Significance
Learning and memory are caused by changes in strength of communication between neurons at synapses. Both brief changes (short-term plasticity) and long-lasting changes (long-term plasticity) are important. Synaptic transmission is initiated by calcium channels, which are regulated by calcium-sensor proteins that induce short-term synaptic plasticity. We studied genetically modified mice with a mutation in the binding site for calcium-sensor proteins on calcium channels, which alters short-term synaptic plasticity. Surprisingly, we found that synapses in the hippocampus of these mice also have impaired long-term potentiation. In addition, these mutant mice have impaired spatial learning and memory. Our results show that disruption of calcium-channel regulation by calcium-sensor proteins alters both short-term and long-term plasticity, and these changes impair spatial learning and memory.
Keywords: calcium channel, calmodulin, synaptic plasticity, calcium sensor proteins, hippocampus
Abstract
Many forms of short-term synaptic plasticity rely on regulation of presynaptic voltage-gated Ca2+ type 2.1 (CaV2.1) channels. However, the contribution of regulation of CaV2.1 channels to other forms of neuroplasticity and to learning and memory are not known. Here we have studied mice with a mutation (IM-AA) that disrupts regulation of CaV2.1 channels by calmodulin and related calcium sensor proteins. Surprisingly, we find that long-term potentiation (LTP) of synaptic transmission at the Schaffer collateral-CA1 synapse in the hippocampus is substantially weakened, even though this form of synaptic plasticity is thought to be primarily generated postsynaptically. LTP in response to θ-burst stimulation and to 100-Hz tetanic stimulation is much reduced. However, a normal level of LTP can be generated by repetitive 100-Hz stimulation or by depolarization of the postsynaptic cell to prevent block of NMDA-specific glutamate receptors by Mg2+. The ratio of postsynaptic responses of NMDA-specific glutamate receptors to those of AMPA-specific glutamate receptors is decreased, but the postsynaptic current from activation of NMDA-specific glutamate receptors is progressively increased during trains of stimuli and exceeds WT by the end of 1-s trains. Strikingly, these impairments in long-term synaptic plasticity and the previously documented impairments in short-term synaptic plasticity in IM-AA mice are associated with pronounced deficits in spatial learning and memory in context-dependent fear conditioning and in the Barnes circular maze. Thus, regulation of CaV2.1 channels by calcium sensor proteins is required for normal short-term synaptic plasticity, LTP, and spatial learning and memory in mice.
Activity-dependent modification of synaptic strength in synapses in the central nervous system is important for hippocampal-dependent information processing and for spatial learning and memory (1). Short-term and long-term modifications in synaptic strength are regulated by the frequency and pattern of presynaptic spiking (2–5). Regulation of voltage-gated Ca2+ channel type 2.1 (CaV2.1) by calmodulin (CaM) and related Ca2+ sensor (CaS) proteins causes Ca2+-dependent facilitation and inactivation of P/Q-type Ca2+ currents (6–12) that results in short-term facilitation and rapid depression of synaptic transmission (9, 12–14). Deletion of the gene encoding CaV2.1 channels (15) or mutation of their CaS protein binding domain (12–14) impairs short-term synaptic plasticity. Although regulation of CaV2.1 channels contributes to short-term synaptic plasticity in multiple types of synapses, the functional role of this form of synaptic regulation in learning and memory is unknown.
Long-term potentiation (LTP) of synaptic transmission in the hippocampus is thought to be important for spatial learning and memory (16). High-frequency stimuli induce LTP, which depends on postsynaptic Ca2+ entry via NMDA-specific glutamate receptors and activation of calcium/calmodulin-dependent protein kinase II (17, 18). These events cause increased activity of AMPA-specific glutamate receptors via both direct phosphorylation and enhanced trafficking to the postsynaptic membrane (19). Presynaptic signals may also contribute to this series of events by strengthening glutamatergic transmission at hippocampal synapses (20); however, at the outset of these experiments, we did not expect that changes in short-term synaptic plasticity would have major effects on LTP.
To further explore the role of CaV2.1 channel regulation by CaS proteins in spatial learning and memory, we used knockin mice with paired alanine substitutions for the isoleucine and methionine residues in the IQ-like motif (IM-AA) in their C-terminal domain (21), and we studied LTP and spatial learning and memory in IM-AA mice. In addition to the previously documented alterations in short-term facilitation in Schaffer collateral (SC)-CA1 synapses in acute hippocampal slices (21), we found major deficits in LTP and in spatial learning and memory, which reveal unexpected connections among presynaptic neuroplasticity, postsynaptic LTP, and spatial learning and memory.
