Abstract
Atrial fibrillation (AF) and heart failure (HF) are common conditions that frequently coexist. Both conditions share risk factors, are associated with increased morbidity and mortality, and may worsen the other. The presence of heart failure and symptoms associated with it may influence both the approach to management (i.e., rate versus rhythm control) and the treatment options available for AF patients. The presence of HF increases the stroke risk with atrial fibrillation, and thromboembolic risk reduction is paramount. Some patients with HF tolerate AF poorly and therefore , a rhythm control strategy may be preferred. More insight into the success rates with catheter ablation in heart failure has been gleaned from recent studies.
Introduction
Atrial fibrillation and heart failure have been recognized as the 2 epidemics of modern cardiovascular medicine.[1] In an analysis of the Framingham Heart Study, atrial fibrillation (AF) and heart failure (HF) have been associated with each other, as the presence of either one increases the risk of developing the other and also increases the mortality risk associated with the other.[2] The incidence and prevalence of AF are increasing, even after adjustment for aging of the population, and the prevalence of HF is increasing as improved therapies are prolonging survival.[1,3-4] The risk of AF increases 4.5- to 5.9-fold in the presence of HF, and HF is a more powerful risk factor for AF than advanced age, valvular heart disease, hypertension, diabetes mellitus, or prior myocardial infarction.[5,6] AF prevalence increases as HF severity worsens. AF has been estimated to occur in 5 to 10% of patients with mild HF, 10 to 26% with moderate disease, and up to 50% with advanced HF.[7-12] Overall, patients with HF develop AF at a rate of 6 to 8% per year, and AF is present in > 15% of HF patients.
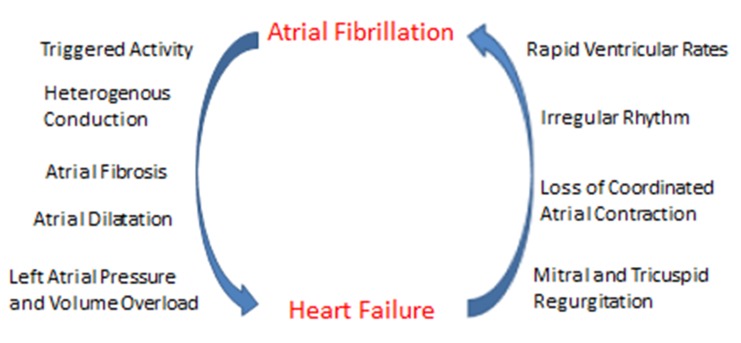
Controversy exists as to the prognostic significance of AF in heart failure, although a negative impact is presumed. AF may negatively affect outcomes in HF through adverse hemodynamic changes, heightened thromboembolic risk, and exposure of patients to the harmful effects of AF therapies (e.g., antiarrhythmic drugs and anticoagulants).[7-9] Heart failure also facilitates atrial remodeling, which promotes the development and maintenance of AF (figure 1). Studies of HF patients with and without systolic dysfunction have suggested an association between baseline AF and greater long-term morbidity, mortality, and/or hospitalization for HF.[13-16] A retrospective analysis of SOLVD, for instance, which enrolled 6500 patients with left ventricular ejection fraction < 35%, found baseline AF to be an independent predictor for all-cause mortality, progressive pump failure, and the combined end point of death or hospitalization for heart failure.[13] A more recent analysis of a multicenter cohort of adults with HF found preexisting and incident AF were associated with higher rates of ischemic stroke, hospitalization for HF, and death.[17] The associations of AF with these adverse outcomes occurred similarly for patients with reduced as well as preserved systolic function. Despite data from retrospective and observational studies suggesting AF worsens HF prognosis, the complexities of both conditions make it difficult to determine whether AF is an independent risk factor for mortality or rather is indicative of disease severity.
Figure 1. Mechanisms underlying the complex interplay between atrial fibrillation and heart failure.
Among patients with AF and HF, the timing of the development of these conditions may have prognostic implications. A recent study assessed the incidence of subsequent hospitalization or all-cause mortality among 182 consecutive patients hospitalized with AF and HF.[18] Outcomes were analyzed based upon whether patients developed AF before or concurrent with HF as opposed to those who had HF prior to onset of AF. Over an approximate 16-month follow-up period, patients who had HF prior to the development of AF had worse outcomes with more repeat hospitalizations and increased mortality. The results suggest that HF patients who develop AF may have more severe underlying cardiac structural abnormalities and worse prognosis compared with AF patients who later develop HF. In addition, the development of AF in a HF patient may be a marker of disease progression.
Clinical Management Of Atrial Fibrillation In Heart Failure Patients
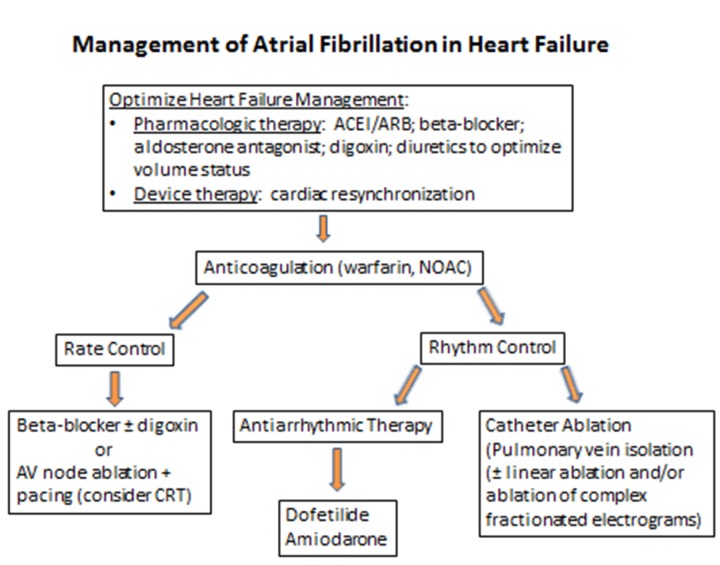
The American College of Cardiology/American Heart Association/Heart Rhythm Society (ACC/AHA/HRS) updated guidelines on the management of AF have recently been published and provide an extensive referenced document on the evaluation and treatment of AF.[19] Similar to patients without HF, the primary tenets of AF management in HF patients should include: 1) thromboembolic risk assessment and anticoagulation as appropriate; 2) ventricular rate control; and 3) assessment of the need for cardioversion to and maintenance of sinus rhythm. However, several unique issues must be considered when treating HF patients with AF. Some HF patients have implantable cardioverter-defibrillators in place that should be programmed to minimize the risk of inappropriate therapies. Because most patients with structural heart disease are on multiple medications, a careful review of the medication history is important to prevent overdosage and adverse drug interactions. In addition, HF treatments should be optimized for AF therapies to be most effective (figure 2). This should include guideline-directed medical therapy (e.g., angiotensin-converting enzyme inhibitors or angiotensin receptor blockers; beta-blockers with proven efficacy in heart failure; and aldosterone antagonsists/diuretics when appropriate) as well as device-based therapy (e.g., cardiac resynchronization).
Figure 2. Overview of management considerations for patients with atrial fibrillation and heart failure. (ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; CRT, cardiac resynchronization therapy; NOAC, novel oral anticoagulant).
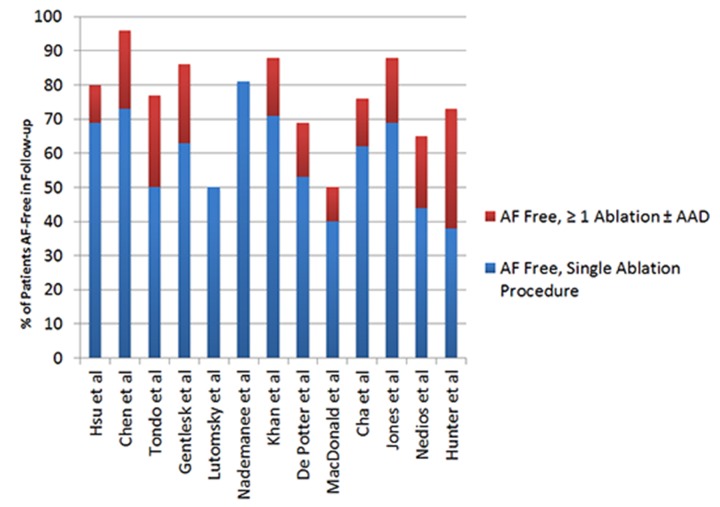
Figure 3. Published success rates of AF ablation in patients with heart failure (with and without concomitant antiarrhythmic therapy).
Stroke Prevention
As outlined in the CHADS2 index, HF and/or LVEF < 35% is a risk factor for stroke in AF.[19] The CHA2DS2-VASc scoring system has been developed as an alternative scoring system for stroke risk stratification. It continues to include HF as a stroke risk factor but also incorporates additional stroke risk factors not included in the traditional CHADS score (e.g., vascular disease; age 65 – 75 years; female gender). The CHA2DS2-VASc score has been found superior to the CHADS2 score in predicting stroke risk in AF and is particularly helpful in determining which patients are truly “low” risk and in whom anticoagulation may be withheld.[20-22] Recent AF guidelines recommend the CHA2DS2-VASc scoring system for stroke risk stratification and advocate systemic anticoagulation in patients with a score ≥ 1.[19,23] Because heart failure patients often have additional stroke risk factors, our practice is to routinely recommend systemic anticoagulation for patients with HF in the absence of contraindications.
