Abstract
Introduction:
The left atrial posterior wall has been shown to play na important role in AF pathophysiology.
Objective:
Evaluate the efficacy of an ablation strategy designed to completely isolate the LA posterior wall, on top of PV isolation. Methods and Results: 25 pts (72% male age 65 ± 12 years) undergoing AF ablation for persistent or long term persistent AF. Mean AF duration was 11 ± 3 months and mean LA diameter was 4.8 ± 0.4 mm. After complete PVI, a “Roof Line” was created between the top of each contralateral set of lesions and a “floor line” closed the posterior wall in a “Box” fashion, connecting the bottom of each set of contralateral lesions. After an average follow-up of 16 ± 2 months, 20 patients (80%) were free of any atrial arrhythmia recurrences (18 of whom off drugs). Five patients (20%) had sustained atypical flutter and required a new ablation procedure. All these patients had mitral isthmus dependent flutters and no electrical conduction in the PVs or posterior wall were detected.
Conclusions:
Complete LA posterior wall isolation on top of PV is associated with good outcomes in patients with persistent and long-standing persistent AF when performed using meticulous bidirectional isolation criteria and adenosine infusion. Recurrences occur predominately as perimitral flutter, without gaps in the posterior wall.
Introduction
Pulmonary vein isolation (PVI) is an stablished procedure to treat paroxysmal atrial fibrillation (AF), reaching success rates of up to 80%.[1] However, as the ablation techniques evolved, other left atrial structures proved to play a significant role in maintaining atrial fibrillation, especially in longstanding AF [2,3]. Fragmented potentials originated from left atrial (LA) roof, posterior wall (figure 1), and atrial septum became targets for ablation. Linear radiofrequency (RF) lesions in left atrial roof proved to be effective both in paroxysmal and persistent AF.[4]However, the alternative approach to eliminate fragmented potentials in the posterior wall still depends on extensive mapping and point-by-point RF lesions, leading to a longer procedure with no proof of complete posterior wall isolation and possibly creating new substrates to macroreentry.
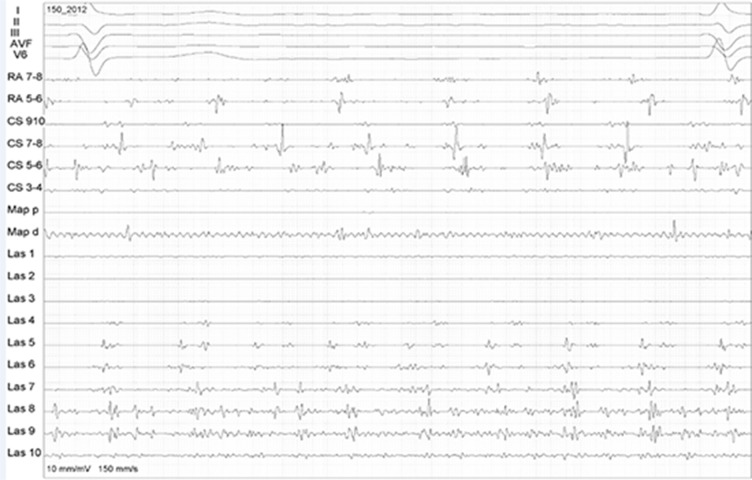
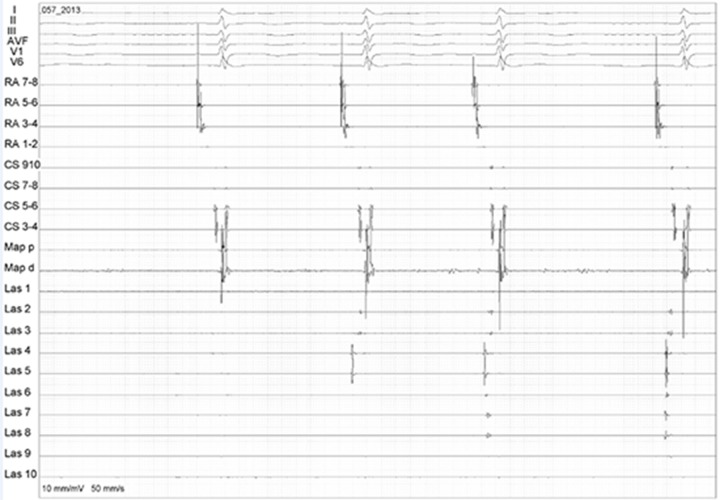
Figure 1. Complex atrial fragmented potentials in the LA posterior wall during AF are shown by the circular mapping catheter (Las 1-10).
