Abstract
Current approaches for the ablation of atrial fibrillation are often effective, but only partially rooted in mechanistic understanding. Accordingly, they are unable to predict whether a given patient will or will not do well, or which lesions sets should or should not be performed – in any given patient. This goal would require clearer mechanistic definition of what sustains AF after it has been triggered (i.e. electrophysiological substrates). There are two schools of thought. The first proposes disorganized activity that self-sustains with no ‘driver’, and the second describes drivers that then cause disorganization. Interestingly, these mechanisms can be separated in human studies by mapping approach – proponents of the disorganized hypothesis studying small atrial areas at high resolution, and proponents of the driver model studying wide fields-of-view at varying resolutions. Focal impulse and rotor modulation (FIRM) mapping combines a wide field of view with physiologically based signal filtering and phase analysis, and has revealed that human AF is often sustained by rotors. In the CONFIRM Trial, targeting stable AF rotors/sources for ablation improved the single-procedure efficacy for paroxysmal and persistent AF over conventional ablation alone, as now confirmed by independent laboratories. FIRM mapping gives a mechanistic foundation to predict whether any selected lesions should intersect AF sources in any given patient and which mechanisms may cause recurrence. Rotors of varying characteristics have now been shown by many groups. These insights have reinvigorated interest in AF mapping, and rationalizing these findings will likely translate into improved therapy for our patients.
Introduction
Atrial fibrillation (AF) is ‘the most common sustained arrhythmia’ with an increasing impact on global health.[1] Whatever the precipitant – and there is increasing opinion to refute ‘lone’ AF[2] – the consequences are severe, with thromboembolic disease in the form of strokes and death being the most devastating.
Catheter ablation promises durable elimination of AF, yet there is considerable room for improvement as single procedure success rates for paroxysmal AF are only 50-60% in recent multicenter trials and lower for persistent AF.[3] Recent evidence suggests that this reflects incomplete understanding of ablation targets as well as ineffective delivery of ablation lesions or disease progression. In this review we shall outline mechanistic insights into AF guided by focal impulse and rotor modulation (FIRM), to address the question of whether this physiologically-tailored approach offers real progress over existing mapping approaches in the field.
Current Concepts In AF
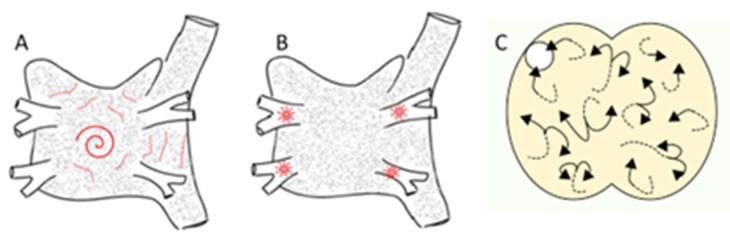
The mechanisms underpinning AF have been the subject of speculation and intense research for well over a century. Classical teaching requires a trigger to encounter a substrate that sustains an arrhythmia, and this applies equally to AF. There are three main mechanistic theories for AF substrates (figure 1), discussed in chronological order:
Figure 1. Proposed mechanisms in AF. A) spiral wave re-entry with peripheral fibrillatory conduction. B) focal discharges from the pulmonary veins. C) multiple wavelets. Adapted from.[12,52].
Re-entry
The requirement of re-entry, or circus movement of an impulse to maintain AF was first suggested by Sir Thomas Lewis as early as 1920. Re-entry has certain pre-requisites, all of which are present in AF. Re-entry is affected by the nature of the obstacle circumscribed by the wavefront (anatomical or functional) and by the shape of the wavefront itself. Re-entry around an anatomical obstacle, such as in atrial flutter, is rarely if ever demonstrated in AF, and so it is unclear if ‘macro-reentrant’ circuits described in early human AF mapping are indeed anatomical.[4] Functional reentry by the leading circle hypothesis[5] suggests that a wavefront encircles a region of tissue that remains refractory because constant input from reentry keeps it continuously depolarized.
A rotor is a specific form of functional reentry, first shown in isolated fibrillating ventricular muscle using optical mapping,[6] that pivots around a “core” with extreme conduction slowing. Jalife subsequently showed that AF in a sheep model can be caused by spiral wave re-entry around a central rotor that spins rapidly to produce complex fibrillatory patterns.[7] Of note, rotors may superficially resemble macro-reentry – but are quite different. In macro-reentry, a stable circuit revolves around an inert obstacle. In AF, the rotor core (‘singularity’) is the primary mechanism, and emanating spiral waves rotate in variable fashion and break down into disorganized AF (‘fibrillatory conduction’). In addition, the rotor core precesses (‘wobbles’), as described by Davidenko and Jalife in animals in 1992[6] and recently in humans.[8]
Focal Discharges
Focal discharges were produced experimentally by Scherf by the application of aconitine[9] and implicated in atrial arrhythmogenesis, with the caveat that such discharges would have to sustain for years to drive persistent AF. The importance of focal beats to AF underwent a revolution in 1998 with the demonstration that pulmonary veins may harbor rapidly firing sources in patients with repetitive frequent paroxysms of AF,[10] contradicting the concept of multiple wavelets with no driver (see next section), in whom focal ablation could eliminate AF.[11] This work led to current approaches for pulmonary vein isolation for AF.[12]
Multiple Wavelets
In the late 1950s Gordon Moe used computational and canine models of AF to show that multiple small wavelets meandering through the atrium could sustain AF[13] given a minimum mass of tissue to propagate. He initially suggested the need for 20-30 wavelets, with trajectories based on regional refractory periods. Allessie subsequently reported multiple meandering wavelets in elegant animal and human studies, using high-density epicardial and endocardial mapping plaques,[5,14-16] albeit of small atrial areas and without proving that this disorganization drives AF. Recent attempts to recreate Moe’s original work have questioned his original interpretation, suggesting instead that focal drivers may cause vagally induced AF in the canine heart.[17] These possibly model-dependent results may explain the dichotomy of opinion that exists today for human AF.
The Applicability Of Existing Cardiac Mapping To AF
Traditional mapping approaches have been applied to AF, but with often contradictory and often unsatisfying clinical results.