Options for systemic anticoagulation include warfarin and the novel oral anticoagulants (NOACs) dabigatran, rivaroxaban, and apixaban. A substudy of RE-LY found the overall benefits for stroke prevention, as well as risks of major and intracranial bleeding, were similar with dabigatran and warfarin in 4904 patients with HF compared to those without HF.[24] Among the 14264 patients randomized to rivaroxaban versus warfarin in ROCKET-AF, 9033 had heart failure or reduced ejection fraction.[25] There were no statistically significant differences between treatments in patients with or without HF. ARISTOTLE randomized 18201 patients with atrial fibrillation and at least 1 additional stroke risk factor to apixaban versus dose-adjusted warfarin.[26] Symptomatic heart failure without left ventricular systolic dysfunction was present in 3207 patients, and 2736 had left ventricular systolic dysfunction with or without symptoms of heart failure.[27] Retrospective analysis of these subgroups demonstrated patients with LV dysfunction (with or without HF) had a higher thromboembolic risk compared with those who had heart failure with preserved LV function and patients without either HF or LV dysfunction. Importantly, apixaban reduced the risk of stroke and thromboembolic events more than warfarin in all 3 patient groups. From the available data, it appears that the novel anticoagulants are at least as effective as, if not superior to, warfarin for prevention of stroke and embolic events in patients with heart failure. An important caveat, however, is the NOAC studies were underpowered to detect statistically significant differences among subgroups.
A number of left atrial appendage closure procedures are being developed as alternatives to warfarin for patients who cannot receive systemic anticoagulation. Options for left atrial appendage occlusion include percutaneous procedures such as WATCHMAN (Boston Scientific, Natick, MA) and LARIAT (SentreHEART, Inc., Redwood City, CA) as well as surgical removal or occlusion such as with the thoracoscopic AtriClip device(AtriCure, West Chester, OH). The PROTECT AF Trial randomized approximately 700 patients to left atrial appendage occlusion with the WATCHMAN device versus warfarin, and the Continued Access Protocol (CAP) registry was a subsequent nonrandomized registry including 460 patients undergoing Watchman implantation.[28] Exclusion criteria for PROTECT AF included NYHA Class IV heart failure and LVEF < 30%. Among the patients randomized in PROTECT AF and CAP, approximately 27% and 19% had symptomatic heart failure, respectively. We do not have data at the present time regarding outcomes in HF patients. Consequently, we cannot judge the effectiveness of the WATCHMAN device for stroke prevention in AF patients with heart failure. Similarly, we have no data on the LARIAT, AtriClip, or other left atrial appendage occlusion procedures for stroke prevention in HF patients with AF.
Ventricular Rate Control
Adequate control of the ventricular response to AF improves symptoms by alleviating the negative hemodynamic effects of rapid rates. Left ventricular function may improve with adequate rate control if the LV dysfunction is due to persistent tachycardia.[29] Recent guidelines suggest a lenient rate-control strategy (resting HR < 110 bpm) is reasonable as long as patients remain asymptomatic and LV systolic function is preserved with no mention of appropriate rate control criteria for patients with heart failure.[19] Guidelines advocate more stringent rate control for symptomatic patients (HR < 80 bpm at rest, < 110 bpm with moderate exertion). RACE II (Rate Control Efficacy in Permanent Atrial Fibrillation: A Comparison Between Lenient Versus Strict Rate Control II) found no significant difference in HF events between patients randomized to strict (resting HR < 80 bpm; < 110 bpm with moderate exercise) or lenient (resting HR < 110 bpm) rate control.[30] Further evidence is required to define the appropriate heart rate goal for ambulatory patients with HF and AF. In the absence of additional data, we believe a lenient approach is a reasonable starting point for most patients. Patients with refractory symptoms or LV dysfunction believed due to elevated heart rates would then be candidates for a trial of strict rate control.
Pharmacologic options for controlling the ventricular response to AF include β-blockers, nondihydropyridine calcium channel blockers, and digoxin. Digoxin primarily slows the ventricular rate by increasing parasympathetic tone on the atrioventricular node. Conditions associated with high sympathetic tone, such as heart failure, may easily overcome this effect, rendering digoxin frequently ineffective as monotherapy. Thus, additional medications are often required for adequate rate control in patients with heart failure. Controversy exists regarding the impact of digoxin on mortality in AF patients with and without heart failure. A post hoc analysis of the Digitalis Investigation Group (DIG) trial found an increased risk of death among women, but not men, treated with digoxin.[31] Another review of the DIG trial data assessed outcomes based upon serum digoxin concentration independent of gender.[32] Among 5548 patients followed over an average of 40 months, patients with a serum digoxin concentration 0.5 – 0.9 ng/ml had reduced mortality and hospitalizations. Higher digoxin concentrations were associated with reduced HF hospitalization with no effect on mortality. Post hoc analyses of AFFIRM also provide conflicting data with regard to the effect of digoxin on mortality among patients with AF. One post hoc analysis of AFFIRM found digoxin was associated with a significant increase in all-cause mortality in patients with AF, regardless of gender or the presence or absence of HF.[33] Another post hoc study of AFFIRM data used propensity scoring to assess the effect of digoxin on mortality and found no evidence of increased mortality or hospitalization among patients taking digoxin as baseline initial therapy.[34] We rarely use digoxin as monotherapy to control the ventricular response to AF but occasionally add it to beta-blocker therapy if additional slowing of the ventricular rate is needed. If digoxin is used, the serum concentration should be monitored due to the drug’s narrow therapeutic window.
In patients who have heart failure with preserved LV systolic function, calcium channel antagonists or β-blockers may be used as first line therapy. In multiple studies of HF patients with reduced systolic function, long-term use of β-blockers has been shown to lessen the symptoms of HF and reduce the risk of death or hospitalization.[35-37] We therefore prefer β-blockers for long-term rate control in patients with both HF and AF. Carvedilol improves LVEF with a trend toward fewer deaths and HF hospitalizations in patients with concomitant AF and HF and may therefore be the preferred β-blocker for patients with both conditions.[38] In addition, guidelines for heart failure management recommend against the use of calcium channel antagonists in patients with AF and systolic dysfunction.[39] The combination of a β-blocker and digoxin may be more effective than a single agent and should be considered if β-blockade alone does not control the ventricular rate.[40] It is prudent not to initiate β-blockers in the acute decompensated state and rather start therapy once the volume status is optimized unless the heart failure exacerbation is presumably due to an uncontrolled ventricular response to AF.
A nonpharmacologic method to achieve long-term rate control is catheter ablation of the atrioventricular node and implantation of a permanent pacemaker. This strategy has been shown to improve LV function, exercise capacity, and quality of life in patients with medically-refractory AF.[41] Chronic right ventricular pacing, however, creates a dyssynchronous pattern of ventricular activation that may worsen HF. Thus, for patients with baseline LV function ≤ 45% or mild to moderate heart failure symptoms at baseline, it is preferable to implant a biventricular pacing system at the time of atrioventricular junction ablation to avoid chronic right ventricular pacing alone.[42,43] Catheter ablation of AF (pulmonary vein isolation) has been compared against AVN ablation with biventricular pacing in patients with drug-refractory AF.[44] In this study, greater improvements in LV function, exercise tolerance, and quality of life were more often observed among 41 patients who underwent catheter ablation compared with 40 patients who underwent AVN ablation with biventricular pacing over 6 months’ follow-up. Additional evidence in support of catheter ablation was provided by an observational nested case-control study in which improved survival was associated with pulmonary vein isolation (146 patients) compared with AV junction ablation (101 patients) or anti-arrhythmic therapy/cardioversion (205 patients) over a 7-year follow-up period.[45] The study results are confounded by the non-randomized selection of therapy. In our practice, we generally reserve AV junction ablation with pacing for patients who have failed or not tolerated antiarrhythmic therapy and, typically, at least one attempt at PVI. If catheter ablation of the AV junction is considered for a patient with heart failure, a resynchronization device should be strongly considered.
Rhythm Control
Data from prospective, randomized -controlled trials demonstrating a survival advantage with maintenance of sinus rhythm in HF are lacking. The AFFIRM and RACE trials found maintenance of sinus rhythm in mixed AF populations provided no benefit with a trend toward harm.[46,47] Extrapolation of these results to patients with HF must be done with caution because only a small percentage of patients in both trials had reduced LV function or HF symptoms at baseline. For instance, a subset analysis of AFFIRM found no significant improvement in mortality, hospitalization, and New York Heart Association class with rhythm control among patients with LV dysfunction, although only 339 patients had symptoms ≥ New York Heart Association class II.[48] Other reports, however, have suggested an association between sinus rhythm and improved survival in HF patients. An analysis of the Congestive Heart Failure Survival Trial of Antiarrhythmic Therapy (CHF-STAT) found improved survival among 51 patients treated with amiodarone who converted to, and maintained, sinus rhythm compared with 52 patients in the placebo arm.[49] Maintenance of sinus rhythm in patients with LV function < 35% was also associated with a significant reduction in mortality in the Danish Investigations of Arrhythmia and Mortality on Dofetilide (DIAMOND) trials.[44-50] Among the 3028 patients enrolled in the 2 DIAMOND studies, 506 had AF or atrial flutter at baseline. Cardioversion occurred in 148 dofetilide and 86 placebo-treated patients. The mortality benefit associated with maintenance of sinus rhythm was present in both the dofetilide and placebo groups.
The Atrial Fibrillation and Congestive Heart Failure (AF-CHF) trial was the first prospective randomized trial comparing rate and rhythm control in HF patients.[51] The study randomized 1376 patients with LV ejection fraction < 35%, HF symptoms, and paroxysmal or persistent AF to either rhythm control (primarily amiodarone) or rate control (mostly β-blockers). At a mean follow-up of 37 months, there was no significant difference in the primary outcome of death from cardiovascular causes between the rhythm and rate control groups (27 and 25%, respectively) by intention-to-treat analysis. There was also no advantage with regard to stroke prevention or HF hospitalization in the rhythm control group. The AF-CHF trial therefore appears to extend the general findings of AFFIRM to patients with HF.