Surgical treatment of longstanding atrial fibrillation, “The Cox maze technique”, emphasizes the role of posterior wall isolation in the maintenance of sinus rhythm,[5] and advocates the use of the Box Lesion approach, where the whole posterior wall and the pulmonary veins are isolated by cut and sew techniques.[6]Recently, some new devices have been used to perform single-shot epicardial unipolar radiofrequency pulmonary vein ablation. These devices are designed to perform a single linear lesion around the whole posterior wall surrounding the pulmonary veins.[7]Open atrium surgical RF ablation uses a similar approach to the pulmonary veins and posterior wall, using a bipolar RF energy device.[8]This article describes our experience with catheter ablation for posterior wall isolation and discusses the pros and cons of linear posterior wall isolation in addition to PVI in the maintenance of sinus rhythm.
Methods
Between January and December 2013, 25 patients (72% male, age 65 ± 12 years) with persistent or longstanding persistent AF refractory to at least one antiarrhythmic underwent catheter ablation. Mean AF duration was 11 ± 3 months. Patients using warfarin were oriented to maintain its use and ablation was performed on therapeutic INR (between 2.0 and 3.0). Patients using novel anticoagulants (Rivaroxaban or Dabigatran) were oriented to withdraw these drugs 24-48 hours before the procedure. All patients had evidence of left atrial enlargement (mean LA diameter 4.8 ± 0.4 mm). The procedure has been performed under general anesthesia and during systemic anticoagulation with heparin, maintaining activated clotting times between 350-400s before the first transeptal puncture.
An electroanatomical mapping system (Carto 3 - Biosense Webster, Diamond Bar, CA or NaVx Velocity, St. Jude Medical, Sylmar – CA) was used to create a 3D model of left atrium, subsequently merged into a previously acquired CT scan, obtained 2 to 5 days before the procedure in order to ensure fusion reliability.
After the two transeptal punctures, performed under fluoroscopy and intracardiac echocardiography guidance, a circular mapping catheter was placed inside the pulmonary veins and a wide antral ablation line was created around each pair of ipsilateral pulmonary veins using a cooled tip ablation catheter (temperature-controlled – 43ºC, 30-35W with 30ml/min infusion rate). After complete PVI, a “Roof Line” was created between the top of each contralateral set of lesions and a “floor line” closed the posterior wall in a “Box” fashion, connecting the bottom of each set of contralateral lesions (Figure 2). The end-point of the procedure was complete isolation of the posterior wall, characterized by electrical silence (Figure 3) or dissociated potentials (Figure 4) in the posterior wall using the multiple poles of the circular mapping catheter, and the inability capture the atrium by pacing in the posterior wall after return to sinus rhythm (Figure 5,Figure 6). Power titration was performed according to esophageal temperature and RF lesions interrupted when ≥ 39º was reached.
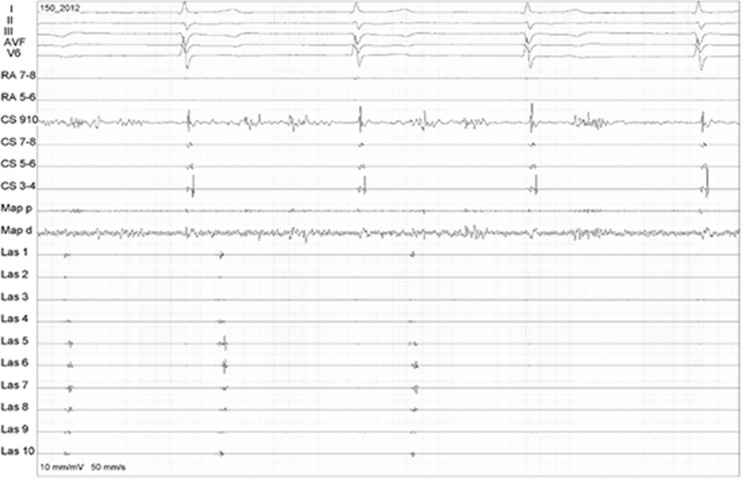
Figure 2. Electroanatomical map during linear lesions in the inferior part of the box lesion set while the circular mapping catheter is monitoring the posterior wall EGMs to assess isolation (entry block).
