Abstract
Atrial fibrillation ablation has evolved considerably over the last few years. In this article we review current and past catheter ablation techniques, with a special focus on new simplified systems that allow a faster and simpler procedure, so called “one-shot” atrial fibrillation ablation.
Introduction
Atrial fibrillation (AF) is the most important tachyarrhytmia in the developed world, due to its high prevalence, incidence and impact on morbidity and mortality.[1-3] Ablation is the only potentially curative therapy for this disease.
AF Ablation: The First Steps
AF ablation originally developed as a surgical procedure consisting of a series of trans-mural incisions on the left atrium (LA), first performed by James Cox in 1987, creating an atrial “maze”, and thus named the Cox-Maze procedure. The procedure evolved until what is known today as the Cox-Maze III.[4] Due to its reported high rate of efficacy, with freedom from AF at 12 months > 90%,[5] this procedure has been advocated by many as the gold-standard in AF ablation.[5-7] However, despite the fact that a less aggressive modification of this procedure, using other techniques such as radiofrequency (RF) and cryoablation was developed - the Cox-Maze IV procedure[8] - published experience is limited, and the procedure still heralds an invasive surgical approach.
Pulmonary Veins As Triggers For AF: The Cornerstone Of Ablation
Despite the fact that the Cox-Maze procedure used incisions around the pulmonary veins (PV), it wasn’t until the 1990’s, with the pivotal work by Haïssaguerre et al,[9] that its major role in the initiation of AF was clearly recognized. His team established that focal ectopic beats originating in the PV could trigger AF (representing 94% of atrial ectopic beats foci), and that catheter focal ablation of the pulmonary veins could halt its initiation. This discovery spawned the beginning of the catheter-based procedure for the ablation of AF.
One initial strategy consisted of pulmonary vein disconnection by isolation of the pulmonary vein ostium using a guiding loop shaped multipolar catheter (Lasso, Biosense-Webster). Using this strategy, Haisaguerre et al yielded a 71% freedom from AF and AAD at 8 ± 5 months post-procedure.[10] A similar technique, using intra-cardiac echocardiography, to precisely determine the PV antrum, was also developed by Natale et al.[11] Kuck et al also developed a strategy consisting of circumferential PV isolation, yet isolating not each PV individually, but rather the LA around the PVs, i.e. the PVs antrum. This technique (known as the Double Lasso) used the catheter around each pair of ipsi-lateral PVs. Using this method, 31 (75.6%) of 41 patients were free of AF and AAD 4 weeks after the procedure. Repeat procedure was performed later in an additional 8 patients, resulting in a total of 95.1% of patients free of PAF after ablation (mean 131±12 days of follow-up).[12]
Pappone et al[13] used a new ablative technique by creating circumferential lesions around the PV ostia, based on an LA anatomic map created by a 3D non-fluoroscopic mapping system (CARTO; Biosense Webster). In a large series of 251 consecutive patients with paroxysmal (n=179) or persistent (n=72) AF, the procedure had an AF freedom (mean follow-up 10.4±4.5 months) of 85% in patients with paroxysmal AF and 69% in patients with persistent AF.[14]
A different approach, also using a single trans-septal puncture, has been described, the so called pace and ablate technique. Using this strategy, PV isolation is carried out by simultaneous pacing and ablation allowing the confirmation of PV bidirectional block using a single tip catheter.[15]
Therefore, in spite of these different ablation techniques, the common goal in paroxysmal atrial fibrillation ablation is the complete isolation of the PVs.
The AF Ablation Registry undertaken in 2009 provided very important data on the usefulness and safety of these procedures. Overall, a total of 85 centers, comprising 20.825 ablations and around 16.309 patients, were included. Freedom from AF and AAD was observed in 70% of patients and another 10% were free from AF using previously ineffective AAD, over a mean 18 months of follow-up. Major complications occurred in 4.5% of patients. The most common procedures were PVs isolation, be it circumferential ablation (48.2%) or Lasso-guided ostial electric disconnection (27.4%). The net clinical benefit was 80%.[16]
The publication of this registry and of several randomized trials favorably comparing catheter ablation with anti-arrhythmic drug treatment lead the acceptance of pulmonary vein isolation (PVI) as a first line PAF treatment, with a Class I, level A indication in the HRS/EHRA/ECAS consensus for “PAF refractory or intolerant to at least one Class 1 or 3 anti-arrhythmic medication”.[17]
A Simplified Approach To Pulmonary Vein Isolation: One-Shot AF Ablation?
