heart Rhythm. 2013 Jan;10 (1):22–8. doi: 10.1016/j.hrthm.2012.08.044. [DOI] [PubMed] [Google Scholar]
- 62.La Meir Mark, Gelsomino Sandro, Lucà Fabiana, Pison Laurant, Parise Orlando, Colella Andrea, Gensini Gian Franco, Crijns Harry, Wellens Francis, Maessen Jos G. Minimally invasive surgical treatment of lone atrial fibrillation: early results of hybrid versus standard minimally invasive approach employing radiofrequency sources. Int. J. Cardiol. 2013 Aug 20;167 (4):1469–75. doi: 10.1016/j.ijcard.2012.04.044. [DOI] [PubMed] [Google Scholar]
- 63.Kurfirst Vojtěch, Mokraček Aleš, Bulava Alan, Čanadyova Júlia, Haniš Jiři, Pešl Ladislav. Two-staged hybrid treatment of persistent atrial fibrillation: short-term single-centre results. Interact Cardiovasc Thorac Surg. 2014 Apr;18 (4):451–6. doi: 10.1093/icvts/ivt538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee Hee Moon, Chung Su Ryeun, Jeong Dong Seop. Initial experience with total thoracoscopic ablation. Korean J Thorac Cardiovasc Surg. 2014 Feb;47 (1):1–5. doi: 10.5090/kjtcs.2014.47.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pison Laurent, Gelsomino Sandro, Lucà Fabiana, Parise Orlando, Maessen Jos G, Crijns Harry J G M, La Meir Mark. Effectiveness and safety of simultaneous hybrid thoracoscopic and endocardial catheter ablation of lone atrial fibrillation. Ann Cardiothorac Surg. 2014 Jan;3 (1):38–44. doi: 10.3978/j.issn.2225-319X.2013.12.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Geršak Borut, Zembala Michael O, Müller Dirk, Folliguet Thierry, Jan Matevz, Kowalski Oskar, Erler Stefan, Bars Clement, Robic Boris, Filipiak Krzysztof, Wimmer-Greinecker Gerhard. European experience of the convergent atrial fibrillation procedure: multicenter outcomes in consecutive patients. J. Thorac. Cardiovasc. Surg. 2014 Apr;147 (4):1411–6. doi: 10.1016/j.jtcvs.2013.06.057. [DOI] [PubMed] [Google Scholar]
- 67.Kiser Andy C, Landers Mark D, Boyce Ker, Sinkovec Matjaz, Pernat Andrej, Geršak Borut. Simultaneous catheter and epicardial ablations enable a comprehensive atrial fibrillation procedure. Innovations (Phila) 2011 Jul;6 (4):243–7. doi: 10.1097/IMI.0b013e31822ca15c. [DOI] [PubMed] [Google Scholar]
- 68.Gersak Borut, Pernat Andrej, Robic Boris, Sinkovec Matjaz. Low rate of atrial fibrillation recurrence verified by implantable loop recorder monitoring following a convergent epicardial and endocardial ablation of atrial fibrillation. J. Cardiovasc. Electrophysiol. 2012 Oct;23 (10):1059–66. doi: 10.1111/j.1540-8167.2012.02355.x. [DOI] [PubMed] [Google Scholar]
- 69.Zembala Michał, Filipiak Krzysztof, Kowalski Oskar, Boidol Joanna, Sokal Adam, Lenarczyk Radosław, Niklewski Tomasz, Garbacz Marcin, Nadziakiewicz Paweł, Kalarus Zbigniew, Zembala Marian. Minimally invasive hybrid ablation procedure for the treatment of persistent atrial fibrillation: one year results. Kardiol Pol. 2012;70 (8):819–28. [PubMed] [Google Scholar]
- 70.Muneretto Claudio, Bisleri Gianluigi, Bontempi Luca, Curnis Antonio. Durable staged hybrid ablation with thoracoscopic and percutaneous approach for treatment of long-standing atrial fibrillation: a 30-month assessment with continuous monitoring. J. Thorac. Cardiovasc. Surg. 2012 Dec;144 (6):1460–5. doi: 10.1016/j.jtcvs.2012.08.069. [DOI] [PubMed] [Google Scholar]
- 71.Gilligan DM, Joyner CA, Bundy GM. Multidisciplinary collaboration for the treatment of atrial fibrillation: convergent procedure outcomes from a single center. J Innov CRM. 2013;0:1396–1403. [Google Scholar]
- 72.Civello KC, Smith CA, Boedefeld W. Combined endocardial and epicardial ablation for symptomatic atrial fibrillation: single center experience in 100+ consecutive patients. J Innov CRM. 2013;0:1–7. [Google Scholar]
- 73.Thosani AJ, Gerczuk P, Liu E, Belden W, Moraca R. Closed chest convergent epicardial-endocardial ablation of non-paroxysmal atrial fibrillation - a case series and literature review. Arrhythmia and Electrophysiology Review. 2013;2:65–68. doi: 10.15420/aer.2013.2.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Robinson MC, Chiravuri M, McPherson C, Winslow R. Maximizing ablation, limiting invasiveness, and being realistic about atrial fibrillation: the convergent hybrid ablation procedure for advanced AF. EP Lab Digest. 2013;13:0–0. [Google Scholar]
- 75.January Craig T, Wann L Samuel, Alpert Joseph S, Calkins Hugh, Cigarroa Joaquin E, Cleveland Joseph C, Conti Jamie B, Ellinor Patrick T, Ezekowitz Michael D, Field Michael E, Murray Katherine T, Sacco Ralph L, Stevenson William G, Tchou Patrick J, Tracy Cynthia M, Yancy Clyde W. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2014 Dec 2;64 (21):e1–76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 76.Pinho-Gomes Ana C, Amorim Mário J, Oliveira Sílvia M, Leite-Moreira Adelino F. Surgical treatment of atrial fibrillation: an updated review. Eur J Cardiothorac Surg. 2014 Aug;46 (2):167–78. doi: 10.1093/ejcts/ezt584. [DOI] [PubMed] [Google Scholar]
- 77.Calkins Hugh, Kuck Karl Heinz, Cappato Riccardo, Brugada Josep, Camm A John, Chen Shih-Ann, Crijns Harry J G, Damiano Ralph J, Davies D Wyn, DiMarco John, Edgerton James, Ellenbogen Kenneth, Ezekowitz Michael D, Haines David E, Haissaguerre Michel, Hindricks Gerhard, Iesaka Yoshito, Jackman Warren, Jalife José, Jais Pierre, Kalman Jonathan, Keane David, Kim Young-Hoon, Kirchhof Paulus, Klein George, Kottkamp Hans, Kumagai Koichiro, Lindsay Bruce D, Mansour Moussa, Marchlinski Francis E, McCarthy Patrick M, Mont J Lluis, Morady Fred, Nademanee Koonlawee, Nakagawa Hiroshi, Natale Andrea, Nattel Stanley, Packer Douglas L, Pappone Carlo, Prystowsky Eric, Raviele Antonio, Reddy Vivek, Ruskin Jeremy N, Shemin Richard J, Tsao Hsuan-Ming, Wilber David. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm. 2012 Apr;9 (4):632–696.e21. doi: 10.1016/j.hrthm.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 78.Marrouche Nassir F, Wilber David, Hindricks Gerhard, Jais Pierre, Akoum Nazem, Marchlinski Francis, Kholmovski Eugene, Burgon Nathan, Hu Nan, Mont Lluis, Deneke Thomas, Duytschaever Mattias, Neumann Thomas, Mansour Moussa, Mahnkopf Christian, Herweg Bengt, Daoud Emile, Wissner Erik, Bansmann Paul, Brachmann Johannes. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. JAMA. 2014 Feb 5;311 (5):498–506. doi: 10.1001/jama.2014.3. [DOI] [PubMed] [Google Scholar]
- 79.Katritsis D, Ioannidis J P, Anagnostopoulos C E, Sarris G E, Giazitzoglou E, Korovesis S, Camm A J. Identification and catheter ablation of extracardiac and intracardiac components of ligament of Marshall tissue for treatment of paroxysmal atrial fibrillation. J. Cardiovasc. Electrophysiol. 2001 Jul;12 (7):750–8. doi: 10.1046/j.1540-8167.2001.00750.x. [DOI] [PubMed] [Google Scholar]
- 80.Oral Hakan, Chugh Aman, Scharf Christoph, Hall Burr, Cheung Peter, Veerareddy Srikar, Daneshvar Gerald F, Pelosi Frank, Morady Fred. Pulmonary vein isolation for vagotonic, adrenergic, and random episodes of paroxysmal atrial fibrillation. J. Cardiovasc. Electrophysiol. 2004 Apr;15 (4):402–6. doi: 10.1046/j.1540-8167.2004.03432.x. [DOI] [PubMed] [Google Scholar]
- 81.Pappone Carlo, Santinelli Vincenzo, Manguso Francesco, Vicedomini Gabriele, Gugliotta Filippo, Augello Giuseppe, Mazzone Patrizio, Tortoriello Valter, Landoni Giovanni, Zangrillo Alberto, Lang Christopher, Tomita Takeshi, Mesas Cézar, Mastella Elio, Alfieri Ottavio. Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation. 2004 Jan 27;109 (3):327–34. doi: 10.1161/01.CIR.0000112641.16340.C7. [DOI] [PubMed] [Google Scholar]
- 82.Scherlag Benjamin J, Nakagawa Hiroshi, Jackman Warren M, Yamanashi William S, Patterson Eugene, Po Sunny, Lazzara Ralph. Electrical stimulation to identify neural elements on the heart: their role in atrial fibrillation. J Interv Card Electrophysiol. 2005 Aug;13 Suppl 1 ():37–42. doi: 10.1007/s10840-005-2492-2. [DOI] [PubMed] [Google Scholar]
- 83.Lellouche Nicolas, Buch Eric, Celigoj Andrew, Siegerman Carin, Cesario David, De Diego Carlos, Mahajan Aman, Boyle Noel G, Wiener Isaac, Garfinkel Alan, Shivkumar Kalyanam. Functional characterization of atrial electrograms in sinus rhythm delineates sites of parasympathetic innervation in patients with paroxysmal atrial fibrillation. J. Am. Coll. Cardiol. 2007 Oct 2;50 (14):1324–31. doi: 10.1016/j.jacc.2007.03.069. [DOI] [PubMed] [Google Scholar]
- 84.Pokushalov Evgeny, Romanov Alex, Shugayev Pavel, Artyomenko Sergey, Shirokova Natalya, Turov Alex, Katritsis Demosthenes G. Selective ganglionated plexi ablation for paroxysmal atrial fibrillation. Heart Rhythm. 2009 Sep;6 (9):1257–64. doi: 10.1016/j.hrthm.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 85.Katritsis Demosthenes, Giazitzoglou Eleftherios, Sougiannis Demetrios, Voridis Eutychios, Po Sunny S. Complex fractionated atrial electrograms at anatomic sites of ganglionated plexi in atrial fibrillation. Europace. 2009 Mar;11 (3):308–15. doi: 10.1093/europace/eup036. [DOI] [PubMed] [Google Scholar]
- 86.Nakagawa Hiroshi, Scherlag Benjamin J, Patterson Eugene, Ikeda Atsuhsi, Lockwood Deborah, Jackman Warren M. Pathophysiologic basis of autonomic ganglionated plexus ablation in patients with atrial fibrillation. Heart Rhythm. 2009 Dec;6 (12 Suppl):S26–34. doi: 10.1016/j.hrthm.2009.07.029. [DOI] [PubMed] [Google Scholar]
- 87.Po Sunny S, Nakagawa Hiroshi, Jackman Warren M. Localization of left atrial ganglionated plexi in patients with atrial fibrillation. J. Cardiovasc. Electrophysiol. 2009 Oct;20 (10):1186–9. doi: 10.1111/j.1540-8167.2009.01515.x. [DOI] [PubMed] [Google Scholar]
- 88.Calò Leonardo, Rebecchi Marco, Sciarra Luigi, De Luca Lucia, Fagagnini Alessandro, Zuccaro Lorenzo Maria, Pitrone Pietro, Dottori Serena, Porfirio Maurizio, de Ruvo Ermenegildo, Lioy Ernesto. Catheter ablation of right atrial ganglionated plexi in patients with vagal paroxysmal atrial fibrillation. Circ Arrhythm Electrophysiol. 2012 Feb;5 (1):22–31. doi: 10.1161/CIRCEP.111.964262. [DOI] [PubMed] [Google Scholar]
- 89.Kondo Yusuke, Ueda Marehiko, Watanabe Michiko, Ishimura Masayuki, Kajiyama Takatsugu, Hashiguchi Naotaka, Kanaeda Tomonori, Nakano Masahiro, Hiranuma Yasunori, Ishizaka Toru, Matsumiya Goro, Kobayashi Yoshio. Identification of left atrial ganglionated plexi by dense epicardial mapping as ablation targets for the treatment of concomitant atrial fibrillation. Pacing Clin Electrophysiol. 2013 Nov;36 (11):1336–41. doi: 10.1111/pace.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Noheria Amit, Patel Sandeep M, Mirzoyev Sultan, Madhavan Malini, Friedman Paul A, Packer Douglas L, Daly Richard C, Kushwaha Sudhir S, Edwards Brooks S, Asirvatham Samuel J. Decreased postoperative atrial fibrillation following cardiac transplantation: the significance of autonomic denervation. Pacing Clin Electrophysiol. 2013 Jun;36 (6):741–7. doi: 10.1111/pace.12102. [DOI] [PubMed] [Google Scholar]
- 91.Pokushalov Evgeny, Romanov Alexandr, Katritsis Demosthenes G, Artyomenko Sergey, Shirokova Natalya, Karaskov Alexandr, Mittal Suneet, Steinberg Jonathan S. Ganglionated plexus ablation vs linear ablation in patients undergoing pulmonary vein isolation for persistent/long-standing persistent atrial fibrillation: a randomized comparison. Heart Rhythm. 2013 Sep;10 (9):1280–6. doi: 10.1016/j.hrthm.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 92.Katritsis Demosthenes G, Pokushalov Evgeny, Romanov Alexander, Giazitzoglou Eleftherios, Siontis George C M, Po Sunny S, Camm A John, Ioannidis John P A. Autonomic denervation added to pulmonary vein isolation for paroxysmal atrial fibrillation: a randomized clinical trial. J. Am. Coll. Cardiol. 2013 Dec 17;62 (24):2318–25. doi: 10.1016/j.jacc.2013.06.053. [DOI] [PubMed] [Google Scholar]
- 93.Al-Atassi Talal, Toeg Hadi, Malas Tarek, Lam Buu-Khanh. Mapping and ablation of autonomic ganglia in prevention of postoperative atrial fibrillation in coronary surgery: MAAPPAFS atrial fibrillation randomized controlled pilot study. Can J Cardiol. 2014 Oct;30 (10):1202–7. doi: 10.1016/j.cjca.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 94.Kurotobi Toshiya, Shimada Yoshihisa, Kino Naoto, Ito Kazato, Tonomura Daisuke, Yano Kentaro, Tanaka Chiharu, Yoshida Masataka, Tsuchida Takao, Fukumoto Hitoshi. Features of intrinsic ganglionated plexi in both atria after extensive pulmonary isolation and their clinical significance after catheter ablation in patients with atrial fibrillation. Heart Rhythm. 2015 Mar;12 (3):470–6. doi: 10.1016/j.hrthm.2014.11.033. [DOI] [PubMed] [Google Scholar]
- 95.Stavrakis Stavros, Humphrey Mary Beth, Scherlag Benjamin J, Hu Yanqing, Jackman Warren M, Nakagawa Hiroshi, Lockwood Deborah, Lazzara Ralph, Po Sunny S. Low-level transcutaneous electrical vagus nerve stimulation suppresses atrial fibrillation. J. Am. Coll. Cardiol. 2015 Mar 10;65 (9):867–75. doi: 10.1016/j.jacc.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yalcin Muhammed Ulvi, Gurses Kadri Murat, Kocyigit Duygu, Kesikli Sacit Altug, Dural Muhammet, Evranos Banu, Yorgun Hikmet, Sahiner Levent, Kaya Ergun Baris, Oto Mehmet Ali, Guc Dicle, Aytemir Kudret, Ozer Necla. Cardiac Autoantibody Levels Predict Recurrence Following Cryoballoon-Based Pulmonary Vein Isolation in Paroxysmal Atrial Fibrillation Patients. J. Cardiovasc. Electrophysiol. 2015 Jun;26 (6):615–21. doi: 10.1111/jce.12665. [DOI] [PubMed] [Google Scholar]
- 97.Jongnarangsin Krit, Chugh Aman, Good Eric, Mukerji Siddharth, Dey Sujoya, Crawford Thomas, Sarrazin Jean F, Kuhne Michael, Chalfoun Nagib, Wells Darryl, Boonyapisit Warangkna, Pelosi Frank, Bogun Frank, Morady Fred, Oral Hakan. Body mass index, obstructive sleep apnea, and outcomes of catheter ablation of atrial fibrillation. J. Cardiovasc. Electrophysiol. 2008 Jul;19 (7):668–72. doi: 10.1111/j.1540-8167.2008.01118.x. [DOI] [PubMed] [Google Scholar]
- 98.Tang Ri-Bo, Dong Jian-Zeng, Liu Xing-Peng, Long De-Yong, Yu Rong-Hui, Kalifa Jérôme, Ma Chang-Sheng. Metabolic syndrome and risk of recurrence of atrial fibrillation after catheter ablation. Circ. J. 2009 Mar;73 (3):438–43. doi: 10.1253/circj.cj-08-0832. [DOI] [PubMed] [Google Scholar]
- 99.Bhargava Mandeep, Di Biase Luigi, Mohanty Prasant, Prasad Subramanyam, Martin David O, Williams-Andrews Michelle, Wazni Oussama M, Burkhardt J David, Cummings Jennifer E, Khaykin Yaariv, Verma Atul, Hao Steven, Beheiry Salwa, Hongo Richard, Rossillo Antonio, Raviele Antonio, Bonso Aldo, Themistoclakis Sakis, Stewart Kelly, Saliba Walid I, Schweikert Robert A, Natale Andrea. Impact of type of atrial fibrillation and repeat catheter ablation on long-term freedom from atrial fibrillation: results from a multicenter study. Heart Rhythm. 2009 Oct;6 (10):1403–12. doi: 10.1016/j.hrthm.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 100.Jongnarangsin Krit, Suwanagool Arisara, Chugh Aman, Crawford Thomas, Good Eric, Pelosi Frank, Bogun Frank, Oral Hakan, Morady Fred. Effect of catheter ablation on progression of paroxysmal atrial fibrillation. J. Cardiovasc. Electrophysiol. 2012 Jan;23 (1):9–14. doi: 10.1111/j.1540-8167.2011.02137.x. [DOI] [PubMed] [Google Scholar]
- 101.Mohanty Sanghamitra, Mohanty Prasant, Di Biase Luigi, Bai Rong, Pump Agnes, Santangeli Pasquale, Burkhardt David, Gallinghouse Joseph G, Horton Rodney, Sanchez Javier E, Bailey Shane, Zagrodzky Jason, Natale Andrea. Impact of metabolic syndrome on procedural outcomes in patients with atrial fibrillation undergoing catheter ablation. J. Am. Coll. Cardiol. 2012 Apr 3;59 (14):1295–301. doi: 10.1016/j.jacc.2011.11.051. [DOI] [PubMed] [Google Scholar]
- 102.Chao Tze-Fan, Tsao Hsuan-Ming, Lin Yenn-Jiang, Tsai Chin-Feng, Lin Wei-Shiang, Chang Shih-Lin, Lo Li-Wei, Hu Yu-Feng, Tuan Ta-Chuan, Suenari Kazuyoshi, Li Cheng-Hung, Hartono Beny, Chang Hung-Yu, Ambrose Kibos, Wu Tsu-Juey, Chen Shih-Ann. Clinical outcome of catheter ablation in patients with nonparoxysmal atrial fibrillation: results of 3-year follow-up. Circ Arrhythm Electrophysiol. 2012 Jun 1;5 (3):514–20. doi: 10.1161/CIRCEP.111.968032. [DOI] [PubMed] [Google Scholar]
- 103.Chao Tze-Fan, Ambrose Kibos, Tsao Hsuan-Ming, Lin Yenn-Jiang, Chang Shih-Lin, Lo Li-Wei, Hu Yu-Feng, Tuan Ta-Chuan, Suenari Kazuyoshi, Li Cheng-Hung, Hartono Beny, Chang Hung-Yu, Wu Tsu-Juey, Chen Shih-Ann. Relationship between the CHADS(2) score and risk of very late recurrences after catheter ablation of paroxysmal atrial fibrillation. Heart Rhythm. 2012 Aug;9 (8):1185–91. doi: 10.1016/j.hrthm.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 104.Fein Adam S, Shvilkin Alexei, Shah Dhaval, Haffajee Charles I, Das Saumya, Kumar Kapil, Kramer Daniel B, Zimetbaum Peter J, Buxton Alfred E, Josephson Mark E, Anter Elad. Treatment of obstructive sleep apnea reduces the risk of atrial fibrillation recurrence after catheter ablation. J. Am. Coll. Cardiol. 2013 Jul 23;62 (4):300–5. doi: 10.1016/j.jacc.2013.03.052. [DOI] [PubMed] [Google Scholar]
- 105.Pathak Rajeev K, Middeldorp Melissa E, Lau Dennis H, Mehta Abhinav B, Mahajan Rajiv, Twomey Darragh, Alasady Muayad, Hanley Lorraine, Antic Nicholas A, McEvoy R Doug, Kalman Jonathan M, Abhayaratna Walter P, Sanders Prashanthan. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study. J. Am. Coll. Cardiol. 2014 Dec 2;64 (21):2222–31. doi: 10.1016/j.jacc.2014.09.028. [DOI] [PubMed] [Google Scholar]