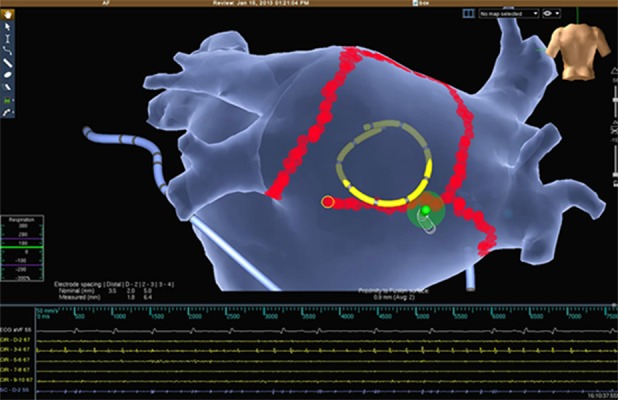
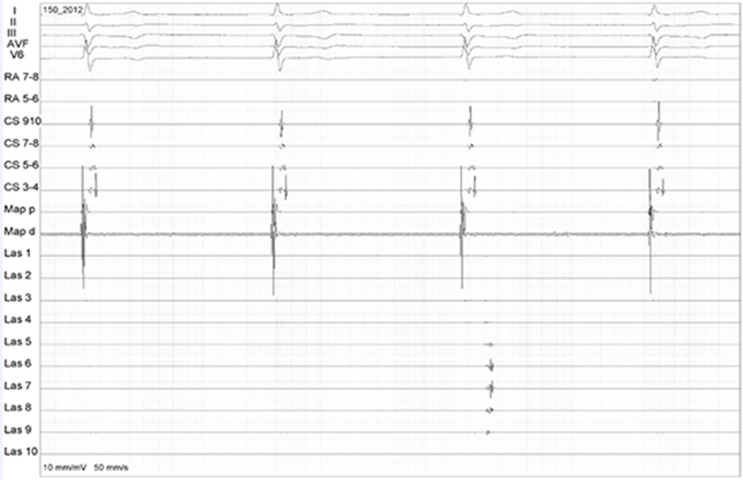
Figure 3. Posterior wall isolation during upon linear lesion completion. The EGMs in the circular catheter suddenly disappear (arrow).
Figure 4. Dissociated potentials in the posterior wall after ablation (arrow), confirming isolation.
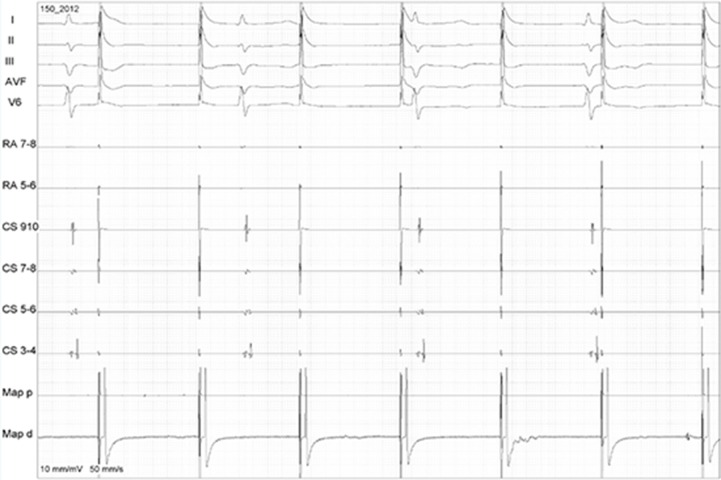
Figure 5. Demonstration of exit block after sinus rhythm restoration. The ablation catheter is placed inside the box and pacing can’t capture the LA.
