Abstract
Ventricular arrhythmias (VAs) arising from the right ventricular outflow tract (RVOT) are a common and heterogeneous entity. Idiopathic right ventricular arrhythmias (IdioVAs) are generally benign, with excellent ablation outcomes and long-term arrhythmia-free survival, and must be distinguished from other conditions associated with VAs arising from the right ventricle: the differential diagnosis with arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D) is therefore crucial because VAs are one of the most important causes of sudden cardiac death (SCD) in young individuals even with early stage of the disease. Radiofrequency catheter ablation (RFCA) is a current option for the treatment of VAs but important differences must be considered in terms of indication, purposes and procedural strategies in the treatment of the two conditions. In this review, we comprehensively discuss clinical and electrophysiological features, diagnostic and therapeutic techniques in a compared analysis of these two entities.
Keywords: Right Ventricular Outflow Tract, Idiopathic Ventricular Arrhythmias, Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia, Three-Dimensional Electroanatomical Mapping, Cardiac Magnetic Resonance Imaging, Catheter Ablation
Introduction
The right ventricular outflow tract (RVOT) is the most common origin of non-ischemic ventricular arrhythmias (VAs). RVOT VAs - including premature ventricular contractions (PVCs) and non-sustained or sustained ventricular tachycardia (VT) - are the expression of two different entities, both in terms of natural history and in terms of therapeutic management: idiopathic right ventricular arrhythmias (IdioVAs) and arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D).[1,2]
IdioVAs is a benign condition that occurs in young adults without structural heart disease, traditionally considered a primary electrical disease, responsive to medical and ablative therapy.[3,4] ARVC/D is a autosomal dominant genetically determined heart muscle disorder characterized by pathological fibro-fatty replacement of the right ventricular (RV) myocardium, that leads to VAs, RV dysfunction and sudden cardiac death (SCD).[5-7] In the early stage of the disease, structural changes may be absent or subtle, progressively affecting localized areas of the RV, typically the inflow tract, outflow tract, or apex of the RV, the so-called “triangle of dysplasia”.[8] However, the subsequent involvement of other regions of the RV is common.[9] Whilst in the past the involvement of the left ventricle (LV) was considered an expression of the final stage of the disease, known as “biventricular failure phase”, it is currently recognized that ARVC/D can present with isolated or predominant involvement of LV since the early stages.[10] Clinical and genetic characterization of families demonstrated 3 different patterns of ARVC/D: classic pattern with predominant RV disease; left dominant pattern, with early and prominent LV disease; bi-ventricular pattern, with synchronous involvement of both ventricles.[11] The diagnosis of ARVC/D relies on the demonstration of structural, functional, and electrophysiological abnormalities, as defined by the 2010 modified Task Force criteria adapted to improve diagnostic sensitivity.[12]
Radiofrequency catheter ablation (RFCA) is a highly effective treatment for symptomatic patients with IdioVAs, whilst the role of ablation in ARVC/D is not definitively curative. As such, differentiating between the two conditions is essential because different procedural endpoints should be defined and different ablation strategies are required.[13-15]
Electrophysiological Properties Of The Embryonic Outflow Tract Related To Ventricular Arrhythmias In Adults
Anatomically, four portions of RVOT are described: rightward, also called free wall, anterior, leftward, and posterior. The myocardium is relatively thin in the rightward, anterior, and leftward portions of the RVOT, whilst the posterior infundibular part is the thickest[16] (Figure 1). In the developing embryonic heart, the presence of "transitional zones", with slow conducting properties, seems to be related to the presumptive cardiac conduction system.[17] One of these transitional zones is found in the myocardial outflow tract. Several markers related to the developing cardiac conduction system have been described in these zones,[18] including the Hyperpolarization-activated cyclic nucleotide-gated channel 4 (HCN4), that is responsible for the current in the sino-atrial node (If).[19] The expression of HCN4 among other markers (such as CCS-lacZ and MinK-lacZ[20,21]), in RVOT of the developing heart may explain the occurrence of arrhythmias in the adult heart. Re-expression of an embryonic phenotype, or embryonic remnants of tissue may provide the arrhythmogenic potential of this area.[22]
Figure 1. RVOT can be thought of as a cylinder that wraps around LVOT. RVOT courses anterior to the LVOT, from a rightward inferior to a leftward superior direction, such that the pulmonary valve lies to the left and anteriorly to the aortic valve. The pulmonary annulus is cephalad to the aortic annulus thus the structure immediately anterior to the aortic valve, specifically the right coronary cusp and part of the left coronary cusp, is the posterior muscular infundibular portion of the RVOT.
Clinical Presentation And ECG Of Ventricular Arrhythmias In Patients With IdioVAs And ARVC/D
Clinical presentation of IdioVAs typically includes palpitations, dizziness, related to frequent PVCs or non-sustained VT. Less frequently, physical exercise or emotional stress can lead to sustained VT that may be occasionally the cause for syncope. On the other hand, clinical presentation of ARVC/D may be variable: palpitations, but also syncope, cardiac arrest or SCD have been reported. Traditionally, 3 different phases have been distinguished. In the early “concealed phase”, patients are commonly asymptomatic, with minor VAs and subtle RV structural changes. However, risk of SCD is reported particularly during exercise.[5] In the second phase, “overt electrical phase”, patients present symptomatic VAs and morphological RV abnormalities detected by imaging. Finally, the third phase is the “end-stage-disease”, often indistinguishable from dilated cardiomyopathy.[23]
Several ECG markers related to ARVC/D have been described.[24-29] According to the Revised Task Force criteria, summarized in Table 1, evidence of negative precordial T-wave in V1-V3 or beyond and epsilon wave in V1-V3 on sinus rhythm are major criteria.[12]
Table 1. . Revised Task Force (TF) criteria of 2010.[12].
RV = right ventricle, PLAX = parasternal long-axis, RVOT = right ventricular outflow tract, BSA = body surface area, PSAX = parasternal short axis, RVEDV = right ventricular end-diastolic volume, EMB = endomyocardial biopsy, RBBB = right bundle-branch block, SAECG = signal-averaged ECG, LBBB = left bundle-branch block, SCD = sudden cardiac death
| Global or regional dysfunction and structural alterations | |
|---|---|
| Major Criteria | |
| - 2D echo | Regional RV akinesia, dyskinesia, or aneurysm and 1 of the following (end diastole): |
| PLAX RVOT ≥32 mm (corrected [PLAX/BSA] ≥19 mm/m2) | |
| PSAX RVOT ≥36 mm (corrected [PSAX/BSA] ≥21 mm/m2) | |
| or fractional area change ≤33% | |
| - MRI | Regional RV akinesia or dyskinesia or dyssynchronous RV contraction and 1 of the following: |
| Ratio of RVEDV to BSA ≥110 mL/m2 (male) or ≥100 mL/m2 (female) | |
| or RV ejection fraction ≤40% | |
| - RV angiography | Regional RV akinesia, dyskinesia, or aneurysm |
| Minor Criteria | |
| - 2D echo | Regional RV akinesia or dyskinesia and 1 of the following (end diastole): |