- 106.Husser Daniela, Adams Volker, Piorkowski Christopher, Hindricks Gerhard, Bollmann Andreas. Chromosome 4q25 variants and atrial fibrillation recurrence after catheter ablation. J. Am. Coll. Cardiol. 2010 Feb 23;55 (8):747–53. doi: 10.1016/j.jacc.2009.11.041. [DOI] [PubMed] [Google Scholar]
- 107.Shoemaker M Benjamin, Bollmann Andreas, Lubitz Steven A, Ueberham Laura, Saini Harsimran, Montgomery Jay, Edwards Todd, Yoneda Zachary, Sinner Moritz F, Arya Arash, Sommer Philipp, Delaney Jessica, Goyal Sandeep K, Saavedra Pablo, Kanagasundram Arvindh, Whalen S Patrick, Roden Dan M, Hindricks Gerhard, Ellis Christopher R, Ellinor Patrick T, Darbar Dawood, Husser Daniela. Common genetic variants and response to atrial fibrillation ablation. Circ Arrhythm Electrophysiol. 2015 Apr;8 (2):296–302. doi: 10.1161/CIRCEP.114.001909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stiles Martin K, John Bobby, Wong Christopher X, Kuklik Pawel, Brooks Anthony G, Lau Dennis H, Dimitri Hany, Roberts-Thomson Kurt C, Wilson Lauren, De Sciscio Paolo, Young Glenn D, Sanders Prashanthan. Paroxysmal lone atrial fibrillation is associated with an abnormal atrial substrate: characterizing the "second factor". J. Am. Coll. Cardiol. 2009 Apr 7;53 (14):1182–91. doi: 10.1016/j.jacc.2008.11.054. [DOI] [PubMed] [Google Scholar]
- 109.Inoue Koichi, Kurotobi Toshiya, Kimura Ryusuke, Toyoshima Yuko, Itoh Norihisa, Masuda Masaharu, Higuchi Yoshiharu, Date Motoo, Koyama Yasushi, Okamura Atsunori, Iwakura Katsuomi, Fujii Kenshi. Trigger-based mechanism of the persistence of atrial fibrillation and its impact on the efficacy of catheter ablation. Circ Arrhythm Electrophysiol. 2012 Apr;5 (2):295–301. doi: 10.1161/CIRCEP.111.964080. [DOI] [PubMed] [Google Scholar]
- 110.Kiaii Bob, Fox Stephanie, Chase Lindsay, Fernandes Michaela, Stitt Larry W, Guo Ray, Quantz Mackenzie, Chu Michael W, Koka Pavan, McClure R Scott, McKenzie F Neil, Klein George J, Novick Richard J, Skanes Allan C. Postoperative atrial fibrillation is not pulmonary vein dependent: results from a randomized trial. Heart Rhythm. 2015 Apr;12 (4):699–705. doi: 10.1016/j.hrthm.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 111.Sanders Prashanthan, Berenfeld Omer, Hocini Mélèze, Jaïs Pierre, Vaidyanathan Ravi, Hsu Li-Fern, Garrigue Stéphane, Takahashi Yoshihide, Rotter Martin, Sacher Fréderic, Scavée Christophe, Ploutz-Snyder Robert, Jalife José, Haïssaguerre Michel. Spectral analysis identifies sites of high-frequency activity maintaining atrial fibrillation in humans. Circulation. 2005 Aug 9;112 (6):789–97. doi: 10.1161/CIRCULATIONAHA.104.517011. [DOI] [PubMed] [Google Scholar]
- 112.Jarman Julian W E, Wong Tom, Kojodjojo Pipin, Spohr Hilmar, Davies Justin E, Roughton Michael, Francis Darrel P, Kanagaratnam Prapa, Markides Vias, Davies D Wyn, Peters Nicholas S. Spatiotemporal behavior of high dominant frequency during paroxysmal and persistent atrial fibrillation in the human left atrium. Circ Arrhythm Electrophysiol. 2012 Aug 1;5 (4):650–8. doi: 10.1161/CIRCEP.111.967992. [DOI] [PubMed] [Google Scholar]
- 113.Atienza Felipe, Almendral Jesús, Ormaetxe José Miguel, Moya Angel, Martínez-Alday Jesús Daniel, Hernández-Madrid Antonio, Castellanos Eduardo, Arribas Fernando, Arias Miguel Ángel, Tercedor Luis, Peinado Rafael, Arcocha Maria Fe, Ortiz Mercedes, Martínez-Alzamora Nieves, Arenal Angel, Fernández-Avilés Francisco, Jalife José. Comparison of radiofrequency catheter ablation of drivers and circumferential pulmonary vein isolation in atrial fibrillation: a noninferiority randomized multicenter RADAR-AF trial. J. Am. Coll. Cardiol. 2014 Dec 16;64 (23):2455–67. doi: 10.1016/j.jacc.2014.09.053. [DOI] [PubMed] [Google Scholar]
- 114.Oral Hakan, Chugh Aman, Good Eric, Crawford Thomas, Sarrazin Jean F, Kuhne Michael, Chalfoun Nagib, Wells Darryl, Boonyapisit Warangkna, Gadeela Nitesh, Sankaran Sundar, Kfahagi Ayman, Jongnarangsin Krit, Pelosi Frank, Bogun Frank, Morady Fred. Randomized evaluation of right atrial ablation after left atrial ablation of complex fractionated atrial electrograms for long-lasting persistent atrial fibrillation. Circ Arrhythm Electrophysiol. 2008 Apr;1 (1):6–13. doi: 10.1161/CIRCEP.107.748780. [DOI] [PubMed] [Google Scholar]
- 115.Hocini Mélèze, Nault Isabelle, Wright Matthew, Veenhuyzen George, Narayan Sanjiv M, Jaïs Pierre, Lim Kang-Teng, Knecht Sébastien, Matsuo Seiichiro, Forclaz Andrei, Miyazaki Shinsuke, Jadidi Amir, O'Neill Mark D, Sacher Frédéric, Clémenty Jacques, Haïssaguerre Michel. Disparate evolution of right and left atrial rate during ablation of long-lasting persistent atrial fibrillation. J. Am. Coll. Cardiol. 2010 Mar 9;55 (10):1007–16. doi: 10.1016/j.jacc.2009.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Garrey WE. The nature of fibrillary contraction of the heart: its relation to tissue mass and form. The American journal of physiology. 1994;33:397–414. [Google Scholar]
- 117.MOE G K, RHEINBOLDT W C, ABILDSKOV J A. A COMPUTER MODEL OF ATRIAL FIBRILLATION. Am. Heart J. 1964 Feb;67 ():200–20. doi: 10.1016/0002-8703(64)90371-0. [DOI] [PubMed] [Google Scholar]
- 118.Byrd Gregory D, Prasad Sandip M, Ripplinger Crystal M, Cassilly T Ryan, Schuessler Richard B, Boineau John P, Damiano Ralph J. Importance of geometry and refractory period in sustaining atrial fibrillation: testing the critical mass hypothesis. Circulation. 2005 Aug 30;112 (9 Suppl):I7–13. doi: 10.1161/CIRCULATIONAHA.104.526210. [DOI] [PubMed] [Google Scholar]
- 119.Asirvatham Samuel J, Jiao Zhen. What causes atrial fibrillation and why do we fail with ablation?: insights from metabolic syndrome. J. Am. Coll. Cardiol. 2012 Apr 3;59 (14):1302–3. doi: 10.1016/j.jacc.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 120.Kottkamp Hans, Bender Roderich, Berg Jan. Catheter ablation of atrial fibrillation: how to modify the substrate? J. Am. Coll. Cardiol. 2015 Jan 20;65 (2):196–206. doi: 10.1016/j.jacc.2014.10.034. [DOI] [PubMed] [Google Scholar]
- 121.Jaïs Pierre, Hocini Mélèze, Sanders Prashanthan, Hsu Li-Fern, Takahashi Yoshihide, Rotter Martin, Rostock Thomas, Sacher Frédéric, Clementy Jacques, Haissaguerre Michel. Long-term evaluation of atrial fibrillation ablation guided by noninducibility. Heart Rhythm. 2006 Feb;3 (2):140–5. doi: 10.1016/j.hrthm.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 122.Oral Hakan, Chugh Aman, Good Eric, Sankaran Sundar, Reich Stephen S, Igic Petar, Elmouchi Darryl, Tschopp David, Crawford Thomas, Dey Sujoya, Wimmer Alan, Lemola Kristina, Jongnarangsin Krit, Bogun Frank, Pelosi Frank, Morady Fred. A tailored approach to catheter ablation of paroxysmal atrial fibrillation. Circulation. 2006 Apr 18;113 (15):1824–31. doi: 10.1161/CIRCULATIONAHA.105.601898. [DOI] [PubMed] [Google Scholar]
- 123.Cuculich Phillip S, Wang Yong, Lindsay Bruce D, Faddis Mitchell N, Schuessler Richard B, Damiano Ralph J, Li Li, Rudy Yoram. Noninvasive characterization of epicardial activation in humans with diverse atrial fibrillation patterns. Circulation. 2010 Oct 5;122 (14):1364–72. doi: 10.1161/CIRCULATIONAHA.110.945709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yoshida Kentaro, Rabbani Amir B, Oral Hakan, Bach David, Morady Fred, Chugh Aman. Left atrial volume and dominant frequency of atrial fibrillation in patients undergoing catheter ablation of persistent atrial fibrillation. J Interv Card Electrophysiol. 2011 Nov;32 (2):155–61. doi: 10.1007/s10840-011-9590-0. [DOI] [PubMed] [Google Scholar]
- 125.Rostock Thomas, Salukhe Tushar V, Steven Daniel, Drewitz Imke, Hoffmann Boris A, Bock Karsten, Servatius Helge, Müllerleile Kai, Sultan Arian, Gosau Nils, Meinertz Thomas, Wegscheider Karl, Willems Stephan. Long-term single- and multiple-procedure outcome and predictors of success after catheter ablation for persistent atrial fibrillation. Heart Rhythm. 2011 Sep;8 (9):1391–7. doi: 10.1016/j.hrthm.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 126.Narayan Sanjiv M, Krummen David E, Shivkumar Kalyanam, Clopton Paul, Rappel Wouter-Jan, Miller John M. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation) trial. J. Am. Coll. Cardiol. 2012 Aug 14;60 (7):628–36. doi: 10.1016/j.jacc.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rostock Thomas, Salukhe Tushar V, Hoffmann Boris A, Steven Daniel, Berner Imke, Müllerleile Kai, Theis Cathrin, Bock Karsten, Servatius Helge, Sultan Arian, Willems Stephan. Prognostic role of subsequent atrial tachycardias occurring during ablation of persistent atrial fibrillation: a prospective randomized trial. Circ Arrhythm Electrophysiol. 2013 Dec;6 (6):1059–65. doi: 10.1161/CIRCEP.113.001019. [DOI] [PubMed] [Google Scholar]
- 128.Haissaguerre Michel, Hocini Meleze, Denis Arnaud, Shah Ashok J, Komatsu Yuki, Yamashita Seigo, Daly Matthew, Amraoui Sana, Zellerhoff Stephan, Picat Marie-Quitterie, Quotb Adam, Jesel Laurence, Lim Han, Ploux Sylvain, Bordachar Pierre, Attuel Guillaume, Meillet Valentin, Ritter Philippe, Derval Nicolas, Sacher Frederic, Bernus Olivier, Cochet Hubert, Jais Pierre, Dubois Remi. Driver domains in persistent atrial fibrillation. Circulation. 2014 Aug 12;130 (7):530–8. doi: 10.1161/CIRCULATIONAHA.113.005421. [DOI] [PubMed] [Google Scholar]
- 129.Scherr Daniel, Khairy Paul, Miyazaki Shinsuke, Aurillac-Lavignolle Valerie, Pascale Patrizio, Wilton Stephen B, Ramoul Khaled, Komatsu Yuki, Roten Laurent, Jadidi Amir, Linton Nick, Pedersen Michala, Daly Matthew, O'Neill Mark, Knecht Sebastien, Weerasooriya Rukshen, Rostock Thomas, Manninger Martin, Cochet Hubert, Shah Ashok J, Yeim Sunthareth, Denis Arnaud, Derval Nicolas, Hocini Meleze, Sacher Frederic, Haissaguerre Michel, Jais Pierre. Five-year outcome of catheter ablation of persistent atrial fibrillation using termination of atrial fibrillation as a procedural endpoint. Circ Arrhythm Electrophysiol. 2015 Feb;8 (1):18–24. doi: 10.1161/CIRCEP.114.001943. [DOI] [PubMed] [Google Scholar]
- 130.Chugh Aman. Atrial tachycardia after ablation of persistent atrial fibrillation: is it us or them? Circ Arrhythm Electrophysiol. 2013 Dec;6 (6):1047–9. doi: 10.1161/CIRCEP.113.001168. [DOI] [PubMed] [Google Scholar]
- 131.Chugh Aman. Catheter ablation of persistent atrial fibrillation: how much is enough? Circ Arrhythm Electrophysiol. 2015 Feb;8 (1):2–4. doi: 10.1161/CIRCEP.115.002674. [DOI] [PubMed] [Google Scholar]
- 132.Nathan H, Gloobe H. Myocardial atrio-venous junctions and extensions (sleeves) over the pulmonary and caval veins. Anatomical observations in various mammals. Thorax. 1970 May;25 (3):317–24. doi: 10.1136/thx.25.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.DeSimone Christopher V, De Simone Christopher V, Noheria Amit, Lachman Nirusha, Edwards William D, Gami Apoor S, Maleszewski Joseph J, Friedman Paul A, Munger Thomas M, Hammill Stephen C, Packer Douglas L, Asirvatham Samuel J. Myocardium of the superior vena cava, coronary sinus, vein of Marshall, and the pulmonary vein ostia: gross anatomic studies in 620 hearts. J. Cardiovasc. Electrophysiol. 2012 Dec;23 (12):1304–9. doi: 10.1111/j.1540-8167.2012.02403.x. [DOI] [PubMed] [Google Scholar]
- 134.Haïssaguerre M, Jaïs P, Shah D C, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N. Engl. J. Med. 1998 Sep 3;339 (10):659–66. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 135.Chen S A, Hsieh M H, Tai C T, Tsai C F, Prakash V S, Yu W C, Hsu T L, Ding Y A, Chang M S. Initiation of atrial fibrillation by ectopic beats originating from the pulmonary veins: electrophysiological characteristics, pharmacological responses, and effects of radiofrequency ablation. Circulation. 1999 Nov 2;100 (18):1879–86. doi: 10.1161/01.cir.100.18.1879. [DOI] [PubMed] [Google Scholar]
- 136.Robbins I M, Colvin E V, Doyle T P, Kemp W E, Loyd J E, McMahon W S, Kay G N. Pulmonary vein stenosis after catheter ablation of atrial fibrillation. Circulation. 1998 Oct 27;98 (17):1769–75. doi: 10.1161/01.cir.98.17.1769. [DOI] [PubMed] [Google Scholar]
- 137.Gerstenfeld E P, Guerra P, Sparks P B, Hattori K, Lesh M D. Clinical outcome after radiofrequency catheter ablation of focal atrial fibrillation triggers. J. Cardiovasc. Electrophysiol. 2001 Aug;12 (8):900–8. doi: 10.1046/j.1540-8167.2001.00900.x. [DOI] [PubMed] [Google Scholar]
- 138.Yu W C, Hsu T L, Tai C T, Tsai C F, Hsieh M H, Lin W S, Lin Y K, Tsao H M, Ding Y A, Chang M S, Chen S A. Acquired pulmonary vein stenosis after radiofrequency catheter ablation of paroxysmal atrial fibrillation. J. Cardiovasc. Electrophysiol. 2001 Aug;12 (8):887–92. doi: 10.1046/j.1540-8167.2001.00887.x. [DOI] [PubMed] [Google Scholar]
- 139.Haïssaguerre M, Shah D C, Jaïs P, Hocini M, Yamane T, Deisenhofer I, Chauvin M, Garrigue S, Clémenty J. Electrophysiological breakthroughs from the left atrium to the pulmonary veins. Circulation. 2000 Nov 14;102 (20):2463–5. doi: 10.1161/01.cir.102.20.2463. [DOI] [PubMed] [Google Scholar]
- 140.Oral Hakan, Knight Bradley P, Ozaydin Mehmet, Chugh Aman, Lai Steve W K, Scharf Christoph, Hassan Sohail, Greenstein Radmira, Han Jihn D, Pelosi Frank, Strickberger S Adam, Morady Fred. Segmental ostial ablation to isolate the pulmonary veins during atrial fibrillation: feasibility and mechanistic insights. Circulation. 2002 Sep 3;106 (10):1256–62. doi: 10.1161/01.cir.0000027821.55835.00. [DOI] [PubMed] [Google Scholar]
- 141.Pappone C, Rosanio S, Oreto G, Tocchi M, Gugliotta F, Vicedomini G, Salvati A, Dicandia C, Mazzone P, Santinelli V, Gulletta S, Chierchia S. Circumferential radiofrequency ablation of pulmonary vein ostia: A new anatomic approach for curing atrial fibrillation. Circulation. 2000 Nov 21;102 (21):2619–28. doi: 10.1161/01.cir.102.21.2619. [DOI] [PubMed] [Google Scholar]
- 142.Pappone C, Oreto G, Rosanio S, Vicedomini G, Tocchi M, Gugliotta F, Salvati A, Dicandia C, Calabrò M P, Mazzone P, Ficarra E, Di Gioia C, Gulletta S, Nardi S, Santinelli V, Benussi S, Alfieri O. Atrial electroanatomic remodeling after circumferential radiofrequency pulmonary vein ablation: efficacy of an anatomic approach in a large cohort of patients with atrial fibrillation. Circulation. 2001 Nov 20;104 (21):2539–44. doi: 10.1161/hc4601.098517. [DOI] [PubMed] [Google Scholar]
- 143.Kanagaratnam L, Tomassoni G, Schweikert R, Pavia S, Bash D, Beheiry S, Lesh M, Niebauer M, Saliba W, Chung M, Tchou P, Natale A. Empirical pulmonary vein isolation in patients with chronic atrial fibrillation using a three-dimensional nonfluoroscopic mapping system: long-term follow-up. Pacing Clin Electrophysiol. 2001 Dec;24 (12):1774–9. doi: 10.1046/j.1460-9592.2001.01774.x. [DOI] [PubMed] [Google Scholar]
- 144.Oral Hakan, Ozaydin Mehmet, Tada Hiroshi, Chugh Aman, Scharf Christoph, Hassan Sohail, Lai Steve, Greenstein Radmira, Pelosi Frank, Knight Bradley P, Strickberger S Adam, Morady Fred. Mechanistic significance of intermittent pulmonary vein tachycardia in patients with atrial fibrillation. J. Cardiovasc. Electrophysiol. 2002 Jul;13 (7):645–50. doi: 10.1046/j.1540-8167.2002.00645.x. [DOI] [PubMed] [Google Scholar]
- 145.Willems Stephan, Weiss Christian, Risius Tim, Rostock Thomas, Hoffmann Matthias, Ventura Rodolfo, Meinertz Thomas. Dissociated activity and pulmonary vein fibrillation following functional disconnection: impact for the arrhythmogenesis of focal atrial fibrillation. Pacing Clin Electrophysiol. 2003 Jun;26 (6):1363–70. doi: 10.1046/j.1460-9592.2003.t01-1-00195.x. [DOI] [PubMed] [Google Scholar]
- 146.Ouyang Feifan, Bänsch Dietmar, Ernst Sabine, Schaumann Anselm, Hachiya Hitoshi, Chen Minglong, Chun Julian, Falk Peter, Khanedani Afsaneh, Antz Matthias, Kuck Karl-Heinz. Complete isolation of left atrium surrounding the pulmonary veins: new insights from the double-Lasso technique in paroxysmal atrial fibrillation. Circulation. 2004 Oct 12;110 (15):2090–6. doi: 10.1161/01.CIR.0000144459.37455.EE. [DOI] [PubMed] [Google Scholar]
- 147.Marchlinski Francis E, Callans David, Dixit Sanjay, Gerstenfeld Edward P, Rho Robert, Ren Jian-Fang, Zado Erica. Efficacy and safety of targeted focal ablation versus PV isolation assisted by magnetic electroanatomic mapping. J. Cardiovasc. Electrophysiol. 2003 Apr;14 (4):358–65. doi: 10.1046/j.1540-8167.2003.02468.x. [DOI] [PubMed] [Google Scholar]
- 148.Nakashima Hideko, Kumagai Koichiro, Noguchi Hiroo, Tojo Hideaki, Yasuda Tomoo, Saku Keijiro. Evaluation of the recurrence of atrial fibrillation after pulmonary venous ablation. J Cardiol. 2002 Sep;40 (3):87–94. [PubMed] [Google Scholar]
- 149.Takahashi Atsushi, Iesaka Yoshito, Takahashi Yoshihide, Takahashi Ryoko, Kobayashi Kenzaburo, Takagi Katsumasa, Kuboyama Osamu, Nishimori Takeo, Takei Hidenobu, Amemiya Hiroshi, Fujiwara Hideomi, Hiraoka Masayasu. Electrical connections between pulmonary veins: implication for ostial ablation of pulmonary veins in patients with paroxysmal atrial fibrillation. Circulation. 2002 Jun 25;105 (25):2998–3003. doi: 10.1161/01.cir.0000019585.91146.ab. [DOI] [PubMed] [Google Scholar]
- 150.Cabrera José Angel, Ho Siew Yen, Climent Vicente, Fuertes Beatriz, Murillo Margarita, Sánchez-Quintana Damián. Morphological evidence of muscular connections between contiguous pulmonary venous orifices: relevance of the interpulmonary isthmus for catheter ablation in atrial fibrillation. Heart Rhythm. 2009 Aug;6 (8):1192–8. doi: 10.1016/j.hrthm.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 151.Shah Dipen, Haissaguerre Michel, Jais Pierre, Hocini Meleze. Nonpulmonary vein foci: do they exist? Pacing Clin Electrophysiol. 2003 Jul;26 (7 Pt 2):1631–5. doi: 10.1046/j.1460-9592.2003.t01-1-00243.x. [DOI] [PubMed] [Google Scholar]