An additional study (CAFÉ-II) randomly assigned 61 patients with chronic heart failure and persistent AF to either a rate or rhythm control strategy.[52] Patients in the rhythm control arm were treated with amiodarone for 3 months followed by cardioversion after which amiodarone was continued to maintain sinus rhythm. Both groups were treated with goal heart rate < 80 bpm at rest and < 110 bpm with exertion when in AF. At 1 year follow-up, 66% of patients in the rhythm control arm were in sinus rhythm. There were no significant differences in NYHA class and exercise capacity between the 2 groups, but patients assigned to rhythm control had improved LV function and quality of life compared to patients assigned to rate control.
In the absence of randomized trial data demonstrating a survival advantage with maintaining sinus rhythm in HF patients, the decision to adopt a rhythm control approach is driven largely by symptoms. Some patients, particularly those with structural heart disease and/or heart failure, tolerate AF poorly (i.e., develop hemodynamic instability or pulmonary edema or experience rapid heart rates that are difficult to control , and a rhythm control strategy may be preferred. Specific situations in which this may be the case include AF complicating hypertrophic cardiomyopathy (loss of atrial transport function and rapid ventricular rates may lead to hemodynamic instability and advanced symptoms); valvular heart disease, particularly mitral stenosis; and perhaps certain obstructive congenital heart lesions. For any HF patient with AF who has at least mild symptoms, our preference is to try and maintain sinus rhythm with the thought that maintaining AV synchrony will help alleviate symptoms.
Antiarrhythmic Therapy
Rhythm control in HF patients with AF is challenging with fewer available antiarrhythmic options due to the potential for proarrhythmia in patients with structural heart disease. In addition, patients with HF are often on additional medical therapies placing them at risk for drug interactions and greater risk of side effects. Renal insufficiency is also common in HF patients which may result in deayed clearance of antiarrhythmic drugs thereby increasing the risk for proarrhythmia and toxicity. The primary pharmacological agents for rhythm control in patients with AF and HF are the class III antiarrhythmic drugs. Amiodarone has the greatest efficacy with regard to maintenance of sinus rhythm, although the noncardiac toxicities of the drug limit its widespread use.[19,23,53] Amiodarone may cause bradycardia and prolongation of the QT interval but rarely causes ventricular proarrhythmia. It is worth noting, however, that patients with NYHA Class III symptoms randomized to amiodarone in the SCD-HeFT trial had increased mortality relative to placebo.[54] The reasons for this finding are unclear, and it has not been our practice to withhold amiodarone from such patients.
The DIAMOND congestive heart failure trial found dofetilide reasonably safe and effective in HF patients.[55] Dofetilide was more effective than placebo in maintaining sinus rhythm with no adverse effect on all-cause mortality but resulted in a lower combined end point of mortality and HF hospitalization. Dronedarone is another potential agent for rhythm control in AF. It is modestly effective in maintaining sinus rhythm and, when AF does occur, has ventricular rate-slowing properties. In ATHENA, which included a mixed population with paroxysmal and persistent AF, dronedarone reduced the primary end point (composite of hospitalization due to cardiovascular events and death) as well as deaths from cardiovascular causes, primarily as a result of a reduction in arrhythmic death.[50-56] The study enrolled 21% with a history of NYHA class II or III symptoms, and 12% had LV ejection fraction < 45%. Patients with HF who received dronedarone had a benefit similar to that of the entire group. The drug should not be used, however, in patients with clinically significant NYHA class III or IV heart failure or those with a recent hospitalization for heart failure in the preceding 4 weeks, nor should it be used for rate control in patients with permanent atrial fibrillation because of increased mortality and adverse events.[19,57,58] SWORD (Survival with Oral d-Sotalol), a trial of d-sotalol in patients with LV ejection fraction ≤ 40% post myocardial infarction demonstrated increased mortality with d-sotalol compared with placebo.[59] SWORD was not a study looking specifically at AF patients and maintenance of sinus rhythm, but it does raise concern about the use of sotalol in patients with HF post-myocardial infarction. Class Ia and Ic agents have negative inotropic effects and the potential for proarrhythmia in patients with HF and should thus be avoided.[19]
Catheter Ablation
With limited antiarrhythmic options in HF patients and data from multiple studies demonstrating superiority of catheter ablation over antiarrhythmic therapy in mixed populations, catheter ablation is an option to achieve long-term rhythm control.[60-62] Data from multiple studies suggest AF ablation is as effective in those with HF as without, and catheter ablation with maintenance of sinus rhythm has been shown to result in improvements in left ventricular ejection fraction, NYHA class, and quality of life.[63-75]
Data From Nonrandomized Studies
Much of the early data on AF ablation in HF patients come from nonrandomized prospective or observational studies (table 1). One of the first reports of AF ablation in HF patients was a case control trial that examined AF ablation in 58 patients with EF <45% and NYHA class II or greater compared with 58 matched patients with normal EF.[63] The ablation procedure primarily consisted of pulmonary vein isolation as well as additional linear ablation in most, and endpoints included maintenance of sinus rhythm, ejection fraction, ventricular dimensions, exercise capacity, and quality of life. Patients with HF had greater improvements in all of the aforementioned indices. Interestingly, improvements in ejection fraction were seen even in patients with adequate rate control prior to the procedure. After a mean follow-up of 12 months, 69 and 71% of the HF and non-HF patients, respectively, were in sinus rhythm without concomitant antiarrhythmic therapy. With the addition of previously ineffective AADs, the success rates improved to 78 and 84 %, respectively.
Table 1. Clinical characteristics and outcome of catheter ablation in patients with reduced systolic function.
AAD = antiarrhythmic drug; Abl = ablation; AF = atrial fibrillation; CFAEs = complex fractionated atrial electrograms; CHD = coronary heart disease; Consec = consecutive; mos = months; CS = coronary sinus; CTI = cavotricuspid isthmus; EF = ejection fraction; LA = left atrium; NR = not reported; LVEF = left ventricular ejection fraction; No. = number; NS = not significant; NYHA = New York Heart Association; Observ = observational; PAF = paroxysmal atrial fibrillation; Post = posterior; Prosp = prospective; Pt = patient; PVI = pulmonary vein isolation; QOL = quality of life; RA = right atrium; RCT = randomized controlled trial; Retro = retrospective; WACA = wide area circumferential ablation; † = EF improved among patients with sustained sinus rhythm; * = quality of life among catheter ablation patients compared with control;
| Hsu et al. (2004) | Chen et al. (2004) | Tondo, et al. (2006) | Gentlesk et al. (2007) | Lutomsky et al. (2008) | Nademanee, et al. (2008) | Khan et al. (2008) | De Potter et al. (2010) | MacDonald et al. (2011) | Cha et al. (2011) | Jones et al. (2013) | Nedios et al. (2014) | Hunter et al. (2014) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Location | France | USA | Italy | USA | Germany | USA | Multi-center | Spain | UK (2 centers) | USA | UK | Germany, Greece | UK |
| Study design | Consec case control | Retro case series | Case control | Retro observ | Prospective, nonrandom | Observ | RCT: RFA vs AV nodal abl + CRT (PABA-CHF) | Case-control | RCT (Abl vs rate control) | Prosp, cohort | RCT (Abl vs rate control) | Retro, case-control | RCT (Abl vs rate control) |
| No. Ablated | 58 | 94 | 40 | 67 | 18 | 674 | 41 | 36 | 22 | 111 | 26 | 69 | 26 |
| Pt profile | |||||||||||||
| - Age | 56 | 57 | 57 | 54 | 56 | 45 | 60 | 51 | 62.3 | 55 | 64 | 60 | 55 |
| - LVEF | 35% | 36% | 33% | 42% | 41% | NR | 27% | 41% | 36% | 35% | 22% | 31% | 32% |
| - NYHA Class | 2.3 (mean) | 3 (68%) | 2.8 | NR | NR | 2 or 3 | NR | 3 (91%) | NR | 2.4 | 2.4 | 2.6 | |
| -Etiology | CHD 21% | CHD 86% | CHD 25% | CHD 18% | CHD 17% | CHD 21% | CHD 73% | CHD 9% | CHD 50% | CHD 13% | CHD 38% | CHD 38% | CHD 23% |
| - AF duration | 80 mos | 72 mos | 36 mos | 72 mos | NR | 40 mos | 48 mos | 78 mos | 44 mos | 65 mos | 51 mos | NR | 24 mos continuous |
| - PAF, % | 9 | 43 | 25 | 47 | 100 | 0 | 49 | 42 | 0 | 31 | 0 | 33 | 0 |
| Abl strategy | PVI + roof + mitral isthmus | PVI | PVI, mitral isthmus (85%) + CTI (98%) | PVI | PVI | CFAE | PVI + (lines, CFAEs) | PVI + box isolation of post wall + mitral isthmus | PVI + roof + CFAE + CS ± CTI | PVI (WACA); roof line (59%); mitral isthmus line (68%); Non-PV foci (25%) | PVI + roof and mitral lines; CFAE; CTI line | PVI (roof, mitral and “box” isolation of post wall in 64%) | PVI (WACA); CFAE (RA + LA); roof and mitral lines; CTI line |
| Repeat Abl | 50% | 22% | 13% | 31% | NR | 48% | 20% | 30% | 30% | 20% | 27% | 28% | 54% |
| Adverse events | 4% | 4% | 13% | NR | NR | 4.7% | 12% | 2.7% | 14.8% | 3.6% | 7.7% | 1.4% | 4.7% |
| Follow-up | 12 mos | 14 mos | 14 mos | 20 mos | 6 mos | 28 mos | 6 mos | 14 mos | 6 mos | 12 mos | 12 mos | 28 mos | 12 mos |
| Abl success(≥ 1 Abl ± AAD) | 69% (78%) | 73% (96%) | 50% (87%) | 63% (86%) | 50% | (81%) | 71% (88%) | 53% (69%) | 40% (50%) | 62% (76%) | 69% (88%) | 44% (65%) | 38% (73%) |
| EF improved | + 21% | + 5% (NS) | + 14% | + 14% | +10% | NR | + 8% | + 8% | + 8% | + 21% | + 11% | + 15%† | + 8% |
| 6 minute walk | Better with Abl | NR | NR | NR | NR | NR | Better with Abl | NR | Not improved with Abl | NR | Better with Abl | NR | NR |
| QOL* | Better with Abl | Better with Abl | Better with Abl | NR | NR | NR | Better with Abl | NR | Not improved with Abl | Better with Abl | Better with Abl | NR | Better with Abl |
Another large cohort study compared outcomes in 94 patients with reduced ejection fraction undergoing catheter ablation for AF with a “control” group of 283 patients with preserved EF.[64] The mean ejection fraction in the reduced EF group was 36%, and the primary ablation procedure was PVI with elimination of all PV potentials as detected by a circular mapping catheter placed in each pulmonary vein (linear ablation was rarely done). Success rates were 73 and 87% in the reduced and normal EF groups, respectively. There was no statistically significant improvement in EF, but there was an improvement in quality of life scores among HF patients who maintained sinus rhythm.