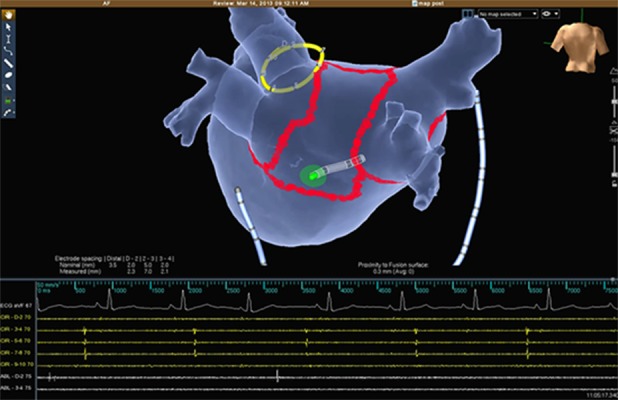
Figure 6. EGMs demonstrating the exit block by pacing (Map – arrows) inside the box lesion set (no LA capture while pacing the posterior wall).
After sinus rhythm resumption, gaps in the lines were mapped (Figure 7) and dormant conduction was checked in the circular catheter by adenosine infusion (Figure 8). We used 18mg rapid bolus while monitoring each pulmonary vein and the posterior wall using the circular mapping catheter. A high dose of intravenous isoproterenol (20 μg/ min) was infused during ten minutes, aiming at triggering ectopic foci located outside the pulmonary veins, which, when present, were mapped and ablated. Finally, the cavotricuspid isthmus was ablated to prevent typical atrial flutter and the superior vena cava was isolated if there wasn’t phrenic nerve capture by the distal dipole of ablation catheter using 20V output.
Figure 7. Gap in the roof ablation line detected by activation mapping during sinus rhythm. Activation in the posterior wall comes from the roof (white) towards the bottom.
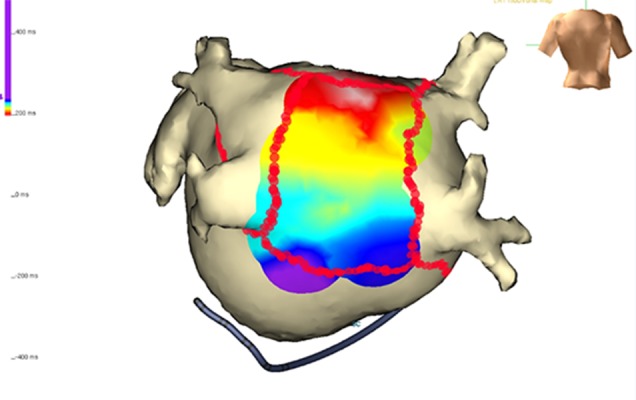
Figure 8. Evaluation of dormant conduction in the ablation lines by adenosine infusion. In this case, transient conduction in the posterior wall is observed (arrow) and further ablation is performed until this is no longer present.
Right after withdrawing the sheaths from the left atrium, systemic heparinization was reverted with protamine to achieve an ACT < 200 seconds. Vascular compression was performed for 20 minutes, and compression was maintained for six hours.
Antiarrhythmic drugs and oral anticoagulation were continued for 1 and 3 months, respectively, after the procedure. Ambulatory follow-up was performed at 1, 3, 6 and 12 months. 7-day holter monitoring and 12 lead ECGs were performed on a regular basis and in case of symptoms.
Results
PVI and posterior wall isolation was achieved in all patients. Dormant conduction was detected in 12 pts (48%) after adenosine challenge and was mapped to the roof (4 pts), inferior (5 pts) and PV circumferential lines (3 pts). Further ablation abolished dormant conduction in all cases by targeting the earliest eletrogram in the circular catheter, which was placed wide open in the posterior wall (25mm), covering almost entirely the box lesion set.
All patients experienced esophageal temperature elevations during RF to perform the inferior line. We stopped ablation whenever it reached 39 degrees and power was titrated down. Several short cycles of RF were performed until the lines were complete and the posterior wall was isolated.