- 152.Macedo Paula G, Kapa Suraj, Mears Jennifer A, Fratianni Amy, Asirvatham Samuel J. Correlative anatomy for the electrophysiologist: ablation for atrial fibrillation. Part I: pulmonary vein ostia, superior vena cava, vein of Marshall. J. Cardiovasc. Electrophysiol. 2010 Jun 1;21 (6):721–30. doi: 10.1111/j.1540-8167.2010.01728.x. [DOI] [PubMed] [Google Scholar]
- 153.Oral Hakan, Scharf Christoph, Chugh Aman, Hall Burr, Cheung Peter, Good Eric, Veerareddy Srikar, Pelosi Frank, Morady Fred. Catheter ablation for paroxysmal atrial fibrillation: segmental pulmonary vein ostial ablation versus left atrial ablation. Circulation. 2003 Nov 11;108 (19):2355–60. doi: 10.1161/01.CIR.0000095796.45180.88. [DOI] [PubMed] [Google Scholar]
- 154.Karch Martin R, Zrenner Bernhard, Deisenhofer Isabel, Schreieck Jürgen, Ndrepepa Gjin, Dong Jun, Lamprecht Katrin, Barthel Petra, Luciani Etienne, Schömig Albert, Schmitt Claus. Freedom from atrial tachyarrhythmias after catheter ablation of atrial fibrillation: a randomized comparison between 2 current ablation strategies. Circulation. 2005 Jun 7;111 (22):2875–80. doi: 10.1161/CIRCULATIONAHA.104.491530. [DOI] [PubMed] [Google Scholar]
- 155.Liu Xingpeng, Dong Jianzeng, Mavrakis Hercules E, Hu Fuli, Long Deyong, Fang Dongping, Yu Ronghui, Tang Ribo, Hao Peng, Lu Chunshan, He Xiaokui, Liu Xiaohui, Vardas Panos E, Ma Changsheng. Achievement of pulmonary vein isolation in patients undergoing circumferential pulmonary vein ablation: a randomized comparison between two different isolation approaches. J. Cardiovasc. Electrophysiol. 2006 Dec;17 (12):1263–70. doi: 10.1111/j.1540-8167.2006.00621.x. [DOI] [PubMed] [Google Scholar]
- 156.Arentz Thomas, Weber Reinhold, Bürkle Gerd, Herrera Claudia, Blum Thomas, Stockinger Jochem, Minners Jan, Neumann Franz Josef, Kalusche Dietrich. Small or large isolation areas around the pulmonary veins for the treatment of atrial fibrillation? Results from a prospective randomized study. Circulation. 2007 Jun 19;115 (24):3057–63. doi: 10.1161/CIRCULATIONAHA.107.690578. [DOI] [PubMed] [Google Scholar]
- 157.Nilsson Brian, Chen Xu, Pehrson Steen, Køber Lars, Hilden Jørgen, Svendsen Jesper H. Recurrence of pulmonary vein conduction and atrial fibrillation after pulmonary vein isolation for atrial fibrillation: a randomized trial of the ostial versus the extraostial ablation strategy. Am. Heart J. 2006 Sep;152 (3):537.e1–8. doi: 10.1016/j.ahj.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 158.Lemola Kristina, Hall Burr, Cheung Peter, Good Eric, Han Jihn, Tamirisa Kamala, Chugh Aman, Bogun Frank, Pelosi Frank, Morady Fred, Oral Hakan. Mechanisms of recurrent atrial fibrillation after pulmonary vein isolation by segmental ostial ablation. Heart Rhythm. 2004 Jul;1 (2):197–202. doi: 10.1016/j.hrthm.2004.03.071. [DOI] [PubMed] [Google Scholar]
- 159.Tamborero David, Mont Lluís, Berruezo Antonio, Guasch Eduard, Rios Jose, Nadal Mercedes, Matiello Maria, Andreu David, Sitges Marta, Brugada Josep. Circumferential pulmonary vein ablation: does use of a circular mapping catheter improve results? A prospective randomized study. Heart Rhythm. 2010 May;7 (5):612–8. doi: 10.1016/j.hrthm.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 160.Zhang Baowei, Zhen Ya, Tao Aibin, Zhang Guohui. Efficacy of selective arrhythmogenic pulmonary veins isolation versus empirical all pulmonary veins isolation for atrial fibrillation: a meta-analysis of randomized and observational studies. J Interv Card Electrophysiol. 2014 Apr;39 (3):233–40. doi: 10.1007/s10840-013-9865-8. [DOI] [PubMed] [Google Scholar]
- 161.Lazar Sorin, Dixit Sanjay, Marchlinski Francis E, Callans David J, Gerstenfeld Edward P. Presence of left-to-right atrial frequency gradient in paroxysmal but not persistent atrial fibrillation in humans. Circulation. 2004 Nov 16;110 (20):3181–6. doi: 10.1161/01.CIR.0000147279.91094.5E. [DOI] [PubMed] [Google Scholar]
- 162.Lo Li-Wei, Tai Ching-Tai, Lin Yenn-Jiang, Chang Shih-Lin, Wongcharoen Wanwarang, Hsieh Ming-Hsiung, Tuan Ta-Chuan, Udyavar Ameya R, Hu Yu-Feng, Chen Yi-Jen, Tsao Hsuan-Ming, Chen Shih-Ann. Mechanisms of recurrent atrial fibrillation: comparisons between segmental ostial versus circumferential pulmonary vein isolation. J. Cardiovasc. Electrophysiol. 2007 Aug;18 (8):803–7. doi: 10.1111/j.1540-8167.2007.00848.x. [DOI] [PubMed] [Google Scholar]
- 163.Yamada Takumi, Murakami Yoshimasa, Okada Taro, Yoshida Naoki, Ninomiya Yuichi, Toyama Junji, Yoshida Yukihiko, Tsuboi Naoya, Inden Yasuya, Hirai Makoto, Murohara Toyoaki, McElderry Hugh T, Epstein Andrew E, Plumb Vance J, Kay G Neal. Non-pulmonary vein epicardial foci of atrial fibrillation identified in the left atrium after pulmonary vein isolation. Pacing Clin Electrophysiol. 2007 Nov;30 (11):1323–30. doi: 10.1111/j.1540-8159.2007.00865.x. [DOI] [PubMed] [Google Scholar]
- 164.Lin Yenn-Jiang, Chang Shih-Lin, Lo Li-Wei, Hu Yu-Feng, Suenari Kazuyoshi, Li Cheng-Hung, Chao Tze-Fan, Chung Fa-Po, Liao Jo-Nan, Hartono Beny, Tso Han-Wen, Tsao Hsuan-Ming, Huang Jin-Long, Kao Tsair, Chen Shih-Ann. A prospective, randomized comparison of modified pulmonary vein isolation versus conventional pulmonary vein isolation in patients with paroxysmal atrial fibrillation. J. Cardiovasc. Electrophysiol. 2012 Nov;23 (11):1155–62. doi: 10.1111/j.1540-8167.2012.02379.x. [DOI] [PubMed] [Google Scholar]
- 165.Suenari Kazuyoshi, Lin Yenn-Jiang, Chang Shih-Lin, Lo Li-Wei, Hu Yu-Feng, Tuan Ta-Chuan, Huang Shih-Yu, Tai Ching-Tai, Nakano Yukiko, Kihara Yasuki, Tsao Hsuan-Ming, Wu Tsu-Juey, Chen Shih-Ann. Relationship between arrhythmogenic pulmonary veins and the surrounding atrial substrate in patients with paroxysmal atrial fibrillation. J. Cardiovasc. Electrophysiol. 2011 Apr;22 (4):405–10. doi: 10.1111/j.1540-8167.2010.01932.x. [DOI] [PubMed] [Google Scholar]
- 166.Jarman Julian W E, Wong Tom, Kojodjojo Pipin, Spohr Hilmar, Davies Justin E R, Roughton Michael, Francis Darrel P, Kanagaratnam Prapa, O'Neill Mark D, Markides Vias, Davies D Wyn, Peters Nicholas S. Organizational index mapping to identify focal sources during persistent atrial fibrillation. J. Cardiovasc. Electrophysiol. 2014 Apr;25 (4):355–63. doi: 10.1111/jce.12352. [DOI] [PubMed] [Google Scholar]
- 167.Deneke Thomas, Khargi Krishna, Müller Klaus-Michael, Lemke Bernd, Mügge Andreas, Laczkovics Axel, Becker Anton E, Grewe Peter H. Histopathology of intraoperatively induced linear radiofrequency ablation lesions in patients with chronic atrial fibrillation. Eur. Heart J. 2005 Sep;26 (17):1797–803. doi: 10.1093/eurheartj/ehi255. [DOI] [PubMed] [Google Scholar]
- 168.Puodziukynas Aras, Kazakevicius Tomas, Vaitkevicius Raimundas, Rysevaite Kristina, Jokubauskas Marius, Saburkina Inga, Sladkeviciute-Dirzinauskiene Vaiva, Dirzinauskas Evaldas, Zabiela Vytautas, Sileikis Vytautas, Plisiene Jurgita, Pauziene Neringa, Zaliunas Remigijus, Jalife José, Pauza Dainius H. Radiofrequency catheter ablation of pulmonary vein roots results in axonal degeneration of distal epicardial nerves. Auton Neurosci. 2012 Apr 3;167 (1-2):61–5. doi: 10.1016/j.autneu.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 169.Pappone Carlo, Augello Giuseppe, Sala Simone, Gugliotta Filippo, Vicedomini Gabriele, Gulletta Simone, Paglino Gabriele, Mazzone Patrizio, Sora Nicoleta, Greiss Isabelle, Santagostino Andreina, LiVolsi Laura, Pappone Nicola, Radinovic Andrea, Manguso Francesco, Santinelli Vincenzo. A randomized trial of circumferential pulmonary vein ablation versus antiarrhythmic drug therapy in paroxysmal atrial fibrillation: the APAF Study. J. Am. Coll. Cardiol. 2006 Dec 5;48 (11):2340–7. doi: 10.1016/j.jacc.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 170.Stabile Giuseppe, Bertaglia Emanuele, Senatore Gaetano, De Simone Antonio, Zoppo Franco, Donnici Giovanni, Turco Pietro, Pascotto Pietro, Fazzari Massimo, Vitale Dino Franco. Catheter ablation treatment in patients with drug-refractory atrial fibrillation: a prospective, multi-centre, randomized, controlled study (Catheter Ablation For The Cure Of Atrial Fibrillation Study). Eur. Heart J. 2006 Jan;27 (2):216–21. doi: 10.1093/eurheartj/ehi583. [DOI] [PubMed] [Google Scholar]
- 171.Forleo Giovanni B, Mantica Massimo, De Luca Lucia, Leo Roberto, Santini Luca, Panigada Stefania, De Sanctis Valerio, Pappalardo Augusto, Laurenzi Francesco, Avella Andrea, Casella Michela, Dello Russo Antonio, Romeo Francesco, Pelargonio Gemma, Tondo Claudio. Catheter ablation of atrial fibrillation in patients with diabetes mellitus type 2: results from a randomized study comparing pulmonary vein isolation versus antiarrhythmic drug therapy. J. Cardiovasc. Electrophysiol. 2009 Jan;20 (1):22–8. doi: 10.1111/j.1540-8167.2008.01275.x. [DOI] [PubMed] [Google Scholar]
- 172.Wilber David J, Pappone Carlo, Neuzil Petr, De Paola Angelo, Marchlinski Frank, Natale Andrea, Macle Laurent, Daoud Emile G, Calkins Hugh, Hall Burr, Reddy Vivek, Augello Giuseppe, Reynolds Matthew R, Vinekar Chandan, Liu Christine Y, Berry Scott M, Berry Donald A. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA. 2010 Jan 27;303 (4):333–40. doi: 10.1001/jama.2009.2029. [DOI] [PubMed] [Google Scholar]
- 173.Packer Douglas L, Kowal Robert C, Wheelan Kevin R, Irwin James M, Champagne Jean, Guerra Peter G, Dubuc Marc, Reddy Vivek, Nelson Linda, Holcomb Richard G, Lehmann John W, Ruskin Jeremy N. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic Front (STOP AF) pivotal trial. J. Am. Coll. Cardiol. 2013 Apr 23;61 (16):1713–23. doi: 10.1016/j.jacc.2012.11.064. [DOI] [PubMed] [Google Scholar]
- 174.Morillo Carlos A, Verma Atul, Connolly Stuart J, Kuck Karl H, Nair Girish M, Champagne Jean, Sterns Laurence D, Beresh Heather, Healey Jeffrey S, Natale Andrea. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of paroxysmal atrial fibrillation (RAAFT-2): a randomized trial. JAMA. 2014 Feb 19;311 (7):692–700. doi: 10.1001/jama.2014.467. [DOI] [PubMed] [Google Scholar]
- 175.Ghanbari Hamid, Başer Kazım, Jongnarangsin Krit, Chugh Aman, Nallamothu Brahmajee K, Gillespie Brenda W, Başer Hatice Duygu, Suwanagool Arisara, Swangasool Arisara, Crawford Thomas, Latchamsetty Rakesh, Good Eric, Pelosi Frank, Bogun Frank, Morady Fred, Oral Hakan. Mortality and cerebrovascular events after radiofrequency catheter ablation of atrial fibrillation. Heart Rhythm. 2014 Sep;11 (9):1503–11. doi: 10.1016/j.hrthm.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 176.Takigawa Masateru, Takahashi Atsushi, Kuwahara Taishi, Okubo Kenji, Takahashi Yoshihide, Watari Yuji, Takagi Katsumasa, Fujino Tadashi, Kimura Shigeki, Hikita Hiroyuki, Tomita Makoto, Hirao Kenzo, Isobe Mitsuaki. Long-term follow-up after catheter ablation of paroxysmal atrial fibrillation: the incidence of recurrence and progression of atrial fibrillation. Circ Arrhythm Electrophysiol. 2014 Apr;7 (2):267–73. doi: 10.1161/CIRCEP.113.000471. [DOI] [PubMed] [Google Scholar]
- 177.Ouyang Feifan, Tilz Roland, Chun Julian, Schmidt Boris, Wissner Erik, Zerm Thomas, Neven Kars, Köktürk Bulent, Konstantinidou Melanie, Metzner Andreas, Fuernkranz Alexander, Kuck Karl-Heinz. Long-term results of catheter ablation in paroxysmal atrial fibrillation: lessons from a 5-year follow-up. Circulation. 2010 Dec 7;122 (23):2368–77. doi: 10.1161/CIRCULATIONAHA.110.946806. [DOI] [PubMed] [Google Scholar]
- 178.Kerr Charles R, Humphries Karin H, Talajic Mario, Klein George J, Connolly Stuart J, Green Martin, Boone John, Sheldon Robert, Dorian Paul, Newman David. Progression to chronic atrial fibrillation after the initial diagnosis of paroxysmal atrial fibrillation: results from the Canadian Registry of Atrial Fibrillation. Am. Heart J. 2005 Mar;149 (3):489–96. doi: 10.1016/j.ahj.2004.09.053. [DOI] [PubMed] [Google Scholar]
- 179.Tsai C F, Tai C T, Hsieh M H, Lin W S, Yu W C, Ueng K C, Ding Y A, Chang M S, Chen S A. Initiation of atrial fibrillation by ectopic beats originating from the superior vena cava: electrophysiological characteristics and results of radiofrequency ablation. Circulation. 2000 Jul 4;102 (1):67–74. doi: 10.1161/01.cir.102.1.67. [DOI] [PubMed] [Google Scholar]
- 180.Arruda Mauricio, Mlcochova Hanka, Prasad Subramanya K, Kilicaslan Fethi, Saliba Walid, Patel Dimpi, Fahmy Tamer, Morales Luis Saenz, Schweikert Robert, Martin David, Burkhardt David, Cummings Jennifer, Bhargava Mandeep, Dresing Thomas, Wazni Oussama, Kanj Mohamed, Natale Andrea. Electrical isolation of the superior vena cava: an adjunctive strategy to pulmonary vein antrum isolation improving the outcome of AF ablation. J. Cardiovasc. Electrophysiol. 2007 Dec;18 (12):1261–6. doi: 10.1111/j.1540-8167.2007.00953.x. [DOI] [PubMed] [Google Scholar]
- 181.Oral Hakan, Ozaydin Mehmet, Chugh Aman, Scharf Christoph, Tada Hiroshi, Hall Burr, Cheung Peter, Pelosi Frank, Knight Bradley P, Morady Fred. Role of the coronary sinus in maintenance of atrial fibrillation. J. Cardiovasc. Electrophysiol. 2003 Dec;14 (12):1329–36. doi: 10.1046/j.1540-8167.2003.03222.x. [DOI] [PubMed] [Google Scholar]
- 182.Cabrera José Angel, Ho Siew Yen, Climent Vicente, Sánchez-Quintana Damián. The architecture of the left lateral atrial wall: a particular anatomic region with implications for ablation of atrial fibrillation. Eur. Heart J. 2008 Feb;29 (3):356–62. doi: 10.1093/eurheartj/ehm606. [DOI] [PubMed] [Google Scholar]
- 183.Hwang C, Wu T J, Doshi R N, Peter C T, Chen P S. Vein of marshall cannulation for the analysis of electrical activity in patients with focal atrial fibrillation. Circulation. 2000 Apr 4;101 (13):1503–5. doi: 10.1161/01.cir.101.13.1503. [DOI] [PubMed] [Google Scholar]
- 184.Chugh Aman, Wimmer Alan, Morady Fred. Elimination of left superior pulmonary vein ostial potentials during radiofrequency ablation at the mitral isthmus. Heart Rhythm. 2007 Jan;4 (1):85–7. doi: 10.1016/j.hrthm.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 185.Oral Hakan, Knight Bradley P, Ozaydin Mehmet, Tada Hiroshi, Chugh Aman, Hassan Sohail, Scharf Christoph, Lai Steve W K, Greenstein Radmira, Pelosi Frank, Strickberger S Adam, Morady Fred. Clinical significance of early recurrences of atrial fibrillation after pulmonary vein isolation. J. Am. Coll. Cardiol. 2002 Jul 3;40 (1):100–4. doi: 10.1016/s0735-1097(02)01939-3. [DOI] [PubMed] [Google Scholar]
- 186.Lellouche Nicolas, Jaïs Pierre, Nault Isabelle, Wright Matthew, Bevilacqua Michela, Knecht Sébastien, Matsuo Seiichiro, Lim Kang-Teng, Sacher Frederic, Deplagne Antoine, Bordachar Pierre, Hocini Mélèze, Haïssaguerre Michel. Early recurrences after atrial fibrillation ablation: prognostic value and effect of early reablation. J. Cardiovasc. Electrophysiol. 2008 Jun;19 (6):599–605. doi: 10.1111/j.1540-8167.2008.01188.x. [DOI] [PubMed] [Google Scholar]
- 187.Richter Bernhard, Gwechenberger Marianne, Socas Ariel, Marx Manfred, Gössinger Heinz David. Frequency of recurrence of atrial fibrillation within 48 hours after ablation and its impact on long-term outcome. Am. J. Cardiol. 2008 Mar 15;101 (6):843–7. doi: 10.1016/j.amjcard.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 188.Arya Arash, Hindricks Gerhard, Sommer Philipp, Huo Yan, Bollmann Andreas, Gaspar Thomas, Bode Kerstin, Husser Daniela, Kottkamp Hans, Piorkowski Christopher. Long-term results and the predictors of outcome of catheter ablation of atrial fibrillation using steerable sheath catheter navigation after single procedure in 674 patients. Europace. 2010 Feb;12 (2):173–80. doi: 10.1093/europace/eup331. [DOI] [PubMed] [Google Scholar]
- 189.Andrade Jason G, Macle Laurent, Khairy Paul, Khaykin Yaariv, Mantovan Roberto, De Martino Giuseppe, Chen Jian, Morillo Carlos A, Novak Paul, Guerra Peter G, Nair Girish, Torrecilla Esteban G, Verma Atul. Incidence and significance of early recurrences associated with different ablation strategies for AF: a STAR-AF substudy. J. Cardiovasc. Electrophysiol. 2012 Dec;23 (12):1295–301. doi: 10.1111/j.1540-8167.2012.02399.x. [DOI] [PubMed] [Google Scholar]
- 190.Andrade Jason G, Khairy Paul, Macle Laurent, Packer Doug L, Lehmann John W, Holcomb Richard G, Ruskin Jeremy N, Dubuc Marc. Incidence and significance of early recurrences of atrial fibrillation after cryoballoon ablation: insights from the multicenter Sustained Treatment of Paroxysmal Atrial Fibrillation (STOP AF) Trial. Circ Arrhythm Electrophysiol. 2014 Feb;7 (1):69–75. doi: 10.1161/CIRCEP.113.000586. [DOI] [PubMed] [Google Scholar]
- 191.Baman Timir S, Gupta Sanjaya K, Billakanty Sreedhar R, Ilg Karl J, Good Eric, Crawford Thomas, Jongnarangsin Krit, Ebinger Matt, Pelosi Frank, Bogun Frank, Chugh Aman, Morady Fred, Oral Hakan. Time to cardioversion of recurrent atrial arrhythmias after catheter ablation of atrial fibrillation and long-term clinical outcome. J. Cardiovasc. Electrophysiol. 2009 Dec;20 (12):1321–5. doi: 10.1111/j.1540-8167.2009.01553.x. [DOI] [PubMed] [Google Scholar]
- 192.Leong-Sit Peter, Roux Jean-Francois, Zado Erica, Callans David J, Garcia Fermin, Lin David, Marchlinski Francis E, Bala Rupa, Dixit Sanjay, Riley Michael, Hutchinson Mathew D, Cooper Joshua, Russo Andrea M, Verdino Ralph, Gerstenfeld Edward P. Antiarrhythmics after ablation of atrial fibrillation (5A Study): six-month follow-up study. Circ Arrhythm Electrophysiol. 2011 Feb;4 (1):11–4. doi: 10.1161/CIRCEP.110.955393. [DOI] [PubMed] [Google Scholar]