Additional case-control and observational studies have evaluated outcomes of AF ablation in patients with heart failure.[65-68,72,74] Most are small studies that included patients with non-paroxysmal AF with mean AF durations ranging from 40 – 80 months. The studies are quite heterogeneous with regard to ablation method (PVI vs PVI with linear lesions vs CFAE); duration of post-ablation follow-up (6 to 28 months); methods for detecting AF recurrences; and primary endpoints. However, there are several common themes among the studies comparing outcomes among patients with and without LV systolic dysfunction. The two groups have similar outcomes with regard to maintenance of sinus rhythm post-ablation, although patients with systolic dysfunction tend to require more procedures to achieve this endpoint. The studies have also generally reported improvements in ejection fraction, quality of life, and exercise capacity in patients who undergo catheter ablation for AF and maintain sinus rhythm.
Data From Randomized Studies
There is limited data from randomized, controlled trials regarding the role of catheter ablation for maintenance of sinus rhythm in patients with HF. Only 115 total patients have been randomly assigned to catheter ablation in 4 recent studies comparing catheter ablation to either AV-node ablation with biventricular pacing (1 study) or rate control (3 studies).[44,71,73,75] PABA-CHF randomly assigned 41 patients with drug-refractory AF and heart failure to pulmonary vein isolation and 40 to AV-node ablation with biventricular pacing.[44] Over a 6-month follow-up period, 71% of patients who underwent catheter ablation were free of AF without antiarrhythmic drugs and 88% were AF-free with a combination of catheter ablation and antiarrhythmic therapy. Patients assigned to catheter ablation had improvements in quality of life, exercise capacity, and ejection fraction (35 vs 28%, p < 0.001). The study suggests catheter ablation is superior to a strategy of AV-node ablation with biventricular pacing in patients with drug-refractory AF and HF.
Several randomized trials have compared catheter ablation versus rate-control in patients with HF and drug-refractory AF.[71,73,75] Optimal rate control has been defined as a HR < 80 bpm at rest and < 110 bpm with exertion. Two of the studies demonstrated improvements in ejection fraction, functional capacity, and heart failure symptoms in patients treated with catheter ablation. A study by MacDonald et al. randomly comparing catheter ablation (22 patients) to rate-control (19 patients) failed to demonstrate improvements in the same endpoints among patients assigned to catheter ablation.
Catheter Ablation In Patients With Heart Failure With Preserved Ejection Function (HFPEF)
here are limited data regarding the efficacy and outcome of catheter ablation of AF in patients with heart failure with preserved ejection fraction (i.e., diastolic dysfunction). There are no published data from randomized trials in this patient population. The largest study of catheter ablation in patients with HFPEF was a prospective cohort study comparing outcomes among 157 patients with HFPEF, 111 patients with systolic dysfunction, and 100 patients with normal LV function.[72] Among patients with HFPEF, 76% maintained sinus rhythm 1 year post-ablation with or without antiarrhythmic therapy (62% maintained sinus rhythm post-ablation without an antiarrhythmic drug). Patients who maintained sinus rhythm had improved quality of life and functional capacity although the recurrence rate was higher (1.7-fold) compared with patients with normal LV function. An improvement in diastolic dysfunction grade as assessed by echocardiography was observed in 30% post-ablation.
An additional cohort study assessed outcomes of catheter ablation for AF in 74 patients with HFPEF.[76] Over 34-month follow-up, AF-free rates were 27% after a single procedure, 45% after multiple procedures, and 73% after multiple procedures with the assistance of antiarrhythmic therapy. Shorter duration of AF and absence of hypertension were associated with better ablation outcomes. A higher recurrence rate post-ablation was also found in a smaller cohort study comparing ablation outcomes among 29 patients with HFPEF and 51 patients without heart failure.[77]
Importantly, worsening of left ventricular diastolic dysfunction has been reported after catheter ablation for atrial fibrillation.[78] A report of 70 consecutive patients undergoing pulmonary vein isolation for AF, 27 of whom had HFPEF at baseline, identified worse diastolic dysfunction post-ablation in 27%. Worsening of diastolic dysfunction directly correlated with increased ablation time.
Conclusion
Atrial fibrillation occurs commonly among heart failure patients including those with reduced and preserved systolic function. The primary tenets of management include control of the ventricular rate, systemic anticoagulation as guided by the CHADS-VASc score, and determination of the need for restoration and maintenance of sinus rhythm. There is a general lack of evidence from randomized, controlled trials demonstrating a survival advantage with maintenance of sinus rhythm in HF patients. Consequently, the decision to adopt a rhythm control approach is driven largely by symptoms. Patients who tolerate AF poorly or have persistent symptoms despite adequate rate control should be considered for rhythm control strategies.
Options for rhythm control in HF patients are limited due to the potential pro-arrhythmia associated with certain antiarrhythmic drugs. Outcomes of catheter ablation for AF in HF patients are mixed, although several common themes may be derived from the data. First, there have been very few randomized, controlled trials evaluating catheter ablation of AF in heart failure patients. The number of patients enrolled is small which limits the conclusions that can be drawn. Among randomized and nonrandomized trials of AF ablation in heart failure, most patients had persistent AF with AF durations of 24 to 48 months prior to ablation. Catheter ablation consisted of pulmonary vein isolation alone in some studies whereas additional ablation (e.g., CFAE, linear ablation) was performed in others. From the available data, additional ablation beyond PVI does not appear to affect recurrence or long-term success rates (table 1). Earlier studies reported success rates similar to patients without structural heart disease, but recent studies report lower success rates and more repeat ablation procedures (13 – 54%) in heart failure patients with and without LV systolic dysfunction. Among patients who maintain sinus rhythm long-term after ablation, there appears to be general improvement in quality of life, exercise capacity, and left ventricular function compared with patients treated medically or with AV-node ablation and biventricular pacing.
Unresolved questions regarding catheter ablation of AF in heart failure patients include: Which patients with HF and AF are the best candidates for catheter ablation? Presumably patients with AF who subsequently develop HF, particularly those with a tachycardia-related myopathy, would have favorable outcomes post-ablation with maintenance of sinus rhythm. Would catheter ablation earlier in the course of disease improve long-term outcomes? How much ablation should be performed (i.e., PVI alone PVI plus non-pulmonary vein triggers PVI + linear ablation + CFAE)? In an era of increasing accountability for expenditures, is catheter ablation the most cost effective approach, particularly if more than one procedure may be necessary? Several prospective randomized trials have been initiated which will hopefully address some of these questions (CASTLE-AF, AMICA, and RAFT-AF).[79] When catheter ablation is performed, careful consideration should be given to the extent of ablation due to the potential for worsening of LV diastolic function and LA transport function which can have potentially serious complications in patients with baseline heart failure.[78,80]
Disclosures
None.