No intra-procedural complications were detected. Mean left atrial ablation time was of 40±9 min. Reversion to sinus rhythm during ablation occurred in 4 patients (20%). Sinus rhythm was restored by electrical cardioversion in all others. The decision to stop or maintain long term oral anticoagulation was taken using our post-AF ablation management protocol.[9]
After an average follow-up of 16 ± 2 months, 20 patients (80%) were free of any atrial arrhythmia recurrences (18 of whom off drugs). Five patients (20%) had sustained atypical flutter and required a new ablation procedure. All these patients had mitral isthmus dependent flutters and no electrical conduction in the PVs or posterior wall were detected. An endocardial and coronary sinus mitral isthmus line interrupted the arrhythmia in all. 2 patients (40%) had atrial flutter recurrences and were rescheduled for a new ablation procedure.
No complications were detected during follow-up and specifically we had not observed an increase in esophageal symptoms due to the more extensive posterior RF lesions.
Discussion
PVI is the cornerstone of catheter ablation of AF,[1] being effective in elimination of the initiation triggers[10] in paroxysmal AF, with a success rates ranging from 60% to 80%. The most common technique for PVI includes lesions placed at the antral level, far from the pulmonary veins.[11,12] However, in long standing AF PVI is frequently not enough to prevent AF recurrences and further linear lesions are required. The role of these in paroxysmal AF have been studied[4] but due to different techniques and outcomes evaluated, lack of confidence about the use of these linear lesions arose among electrophysiologists.
Tamborero et al[13] reported a randomized comparison between 120 patients with paroxysmal and persistent AF submitted to AF ablation through wide antral PVI with or without superior and inferior lines connecting the contralateral lesions, creating a box lesion that isolated the whole posterior wall. After 9.8 months of follow-up there was no difference in arrhythmia-free survival between the two strategies. However, non-paroxysmal AF patients accounted for less than 30% of cases. Furthermore, isolation of at least one pulmonary vein or the posterior wall was unsuccessful in 9% of the patients.
In our series, all patients had persistent or long standing persistent AF with LA remodeling. In this scenario, the role of the posterior wall in AF maintenance might be important. Recently, Sanders and colleagues[14] have reported a long term evaluation of 27 chronic AF cases with successful isolation of the posterior wall and PVs. The procedure was performed in a very similar fashion as we did, with PVs isolated in pairs and connected by roof and floor lines. The AF-free rate after the first procedure was 44%. After 21 months of follow up, 63% of patients maintained sinus rhythm without antiarrhythmic drugs. We further tested the lines by pacing maneuvers and adenosine infusion – whenever transient conduction was detected, further ablation lesions were placed until its elimination.
Although the ablation approach we used seemed rational, because of proven entry and exit block, different strategies have also shown positive outcomes. Lim and colleagues[15] showed that a single ring ablation around the four PVs, passing through the anterior aspect of the veins, left atrial roof and lower posterior wall is an option to wide antral PVI, with lower 2-year AF recurrence rates (74% vs. 61%, respectively). When the recurrences of organized atrial tachyarrhythmias were evaluated, the group undergoing single ring isolation plus a mitral isthmus line had the best 2-year arrhythmia-free rate (71% vs. 60%). This speaks for a possible role of left posterior wall in maintaining AF despite PVI. Another advantage of this technique is the relatively low use of RF in the posterior wall, with less risk for esophageal termal injury.[16]
Providencia and colleagues[17] reported the use of a single ring left atrial posterior wall isolation in a patient with an anusual anatomical variant of PV drainage. In this case, a common inferior trunk posed difficulties to a traditional approach. So, a triangle-shaped single lesion encircling all PVs (right superior, left superior and the common inferior trunk) allowed complete isolation, maintaining sinus rhythm after 3 months of follow –up.
The impact of a potential autonomic modulation and changes in sympathovagal balance caused by posterior wall isolation has also been evaluated. Yamaguchi et al[18] performed a series of 92 PVI and posterior wall isolations (82% Paroxysmal AF) using non-contact mapping and undergoing ambulatory holter Heart Rate Variability evaluation at 3, 6 and 12 months after the procedure. Long-term heart rate variability attenuations were observed in all patients without AF recurrence, but not in those with AF recurrences. These findings were maintained at 12 months in patients with no AF recurrence, suggesting a possible contribution of autonomic modulation to sinus rhythm maintenance.
Conclusions
Complete LA posterior wall isolation on top of PVI is associated with good outcomes in patients with persistent and long-standing persistent AF when performed using meticulous bidirectional isolation criteria and adenosine infusion.