AF ablation conventional techniques using RF irrigated catheters and point-by-point ablation are complex, lengthy procedures which require extensive LA mapping and the use of multiple catheters. Also, gaps in the ablation lines can occur. The potential complications of collateral esophageal damage and pulmonary vein ostial stenosis are noteworthy as well. All of these result in a slow learning curve, with success and safety quite dependent on operator experience. Therefore, the use of a simpler, faster and less expensive technique for PV isolation is desirable.
In the last few years, efforts have focused on developing ablation techniques which overcome these limitations, namely the so-called single-shot ablation with the possibility of a single trans-septal puncture and the simultaneous application of energy in order to perform easier, faster and safer procedures with less complications and increased success. The types of energy studied for this purpose were radiofrequency (initially with the PVAC system and more recently the nMarq system), cryo-energy, laser and ultrasound.
The PVAC System
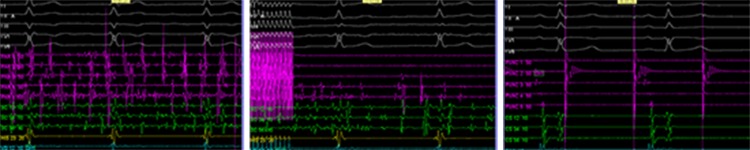
One of the new catheters is the pulmonary vein ablation catheter (PVAC, Medtronic), a decapolar, circular, over the wire mapping and ablation catheter designed for pulmonary vein (PV) isolation (figure 1). It is a non-irrigated catheter, with duty cycling to facilitate cooling. It has a lower input power (10 W), temperature sensor in each electrode, and also an automatic power adjustment to control temperature in each electrode pair with the capacity to shut off in case of suboptimal application. The application of energy can shift from unipolar (deeper lesion) to bipolar (more contiguous lesion). Figure 2 depicts intra-procedural electrograms before, during and after PV isolation.
Figure 1. Fluroscopy imaging of the PVAC catheter in the left inferior pulmonary vein (arrow).
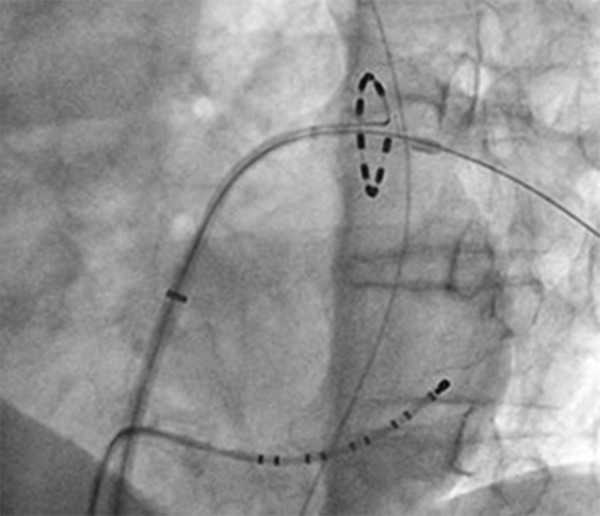
Figure 2. Intra-procedural electrograms before (A), during (B) and after (C) ablation (the latter also depicting PV pacing after obtaining sinus rhythm with no atrial capture, therefore confirming PV isolation).