References
- Braunwald E. Shattuck lecture--cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N. Engl. J. Med. 1997 Nov 6;337 (19):1360–9. doi: 10.1056/NEJM199711063371906. [DOI] [PubMed] [Google Scholar]
- Wang Thomas J, Larson Martin G, Levy Daniel, Vasan Ramachandran S, Leip Eric P, Wolf Philip A, D'Agostino Ralph B, Murabito Joanne M, Kannel William B, Benjamin Emelia J. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003 Jun 17;107 (23):2920–5. doi: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- Wolf P A, Benjamin E J, Belanger A J, Kannel W B, Levy D, D'Agostino R B. Secular trends in the prevalence of atrial fibrillation: The Framingham Study. Am. Heart J. 1996 Apr;131 (4):790–5. doi: 10.1016/s0002-8703(96)90288-4. [DOI] [PubMed] [Google Scholar]
- Levy Daniel, Kenchaiah Satish, Larson Martin G, Benjamin Emelia J, Kupka Michelle J, Ho Kalon K L, Murabito Joanne M, Vasan Ramachandran S. Long-term trends in the incidence of and survival with heart failure. N. Engl. J. Med. 2002 Oct 31;347 (18):1397–402. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- Kannel W B, Abbott R D, Savage D D, McNamara P M. Epidemiologic features of chronic atrial fibrillation: the Framingham study. N. Engl. J. Med. 1982 Apr 29;306 (17):1018–22. doi: 10.1056/NEJM198204293061703. [DOI] [PubMed] [Google Scholar]
- Benjamin E J, Levy D, Vaziri S M, D'Agostino R B, Belanger A J, Wolf P A. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994 Mar 16;271 (11):840–4. [PubMed] [Google Scholar]
- Maisel William H, Stevenson Lynne Warner. Atrial fibrillation in heart failure: epidemiology, pathophysiology, and rationale for therapy. Am. J. Cardiol. 2003 Mar 20;91 (6A):2D–8D. doi: 10.1016/s0002-9149(02)03373-8. [DOI] [PubMed] [Google Scholar]
- Ehrlich Joachim R, Nattel Stanley, Hohnloser Stefan H. Atrial fibrillation and congestive heart failure: specific considerations at the intersection of two common and important cardiac disease sets. J. Cardiovasc. Electrophysiol. 2002 Apr;13 (4):399–405. doi: 10.1046/j.1540-8167.2002.00399.x. [DOI] [PubMed] [Google Scholar]
- Seiler J, Stevenson WG. Atrial fibrillation in congestive heart failure. Cardiol Rev . 2010;8:38–50. doi: 10.1097/CRD.0b013e3181c21cff. [DOI] [PubMed] [Google Scholar]
- Krum Henry, Gilbert Richard E. Demographics and concomitant disorders in heart failure. Lancet. 2003 Jul 12;362 (9378):147–58. doi: 10.1016/S0140-6736(03)13869-X. [DOI] [PubMed] [Google Scholar]
- Anter Elad, Jessup Mariell, Callans David J. Atrial fibrillation and heart failure: treatment considerations for a dual epidemic. Circulation. 2009 May 12;119 (18):2516–25. doi: 10.1161/CIRCULATIONAHA.108.821306. [DOI] [PubMed] [Google Scholar]
- Darby Andrew E, Dimarco John P. Management of atrial fibrillation in patients with structural heart disease. Circulation. 2012 Feb 21;125 (7):945–57. doi: 10.1161/CIRCULATIONAHA.111.019935. [DOI] [PubMed] [Google Scholar]
- Dries D L, Exner D V, Gersh B J, Domanski M J, Waclawiw M A, Stevenson L W. Atrial fibrillation is associated with an increased risk for mortality and heart failure progression in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: a retrospective analysis of the SOLVD trials. Studies of Left Ventricular Dysfunction. J. Am. Coll. Cardiol. 1998 Sep;32 (3):695–703. doi: 10.1016/s0735-1097(98)00297-6. [DOI] [PubMed] [Google Scholar]
- Swedberg Karl, Olsson Lars G, Charlesworth Andrew, Cleland John, Hanrath Peter, Komajda Michel, Metra Marco, Torp-Pedersen Christian, Poole-Wilson Philip. Prognostic relevance of atrial fibrillation in patients with chronic heart failure on long-term treatment with beta-blockers: results from COMET. Eur. Heart J. 2005 Jul;26 (13):1303–8. doi: 10.1093/eurheartj/ehi166. [DOI] [PubMed] [Google Scholar]
- Pedersen Ole Dyg, Bagger Henning, Køber Lars, Torp-Pedersen Christian. Impact of congestive heart failure and left ventricular systolic function on the prognostic significance of atrial fibrillation and atrial flutter following acute myocardial infarction. Int. J. Cardiol. 2005 Apr 8;100 (1):65–71. doi: 10.1016/j.ijcard.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Olsson Lars G, Swedberg Karl, Ducharme Anique, Granger Christopher B, Michelson Eric L, McMurray John J V, Puu Margareta, Yusuf Salim, Pfeffer Marc A. Atrial fibrillation and risk of clinical events in chronic heart failure with and without left ventricular systolic dysfunction: results from the Candesartan in Heart failure-Assessment of Reduction in Mortality and morbidity (CHARM) program. J. Am. Coll. Cardiol. 2006 May 16;47 (10):1997–2004. doi: 10.1016/j.jacc.2006.01.060. [DOI] [PubMed] [Google Scholar]
- McManus David D, Hsu Grace, Sung Sue Hee, Saczynski Jane S, Smith David H, Magid David J, Gurwitz Jerry H, Goldberg Robert J, Go Alan S. Atrial fibrillation and outcomes in heart failure with preserved versus reduced left ventricular ejection fraction. J Am Heart Assoc. 2013 Feb;2 (1):e005694. doi: 10.1161/JAHA.112.005694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit Marcelle D, Moes Marjolein L, Maass Alexander H, Achekar Ismaël D, Van Geel Peter P, Hillege Hans L, van Veldhuisen Dirk J, Van Gelder Isabelle C. The importance of whether atrial fibrillation or heart failure develops first. Eur. J. Heart Fail. 2012 Sep;14 (9):1030–40. doi: 10.1093/eurjhf/hfs097. [DOI] [PubMed] [Google Scholar]
- January Craig T, Wann L Samuel, Alpert Joseph S, Calkins Hugh, Cleveland Joseph C, Cigarroa Joaquin E, Conti Jamie B, Ellinor Patrick T, Ezekowitz Michael D, Field Michael E, Murray Katherine T, Sacco Ralph L, Stevenson William G, Tchou Patrick J, Tracy Cynthia M, Yancy Clyde W. 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2014 Mar 28;64(21):e1–76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- Olesen Jonas Bjerring, Torp-Pedersen Christian, Hansen Morten Lock, Lip Gregory Y H. The value of the CHA2DS2-VASc score for refining stroke risk stratification in patients with atrial fibrillation with a CHADS2 score 0-1: a nationwide cohort study. Thromb. Haemost. 2012 Jun;107 (6):1172–9. doi: 10.1160/TH12-03-0175. [DOI] [PubMed] [Google Scholar]
- Mason Pamela K, Lake Douglas E, DiMarco John P, Ferguson John D, Mangrum J Michael, Bilchick Kenneth, Moorman Liza P, Moorman J Randall. Impact of the CHA2DS2-VASc score on anticoagulation recommendations for atrial fibrillation. Am. J. Med. 2012 Jun;125 (6):603.e1–6. doi: 10.1016/j.amjmed.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane Deirdre A, Lip Gregory Y H. Use of the CHA(2)DS(2)-VASc and HAS-BLED scores to aid decision making for thromboprophylaxis in nonvalvular atrial fibrillation. Circulation. 2012 Aug 14;126 (7):860–5. doi: 10.1161/CIRCULATIONAHA.111.060061. [DOI] [PubMed] [Google Scholar]
- Camm A John, Lip Gregory Y H, De Caterina Raffaele, Savelieva Irene, Atar Dan, Hohnloser Stefan H, Hindricks Gerhard, Kirchhof Paulus. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur. Heart J. 2012 Nov;33 (21):2719–47. doi: 10.1093/eurheartj/ehs253. [DOI] [PubMed] [Google Scholar]
- Ferreira Jorge, Ezekowitz Michael D, Connolly Stuart J, Brueckmann Martina, Fraessdorf Mandy, Reilly Paul A, Yusuf Salim, Wallentin Lars. Dabigatran compared with warfarin in patients with atrial fibrillation and symptomatic heart failure: a subgroup analysis of the RE-LY trial. Eur. J. Heart Fail. 2013 Sep;15 (9):1053–61. doi: 10.1093/eurjhf/hft111. [DOI] [PubMed] [Google Scholar]
