Abstract
AF ablation can be curative or reduce the symptoms of AF. However, success rates are suboptimal while procedure times can be long. Since AF ablation depends on excellent tip to tissue contact, ease of use and signal quality, a new ablation catheter (FlexAbilityTM Ablation Catheter, St Jude Medical, St Paul, MN) was designed for use during complex ablation. We report an initial case of its use during paroxysmal AF.
Introduction
We report the case of a 64-year-old woman who was referred to our institution for paroxysmal AF ablation, in which we used a novel ablation catheter (FlexAbilityTM Ablation Catheter, St Jude Medical). The patient had paroxysmal atrial fibrillation for 12 years. She had between 2 and 3 AF symptomatic episodes every month that last from 30 min to 2 hours that terminate without cardioversion. Symptoms included palpitations, shortness of breath, and sometimes syncopal episodes. Different combinations of antiarrhythmic drugs: flecanide, bisoprolol, propafenone and amiodarone were attempted without a significant improvement.
The patient had no underlying heart disease. The last TTE showed a normal left ventricular ejection fraction (60%), no significant valvular disease, and a mildly enlarged LA (17cm2). She was admitted in our department for paroxysmal AF ablation considering that she has short, recurrent, symptomatic, drug-resistant paroxysmal AF. The patient was treated with oral anticoagulation (INR between 2 and 3) since 2002. A trans-esophageal echocardiogram was performed the day prior to the ablation and found a contractile left atrial appendage with no thrombus. Left atrial septum was normal without persistent foramen ovale.
Electrophysiologic Study
An electrophysiology study was performed with patient in the postabsorptive state under light sedation. All antiarrhythmics were discontinued 5 half-life periods. Oral anticoagulation was replaced by heparin 48 hours before and ceased 6 hours before the procedure. After transseptal access, an intravenous bolus of heparin (0.5 mg/kg body wt.) was administered.
A 6-French decapolar catheter (2-5-2mm, Xtrem, ELA Medical, Montrouge, France) was advanced from the right femoral vein and placed in the coronary sinus (CS). After performing the transseptal catheterization, pulmonary veins were mapped with a decapolar circular mapping catheter (Reflexion™ Spiral Catheter, St Jude Medical).
Ablation Catheter
Ablation was performed by using a novel 4-mm, flexible irrigated-tip catheter with 1-4-1 mm interelectrode spacing (FlexAbilityTM Ablation Catheter, uni-directional F curve, St Jude Medical). The ablation catheter uses a flexible tip (Figure 1) designed to conform to the cardiac anatomy. The tip was also designed to provide improved signal quality and better irrigation in the direction of the tissue being ablated. The shaft was designed to improve the ability to get to challenging anatomy.
Figure 1. FlexAbilityTM Catheter tip and handles.
Pulmonary Vein Isolation
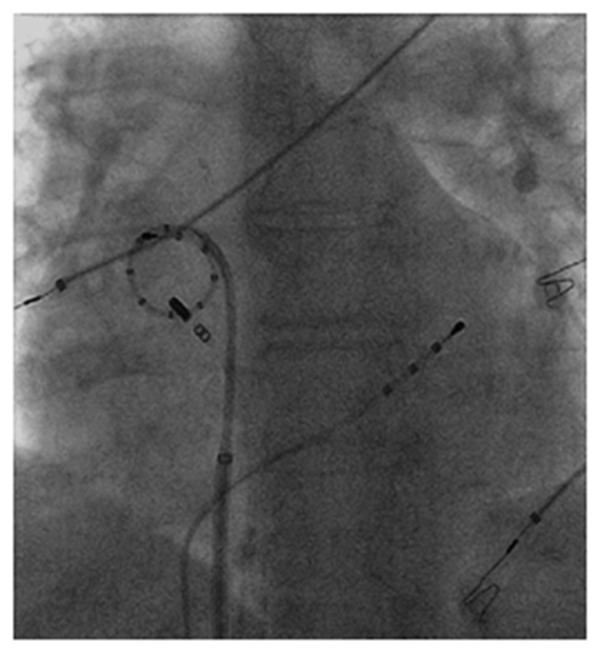
The patient presented into the lab in atrial fibrillation. The techniques used for segmental ostial PV isolation was previously described.[1] The catheter was advanced via transseptal access and manipulated within the left atrium without the aid of a long sheath. Ablation was performed at 1 cm from the ostium of both right PVs as well as the posterior and superior aspects of the left PVs (to minimize any risk of PV stenosis). Ablation continued circumferentially around the PV during ongoing AF to achieve electrical isolation of the PVs. The catheter maintained stable tissue contact and was easy to use navigate around the RIPV (Figure 2).