Initial results were from a single center trial that used this catheter in a population of 98 patients with paroxysmal AF. Freedom from AF was 83% at 6 months. Mean procedure time was 84 ± 29 minutes, and fluoroscopy time 18 ± 8 minutes.[18]
Another trial included 102 consecutive patients with either paroxysmal or persistent drug refractory AF. Freedom from AF and AAD at a mean follow-up of 12.2 +/- 3.9 months was 60.8%. Total mean procedure time was 139 ± 38 minutes and mean fluoroscopy time 17 ± 12 min.[19] Other similar trial showed identical results.[20]
A multicenter trial in 5 European centers comprising 50 patients with persistent atrial fibrillation assessed the efficacy and safety of this procedure. Success rates at 6 months showed freedom from AF with AAD of 80% and freedom from AF alone in 64%. At 20 ± 4 months after the last procedure, 66% of patients had a >80% reduction in AF burden, with 45% free of AF and with AAD. Repeat procedure was required in 50% of cases. Procedure time and fluoroscopy durations were 155 ± 40 min and 55 ± 35 minutes, respectively. No adverse events were noted.[21]
A recent systematic review and meta-analysis of this type of procedures, which included patients with paroxysmal AF or short-lasting persistent AF, reported a 98.9% acute procedural success, a 6-month 81,4% success and a 12 month 59.6% success.[22]
Finally, a randomized trial compared the clinical outcome of pulmonary vein (PV) isolation using a multipolar circular ablation catheter (PVAC group), with conventional PV isolation using an irrigated-tip ablation catheter and the CARTO mapping system. This trial included 102 patients with paroxysmal atrial fibrillation. The efficacy regarding AF ablation was similar in both groups, yet the total procedural and fluoroscopy times were close to half in the PVAC group.[23]
Despite these favorable results, the PVAC catheter ablation technique is not free of problems. Due to its non-irrigated technology, this catheter may be more prone to clot formation during ablation as showed by Gaita et al.[24] A comparative study reported a higher incidence of subclinical cardioembolic cerebrocascular events detected by MRI. Specifically, 4.3% of the irrigated RF group of patients developed this complication, while 37.5% of the PVAC group developed such an event. Clinical neurological examination, however, was normal in both groups.[25] Although the actual clinical significance of these events remains to be defined, the results certainly warrant caution.
In order to overcome this limitation, a trial with specific procedural changes was carried out. The procedure was undertaken under therapeutic vitamin K antagonist and heparin for an activated clotting time > 350 s, the catheter was loaded into the introducer while submerged to prevent air entrance, and the distal or proximal electrode of the circular catheter was shut off to prevent bipolar RF interaction. Using this strategy, the rate of subclinical cerebral embolism was reduced to a much lower 1.7%.[26]
Thus, the PVAC procedure offers the advantage of similar efficacy, with simpler, cheaper and faster procedures. It is, however, limited by anatomical variations such as the presence of accessory pulmonary veins or common pulmonary ostium, and possibly a higher rate of cerebrovascular embolic events.
The nMARQ: All In One?
The nMARQ® (Biosense Webster) is a RF irrigated circular multipolar catheter which allows for simultaneous irrigation, mapping and ablation. The catheter is integrated into the CARTO electro-anatomic mapping system. It is currently under research in a randomized trial since March 2013. Primary completion date is estimated in September 2015 while study completion is expected by September 2018 (“nMARQTM Pulmonary Vein Isolation System for the Treatment of Paroxysmal Atrial Fibrillation”). Preliminary results of this multicenter Revolution Study have been presented in 160 AF patients with an acute success of 98.7%. The mean procedure time was 172 ± 58 min, the fluoroscopy time 26 ± 13 min and the ablation time 91 ± 46 min. After a mean follow up of 8 months the success rate was 70.8%. Similar results were reported in a single center experience[28] in 43 patients with 98% of acute success with a procedure duration of 133 minutes, without any clinical complication. A mean of 4.8 RF applications per vein was needed for effective PVI. Cerebral MRI and esophagus endoscopy were performed in all patients to identify potential collateral damage. Endoscopy identified sub-clinical thermal esophageal lesions in 14 patients. A total of 26 silent cerebral lesions occurred in 14 patients (mean diameter of 2.3 mm). Deneke et al also reported one fatal case of esophago-pericardial fistula using this technique.[29]
In conclusion, although nMarq technology efficacy seems very promising, long-term results are still lacking and its safety profile needs further investigation.
Cryoballoon Ablation
Another single-delivery approach to PVI is the cryoballoon (Artic Front®, Medtronic) ablation. This technique relies on a nitrogen balloon capable of reaching temperatures of -50ºC. The ballon is inserted into the ostium of the PV allowing a one-shot delivery. A more recent development includes a multipolar wire distal to the balloon to allow electrogram recording and evaluation of PV isolation during the energy application.
A recently published randomized controlled trial (STOP-AF) evaluated this technique in a population of 245 patients with paroxysmal symptomatic AF refractory to at least one AAD. Patients were randomized in a 2:1 fashion to cryoballoon ablation or drug therapy, but an additional 65 patients crossed over, resulting in a total 228 patients in the cryoablation group. Freedom from AF at 12 months was achieved in 63.4 ± 4.1 % (in intention to treat) patients and 61.6 ± 7.3% of crossover patients. Mean procedure duration time, including 31 repeat procedures, was 371 minutes, total cryoablation time was 66 minutes and fluoroscopy time was 63 minutes. Regarding adverse effects, PV stenosis occurred in 3.1% of patients, phrenic nerve palsy in 12.3% (with the majority resolving within 1 year, resulting in a 1.8% persistent palsy), and procedure related stroke in 0.4% of patients.[30]
Thus, the cryoablation technique appears to be another promising one-shot ablation technique, with similar advantages to the PVAC catheter, albeit with a greater risk of phrenic nerve palsy, yet an apparently reduced risk of stroke.