- Patel Manesh R, Mahaffey Kenneth W, Garg Jyotsna, Pan Guohua, Singer Daniel E, Hacke Werner, Breithardt Günter, Halperin Jonathan L, Hankey Graeme J, Piccini Jonathan P, Becker Richard C, Nessel Christopher C, Paolini John F, Berkowitz Scott D, Fox Keith A A, Califf Robert M. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N. Engl. J. Med. 2011 Sep 8;365 (10):883–91. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- Granger Christopher B, Alexander John H, McMurray John J V, Lopes Renato D, Hylek Elaine M, Hanna Michael, Al-Khalidi Hussein R, Ansell Jack, Atar Dan, Avezum Alvaro, Bahit M Cecilia, Diaz Rafael, Easton J Donald, Ezekowitz Justin A, Flaker Greg, Garcia David, Geraldes Margarida, Gersh Bernard J, Golitsyn Sergey, Goto Shinya, Hermosillo Antonio G, Hohnloser Stefan H, Horowitz John, Mohan Puneet, Jansky Petr, Lewis Basil S, Lopez-Sendon Jose Luis, Pais Prem, Parkhomenko Alexander, Verheugt Freek W A, Zhu Jun, Wallentin Lars. Apixaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2011 Sep 15;365 (11):981–92. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- McMurray John J V, Ezekowitz Justin A, Lewis Basil S, Gersh Bernard J, van Diepen Sean, Amerena John, Bartunek Jozef, Commerford Patrick, Oh Byung-Hee, Harjola Veli-Pekka, Al-Khatib Sana M, Hanna Michael, Alexander John H, Lopes Renato D, Wojdyla Daniel M, Wallentin Lars, Granger Christopher B. Left ventricular systolic dysfunction, heart failure, and the risk of stroke and systemic embolism in patients with atrial fibrillation: insights from the ARISTOTLE trial. Circ Heart Fail. 2013 May;6 (3):451–60. doi: 10.1161/CIRCHEARTFAILURE.112.000143. [DOI] [PubMed] [Google Scholar]
- Reddy Vivek Y, Holmes David, Doshi Shephal K, Neuzil Petr, Kar Saibal. Safety of percutaneous left atrial appendage closure: results from the Watchman Left Atrial Appendage System for Embolic Protection in Patients with AF (PROTECT AF) clinical trial and the Continued Access Registry. Circulation. 2011 Feb 1;123 (4):417–24. doi: 10.1161/CIRCULATIONAHA.110.976449. [DOI] [PubMed] [Google Scholar]
- Grogan M, Smith H C, Gersh B J, Wood D L. Left ventricular dysfunction due to atrial fibrillation in patients initially believed to have idiopathic dilated cardiomyopathy. Am. J. Cardiol. 1992 Jun 15;69 (19):1570–3. doi: 10.1016/0002-9149(92)90705-4. [DOI] [PubMed] [Google Scholar]
- Van Gelder Isabelle C, Groenveld Hessel F, Crijns Harry J G M, Tuininga Ype S, Tijssen Jan G P, Alings A Marco, Hillege Hans L, Bergsma-Kadijk Johanna A, Cornel Jan H, Kamp Otto, Tukkie Raymond, Bosker Hans A, Van Veldhuisen Dirk J, Van den Berg Maarten P. Lenient versus strict rate control in patients with atrial fibrillation. N. Engl. J. Med. 2010 Apr 15;362 (15):1363–73. doi: 10.1056/NEJMoa1001337. [DOI] [PubMed] [Google Scholar]
- Rathore Saif S, Wang Yongfei, Krumholz Harlan M. Sex-based differences in the effect of digoxin for the treatment of heart failure. N. Engl. J. Med. 2002 Oct 31;347 (18):1403–11. doi: 10.1056/NEJMoa021266. [DOI] [PubMed] [Google Scholar]
- Ahmed Ali, Rich Michael W, Love Thomas E, Lloyd-Jones Donald M, Aban Inmaculada B, Colucci Wilson S, Adams Kirkwood F, Gheorghiade Mihai. Digoxin and reduction in mortality and hospitalization in heart failure: a comprehensive post hoc analysis of the DIG trial. Eur. Heart J. 2006 Jan;27 (2):178–86. doi: 10.1093/eurheartj/ehi687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitbeck Matthew G, Charnigo Richard J, Khairy Paul, Ziada Khaled, Bailey Alison L, Zegarra Milagros M, Shah Jignesh, Morales Gustavo, Macaulay Tracy, Sorrell Vincent L, Campbell Charles L, Gurley John, Anaya Paul, Nasr Hafez, Bai Rong, Di Biase Luigi, Booth David C, Jondeau Guillaume, Natale Andrea, Roy Denis, Smyth Susan, Moliterno David J, Elayi Claude S. Increased mortality among patients taking digoxin--analysis from the AFFIRM study. Eur. Heart J. 2013 May;34 (20):1481–8. doi: 10.1093/eurheartj/ehs348. [DOI] [PubMed] [Google Scholar]
- Gheorghiade Mihai, Fonarow Gregg C, van Veldhuisen Dirk J, Cleland John G F, Butler Javed, Epstein Andrew E, Patel Kanan, Aban Inmaculada B, Aronow Wilbert S, Anker Stefan D, Ahmed Ali. Lack of evidence of increased mortality among patients with atrial fibrillation taking digoxin: findings from post hoc propensity-matched analysis of the AFFIRM trial. Eur. Heart J. 2013 May;34 (20):1489–97. doi: 10.1093/eurheartj/eht120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjalmarson A, Goldstein S, Fagerberg B, Wedel H, Waagstein F, Kjekshus J, Wikstrand J, El Allaf D, Vítovec J, Aldershvile J, Halinen M, Dietz R, Neuhaus K L, Jánosi A, Thorgeirsson G, Dunselman P H, Gullestad L, Kuch J, Herlitz J, Rickenbacher P, Ball S, Gottlieb S, Deedwania P. Effects of controlled-release metoprolol on total mortality, hospitalizations, and well-being in patients with heart failure: the Metoprolol CR/XL Randomized Intervention Trial in congestive heart failure (MERIT-HF). MERIT-HF Study Group. JAMA. 2000 Mar 8;283 (10):1295–302. doi: 10.1001/jama.283.10.1295. [DOI] [PubMed] [Google Scholar]
- Leizorovicz Alain, Lechat Philippe, Cucherat Michel, Bugnard Françoise. Bisoprolol for the treatment of chronic heart failure: a meta-analysis on individual data of two placebo-controlled studies--CIBIS and CIBIS II. Cardiac Insufficiency Bisoprolol Study. Am. Heart J. 2002 Feb;143 (2):301–7. doi: 10.1067/mhj.2002.120768. [DOI] [PubMed] [Google Scholar]
- Packer Milton, Fowler Michael B, Roecker Ellen B, Coats Andrew J S, Katus Hugo A, Krum Henry, Mohacsi Paul, Rouleau Jean L, Tendera Michal, Staiger Christoph, Holcslaw Terry L, Amann-Zalan Ildiko, DeMets David L. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation. 2002 Oct 22;106 (17):2194–9. doi: 10.1161/01.cir.0000035653.72855.bf. [DOI] [PubMed] [Google Scholar]
- Joglar J A, Acusta A P, Shusterman N H, Ramaswamy K, Kowal R C, Barbera S J, Hamdan M H, Page R L. Effect of carvedilol on survival and hemodynamics in patients with atrial fibrillation and left ventricular dysfunction: retrospective analysis of the US Carvedilol Heart Failure Trials Program. Am. Heart J. 2001 Sep;142 (3):498–501. doi: 10.1067/mhj.2001.117318. [DOI] [PubMed] [Google Scholar]
- Hunt Sharon Ann, Abraham William T, Chin Marshall H, Feldman Arthur M, Francis Gary S, Ganiats Theodore G, Jessup Mariell, Konstam Marvin A, Mancini Donna M, Michl Keith, Oates John A, Rahko Peter S, Silver Marc A, Stevenson Lynne Warner, Yancy Clyde W. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009 Apr 14;119 (14):e391–479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- Farshi R, Kistner D, Sarma J S, Longmate J A, Singh B N. Ventricular rate control in chronic atrial fibrillation during daily activity and programmed exercise: a crossover open-label study of five drug regimens. J. Am. Coll. Cardiol. 1999 Feb;33 (2):304–10. doi: 10.1016/s0735-1097(98)00561-0. [DOI] [PubMed] [Google Scholar]
- Wood M A, Brown-Mahoney C, Kay G N, Ellenbogen K A. Clinical outcomes after ablation and pacing therapy for atrial fibrillation : a meta-analysis. Circulation. 2000 Mar 14;101 (10):1138–44. doi: 10.1161/01.cir.101.10.1138. [DOI] [PubMed] [Google Scholar]
- Vaidya VR, Shen WK, Asirvatham SJ. Atrioventricular nodal ablation and pacemaker therapy: role in the management of atrial fibrillation. Journal of Innovations in Cardiac Rhythm Management . 2013;4:1136–1144. [Google Scholar]
- Doshi Rahul N, Daoud Emile G, Fellows Christopher, Turk Kyong, Duran Aurelio, Hamdan Mohamed H, Pires Luis A. Left ventricular-based cardiac stimulation post AV nodal ablation evaluation (the PAVE study). J. Cardiovasc. Electrophysiol. 2005 Nov;16 (11):1160–5. doi: 10.1111/j.1540-8167.2005.50062.x. [DOI] [PubMed] [Google Scholar]
- Khan Mohammed N, Jaïs Pierre, Cummings Jennifer, Di Biase Luigi, Sanders Prashanthan, Martin David O, Kautzner Josef, Hao Steven, Themistoclakis Sakis, Fanelli Raffaele, Potenza Domenico, Massaro Raimondo, Wazni Oussama, Schweikert Robert, Saliba Walid, Wang Paul, Al-Ahmad Amin, Beheiry Salwa, Santarelli Pietro, Starling Randall C, Dello Russo Antonio, Pelargonio Gemma, Brachmann Johannes, Schibgilla Volker, Bonso Aldo, Casella Michela, Raviele Antonio, Haïssaguerre Michel, Natale Andrea. Pulmonary-vein isolation for atrial fibrillation in patients with heart failure. N. Engl. J. Med. 2008 Oct 23;359 (17):1778–85. doi: 10.1056/NEJMoa0708234. [DOI] [PubMed] [Google Scholar]