Activation Mapping
This approach maps activation across the heart, identified when voltage or another parameter (e.g. its first derivative) crosses a threshold. The speed and direction of wavefront propagation can be inferred from these maps, but only when wavefronts are spatially coherent. Notably, varying activation of any given electrode by multiple, possibly colliding and varying wavefronts, as in AF, limits the insights available from activation mapping. Activation mapping from clinical electrograms is also problematic, whether the electrogram is complex or not, because it is sensitive to errors in assigning ‘local activation time’ in AF from far field activity or missed local activity.[18] Accordingly, maps based upon action potentials often show considerable organization,[19] while maps based upon unipolar or bipolar electrograms often show highly complex patterns that have been used as evidence in support of multiple wavelet re-entry.[20]
Entrainment Mapping
Entrainment mapping is specifically designed to reveal and penetrate an excitable gap in stable re-entrant tachyarrhythmias, as in ventricular tachycardia[21] and atrial flutter.[22] This approach is of limited value in AF, since functional reentry or rotors have no excitable gap per se.
Frequency Mapping
As opposed to time domain analyses (voltage and activation mapping), frequency domain analysis uses approaches such as the Fast Fourier Transform to reveal the frequency content of signals a surrogate of rate. This approach can deal with complex electrograms, and has been used to construct dominant frequency (DF) maps in AF that often reveal stable gradients that track the natural history of disease.[23] Notably, animal models of AF exhibit stable drivers with a high DF,[24] and similar spatial gradients have been observed in human AF that may be successful ablation targets.[25] Again, DF analysis may be less affected by noise and artifact if applied to action potentials rather than clinical bipolar signals,[26,27] and thus may be combined with other methods to probe the AF substrate.
Phase Mapping
Phase mapping is a specific mathematical approach that identifies regions of tissue based on their spatial and temporal periodicity, and can identify periodic rotations (i.e. rotors) even in a complex non-coherent milieu. Seminal work by Gray, Jalife et al.[28] used this approach to greatly enhance the detection of rotors from optically mapped atrial fibrillation in the sheep. Whilst rotors were first demonstrated in AF using this approach in animal models (figure 2), the toxicity of potentiometric dyes and mechano-electric uncouplers – both prerequisites for optical mapping – currently preclude their use in humans. However, FIRM mapping was based upon the insights derived from optical mapping applied to human AF.
Figure 2. Phase mapping from an isolated sheep heart. A) Optical action potentials recorded using voltage sensitive dyes. B) Phase plots of F(t-2) vs. F(t), where time, t, is the frame number and 2 is the number of lagging frames. The colorbar represents the variation in phase. Two-dimensional phase map of a clockwise rotor during AF. From.[53].
FIRM – A New Approach To Map Human AF
Development Of FIRM
FIRM mapping provides panoramic mapping of the atria, to avoid missing localized regions of interest, with sufficient spatial resolution to identify AF rotors or focal sources, enhanced by physiologically-based noise filtering. First, we mapped human AF globally, in contrast to prior mapping of 1-10 cm2 areas[29,30] that were assumed to represent the entire atrial surfaces (areas of 110-138 cm2[31]). Rather than use non-contact systems[32] we selected contact recordings. Basket catheters map the majority of each atrium, at 4-10 mm resolution that is theoretically capable[33] of mapping the smallest human reentrant circuits of 4-5 cm perimeter.[34,35] Clinically, ablation lesions of ≈7 mm diameter also provide a relevant ‘resolution’ requirement.
Second, in developing FIRM we used monophasic action potentials (MAP),[36] since AF signals are poorly represented by bipolar signals[18] due to potential AF signal cancellation or artifact between non-coherent waves on each pole of a bipole or between a unipolar recording and its indifferent pole. MAPs represent local activation and recovery more accurately than clinical alternatives.[37] Third, we developed signal processing methods to filter AF signals based on the dynamics of repolarization and conduction in human atria.[26,37-39]
Practical Approach
A commercially available basket catheter (FIRMap, Topera, Palo Alto, CA; Constellation, Boston Scientific, MA) is used to map AF. Ideally, the basket covers >80-90% of the atria, and typically the basket catheter is sized to cover the left atrium. Suboptimal basket deployment (electrode non-contact) may cause AF sources to be missed. Mapping is performed first in right atrium, followed by FIRM-guided elimination of rotors/focal sources. This process is then repeated in the left atrium.
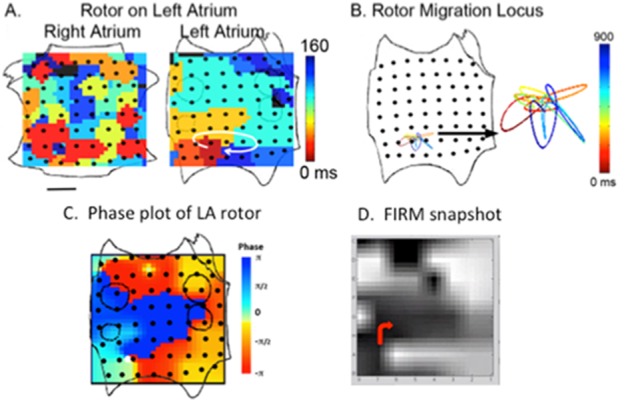
FIRM mapping produces movies of AF propagation that are the primary tool during a case. Snapshots (isochrones) shown in manuscripts are for illustration, and are not used to target ablation since they do not depict rotor precession during and between cycles nor the complexity of the fibrillatory milieu. In FIRM maps, the LA is opened at its “equator” through the mitral valve, and the RA opened along a central meridian through the tricuspid valve (figure 3).
Figure 3. Stages in FIRM mapping. Right atrium basket is opened vertically on tricuspid valve and left atrium is opened horizontally along mitral valve. A) Rotor in left atrium visualized with isochrones. B) Clockwise rotation and precession of rotor over a small locus. C) Phase plot of same rotor showing convergence of phase at rotor core. D) Snapshot from FIRM movie depicting phase and direction of rotor in red. From.[36].
Clinically, AF rotors or focal drivers observed in FIRM maps are diagnosed as sources and targeted for ablation only if they remain in reproducible locations (i.e. spatial stability) for minutes (i.e. temporal stability). This definition excludes transient activity that we considered would be difficult to ablate. This observation also increases our confidence in FIRM maps, as it corroborates data showing spatially stable gradients and propagation patterns in several studies of human AF.
The total FIRM-guided ablation time for the typical 2-3 sources in any given patient is 15-20 minutes. Ablation is performed over each source precession area of ≈2-3 cm2 on phase mapping, requiring an average of 5-10 ablation lesions.[40] The endpoint of FIRM-guided ablation is source elimination on repeat FIRM-maps. Once all right atrial rotors or focal sources are eliminated, this process is repeated in left atrium.