| PLAX RVOT ≥29 to <32 mm (corrected [PLAX/BSA] ≥16 to <19 mm/m2) | |
| PSAX RVOT ≥32 to <36 mm (corrected [PSAX/BSA] ≥18 to <21 mm/m2) | |
| or fractional area change >33 to ≤40% | |
| - MRI | Regional RV akinesia or dyskinesia or dyssynchronous RV contraction and 1 of the following: |
| Ratio of RVEDV to BSA ≥100 to <110 mL/m2 (male) or ≥90 to <100 mL/m2 (female) | |
| or RV ejection fraction > 40 to ≤45% | |
| Tissue characterization of wall | |
| Major Criteria | Residual myocytes <60% by morphometric analysis (or <0% if estimated), with fibrous replacement of the RV free wall myocardium in ≥1 sample, with or without fatty replacement of tissue on EMB |
| Minor Criteria | Residual myocytes 60% to 75% by morphometric analysis (or 50% to 65% if estimated), with fibrous replacement of the RV free wall myocardium in ≥1 sample, with or without fatty replacement of tissue on EMB |
| Repolarization abnormalities | |
| Major Criteria | Inverted T waves in right precordial leads (V1, V2, and V3) or beyond in individuals >14 years of age (in the absence of complete RBBB QRS ≥120 ms) |
| Minor Criteria | Inverted T waves in leads V1 and V2 in individuals >14 years of age (in the absence of complete RBBB) or in V4, V5, or V6 |
| Inverted T waves in leads V1, V2, V3, and V4 in individuals >14 years of age in the presence of complete RBBB | |
| Depolarization/conduction abnormalities | |
| Major Criteria | Epsilon wave (reproducible low-amplitude signals between end of QRS complex to onset of the T wave) in the right precordial leads (V1 to V3) |
| Minor Criteria | Late potentials by SAECG in ≥1 of 3 parameters in the absence of a QRS duration of ≥110 ms on the standard ECG |
| Filtered QRS duration (fQRS) ≥114 ms | |
| Duration of terminal QRS <40 μV (low-amplitude signal duration) ≥38 ms | |
| Root-mean-square voltage of terminal 40 ms ≤20 μV | |
| Terminal activation duration of QRS ≥55 ms measured from the nadir of the S wave to the end of the QRS, including R’, in V1, V2, or V3, in the absence of complete RBBB | |
| Arrhythmias | |
| Major Criteria | Non-sustained or sustained VT of LBBB morphology with superior axis (negative or indeterminate QRS in leads II, III, and aVF and positive in lead aVL |
| Minor Criteria | Non-sustained or sustained VT of RV outflow configuration, LBBB morphology with inferior axis (positive QRS in leads II, III, and aVF and negative in lead aVL) or of unknown axis) |
| >500 ventricular extrasystoles per 24 hours (Holter) | |
| Family History | |
| Major Criteria | ARVC/D confirmed in a first-degree relative who meets current TF criteria |
| ARVC/D confirmed pathologically at autopsy or surgery in a first-degree relative | |
| Identification of a pathogenic mutation categorized as associated or probably associated with ARVC/D in the patient under evaluation | |
| Minor Criteria | History of ARVC/D in a first-degree relative in whom it is not possible or practical to determine whether the family member meets current TF criteria |
| Premature SCD (<35 years of age) due to suspected ARVC/D in a first-degree relative | |
| ARVC/D confirmed pathologically or by current TF criteria in a second-degree relative |
Regarding presentation with Vas, the typical inferior axis / left bundle branch block (LBBB) pattern is common in more than 90% of IdioVAs patients. Differently, ARVC/D patients present with inferior axis as well as intermediate axis or superior axis VAs.[30] Recently, Hoffmayer et al. compared ARVC/D and IdioVAs patients in a population presenting with the same inferior axis / LBBB ECG morphology, finding several distinguishing criteria between the two conditions. With regard to the diagnosis of ARVD/C, QRS duration ≥ 120 ms in lead I was highly sensitive (88%), whereas a “notching” on QRS upstroke (88%), multiple QRS notching in different leads (88%), earliest QRS onset in V1 (90%), late (V5; 90%) and very late (V6; 100%) precordial transition were all highly specific criteria.[31]
For clinical purposes, ECG characteristics of different sites of RVOT and the left ventricular outflow tract (LVOT) VAs are given in Table 2.[32,33]
Table 2. Outflow tract VAs origin based on QRS pattern. ECG differentiation of a RVOT from a LVOT origin VAs is based on the R/S precordial transition.[32,33].
LBBB = left bundle branch block; LCC = left coronary cusp; RCC = right coronary cusp; AMC = aorto-mitral continuity.
| Right ventricle | BBB | Axis | Precordial transition | V1 | V6 | other |
|---|---|---|---|---|---|---|
| RVOT septal | LBBB | Inferior | ≤ V3 | rS | R | (-) polarity of lead I in anterior and leftward location; (+) polarity in posterior and rightward location; multiphasic polarity in midway location. |
| RVOT free-wall | LBBB | Inferior | ≥ V3 | rS | R | (-) polarity of lead I in anterior and leftward location; (+) polarity in posterior and rightward location; multiphasic polarity in midway location. Notching in lead II, III and aVF. |
| LCC | LBBB | Inferior | ≤ V2 | QS/QR | R | |
| RCC | LBBB | Inferior | ≥ V3 | Multiphasic “M” or “W” | R | |
| AMC | “RBBB” | Inferior | ≤ V3 | qR | R |
In the Revised Task Force criteria, Signal Averaged Electrocardiography (SAECG) is a minor criterion of ARVC/D.[12] However, O’Donnell and coworkers found that late potentials (LPs) on the SAECG were not present in any patient with RVOT tachycardia but were present in 78% of the patients with ARVC/D.[34] Moreover, in a recent non-invasive ECG study, the SAECG parameters and the frequency components recorded from the wavelet-transformed ECG were compared between three different groups: IdioVAs, ARVC/D and Brugada syndrome. Focusing on the first two entities, LPs were positive in all of ARVC/D patients whilst were negative in all of IdioVAs patients and high-frequency components (80-150 Hz) were developed in ARVC/D but not in IdioVAs.[35]
Electrophysiological Differences Between IdioVAs And Ventricular Arrhythmias In ARVC/D
Taking into account the different underlying substrate of the two entities, electrophysiological findings help to differentiate IdioVAs from VAs in the setting of ARVC/D.
Different groups compared electrophysiological findings in patients with IdioVAs and ARVC/D reporting similar results.[30,34] IdioVAs are due to cyclic AMP mediated triggered activity. Thus, it is a focal form of tachycardia, frequently presenting in form of non-sustained arrhythmias, sensitive to catecholamine infusion (i.e. epinephrine, phenylephrine or isoproterenol) and burst stimulation; for the same reason IdioVAs are monomorphic with the common inferior axis – LBBB morphology and present a presystolic high amplitude local electrogram.[36,37]
VAs in context of ARVC/D are the expression of slow conduction areas within the diseased myocardium that allow continuous electrical activity, fragmented diastolic potentials (Figure 2) in an expanded area, creating a circuit pathway. For this reason, programmed ventricular stimulation is more effective to induce VT in ARVC/D patients compared with IdioVAs.[38,39] Moreover, the evidence of different reentry pathways justifies the inducibility of multiple VT morphologies that may be common in ARVC/D patients.
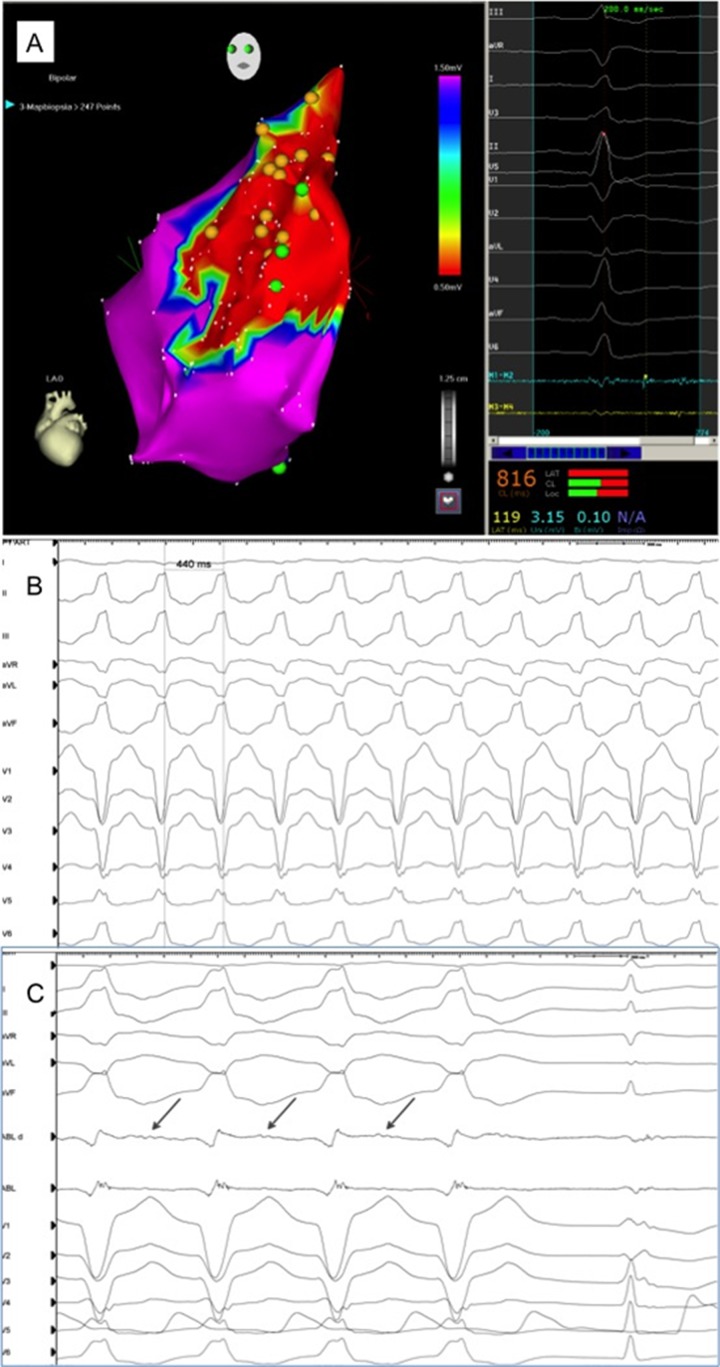
Figure 2. Electroanatomical endocardial 3D mapping shows a large diseased area in the antero-septal portion of the RVOT (<0.5 mV), where fragmented low-voltage electrograms are detected (Panel A, left and right), diagnostic of arrhythmogenic right ventricular cardiomyopathy/dysplasia. VT 12-lead ECG (Panel B) and intracardiac electrograms (Panel C) are shown: radiofrequency delivery at the site of interest, where a diastolic fragmented activity is recorded, immediately terminates the VT.