- 193.Roux Jean-François, Zado Erica, Callans David J, Garcia Fermin, Lin David, Marchlinski Francis E, Bala Rupa, Dixit Sanjay, Riley Michael, Russo Andrea M, Hutchinson Mathew D, Cooper Joshua, Verdino Ralph, Patel Vickas, Joy Parijat S, Gerstenfeld Edward P. Antiarrhythmics After Ablation of Atrial Fibrillation (5A Study). Circulation. 2009 Sep 22;120 (12):1036–40. doi: 10.1161/CIRCULATIONAHA.108.839639. [DOI] [PubMed] [Google Scholar]
- 194.Hayashi Meiso, Miyauchi Yasushi, Iwasaki Yu-ki, Yodogawa Kenji, Tsuboi Ippei, Uetake Shunsuke, Hayashi Hiroshi, Takahashi Kenta, Shimizu Wataru. Three-month lower-dose flecainide after catheter ablation of atrial fibrillation. Europace. 2014 Aug;16 (8):1160–7. doi: 10.1093/europace/euu041. [DOI] [PubMed] [Google Scholar]
- 195.Darkner Stine, Chen Xu, Hansen Jim, Pehrson Steen, Johannessen Arne, Nielsen Jonas Bille, Svendsen Jesper Hastrup. Recurrence of arrhythmia following short-term oral AMIOdarone after CATheter ablation for atrial fibrillation: a double-blind, randomized, placebo-controlled study (AMIO-CAT trial). Eur. Heart J. 2014 Dec 14;35 (47):3356–64. doi: 10.1093/eurheartj/ehu354. [DOI] [PubMed] [Google Scholar]
- 196.Pokushalov Evgeny, Romanov Alexander, De Melis Mirko, Artyomenko Sergey, Baranova Vera, Losik Denis, Bairamova Sevda, Karaskov Alexander, Mittal Suneet, Steinberg Jonathan S. Progression of atrial fibrillation after a failed initial ablation procedure in patients with paroxysmal atrial fibrillation: a randomized comparison of drug therapy versus reablation. Circ Arrhythm Electrophysiol. 2013 Aug;6 (4):754–60. doi: 10.1161/CIRCEP.113.000495. [DOI] [PubMed] [Google Scholar]
- 197.Gerstenfeld Edward P, Callans David J, Dixit Sanjay, Zado Erica, Marchlinski Francis E. Incidence and location of focal atrial fibrillation triggers in patients undergoing repeat pulmonary vein isolation: implications for ablation strategies. J. Cardiovasc. Electrophysiol. 2003 Jul;14 (7):685–90. doi: 10.1046/j.1540-8167.2003.03013.x. [DOI] [PubMed] [Google Scholar]
- 198.Nanthakumar Kumaraswamy, Plumb Vance J, Epstein Andrew E, Veenhuyzen George D, Link Dale, Kay G Neal. Resumption of electrical conduction in previously isolated pulmonary veins: rationale for a different strategy? Circulation. 2004 Mar 16;109 (10):1226–9. doi: 10.1161/01.CIR.0000121423.78120.49. [DOI] [PubMed] [Google Scholar]
- 199.Verma Atul, Kilicaslan Fethi, Pisano Ennio, Marrouche Nassir F, Fanelli Raffaele, Brachmann Johannes, Geunther Jens, Potenza Domenico, Martin David O, Cummings Jennifer, Burkhardt J David, Saliba Walid, Schweikert Robert A, Natale Andrea. Response of atrial fibrillation to pulmonary vein antrum isolation is directly related to resumption and delay of pulmonary vein conduction. Circulation. 2005 Aug 2;112 (5):627–35. doi: 10.1161/CIRCULATIONAHA.104.533190. [DOI] [PubMed] [Google Scholar]
- 200.Miyazaki Shinsuke, Kuwahara Taishi, Kobori Atsushi, Takahashi Yoshihide, Takei Asumi, Sato Akira, Isobe Mitsuaki, Takahashi Atsushi. Long-term clinical outcome of extensive pulmonary vein isolation-based catheter ablation therapy in patients with paroxysmal and persistent atrial fibrillation. Heart. 2011 Apr;97 (8):668–73. doi: 10.1136/hrt.2009.186874. [DOI] [PubMed] [Google Scholar]
- 201.Ganesan Anand N, Shipp Nicholas J, Brooks Anthony G, Kuklik Pawel, Lau Dennis H, Lim Han S, Sullivan Thomas, Roberts-Thomson Kurt C, Sanders Prashanthan. Long-term outcomes of catheter ablation of atrial fibrillation: a systematic review and meta-analysis. J Am Heart Assoc. 2013 Apr;2 (2) doi: 10.1161/JAHA.112.004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.McGann Christopher J, Kholmovski Eugene G, Oakes Robert S, Blauer Joshua J E, Daccarett Marcos, Segerson Nathan, Airey Kelly J, Akoum Nazem, Fish Eric, Badger Troy J, DiBella Edward V R, Parker Dennis, MacLeod Rob S, Marrouche Nassir F. New magnetic resonance imaging-based method for defining the extent of left atrial wall injury after the ablation of atrial fibrillation. J. Am. Coll. Cardiol. 2008 Oct 7;52 (15):1263–71. doi: 10.1016/j.jacc.2008.05.062. [DOI] [PubMed] [Google Scholar]
- 203.Peters Dana C, Wylie John V, Hauser Thomas H, Nezafat Reza, Han Yuchi, Woo Jeong Joo, Taclas Jason, Kissinger Kraig V, Goddu Beth, Josephson Mark E, Manning Warren J. Recurrence of atrial fibrillation correlates with the extent of post-procedural late gadolinium enhancement: a pilot study. JACC Cardiovasc Imaging. 2009 Mar;2 (3):308–16. doi: 10.1016/j.jcmg.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Badger Troy J, Daccarett Marcos, Akoum Nazem W, Adjei-Poku Yaw A, Burgon Nathan S, Haslam Thomas S, Kalvaitis Saul, Kuppahally Suman, Vergara Gaston, McMullen Lori, Anderson Paul A, Kholmovski Eugene, MacLeod Rob S, Marrouche Nassir F. Evaluation of left atrial lesions after initial and repeat atrial fibrillation ablation: lessons learned from delayed-enhancement MRI in repeat ablation procedures. Circ Arrhythm Electrophysiol. 2010 Jun;3 (3):249–59. doi: 10.1161/CIRCEP.109.868356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Arujuna Aruna, Karim Rashed, Caulfield Dennis, Knowles Benjamin, Rhode Kawal, Schaeffter Tobias, Kato Bernet, Rinaldi C Aldo, Cooklin Michael, Razavi Reza, O'Neill Mark D, Gill Jaswinder. Acute pulmonary vein isolation is achieved by a combination of reversible and irreversible atrial injury after catheter ablation: evidence from magnetic resonance imaging. Circ Arrhythm Electrophysiol. 2012 Aug 1;5 (4):691–700. doi: 10.1161/CIRCEP.111.966523. [DOI] [PubMed] [Google Scholar]
- 206.Schmitt Claus, Ndrepepa Gjin, Weber Stefan, Schmieder Sebastian, Weyerbrock Sonja, Schneider Michael, Karch Martin R, Deisenhofer Isabel, Schreieck Jürgen, Zrenner Bernhard, Schömig Albert. Biatrial multisite mapping of atrial premature complexes triggering onset of atrial fibrillation. Am. J. Cardiol. 2002 Jun 15;89 (12):1381–7. doi: 10.1016/s0002-9149(02)02350-0. [DOI] [PubMed] [Google Scholar]
- 207.Lin Wei-Shiang, Tai Ching-Tai, Hsieh Ming-Hsiung, Tsai Chin-Feng, Lin Yung-Kuo, Tsao Hsuan-Ming, Huang Jin-Long, Yu Wen-Chung, Yang Shih-Ping, Ding Yu-An, Chang Mau-Song, Chen Shih-Ann. Catheter ablation of paroxysmal atrial fibrillation initiated by non-pulmonary vein ectopy. Circulation. 2003 Jul 1;107 (25):3176–83. doi: 10.1161/01.CIR.0000074206.52056.2D. [DOI] [PubMed] [Google Scholar]
- 208.Valles Ermengol, Fan Roger, Roux Jean François, Liu Christopher F, Harding John D, Dhruvakumar Sandhya, Hutchinson Mathew D, Riley Michael, Bala Rupa, Garcia Fermin C, Lin David, Dixit Sanjay, Callans David J, Gerstenfeld Edward P, Marchlinski Francis E. Localization of atrial fibrillation triggers in patients undergoing pulmonary vein isolation: importance of the carina region. J. Am. Coll. Cardiol. 2008 Oct 21;52 (17):1413–20. doi: 10.1016/j.jacc.2008.07.025. [DOI] [PubMed] [Google Scholar]
- 209.Kurotobi Toshiya, Iwakura Katsuomi, Inoue Koichi, Kimura Ryusuke, Okamura Atsunori, Koyama Yasushi, Tosyoshima Yuko, Ito Norihisa, Fujii Kenshi. Multiple arrhythmogenic foci associated with the development of perpetuation of atrial fibrillation. Circ Arrhythm Electrophysiol. 2010 Feb;3 (1):39–45. doi: 10.1161/CIRCEP.109.885095. [DOI] [PubMed] [Google Scholar]
- 210.Di Biase Luigi, Burkhardt J David, Mohanty Prasant, Sanchez Javier, Mohanty Sanghamitra, Horton Rodney, Gallinghouse G Joseph, Bailey Shane M, Zagrodzky Jason D, Santangeli Pasquale, Hao Steven, Hongo Richard, Beheiry Salwa, Themistoclakis Sakis, Bonso Aldo, Rossillo Antonio, Corrado Andrea, Raviele Antonio, Al-Ahmad Amin, Wang Paul, Cummings Jennifer E, Schweikert Robert A, Pelargonio Gemma, Dello Russo Antonio, Casella Michela, Santarelli Pietro, Lewis William R, Natale Andrea. Left atrial appendage: an underrecognized trigger site of atrial fibrillation. Circulation. 2010 Jul 13;122 (2):109–18. doi: 10.1161/CIRCULATIONAHA.109.928903. [DOI] [PubMed] [Google Scholar]
- 211.Corrado Andrea, Bonso Aldo, Madalosso Michela, Rossillo Antonio, Themistoclakis Sakis, Di Biase Luigi, Natale Andrea, Raviele Antonio. Impact of systematic isolation of superior vena cava in addition to pulmonary vein antrum isolation on the outcome of paroxysmal, persistent, and permanent atrial fibrillation ablation: results from a randomized study. J. Cardiovasc. Electrophysiol. 2010 Jan;21 (1):1–5. doi: 10.1111/j.1540-8167.2009.01577.x. [DOI] [PubMed] [Google Scholar]
- 212.Da Costa Antoine, Levallois Marie, Romeyer-Bouchard Cécile, Bisch Laurence, Gate-Martinet Alexis, Isaaz Karl. Remote-controlled magnetic pulmonary vein isolation combined with superior vena cava isolation for paroxysmal atrial fibrillation: a prospective randomized study. Arch Cardiovasc Dis. 2015 Mar;108 (3):163–71. doi: 10.1016/j.acvd.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 213.Wang Xin-Hua, Liu Xu, Sun Yu-Min, Shi Hai-Feng, Zhou Li, Gu Jia-Ning. Pulmonary vein isolation combined with superior vena cava isolation for atrial fibrillation ablation: a prospective randomized study. Europace. 2008 May;10 (5):600–5. doi: 10.1093/europace/eun077. [DOI] [PubMed] [Google Scholar]
- 214.Deisenhofer Isabel, Estner Heidi, Reents Tilko, Fichtner Stephanie, Bauer Axel, Wu Jinjin, Kolb Christof, Zrenner Bernhard, Schmitt Claus, Hessling Gabriele. Does electrogram guided substrate ablation add to the success of pulmonary vein isolation in patients with paroxysmal atrial fibrillation? A prospective, randomized study. J. Cardiovasc. Electrophysiol. 2009 May;20 (5):514–21. doi: 10.1111/j.1540-8167.2008.01379.x. [DOI] [PubMed] [Google Scholar]
- 215.Di Biase Luigi, Elayi Claude S, Fahmy Tamer S, Martin David O, Ching Chi Keong, Barrett Conor, Bai Rong, Patel Dimpi, Khaykin Yaariv, Hongo Richard, Hao Steven, Beheiry Salwa, Pelargonio Gemma, Dello Russo Antonio, Casella Michela, Santarelli Pietro, Potenza Domenico, Fanelli Raffaele, Massaro Raimondo, Wang Paul, Al-Ahmad Amin, Arruda Mauricio, Themistoclakis Sakis, Bonso Aldo, Rossillo Antonio, Raviele Antonio, Schweikert Robert A, Burkhardt David J, Natale Andrea. Atrial fibrillation ablation strategies for paroxysmal patients: randomized comparison between different techniques. Circ Arrhythm Electrophysiol. 2009 Apr;2 (2):113–9. doi: 10.1161/CIRCEP.108.798447. [DOI] [PubMed] [Google Scholar]
- 216.Sawhney Navinder, Anousheh Ramtin, Chen Wei, Feld Gregory K. Circumferential pulmonary vein ablation with additional linear ablation results in an increased incidence of left atrial flutter compared with segmental pulmonary vein isolation as an initial approach to ablation of paroxysmal atrial fibrillation. Circ Arrhythm Electrophysiol. 2010 Jun;3 (3):243–8. doi: 10.1161/CIRCEP.109.924878. [DOI] [PubMed] [Google Scholar]
- 217.Hayward Robert M, Upadhyay Gaurav A, Mela Theofanie, Ellinor Patrick T, Barrett Conor D, Heist E Kevin, Verma Atul, Choudhry Niteesh K, Singh Jagmeet P. Pulmonary vein isolation with complex fractionated atrial electrogram ablation for paroxysmal and nonparoxysmal atrial fibrillation: A meta-analysis. Heart Rhythm. 2011 Jul;8 (7):994–1000. doi: 10.1016/j.hrthm.2011.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Mun Hee-Sun, Joung Boyoung, Shim Jaemin, Hwang Hye Jin, Kim Jong Youn, Lee Moon-Hyoung, Pak Hui-Nam. Does additional linear ablation after circumferential pulmonary vein isolation improve clinical outcome in patients with paroxysmal atrial fibrillation? Prospective randomised study. Heart. 2012 Mar;98 (6):480–4. doi: 10.1136/heartjnl-2011-301107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Wu Shao-Hui, Jiang Wei-Feng, Gu Jun, Zhao Liang, Wang Yuan-Long, Liu Yu-Gang, Zhou Li, Gu Jia-Ning, Xu Kai, Liu Xu. Benefits and risks of additional ablation of complex fractionated atrial electrograms for patients with atrial fibrillation: a systematic review and meta-analysis. Int. J. Cardiol. 2013 Oct 25;169 (1):35–43. doi: 10.1016/j.ijcard.2013.08.083. [DOI] [PubMed] [Google Scholar]
- 220.Arbelo Elena, Guiu Esther, Ramos Pablo, Bisbal Felipe, Borras Roger, Andreu David, Tolosana José María, Berruezo Antonio, Brugada Josep, Mont Lluís. Benefit of left atrial roof linear ablation in paroxysmal atrial fibrillation: a prospective, randomized study. J Am Heart Assoc. 2014 Oct;3 (5) doi: 10.1161/JAHA.114.000877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kim Tae-Hoon, Park Junbeom, Park Jin-Kyu, Uhm Jae-Sun, Joung Boyoung, Hwang Chun, Lee Moon-Hyoung, Pak Hui-Nam. Linear ablation in addition to circumferential pulmonary vein isolation (Dallas lesion set) does not improve clinical outcome in patients with paroxysmal atrial fibrillation: a prospective randomized study. Europace. 2015 Mar;17 (3):388–95. doi: 10.1093/europace/euu245. [DOI] [PubMed] [Google Scholar]
- 222.Nührich Jana Mareike, Steven Daniel, Berner Imke, Rostock Thomas, Hoffmann Boris, Servatius Helge, Sultan Arian, Lüker Jakob, Treszl András, Wegscheider Karl, Willems Stephan. Impact of biatrial defragmentation in patients with paroxysmal atrial fibrillation: results from a randomized prospective study. Heart Rhythm. 2014 Sep;11 (9):1536–42. doi: 10.1016/j.hrthm.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 223.Fichtner S, Sparn K, Reents T, Ammar S, Semmler V, Dillier R, Buiatti A, Kathan S, Hessling G, Deisenhofer I. Recurrence of paroxysmal atrial fibrillation after pulmonary vein isolation: is repeat pulmonary vein isolation enough? A prospective, randomized trial. Europace : European pacing, arrhythmias, and cardiac electrophysiology. journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2015;0:0–0. doi: 10.1093/europace/euu389. [DOI] [PubMed] [Google Scholar]
- 224.Chugh Aman, Oral Hakan, Lemola Kristina, Hall Burr, Cheung Peter, Good Eric, Tamirisa Kamala, Han Jihn, Bogun Frank, Pelosi Frank, Morady Fred. Prevalence, mechanisms, and clinical significance of macroreentrant atrial tachycardia during and following left atrial ablation for atrial fibrillation. Heart Rhythm. 2005 May;2 (5):464–71. doi: 10.1016/j.hrthm.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 225.Oral Hakan, Knight Bradley P, Tada Hiroshi, Ozaydin Mehmet, Chugh Aman, Hassan Sohail, Scharf Christoph, Lai Steve W K, Greenstein Radmira, Pelosi Frank, Strickberger S Adam, Morady Fred. Pulmonary vein isolation for paroxysmal and persistent atrial fibrillation. Circulation. 2002 Mar 5;105 (9):1077–81. doi: 10.1161/hc0902.104712. [DOI] [PubMed] [Google Scholar]
- 226.Elayi Claude S, Verma Atul, Di Biase Luigi, Ching Chi Keong, Patel Dimpi, Barrett Conor, Martin David, Rong Bai, Fahmy Tamer S, Khaykin Yaariv, Hongo Richard, Hao Steven, Pelargonio Gemma, Dello Russo Antonio, Casella Michela, Santarelli Pietro, Potenza Domenico, Fanelli Raffaele, Massaro Raimondo, Arruda Mauricio, Schweikert Robert A, Natale Andrea. Ablation for longstanding permanent atrial fibrillation: results from a randomized study comparing three different strategies. Heart Rhythm. 2008 Dec;5 (12):1658–64. doi: 10.1016/j.hrthm.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 227.Tilz Roland Richard, Chun K R Julian, Schmidt Boris, Fuernkranz Alexander, Wissner Erik, Koester Ilka, Baensch Dietmar, Boczor Sigrid, Koektuerk Buelent, Metzner Andreas, Zerm Thomas, Ernst Sabine, Antz Matthias, Kuck Karl-Heinz, Ouyang Feifan. Catheter ablation of long-standing persistent atrial fibrillation: a lesson from circumferential pulmonary vein isolation. J. Cardiovasc. Electrophysiol. 2010 Oct;21 (10):1085–93. doi: 10.1111/j.1540-8167.2010.01799.x. [DOI] [PubMed] [Google Scholar]
- 228.Parkash Ratika, Tang Anthony S L, Sapp John L, Wells George. Approach to the catheter ablation technique of paroxysmal and persistent atrial fibrillation: a meta-analysis of the randomized controlled trials. J. Cardiovasc. Electrophysiol. 2011 Jul;22 (7):729–38. doi: 10.1111/j.1540-8167.2011.02010.x. [DOI] [PubMed] [Google Scholar]
- 229.Willems Stephan, Klemm Hanno, Rostock Thomas, Brandstrup Benedikt, Ventura Rodolfo, Steven Daniel, Risius Tim, Lutomsky Boris, Meinertz Thomas. Substrate modification combined with pulmonary vein isolation improves outcome of catheter ablation in patients with persistent atrial fibrillation: a prospective randomized comparison. Eur. Heart J. 2006 Dec;27 (23):2871–8. doi: 10.1093/eurheartj/ehl093. [DOI] [PubMed] [Google Scholar]
- 230.Akoum Nazem, Wilber David, Hindricks Gerhard, Jais Pierre, Cates Josh, Marchlinski Francis, Kholmovski Eugene, Burgon Nathan, Hu Nan, Mont Lluis, Deneke Thomas, Duytschaever Mattias, Neumann Thomas, Mansour Moussa, Mahnkopf Christian, Hutchinson Mathew, Herweg Bengt, Daoud Emile, Wissner Erik, Brachmann Johannes, Marrouche Nassir F. MRI Assessment of Ablation-Induced Scarring in Atrial Fibrillation: Analysis from the DECAAF Study. J. Cardiovasc. Electrophysiol. 2015 May;26 (5):473–80. doi: 10.1111/jce.12650. [DOI] [PubMed] [Google Scholar]
- 231.Chang Hung-Yu, Lo Li-Wei, Lin Yenn-Jiang, Chang Shih-Lin, Hu Yu-Feng, Li Cheng-Hung, Chao Tze-Fan, Chung Fa-Po, Ha Trung Le, Singhal Rahul, Chong Eric, Yin Wei-Hsian, Tsao Hsuan-Ming, Hsieh Ming-Hsiung, Chen Shih-Ann. Long-term outcome of catheter ablation in patients with atrial fibrillation originating from nonpulmonary vein ectopy. J. Cardiovasc. Electrophysiol. 2013 Mar;24 (3):250–8. doi: 10.1111/jce.12036. [DOI] [PubMed] [Google Scholar]