Figure 2. AP fluoroscopy view demonstrating catheter stability at the inferior margin of the RIPV.
A combination of point lesions (30 to 60 sec per point) and drag lesions were utilized. The irrigation pump (Cool PointTM Pump, St Jude Medical) was set to a constant flow rate of 13 mL/min during radiofrequency (RF) applications and 2mL/min between applications. RF energy was delivered with the generator (AmpereTM Generator, St Jude Medical) programmed in power control with a temperature limit of 45°C. Typically Energy was delivered within the first few millimeters of the anterior portion of the left pulmonary veins to achieve catheter stability and effective disconnection (Figure 3). The catheter was then rotated clockwise to extend the lesion posteriorly to the LIPV ostium. Radiofrequency (RF) energy was prolonged when a change occurred in activation/ morphology of the PV potentials. The stability of the catheter was monitored during RF applications using electrograms and intermittent fluoroscopy.
Figure 3. LAO fluoroscopy view demonstrating ablation catheter stability at the LSPV-LAA ridge.
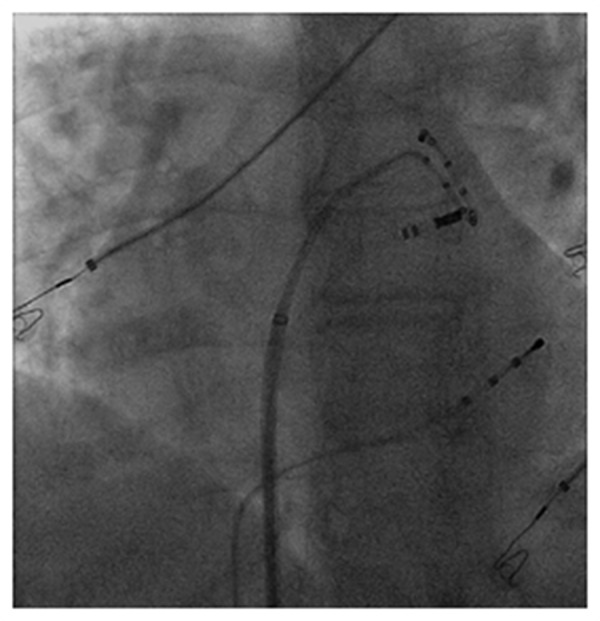
It is often challenging to achieve stability at the LSPV-LAA ridge but the catheter shaft stiffness with the flexible tip helped address this challenge. We felt that when a counter-clockwise rotation was applied to the catheter, the torque was transmitted directly to the catheter tip on the ridge. Additionally the tip conformed to the tissue better than conventional rigid tips (Figure 3).
Electrogram Analysis
Surface electrocardiograms and bipolar intracardiac electrograms were continuously monitored with the use of Lab-System Pro (Bard Electrophysiology, Lowell, MA, USA). Signals were sampled at 1 kHz, and ECG's were filtered at 0.1–50 Hz and 30–250 Hz for all intracardiac signals, displayed at an amplification of 0.1 mV/cm and no 50/60 Hz notch filter.
The FlexAbility Ablation Catheter has a unique 1-4-1 inter-electrode spacing configuration with 4 mm distal tip electrode and 1 mm electrode bands. Given the unique electrode configuration, electrogram quality from bipolar and unipolar electrograms were carefully assessed both prior to and during RF energy application.