Imaging - Cardiac Magnetic Resonance
Cardiac magnetic resonance (CMR) imaging is increasingly used as a standard technique for imaging of RV structure and function. Indeed, CMR is the most reliable modality available for the quantification of ventricular size and volume and for the detection of RV morphological abnormalities due to its capacity to perform tissue characterization.
Although IdioVAs occur in patients with no structural heart disease, several groups reported that CMR imaging detected structural abnormalities in patients with IdioVAs, which are similar to those seen in the early stages of ARVC/D. CMR showed subtle areas of diminished wall motion and suggested that mild structural abnormalities may be present.[40-43] These findings made the differentiation of ARVC/D and RVOT tachycardia at the time of initial diagnosis more difficult in some patients. In clinical routine CMR imaging is of fundamental value in the diagnosis of ARVC/D; however, a suspicion of ARVC/D cannot be confirmed or excluded based on CMR imaging alone.
On the other hand, it is well established that the typical morphological features of ARVC/D are RV dilatation and/or dysfunction, wall motion abnormalities, diastolic bulging, wall thinning, reduced systolic thickening and trabecular disarray.[44] Moreover, CMR has the unique ability to detect diffuse or segmental replacement of myocardium in the RV free wall by fibro-fatty tissue.[45] Delayed enhancement (DE) CMR is effective in detecting the presence, location, and extent of myocardial scarring. DE has been described in areas of fibro-fatty myocardial changes in patients with ARVC/D.[46] Tandri et al.[47] first reported an excellent correlation between DE and histopathological diagnosis of fibro-fatty infiltration in patients with ARVC/D. Moreover, they showed that the presence of RV DE was predictive of inducible VT during electrophysiological study. Another group reported DE in 88% of patients studied with ARVC/D; DE predominantly involved the RV free wall but also affected the RV side of the ventricular septum and, in most of the patients, it was associated with regional contraction abnormality.[48] A significant inter-observer variability in the interpretation of qualitative findings and segmental contraction analysis of the RV free wall was reported: CMR was implicated in the overdiagnosis of ARVC/D based on the low specificity of qualitative findings, such as increased intramyocardial fat and wall thinning.[49] A CMR study demonstrated a 93.1% prevalence of RV wall motion abnormalities in healthy subjects, including areas of apparent dyskinesia (75.9%) and bulging (27.6%).[50] These data indicate that conventional CMR may lead to misdiagnosis of ARVC/D by showing equivocal morphofunctional RV abnormalities. Noteworthily, even if CMR is considered an important test for ARVC/D diagnosis may not detect the initial phases of the disease.
The Adjunctive Role Of Three-Dimensional Electroanatomical Voltage Mapping
Three-dimensional electroanatomical voltage mapping (3D-EAVM) using CARTO system (Biosense-Webster, Diamond Bar, CA, USA) is able to unmask subtle structural heart disease in patients with VAs and an apparently normal heart, despite a thorough noninvasive evaluation, including CMR.[51] Specifically, 3D-EAVM accurately identifies and characterizes low-voltage regions (“electroanatomical scar”) that, in patients with ARVC/D, correspond to areas of myocardial depletion and correlate with the histopathological finding of myocardial atrophy and fibro-fatty replacement confirmed at endomyocardial biopsy (EMB).[52-55] Boulos et al.[56] compared electroanatomical findings in patients with an ultimate diagnosis of IdioVAs with those in patients who had established ARVC/D. They found that mapping results were in concordance with previous clinical diagnosis, by showing normal voltages in the IdioVAs group and abnormal low-amplitude areas in ARVC/D patients.[56] These results were however not confirmed by the histopathological study.[56] Differently, Corrado et al.[57] demonstrated that some patients with RVOT VT in the absence of RV dilatation and/or dysfunction showed electroanatomical scar in the RVOT corresponding to histopathological features diagnostic of early ARVC/D, like fibro-fatty myocardial replacement, conditioning malignant arrhythmic course. This was consistent with the current perspective on the ARVC/D natural history, and with an early “concealed” phase with subtle RV structural changes that can be identified by 3D-EAVM with a high degree of sensitivity of 100% and specificity of 95%.[57] Santangeli et al.[58] compared CMR with 3D-EAVM for scar identification in patients with RV arrhythmias and structural heart disease evidenced at EMB, confirming that 3D-EAVM is more sensitive than DE-CMR, particularly in cases of small scars, and should be used as mapping guide for EMB. Their conclusion was that 3D-EAVM with EMB should be considered when the clinical suspicion is high, because absence of DE does not reliably rule out abnormal myocardial substrates.[58]
Recently, Perazzolo Marra and co-workers confirmed that currently available DE-CMR visualizes RV scars unsatisfactorily: based on their findings, it seems that DE-CMR and 3D-EAVM should not be considered alternative imaging tools in ARVC/D patients, but they should be used synergistically to combine their strategic diagnostic and prognostic information regarding quantitative evaluation of RV function and assessment of arrhythmogenic myocardial substrate.[59] A new technique to predict the presence and extension of epicardial involvement in patients with ARVC/D undergoing endocardial EAVM was proposed by the Marchlinski’s group. This technique shows that, in patients with limited endocardial substrate, endocardial unipolar intracardiac electrograms (EGMs) <5.5 mV well correlate with electrogram abnormalities detected from the epicardial aspect in patients with ARVC/D:[61] therefore endocardial unipolar electrogram abnormalities may represent the clue of an early “epicardial” disease, that requires further investigation (Figure 3).
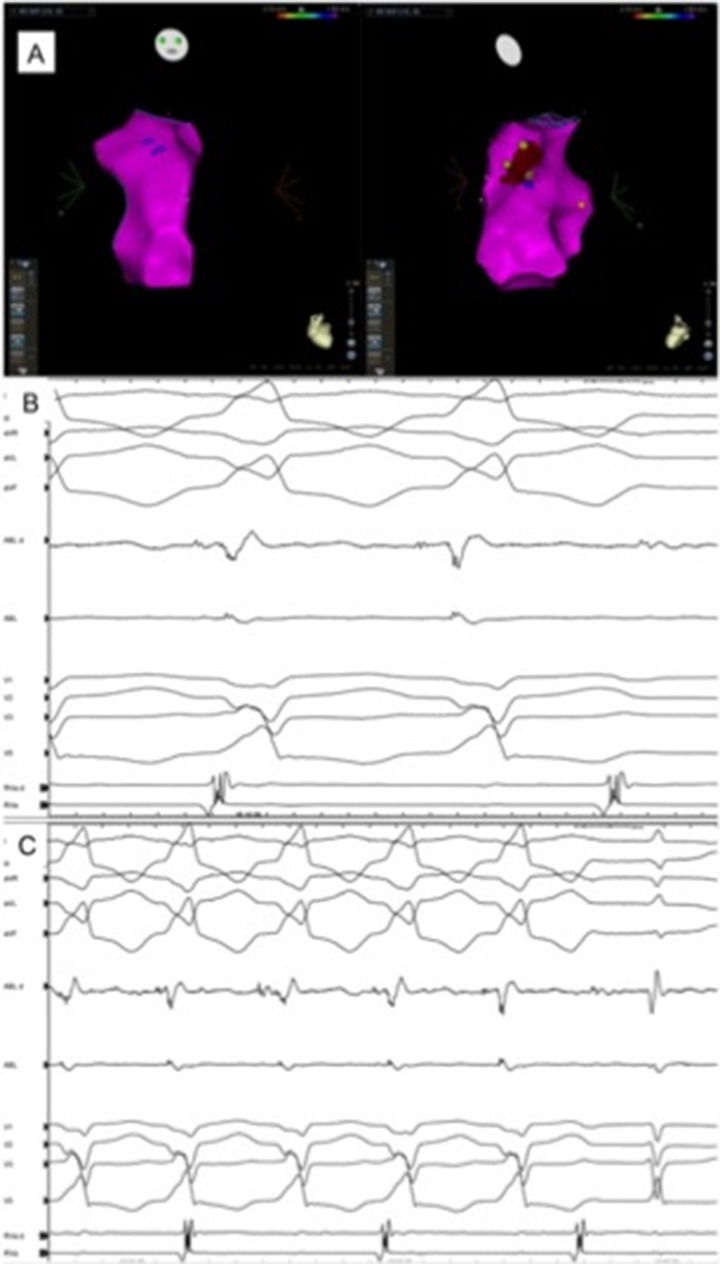
Figure 3. Electroanatomical bipolar endocardial mapping (left anterior oblique, LAO) and right anterior oblique, RAO) views, Panel A and B) is characterized by normal voltage electrograms in the entire right ventricular chamber. Unipolar electrogram analysis shows however in the anterior aspect of the ventricle/RVOT a narrow area where abnormal electrograms (red; <5.5mV) are recorded; this evidence suggests epicardial substrate abnormalities as an initial stage of the disease, that are confirmed by direct epicardial bipolar mapping (<1.0mV).