- 232.Yokokawa Miki, Chugh Aman, Ulfarsson Magnus, Takaki Hiroshi, Han Li, Yoshida Kentaro, Sugimachi Masaru, Morady Fred, Oral Hakan. Effect of linear ablation on spectral components of atrial fibrillation. Heart Rhythm. 2010 Dec;7 (12):1732–7. doi: 10.1016/j.hrthm.2010.05.040. [DOI] [PubMed] [Google Scholar]
- 233.Razavi Mehdi, Zhang Shulong, Delapasse Scott, Yang Donghui, Ai Tomohiko, Kar Biswajit, Younis George, Rasekh Abdi, Cheng Jie. The effects of pulmonary vein isolation on the dominant frequency and organization of coronary sinus electrical activity during permanent atrial fibrillation. Pacing Clin Electrophysiol. 2006 Nov;29 (11):1201–8. doi: 10.1111/j.1540-8159.2006.00524.x. [DOI] [PubMed] [Google Scholar]
- 234.Verma Atul, Mantovan Roberto, Macle Laurent, De Martino Guiseppe, Chen Jian, Morillo Carlos A, Novak Paul, Calzolari Vittorio, Guerra Peter G, Nair Girish, Torrecilla Esteban G, Khaykin Yaariv. Substrate and Trigger Ablation for Reduction of Atrial Fibrillation (STAR AF): a randomized, multicentre, international trial. Eur. Heart J. 2010 Jun;31 (11):1344–56. doi: 10.1093/eurheartj/ehq041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Wynn Gareth J, Das Moloy, Bonnett Laura J, Panikker Sandeep, Wong Tom, Gupta Dhiraj. Efficacy of catheter ablation for persistent atrial fibrillation: a systematic review and meta-analysis of evidence from randomized and nonrandomized controlled trials. Circ Arrhythm Electrophysiol. 2014 Oct;7 (5):841–52. doi: 10.1161/CIRCEP.114.001759. [DOI] [PubMed] [Google Scholar]
- 236.Kainuma Satoshi, Masai Takafumi, Yoshitatsu Masao, Miyagawa Shigeru, Yamauchi Takashi, Takeda Koji, Morii Eiichi, Sawa Yoshiki. Advanced left-atrial fibrosis is associated with unsuccessful maze operation for valvular atrial fibrillation. Eur J Cardiothorac Surg. 2011 Jul;40 (1):61–9. doi: 10.1016/j.ejcts.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 237.Hartono Beny, Lo Li-Wei, Cheng Chen-Chuan, Lin Yenn-Jiang, Chang Shih-Lin, Hu Yu-Feng, Suenari Kazuyoshi, Li Cheng Hung, Chao Tze-Fan, Liu Shuen-Hsin, Niu Ya-Lei, Chang Hung-Yu, Ambrose Kibos, Yu Wen-Chung, Hsu Tsui-Lieh, Chen Shih-Ann. A novel finding of the atrial substrate properties and long-term results of catheter ablation in chronic atrial fibrillation patients with left atrial spontaneous echo contrast. J. Cardiovasc. Electrophysiol. 2012 Mar;23 (3):239–46. doi: 10.1111/j.1540-8167.2011.02170.x. [DOI] [PubMed] [Google Scholar]
- 238.Fiala Martin, Bulková Veronika, Škňouřil Libor, Nevřalová Renáta, Toman Ondřej, Januška Jaroslav, Špinar Jindřich, Wichterle Dan. Sinus rhythm restoration and arrhythmia noninducibility are major predictors of arrhythmia-free outcome after ablation for long-standing persistent atrial fibrillation: a prospective study. Heart Rhythm. 2015 Apr;12 (4):687–98. doi: 10.1016/j.hrthm.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 239.Budera Petr, Straka Zbyněk, Osmančík Pavel, Vaněk Tomáš, Jelínek Štěpán, Hlavička Jan, Fojt Richard, Červinka Pavel, Hulman Michal, Šmíd Michal, Malý Marek, Widimský Petr. Comparison of cardiac surgery with left atrial surgical ablation vs. cardiac surgery without atrial ablation in patients with coronary and/or valvular heart disease plus atrial fibrillation: final results of the PRAGUE-12 randomized multicentre study. Eur. Heart J. 2012 Nov;33 (21):2644–52. doi: 10.1093/eurheartj/ehs290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Yoshida Kentaro, Chugh Aman, Good Eric, Crawford Thomas, Myles James, Veerareddy Srikar, Billakanty Sreedhar, Wong Wai S, Ebinger Matthew, Pelosi Frank, Jongnarangsin Krit, Bogun Frank, Morady Fred, Oral Hakan. A critical decrease in dominant frequency and clinical outcome after catheter ablation of persistent atrial fibrillation. Heart Rhythm. 2010 Mar;7 (3):295–302. doi: 10.1016/j.hrthm.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 241.Nagamoto Yasutsugu, Park Jae-Seok, Tanubudi Daniel, Ko Yiu-Kwan, Ban Ji-Eun, Kwak Jae-Jin, Choi Jong-Il, Lim Hong-Euy, Park Sang-Weon, Kim Young-Hoon. Clinical significance of induced atrial tachycardia after termination of longstanding persistent atrial fibrillation using a stepwise approach. J. Cardiovasc. Electrophysiol. 2012 Nov;23 (11):1171–8. doi: 10.1111/j.1540-8167.2012.02382.x. [DOI] [PubMed] [Google Scholar]
- 242.Elayi Claude S, Di Biase Luigi, Barrett Conor, Ching Chi Keong, al Aly Moataz, Lucciola Maria, Bai Rong, Horton Rodney, Fahmy Tamer S, Verma Atul, Khaykin Yaariv, Shah Jignesh, Morales Gustavo, Hongo Richard, Hao Steven, Beheiry Salwa, Arruda Mauricio, Schweikert Robert A, Cummings Jennifer, Burkhardt J David, Wang Paul, Al-Ahmad Amin, Cauchemez Bruno, Gaita Fiorenzo, Natale Andrea. Atrial fibrillation termination as a procedural endpoint during ablation in long-standing persistent atrial fibrillation. Heart Rhythm. 2010 Sep;7 (9):1216–23. doi: 10.1016/j.hrthm.2010.01.038. [DOI] [PubMed] [Google Scholar]
- 243.Komatsu Yuki, Taniguchi Hiroshi, Miyazaki Shinsuke, Nakamura Hiroaki, Kusa Shigeki, Uchiyama Takashi, Kakita Ken, Kakuta Tsunekazu, Hachiya Hitoshi, Iesaka Yoshito. Impact of atrial fibrillation termination on clinical outcome after ablation in relation to the duration of persistent atrial fibrillation. Pacing Clin Electrophysiol. 2012 Dec;35 (12):1436–43. doi: 10.1111/pace.12009. [DOI] [PubMed] [Google Scholar]
- 244.Fassini Gaetano, Riva Stefania, Chiodelli Roberta, Trevisi Nicola, Berti Marco, Carbucicchio Corrado, Maccabelli Giuseppe, Giraldi Francesco, Bella Paolo Della. Left mitral isthmus ablation associated with PV Isolation: long-term results of a prospective randomized study. J. Cardiovasc. Electrophysiol. 2005 Nov;16 (11):1150–6. doi: 10.1111/j.1540-8167.2005.50192.x. [DOI] [PubMed] [Google Scholar]
- 245.Haïssaguerre Michel, Sanders Prashanthan, Hocini Mélèze, Takahashi Yoshihide, Rotter Martin, Sacher Frederic, Rostock Thomas, Hsu Li-Fern, Bordachar Pierre, Reuter Sylvain, Roudaut Raymond, Clémenty Jacques, Jaïs Pierre. Catheter ablation of long-lasting persistent atrial fibrillation: critical structures for termination. J. Cardiovasc. Electrophysiol. 2005 Nov;16 (11):1125–37. doi: 10.1111/j.1540-8167.2005.00307.x. [DOI] [PubMed] [Google Scholar]
- 246.Gaita Fiorenzo, Caponi Domenico, Scaglione Marco, Montefusco Antonio, Corleto Antonella, Di Monte Fernando, Coin Daniele, Di Donna Paolo, Giustetto Carla. Long-term clinical results of 2 different ablation strategies in patients with paroxysmal and persistent atrial fibrillation. Circ Arrhythm Electrophysiol. 2008 Oct;1 (4):269–75. doi: 10.1161/CIRCEP.108.774885. [DOI] [PubMed] [Google Scholar]
- 247.Kim Jin-Seok, Shin Seung Yong, Na Jin Oh, Choi Cheol Ung, Kim Seong Hwan, Kim Jin Won, Kim Eung Ju, Rha Seung-Woon, Park Chang Gyu, Seo Hong Seog, Oh Dong Joo, Hwang Chun, Lim Hong Euy. Does isolation of the left atrial posterior wall improve clinical outcomes after radiofrequency catheter ablation for persistent atrial fibrillation?: A prospective randomized clinical trial. Int. J. Cardiol. 2015 Feb 15;181 ():277–83. doi: 10.1016/j.ijcard.2014.12.035. [DOI] [PubMed] [Google Scholar]
- 248.Bai Rong, Di Biase Luigi, Mohanty Prasant, Trivedi Chintan, Dello Russo Antonio, Themistoclakis Sakis, Casella Michela, Santarelli Pietro, Fassini Gaetano, Santangeli Pasquale, Mohanty Sanghamitra, Rossillo Antonio, Pelargonio Gemma, Horton Rodney, Sanchez Javier, Gallinghouse Joseph, Burkhardt J David, Ma Chang-Sheng, Tondo Claudio, Natale Andrea. Proven isolation of the pulmonary vein antrum with or without left atrial posterior wall isolation in patients with persistent atrial fibrillation. Heart Rhythm. 2016 Jan;13 (1):132–40. doi: 10.1016/j.hrthm.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 249.Nademanee Koonlawee, McKenzie John, Kosar Erol, Schwab Mark, Sunsaneewitayakul Buncha, Vasavakul Thaveekiat, Khunnawat Chotikorn, Ngarmukos Tachapong. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J. Am. Coll. Cardiol. 2004 Jun 2;43 (11):2044–53. doi: 10.1016/j.jacc.2003.12.054. [DOI] [PubMed] [Google Scholar]
- 250.Oral Hakan, Chugh Aman, Lemola Kristina, Cheung Peter, Hall Burr, Good Eric, Han Jihn, Tamirisa Kamala, Bogun Frank, Pelosi Frank, Morady Fred. Noninducibility of atrial fibrillation as an end point of left atrial circumferential ablation for paroxysmal atrial fibrillation: a randomized study. Circulation. 2004 Nov 2;110 (18):2797–801. doi: 10.1161/01.CIR.0000146786.87037.26. [DOI] [PubMed] [Google Scholar]
- 251.Lin Yenn-Jiang, Tai Ching-Tai, Chang Shih-Lin, Lo Li-Wei, Tuan Ta-Chuan, Wongcharoen Wanwarang, Udyavar Ameya R, Hu Yu-Feng, Chang Chien-Jung, Tsai Wen-Chin, Kao Tsair, Higa Satoshi, Chen Shih-Ann. Efficacy of additional ablation of complex fractionated atrial electrograms for catheter ablation of nonparoxysmal atrial fibrillation. J. Cardiovasc. Electrophysiol. 2009 Jun;20 (6):607–15. doi: 10.1111/j.1540-8167.2008.01393.x. [DOI] [PubMed] [Google Scholar]
- 252.Bai Rong, Di Biase Luigi, Mohanty Prasant, Dello Russo Antonio, Casella Michela, Pelargonio Gemma, Themistoclakis Sakis, Mohanty Sanghamitra, Elayi Claude S, Sanchez Javier, Burkhardt J David, Horton Rodney, Gallinghouse G Joseph, Bailey Shane M, Bonso Aldo, Beheiry Salwa, Hongo Richard H, Raviele Antonio, Tondo Claudio, Natale Andrea. Ablation of perimitral flutter following catheter ablation of atrial fibrillation: impact on outcomes from a randomized study (PROPOSE). J. Cardiovasc. Electrophysiol. 2012 Feb;23 (2):137–44. doi: 10.1111/j.1540-8167.2011.02182.x. [DOI] [PubMed] [Google Scholar]
- 253.Kumagai Koji, Sakamoto Tamotsu, Nakamura Keijiro, Nishiuchi Suguru, Hayano Mamoru, Hayashi Tatsuya, Sasaki Takehito, Aonuma Kazutaka, Oshima Shigeru. Combined dominant frequency and complex fractionated atrial electrogram ablation after circumferential pulmonary vein isolation of atrial fibrillation. J. Cardiovasc. Electrophysiol. 2013 Sep;24 (9):975–83. doi: 10.1111/jce.12166. [DOI] [PubMed] [Google Scholar]
- 254.Lemola Kristina, Chartier Denis, Yeh Yung-Hsin, Dubuc Marc, Cartier Raymond, Armour Andrew, Ting Michael, Sakabe Masao, Shiroshita-Takeshita Akiko, Comtois Philippe, Nattel Stanley. Pulmonary vein region ablation in experimental vagal atrial fibrillation: role of pulmonary veins versus autonomic ganglia. Circulation. 2008 Jan 29;117 (4):470–7. doi: 10.1161/CIRCULATIONAHA.107.737023. [DOI] [PubMed] [Google Scholar]
- 255.Narayan Sanjiv M, Patel Jigar, Mulpuru Siva, Krummen David E. Focal impulse and rotor modulation ablation of sustaining rotors abruptly terminates persistent atrial fibrillation to sinus rhythm with elimination on follow-up: a video case study. Heart Rhythm. 2012 Sep;9 (9):1436–9. doi: 10.1016/j.hrthm.2012.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Jaïs Pierre, Hocini Mélèze, Hsu Li-Fern, Sanders Prashanthan, Scavee Christophe, Weerasooriya Rukshen, Macle Laurent, Raybaud Florence, Garrigue Stéphane, Shah Dipen C, Le Metayer Philippe, Clémenty Jacques, Haïssaguerre Michel. Technique and results of linear ablation at the mitral isthmus. Circulation. 2004 Nov 9;110 (19):2996–3002. doi: 10.1161/01.CIR.0000146917.75041.58. [DOI] [PubMed] [Google Scholar]
- 257.Yokokawa Miki, Sundaram Baskaran, Garg Anubhav, Stojanovska Jadranka, Oral Hakan, Morady Fred, Chugh Aman. Impact of mitral isthmus anatomy on the likelihood of achieving linear block in patients undergoing catheter ablation of persistent atrial fibrillation. Heart Rhythm. 2011 Sep;8 (9):1404–10. doi: 10.1016/j.hrthm.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 258.Hocini Mélèze, Shah Ashok J, Nault Isabelle, Rivard Lena, Linton Nick, Narayan Sanjiv, Myiazaki Shinsuke, Jadidi Amir S, Knecht Sébastien, Scherr Daniel, Wilton Stephen B, Roten Laurent, Pascale Patrizio, Pedersen Michala, Derval Nicolas, Sacher Frédéric, Jaïs Pierre, Clémenty Jacques, Haïssaguerre Michel. Mitral isthmus ablation with and without temporary spot occlusion of the coronary sinus: a randomized clinical comparison of acute outcomes. J. Cardiovasc. Electrophysiol. 2012 May;23 (5):489–96. doi: 10.1111/j.1540-8167.2011.02248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.D'Avila Andre, Thiagalingam Aravinda, Foley Lori, Fox Melodie, Ruskin Jeremy N, Reddy Vivek Y. Temporary occlusion of the great cardiac vein and coronary sinus to facilitate radiofrequency catheter ablation of the mitral isthmus. J. Cardiovasc. Electrophysiol. 2008 Jun;19 (6):645–50. doi: 10.1111/j.1540-8167.2008.01185.x. [DOI] [PubMed] [Google Scholar]
- 260.Sawhney Navinder, Anand Kislay, Robertson Clare E, Wurdeman Taylor, Anousheh Ramtin, Feld Gregory K. Recovery of mitral isthmus conduction leads to the development of macro-reentrant tachycardia after left atrial linear ablation for atrial fibrillation. Circ Arrhythm Electrophysiol. 2011 Dec;4 (6):832–7. doi: 10.1161/CIRCEP.111.964817. [DOI] [PubMed] [Google Scholar]
- 261.Verma Atul, Jiang Chen-yang, Betts Timothy R, Chen Jian, Deisenhofer Isabel, Mantovan Roberto, Macle Laurent, Morillo Carlos A, Haverkamp Wilhelm, Weerasooriya Rukshen, Albenque Jean-Paul, Nardi Stefano, Menardi Endrj, Novak Paul, Sanders Prashanthan. Approaches to catheter ablation for persistent atrial fibrillation. N. Engl. J. Med. 2015 May 7;372 (19):1812–22. doi: 10.1056/NEJMoa1408288. [DOI] [PubMed] [Google Scholar]
- 262.Wazni Oussama, Marrouche Nassir F, Martin David O, Gillinov A Marc, Saliba Walid, Saad Eduardo, Klein Allan, Bhargava Mandeep, Bash Dianna, Schweikert Robert, Erciyes Demet, Abdul-Karim Ahmad, Brachman Johannes, Gunther Jens, Pisano Ennio, Potenza Domenico, Fanelli Raffaele, Natale Andrea. Randomized study comparing combined pulmonary vein-left atrial junction disconnection and cavotricuspid isthmus ablation versus pulmonary vein-left atrial junction disconnection alone in patients presenting with typical atrial flutter and atrial fibrillation. Circulation. 2003 Nov 18;108 (20):2479–83. doi: 10.1161/01.CIR.0000101684.88679.AB. [DOI] [PubMed] [Google Scholar]
- 263.Pontoppidan J, Nielsen J C, Poulsen S H, Jensen H K, Walfridsson H, Pedersen A K, Hansen P S. Prophylactic cavotricuspid isthmus block during atrial fibrillation ablation in patients without atrial flutter: a randomised controlled trial. Heart. 2009 Jun;95 (12):994–9. doi: 10.1136/hrt.2008.153965. [DOI] [PubMed] [Google Scholar]
- 264.Chugh Aman, Latchamsetty Rakesh, Oral Hakan, Elmouchi Darryl, Tschopp David, Reich Scott, Igic Petar, Lemerand Tammy, Good Eric, Bogun Frank, Pelosi Frank, Morady Fred. Characteristics of cavotricuspid isthmus-dependent atrial flutter after left atrial ablation of atrial fibrillation. Circulation. 2006 Feb 7;113 (5):609–15. doi: 10.1161/CIRCULATIONAHA.105.580936. [DOI] [PubMed] [Google Scholar]
- 265.Shah Dipen C, Sunthorn Henri, Burri Haran, Gentil-Baron Pascale. Evaluation of an individualized strategy of cavotricuspid isthmus ablation as an adjunct to atrial fibrillation ablation. J. Cardiovasc. Electrophysiol. 2007 Sep;18 (9):926–30. doi: 10.1111/j.1540-8167.2007.00896.x. [DOI] [PubMed] [Google Scholar]
- 266.Phan Kevin, Xie Ashleigh, Tsai Yi-Chin, Kumar Narendra, La Meir Mark, Yan Tristan D. Biatrial ablation vs. left atrial concomitant surgical ablation for treatment of atrial fibrillation: a meta-analysis. Europace. 2015 Jan;17 (1):38–47. doi: 10.1093/europace/euu220. [DOI] [PubMed] [Google Scholar]
- 267.Oral Hakan, Chugh Aman, Yoshida Kentaro, Sarrazin Jean F, Kuhne Michael, Crawford Thomas, Chalfoun Nagib, Wells Darryl, Boonyapisit Warangkna, Veerareddy Srikar, Billakanty Sreedhar, Wong Wai S, Good Eric, Jongnarangsin Krit, Pelosi Frank, Bogun Frank, Morady Fred. A randomized assessment of the incremental role of ablation of complex fractionated atrial electrograms after antral pulmonary vein isolation for long-lasting persistent atrial fibrillation. J. Am. Coll. Cardiol. 2009 Mar 3;53 (9):782–9. doi: 10.1016/j.jacc.2008.10.054. [DOI] [PubMed] [Google Scholar]
- 268.Nakahara Shiro, Kamijima Tohru, Hori Yuichi, Tsukada Naofumi, Okano Akiko, Takayanagi Kan. Substrate modification by adding ablation of localized complex fractionated electrograms after stepwise linear ablation in persistent atrial fibrillation. J Interv Card Electrophysiol. 2014 Mar;39 (2):121–9. doi: 10.1007/s10840-013-9848-9. [DOI] [PubMed] [Google Scholar]
- 269.Lemola Kristina, Ting Michael, Gupta Priya, Anker Jeffrey N, Chugh Aman, Good Eric, Reich Scott, Tschopp David, Igic Petar, Elmouchi Darryl, Jongnarangsin Krit, Bogun Frank, Pelosi Frank, Morady Fred, Oral Hakan. Effects of two different catheter ablation techniques on spectral characteristics of atrial fibrillation. J. Am. Coll. Cardiol. 2006 Jul 18;48 (2):340–8. doi: 10.1016/j.jacc.2006.04.053. [DOI] [PubMed] [Google Scholar]
- 270.Takahashi Yoshihide, O'Neill Mark D, Hocini Mélèze, Dubois Rémi, Matsuo Seiichiro, Knecht Sébastien, Mahapatra Srijoy, Lim Kang-Teng, Jaïs Pierre, Jonsson Anders, Sacher Frédéric, Sanders Prashanthan, Rostock Thomas, Bordachar Pierre, Clémenty Jacques, Klein George J, Haïssaguerre Michel. Characterization of electrograms associated with termination of chronic atrial fibrillation by catheter ablation. J. Am. Coll. Cardiol. 2008 Mar 11;51 (10):1003–10. doi: 10.1016/j.jacc.2007.10.056. [DOI] [PubMed] [Google Scholar]