Recurrences occur predominately as perimitral flutter, without gaps in the posterior wall, suggesting that the previously reported increased risk of organized atrial tachyarrhythmias after posterior wall box isolation may be related to incomplete posterior lines. However, the value of prophylactic mitral isthmus line complementing the box lesion at the first procedure is yet to be proven.
Disclosures
None.
References
- Raviele Antonio, Natale Andrea, Calkins Hugh, Camm John A, Cappato Riccardo, Ann Chen Shih, Connolly Stuart J, Damiano Ralph, DE Ponti Roberto, Edgerton James R, Haïssaguerre Michel, Hindricks Gerhard, Ho Siew Y, Jalife José, Kirchhof Paulus, Kottkamp Hans, Kuck Karl H, Marchlinski Francis E, Packer Douglas L, Pappone Carlo, Prystowsky Eric, Reddy Vivek K, Themistoclakis Sakis, Verma Atul, Wilber David J, Willems Stephan. Venice Chart international consensus document on atrial fibrillation ablation: 2011 update. J. Cardiovasc. Electrophysiol. 2012 Aug;23 (8):890–923. doi: 10.1111/j.1540-8167.2012.02381.x. [DOI] [PubMed] [Google Scholar]
- Todd Derick M, Skanes Allan C, Guiraudon Gerard, Guiraudon Colette, Krahn Andrew D, Yee Raymond, Klein George J. Role of the posterior left atrium and pulmonary veins in human lone atrial fibrillation: electrophysiological and pathological data from patients undergoing atrial fibrillation surgery. Circulation. 2003 Dec 23;108 (25):3108–14. doi: 10.1161/01.CIR.0000104567.72914.BF. [DOI] [PubMed] [Google Scholar]
- Mandapati R, Skanes A, Chen J, Berenfeld O, Jalife J. Stable microreentrant sources as a mechanism of atrial fibrillation in the isolated sheep heart. Circulation. 2000 Jan 18;101 (2):194–9. doi: 10.1161/01.cir.101.2.194. [DOI] [PubMed] [Google Scholar]
- Hocini Mélèze, Jaïs Pierre, Sanders Prashanthan, Takahashi Yoshihide, Rotter Martin, Rostock Thomas, Hsu Li-Fern, Sacher Frédéric, Reuter Sylvain, Clémenty Jacques, Haïssaguerre Michel. Techniques, evaluation, and consequences of linear block at the left atrial roof in paroxysmal atrial fibrillation: a prospective randomized study. Circulation. 2005 Dec 13;112 (24):3688–96. doi: 10.1161/CIRCULATIONAHA.105.541052. [DOI] [PubMed] [Google Scholar]
- Cox J L, Schuessler R B, D'Agostino H J, Stone C M, Chang B C, Cain M E, Corr P B, Boineau J P. The surgical treatment of atrial fibrillation. III. Development of a definitive surgical procedure. J. Thorac. Cardiovasc. Surg. 1991 Apr;101 (4):569–83. [PubMed] [Google Scholar]
- Voeller Rochus K, Bailey Marci S, Zierer Andreas, Lall Shelly C, Sakamoto Shun-ichiro, Aubuchon Kristen, Lawton Jennifer S, Moazami Nader, Huddleston Charles B, Munfakh Nabil A, Moon Marc R, Schuessler Richard B, Damiano Ralph J. Isolating the entire posterior left atrium improves surgical outcomes after the Cox maze procedure. J. Thorac. Cardiovasc. Surg. 2008 Apr;135 (4):870–7. doi: 10.1016/j.jtcvs.2007.10.063. [DOI] [PubMed] [Google Scholar]
- Sternik Leonid, Ghosh Probal, Luria David, Glikson Michael, Shpigelshtein Daniel, Malachy Ateret, Raanani Ehud. Mid-term results of the 'hybrid maze': a combination of bipolar radiofrequency and cryoablation for surgical treatment of atrial fibrillation. J. Heart Valve Dis. 2006 Sep;15 (5):664–70. [PubMed] [Google Scholar]