Radiofrequency Catheter Ablation In IdioVAs And ARVC/D
RFCA of IdioVAs arising from the RVOT is based on two main methods of mapping: activation mapping and pace-mapping. In addition, both available 3D-EAVM systems, CARTOTM (Biosense-Webster, Diamond Bar, CA, USA) or NavXTM (St. Jude Medical, St. Paul, MN, USA), are widely used to relate the anatomy to the mapping data (Figure 4, panel A). During activation mapping, the earliest intracardiac bipolar electrogram recorded from the mapping catheter is compared with the surface QRS onset, with an expected advance of 20 to 40 milliseconds (Figure 4, panel B-C); a sharp “QS” deflection at the unipolar recording should confirm the site of origin of the arrhythmia. Of note, unipolar EGMs have been related to a high sensitivity for successful ablation sites, but may also be recorded at unsuccessful sites up to 11 mm from the site of origin.[26,62-64] Pacemapping has been proposed to confirm the activation mapping findings and in particular if PVCs are not frequent or VT is not inducible. It should be performed using as little current as needed to reliably capture and at the same cycle length of VT or similar, and perfect match on a 12-lead ECG with regard to spontaneous arrhythmias is mandatory.[65-67]
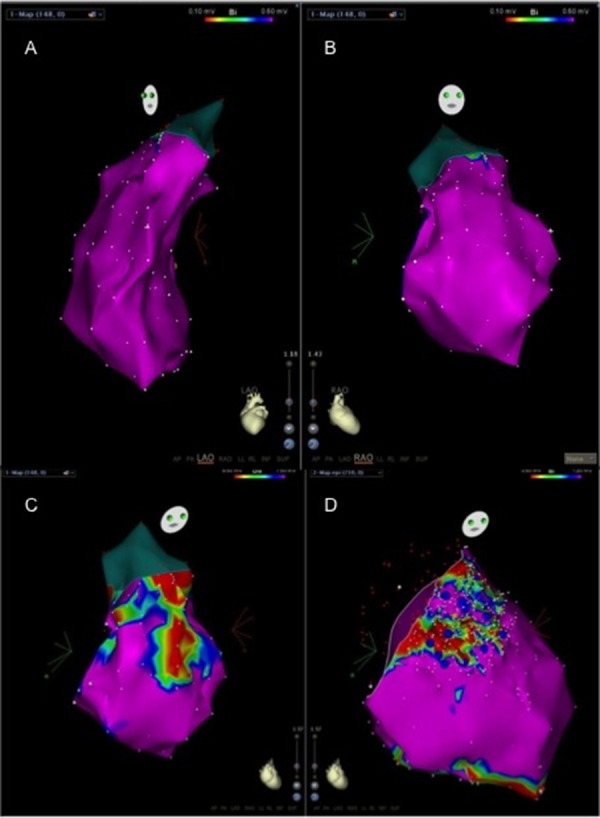
Figure 4. Electroanatomical endocardial 3D mapping (2 projections of the right ventricle) shows normal electrograms confirming the idiopathic nature of the arrhythmia (Panel A). The 3D shell is used for navigation to precisely define earliest ventricular activation that is the target area for radiofrequency delivery (red dots). Intracardiac recording at the site of ablation shows an early presystolic electrogram (Panel B), where radiofrequency delivery instantaneously terminates the VT (exit block is shown) (Panel B and C).
Accordingly to the available published data in respect of the outcome of RFCA for IdioVAs, acute procedural success was reported in 93% of patients, with about 5% of recurrence risk.[68] Serious complications were described in approximately 1% of patients, primarily related to myocardial perforation. For this reason, the integration with the new available technologies, such as contact force information, is mandatory to reduce the risk of perforation mostly due to the relatively thin structure of the RVOT.[16]
RFCA of VAs in ARVC/D patients is not considered curative and thus is not a first-line therapy.[13-15] The results of RFCA in the setting of ARVC/D-related VAs substantially vary among the several single-center experiences, reflecting different procedural strategies and mapping techniques. No VT recurrences were reported in 89% of patients treated by the Marchlinski’s group during a 27±22 months of follow-up.[53] Differently, Verma et al.[55] achieved a short-term success in 82% of patients, with a VT recurrence at 1, 2, and 3 years of follow-up in 23%, 27%, and 47% of cases, respectively. Also Dalal et al.[14] reported unsatisfactory VT recurrence-free survival rates at 1.5, 5 and 14 months in 75%, 50% and 25% of patients, respectively.[14] Noteworthily, only an endocardial approach was used in most of the papers published.[13,14,53,55,69-71] These unsatisfactory results achieved with RFCA may be related to an inadequate characterization of the substrate. Considering the diffuse substrate of ARVC/D and the predominant epicardial involvement and taking into account the relevant incidence of failure using an endocardial-only approach, Garcia et al.[60] described the role of a combined endo-epicardial substrate-based ablation approach to improve the outcomes in ARVC/D patient population. In this study the feasibility of endo-epicardial substrate–based approach to improve arrhythmia control was demonstrated, underlying importance of targeting the epicardium to further optimize long-term clinical outcome.[60] During a mean follow-up of 18±12 months from RFCA, 77% of patients have no VT recurrence.[60] Bai and coworkers[72] interestingly suggested that the endo-epicardial approach not only increased long-term arrhythmia-free survival but was more likely to result in discontinuation of antiarrhythmic drugs. Berruezo et al.[73] showed that a combined endo-epicardial mapping reveals larger epicardial substrate in patients with ARVC/D, confirming the low efficacy of the endocardial-only strategy. Moreover, it was demonstrated that using the endo-epicardial approach including scar dechannelling technique is possible to achieve a high acute success rate with low incidence of recurrence during follow-up.[73]
RFCA results in a significant reduction in the VT burden among patients with ARVC/D, regardless of ablation strategy. However, despite the better results using the epicardial approach, recurrence rates remain considerable as shown by Philips et al.[74] In their series of 87 ARVC/D patients undergoing RFCA, they reported a cumulative freedom from VT after epicardial ablation of 64% and 45% at 1 and 5 years, which was significantly longer than with the endocardial approach.[74]
Conclusion
IdioVAs and ARVC/D, even at the early stage, are fundamentally different entities. Nevertheless, clinical presentation is not unequivocal, so that, particularly in early stage of ARVC/D, non-invasive diagnostic tools may direct towards one or the other suspected diagnosis. ECG differences in sinus rhythm between IdioVAs and early stage ARVC/D may be unremarkable, whilst, during VAs different ECG and intracardiac findings have been identified to assist in the differential diagnosis. Currently available imaging techniques are of fundamental importance to recognize RV structural and functional abnormalities and the combined information derived from CMR and 3D-EAVM represent the most effective tool for the identification of myocardial abnormalities in early stages of the disease. The role of RFCA is well established in the setting of IdioVAs, whilst more and more evidences support the use of a combined endo-epicardial substrate-based approach to effectively control the arrhythmic burden in ARVD patients, modifying the course of the disease in a prognostic way.