- 271.Lin Yenn-Jiang, Tai Ching-Tai, Kao Tsair, Chang Shih-Lin, Lo Li-Wei, Tuan Ta-Chuan, Udyavar Ameya R, Wongcharoen Wanwarang, Hu Yu-Feng, Tso Han-Wen, Tsai Wen-Chin, Chang Chien-Jung, Ueng Kuo-Chang, Higa Satoshi, Chen Shih-Ann. Spatiotemporal organization of the left atrial substrate after circumferential pulmonary vein isolation of atrial fibrillation. Circ Arrhythm Electrophysiol. 2009 Jun;2 (3):233–41. doi: 10.1161/CIRCEP.108.812024. [DOI] [PubMed] [Google Scholar]
- 272.Hunter Ross J, Diab Ihab, Tayebjee Muzahir, Richmond Laura, Sporton Simon, Earley Mark J, Schilling Richard J. Characterization of fractionated atrial electrograms critical for maintenance of atrial fibrillation: a randomized, controlled trial of ablation strategies (the CFAE AF trial). Circ Arrhythm Electrophysiol. 2011 Oct;4 (5):622–9. doi: 10.1161/CIRCEP.111.962928. [DOI] [PubMed] [Google Scholar]
- 273.Verma Atul, Sanders Prashanthan, Champagne Jean, Macle Laurent, Nair Girish M, Calkins Hugh, Wilber David J. Selective complex fractionated atrial electrograms targeting for atrial fibrillation study (SELECT AF): a multicenter, randomized trial. Circ Arrhythm Electrophysiol. 2014 Feb;7 (1):55–62. doi: 10.1161/CIRCEP.113.000890. [DOI] [PubMed] [Google Scholar]
- 274.Tuan Jiun, Jeilan Mohamed, Kundu Suman, Nicolson Will, Chung Irene, Stafford Peter J, Ng G André. Regional fractionation and dominant frequency in persistent atrial fibrillation: effects of left atrial ablation and evidence of spatial relationship. Europace. 2011 Nov;13 (11):1550–6. doi: 10.1093/europace/eur174. [DOI] [PubMed] [Google Scholar]
- 275.Han Seong Woo, Shin Seung Yong, Im Sung Il, Na Jin Oh, Choi Cheol Ung, Kim Seong Hwan, Kim Jin Won, Kim Eung Ju, Rha Seung-Woon, Park Chang Gyu, Seo Hong Seog, Oh Dong Joo, Hwang Chun, Lim Hong Euy. Does the amount of atrial mass reduction improve clinical outcomes after radiofrequency catheter ablation for long-standing persistent atrial fibrillation? Comparison between linear ablation and defragmentation. Int. J. Cardiol. 2014 Jan 15;171 (1):37–43. doi: 10.1016/j.ijcard.2013.11.041. [DOI] [PubMed] [Google Scholar]
- 276.Estner Heidi L, Hessling Gabriele, Biegler Roman, Schreieck Juergen, Fichtner Stephanie, Wu Jinjin, Jilek Clemens, Zrenner Bernhard, Ndrepepa Gjin, Schmitt Claus, Deisenhofer Isabel. Complex fractionated atrial electrogram or linear ablation in patients with persistent atrial fibrillation--a prospective randomized study. Pacing Clin Electrophysiol. 2011 Aug;34 (8):939–48. doi: 10.1111/j.1540-8159.2011.03100.x. [DOI] [PubMed] [Google Scholar]
- 277.Haïssaguerre Michel, Hocini Mélèze, Sanders Prashanthan, Takahashi Yoshihide, Rotter Martin, Sacher Frederic, Rostock Thomas, Hsu Li-Fern, Jonsson Anders, O'Neill Mark D, Bordachar Pierre, Reuter Sylvain, Roudaut Raymond, Clémenty Jacques, Jaïs Pierre. Localized sources maintaining atrial fibrillation organized by prior ablation. Circulation. 2006 Feb 7;113 (5):616–25. doi: 10.1161/CIRCULATIONAHA.105.546648. [DOI] [PubMed] [Google Scholar]
- 278.Dibs Samer R, Ng Jason, Arora Rishi, Passman Rod S, Kadish Alan H, Goldberger Jeffrey J. Spatiotemporal characterization of atrial activation in persistent human atrial fibrillation: multisite electrogram analysis and surface electrocardiographic correlations--a pilot study. Heart Rhythm. 2008 May;5 (5):686–93. doi: 10.1016/j.hrthm.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 279.Narayan Sanjiv M, Krummen David E, Rappel Wouter-Jan. Clinical mapping approach to diagnose electrical rotors and focal impulse sources for human atrial fibrillation. J. Cardiovasc. Electrophysiol. 2012 May;23 (5):447–54. doi: 10.1111/j.1540-8167.2012.02332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Narayan Sanjiv M, Krummen David E, Clopton Paul, Shivkumar Kalyanam, Miller John M. Direct or coincidental elimination of stable rotors or focal sources may explain successful atrial fibrillation ablation: on-treatment analysis of the CONFIRM trial (Conventional ablation for AF with or without focal impulse and rotor modulation). J. Am. Coll. Cardiol. 2013 Jul 9;62 (2):138–47. doi: 10.1016/j.jacc.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Narayan Sanjiv M, Baykaner Tina, Clopton Paul, Schricker Amir, Lalani Gautam G, Krummen David E, Shivkumar Kalyanam, Miller John M. Ablation of rotor and focal sources reduces late recurrence of atrial fibrillation compared with trigger ablation alone: extended follow-up of the CONFIRM trial (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation). J. Am. Coll. Cardiol. 2014 May 6;63 (17):1761–8. doi: 10.1016/j.jacc.2014.02.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Jones Aled R, Krummen David E, Narayan Sanjiv M. Non-invasive identification of stable rotors and focal sources for human atrial fibrillation: mechanistic classification of atrial fibrillation from the electrocardiogram. Europace. 2013 Sep;15 (9):1249–58. doi: 10.1093/europace/eut038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Guillem Maria S, Climent Andreu M, Millet Jose, Arenal Ángel, Fernández-Avilés Francisco, Jalife José, Atienza Felipe, Berenfeld Omer. Noninvasive localization of maximal frequency sites of atrial fibrillation by body surface potential mapping. Circ Arrhythm Electrophysiol. 2013 Apr;6 (2):294–301. doi: 10.1161/CIRCEP.112.000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Misiri Juna, Asirvatham Samuel J. Can we isolate the pulmonary veins? Heart Rhythm. 2014 Apr;11 (4):557–8. doi: 10.1016/j.hrthm.2013.12.035. [DOI] [PubMed] [Google Scholar]
- 285.Scharf Christoph, Sneider Michael, Case Ian, Chugh Aman, Lai Steve W K, Pelosi Frank, Knight Bradley P, Kazerooni Ella, Morady Fred, Oral Hakan. Anatomy of the pulmonary veins in patients with atrial fibrillation and effects of segmental ostial ablation analyzed by computed tomography. J. Cardiovasc. Electrophysiol. 2003 Feb;14 (2):150–5. doi: 10.1046/j.1540-8167.2003.02444.x. [DOI] [PubMed] [Google Scholar]
- 286.Markides Vias, Schilling Richard J, Ho Siew Yen, Chow Anthony W C, Davies D Wyn, Peters Nicholas S. Characterization of left atrial activation in the intact human heart. Circulation. 2003 Feb 11;107 (5):733–9. doi: 10.1161/01.cir.0000048140.31785.02. [DOI] [PubMed] [Google Scholar]
- 287.Chang Shih-Lin, Tai Ching-Tai, Lin Yenn-Jiang, Wongcharoen Wanwarang, Lo Li-Wei, Lee Kun-Tai, Chang Sheng-Hsiung, Tuan Ta-Chuan, Chen Yi-Jen, Hsieh Ming-Hsiung, Tsao Hsuan-Ming, Wu Mei-Han, Sheu Ming-Huei, Chang Cheng-Yen, Chen Shih-Ann. The role of left atrial muscular bundles in catheter ablation of atrial fibrillation. J. Am. Coll. Cardiol. 2007 Sep 4;50 (10):964–73. doi: 10.1016/j.jacc.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 288.Roberts-Thomson Kurt C, Stevenson Irene, Kistler Peter M, Haqqani Haris M, Spence Steven J, Goldblatt John C, Sanders Prashanthan, Kalman Jonathan M. The role of chronic atrial stretch and atrial fibrillation on posterior left atrial wall conduction. Heart Rhythm. 2009 Aug;6 (8):1109–17. doi: 10.1016/j.hrthm.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 289.Tagawa M, Higuchi K, Chinushi M, Washizuka T, Ushiki T, Ishihara N, Aizawa Y. Myocardium extending from the left atrium onto the pulmonary veins: a comparison between subjects with and without atrial fibrillation. Pacing Clin Electrophysiol. 2001 Oct;24 (10):1459–63. doi: 10.1046/j.1460-9592.2001.01459.x. [DOI] [PubMed] [Google Scholar]
- 290.Tanaka Kazuhiko, Zlochiver Sharon, Vikstrom Karen L, Yamazaki Masatoshi, Moreno Javier, Klos Matthew, Zaitsev Alexey V, Vaidyanathan Ravi, Auerbach David S, Landas Steve, Guiraudon Gérard, Jalife José, Berenfeld Omer, Kalifa Jérôme. Spatial distribution of fibrosis governs fibrillation wave dynamics in the posterior left atrium during heart failure. Circ. Res. 2007 Oct 12;101 (8):839–47. doi: 10.1161/CIRCRESAHA.107.153858. [DOI] [PubMed] [Google Scholar]
- 291.Jadidi Amir S, Cochet Hubert, Shah Ashok J, Kim Steven J, Duncan Edward, Miyazaki Shinsuke, Sermesant Maxime, Lehrmann Heiko, Lederlin Matthieu, Linton Nick, Forclaz Andrei, Nault Isabelle, Rivard Lena, Wright Matthew, Liu Xingpeng, Scherr Daniel, Wilton Stephen B, Roten Laurent, Pascale Patrizio, Derval Nicolas, Sacher Frédéric, Knecht Sebastien, Keyl Cornelius, Hocini Mélèze, Montaudon Michel, Laurent Francois, Haïssaguerre Michel, Jaïs Pierre. Inverse relationship between fractionated electrograms and atrial fibrosis in persistent atrial fibrillation: combined magnetic resonance imaging and high-density mapping. J. Am. Coll. Cardiol. 2013 Aug 27;62 (9):802–12. doi: 10.1016/j.jacc.2013.03.081. [DOI] [PubMed] [Google Scholar]
- 292.Scherlag Benjamin J, Yamanashi William, Patel Utpal, Lazzara Ralph, Jackman Warren M. Autonomically induced conversion of pulmonary vein focal firing into atrial fibrillation. J. Am. Coll. Cardiol. 2005 Jun 7;45 (11):1878–86. doi: 10.1016/j.jacc.2005.01.057. [DOI] [PubMed] [Google Scholar]
- 293.Hunter Ross J, Liu Yankai, Lu Yiling, Wang Wen, Schilling Richard J. Left atrial wall stress distribution and its relationship to electrophysiologic remodeling in persistent atrial fibrillation. Circ Arrhythm Electrophysiol. 2012 Apr;5 (2):351–60. doi: 10.1161/CIRCEP.111.965541. [DOI] [PubMed] [Google Scholar]
- 294.Yoshida Kentaro, Ulfarsson Magnus, Oral Hakan, Crawford Thomas, Good Eric, Jongnarangsin Krit, Bogun Frank, Pelosi Frank, Jalife Jose, Morady Fred, Chugh Aman. Left atrial pressure and dominant frequency of atrial fibrillation in humans. Heart Rhythm. 2011 Feb;8 (2):181–7. doi: 10.1016/j.hrthm.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Munger Thomas M, Dong Ying-Xue, Masaki Mitsuru, Oh Jae K, Mankad Sunil V, Borlaug Barry A, Asirvatham Samuel J, Shen Win-Kuang, Lee Hon-Chi, Bielinski Suzette J, Hodge David O, Herges Regina M, Buescher Traci L, Wu Jia-Hui, Ma Changsheng, Zhang Yanhua, Chen Peng-Sheng, Packer Douglas L, Cha Yong-Mei. Electrophysiological and hemodynamic characteristics associated with obesity in patients with atrial fibrillation. J. Am. Coll. Cardiol. 2012 Aug 28;60 (9):851–60. doi: 10.1016/j.jacc.2012.03.042. [DOI] [PubMed] [Google Scholar]
- 296.Takahashi Yoshihide, O'Neill Mark D, Hocini Méléze, Reant Patricia, Jonsson Anders, Jaïs Pierre, Sanders Prashanthan, Rostock Thomas, Rotter Martin, Sacher Frédéric, Laffite Stephane, Roudaut Raymond, Clémenty Jacques, Haïssaguerre Michel. Effects of stepwise ablation of chronic atrial fibrillation on atrial electrical and mechanical properties. J. Am. Coll. Cardiol. 2007 Mar 27;49 (12):1306–14. doi: 10.1016/j.jacc.2006.11.033. [DOI] [PubMed] [Google Scholar]
- 297.Rolf Sascha, Kircher Simon, Arya Arash, Eitel Charlotte, Sommer Philipp, Richter Sergio, Gaspar Thomas, Bollmann Andreas, Altmann David, Piedra Carlos, Hindricks Gerhard, Piorkowski Christopher. Tailored atrial substrate modification based on low-voltage areas in catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol. 2014 Oct;7 (5):825–33. doi: 10.1161/CIRCEP.113.001251. [DOI] [PubMed] [Google Scholar]
- 298.Kofune Masayoshi, Okumura Yasuo, Watanabe Ichiro, Nagashima Koichi, Sonoda Kazumasa, Mano Hiroaki, Kogawa Rikitake, Sasaki Naoko, Ohkubo Kimie, Nakai Toshiko, Nikaido Mizuki, Hirayama Atsushi. Comparative distribution of complex fractionated atrial electrograms, high dominant frequency (HDF) sites during atrial fibrillation and HDF sites during sinus rhythm. J Interv Card Electrophysiol. 2013 Apr;36 (3):297–306. doi: 10.1007/s10840-012-9748-4. [DOI] [PubMed] [Google Scholar]
- 299.Lee Geoffrey, Roberts-Thomson Kurt, Madry Andrew, Spence Steven, Teh Andrew, Heck Patrick M, Kumar Saurabh, Kistler Peter M, Morton Joseph B, Sanders Prashanthan, Kalman Jonathan M. Relationship among complex signals, short cycle length activity, and dominant frequency in patients with long-lasting persistent AF: a high-density epicardial mapping study in humans. Heart Rhythm. 2011 Nov;8 (11):1714–9. doi: 10.1016/j.hrthm.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 300.Nakahara Shiro, Toratani Noritaka, Nakamura Hidehiko, Higashi Akihiro, Takayanagi Kan. Spatial relationship between high-dominant-frequency sites and the linear ablation line in persistent atrial fibrillation: its impact on complex fractionated electrograms. Europace. 2013 Feb;15 (2):189–97. doi: 10.1093/europace/eus290. [DOI] [PubMed] [Google Scholar]
- 301.Singh Sheldon M, Heist E Kevin, Koruth Jacob S, Barrett Conor D, Ruskin Jeremy N, Mansour Moussa C. The relationship between electrogram cycle length and dominant frequency in patients with persistent atrial fibrillation. J. Cardiovasc. Electrophysiol. 2009 Dec;20 (12):1336–42. doi: 10.1111/j.1540-8167.2009.01580.x. [DOI] [PubMed] [Google Scholar]
- 302.Ghoraani B, Dalvi R, Gizurarson S, Das M, Ha A, Suszko A, Krishnan S, Chauhan V S. Localized rotational activation in the left atrium during human atrial fibrillation: relationship to complex fractionated atrial electrograms and low-voltage zones. Heart Rhythm. 2013 Dec;10 (12):1830–8. doi: 10.1016/j.hrthm.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 303.Salinet João L, Tuan Jiun H, Sandilands Alistair J, Stafford Peter J, Schlindwein Fernando S, Ng G André. Distinctive patterns of dominant frequency trajectory behavior in drug-refractory persistent atrial fibrillation: preliminary characterization of spatiotemporal instability. J. Cardiovasc. Electrophysiol. 2014 Apr;25 (4):371–9. doi: 10.1111/jce.12331. [DOI] [PubMed] [Google Scholar]
- 304.Miller John M, Kowal Robert C, Swarup Vijay, Daubert James P, Daoud Emile G, Day John D, Ellenbogen Kenneth A, Hummel John D, Baykaner Tina, Krummen David E, Narayan Sanjiv M, Reddy Vivek Y, Shivkumar Kalyanam, Steinberg Jonathan S, Wheelan Kevin R. Initial independent outcomes from focal impulse and rotor modulation ablation for atrial fibrillation: multicenter FIRM registry. J. Cardiovasc. Electrophysiol. 2014 Sep;25 (9):921–9. doi: 10.1111/jce.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Hong Kimberly N, Russo Mark J, Liberman Elyse A, Trzebucki Alex, Oz Mehmet C, Argenziano Michael, Williams Mathew R. Effect of epicardial fat on ablation performance: a three-energy source comparison. J Card Surg. 2007 Nov 28;22 (6):521–4. doi: 10.1111/j.1540-8191.2007.00454.x. [DOI] [PubMed] [Google Scholar]
- 306.Berjano Enrique J, Hornero Fernando. Thermal-electrical modeling for epicardial atrial radiofrequency ablation. IEEE Trans Biomed Eng. 2004 Aug;51 (8):1348–57. doi: 10.1109/TBME.2004.827545. [DOI] [PubMed] [Google Scholar]
- 307.Sakamoto Shun-ichiro, Voeller Rochus K, Melby Spencer J, Lall Shelly C, Chang Nai-lun, Schuessler Richard B, Damiano Ralph J. Surgical ablation for atrial fibrillation: the efficacy of a novel bipolar pen device in the cardioplegically arrested and beating heart. J. Thorac. Cardiovasc. Surg. 2008 Nov;136 (5):1295–301. doi: 10.1016/j.jtcvs.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Beinart Roy, Abbara Suhny, Blum Andrew, Ferencik Maros, Heist Kevin, Ruskin Jeremy, Mansour Moussa. Left atrial wall thickness variability measured by CT scans in patients undergoing pulmonary vein isolation. J. Cardiovasc. Electrophysiol. 2011 Nov;22 (11):1232–6. doi: 10.1111/j.1540-8167.2011.02100.x. [DOI] [PubMed] [Google Scholar]
- 309.Lemola Kristina, Sneider Michael, Desjardins Benoit, Case Ian, Han Jihn, Good Eric, Tamirisa Kamala, Tsemo Ariane, Chugh Aman, Bogun Frank, Pelosi Frank, Kazerooni Ella, Morady Fred, Oral Hakan. Computed tomographic analysis of the anatomy of the left atrium and the esophagus: implications for left atrial catheter ablation. Circulation. 2004 Dec 14;110 (24):3655–60. doi: 10.1161/01.CIR.0000149714.31471.FD. [DOI] [PubMed] [Google Scholar]
- 310.den Uijl Dennis W, Tops Laurens F, Delgado Victoria, Schuijf Joanne D, Kroft Lucia J M, de Roos Albert, Boersma Eric, Trines Serge A, Zeppenfeld Katja, Schalij Martin J, Bax Jeroen J. Effect of pulmonary vein anatomy and left atrial dimensions on outcome of circumferential radiofrequency catheter ablation for atrial fibrillation. Am. J. Cardiol. 2011 Jan 15;107 (2):243–9. doi: 10.1016/j.amjcard.2010.08.069. [DOI] [PubMed] [Google Scholar]
- 311.McLellan Alex J A, Ling Liang-han, Ruggiero Diego, Wong Michael C G, Walters Tomos E, Nisbet Ashley, Shetty Anoop K, Azzopardi Sonia, Taylor Andrew J, Morton Joseph B, Kalman Jonathan M, Kistler Peter M. Pulmonary vein isolation: the impact of pulmonary venous anatomy on long-term outcome of catheter ablation for paroxysmal atrial fibrillation. Heart Rhythm. 2014 Apr;11 (4):549–56. doi: 10.1016/j.hrthm.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 312.Avitall B, Mughal K, Hare J, Helms R, Krum D. The effects of electrode-tissue contact on radiofrequency lesion generation. Pacing Clin Electrophysiol. 1997 Dec;20 (12 Pt 1):2899–910. doi: 10.1111/j.1540-8159.1997.tb05458.x. [DOI] [PubMed] [Google Scholar]
- 313.Okumura Yasuo, Johnson Susan B, Bunch T Jared, Henz Benhur D, O'Brien Christine J, Packer Douglas L. A systematical analysis of in vivo contact forces on virtual catheter tip/tissue surface contact during cardiac mapping and intervention. J. Cardiovasc. Electrophysiol. 2008 Jun;19 (6):632–40. doi: 10.1111/j.1540-8167.2008.01135.x. [DOI] [PubMed] [Google Scholar]
- 314.Hindricks G, Haverkamp W, Gülker H, Rissel U, Budde T, Richter K D, Borggrefe M, Breithardt G. Radiofrequency coagulation of ventricular myocardium: improved prediction of lesion size by monitoring catheter tip temperature. Eur. Heart J. 1989 Nov;10 (11):972–84. doi: 10.1093/oxfordjournals.eurheartj.a059422. [DOI] [PubMed] [Google Scholar]