- Sie H T, Beukema W P, Ramdat Misier A R, Elvan A, Ennema J J, Wellens H J. The radiofrequency modified maze procedure. A less invasive surgical approach to atrial fibrillation during open-heart surgery. Eur J Cardiothorac Surg. 2001 Apr;19 (4):443–7. doi: 10.1016/s1010-7940(01)00595-4. [DOI] [PubMed] [Google Scholar]
- Saad Eduardo B, d'Avila André, Costa Ieda P, Aryana Arash, Slater Charles, Costa Rodrigo E, Inácio Luiz A, Maldonado Paulo, Neto Dario M, Camiletti Angelina, Camanho Luiz E, Polanczyk Carisi A. Very low risk of thromboembolic events in patients undergoing successful catheter ablation of atrial fibrillation with a CHADS2 score ≤3: a long-term outcome study. Circ Arrhythm Electrophysiol. 2011 Oct;4 (5):615–21. doi: 10.1161/CIRCEP.111.963231. [DOI] [PubMed] [Google Scholar]
- Haïssaguerre M, Jaïs P, Shah D C, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N. Engl. J. Med. 1998 Sep 3;339 (10):659–66. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- Koichiro Kumagai. Box Isolation for Atrial Fibrillation. Journal of Arrhythmia. 2011;0:255–267. [Google Scholar]
- Verma Atul. The techniques for catheter ablation of paroxysmal and persistent atrial fibrillation: a systematic review. Curr. Opin. Cardiol. 2011 Jan;26 (1):17–24. doi: 10.1097/HCO.0b013e3283413925. [DOI] [PubMed] [Google Scholar]
- Tamborero David, Mont Lluís, Berruezo Antonio, Matiello Maria, Benito Begoña, Sitges Marta, Vidal Barbara, de Caralt Teresa M, Perea Rosario J, Vatasescu Radu, Brugada Josep. Left atrial posterior wall isolation does not improve the outcome of circumferential pulmonary vein ablation for atrial fibrillation: a prospective randomized study. Circ Arrhythm Electrophysiol. 2009 Feb;2 (1):35–40. doi: 10.1161/CIRCEP.108.797944. [DOI] [PubMed] [Google Scholar]
- Sanders Prashanthan, Hocini Mélèze, Jaïs Pierre, Sacher Fréderic, Hsu Li-Fern, Takahashi Yoshihide, Rotter Martin, Rostock Thomas, Nalliah Chrishan J, Clémenty Jacques, Haïssaguerre Michel. Complete isolation of the pulmonary veins and posterior left atrium in chronic atrial fibrillation. Long-term clinical outcome. Eur. Heart J. 2007 Aug;28 (15):1862–71. doi: 10.1093/eurheartj/ehl548. [DOI] [PubMed] [Google Scholar]
- Lim Toon Wei, Koay Choon Hiang, McCall Rebecca, See Valerie A, Ross David L, Thomas Stuart P. Atrial arrhythmias after single-ring isolation of the posterior left atrium and pulmonary veins for atrial fibrillation: mechanisms and management. Circ Arrhythm Electrophysiol. 2008 Jun 1;1 (2):120–6. doi: 10.1161/CIRCEP.108.769752. [DOI] [PubMed] [Google Scholar]
- Thomas Stuart P, Lim Toon Wei, McCall Rebecca, Seow Swee-Chong, Ross David L. Electrical isolation of the posterior left atrial wall and pulmonary veins for atrial fibrillation: feasibility of and rationale for a single-ring approach. Heart Rhythm. 2007 Jun;4 (6):722–30. doi: 10.1016/j.hrthm.2007.01.034. [DOI] [PubMed] [Google Scholar]
- Providência Rui, Combes Stéphane, Albenque Jean-Paul. Adjusting treatment to pulmonary vein rare anatomic variants: a box lesion for the ablation of atrial fibrillation in a patient with an atypical common inferior trunk. Europace. 2013 Oct;15 (10):1420. doi: 10.1093/europace/eut137. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Yoshio, Kumagai Koichiro, Nakashima Hideko, Saku Keijiro. Long-term effects of box isolation on sympathovagal balance in atrial fibrillation. Circ. J. 2010 Jun;74 (6):1096–103. doi: 10.1253/circj.cj-09-0899. [DOI] [PubMed] [Google Scholar]