Disclosures
None.
References
- 1.Buxton A E, Waxman H L, Marchlinski F E, Simson M B, Cassidy D, Josephson M E. Right ventricular tachycardia: clinical and electrophysiologic characteristics. Circulation. 1983 Nov;68 (5):917–27. doi: 10.1161/01.cir.68.5.917. [DOI] [PubMed] [Google Scholar]
- 2.Lerman B B, Stein K, Engelstein E D, Battleman D S, Lippman N, Bei D, Catanzaro D. Mechanism of repetitive monomorphic ventricular tachycardia. Circulation. 1995 Aug 1;92 (3):421–9. doi: 10.1161/01.cir.92.3.421. [DOI] [PubMed] [Google Scholar]
- 3.Lemery R, Brugada P, Bella P D, Dugernier T, van den Dool A, Wellens H J. Nonischemic ventricular tachycardia. Clinical course and long-term follow-up in patients without clinically overt heart disease. Circulation. 1989 May;79 (5):990–9. doi: 10.1161/01.cir.79.5.990. [DOI] [PubMed] [Google Scholar]
- 4.Goy J J, Tauxe F, Fromer M, Schläpfer J, Vogt P, Kappenberger L. Ten-years follow-up of 20 patients with idiopathic ventricular tachycardia. Pacing Clin Electrophysiol. 1990 Sep;13 (9):1142–7. doi: 10.1111/j.1540-8159.1990.tb02172.x. [DOI] [PubMed] [Google Scholar]
- 5.Thiene G, Nava A, Corrado D, Rossi L, Pennelli N. Right ventricular cardiomyopathy and sudden death in young people. N. Engl. J. Med. 1988 Jan 21;318 (3):129–33. doi: 10.1056/NEJM198801213180301. [DOI] [PubMed] [Google Scholar]
- 6.Basso Cristina, Corrado Domenico, Marcus Frank I, Nava Andrea, Thiene Gaetano. Arrhythmogenic right ventricular cardiomyopathy. Lancet. 2009 Apr 11;373 (9671):1289–300. doi: 10.1016/S0140-6736(09)60256-7. [DOI] [PubMed] [Google Scholar]
- 7.Basso C, Thiene G, Corrado D, Angelini A, Nava A, Valente M. Arrhythmogenic right ventricular cardiomyopathy. Dysplasia, dystrophy, or myocarditis? Circulation. 1996 Sep 1;94 (5):983–91. doi: 10.1161/01.cir.94.5.983. [DOI] [PubMed] [Google Scholar]
- 8.Marcus F I, Fontaine G H, Guiraudon G, Frank R, Laurenceau J L, Malergue C, Grosgogeat Y. Right ventricular dysplasia: a report of 24 adult cases. Circulation. 1982 Feb;65 (2):384–98. doi: 10.1161/01.cir.65.2.384. [DOI] [PubMed] [Google Scholar]
- 9.Corrado D, Basso C, Thiene G, McKenna W J, Davies M J, Fontaliran F, Nava A, Silvestri F, Blomstrom-Lundqvist C, Wlodarska E K, Fontaine G, Camerini F. Spectrum of clinicopathologic manifestations of arrhythmogenic right ventricular cardiomyopathy/dysplasia: a multicenter study. J. Am. Coll. Cardiol. 1997 Nov 15;30 (6):1512–20. doi: 10.1016/s0735-1097(97)00332-x. [DOI] [PubMed] [Google Scholar]
- 10.Basso Cristina, Corrado Domenico, Bauce Barbara, Thiene Gaetano. Arrhythmogenic right ventricular cardiomyopathy. Circ Arrhythm Electrophysiol. 2012 Dec;5 (6):1233–46. doi: 10.1161/CIRCEP.111.962035. [DOI] [PubMed] [Google Scholar]
- 11.Sen-Chowdhry Srijita, Syrris Petros, Ward Deirdre, Asimaki Angeliki, Sevdalis Elias, McKenna William J. Clinical and genetic characterization of families with arrhythmogenic right ventricular dysplasia/cardiomyopathy provides novel insights into patterns of disease expression. Circulation. 2007 Apr 3;115 (13):1710–20. doi: 10.1161/CIRCULATIONAHA.106.660241. [DOI] [PubMed] [Google Scholar]
- 12.Marcus Frank I, McKenna William J, Sherrill Duane, Basso Cristina, Bauce Barbara, Bluemke David A, Calkins Hugh, Corrado Domenico, Cox Moniek G P J, Daubert James P, Fontaine Guy, Gear Kathleen, Hauer Richard, Nava Andrea, Picard Michael H, Protonotarios Nikos, Saffitz Jeffrey E, Sanborn Danita M Yoerger, Steinberg Jonathan S, Tandri Harikrishna, Thiene Gaetano, Towbin Jeffrey A, Tsatsopoulou Adalena, Wichter Thomas, Zareba Wojciech. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation. 2010 Apr 6;121 (13):1533–41. doi: 10.1161/CIRCULATIONAHA.108.840827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aliot Etienne M, Stevenson William G, Almendral-Garrote Jesus Ma, Bogun Frank, Calkins C Hugh, Delacretaz Etienne, Bella Paolo Della, Hindricks Gerhard, Jaïs Pierre, Josephson Mark E, Kautzner Josef, Kay G Neal, Kuck Karl-Heinz, Lerman Bruce B, Marchlinski Francis, Reddy Vivek, Schalij Martin-Jan, Schilling Richard, Soejima Kyoko, Wilber David. EHRA/HRS Expert Consensus on Catheter Ablation of Ventricular Arrhythmias: developed in a partnership with the European Heart Rhythm Association (EHRA), a Registered Branch of the European Society of Cardiology (ESC), and the Heart Rhythm Society (HRS); in collaboration with the American College of Cardiology (ACC) and the American Heart Association (AHA). Europace. 2009 Jun;11 (6):771–817. doi: 10.1093/europace/eup098. [DOI] [PubMed] [Google Scholar]
- 14.Dalal Darshan, Jain Rahul, Tandri Harikrishna, Dong Jun, Eid Shaker M, Prakasa Kalpana, Tichnell Crystal, James Cynthia, Abraham Theodore, Russell Stuart D, Sinha Sunil, Judge Daniel P, Bluemke David A, Marine Joseph E, Calkins Hugh. Long-term efficacy of catheter ablation of ventricular tachycardia in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy. J. Am. Coll. Cardiol. 2007 Jul 31;50 (5):432–40. doi: 10.1016/j.jacc.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 15.Arbelo Elena, Josephson Mark E. Ablation of ventricular arrhythmias in arrhythmogenic right ventricular dysplasia. J. Cardiovasc. Electrophysiol. 2010 Apr;21 (4):473–86. doi: 10.1111/j.1540-8167.2009.01694.x. [DOI] [PubMed] [Google Scholar]
- 16.Asirvatham Samuel J. Correlative anatomy for the invasive electrophysiologist: outflow tract and supravalvar arrhythmia. J. Cardiovasc. Electrophysiol. 2009 Aug;20 (8):955–68. doi: 10.1111/j.1540-8167.2009.01472.x. [DOI] [PubMed] [Google Scholar]
- 17.de Jong F, Opthof T, Wilde A A, Janse M J, Charles R, Lamers W H, Moorman A F. Persisting zones of slow impulse conduction in developing chicken hearts. Circ. Res. 1992 Aug;71 (2):240–50. doi: 10.1161/01.res.71.2.240. [DOI] [PubMed] [Google Scholar]
- 18.Jongbloed M R M, Mahtab E A F, Blom N A, Schalij M J, Gittenberger-de Groot A C. Development of the cardiac conduction system and the possible relation to predilection sites of arrhythmogenesis. ScientificWorldJournal. 2008;8:239–69. doi: 10.1100/tsw.2008.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vicente-Steijn Rebecca, Passier Robert, Wisse Lambertus J, Schalij Martin J, Poelmann Robert E, Gittenberger-de Groot Adriana C, Jongbloed Monique R M. Funny current channel HCN4 delineates the developing cardiac conduction system in chicken heart. Heart Rhythm. 2011 Aug;8 (8):1254–63. doi: 10.1016/j.hrthm.2011.03.043. [DOI] [PubMed] [Google Scholar]
- 20.Jongbloed Monique R M, Schalij Martin J, Poelmann Robert E, Blom Nico A, Fekkes Madelon L, Wang Zhiyong, Fishman Glenn I, Gittenberger-De Groot Adriana C. Embryonic conduction tissue: a spatial correlation with adult arrhythmogenic areas. J. Cardiovasc. Electrophysiol. 2004 Mar;15 (3):349–55. doi: 10.1046/j.1540-8167.2004.03487.x. [DOI] [PubMed] [Google Scholar]