Ballon-Based Laser
This technique uses a laser balloon aproach (HeartLight®, CardioFocus). The clinical trial Pivotal Clinical Study of the CardioFocus Endoscopic Ablation System - Adaptive Contact (EAS-AC) (HeartLight) in Patients With Paroxysmal Atrial Fibrillation (PAF) is currently underway.[31] Trial results are expected at the end of 2014. This technique is therefore still considered as a research procedure, not yet ready for widespread clinical use.
Ballon-Based High-Intensity Ultrasound
This is a balloon-based ablation technique which, despite similar success rates to traditional RF ablation, was abandoned because of a high rate of esophageal colateral damage, with documentation of atrio-esophageal fistulae.[32]
The PVAC Experience: Data From Hospital De Santa Maria
Our initial experience with the PVAC system included 30 patients with paroxysmal or persistent AF, submitted to PV isolation from March 2009 to October 2010. The acute success for PVI was 94.7%, (108 of 114 ablated pulmonary veins, after 41 ± 17 RF applications) with procedure and fluoroscopy duration of 170 ± 73 min and 35 ± 17 min respectively. There were no acute clinical complications. After a follow-up of 13 ± 5 months, 69% of our patients were free from AF.
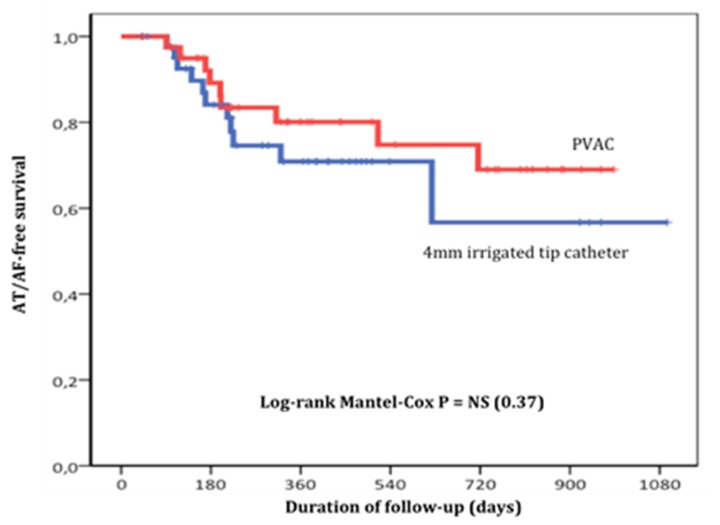
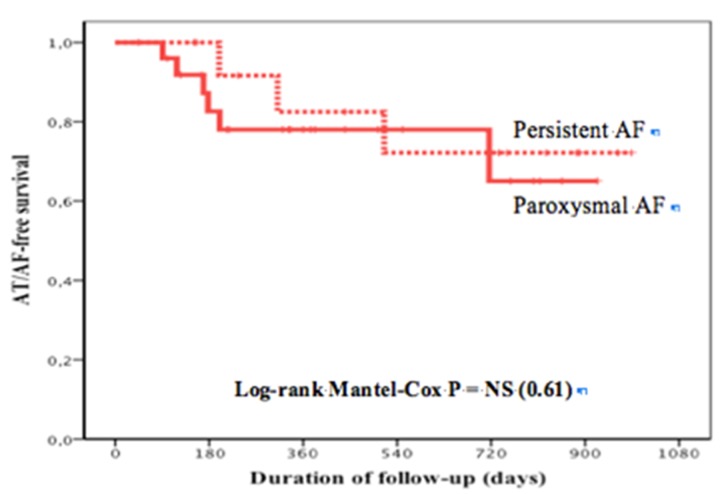
These encouraging results prompted the initiation of a comparative randomized trial between the PVAC system and the classical point-by-point 4 mm-tip irrigated-catheter ablation with electro-anatomic mapping, currently underway in our institution. The study population includes patients with paroxysmal or short-lasting (less than 1 year) persistent atrial fibrillation. All patients are submitted to cardiac MRI to evaluate left atrium and pulmonary vein anatomy and extent of atrium fibrosis. AF ablation success is evaluated with clinical and 7 day event recorder at 3, 6 and 12 months. Thus far we enrolled 102 patients (50 in the PVAC group and 52 in the conventional group), and the survival curves free of AF between the two techniques are similar between 70-80% at 1 year (Fig. 3). Among PVAC group, there was no difference in the ablation result between patients with persistent or paroxysmal AF (Fig. 4).