Clinical Outcomes of FIRM Guided Ablation
Confirm
The scientific proof of any mechanism requires intervention to eliminate said mechanism. The CONFIRM trial (CONventional ablation with or without Focal Impulse and Rotor Modulation)[41] enrolled 92 subjects undergoing 107 consecutive ablation procedures for drug-refractory AF under an IRB-approved protocol. Cases were prospectively enrolled in a two-arm design: FIRM-guided patients underwent ablation at sources followed by conventional ablation (n=36), whereas FIRM-blinded patients underwent conventional ablation only (n=71) with FIRM mapping performed off-line and not used to guide ablation. This was a tertiary care AF population, with persistent AF present in 81% of the FIRM-guided group, and in 66% of the FIRM-blinded group. The primary long-term endpoint was defined as freedom from AF for up to two years after a single procedure, defined as <1% burden using continuous implanted ECG monitors (monitoring 100% of 1 year), or AF <30 seconds on quarterly event monitors (monitoring 7.8% of 1 year).
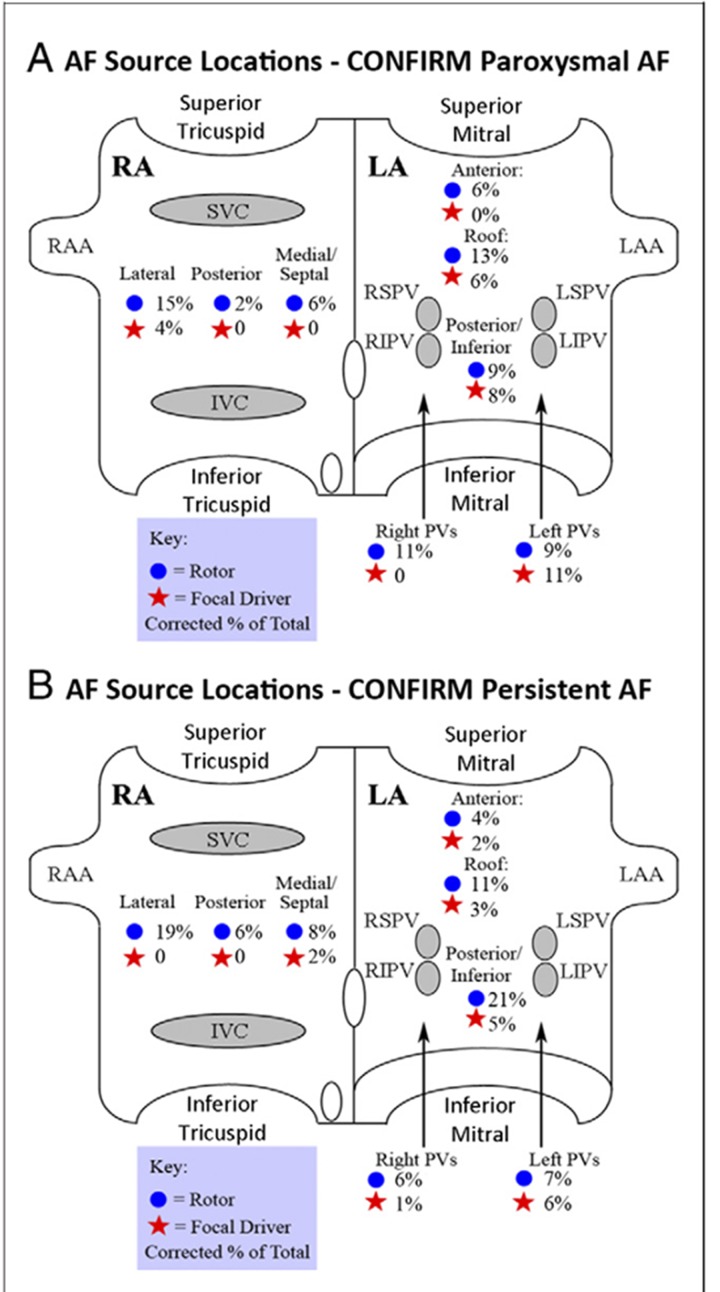
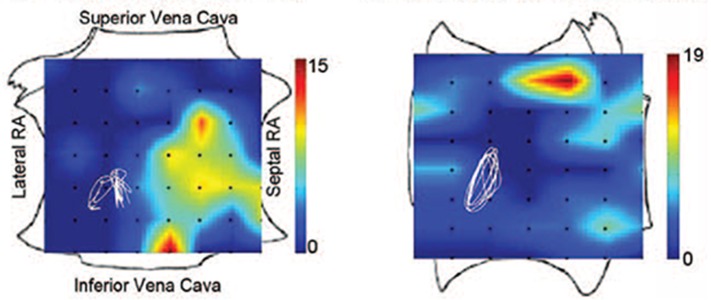
AF sources were documented in 97% of cases (98 of 101) with sustained AF, each subject demonstrating 2.1±1.0 sources (median 2, interquartile range [IQR] 1-3). The number of simultaneous AF sources was higher in persistent than paroxysmal AF (2.2±1.0 vs. 1.7±0.9; median 2.0 vs. 1.0; p=0.03). Notably, stable AF sources lay at diverse patient-specific locations (figure 4) in the LA (76%) and RA (24%). Left atrial sources were found at widespread locations, including sites outside the PVs, as well as the posterior, inferior, and roof regions. RA sources included the inferolateral, posterior, and septal regions typically away from the superior vena cava.
Figure 4. Location of focal sources and rotors in patients enrolled in the CONFIRM trial in A) paroxysmal and B) persistent AF. From.[41].
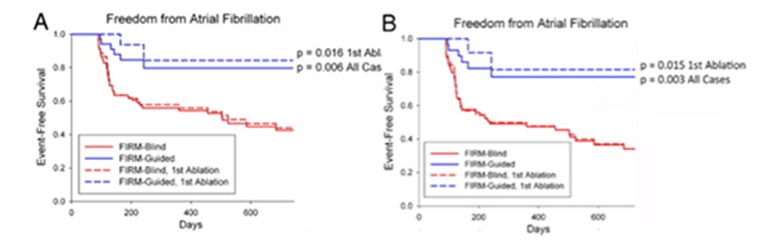
By intention-to-treat analysis, freedom from AF after a single procedure was higher for FIRM-guided than conventional ablation (82.4% vs. 44.9%; p= 0.001) after a median of 273 days follow-up censored at first recurrence (IQR: 132-681 days). Freedom after a single procedure from any atrial tachyarrhythmia was also higher in FIRM-guided than FIRM-blinded cases. These results were achieved despite more rigorous follow-up in FIRM-guided than FIRM-blinded patients (figure 5).