- 315.Nakagawa H, Wittkampf F H, Yamanashi W S, Pitha J V, Imai S, Campbell B, Arruda M, Lazzara R, Jackman W M. Inverse relationship between electrode size and lesion size during radiofrequency ablation with active electrode cooling. Circulation. 1998 Aug 4;98 (5):458–65. doi: 10.1161/01.cir.98.5.458. [DOI] [PubMed] [Google Scholar]
- 316.Chan Rodrigo C, Johnson Susan B, Seward James B, Packer Douglas L. The effect of ablation electrode length and catheter tip to endocardial orientation on radiofrequency lesion size in the canine right atrium. Pacing Clin Electrophysiol. 2002 Jan;25 (1):4–13. doi: 10.1046/j.1460-9592.2002.00004.x. [DOI] [PubMed] [Google Scholar]
- 317.Wood Mark A, Goldberg Scott M, Parvez Babar, Pathak Vishesh, Holland Kristen, Ellenbogen Amy L, Han Frederick T, Alexander Daniel, Lau Melissa, Reshko Leonid, Goel Aneesh. Effect of electrode orientation on lesion sizes produced by irrigated radiofrequency ablation catheters. J. Cardiovasc. Electrophysiol. 2009 Nov;20 (11):1262–8. doi: 10.1111/j.1540-8167.2009.01538.x. [DOI] [PubMed] [Google Scholar]
- 318.Nakagawa H, Yamanashi W S, Pitha J V, Arruda M, Wang X, Ohtomo K, Beckman K J, McClelland J H, Lazzara R, Jackman W M. Comparison of in vivo tissue temperature profile and lesion geometry for radiofrequency ablation with a saline-irrigated electrode versus temperature control in a canine thigh muscle preparation. Circulation. 1995 Apr 15;91 (8):2264–73. doi: 10.1161/01.cir.91.8.2264. [DOI] [PubMed] [Google Scholar]
- 319.d'Avila André, Houghtaling Christopher, Gutierrez Paulo, Vragovic Olivera, Ruskin Jeremy N, Josephson Mark E, Reddy Vivek Y. Catheter ablation of ventricular epicardial tissue: a comparison of standard and cooled-tip radiofrequency energy. Circulation. 2004 May 18;109 (19):2363–9. doi: 10.1161/01.CIR.0000128039.87485.0B. [DOI] [PubMed] [Google Scholar]
- 320.Fuller Ithiel A, Wood Mark A. Intramural coronary vasculature prevents transmural radiofrequency lesion formation: implications for linear ablation. Circulation. 2003 Apr 8;107 (13):1797–803. doi: 10.1161/01.CIR.0000058705.97823.F4. [DOI] [PubMed] [Google Scholar]
- 321.Mesas Cézar Eumann, Augello Giuseppe, Lang Christopher Charles Edward, Gugliotta Filippo, Vicedomini Gabriele, Sora Nicoleta, De Paola Angelo Amato Vincenzo, Pappone Carlo. Electroanatomic remodeling of the left atrium in patients undergoing repeat pulmonary vein ablation: mechanistic insights and implications for ablation. J. Cardiovasc. Electrophysiol. 2006 Dec;17 (12):1279–85. doi: 10.1111/j.1540-8167.2006.00654.x. [DOI] [PubMed] [Google Scholar]
- 322.Neuzil Petr, Reddy Vivek Y, Kautzner Josef, Petru Jan, Wichterle Dan, Shah Dipen, Lambert Hendrik, Yulzari Aude, Wissner Erik, Kuck Karl-Heinz. Electrical reconnection after pulmonary vein isolation is contingent on contact force during initial treatment: results from the EFFICAS I study. Circ Arrhythm Electrophysiol. 2013 Apr;6 (2):327–33. doi: 10.1161/CIRCEP.113.000374. [DOI] [PubMed] [Google Scholar]
- 323.Makimoto Hisaki, Lin Tina, Rillig Andreas, Metzner Andreas, Wohlmuth Peter, Arya Anita, Antz Matthias, Mathew Shibu, Deiss Sebastian, Wissner Erik, Rausch Peter, Bardyszewski Aleksander, Kamioka Masashi, Li Xuping, Kuck Karl-Heinz, Ouyang Feifan, Tilz Roland Richard. In vivo contact force analysis and correlation with tissue impedance during left atrial mapping and catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol. 2014 Feb;7 (1):46–54. doi: 10.1161/CIRCEP.113.000556. [DOI] [PubMed] [Google Scholar]
- 324.Schwartzman D, Ren J F, Devine W A, Callans D J. Cardiac swelling associated with linear radiofrequency ablation in the atrium. J Interv Card Electrophysiol. 2001 Jun;5 (2):159–66. doi: 10.1023/a:1011477408021. [DOI] [PubMed] [Google Scholar]
- 325.Kowalski Marcin, Grimes Margaret M, Perez Francisco J, Kenigsberg David N, Koneru Jayanthi, Kasirajan Vigneshwar, Wood Mark A, Ellenbogen Kenneth A. Histopathologic characterization of chronic radiofrequency ablation lesions for pulmonary vein isolation. J. Am. Coll. Cardiol. 2012 Mar 6;59 (10):930–8. doi: 10.1016/j.jacc.2011.09.076. [DOI] [PubMed] [Google Scholar]
- 326.Yamane Teiichi, Matsuo Seiichiro, Date Taro, Lellouche Nicolas, Hioki Mika, Narui Ryosuke, Ito Keiichi, Tanigawa Shin-ichi, Yamashita Seigo, Tokuda Michifumi, Yoshida Hiroshi, Inada Keiichi, Shibayama Kenri, Miyanaga Satoru, Miyazaki Hidekazu, Abe Kunihiko, Sugimoto Ken-ichi, Yoshimura Michihiro. Repeated provocation of time- and ATP-induced early pulmonary vein reconnections after pulmonary vein isolation: eliminating paroxysmal atrial fibrillation in a single procedure. Circ Arrhythm Electrophysiol. 2011 Oct;4 (5):601–8. doi: 10.1161/CIRCEP.110.960138. [DOI] [PubMed] [Google Scholar]
- 327.Arentz Thomas, Macle Laurent, Kalusche Dietrich, Hocini Mélèze, Jais Pierre, Shah Dipen, Haissaguerre Michel. "Dormant" pulmonary vein conduction revealed by adenosine after ostial radiofrequency catheter ablation. J. Cardiovasc. Electrophysiol. 2004 Sep;15 (9):1041–7. doi: 10.1046/j.1540-8167.2004.04031.x. [DOI] [PubMed] [Google Scholar]
- 328.Andrade Jason G, Pollak Scott J, Monir George, Khairy Paul, Dubuc Marc, Roy Denis, Talajic Mario, Deyell Marc, Rivard Léna, Thibault Bernard, Guerra Peter G, Nattel Stanley, Macle Laurent. Pulmonary vein isolation using a pace-capture-guided versus an adenosine-guided approach: effect on dormant conduction and long-term freedom from recurrent atrial fibrillation--a prospective study. Circ Arrhythm Electrophysiol. 2013 Dec;6 (6):1103–8. doi: 10.1161/CIRCEP.113.000454. [DOI] [PubMed] [Google Scholar]
- 329.Steven Daniel, Sultan Arian, Reddy Vivek, Luker Jakob, Altenburg Manuel, Hoffmann Boris, Rostock Thomas, Servatius Helge, Stevenson William G, Willems Stephan, Michaud Gregory F. Benefit of pulmonary vein isolation guided by loss of pace capture on the ablation line: results from a prospective 2-center randomized trial. J. Am. Coll. Cardiol. 2013 Jul 2;62 (1):44–50. doi: 10.1016/j.jacc.2013.03.059. [DOI] [PubMed] [Google Scholar]
- 330.Reddy Vivek Y, Shah Dipen, Kautzner Josef, Schmidt Boris, Saoudi Nadir, Herrera Claudia, Jaïs Pierre, Hindricks Gerhard, Peichl Petr, Yulzari Aude, Lambert Hendrik, Neuzil Petr, Natale Andrea, Kuck Karl-Heinz. The relationship between contact force and clinical outcome during radiofrequency catheter ablation of atrial fibrillation in the TOCCATA study. Heart Rhythm. 2012 Nov;9 (11):1789–95. doi: 10.1016/j.hrthm.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 331.Natale Andrea, Reddy Vivek Y, Monir George, Wilber David J, Lindsay Bruce D, McElderry H Thomas, Kantipudi Charan, Mansour Moussa C, Melby Daniel P, Packer Douglas L, Nakagawa Hiroshi, Zhang Baohui, Stagg Robert B, Boo Lee Ming, Marchlinski Francis E. Paroxysmal AF catheter ablation with a contact force sensing catheter: results of the prospective, multicenter SMART-AF trial. J. Am. Coll. Cardiol. 2014 Aug 19;64 (7):647–56. doi: 10.1016/j.jacc.2014.04.072. [DOI] [PubMed] [Google Scholar]
- 332.Andrade Jason G, Monir George, Pollak Scott J, Khairy Paul, Dubuc Marc, Roy Denis, Talajic Mario, Deyell Marc, Rivard Léna, Thibault Bernard, Guerra Peter G, Nattel Stanley, Macle Laurent. Pulmonary vein isolation using "contact force" ablation: the effect on dormant conduction and long-term freedom from recurrent atrial fibrillation--a prospective study. Heart Rhythm. 2014 Nov;11 (11):1919–24. doi: 10.1016/j.hrthm.2014.07.033. [DOI] [PubMed] [Google Scholar]
- 333.Miller Marc A, d'Avila Andre, Dukkipati Srinivas R, Koruth Jacob S, Viles-Gonzalez Juan, Napolitano Craig, Eggert Charles, Fischer Avi, Gomes Joseph A, Reddy Vivek Y. Acute electrical isolation is a necessary but insufficient endpoint for achieving durable PV isolation: the importance of closing the visual gap. Europace. 2012 May;14 (5):653–60. doi: 10.1093/europace/eus048. [DOI] [PubMed] [Google Scholar]
- 334.Anter Elad, Tschabrunn Cory M, Contreras-Valdes Fernando M, Buxton Alfred E, Josephson Mark E. Radiofrequency ablation annotation algorithm reduces the incidence of linear gaps and reconnection after pulmonary vein isolation. Heart Rhythm. 2014 May;11 (5):783–90. doi: 10.1016/j.hrthm.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 335.Lachman Nirusha, Syed Faisal F, Habib Ammar, Kapa Suraj, Bisco Susan E, Venkatachalam K L, Asirvatham Samuel J. Correlative anatomy for the electrophysiologist, Part I: the pericardial space, oblique sinus, transverse sinus. J. Cardiovasc. Electrophysiol. 2010 Dec;21 (12):1421–6. doi: 10.1111/j.1540-8167.2010.01872.x. [DOI] [PubMed] [Google Scholar]
- 336.Aoyama Hiroshi, Nakagawa Hiroshi, Pitha Jan V, Khammar George S, Chandrasekaran Krishnaswamy, Matsudaira Kagari, Yagi Tetsuo, Yokoyama Katsuaki, Lazzara Ralph, Jackman Warren M. Comparison of cryothermia and radiofrequency current in safety and efficacy of catheter ablation within the canine coronary sinus close to the left circumflex coronary artery. J. Cardiovasc. Electrophysiol. 2005 Nov;16 (11):1218–26. doi: 10.1111/j.1540-8167.2005.50126.x. [DOI] [PubMed] [Google Scholar]
- 337.Thomas Stuart P, Guy Duncan J R, Boyd Anita C, Eipper Vicki E, Ross David L, Chard Richard B. Comparison of epicardial and endocardial linear ablation using handheld probes. Ann. Thorac. Surg. 2003 Feb;75 (2):543–8. doi: 10.1016/s0003-4975(02)04314-x. [DOI] [PubMed] [Google Scholar]
- 338.Stojanovska Jadranka, Kazerooni Ella A, Sinno Mohamad, Gross Barry H, Watcharotone Kuanwong, Patel Smita, Jacobson Jon A, Oral Hakan. Increased epicardial fat is independently associated with the presence and chronicity of atrial fibrillation and radiofrequency ablation outcome. Eur Radiol. 2015 Aug;25 (8):2298–309. doi: 10.1007/s00330-015-3643-1. [DOI] [PubMed] [Google Scholar]
- 339.Prasad Sunil M, Maniar Hersh S, Schuessler Richard B, Damiano Ralph J. Chronic transmural atrial ablation by using bipolar radiofrequency energy on the beating heart. J. Thorac. Cardiovasc. Surg. 2002 Oct;124 (4):708–13. doi: 10.1067/mtc.2002.125057. [DOI] [PubMed] [Google Scholar]
- 340.La Meir Mark, Gelsomino Sandro, Lucà Fabiana, Lorusso Roberto, Gensini Gian Franco, Pison Laurant, Wellens Francis, Maessen Jos. Minimally invasive thoracoscopic hybrid treatment of lone atrial fibrillation: early results of monopolar versus bipolar radiofrequency source. Interact Cardiovasc Thorac Surg. 2012 Apr;14 (4):445–50. doi: 10.1093/icvts/ivr142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Voeller Rochus K, Zierer Andreas, Lall Shelly C, Sakamoto Shun-ichiro, Schuessler Richard B, Damiano Ralph J. Efficacy of a novel bipolar radiofrequency ablation device on the beating heart for atrial fibrillation ablation: a long-term porcine study. J. Thorac. Cardiovasc. Surg. 2010 Jul;140 (1):203–8. doi: 10.1016/j.jtcvs.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Coumel P, Attuel P, Lavallée J, Flammang D, Leclercq J F, Slama R. [The atrial arrhythmia syndrome of vagal origin]. Arch Mal Coeur Vaiss. 1978 Jun;71 (6):645–56. [PubMed] [Google Scholar]
- 343.Vaitkevicius Raimundas, Saburkina Inga, Rysevaite Kristina, Vaitkeviciene Inga, Pauziene Neringa, Zaliunas Remigijus, Schauerte Patrick, Jalife José, Pauza Dainius H. Nerve supply of the human pulmonary veins: an anatomical study. Heart Rhythm. 2009 Feb;6 (2):221–8. doi: 10.1016/j.hrthm.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 344.Kapa Suraj, Venkatachalam K L, Asirvatham Samuel J. The autonomic nervous system in cardiac electrophysiology: an elegant interaction and emerging concepts. Cardiol Rev. 2010 Oct 12;18 (6):275–84. doi: 10.1097/CRD.0b013e3181ebb152. [DOI] [PubMed] [Google Scholar]
- 345.Patterson Eugene, Po Sunny S, Scherlag Benjamin J, Lazzara Ralph. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm. 2005 Jun;2 (6):624–31. doi: 10.1016/j.hrthm.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 346.Onorati Francesco, Curcio Antonio, Santarpino Giuseppe, Torella Daniele, Mastroroberto Pasquale, Tucci Luigi, Indolfi Ciro, Renzulli Attilio. Routine ganglionic plexi ablation during Maze procedure improves hospital and early follow-up results of mitral surgery. J. Thorac. Cardiovasc. Surg. 2008 Aug;136 (2):408–18. doi: 10.1016/j.jtcvs.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 347.Chugh Aman. Ganglionated plexus ablation in patients undergoing pulmonary vein isolation for paroxysmal atrial fibrillation: here we go again. J. Am. Coll. Cardiol. 2013 Dec 17;62 (24):2326–8. doi: 10.1016/j.jacc.2013.07.055. [DOI] [PubMed] [Google Scholar]
- 348.Zhou Qina, Hou Yuemei, Yang Shanglei. A meta-analysis of the comparative efficacy of ablation for atrial fibrillation with and without ablation of the ganglionated plexi. Pacing Clin Electrophysiol. 2011 Dec;34 (12):1687–94. doi: 10.1111/j.1540-8159.2011.03220.x. [DOI] [PubMed] [Google Scholar]
- 349.Oh Seil, Zhang Youhua, Bibevski Steve, Marrouche Nassir F, Natale Andrea, Mazgalev Todor N. Vagal denervation and atrial fibrillation inducibility: epicardial fat pad ablation does not have long-term effects. Heart Rhythm. 2006 Jun;3 (6):701–8. doi: 10.1016/j.hrthm.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 350.Mao Jun, Yin Xiandong, Zhang Ying, Yan Qian, Dong Jianzeng, Ma Changsheng, Liu Xingpeng. Ablation of epicardial ganglionated plexi increases atrial vulnerability to arrhythmias in dogs. Circ Arrhythm Electrophysiol. 2014 Aug;7 (4):711–7. doi: 10.1161/CIRCEP.113.000799. [DOI] [PubMed] [Google Scholar]
- 351.Syed Faisal F, DeSimone Christopher V, Friedman Paul A, Asirvatham Samuel J. Left atrial appendage exclusion for atrial fibrillation. Cardiol Clin. 2014 Nov;32 (4):601–25. doi: 10.1016/j.ccl.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Cox J L, Ad N, Palazzo T. Impact of the maze procedure on the stroke rate in patients with atrial fibrillation. J. Thorac. Cardiovasc. Surg. 1999 Nov;118 (5):833–40. doi: 10.1016/s0022-5223(99)70052-8. [DOI] [PubMed] [Google Scholar]
- 353.Buber Jonathan, Luria David, Sternik Leonid, Raanani Ehud, Feinberg Micha S, Goldenberg Ilan, Nof Eyal, Gurevitz Osnat, Eldar Michael, Glikson Michael, Kuperstein Rafael. Left atrial contractile function following a successful modified Maze procedure at surgery and the risk for subsequent thromboembolic stroke. J. Am. Coll. Cardiol. 2011 Oct 4;58 (15):1614–21. doi: 10.1016/j.jacc.2011.05.051. [DOI] [PubMed] [Google Scholar]
- 354.Bunch T Jared, Crandall Brian G, Weiss J Peter, May Heidi T, Bair Tami L, Osborn Jeffrey S, Anderson Jeffrey L, Muhlestein Joseph B, Horne Benjamin D, Lappe Donald L, Day John D. Patients treated with catheter ablation for atrial fibrillation have long-term rates of death, stroke, and dementia similar to patients without atrial fibrillation. J. Cardiovasc. Electrophysiol. 2011 Aug;22 (8):839–45. doi: 10.1111/j.1540-8167.2011.02035.x. [DOI] [PubMed] [Google Scholar]
- 355.Di Biase Luigi, Santangeli Pasquale, Anselmino Matteo, Mohanty Prasant, Salvetti Ilaria, Gili Sebastiano, Horton Rodney, Sanchez Javier E, Bai Rong, Mohanty Sanghamitra, Pump Agnes, Cereceda Brantes Mauricio, Gallinghouse G Joseph, Burkhardt J David, Cesarani Federico, Scaglione Marco, Natale Andrea, Gaita Fiorenzo. Does the left atrial appendage morphology correlate with the risk of stroke in patients with atrial fibrillation? Results from a multicenter study. J. Am. Coll. Cardiol. 2012 Aug 7;60 (6):531–8. doi: 10.1016/j.jacc.2012.04.032. [DOI] [PubMed] [Google Scholar]
- 356.Holmes David R, Reddy Vivek Y, Turi Zoltan G, Doshi Shephal K, Sievert Horst, Buchbinder Maurice, Mullin Christopher M, Sick Peter. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. 2009 Aug 15;374 (9689):534–42. doi: 10.1016/S0140-6736(09)61343-X. [DOI] [PubMed] [Google Scholar]
- 357.Tsai Yi-Chin, Phan Kevin, Munkholm-Larsen Stine, Tian David H, La Meir Mark, Yan Tristan D. Surgical left atrial appendage occlusion during cardiac surgery for patients with atrial fibrillation: a meta-analysis. Eur J Cardiothorac Surg. 2015 May;47 (5):847–54. doi: 10.1093/ejcts/ezu291. [DOI] [PubMed] [Google Scholar]
- 358.Syed Faisal F, Asirvatham Samuel J. Left atrial appendage as a target for reducing strokes: justifiable rationale? Safe and effective approaches? Heart Rhythm. 2011 Feb;8 (2):194–8. doi: 10.1016/j.hrthm.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 359.Tabata T, Oki T, Yamada H, Iuchi A, Ito S, Hori T, Kitagawa T, Kato I, Kitahata H, Oshita S. Role of left atrial appendage in left atrial reservoir function as evaluated by left atrial appendage clamping during cardiac surgery. Am. J. Cardiol. 1998 Feb 1;81 (3):327–32. doi: 10.1016/s0002-9149(97)00903-x. [DOI] [PubMed] [Google Scholar]
- 360.Lee Chee-Hoon, Kim Joon Bum, Jung Sung-Ho, Choo Suk Jung, Chung Cheol Hyun, Lee Jae Won. Left atrial appendage resection versus preservation during the surgical ablation of atrial fibrillation. Ann. Thorac. Surg. 2014 Jan;97 (1):124–32. doi: 10.1016/j.athoracsur.2013.07.073. [DOI] [PubMed] [Google Scholar]