Figure 3. Kaplan-Meier curves depicting survival free from atrial tachycardia or AF.
Figure 4. Kaplan-Meier curves depicting survival free from atrial tachycardia or AF between patients with paroxysmal or persistent AF who underwent PVAC abation.
One-Shot Ablation For PVI: Conclusion
PV isolation is the cornerstone of paroxysmal or short-lasting persistent atrial fibrillation ablation. Single-shot devices have several theoretical advantages over conventional ablation. Their use is simpler, procedures can be faster and the learning curve is steeper, making operator experience dependency less of an issue. Several devices with different forms of energy are available in the market but some are still undergoing research studies (for example the laser-based balloon technology). Studies have showed that single-shot devices have equal success compared t o the conventional ablation and can be easier and faster in achieving PVI.
However, the presence of complications, mainly collateral damaged is noteworthy and may somehow be related to the energy source: esophageal damage with high-focus US, phrenic-nerve palsy with cryoenergy-balloon and cerebral ischemic lesions with non-irrigated RF energy. And despite its theoretically superior safety profile, both cerebral embolization and esophageal lesions can also occur with irrigated RF energy.
This type of devices can also face difficulties with left atrium anatomic variations (for example common pulmonary vein ostium or the presence of accessory pulmonary veins) or if there is a need for additional linear or focal lesions to complete AF ablation, after PV isolation. For example, if rotor, complex fractionated atrial electrograms, ganglionated plexus or right isthmus ablation are required during the procedure, tip catheters must still be used. Finally, other atrial arrhytmias may occur following AF ablation, such as left atrium macro-reentrant tachycardia, that also require the use of conventional tip catheters.
In spite of these limitations, the advantages of single-shot devices are very significant, and we believe they will be the dominant technology for PV isolation in the near future.
Disclosures
None.
References
- Go A S, Hylek E M, Phillips K A, Chang Y, Henault L E, Selby J V, Singer D E. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001 May 9;285 (18):2370–5. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- Kirchhof Paulus, Auricchio Angelo, Bax Jeroen, Crijns Harry, Camm John, Diener Hans-Christoph, Goette Andreas, Hindricks Gerd, Hohnloser Stefan, Kappenberger Lukas, Kuck Karl-Heinz, Lip Gregory Y H, Olsson Bertil, Meinertz Thomas, Priori Silvia, Ravens Ursula, Steinbeck Gerhard, Svernhage Elisabeth, Tijssen Jan, Vincent Alphons, Breithardt Günter. Outcome parameters for trials in atrial fibrillation: executive summary. Eur. Heart J. 2007 Nov;28 (22):2803–17. doi: 10.1093/eurheartj/ehm358. [DOI] [PubMed] [Google Scholar]
- Stewart S, Hart C L, Hole D J, McMurray J J. Population prevalence, incidence, and predictors of atrial fibrillation in the Renfrew/Paisley study. Heart. 2001 Nov;86 (5):516–21. doi: 10.1136/heart.86.5.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J L, Schuessler R B, D'Agostino H J, Stone C M, Chang B C, Cain M E, Corr P B, Boineau J P. The surgical treatment of atrial fibrillation. III. Development of a definitive surgical procedure. J. Thorac. Cardiovasc. Surg. 1991 Apr;101 (4):569–83. [PubMed] [Google Scholar]