Figure 5. Cumulative freedom in CONFIRM from atrial fibrillation, in all cases and in those at first ablation for (A) the entire population and (B) the population off anti-arrhythmic medications. From.[43].
Very long-term analysis of all patients in CONFIRM recently showed that the benefits of FIRM plus PVI are maintained at 3 years (median follow up 873 days) compared to the expected attrition in success with PVI alone (figure 6).[42]
Figure 6. Very long term results from CONFIRM trial. From.[42].
On-Treatment Analysis Of CONFIRM
The mapping of rotors and focal sources in all patients in CONFIRM enabled an on-treatment analysis of AF source ablation, i.e. to determine outcome when sources were ablated by any means (directly by FIRM or coincidentally by anatomical lesions) or not. In this on-treatment analysis, freedom from AF was highest when all sources were eliminated, intermediate when some were eliminated, and lowest when all sources were missed.[43] This study suggests that rotor elimination was the key to successful AF ablation. This mechanistic concept was tested prospectively in early data from the PRECISE trial (Precise Rotor Elimination without Concomitant pulmonary vein Isolation for the Successful Elimination of Paroxysmal AF), a multicenter single-arm trial of FIRM ablation at sources only (without PVI). Preliminary results from PRECISE showed that FIRM-only ablation provided >80% elimination of paroxysmal AF.[44] These results support FIRM-mapped AF rotors and focal sources as a primary mechanism for AF.
Multicenter FIRM Registry
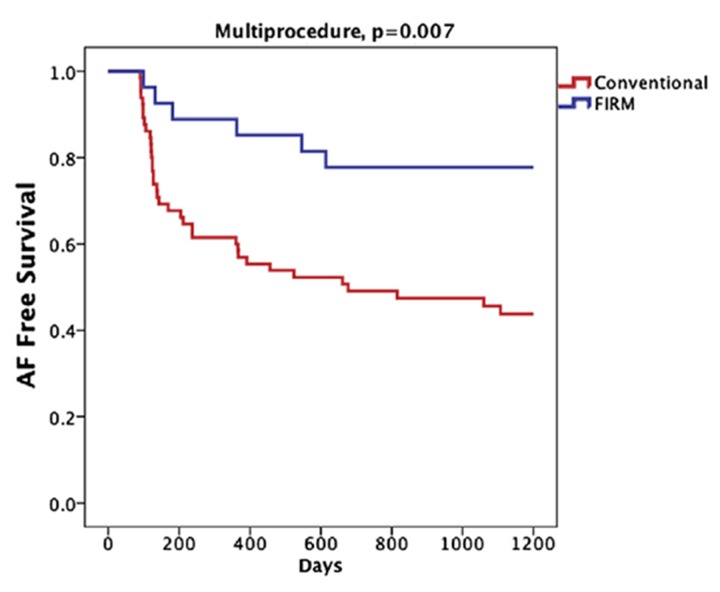
Independent centers have now confirmed acute[45] and long-term outcomes from FIRM-guided ablation. In a recent prospective evaluation, Miller et al.[40] examined the first n=78 consecutive patients undergoing FIRM guided ablation for AF with >1 year follow-up at 10 centers, excluding the original San Diego center. The population had age 61±10 years, n=23 had paroxysmal AF, n=48 had persistent AF and n=7 had longstanding persistent AF. All patients exhibited AF sources, for an average of 2.3±0.9 concurrent AF rotors or focal sources per patient. Patients were treated by ablation of all sources, requiring a total of 16.6±11.7 minutes, followed by conventional ablation. On >1 Year follow up with a 3 month blanking period and no repeat procedures (median time to 1st recurrence: 245 days, IQR 145-354), single-procedure freedom from AF was 80.5%, similar for persistent and paroxysmal AF (p=0.89). The authors concluded that FIRM-guided ablation has a short learning time, and that elimination of AF rotors and focal sources produced freedom-from-AF of ≈80% at 1 year at centers new to FIRM. No safety issues were identified, with no reported cases of thromboembolism or perforation related to basket catheters.
Limitations Of FIRM Mapping
FIRM mapping requires a learning curve to interpret the visual propagation videos which are the primary guide to ablation targets, although in a multicenter study centers were early in their experience.[45] Whilst the basket catheter is typically stable within the atria, registration errors if the basket moves between mapping and ablation can affect results and so steps must be taken to track position (e.g. biplane cineangiography or electroanatomic mapping).
Contact of the basket with the atrial wall is a major concern, and must be optimized prior to FIRM mapping that may require basket repositioning or even resizing. Many authors[46,47] recognize that atria >55-60 mm diameter currently pose a problem (this is the current size of the Constellation basket). New basket designs may reduce this limitation. We use fluoroscopy, intracardiac echo (if already used) and electrogram quality to determine contact. Basket coverage may under-represent certain areas, notably inter-atrial septum, appendage and vena cava, due to splaying of the splines and unequal electrode spacing, that can also be accommodated by repositioning the basket.
Future Directions
Comparison with Non-FIRM methods to detect rotational circuits in AF
Since CONFIRM was reported, several groups have shown rotational activity and rotors in human AF.[30,48,49] These reports used various mapping techniques, some using non-contact body surface electrodes to create virtual electrograms[48] and others using various analyses of contact mapping including traditional activation mapping – despite its caveats in AF. It is not surprising that these divergent approaches different characteristics of rotation, including case reports of rotors[48] which repeatedly return to spatially circumscribed areas in the atria. Other groups have detected only focal sources.[47] As with FIRM, validation of each new tool will require mapping before/after each localized ablation set to confirm that the feature was abolished. This introduces an unanticipated limitation of the use of AF termination during non map-guided ablation. Without defining a mechanistic target or mapping it pre/post ablation, it is unclear if AF termination after potentially widespread and lengthy ablation (>40-50 minutes) reflects just the last lesion(s) or some cumulative aspects of prior atrial modification. Such termination is thus difficult to compare to results from driver-focused localized intervention. Studies comparing these strategies – by continuous mapping – are needed. Such studies are ongoing.[50]
Integrating FIRM with existing concepts of AF
The identification of stable rotors in human AF by FIRM mapping reconciles diverse clinical observations. How do these ideas fit with existing models? What role is there for other substrate and trigger based methods of AF ablation? If rotors are a prevalent mechanism for AF maintenance, then there should be a more robust electrogram surrogate for these initiating processes than currently proven. In particular, this may be based on stereotypic changes in conduction and wavefront propagation that appear to precede AF rotor formation. However, in CONFIRM there was no relationship between rotor sites and electrogram fractionation (figure 7), and so novel electrogram indices of AF driver sites are needed.