- 21.Kondo Richard P, Anderson Robert H, Kupershmidt Sabina, Roden Dan M, Evans Sylvia M. Development of the cardiac conduction system as delineated by minK-lacZ. J. Cardiovasc. Electrophysiol. 2003 Apr;14 (4):383–91. doi: 10.1046/j.1540-8167.2003.02467.x. [DOI] [PubMed] [Google Scholar]
- 22.Calvo Naiara, Jongbloed Monique, Zeppenfeld Katja. Radiofrequency catheter ablation of idiopathic right ventricular outflow tract arrhythmias. Indian Pacing Electrophysiol J. 2013 Jan;13 (1):14–33. doi: 10.1016/s0972-6292(16)30585-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thiene G, Nava A, Angelini A, Daliento L, Scognamiglio R, Corrado D. Anatomoclinical aspects of arrhythmogenic right ventricular cardiomyopathy. In Advances in cardiomyopathies. Baroldi G, Camerini F, Goodwin JF, eds. . Milano: Springer Verlag. 1990;0:397–408. [Google Scholar]
- 24.McKenna W J, Thiene G, Nava A, Fontaliran F, Blomstrom-Lundqvist C, Fontaine G, Camerini F. Diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Task Force of the Working Group Myocardial and Pericardial Disease of the European Society of Cardiology and of the Scientific Council on Cardiomyopathies of the International Society and Federation of Cardiology. Br Heart J. 1994 Mar;71 (3):215–8. doi: 10.1136/hrt.71.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turrini P, Corrado D, Basso C, Nava A, Bauce B, Thiene G. Dispersion of ventricular depolarization-repolarization: a noninvasive marker for risk stratification in arrhythmogenic right ventricular cardiomyopathy. Circulation. 2001 Jun 26;103 (25):3075–80. doi: 10.1161/01.cir.103.25.3075. [DOI] [PubMed] [Google Scholar]
- 26.Fontaine G, Fontaliran F, Hébert J L, Chemla D, Zenati O, Lecarpentier Y, Frank R. Arrhythmogenic right ventricular dysplasia. Annu. Rev. Med. 1999;50 ():17–35. doi: 10.1146/annurev.med.50.1.17. [DOI] [PubMed] [Google Scholar]
- 27.Peters Stefan, Trümmel Martina. Diagnosis of arrhythmogenic right ventricular dysplasia-cardiomyopathy: value of standard ECG revisited. Ann Noninvasive Electrocardiol. 2003 Jul;8 (3):238–45. doi: 10.1046/j.1542-474X.2003.08312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nasir Khurram, Bomma Chandra, Tandri Harikrishna, Roguin Ariel, Dalal Darshan, Prakasa Kalpana, Tichnell Crystal, James Cynthia, Spevak Phillip J, Jspevak Phillip, Marcus Frank, Calkins Hugh. Electrocardiographic features of arrhythmogenic right ventricular dysplasia/cardiomyopathy according to disease severity: a need to broaden diagnostic criteria. Circulation. 2004 Sep 21;110 (12):1527–34. doi: 10.1161/01.CIR.0000142293.60725.18. [DOI] [PubMed] [Google Scholar]
- 29.Migliore Federico, Zorzi Alessandro, Michieli Pierantonio, Perazzolo Marra Martina, Siciliano Mariachiara, Rigato Ilaria, Bauce Barbara, Basso Cristina, Toazza Daniela, Schiavon Maurizio, Iliceto Sabino, Thiene Gaetano, Corrado Domenico. Prevalence of cardiomyopathy in Italian asymptomatic children with electrocardiographic T-wave inversion at preparticipation screening. Circulation. 2012 Jan 24;125 (3):529–38. doi: 10.1161/CIRCULATIONAHA.111.055673. [DOI] [PubMed] [Google Scholar]
- 30.Niroomand F, Carbucicchio C, Tondo C, Riva S, Fassini G, Apostolo A, Trevisi N, Bella P Della. Electrophysiological characteristics and outcome in patients with idiopathic right ventricular arrhythmia compared with arrhythmogenic right ventricular dysplasia. Heart. 2002 Jan;87 (1):41–7. doi: 10.1136/heart.87.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoffmayer Kurt S, Machado Orlando N, Marcus Gregory M, Yang Yanfei, Johnson Colleen J, Ermakov Simon, Vittinghoff Eric, Pandurangi Ulhas, Calkins Hugh, Cannom David, Gear Kathleen C, Tichnell Crystal, Park Young, Zareba Wojciech, Marcus Frank I, Scheinman Melvin M. Electrocardiographic comparison of ventricular arrhythmias in patients with arrhythmogenic right ventricular cardiomyopathy and right ventricular outflow tract tachycardia. J. Am. Coll. Cardiol. 2011 Aug 16;58 (8):831–8. doi: 10.1016/j.jacc.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 32.Dixit Sanjay, Gerstenfeld Edward P, Callans David J, Marchlinski Francis E. Electrocardiographic patterns of superior right ventricular outflow tract tachycardias: distinguishing septal and free-wall sites of origin. J. Cardiovasc. Electrophysiol. 2003 Jan;14 (1):1–7. doi: 10.1046/j.1540-8167.2003.02404.x. [DOI] [PubMed] [Google Scholar]
- 33.Huang SKS, Wood MA. Ablation of ventricular outflow tract tachycardia. In: Huang SKS, Wood MA. Catheter ablation of cardiac arrhythmias. 2 ed. Philadelphia, PA. Saunders Elsevier. 2011;0:446–462. [Google Scholar]
- 34.O'Donnell D, Cox D, Bourke J, Mitchell L, Furniss S. Clinical and electrophysiological differences between patients with arrhythmogenic right ventricular dysplasia and right ventricular outflow tract tachycardia. Eur. Heart J. 2003 May;24 (9):801–10. doi: 10.1016/s0195-668x(02)00654-1. [DOI] [PubMed] [Google Scholar]
- 35.Yodogawa Kenji, Morita Norishige, Kobayashi Yoshinori, Takayama Hideo, Ohara Toshihiko, Seino Yoshihiko, Katoh Takao, Mizuno Kyoichi. A new approach for the comparison of conduction abnormality between arrhythmogenic right ventricular cardiomyopathy/dysplasia and Brugada syndrome. Ann Noninvasive Electrocardiol. 2011 Jul;16 (3):263–9. doi: 10.1111/j.1542-474X.2011.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lerman B B, Belardinelli L, West G A, Berne R M, DiMarco J P. Adenosine-sensitive ventricular tachycardia: evidence suggesting cyclic AMP-mediated triggered activity. Circulation. 1986 Aug;74 (2):270–80. doi: 10.1161/01.cir.74.2.270. [DOI] [PubMed] [Google Scholar]
- 37.Peters NS, Cabo C, Witt AL. Arrhythmogenic mechanisms: automaticity, triggered activity and reentry. In: Zipes DP, Jalife J, editors. Cardiac electrophysiology: from cell to bedside. WB Saunders. Philadelphia. 2000;0:345–56. [Google Scholar]
- 38.Josephson M E, Horowitz L N, Farshidi A, Spielman S R, Michelson E L, Greenspan A M. Sustained ventricular tachycardia: evidence for protected localized reentry. Am. J. Cardiol. 1978 Sep;42 (3):416–24. doi: 10.1016/0002-9149(78)90936-0. [DOI] [PubMed] [Google Scholar]
- 39.Ellison K E, Friedman P L, Ganz L I, Stevenson W G. Entrainment mapping and radiofrequency catheter ablation of ventricular tachycardia in right ventricular dysplasia. J. Am. Coll. Cardiol. 1998 Sep;32 (3):724–8. doi: 10.1016/s0735-1097(98)00292-7. [DOI] [PubMed] [Google Scholar]
- 40.Carlson M D, White R D, Trohman R G, Adler L P, Biblo L A, Merkatz K A, Waldo A L. Right ventricular outflow tract ventricular tachycardia: detection of previously unrecognized anatomic abnormalities using cine magnetic resonance imaging. J. Am. Coll. Cardiol. 1994 Sep;24 (3):720–7. doi: 10.1016/0735-1097(94)90020-5. [DOI] [PubMed] [Google Scholar]