- Gaynor Sydney L, Schuessler Richard B, Bailey Marci S, Ishii Yosuke, Boineau John P, Gleva Marye J, Cox James L, Damiano Ralph J. Surgical treatment of atrial fibrillation: predictors of late recurrence. J. Thorac. Cardiovasc. Surg. 2005 Jan;129 (1):104–11. doi: 10.1016/j.jtcvs.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Prasad Sunil M, Maniar Hersh S, Camillo Cindy J, Schuessler Richard B, Boineau John P, Sundt Thoralf M, Cox James L, Damiano Ralph J. The Cox maze III procedure for atrial fibrillation: long-term efficacy in patients undergoing lone versus concomitant procedures. J. Thorac. Cardiovasc. Surg. 2003 Dec;126 (6):1822–8. doi: 10.1016/s0022-5223(03)01287-x. [DOI] [PubMed] [Google Scholar]
- Johansson Birgitta I, Väärt Oskar, Edvardsson Nils, Nyström Britta, Scherstén Henrik, Karlsson Thomas, Berglin Eva. Low mortality and low rate of perceived and documented arrhythmias after Cox maze III surgery for atrial fibrillation. Pacing Clin Electrophysiol. 2014 Feb;37 (2):147–56. doi: 10.1111/pace.12286. [DOI] [PubMed] [Google Scholar]
- Damiano Ralph J, Bailey Marci. The Cox-Maze IV procedure for lone atrial fibrillation. Multimed Man Cardiothorac Surg. 2007 Jan 1;2007 (723):002758. doi: 10.1510/mmcts.2007.002758. [DOI] [PubMed] [Google Scholar]
- Haïssaguerre M, Jaïs P, Shah D C, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P, Clémenty J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N. Engl. J. Med. 1998 Sep 3;339 (10):659–66. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- Haïssaguerre M, Shah D C, Jaïs P, Hocini M, Yamane T, Deisenhofer I, Chauvin M, Garrigue S, Clémenty J. Electrophysiological breakthroughs from the left atrium to the pulmonary veins. Circulation. 2000 Nov 14;102 (20):2463–5. doi: 10.1161/01.cir.102.20.2463. [DOI] [PubMed] [Google Scholar]
- Verma Atul, Marrouche Nassir F, Natale Andrea. Pulmonary vein antrum isolation: intracardiac echocardiography-guided technique. J. Cardiovasc. Electrophysiol. 2004 Nov;15 (11):1335–40. doi: 10.1046/j.1540-8167.2004.04428.x. [DOI] [PubMed] [Google Scholar]
- Ouyang Feifan, Bänsch Dietmar, Ernst Sabine, Schaumann Anselm, Hachiya Hitoshi, Chen Minglong, Chun Julian, Falk Peter, Khanedani Afsaneh, Antz Matthias, Kuck Karl-Heinz. Complete isolation of left atrium surrounding the pulmonary veins: new insights from the double-Lasso technique in paroxysmal atrial fibrillation. Circulation. 2004 Oct 12;110 (15):2090–6. doi: 10.1161/01.CIR.0000144459.37455.EE. [DOI] [PubMed] [Google Scholar]
- Pappone C, Rosanio S, Oreto G, Tocchi M, Gugliotta F, Vicedomini G, Salvati A, Dicandia C, Mazzone P, Santinelli V, Gulletta S, Chierchia S. Circumferential radiofrequency ablation of pulmonary vein ostia: A new anatomic approach for curing atrial fibrillation. Circulation. 2000 Nov 21;102 (21):2619–28. doi: 10.1161/01.cir.102.21.2619. [DOI] [PubMed] [Google Scholar]
- Pappone C, Oreto G, Rosanio S, Vicedomini G, Tocchi M, Gugliotta F, Salvati A, Dicandia C, Calabrò M P, Mazzone P, Ficarra E, Di Gioia C, Gulletta S, Nardi S, Santinelli V, Benussi S, Alfieri O. Atrial electroanatomic remodeling after circumferential radiofrequency pulmonary vein ablation: efficacy of an anatomic approach in a large cohort of patients with atrial fibrillation. Circulation. 2001 Nov 20;104 (21):2539–44. doi: 10.1161/hc4601.098517. [DOI] [PubMed] [Google Scholar]