Results
IM-AA Mutation in CaV2.1 Channels Impairs LTP of SC-CA1 Pyramidal Cell Synapses.
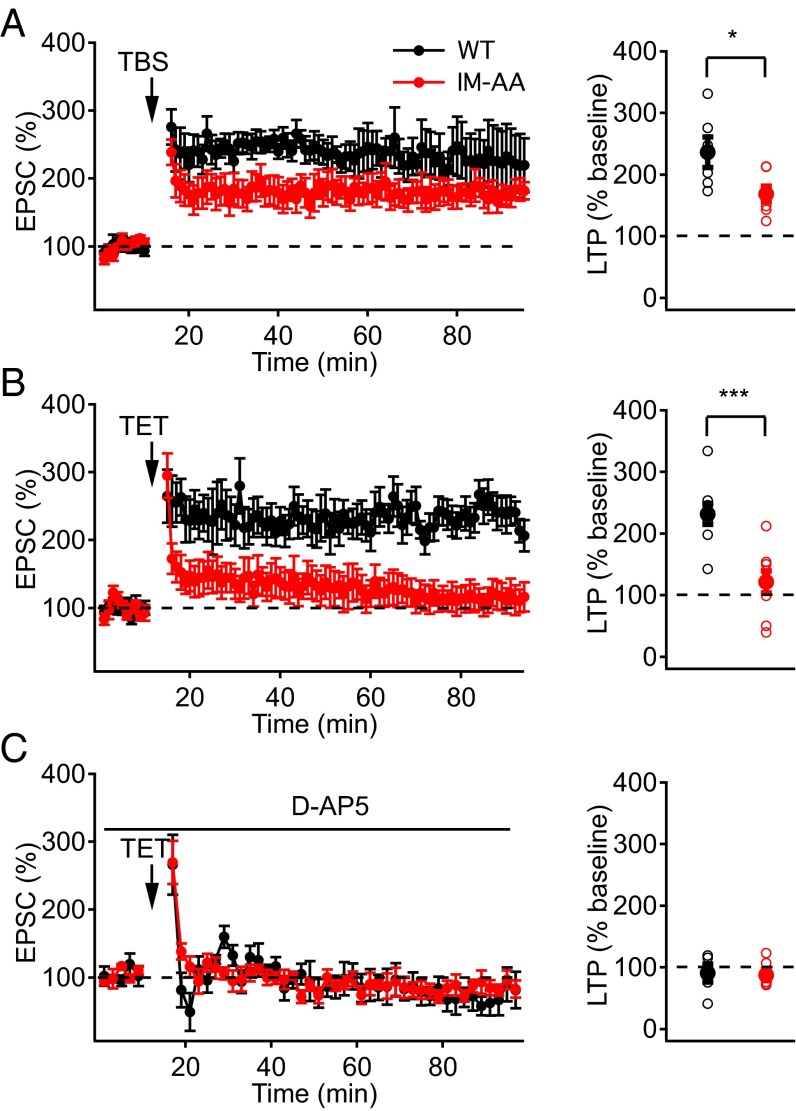
In the acute hippocampal slice preparation, repetitive bursts of stimulation at the frequency of θ-waves elicit LTP in response to a natural stimulus pattern (22, 23). We examined whether IM-AA synapses might have changes in long-term synaptic modulation induced by θ-burst stimulation (TBS) (Materials and Methods). We found that LTP, induced by TBS of SC fibers while holding the membrane potential of postsynaptic CA1 neurons at −40 mV, was significantly reduced in IM-AA synapses compared with WT (Fig. 1A). These results indicate that regulation of CaV2.1 channels by CaS proteins contributes to long-term synaptic plasticity as well as short-term plasticity.
Fig. 1.
IM-AA mutation reduces LTP in SC-CA1 synapses. (A) LTP induced at −40 mV by eight trains of θ-burst stimulation (10 bursts delivered at 5 Hz, each burst consisted of 10 pulses at 100 Hz) from WT (black, n = 6) and IM-AA (red, n = 7) (Left). LTP values from individual cells (open symbols) and average LTP values (solid symbols) from WT (black) and IM-AA (red) (Right). (B) LTP induced by two 100-Hz trains at −60 mV in the presence of 1 μM ω-Ctx, 50 μM picrotoxin, and 10 μM CGP55845 hydrochloride from WT (black, n = 8) and IM-AA (red, n = 8) (Left). (Right) Summarized LTP values as in A. (C) LTP induced by two 100-Hz trains at 0 mV in the presence of 1 μM ω-Ctx, 50 μM picrotoxin, and 10 μM CGP55845 hydrochloride from WT (black, n = 7) and IM-AA (red, n = 6) (Left). (Right) Summarized LTP values as in A. *P < 0.05; ***P < 0.001.