- 41.White R D, Trohman R G, Flamm S D, VanDyke C W, Optican R J, Sterba R, Obuchowski N A, Carlson M D, Tchou P J. Right ventricular arrhythmia in the absence of arrhythmogenic dysplasia: MR imaging of myocardial abnormalities. Radiology. 1998 Jun;207 (3):743–51. doi: 10.1148/radiology.207.3.9609899. [DOI] [PubMed] [Google Scholar]
- 42.Globits S, Kreiner G, Frank H, Heinz G, Klaar U, Frey B, Gössinger H. Significance of morphological abnormalities detected by MRI in patients undergoing successful ablation of right ventricular outflow tract tachycardia. Circulation. 1997 Oct 21;96 (8):2633–40. doi: 10.1161/01.cir.96.8.2633. [DOI] [PubMed] [Google Scholar]
- 43.Markowitz S M, Litvak B L, Ramirez de Arellano E A, Markisz J A, Stein K M, Lerman B B. Adenosine-sensitive ventricular tachycardia: right ventricular abnormalities delineated by magnetic resonance imaging. Circulation. 1997 Aug 19;96 (4):1192–200. doi: 10.1161/01.cir.96.4.1192. [DOI] [PubMed] [Google Scholar]
- 44.Tandri Harikrishna, Calkins Hugh, Nasir Khurram, Bomma Chandra, Castillo Ernesto, Rutberg Julie, Tichnell Crystal, Lima João A C, Bluemke David A. Magnetic resonance imaging findings in patients meeting task force criteria for arrhythmogenic right ventricular dysplasia. J. Cardiovasc. Electrophysiol. 2003 May;14 (5):476–82. doi: 10.1046/j.1540-8167.2003.02560.x. [DOI] [PubMed] [Google Scholar]
- 45.Ricci C, Longo R, Pagnan L, Dalla Palma L, Pinamonti B, Camerini F, Bussani R, Silvestri F. Magnetic resonance imaging in right ventricular dysplasia. Am. J. Cardiol. 1992 Dec 15;70 (20):1589–95. doi: 10.1016/0002-9149(92)90462-8. [DOI] [PubMed] [Google Scholar]
- 46.Bluemke David A, Krupinski Elizabeth A, Ovitt Theron, Gear Kathleen, Unger Evan, Axel Leon, Boxt Lawrence M, Casolo Giancarlo, Ferrari Victor A, Funaki Brian, Globits Sebastian, Higgins Charles B, Julsrud Paul, Lipton Martin, Mawson John, Nygren Anders, Pennell Dudley J, Stillman Arthur, White Richard D, Wichter Thomas, Marcus Frank. MR Imaging of arrhythmogenic right ventricular cardiomyopathy: morphologic findings and interobserver reliability. Cardiology. 2003;99 (3):153–62. doi: 10.1159/000070672. [DOI] [PubMed] [Google Scholar]
- 47.Tandri Harikrishna, Saranathan Manoj, Rodriguez E Rene, Martinez Claudia, Bomma Chandra, Nasir Khurram, Rosen Boas, Lima João A C, Calkins Hugh, Bluemke David A. Noninvasive detection of myocardial fibrosis in arrhythmogenic right ventricular cardiomyopathy using delayed-enhancement magnetic resonance imaging. J. Am. Coll. Cardiol. 2005 Jan 4;45 (1):98–103. doi: 10.1016/j.jacc.2004.09.053. [DOI] [PubMed] [Google Scholar]
- 48.Pfluger Heinz B, Phrommintikul Arintaya, Mariani Justin A, Cherayath Joshi G, Taylor Andrew J. Utility of myocardial fibrosis and fatty infiltration detected by cardiac magnetic resonance imaging in the diagnosis of arrhythmogenic right ventricular dysplasia--a single centre experience. Heart Lung Circ. 2008 Dec;17 (6):478–83. doi: 10.1016/j.hlc.2008.03.085. [DOI] [PubMed] [Google Scholar]
- 49.Tandri Harikrishna, Castillo Ernesto, Ferrari Victor A, Nasir Khurram, Dalal Darshan, Bomma Chandra, Calkins Hugh, Bluemke David A. Magnetic resonance imaging of arrhythmogenic right ventricular dysplasia: sensitivity, specificity, and observer variability of fat detection versus functional analysis of the right ventricle. J. Am. Coll. Cardiol. 2006 Dec 5;48 (11):2277–84. doi: 10.1016/j.jacc.2006.07.051. [DOI] [PubMed] [Google Scholar]
- 50.Sievers Burkhard, Addo Marvin, Franken Ulrich, Trappe Hans-Joachim. Right ventricular wall motion abnormalities found in healthy subjects by cardiovascular magnetic resonance imaging and characterized with a new segmental model. J Cardiovasc Magn Reson. 2004;6 (3):601–8. doi: 10.1081/jcmr-120038528. [DOI] [PubMed] [Google Scholar]
- 51.Dello Russo Antonio, Pieroni Maurizio, Santangeli Pasquale, Bartoletti Stefano, Casella Michela, Pelargonio Gemma, Smaldone Costantino, Bianco Massimiliano, Di Biase Luigi, Bellocci Fulvio, Zeppilli Paolo, Fiorentini Cesare, Natale Andrea, Tondo Claudio. Concealed cardiomyopathies in competitive athletes with ventricular arrhythmias and an apparently normal heart: role of cardiac electroanatomical mapping and biopsy. Heart Rhythm. 2011 Dec;8 (12):1915–22. doi: 10.1016/j.hrthm.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 52.Boulos M, Lashevsky I, Reisner S, Gepstein L. Electroanatomic mapping of arrhythmogenic right ventricular dysplasia. J. Am. Coll. Cardiol. 2001 Dec;38 (7):2020–7. doi: 10.1016/s0735-1097(01)01625-4. [DOI] [PubMed] [Google Scholar]
- 53.Marchlinski Francis E, Zado Erica, Dixit Sanjay, Gerstenfeld Edward, Callans David J, Hsia Henry, Lin David, Nayak Hemal, Russo Andrea, Pulliam Ward. Electroanatomic substrate and outcome of catheter ablative therapy for ventricular tachycardia in setting of right ventricular cardiomyopathy. Circulation. 2004 Oct 19;110 (16):2293–8. doi: 10.1161/01.CIR.0000145154.02436.90. [DOI] [PubMed] [Google Scholar]
- 54.Corrado Domenico, Basso Cristina, Leoni Loira, Tokajuk Barbara, Bauce Barbara, Frigo Gianfranco, Tarantini Giuseppe, Napodano Massimo, Turrini Pietro, Ramondo Angelo, Daliento Luciano, Nava Andrea, Buja Gianfranco, Iliceto Sabino, Thiene Gaetano. Three-dimensional electroanatomic voltage mapping increases accuracy of diagnosing arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation. 2005 Jun 14;111 (23):3042–50. doi: 10.1161/CIRCULATIONAHA.104.486977. [DOI] [PubMed] [Google Scholar]
- 55.Verma Atul, Kilicaslan Fethi, Schweikert Robert A, Tomassoni Gery, Rossillo Antonio, Marrouche Nassir F, Ozduran Volkan, Wazni Oussama M, Elayi Samy C, Saenz Luis C, Minor Stephen, Cummings Jennifer E, Burkhardt J David, Hao Steven, Beheiry Salwa, Tchou Patrick J, Natale Andrea. Short- and long-term success of substrate-based mapping and ablation of ventricular tachycardia in arrhythmogenic right ventricular dysplasia. Circulation. 2005 Jun 21;111 (24):3209–16. doi: 10.1161/CIRCULATIONAHA.104.510503. [DOI] [PubMed] [Google Scholar]