- Sonne Kai, Patel Dimpi, Mohanty Prasant, Armaganijan Luciana, Riedlbauchova Lucie, El-Ali Moataz, Di Biase Luigi, Venkatraman Preeti, Shaheen Mazen, Kozeluhova Marketa, Schweikert Robert, Burkhardt J David, Canby Robert, Wazni Oussama, Saliba Walid, Natale Andrea. Pulmonary vein antrum isolation, atrioventricular junction ablation, and antiarrhythmic drugs combined with direct current cardioversion: survival rates at 7 years follow-up. J Interv Card Electrophysiol. 2009 Nov;26 (2):121–6. doi: 10.1007/s10840-009-9436-1. [DOI] [PubMed] [Google Scholar]
- Wyse D G, Waldo A L, DiMarco J P, Domanski M J, Rosenberg Y, Schron E B, Kellen J C, Greene H L, Mickel M C, Dalquist J E, Corley S D. A comparison of rate control and rhythm control in patients with atrial fibrillation. N. Engl. J. Med. 2002 Dec 5;347 (23):1825–33. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- Van Gelder Isabelle C, Hagens Vincent E, Bosker Hans A, Kingma J Herre, Kamp Otto, Kingma Tsjerk, Said Salah A, Darmanata Julius I, Timmermans Alphons J M, Tijssen Jan G P, Crijns Harry J G M. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N. Engl. J. Med. 2002 Dec 5;347 (23):1834–40. doi: 10.1056/NEJMoa021375. [DOI] [PubMed] [Google Scholar]
- Freudenberger Ronald S, Wilson Alan C, Kostis John B. Comparison of rate versus rhythm control for atrial fibrillation in patients with left ventricular dysfunction (from the AFFIRM Study). Am. J. Cardiol. 2007 Jul 15;100 (2):247–52. doi: 10.1016/j.amjcard.2007.02.101. [DOI] [PubMed] [Google Scholar]
- Deedwania P C, Singh B N, Ellenbogen K, Fisher S, Fletcher R, Singh S N. Spontaneous conversion and maintenance of sinus rhythm by amiodarone in patients with heart failure and atrial fibrillation: observations from the veterans affairs congestive heart failure survival trial of antiarrhythmic therapy (CHF-STAT). The Department of Veterans Affairs CHF-STAT Investigators. Circulation. 1998 Dec 8;98 (23):2574–9. doi: 10.1161/01.cir.98.23.2574. [DOI] [PubMed] [Google Scholar]
- Pedersen Ole Dyg, Brendorp Bente, Elming Hanne, Pehrson Steen, Køber Lars, Torp-Pedersen Christian. Does conversion and prevention of atrial fibrillation enhance survival in patients with left ventricular dysfunction? Evidence from the Danish Investigations of Arrhythmia and Mortality ON Dofetilide/(DIAMOND) study. Card Electrophysiol Rev. 2003 Sep;7 (3):220–4. doi: 10.1023/B:CEPR.0000012386.82055.81. [DOI] [PubMed] [Google Scholar]
- Roy Denis, Talajic Mario, Nattel Stanley, Wyse D George, Dorian Paul, Lee Kerry L, Bourassa Martial G, Arnold J Malcolm O, Buxton Alfred E, Camm A John, Connolly Stuart J, Dubuc Marc, Ducharme Anique, Guerra Peter G, Hohnloser Stefan H, Lambert Jean, Le Heuzey Jean-Yves, O'Hara Gilles, Pedersen Ole Dyg, Rouleau Jean-Lucien, Singh Bramah N, Stevenson Lynne Warner, Stevenson William G, Thibault Bernard, Waldo Albert L. Rhythm control versus rate control for atrial fibrillation and heart failure. N. Engl. J. Med. 2008 Jun 19;358 (25):2667–77. doi: 10.1056/NEJMoa0708789. [DOI] [PubMed] [Google Scholar]
- Shelton R J, Clark A L, Goode K, Rigby A S, Houghton T, Kaye G C, Cleland J G F. A randomised, controlled study of rate versus rhythm control in patients with chronic atrial fibrillation and heart failure: (CAFE-II Study). Heart. 2009 Jun;95 (11):924–30. doi: 10.1136/hrt.2008.158931. [DOI] [PubMed] [Google Scholar]
- Weinfeld M S, Drazner M H, Stevenson W G, Stevenson L W. Early outcome of initiating amiodarone for atrial fibrillation in advanced heart failure. J. Heart Lung Transplant. 2000 Jul;19 (7):638–43. doi: 10.1016/s1053-2498(00)00123-6. [DOI] [PubMed] [Google Scholar]
- Bardy Gust H, Lee Kerry L, Mark Daniel B, Poole Jeanne E, Packer Douglas L, Boineau Robin, Domanski Michael, Troutman Charles, Anderson Jill, Johnson George, McNulty Steven E, Clapp-Channing Nancy, Davidson-Ray Linda D, Fraulo Elizabeth S, Fishbein Daniel P, Luceri Richard M, Ip John H. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N. Engl. J. Med. 2005 Jan 20;352 (3):225–37. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- Torp-Pedersen C, Møller M, Bloch-Thomsen P E, Køber L, Sandøe E, Egstrup K, Agner E, Carlsen J, Videbaek J, Marchant B, Camm A J. Dofetilide in patients with congestive heart failure and left ventricular dysfunction. Danish Investigations of Arrhythmia and Mortality on Dofetilide Study Group. N. Engl. J. Med. 1999 Sep 16;341 (12):857–65. doi: 10.1056/NEJM199909163411201. [DOI] [PubMed] [Google Scholar]
- Hohnloser Stefan H, Crijns Harry J G M, van Eickels Martin, Gaudin Christophe, Page Richard L, Torp-Pedersen Christian, Connolly Stuart J. Effect of dronedarone on cardiovascular events in atrial fibrillation. N. Engl. J. Med. 2009 Feb 12;360 (7):668–78. doi: 10.1056/NEJMoa0803778. [DOI] [PubMed] [Google Scholar]
- Køber Lars, Torp-Pedersen Christian, McMurray John J V, Gøtzsche Ole, Lévy Samuel, Crijns Harry, Amlie Jan, Carlsen Jan. Increased mortality after dronedarone therapy for severe heart failure. N. Engl. J. Med. 2008 Jun 19;358 (25):2678–87. doi: 10.1056/NEJMoa0800456. [DOI] [PubMed] [Google Scholar]
- Connolly Stuart J, Camm A John, Halperin Jonathan L, Joyner Campbell, Alings Marco, Amerena John, Atar Dan, Avezum Álvaro, Blomström Per, Borggrefe Martin, Budaj Andrzej, Chen Shih-Ann, Ching Chi Keong, Commerford Patrick, Dans Antonio, Davy Jean-Marc, Delacrétaz Etienne, Di Pasquale Giuseppe, Diaz Rafael, Dorian Paul, Flaker Greg, Golitsyn Sergey, Gonzalez-Hermosillo Antonio, Granger Christopher B, Heidbüchel Hein, Kautzner Josef, Kim June Soo, Lanas Fernando, Lewis Basil S, Merino Jose L, Morillo Carlos, Murin Jan, Narasimhan Calambur, Paolasso Ernesto, Parkhomenko Alexander, Peters Nicholas S, Sim Kui-Hian, Stiles Martin K, Tanomsup Supachai, Toivonen Lauri, Tomcsányi János, Torp-Pedersen Christian, Tse Hung-Fat, Vardas Panos, Vinereanu Dragos, Xavier Denis, Zhu Jun, Zhu Jun-Ren, Baret-Cormel Lydie, Weinling Estelle, Staiger Christoph, Yusuf Salim, Chrolavicius Susan, Afzal Rizwan, Hohnloser Stefan H. Dronedarone in high-risk permanent atrial fibrillation. N. Engl. J. Med. 2011 Dec 15;365 (24):2268–76. doi: 10.1056/NEJMoa1109867. [DOI] [PubMed] [Google Scholar]
- Waldo A L, Camm A J, deRuyter H, Friedman P L, MacNeil D J, Pauls J F, Pitt B, Pratt C M, Schwartz P J, Veltri E P. Effect of d-sotalol on mortality in patients with left ventricular dysfunction after recent and remote myocardial infarction. The SWORD Investigators. Survival With Oral d-Sotalol. Lancet. 1996 Jul 6;348 (9019):7–12. doi: 10.1016/s0140-6736(96)02149-6. [DOI] [PubMed] [Google Scholar]
- Farkowski Michał M. [Commentary to the article: Wilber DJ, Pappone C, Neuzil P et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA, 2010; 303: 333-340]. Kardiol Pol. 2010 May;68 (5):609–11. [PubMed] [Google Scholar]
- Piccini Jonathan P, Lopes Renato D, Kong Melissa H, Hasselblad Vic, Jackson Kevin, Al-Khatib Sana M. Pulmonary vein isolation for the maintenance of sinus rhythm in patients with atrial fibrillation: a meta-analysis of randomized, controlled trials. Circ Arrhythm Electrophysiol. 2009 Dec;2 (6):626–33. doi: 10.1161/CIRCEP.109.856633. [DOI] [PubMed] [Google Scholar]
- Calkins Hugh, Reynolds Matthew R, Spector Peter, Sondhi Manu, Xu Yingxin, Martin Amber, Williams Catherine J, Sledge Isabella. Treatment of atrial fibrillation with antiarrhythmic drugs or radiofrequency ablation: two systematic literature reviews and meta-analyses. Circ Arrhythm Electrophysiol. 2009 Aug;2 (4):349–61. doi: 10.1161/CIRCEP.108.824789. [DOI] [PubMed] [Google Scholar]
- Hsu Li-Fern, Jaïs Pierre, Sanders Prashanthan, Garrigue Stéphane, Hocini Mélèze, Sacher Fréderic, Takahashi Yoshihide, Rotter Martin, Pasquié Jean-Luc, Scavée Christophe, Bordachar Pierre, Clémenty Jacques, Haïssaguerre Michel. Catheter ablation for atrial fibrillation in congestive heart failure. N. Engl. J. Med. 2004 Dec 2;351 (23):2373–83. doi: 10.1056/NEJMoa041018. [DOI] [PubMed] [Google Scholar]