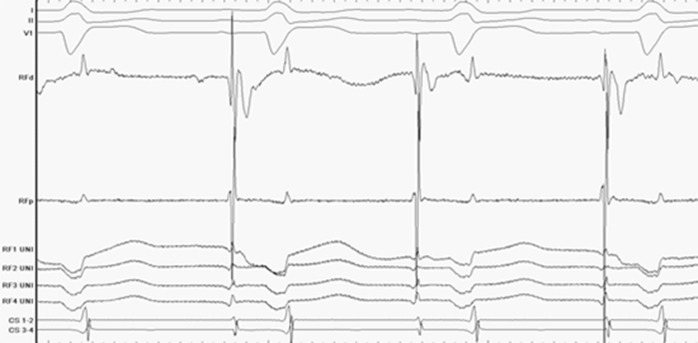
Prior to RF ablation, the bipolar and unipolar signal quality of the ablation catheter electrograms were assessed. The peak-to-peak noise was recorded at .015 mV on the distal ablation electrogram (Figure 4).
Figure 4. Bipolar/unipolar electrograms from the ablation catheter during sinus rhythm.
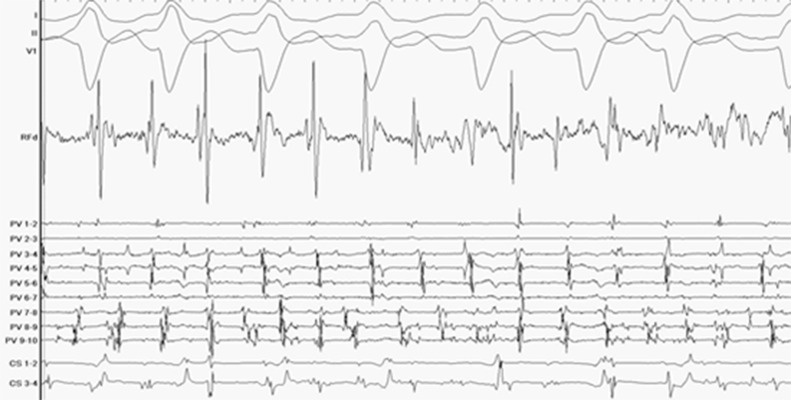
During RF ablation, bipolar electrograms from the distal tip were monitored to confirm stability of the ablation catheter. Additionally, the enhanced signal resolution of the FlexAbility Ablation Catheter during RF energy delivery proved useful to assess the effect of each RF application. During application of RF energy, peak-to-peak noise on the distal ablation electrogram measured .03 mV. (Figure 5).
Figure 5. Electrogram assessment at the LSPV during RF energy delivery while in AF.
Signal quality was exceptionally good during the RF application, which is better than prior catheters.
Procedure Endpoint
The procedure endpoint consisted of PV isolation (PVI) of all four PV's. PVI was achieved by complete elimination or dissociation of all PV potentials as determined by the circular mapping catheter. After PVI, a careful search was performed of the entire circumferential perimeter of each PV, and ablation was performed at sites demonstrating any residual potentials. The final endpoint was demonstrated by entrance and exit block for all pulmonary veins. Subsequent to PVI, no spontaneously occurring premature beats were identified. Procedure time from transseptal access to final PVI was 1 hour 20 min. Total RF duration was 21 minutes. Fluoroscopy time was 19 minutes. There were no complications.
Table 1. Procedure Statistics.
| Patient Age | 64 years |
|---|---|
| Diagnosis | Paroxysmal Atrial Fibrillation |
| Underlying Heart Disease | None |
| LV Ejection Fraction | 60% |
| LA Size | 17 cm2 |
| Procedure Time | 80 min |
Conclusions:
In this initial case report, the FlexAbility Catheter allows for safe and effective PVI with excellent signal quality and maneuverability. Future studies targeting VT and AF will determine if this catheter improves long-term success rates and reduces procedure times.
Disclosure
None.
References
- Haïssaguerre Michel, Sanders Prashanthan, Hocini Mélèze, Hsu Li-Fern, Shah Dipen C, Scavée Christophe, Takahashi Yoshihide, Rotter Martin, Pasquié Jean-Luc, Garrigue Stéphane, Clémenty Jacques, Jaïs Pierre. Changes in atrial fibrillation cycle length and inducibility during catheter ablation and their relation to outcome. Circulation. 2004 Jun 22;109 (24):3007–13. doi: 10.1161/01.CIR.0000130645.95357.97. [DOI] [PubMed] [Google Scholar]