Figure 7. Rotor precession (white) in right (A) and left (B) atrium does not overlap with CFAE areas on NavX map. From.[8].
There are numerous other putative stabilizing mechanisms in human AF,[2] which likely have relevance in relation to the driver/disorganization debate – autonomic innervation, fibrosis, connexin distribution and cellular calcium handling are a few examples of mechanisms that are currently being related to regions of relative organization (possible AF drivers) or disorganization in various studies. It is likely that many of these concepts will converge in the near future – for instance, the locations of autonomic ganglia coincide with some areas ablated during PVI and WACA, and their resultant shortening of action potential duration locally but also widely within the atria may stabilize localized reentry.[51] Clearly, further studies are needed to better link these mechanistic schools of thought.
Summary Of Conventional vs. FIRM Mapping
Table 1. FIRM – Real progress over conventional techniques.
Table 1. FIRM – Real progress over conventional techniques.
| Conventional | FIRM | |
|---|---|---|
| Single procedure success, 1 year | ≈50% | ≈80% |
| Proof of success | Some implanted ECG Intermittent Holter recording | Many (majority) implanted ECG |
| Multicenter | ✔ | 14 centers, ~200 reported cases |
| Ability to predict who will do well/recur? | ✖ | ✔ |
| Ability to explain why patients do well/recur? | ✖ | ✔ |
| Defined patient specific mechanism | Triggers – Sometimes defined Sustainers – rarely defined | Defined consistently. Validated by mapping pre/post ablation. |
| Basic science supports mechanisms | ✔/✖ | ✔ |
| Future work |
|
|
Conclusion
Focal Impulse and Rotor Modulation (FIRM) mapping provides a mechanistic approach to map and ablate AF. Demonstration of rotors and focal sources in AF by FIRM builds upon decades of bench-to-bedside studies, and explains divergent clinical results that are difficult to explain by other hypotheses. The preliminary results of FIRM-only ablation in the PRECISE trial confirm that stable AF rotors are important mechanisms for human AF, and provide a framework to predict which lesion sets may or may not be effective in each individual. The results of the CONFIRM trial and multicenter registry show that adding AF source elimination to trigger ablation provides high single-procedure efficacy on term follow-up. Future studies should explain why FIRM-guided ablation is not always effective, and whether this reflects undetected sources, suboptimal source elimination or other mechanisms. Ongoing randomized clinical trials will define the efficacy of FIRM-guided ablation versus conventional ablation. We are hopeful that renewed focus on the mechanisms of human AF will accelerate progress in our understanding and rapidly translate into better therapies for our patients.
Disclosure
This work was supported by grants to Dr Narayan from NIH (HL 83359, HL103800). Dr. Narayan is co-author of intellectual property owned by the University of California Regents and licensed to Topera Inc. Topera does not sponsor any research, including that presented here. Dr. Narayan holds equity in Topera, and reports having received honoraria from Medtronic, St. Jude Medical, and Biotronik. Drs. Zaman, Lalani, Schricker and Krummen, and Mr. Trikha report no conflicts.
References
- 1.Chugh Sumeet S, Havmoeller Rasmus, Narayanan Kumar, Singh David, Rienstra Michiel, Benjamin Emelia J, Gillum Richard F, Kim Young-Hoon, McAnulty John H, Zheng Zhi-Jie, Forouzanfar Mohammad H, Naghavi Mohsen, Mensah George A, Ezzati Majid, Murray Christopher J L. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014 Feb 25;129 (8):837–47. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wyse DG, Van Gelder IC, Ellinor PT, Go AS, Kalman JM, Narayan SM, Nattel S, Schotten U, Rienstra M. Lone Atrial Fibrillation: Does It Exist? A “White Paper” of the Journal of the American College of Cardiology. J Am Coll Cardiol . 2014;0:0–0. doi: 10.1016/j.jacc.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ganesan Anand N, Shipp Nicholas J, Brooks Anthony G, Kuklik Pawel, Lau Dennis H, Lim Han S, Sullivan Thomas, Roberts-Thomson Kurt C, Sanders Prashanthan. Long-term outcomes of catheter ablation of atrial fibrillation: a systematic review and meta-analysis. J Am Heart Assoc. 2013 Mar 18;2 (2):e004549. doi: 10.1161/JAHA.112.004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cox J L, Canavan T E, Schuessler R B, Cain M E, Lindsay B D, Stone C, Smith P K, Corr P B, Boineau J P. The surgical treatment of atrial fibrillation. II. Intraoperative electrophysiologic mapping and description of the electrophysiologic basis of atrial flutter and atrial fibrillation. J. Thorac. Cardiovasc. Surg. 1991 Mar;101 (3):406–26. [PubMed] [Google Scholar]
- 5.Allessie M A, Bonke F I, Schopman F J. Circus movement in rabbit atrial muscle as a mechanism of trachycardia. Circ. Res. 1973 Jul;33 (1):54–62. [PubMed] [Google Scholar]
- 6.Davidenko J M, Pertsov A V, Salomonsz R, Baxter W, Jalife J. Stationary and drifting spiral waves of excitation in isolated cardiac muscle. Nature. 1992 Jan 23;355 (6358):349–51. doi: 10.1038/355349a0. [DOI] [PubMed] [Google Scholar]