- Eitel Charlotte, Hindricks Gerhard, Sommer Philipp, Gaspar Thomas, Kircher Simon, Wetzel Ulrike, Dagres Nicos, Esato Masahiro, Bollmann Andreas, Husser Daniela, Hilbert Sebastian, Zaker-Shahrak Ruzbeh, Arya Arash, Piorkowski Christopher. Circumferential pulmonary vein isolation and linear left atrial ablation as a single-catheter technique to achieve bidirectional conduction block: the pace-and-ablate approach. Heart Rhythm. 2010;7 (2):157–64. doi: 10.1016/j.hrthm.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Cappato Riccardo, Calkins Hugh, Chen Shih-Ann, Davies Wyn, Iesaka Yoshito, Kalman Jonathan, Kim You-Ho, Klein George, Natale Andrea, Packer Douglas, Skanes Allan, Ambrogi Federico, Biganzoli Elia. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2010 Feb;3 (1):32–8. doi: 10.1161/CIRCEP.109.859116. [DOI] [PubMed] [Google Scholar]
- Calkins Hugh, Kuck Karl Heinz, Cappato Riccardo, Brugada Josep, Camm A John, Chen Shih-Ann, Crijns Harry J G, Damiano Ralph J, Davies D Wyn, DiMarco John, Edgerton James, Ellenbogen Kenneth, Ezekowitz Michael D, Haines David E, Haissaguerre Michel, Hindricks Gerhard, Iesaka Yoshito, Jackman Warren, Jalife José, Jais Pierre, Kalman Jonathan, Keane David, Kim Young-Hoon, Kirchhof Paulus, Klein George, Kottkamp Hans, Kumagai Koichiro, Lindsay Bruce D, Mansour Moussa, Marchlinski Francis E, McCarthy Patrick M, Mont J Lluis, Morady Fred, Nademanee Koonlawee, Nakagawa Hiroshi, Natale Andrea, Nattel Stanley, Packer Douglas L, Pappone Carlo, Prystowsky Eric, Raviele Antonio, Reddy Vivek, Ruskin Jeremy N, Shemin Richard J, Tsao Hsuan-Ming, Wilber David. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm. 2012 Apr;9 (4):632–696.e21. doi: 10.1016/j.hrthm.2011.12.016. [DOI] [PubMed] [Google Scholar]
- Boersma Lucas V A, Wijffels Maurits C E F, Oral Hakan, Wever Eric F D, Morady Fred. Pulmonary vein isolation by duty-cycled bipolar and unipolar radiofrequency energy with a multielectrode ablation catheter. Heart Rhythm. 2008 Dec;5 (12):1635–42. doi: 10.1016/j.hrthm.2008.08.037. [DOI] [PubMed] [Google Scholar]
- Beukema Rypko P, Beukema Willem P, Smit Jaap Jan J, Ramdat Misier Anand R, Delnoij Peter Paul H M, Wellens Hein, Elvan Arif. Efficacy of multi-electrode duty-cycled radiofrequency ablation for pulmonary vein disconnection in patients with paroxysmal and persistent atrial fibrillation. Europace. 2010 Apr;12 (4):502–7. doi: 10.1093/europace/euq023. [DOI] [PubMed] [Google Scholar]
- Wieczorek Marcus, Hoeltgen Reinhard, Akin Elvan, Salili Ali Reza, Oral Hakan, Morady Fred. Results of short-term and long-term pulmonary vein isolation for paroxysmal atrial fibrillation using duty-cycled bipolar and unipolar radiofrequency energy. J. Cardiovasc. Electrophysiol. 2010 Apr;21 (4):399–405. doi: 10.1111/j.1540-8167.2009.01640.x. [DOI] [PubMed] [Google Scholar]
- Scharf Christoph, Boersma Lucas, Davies Wyn, Kanagaratnam Prapa, Peters Nicholas S, Paul Vince, Rowland Edward, Grace Andrew, Fynn Simon, Dang Lam, Oral Hakan, Morady Fred. Ablation of persistent atrial fibrillation using multielectrode catheters and duty-cycled radiofrequency energy. J. Am. Coll. Cardiol. 2009 Oct 6;54 (15):1450–6. doi: 10.1016/j.jacc.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Andrade Jason G, Dubuc Marc, Rivard Lena, Guerra Peter G, Mondesert Blandine, Macle Laurent, Thibault Bernard, Talajic Mario, Roy Denis, Khairy Paul. Efficacy and safety of atrial fibrillation ablation with phased radiofrequency energy and multielectrode catheters. Heart Rhythm. 2012 Feb;9 (2):289–96. doi: 10.1016/j.hrthm.2011.09.009. [DOI] [PubMed] [Google Scholar]