- Chen Michael S, Marrouche Nassir F, Khaykin Yaariv, Gillinov A Marc, Wazni Oussama, Martin David O, Rossillo Antonio, Verma Atul, Cummings Jennifer, Erciyes Demet, Saad Eduardo, Bhargava Mandeep, Bash Dianna, Schweikert Robert, Burkhardt David, Williams-Andrews Michelle, Perez-Lugones Alejandro, Abdul-Karim Ahmad, Saliba Walid, Natale Andrea. Pulmonary vein isolation for the treatment of atrial fibrillation in patients with impaired systolic function. J. Am. Coll. Cardiol. 2004 Mar 17;43 (6):1004–9. doi: 10.1016/j.jacc.2003.09.056. [DOI] [PubMed] [Google Scholar]
- Tondo Claudio, Mantica Massimo, Russo Giovanni, Avella Andrea, De Luca Lucia, Pappalardo Augusto, Fagundes Rafael Lopes, Picchio Edo, Laurenzi Francesco, Piazza Vito, Bisceglia Irma. Pulmonary vein vestibule ablation for the control of atrial fibrillation in patients with impaired left ventricular function. Pacing Clin Electrophysiol. 2006 Sep;29 (9):962–70. doi: 10.1111/j.1540-8159.2006.00471.x. [DOI] [PubMed] [Google Scholar]
- Gentlesk Philip J, Sauer William H, Gerstenfeld Edward P, Lin David, Dixit Sanjay, Zado Erica, Callans David, Marchlinski Francis E. Reversal of left ventricular dysfunction following ablation of atrial fibrillation. J. Cardiovasc. Electrophysiol. 2007 Jan;18 (1):9–14. doi: 10.1111/j.1540-8167.2006.00653.x. [DOI] [PubMed] [Google Scholar]
- Lutomsky Boris A, Rostock Thomas, Koops Andreas, Steven Daniel, Müllerleile Kai, Servatius Helge, Drewitz Imke, Ueberschär Denis, Plagemann Thorsten, Ventura Rodolfo, Meinertz Thomas, Willems Stephan. Catheter ablation of paroxysmal atrial fibrillation improves cardiac function: a prospective study on the impact of atrial fibrillation ablation on left ventricular function assessed by magnetic resonance imaging. Europace. 2008 May;10 (5):593–9. doi: 10.1093/europace/eun076. [DOI] [PubMed] [Google Scholar]
- Nademanee Koonlawee, Schwab Mark C, Kosar Erol M, Karwecki Margaret, Moran Michael D, Visessook Nithi, Michael Anthony Don, Ngarmukos Tachapong. Clinical outcomes of catheter substrate ablation for high-risk patients with atrial fibrillation. J. Am. Coll. Cardiol. 2008 Feb 26;51 (8):843–9. doi: 10.1016/j.jacc.2007.10.044. [DOI] [PubMed] [Google Scholar]
- Khan Mohammed N, Jaïs Pierre, Cummings Jennifer, Di Biase Luigi, Sanders Prashanthan, Martin David O, Kautzner Josef, Hao Steven, Themistoclakis Sakis, Fanelli Raffaele, Potenza Domenico, Massaro Raimondo, Wazni Oussama, Schweikert Robert, Saliba Walid, Wang Paul, Al-Ahmad Amin, Beheiry Salwa, Santarelli Pietro, Starling Randall C, Dello Russo Antonio, Pelargonio Gemma, Brachmann Johannes, Schibgilla Volker, Bonso Aldo, Casella Michela, Raviele Antonio, Haïssaguerre Michel, Natale Andrea. Pulmonary-vein isolation for atrial fibrillation in patients with heart failure. N. Engl. J. Med. 2008 Oct 23;359 (17):1778–85. doi: 10.1056/NEJMoa0708234. [DOI] [PubMed] [Google Scholar]
- De Potter Tom, Berruezo Antonio, Mont Lluis, Matiello Maria, Tamborero David, Santibañez Claudio, Benito Begoña, Zamorano Nibaldo, Brugada Josep. Left ventricular systolic dysfunction by itself does not influence outcome of atrial fibrillation ablation. Europace. 2010 Jan;12 (1):24–9. doi: 10.1093/europace/eup309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald Michael R, Connelly Derek T, Hawkins Nathaniel M, Steedman Tracey, Payne John, Shaw Morag, Denvir Martin, Bhagra Sai, Small Sandy, Martin William, McMurray John J V, Petrie Mark C. Radiofrequency ablation for persistent atrial fibrillation in patients with advanced heart failure and severe left ventricular systolic dysfunction: a randomised controlled trial. Heart. 2011 May;97 (9):740–7. doi: 10.1136/hrt.2010.207340. [DOI] [PubMed] [Google Scholar]
- Cha Yong-Mei, Wokhlu Anita, Asirvatham Samuel J, Shen Win-Kuang, Friedman Paul A, Munger Thomas M, Oh Jae K, Monahan Kristi H, Haroldson Janis M, Hodge David O, Herges Regina M, Hammill Stephen C, Packer Douglas L. Success of ablation for atrial fibrillation in isolated left ventricular diastolic dysfunction: a comparison to systolic dysfunction and normal ventricular function. Circ Arrhythm Electrophysiol. 2011 Oct;4 (5):724–32. doi: 10.1161/CIRCEP.110.960690. [DOI] [PubMed] [Google Scholar]
- Jones David G, Haldar Shouvik K, Hussain Wajid, Sharma Rakesh, Francis Darrel P, Rahman-Haley Shelley L, McDonagh Theresa A, Underwood S Richard, Markides Vias, Wong Tom. A randomized trial to assess catheter ablation versus rate control in the management of persistent atrial fibrillation in heart failure. J. Am. Coll. Cardiol. 2013 May 7;61 (18):1894–903. doi: 10.1016/j.jacc.2013.01.069. [DOI] [PubMed] [Google Scholar]
- Nedios Sotirios, Sommer Philipp, Dagres Nikolaos, Kosiuk Jedrzej, Arya Arash, Richter Sergio, Gaspar Thomas, Kanagkinis Nikolaos, Dinov Borislav, Piorkowski Christopher, Bollmann Andreas, Hindricks Gerhard, Rolf Sascha. Long-term follow-up after atrial fibrillation ablation in patients with impaired left ventricular systolic function: the importance of rhythm and rate control. Heart Rhythm. 2014 Mar;11 (3):344–51. doi: 10.1016/j.hrthm.2013.12.031. [DOI] [PubMed] [Google Scholar]
- Hunter Ross J, Berriman Thomas J, Diab Ihab, Kamdar Ravindu, Richmond Laura, Baker Victoria, Goromonzi Farai, Sawhney Vinit, Duncan Edward, Page Stephen P, Ullah Waqas, Unsworth Beth, Mayet Jamil, Dhinoja Mehul, Earley Mark J, Sporton Simon, Schilling Richard J. A randomized controlled trial of catheter ablation versus medical treatment of atrial fibrillation in heart failure (the CAMTAF trial). Circ Arrhythm Electrophysiol. 2014 Feb;7 (1):31–8. doi: 10.1161/CIRCEP.113.000806. [DOI] [PubMed] [Google Scholar]
- Machino-Ohtsuka Tomoko, Seo Yoshihiro, Ishizu Tomoko, Sugano Akinori, Atsumi Akiko, Yamamoto Masayoshi, Kawamura Ryo, Machino Takeshi, Kuroki Kenji, Yamasaki Hiro, Igarashi Miyako, Sekiguchi Yukio, Aonuma Kazutaka. Efficacy, safety, and outcomes of catheter ablation of atrial fibrillation in patients with heart failure with preserved ejection fraction. J. Am. Coll. Cardiol. 2013 Nov 12;62 (20):1857–65. doi: 10.1016/j.jacc.2013.07.020. [DOI] [PubMed] [Google Scholar]
- Ejima Koichiro, Shoda Morio, Arai Kotaro, Suzuki Atsushi, Yagishita Daigo, Yagishita Yoshimi, Yashiro Bun, Sato Takahiro, Manaka Tetsuyuki, Ashihara Kyomi, Hagiwara Nobuhisa. Impact of diastolic dysfunction on the outcome of catheter ablation in patients with atrial fibrillation. Int. J. Cardiol. 2013 Mar 20;164 (1):88–93. doi: 10.1016/j.ijcard.2011.06.093. [DOI] [PubMed] [Google Scholar]
- Kosiuk Jedrzej, Buchta Piotr, Gaspar Thomas, Arya Arash, Piorkowski Christopher, Rolf Sascha, Sommer Philipp, Husser Daniela, Hindricks Gerhard, Bollmann Andreas. Prevalence and predictors of worsened left ventricular diastolic dysfunction after catheter ablation of atrial fibrillation. Int. J. Cardiol. 2013 Oct 9;168 (4):3613–5. doi: 10.1016/j.ijcard.2013.05.047. [DOI] [PubMed] [Google Scholar]
- Marrouche Nassir F, Brachmann Johannes. Catheter ablation versus standard conventional treatment in patients with left ventricular dysfunction and atrial fibrillation (CASTLE-AF) - study design. Pacing Clin Electrophysiol. 2009 Aug;32 (8):987–94. doi: 10.1111/j.1540-8159.2009.02428.x. [DOI] [PubMed] [Google Scholar]
- Gibson Douglas N, Di Biase Luigi, Mohanty Prasant, Patel Jigar D, Bai Rong, Sanchez Javier, Burkhardt J David, Heywood J Thomas, Johnson Allen D, Rubenson David S, Horton Rodney, Gallinghouse G Joseph, Beheiry Salwa, Curtis Guy P, Cohen David N, Lee Mark Y, Smith Michael R, Gopinath Devi, Lewis William R, Natale Andrea. Stiff left atrial syndrome after catheter ablation for atrial fibrillation: clinical characterization, prevalence, and predictors. Heart Rhythm. 2011 Sep;8 (9):1364–71. doi: 10.1016/j.hrthm.2011.02.026. [DOI] [PubMed] [Google Scholar]