- 7.Jalife José. Déjà vu in the theories of atrial fibrillation dynamics. Cardiovasc. Res. 2011 Mar 1;89 (4):766–75. doi: 10.1093/cvr/cvq364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Narayan Sanjiv M, Shivkumar Kalyanam, Krummen David E, Miller John M, Rappel Wouter-Jan. Panoramic electrophysiological mapping but not electrogram morphology identifies stable sources for human atrial fibrillation: stable atrial fibrillation rotors and focal sources relate poorly to fractionated electrograms. Circ Arrhythm Electrophysiol. 2013 Feb;6 (1):58–67. doi: 10.1161/CIRCEP.111.977264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.SCHERF D. Studies on auricular tachycardia caused by aconitine administration. Proc. Soc. Exp. Biol. Med. 1947 Feb;64 (2):233–9. doi: 10.3181/00379727-64-15754. [DOI] [PubMed] [Google Scholar]
- 10.Haïssaguerre M, Jaïs P, Shah D C, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N. Engl. J. Med. 1998 Sep 3;339 (10):659–66. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 11.Jaïs P, Haïssaguerre M, Shah D C, Chouairi S, Gencel L, Hocini M, Clémenty J. A focal source of atrial fibrillation treated by discrete radiofrequency ablation. Circulation. 1997 Feb 4;95 (3):572–6. doi: 10.1161/01.cir.95.3.572. [DOI] [PubMed] [Google Scholar]
- 12.Calkins Hugh, Kuck Karl Heinz, Cappato Riccardo, Brugada Josep, Camm A John, Chen Shih-Ann, Crijns Harry J G, Damiano Ralph J, Davies D Wyn, DiMarco John, Edgerton James, Ellenbogen Kenneth, Ezekowitz Michael D, Haines David E, Haissaguerre Michel, Hindricks Gerhard, Iesaka Yoshito, Jackman Warren, Jalife Jose, Jais Pierre, Kalman Jonathan, Keane David, Kim Young-Hoon, Kirchhof Paulus, Klein George, Kottkamp Hans, Kumagai Koichiro, Lindsay Bruce D, Mansour Moussa, Marchlinski Francis E, McCarthy Patrick M, Mont J Lluis, Morady Fred, Nademanee Koonlawee, Nakagawa Hiroshi, Natale Andrea, Nattel Stanley, Packer Douglas L, Pappone Carlo, Prystowsky Eric, Raviele Antonio, Reddy Vivek, Ruskin Jeremy N, Shemin Richard J, Tsao Hsuan-Ming, Wilber David. 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace. 2012 Apr;14 (4):528–606. doi: 10.1093/europace/eus027. [DOI] [PubMed] [Google Scholar]
- 13.MOE G K, ABILDSKOV J A. Atrial fibrillation as a self-sustaining arrhythmia independent of focal discharge. Am. Heart J. 1959 Jul;58 (1):59–70. doi: 10.1016/0002-8703(59)90274-1. [DOI] [PubMed] [Google Scholar]
- 14.Eckstein Jens, Maesen Bart, Linz Dominik, Zeemering Stef, van Hunnik Arne, Verheule Sander, Allessie Maurits, Schotten Ulrich. Time course and mechanisms of endo-epicardial electrical dissociation during atrial fibrillation in the goat. Cardiovasc. Res. 2011 Mar 1;89 (4):816–24. doi: 10.1093/cvr/cvq336. [DOI] [PubMed] [Google Scholar]
- 15.Wijffels M C, Kirchhof C J, Dorland R, Allessie M A. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995 Oct 1;92 (7):1954–68. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 16.Konings K T, Kirchhof C J, Smeets J R, Wellens H J, Penn O C, Allessie M A. High-density mapping of electrically induced atrial fibrillation in humans. Circulation. 1994 Apr;89 (4):1665–80. doi: 10.1161/01.cir.89.4.1665. [DOI] [PubMed] [Google Scholar]
- 17.Lee Seungyup, Sahadevan Jayakumar, Khrestian Celeen M, Durand Dominique M, Waldo Albert L. High density mapping of atrial fibrillation during vagal nerve stimulation in the canine heart: restudying the Moe hypothesis. J. Cardiovasc. Electrophysiol. 2013 Mar;24 (3):328–35. doi: 10.1111/jce.12032. [DOI] [PubMed] [Google Scholar]
- 18.Narayan Sanjiv M, Wright Matthew, Derval Nicolas, Jadidi Amir, Forclaz Andrei, Nault Isabelle, Miyazaki Shinsuke, Sacher Frédéric, Bordachar Pierre, Clémenty Jacques, Jaïs Pierre, Haïssaguerre Michel, Hocini Mélèze. Classifying fractionated electrograms in human atrial fibrillation using monophasic action potentials and activation mapping: evidence for localized drivers, rate acceleration, and nonlocal signal etiologies. Heart Rhythm. 2011 Feb;8 (2):244–53. doi: 10.1016/j.hrthm.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pandit Sandeep V, Jalife José. Rotors and the dynamics of cardiac fibrillation. Circ. Res. 2013 Mar 1;112 (5):849–62. doi: 10.1161/CIRCRESAHA.111.300158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allessie Maurits A, de Groot Natasja M S, Houben Richard P M, Schotten Ulrich, Boersma Eric, Smeets Joep L, Crijns Harry J. Electropathological substrate of long-standing persistent atrial fibrillation in patients with structural heart disease: longitudinal dissociation. Circ Arrhythm Electrophysiol. 2010 Dec;3 (6):606–15. doi: 10.1161/CIRCEP.109.910125. [DOI] [PubMed] [Google Scholar]
- 21.Stevenson W G, Khan H, Sager P, Saxon L A, Middlekauff H R, Natterson P D, Wiener I. Identification of reentry circuit sites during catheter mapping and radiofrequency ablation of ventricular tachycardia late after myocardial infarction. Circulation. 1993 Oct;88 (4 Pt 1):1647–70. doi: 10.1161/01.cir.88.4.1647. [DOI] [PubMed] [Google Scholar]
- 22.Waldo A L, MacLean W A, Karp R B, Kouchoukos N T, James T N. Entrainment and interruption of atrial flutter with atrial pacing: studies in man following open heart surgery. Circulation. 1977 Nov;56 (5):737–45. doi: 10.1161/01.cir.56.5.737. [DOI] [PubMed] [Google Scholar]