- 361.Syed Faisal F, Rangu Venu, Bruce Charles J, Johnson Susan B, Danielsen Andrew, Gilles Emily J, Ladewig Dorothy J, Mikell Susan B, Berhow Steven, Wahnschaffe Douglas, Suddendorf Scott H, Asirvatham Samuel J, Friedman Paul A. Percutaneous ligation of the left atrial appendage results in atrial electrical substrate modification. Transl Res. 2015 Mar;165 (3):365–73. doi: 10.1016/j.trsl.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 362.Hocini Mélèze, Shah Ashok J, Nault Isabelle, Sanders Prashanthan, Wright Matthew, Narayan Sanjiv M, Takahashi Yoshihide, Jaïs Pierre, Matsuo Seiichiro, Knecht Sébastien, Sacher Frédéric, Lim Kang-Teng, Clémenty Jacques, Haïssaguerre Michel. Localized reentry within the left atrial appendage: arrhythmogenic role in patients undergoing ablation of persistent atrial fibrillation. Heart Rhythm. 2011 Dec;8 (12):1853–61. doi: 10.1016/j.hrthm.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Yamada Takumi, Murakami Yoshimasa, Yoshida Yukihiko, Okada Taro, Yoshida Naoki, Toyama Junji, Tsuboi Naoya, Inden Yasuya, Hirai Makoto, Murohara Toyoaki, McElderry Hugh T, Epstein Andrew E, Plumb Vance J, Kay G Neal. Electrophysiologic and electrocardiographic characteristics and radiofrequency catheter ablation of focal atrial tachycardia originating from the left atrial appendage. Heart Rhythm. 2007 Oct;4 (10):1284–91. doi: 10.1016/j.hrthm.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 364.Chan Chin Pang, Wong Wai Shun, Pumprueg Satchana, Veerareddy Srikar, Billakanty Sreedhar, Ellis Christopher, Chae Sanders, Buerkel Daniel, Aasbo Johan, Crawford Thomas, Good Eric, Jongnarangsin Krit, Ebinger Matthew, Bogun Frank, Pelosi Frank, Oral Hakan, Morady Fred, Chugh Aman. Inadvertent electrical isolation of the left atrial appendage during catheter ablation of persistent atrial fibrillation. Heart Rhythm. 2010;7 (2):173–80. doi: 10.1016/j.hrthm.2009.10.036. [DOI] [PubMed] [Google Scholar]
- 365.Khan Mohammed N, Jaïs Pierre, Cummings Jennifer, Di Biase Luigi, Sanders Prashanthan, Martin David O, Kautzner Josef, Hao Steven, Themistoclakis Sakis, Fanelli Raffaele, Potenza Domenico, Massaro Raimondo, Wazni Oussama, Schweikert Robert, Saliba Walid, Wang Paul, Al-Ahmad Amin, Beheiry Salwa, Santarelli Pietro, Starling Randall C, Dello Russo Antonio, Pelargonio Gemma, Brachmann Johannes, Schibgilla Volker, Bonso Aldo, Casella Michela, Raviele Antonio, Haïssaguerre Michel, Natale Andrea. Pulmonary-vein isolation for atrial fibrillation in patients with heart failure. N. Engl. J. Med. 2008 Oct 23;359 (17):1778–85. doi: 10.1056/NEJMoa0708234. [DOI] [PubMed] [Google Scholar]
- 366.Ling Liang-Han, Taylor Andrew J, Ellims Andris H, Iles Leah M, McLellan Alex J A, Lee Geoffrey, Kumar Saurabh, Lee Geraldine, Teh Andrew, Medi Caroline, Kaye David M, Kalman Jonathan M, Kistler Peter M. Sinus rhythm restores ventricular function in patients with cardiomyopathy and no late gadolinium enhancement on cardiac magnetic resonance imaging who undergo catheter ablation for atrial fibrillation. Heart Rhythm. 2013 Sep;10 (9):1334–9. doi: 10.1016/j.hrthm.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 367.Tops Laurens F, Den Uijl Dennis W, Delgado Victoria, Marsan Nina Ajmone, Zeppenfeld Katja, Holman Eduard, van der Wall Ernst E, Schalij Martin J, Bax Jeroen J. Long-term improvement in left ventricular strain after successful catheter ablation for atrial fibrillation in patients with preserved left ventricular systolic function. Circ Arrhythm Electrophysiol. 2009 Jun;2 (3):249–57. doi: 10.1161/CIRCEP.108.838748. [DOI] [PubMed] [Google Scholar]
- 368.Stulak John M, Dearani Joseph A, Daly Richard C, Zehr Kenton J, Sundt Thoralf M, Schaff Hartzell V. Left ventricular dysfunction in atrial fibrillation: restoration of sinus rhythm by the Cox-maze procedure significantly improves systolic function and functional status. Ann. Thorac. Surg. 2006 Aug;82 (2):494–500. doi: 10.1016/j.athoracsur.2006.03.075. [DOI] [PubMed] [Google Scholar]
- 369.Tse H F, Lau C P, Yu C M, Lee K L, Michaud G F, Knight B P, Morady F, Strickberger S A. Effect of the implantable atrial defibrillator on the natural history of atrial fibrillation. J. Cardiovasc. Electrophysiol. 1999 Sep;10 (9):1200–9. doi: 10.1111/j.1540-8167.1999.tb00296.x. [DOI] [PubMed] [Google Scholar]
- 370.Igarashi Miyako, Tada Hiroshi, Sekiguchi Yukio, Yamasaki Hiro, Arimoto Takanori, Kuroki Kenji, Machino Takeshi, Murakoshi Nobuyuki, Aonuma Kazutaka. Effect of restoration of sinus rhythm by extensive antiarrhythmic drugs in predicting results of catheter ablation of persistent atrial fibrillation. Am. J. Cardiol. 2010 Jul 1;106 (1):62–8. doi: 10.1016/j.amjcard.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 371.Feinberg M S, Waggoner A D, Kater K M, Cox J L, Lindsay B D, Pérez J E. Restoration of atrial function after the maze procedure for patients with atrial fibrillation. Assessment by Doppler echocardiography. Circulation. 1994 Nov;90 (5 Pt 2):II285–92. [PubMed] [Google Scholar]
- 372.Lönnerholm Stefan, Blomström Per, Nilsson Leif, Blomström-Lundqvist Carina. Long-term effects of the maze procedure on atrial size and mechanical function. Ann. Thorac. Surg. 2008 Mar;85 (3):916–20. doi: 10.1016/j.athoracsur.2007.10.090. [DOI] [PubMed] [Google Scholar]
- 373.Sheldon SH, Bois JP, Stulak JM, Ammash NM, Brady PA, Lin G. Surgical treatment of atrial fibrillation: effects of different techniques on atrial mechanical function. Circulation [abstract] 2013;128:0–0. [Google Scholar]
- 374.Welch Terrence D, Coylewright Megan, Powell Brian D, Asirvatham Samuel J, Gersh Bernard J, Dearani Joseph A, Nishimura Rick A. Symptomatic pulmonary hypertension with giant left atrial v waves after surgical maze procedures: evaluation by comprehensive hemodynamic catheterization. Heart Rhythm. 2013 Dec;10 (12):1839–42. doi: 10.1016/j.hrthm.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 375.Gibson Douglas N, Di Biase Luigi, Mohanty Prasant, Patel Jigar D, Bai Rong, Sanchez Javier, Burkhardt J David, Heywood J Thomas, Johnson Allen D, Rubenson David S, Horton Rodney, Gallinghouse G Joseph, Beheiry Salwa, Curtis Guy P, Cohen David N, Lee Mark Y, Smith Michael R, Gopinath Devi, Lewis William R, Natale Andrea. Stiff left atrial syndrome after catheter ablation for atrial fibrillation: clinical characterization, prevalence, and predictors. Heart Rhythm. 2011 Sep;8 (9):1364–71. doi: 10.1016/j.hrthm.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 376.Cappato Riccardo, Calkins Hugh, Chen Shih-Ann, Davies Wyn, Iesaka Yoshito, Kalman Jonathan, Kim You-Ho, Klein George, Natale Andrea, Packer Douglas, Skanes Allan, Ambrogi Federico, Biganzoli Elia. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2010 Feb;3 (1):32–8. doi: 10.1161/CIRCEP.109.859116. [DOI] [PubMed] [Google Scholar]
- 377.Providência Rui, Marijon Eloi, Combes Stéphane, Bouzeman Abdeslam, Jourda François, Khoueiry Ziad, Cardin Christelle, Combes Nicolas, Boveda Serge, Albenque Jean-Paul. Higher contact-force values associated with better mid-term outcome of paroxysmal atrial fibrillation ablation using the SmartTouch™ catheter. Europace. 2015 Jan;17 (1):56–63. doi: 10.1093/europace/euu218. [DOI] [PubMed] [Google Scholar]
- 378.Squara Fabien, Zhao Alexandre, Marijon Eloi, Latcu Decebal Gabriel, Providencia Rui, Di Giovanni Giacomo, Jauvert Gaël, Jourda Francois, Chierchia Gian-Battista, De Asmundis Carlo, Ciconte Giuseppe, Alonso Christine, Grimard Caroline, Boveda Serge, Cauchemez Bruno, Saoudi Nadir, Brugada Pedro, Albenque Jean-Paul, Thomas Olivier. Comparison between radiofrequency with contact force-sensing and second-generation cryoballoon for paroxysmal atrial fibrillation catheter ablation: a multicentre European evaluation. Europace. 2015 May;17 (5):718–24. doi: 10.1093/europace/euv060. [DOI] [PubMed] [Google Scholar]
- 379.Ciconte Giuseppe, de Asmundis Carlo, Sieira Juan, Conte Giulio, Di Giovanni Giacomo, Mugnai Giacomo, Saitoh Yukio, Baltogiannis Giannis, Irfan Ghazala, Coutiño-Moreno Hugo Enrique, Hunuk Burak, Velagić Vedran, Brugada Pedro, Chierchia Gian-Battista. Single 3-minute freeze for second-generation cryoballoon ablation: one-year follow-up after pulmonary vein isolation. Heart Rhythm. 2015 Apr;12 (4):673–80. doi: 10.1016/j.hrthm.2014.12.026. [DOI] [PubMed] [Google Scholar]
- 380.Wokhlu Anita, Monahan Kristi H, Hodge David O, Asirvatham Samuel J, Friedman Paul A, Munger Thomas M, Bradley David J, Bluhm Christine M, Haroldson Janis M, Packer Douglas L. Long-term quality of life after ablation of atrial fibrillation the impact of recurrence, symptom relief, and placebo effect. J. Am. Coll. Cardiol. 2010 May 25;55 (21):2308–16. doi: 10.1016/j.jacc.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 381.Tilz Roland Richard, Rillig Andreas, Thum Anna-Maria, Arya Anita, Wohlmuth Peter, Metzner Andreas, Mathew Shibu, Yoshiga Yasuhiro, Wissner Erik, Kuck Karl-Heinz, Ouyang Feifan. Catheter ablation of long-standing persistent atrial fibrillation: 5-year outcomes of the Hamburg Sequential Ablation Strategy. J. Am. Coll. Cardiol. 2012 Nov 6;60 (19):1921–9. doi: 10.1016/j.jacc.2012.04.060. [DOI] [PubMed] [Google Scholar]
- 382.Schreiber Doreen, Rostock Thomas, Fröhlich Max, Sultan Arian, Servatius Helge, Hoffmann Boris A, Lüker Jakob, Berner Imke, Schäffer Benjamin, Wegscheider Karl, Lezius Susanne, Willems Stephan, Steven Daniel. Five-year follow-up after catheter ablation of persistent atrial fibrillation using the stepwise approach and prognostic factors for success. Circ Arrhythm Electrophysiol. 2015 Apr;8 (2):308–17. doi: 10.1161/CIRCEP.114.001672. [DOI] [PubMed] [Google Scholar]
- 383.Deshmukh Abhishek, Patel Nileshkumar J, Pant Sadip, Shah Neeraj, Chothani Ankit, Mehta Kathan, Grover Peeyush, Singh Vikas, Vallurupalli Srikanth, Savani Ghanshyambhai T, Badheka Apurva, Tuliani Tushar, Dabhadkar Kaustubh, Dibu George, Reddy Y Madhu, Sewani Asif, Kowalski Marcin, Mitrani Raul, Paydak Hakan, Viles-Gonzalez Juan F. In-hospital complications associated with catheter ablation of atrial fibrillation in the United States between 2000 and 2010: analysis of 93 801 procedures. Circulation. 2013 Nov 5;128 (19):2104–12. doi: 10.1161/CIRCULATIONAHA.113.003862. [DOI] [PubMed] [Google Scholar]
- 384.Shah Rashmee U, Freeman James V, Shilane David, Wang Paul J, Go Alan S, Hlatky Mark A. Procedural complications, rehospitalizations, and repeat procedures after catheter ablation for atrial fibrillation. J. Am. Coll. Cardiol. 2012 Jan 10;59 (2):143–9. doi: 10.1016/j.jacc.2011.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Reynolds Matthew R, Zimetbaum Peter, Josephson Mark E, Ellis Ethan, Danilov Tatyana, Cohen David J. Cost-effectiveness of radiofrequency catheter ablation compared with antiarrhythmic drug therapy for paroxysmal atrial fibrillation. Circ Arrhythm Electrophysiol. 2009 Aug;2 (4):362–9. doi: 10.1161/CIRCEP.108.837294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Blackhouse Gord, Assasi Nazila, Xie Feng, Gaebel Kathryn, Campbell Kaitryn, Healey Jeff S, O'Reilly Daria, Goeree Ron. Cost-effectiveness of catheter ablation for rhythm control of atrial fibrillation. Int J Vasc Med. 2013;2013 () doi: 10.1155/2013/262809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Aronsson Mattias, Walfridsson Håkan, Janzon Magnus, Walfridsson Ulla, Nielsen Jens Cosedis, Hansen Peter Steen, Johannessen Arne, Raatikainen Pekka, Hindricks Gerhard, Kongstad Ole, Pehrson Steen, Englund Anders, Hartikainen Juha, Mortensen Leif Spange, Levin Lars-Åke. The cost-effectiveness of radiofrequency catheter ablation as first-line treatment for paroxysmal atrial fibrillation: results from a MANTRA-PAF substudy. Europace. 2015 Jan;17 (1):48–55. doi: 10.1093/europace/euu188. [DOI] [PubMed] [Google Scholar]
- 388.Chan Paul S, Vijan Sandeep, Morady Fred, Oral Hakan. Cost-effectiveness of radiofrequency catheter ablation for atrial fibrillation. J. Am. Coll. Cardiol. 2006 Jun 20;47 (12):2513–20. doi: 10.1016/j.jacc.2006.01.070. [DOI] [PubMed] [Google Scholar]
- 389.Rodgers M, McKenna C, Palmer S, Chambers D, Van Hout S, Golder S, Pepper C, Todd D, Woolacott N. Curative catheter ablation in atrial fibrillation and typical atrial flutter: systematic review and economic evaluation. Health Technol Assess. 2008 Nov;12 (34):iii–iv, xi-xiii, 1-198. doi: 10.3310/hta12340. [DOI] [PubMed] [Google Scholar]
- 390.McKenna C, Palmer S, Rodgers M, Chambers D, Hawkins N, Golder S, Van Hout S, Pepper C, Todd D, Woolacott N. Cost-effectiveness of radiofrequency catheter ablation for the treatment of atrial fibrillation in the United Kingdom. Heart. 2009 Apr;95 (7):542–9. doi: 10.1136/hrt.2008.147165. [DOI] [PubMed] [Google Scholar]
- 391.Noro Mahito, Kujime Shingo, Ito Naoshi, Enomoto Yoshinari, Nakamura Keijirou, Sakai Tsuyoshi, Sakata Takao, Sugi Kaoru. Cost effectiveness of radiofrequency catheter ablation vs. medical treatment for atrial fibrillation in Japan. -Cost performance for atrial fibrillation-. Circ. J. 2011;75 (8):1860–6. doi: 10.1253/circj.cj-10-0793. [DOI] [PubMed] [Google Scholar]
- 392.Neyt Mattias, Van Brabandt Hans, Devos Carl. The cost-utility of catheter ablation of atrial fibrillation: a systematic review and critical appraisal of economic evaluations. BMC Cardiovasc Disord. 2013;13 () doi: 10.1186/1471-2261-13-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Reynolds Matthew R, Lamotte Mark, Todd Derick, Khaykin Yaariv, Eggington Simon, Tsintzos Stelios, Klein Gunnar. Cost-effectiveness of cryoballoon ablation for the management of paroxysmal atrial fibrillation. Europace. 2014 May;16 (5):652–9. doi: 10.1093/europace/eut380. [DOI] [PubMed] [Google Scholar]
- 394.Boersma Lucas V A, Castella Manuel, van Boven Wimjan, Berruezo Antonio, Yilmaz Alaaddin, Nadal Mercedes, Sandoval Elena, Calvo Naiara, Brugada Josep, Kelder Johannes, Wijffels Maurits, Mont Lluís. Atrial fibrillation catheter ablation versus surgical ablation treatment (FAST): a 2-center randomized clinical trial. Circulation. 2012 Jan 3;125 (1):23–30. doi: 10.1161/CIRCULATIONAHA.111.074047. [DOI] [PubMed] [Google Scholar]
- 395.Anderson Louise H, Black Edward J, Civello Kenneth C, Martinson Melissa S, Kress David C. Cost-effectiveness of the convergent procedure and catheter ablation for non-paroxysmal atrial fibrillation. J Med Econ. 2014 Jul;17 (7):481–91. doi: 10.3111/13696998.2014.911185. [DOI] [PubMed] [Google Scholar]
- 396.Stulak John M, Suri Rakesh M, Burkhart Harold M, Daly Richard C, Dearani Joseph A, Greason Kevin L, Joyce Lyle D, Park Soon J, Schaff Hartzell V. Surgical ablation for atrial fibrillation for two decades: are the results of new techniques equivalent to the Cox maze III procedure? J. Thorac. Cardiovasc. Surg. 2014 May;147 (5):1478–86. doi: 10.1016/j.jtcvs.2013.10.084. [DOI] [PubMed] [Google Scholar]
- 397.Phan Kevin, Xie Ashleigh, Kumar Narendra, Wong Sophia, Medi Caroline, La Meir Mark, Yan Tristan D. Comparing energy sources for surgical ablation of atrial fibrillation: a Bayesian network meta-analysis of randomized, controlled trials. Eur J Cardiothorac Surg. 2015 Aug;48 (2):201–11. doi: 10.1093/ejcts/ezu408. [DOI] [PubMed] [Google Scholar]
- 398.Ellis CR, Richardson TD, Shoemaker MB, Whalen SP, Hoff SJ. Abstract 12964: Staged versus Same-Day Thoracoscopic Hybrid Ablation for Persistent Atrial Fibrillation: Identification of Pulmonary Vein Reconnection Following Surgical Ablation. Circulation. 2014;0:0–0. [Google Scholar]
- 399.Kikuchi Kan, McDonald Amy D, Sasano Tetsuo, Donahue J Kevin. Targeted modification of atrial electrophysiology by homogeneous transmural atrial gene transfer. Circulation. 2005 Jan 25;111 (3):264–70. doi: 10.1161/01.CIR.0000153338.47507.83. [DOI] [PubMed] [Google Scholar]
- 400.Trappe Kerstin, Thomas Dierk, Bikou Olympia, Kelemen Kamilla, Lugenbiel Patrick, Voss Frederik, Becker Rüdiger, Katus Hugo A, Bauer Alexander. Suppression of persistent atrial fibrillation by genetic knockdown of caspase 3: a pre-clinical pilot study. Eur. Heart J. 2013 Jan;34 (2):147–57. doi: 10.1093/eurheartj/ehr269. [DOI] [PubMed] [Google Scholar]
- 401.Soucek Radim, Thomas Dierk, Kelemen Kamilla, Bikou Olympia, Seyler Claudia, Voss Frederik, Becker Rüdiger, Koenen Michael, Katus Hugo A, Bauer Alexander. Genetic suppression of atrial fibrillation using a dominant-negative ether-a-go-go-related gene mutant. Heart Rhythm. 2012 Feb;9 (2):265–72. doi: 10.1016/j.hrthm.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 402.Wu Cheng-Hsueh, Hu Yu-Feng, Chou Chia-Yu, Lin Yenn-Jiang, Chang Shih-Lin, Lo Li-Wei, Tuan Ta-Chuan, Li Cheng-Hung, Chao Tze-Fan, Chung Fa-Po, Liao Jo-Nan, Chen Shih-Ann. Transforming growth factor-β1 level and outcome after catheter ablation for nonparoxysmal atrial fibrillation. Heart Rhythm. 2013 Jan;10 (1):10–5. doi: 10.1016/j.hrthm.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 403.Cabrera-Bueno Fernando, Medina-Palomo Carmen, Ruiz-Salas Amalio, Flores Ana, Rodríguez-Losada Noela, Barrera Alberto, Jiménez-Navarro Manuel, Alzueta Javier. Serum levels of interleukin-2 predict the recurrence of atrial fibrillation after pulmonary vein ablation. Cytokine. 2015 May;73 (1):74–8. doi: 10.1016/j.cyto.2015.01.026. [DOI] [PubMed] [Google Scholar]
- 404.Park Seung-Jung, On Young Keun, Kim June Soo, Choi Jin-Oh, Ju Eun-Seon, Jeong Dong Seop, Park Pyo Won, Jeon Eun-Seok. Transforming growth factor β1-mediated atrial fibrotic activity and the recovery of atrial mechanical contraction after surgical maze procedure. Int. J. Cardiol. 2013 Apr 5;164 (2):232–7. doi: 10.1016/j.ijcard.2013.01.066. [DOI] [PubMed] [Google Scholar]