- 56.Boulos Monther, Lashevsky Ilan, Gepstein Lior. Usefulness of electroanatomical mapping to differentiate between right ventricular outflow tract tachycardia and arrhythmogenic right ventricular dysplasia. Am. J. Cardiol. 2005 Apr 15;95 (8):935–40. doi: 10.1016/j.amjcard.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 57.Corrado Domenico, Basso Cristina, Leoni Loira, Tokajuk Barbara, Turrini Pietro, Bauce Barbara, Migliore Federico, Pavei Andrea, Tarantini Giuseppe, Napodano Massimo, Ramondo Angelo, Buja Gianfranco, Iliceto Sabino, Thiene Gaetano. Three-dimensional electroanatomical voltage mapping and histologic evaluation of myocardial substrate in right ventricular outflow tract tachycardia. J. Am. Coll. Cardiol. 2008 Feb 19;51 (7):731–9. doi: 10.1016/j.jacc.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 58.Santangeli Pasquale, Hamilton-Craig Christian, Dello Russo Antonio, Pieroni Maurizio, Casella Michela, Pelargonio Gemma, Di Biase Luigi, Smaldone Costantino, Bartoletti Stefano, Narducci Maria L, Tondo Claudio, Bellocci Fulvio, Natale Andrea. Imaging of scar in patients with ventricular arrhythmias of right ventricular origin: cardiac magnetic resonance versus electroanatomic mapping. J. Cardiovasc. Electrophysiol. 2011 Dec;22 (12):1359–66. doi: 10.1111/j.1540-8167.2011.02127.x. [DOI] [PubMed] [Google Scholar]
- 59.Marra Martina Perazzolo, Leoni Loira, Bauce Barbara, Corbetti Francesco, Zorzi Alessandro, Migliore Federico, Silvano Maria, Rigato Ilaria, Tona Francesco, Tarantini Giuseppe, Cacciavillani Luisa, Basso Cristina, Buja Gianfranco, Thiene Gaetano, Iliceto Sabino, Corrado Domenico. Imaging study of ventricular scar in arrhythmogenic right ventricular cardiomyopathy: comparison of 3D standard electroanatomical voltage mapping and contrast-enhanced cardiac magnetic resonance. Circ Arrhythm Electrophysiol. 2012 Feb;5 (1):91–100. doi: 10.1161/CIRCEP.111.964635. [DOI] [PubMed] [Google Scholar]
- 60.Garcia Fermin C, Bazan Victor, Zado Erica S, Ren Jian-Fang, Marchlinski Francis E. Epicardial substrate and outcome with epicardial ablation of ventricular tachycardia in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation. 2009 Aug 4;120 (5):366–75. doi: 10.1161/CIRCULATIONAHA.108.834903. [DOI] [PubMed] [Google Scholar]
- 61.Polin Glenn M, Haqqani Haris, Tzou Wendy, Hutchinson Mathew D, Garcia Fermin C, Callans David J, Zado Erica S, Marchlinski Francis E. Endocardial unipolar voltage mapping to identify epicardial substrate in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Heart Rhythm. 2011 Jan;8 (1):76–83. doi: 10.1016/j.hrthm.2010.09.088. [DOI] [PubMed] [Google Scholar]
- 62.Azegami Koji, Wilber David J, Arruda Mauricio, Lin Albert C, Denman Russell A. Spatial resolution of pacemapping and activation mapping in patients with idiopathic right ventricular outflow tract tachycardia. J. Cardiovasc. Electrophysiol. 2005 Aug;16 (8):823–9. doi: 10.1111/j.1540-8167.2005.50041.x. [DOI] [PubMed] [Google Scholar]
- 63.Miller John M, Pezeshkian Nayereh G, Yadav Anil V. Catheter mapping and ablation of right ventricular outflow tract ventricular tachycardia. J. Cardiovasc. Electrophysiol. 2006 Jul;17 (7):800–2. doi: 10.1111/j.1540-8167.2006.00519.x. [DOI] [PubMed] [Google Scholar]
- 64.Merino J L, Jiménez-Borreguero J, Peinado R, Merino S V, Sobrino J A. Unipolar mapping and magnetic resonance imaging of "idiopathic" right ventricular outflow tract ectopy. J. Cardiovasc. Electrophysiol. 1998 Jan;9 (1):84–7. doi: 10.1111/j.1540-8167.1998.tb00870.x. [DOI] [PubMed] [Google Scholar]
- 65.Stevenson William G, Soejima Kyoko. Recording techniques for clinical electrophysiology. J. Cardiovasc. Electrophysiol. 2005 Sep;16 (9):1017–22. doi: 10.1111/j.1540-8167.2005.50155.x. [DOI] [PubMed] [Google Scholar]
- 66.Goyal R, Harvey M, Daoud E G, Brinkman K, Knight B P, Bahu M, Weiss R, Bogun F, Man K C, Strickberger S A, Morady F. Effect of coupling interval and pacing cycle length on morphology of paced ventricular complexes. Implications for pace mapping. Circulation. 1996 Dec 1;94 (11):2843–9. doi: 10.1161/01.cir.94.11.2843. [DOI] [PubMed] [Google Scholar]
- 67.Gerstenfeld Edward P, Dixit Sanjay, Callans David J, Rajawat Yadavendra, Rho Robert, Marchlinski Francis E. Quantitative comparison of spontaneous and paced 12-lead electrocardiogram during right ventricular outflow tract ventricular tachycardia. J. Am. Coll. Cardiol. 2003 Jun 4;41 (11):2046–53. doi: 10.1016/s0735-1097(03)00427-3. [DOI] [PubMed] [Google Scholar]
- 68.Joshi Sandeep, Wilber David J. Ablation of idiopathic right ventricular outflow tract tachycardia: current perspectives. J. Cardiovasc. Electrophysiol. 2005 Sep;16 Suppl 1:S52–8. doi: 10.1111/j.1540-8167.2005.50163.x. [DOI] [PubMed] [Google Scholar]
- 69.Satomi Kazuhiro, Kurita Takashi, Suyama Kazuhiro, Noda Takashi, Okamura Hideo, Otomo Kiyoshi, Shimizu Wataru, Aihara Naohiko, Kamakura Shiro. Catheter ablation of stable and unstable ventricular tachycardias in patients with arrhythmogenic right ventricular dysplasia. J. Cardiovasc. Electrophysiol. 2006 May;17 (5):469–76. doi: 10.1111/j.1540-8167.2006.00434.x. [DOI] [PubMed] [Google Scholar]
- 70.Wijnmaalen Adrianus P, Schalij Martin J, Bootsma Marianne, Kies Philippine, DE Roos Albert, Putter Hein, Bax Jeroen J, Zeppenfeld Katja. Patients with scar-related right ventricular tachycardia: determinants of long-term outcome. J. Cardiovasc. Electrophysiol. 2009 Oct;20 (10):1119–27. doi: 10.1111/j.1540-8167.2009.01516.x. [DOI] [PubMed] [Google Scholar]
- 71.Miljoen Hielko, State Simona, de Chillou Christian, Magnin-Poull Isabelle, Dotto Pierre, Andronache Marius, Abdelaal Ahmed, Aliot Etienne. Electroanatomic mapping characteristics of ventricular tachycardia in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Europace. 2005 Nov;7 (6):516–24. doi: 10.1016/j.eupc.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 72.Bai Rong, Di Biase Luigi, Shivkumar Kalyanam, Mohanty Prasant, Tung Roderick, Santangeli Pasquale, Saenz Luis Carlos, Vacca Miguel, Verma Atul, Khaykin Yariv, Mohanty Sanghamitra, Burkhardt J David, Hongo Richard, Beheiry Salwa, Dello Russo Antonio, Casella Michela, Pelargonio Gemma, Santarelli Pietro, Sanchez Javier, Tondo Claudio, Natale Andrea. Ablation of ventricular arrhythmias in arrhythmogenic right ventricular dysplasia/cardiomyopathy: arrhythmia-free survival after endo-epicardial substrate based mapping and ablation. Circ Arrhythm Electrophysiol. 2011 Aug;4 (4):478–85. doi: 10.1161/CIRCEP.111.963066. [DOI] [PubMed] [Google Scholar]
- 73.Berruezo Antonio, Fernández-Armenta Juan, Mont Lluís, Zeljko Hrvojka, Andreu David, Herczku Csaba, Boussy Tim, Tolosana Jose María, Arbelo Elena, Brugada Josep. Combined endocardial and epicardial catheter ablation in arrhythmogenic right ventricular dysplasia incorporating scar dechanneling technique. Circ Arrhythm Electrophysiol. 2012 Feb;5 (1):111–21. doi: 10.1161/CIRCEP.110.960740. [DOI] [PubMed] [Google Scholar]
- 74.Philips Binu, Madhavan Srinivasa, James Cynthia, Tichnell Crystal, Murray Brittney, Dalal Darshan, Bhonsale Aditya, Nazarian Saman, Judge Daniel P, Russell Stuart D, Abraham Theodore, Calkins Hugh, Tandri Harikrishna. Outcomes of catheter ablation of ventricular tachycardia in arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circ Arrhythm Electrophysiol. 2012 Jun 1;5 (3):499–505. doi: 10.1161/CIRCEP.111.968677. [DOI] [PubMed] [Google Scholar]