- 23.Martins Raphael P, Kaur Kuljeet, Hwang Elliot, Ramirez Rafael J, Willis B Cicero, Filgueiras-Rama David, Ennis Steven R, Takemoto Yoshio, Ponce-Balbuena Daniela, Zarzoso Manuel, O'Connell Ryan P, Musa Hassan, Guerrero-Serna Guadalupe, Avula Uma Mahesh R, Swartz Michael F, Bhushal Sandesh, Deo Makarand, Pandit Sandeep V, Berenfeld Omer, Jalife José. Dominant frequency increase rate predicts transition from paroxysmal to long-term persistent atrial fibrillation. Circulation. 2014 Apr 8;129 (14):1472–82. doi: 10.1161/CIRCULATIONAHA.113.004742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee Geoffrey, Roberts-Thomson Kurt, Madry Andrew, Spence Steven, Teh Andrew, Heck Patrick M, Kumar Saurabh, Kistler Peter M, Morton Joseph B, Sanders Prashanthan, Kalman Jonathan M. Relationship among complex signals, short cycle length activity, and dominant frequency in patients with long-lasting persistent AF: a high-density epicardial mapping study in humans. Heart Rhythm. 2011 Nov;8 (11):1714–9. doi: 10.1016/j.hrthm.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 25.Atienza Felipe, Almendral Jesús, Jalife José, Zlochiver Sharon, Ploutz-Snyder Robert, Torrecilla Esteban G, Arenal Angel, Kalifa Jérôme, Fernández-Avilés Francisco, Berenfeld Omer. Real-time dominant frequency mapping and ablation of dominant frequency sites in atrial fibrillation with left-to-right frequency gradients predicts long-term maintenance of sinus rhythm. Heart Rhythm. 2009 Jan;6 (1):33–40. doi: 10.1016/j.hrthm.2008.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Narayan Sanjiv M, Krummen David E, Kahn Andrew M, Karasik Pamela L, Franz Michael R. Evaluating fluctuations in human atrial fibrillatory cycle length using monophasic action potentials. Pacing Clin Electrophysiol. 2006 Nov;29 (11):1209–18. doi: 10.1111/j.1540-8159.2006.00525.x. [DOI] [PubMed] [Google Scholar]
- 27.Ng Jason, Kadish Alan H, Goldberger Jeffrey J. Technical considerations for dominant frequency analysis. J. Cardiovasc. Electrophysiol. 2007 Jul;18 (7):757–64. doi: 10.1111/j.1540-8167.2007.00810.x. [DOI] [PubMed] [Google Scholar]
- 28.Gray R A, Jalife J, Panfilov A V, Baxter W T, Cabo C, Davidenko J M, Pertsov A M. Mechanisms of cardiac fibrillation. Science. 1995 Nov 17;270 (5239):1222–3. [PubMed] [Google Scholar]
- 29.de Groot Natasja M S, Houben Richard P M, Smeets Joep L, Boersma Eric, Schotten Ulrich, Schalij Martin J, Crijns Harry, Allessie Maurits A. Electropathological substrate of longstanding persistent atrial fibrillation in patients with structural heart disease: epicardial breakthrough. Circulation. 2010 Oct 26;122 (17):1674–82. doi: 10.1161/CIRCULATIONAHA.109.910901. [DOI] [PubMed] [Google Scholar]
- 30.Lee Geoffrey, Kumar Saurabh, Teh Andrew, Madry Andrew, Spence Steven, Larobina Marco, Goldblatt John, Brown Robin, Atkinson Victoria, Moten Simon, Morton Joseph B, Sanders Prashanthan, Kistler Peter M, Kalman Jonathan M. Epicardial wave mapping in human long-lasting persistent atrial fibrillation: transient rotational circuits, complex wavefronts, and disorganized activity. Eur. Heart J. 2014 Jan;35 (2):86–97. doi: 10.1093/eurheartj/eht267. [DOI] [PubMed] [Google Scholar]
- 31.Jadidi Amir S, Cochet Hubert, Shah Ashok J, Kim Steven J, Duncan Edward, Miyazaki Shinsuke, Sermesant Maxime, Lehrmann Heiko, Lederlin Matthieu, Linton Nick, Forclaz Andrei, Nault Isabelle, Rivard Lena, Wright Matthew, Liu Xingpeng, Scherr Daniel, Wilton Stephen B, Roten Laurent, Pascale Patrizio, Derval Nicolas, Sacher Frédéric, Knecht Sebastien, Keyl Cornelius, Hocini Mélèze, Montaudon Michel, Laurent Francois, Haïssaguerre Michel, Jaïs Pierre. Inverse relationship between fractionated electrograms and atrial fibrosis in persistent atrial fibrillation: combined magnetic resonance imaging and high-density mapping. J. Am. Coll. Cardiol. 2013 Aug 27;62 (9):802–12. doi: 10.1016/j.jacc.2013.03.081. [DOI] [PubMed] [Google Scholar]
- 32.Earley Mark J, Abrams Dominic J R, Sporton Simon C, Schilling Richard J. Validation of the noncontact mapping system in the left atrium during permanent atrial fibrillation and sinus rhythm. J. Am. Coll. Cardiol. 2006 Aug 1;48 (3):485–91. doi: 10.1016/j.jacc.2006.04.069. [DOI] [PubMed] [Google Scholar]
- 33.Rappel Wouter-Jan, Narayan Sanjiv M. Theoretical considerations for mapping activation in human cardiac fibrillation. Chaos. 2013 Jun;23 (2):1–11. doi: 10.1063/1.4807098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rensma P L, Allessie M A, Lammers W J, Bonke F I, Schalij M J. Length of excitation wave and susceptibility to reentrant atrial arrhythmias in normal conscious dogs. Circ. Res. 1988 Feb;62 (2):395–410. doi: 10.1161/01.res.62.2.395. [DOI] [PubMed] [Google Scholar]
- 35.Narayan Sanjiv M, Krummen David E, Rappel Wouter-Jan. Clinical mapping approach to diagnose electrical rotors and focal impulse sources for human atrial fibrillation. J. Cardiovasc. Electrophysiol. 2012 May;23 (5):447–54. doi: 10.1111/j.1540-8167.2012.02332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Narayan Sanjiv M, Krummen David E, Enyeart Michael W, Rappel Wouter-Jan. Computational mapping identifies localized mechanisms for ablation of atrial fibrillation. PLoS ONE. 2012;7 (9):e46034. doi: 10.1371/journal.pone.0046034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Narayan Sanjiv M, Bode Frank, Karasik Pamela L, Franz Michael R. Alternans of atrial action potentials during atrial flutter as a precursor to atrial fibrillation. Circulation. 2002 Oct 8;106 (15):1968–73. doi: 10.1161/01.cir.0000037062.35762.b4. [DOI] [PubMed] [Google Scholar]