- Bulava Alan, Haniš Jiří, Sitek David, Ošmera Ondřej, Karpianus Dan, Snorek Michal, Rehoušková Kateřina, Toušek František, Pešl Ladislav. Catheter ablation for paroxysmal atrial fibrillation: a randomized comparison between multielectrode catheter and point-by-point ablation. Pacing Clin Electrophysiol. 2010 Sep;33 (9):1039–46. doi: 10.1111/j.1540-8159.2010.02807.x. [DOI] [PubMed] [Google Scholar]
- Gaita Fiorenzo, Caponi Domenico, Pianelli Martina, Scaglione Marco, Toso Elisabetta, Cesarani Federico, Boffano Carlo, Gandini Giovanni, Valentini Maria Consuelo, De Ponti Roberto, Halimi Franck, Leclercq Jean François. Radiofrequency catheter ablation of atrial fibrillation: a cause of silent thromboembolism? Magnetic resonance imaging assessment of cerebral thromboembolism in patients undergoing ablation of atrial fibrillation. Circulation. 2010 Oct 26;122 (17):1667–73. doi: 10.1161/CIRCULATIONAHA.110.937953. [DOI] [PubMed] [Google Scholar]
- Herrera Siklódy Claudia, Deneke Thomas, Hocini Mélèze, Lehrmann Heiko, Shin Dong-In, Miyazaki Shinsuke, Henschke Susanne, Fluegel Peter, Schiebeling-Römer Jochen, Bansmann Paul M, Bourdias Thomas, Dousset Vincent, Haïssaguerre Michel, Arentz Thomas. Incidence of asymptomatic intracranial embolic events after pulmonary vein isolation: comparison of different atrial fibrillation ablation technologies in a multicenter study. J. Am. Coll. Cardiol. 2011 Aug 9;58 (7):681–8. doi: 10.1016/j.jacc.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Verma Atul, Debruyne Philippe, Nardi Stefano, Deneke Thomas, DeGreef Yves, Spitzer Stefan, Balzer Jörn O, Boersma Lucas. Evaluation and reduction of asymptomatic cerebral embolism in ablation of atrial fibrillation, but high prevalence of chronic silent infarction: results of the evaluation of reduction of asymptomatic cerebral embolism trial. Circ Arrhythm Electrophysiol. 2013 Oct;6 (5):835–42. doi: 10.1161/CIRCEP.113.000612. [DOI] [PubMed] [Google Scholar]
- nMARQTM Pulmonary Vein Isolation System for the Treatment of Paroxysmal Atrial Fibrillation. Clin. Identifier NCT01824394. 0;0:0–0. [Google Scholar]
- Deneke Thomas, Schade Anja, Müller Patrick, Schmitt Rainer, Christopoulos Georgios, Krug Joachim, Szöllösi Geza, Mügge Andreas, Kerber Sebastian, Nentwich Karin. Acute safety and efficacy of a novel multipolar irrigated radiofrequency ablation catheter for pulmonary vein isolation. J. Cardiovasc. Electrophysiol. 2014 Apr;25 (4):339–45. doi: 10.1111/jce.12316. [DOI] [PubMed] [Google Scholar]
- Deneke Thomas, Schade Anja, Diegeler Anno, Nentwich Karin. Esophago-pericardial fistula complicating atrial fibrillation ablation using a novel irrigated radiofrequency multipolar ablation catheter. J. Cardiovasc. Electrophysiol. 2014 Apr;25 (4):442–3. doi: 10.1111/jce.12308. [DOI] [PubMed] [Google Scholar]
- Packer Douglas L, Kowal Robert C, Wheelan Kevin R, Irwin James M, Champagne Jean, Guerra Peter G, Dubuc Marc, Reddy Vivek, Nelson Linda, Holcomb Richard G, Lehmann John W, Ruskin Jeremy N. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic Front (STOP AF) pivotal trial. J. Am. Coll. Cardiol. 2013 Apr 23;61 (16):1713–23. doi: 10.1016/j.jacc.2012.11.064. [DOI] [PubMed] [Google Scholar]
- Pivotal Clinical Study of the CardioFocus Endoscopic Ablation System - . Adaptive Contact (EAS-AC) (HeartLight). 0;0:0–0. [Google Scholar]
- Neven Kars, Metzner Andreas, Schmidt Boris, Ouyang Feifan, Kuck Karl-Heinz. Two-year clinical follow-up after pulmonary vein isolation using high-intensity focused ultrasound (HIFU) and an esophageal temperature-guided safety algorithm. Heart Rhythm. 2012 Mar;9 (3):407–13. doi: 10.1016/j.hrthm.2011.09.072. [DOI] [PubMed] [Google Scholar]