- 38.Franz Michael R, Jamal Sameer M, Narayan Sanjiv M. The role of action potential alternans in the initiation of atrial fibrillation in humans: a review and future directions. Europace. 2012 Nov;14 Suppl 5:v58–v64. doi: 10.1093/europace/eus273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Narayan Sanjiv M, Kazi Dhruv, Krummen David E, Rappel Wouter-Jan. Repolarization and activation restitution near human pulmonary veins and atrial fibrillation initiation: a mechanism for the initiation of atrial fibrillation by premature beats. J. Am. Coll. Cardiol. 2008 Oct 7;52 (15):1222–30. doi: 10.1016/j.jacc.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller John M, Kowal Robert C, Swarup Vijay, Daubert James P, Daoud Emile G, Day John D, Ellenbogen Kenneth A, Hummel John D, Baykaner Tina, Krummen David E, Narayan Sanjiv M, Reddy Vivek Y, Shivkumar Kalyanam, Steinberg Jonathan S, Wheelan Kevin R. Initial independent outcomes from focal impulse and rotor modulation ablation for atrial fibrillation: multicenter FIRM registry. J. Cardiovasc. Electrophysiol. 2014 Sep;25 (9):921–9. doi: 10.1111/jce.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Narayan Sanjiv M, Krummen David E, Shivkumar Kalyanam, Clopton Paul, Rappel Wouter-Jan, Miller John M. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation) trial. J. Am. Coll. Cardiol. 2012 Aug 14;60 (7):628–36. doi: 10.1016/j.jacc.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Narayan Sanjiv M, Baykaner Tina, Clopton Paul, Schricker Amir, Lalani Gautam G, Krummen David E, Shivkumar Kalyanam, Miller John M. Ablation of rotor and focal sources reduces late recurrence of atrial fibrillation compared with trigger ablation alone: extended follow-up of the CONFIRM trial (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation). J. Am. Coll. Cardiol. 2014 May 6;63 (17):1761–8. doi: 10.1016/j.jacc.2014.02.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Narayan Sanjiv M, Krummen David E, Clopton Paul, Shivkumar Kalyanam, Miller John M. Direct or coincidental elimination of stable rotors or focal sources may explain successful atrial fibrillation ablation: on-treatment analysis of the CONFIRM trial (Conventional ablation for AF with or without focal impulse and rotor modulation). J. Am. Coll. Cardiol. 2013 Jul 9;62 (2):138–47. doi: 10.1016/j.jacc.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Narayan SM, Krummen DE, Donsky A, Swarup V, Tomassoni G, Miller JM. Treatment Of Paroxysmal Atrial Fibrillation By Targeted Elimination Of Stable Rotors And Focal Sources Without Pulmonary Vein Isolation: The Precise Rotor Elimination Without Concomitant Pulmonary Vein Isolation For Subsequent Elimination Of PAF (PRECISE). Hear Rhythm. 2013;0:LB01–05. [Google Scholar]
- 45.Shivkumar Kalyanam, Ellenbogen Kenneth A, Hummel John D, Miller John M, Steinberg Jonathan S. Acute termination of human atrial fibrillation by identification and catheter ablation of localized rotors and sources: first multicenter experience of focal impulse and rotor modulation (FIRM) ablation. J. Cardiovasc. Electrophysiol. 2012 Dec;23 (12):1277–85. doi: 10.1111/jce.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmitt Claus, Ndrepepa Gjin, Weber Stefan, Schmieder Sebastian, Weyerbrock Sonja, Schneider Michael, Karch Martin R, Deisenhofer Isabel, Schreieck Jürgen, Zrenner Bernhard, Schömig Albert. Biatrial multisite mapping of atrial premature complexes triggering onset of atrial fibrillation. Am. J. Cardiol. 2002 Jun 15;89 (12):1381–7. doi: 10.1016/s0002-9149(02)02350-0. [DOI] [PubMed] [Google Scholar]
- 47.Ndrepepa Gjin, Schneider Michael A E, Karch Martin R, Weber Stefan, Schreieck Jürgen, Zrenner Bernhard, Schmitt Claus. Impact of atrial fibrillation on the voltage of bipolar signals acquired from the left and right atria. Pacing Clin Electrophysiol. 2003 Apr;26 (4 Pt 1):862–9. doi: 10.1046/j.1460-9592.2003.t01-1-00151.x. [DOI] [PubMed] [Google Scholar]
- 48.Haissaguerre Michel, Hocini Meleze, Shah Ashok J, Derval Nicolas, Sacher Frederic, Jais Pierre, Dubois Remi. Noninvasive panoramic mapping of human atrial fibrillation mechanisms: a feasibility report. J. Cardiovasc. Electrophysiol. 2013 Jun;24 (6):711–7. doi: 10.1111/jce.12075. [DOI] [PubMed] [Google Scholar]
- 49.Ghoraani B, Dalvi R, Gizurarson S, Das M, Ha A, Suszko A, Krishnan S, Chauhan V S. Localized rotational activation in the left atrium during human atrial fibrillation: relationship to complex fractionated atrial electrograms and low-voltage zones. Heart Rhythm. 2013 Dec;10 (12):1830–8. doi: 10.1016/j.hrthm.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 50.Tilz R, Lin T, Rillig A, Bucur T, Makimoto H, Metzner A, Lemes C, Mathew S, Kamioka M, Wissner E, Ouyang F, Kuck K. Locations of human atrial fibrillation rotors: Rotors indentified frequently in the pulmonary veins. Hear Rhythm . 2014;5:PO05–75. [Google Scholar]
- 51.Zhou Jing, Scherlag Benjamin J, Edwards Jeffery, Jackman Warren M, Lazzara Ralph, Po Sunny S. Gradients of atrial refractoriness and inducibility of atrial fibrillation due to stimulation of ganglionated plexi. J. Cardiovasc. Electrophysiol. 2007 Jan;18 (1):83–90. doi: 10.1111/j.1540-8167.2006.00679.x. [DOI] [PubMed] [Google Scholar]
- 52.Narayan S M, Cain M E, Smith J M. Atrial fibrillation. Lancet. 1997 Sep 27;350 (9082):943–50. doi: 10.1016/S0140-6736(97)06359-9. [DOI] [PubMed] [Google Scholar]
- 53.Chen J, Mandapati R, Berenfeld O, Skanes A C, Gray R A, Jalife J. Dynamics of wavelets and their role in atrial fibrillation in the isolated sheep heart. Cardiovasc. Res. 2000 Nov;48 (2):220–32. doi: 10.1016/s0008-6363(00)00177-2. [DOI] [PubMed] [Google Scholar]