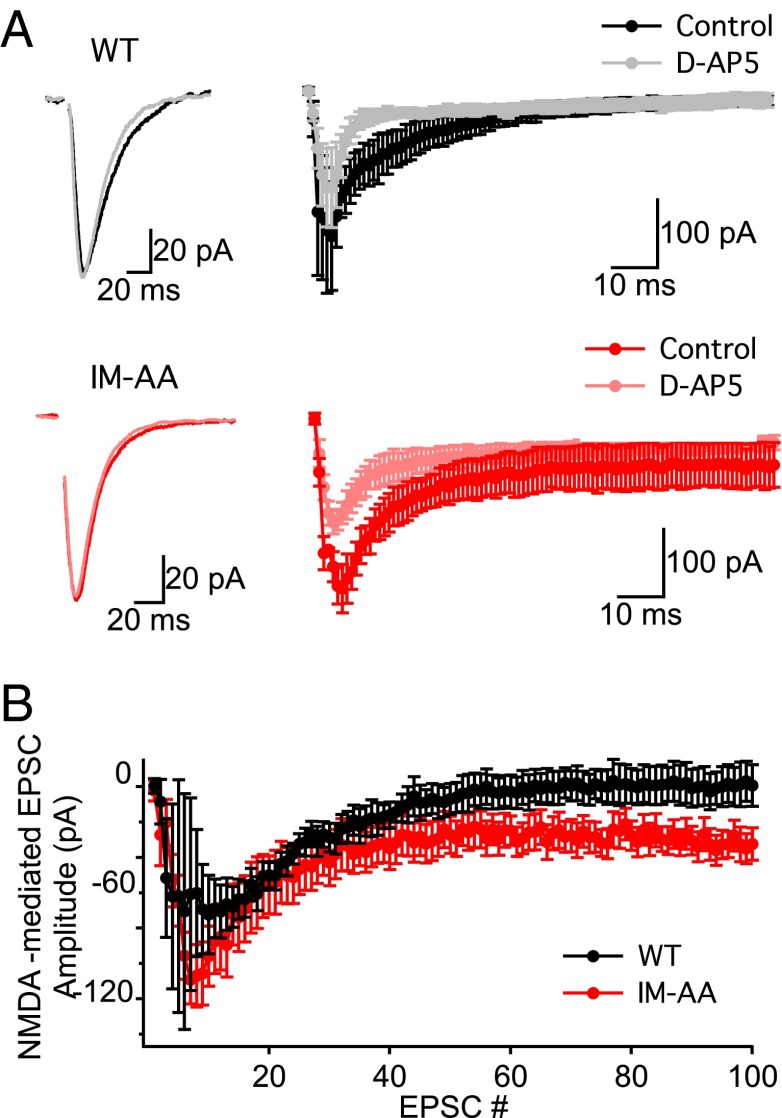
We investigated whether reduced LTP in IM-AA SC-CA1 synapses was specific to the mode of induction of LTP by comparing LTP in WT and IM-AA mice induced by tetanic stimulation. Application of two 100-Hz trains of stimuli to the SC fibers resulted in robust LTP in WT, but not in IM-AA SC-CA1 synapses (Fig. 1B). Potentiation immediately following the tetanus was similar for WT and IM-AA (WT, 265 ± 36%; IM-AA, 295 ± 33%), which is expected because posttetanic potentiation does not involve calcium channel regulation (3, 13). On the other hand, excitatory postsynaptic current (EPSC) amplitude was sustained in WT but decreased rapidly in IM-AA to only 122 ± 18% of the pretetanus value by 60 min after the end of the tetanic stimulus (Fig. 1B). Because LTP in SC-CA1 synapses depends on postsynaptic Ca2+ influx through NMDA-specific glutamate receptors (17, 18), we examined whether application of the NMDA receptor blocker d-AP5 would affect potentiation of the EPSC. We found that d-AP5 completely abolished LTP in both WT and IM-AA synapses (Fig. 1C), consistent with a normal mechanism of induction of LTP dependent on NMDA receptors in IM-AA synapses, which is less effective than in WT.
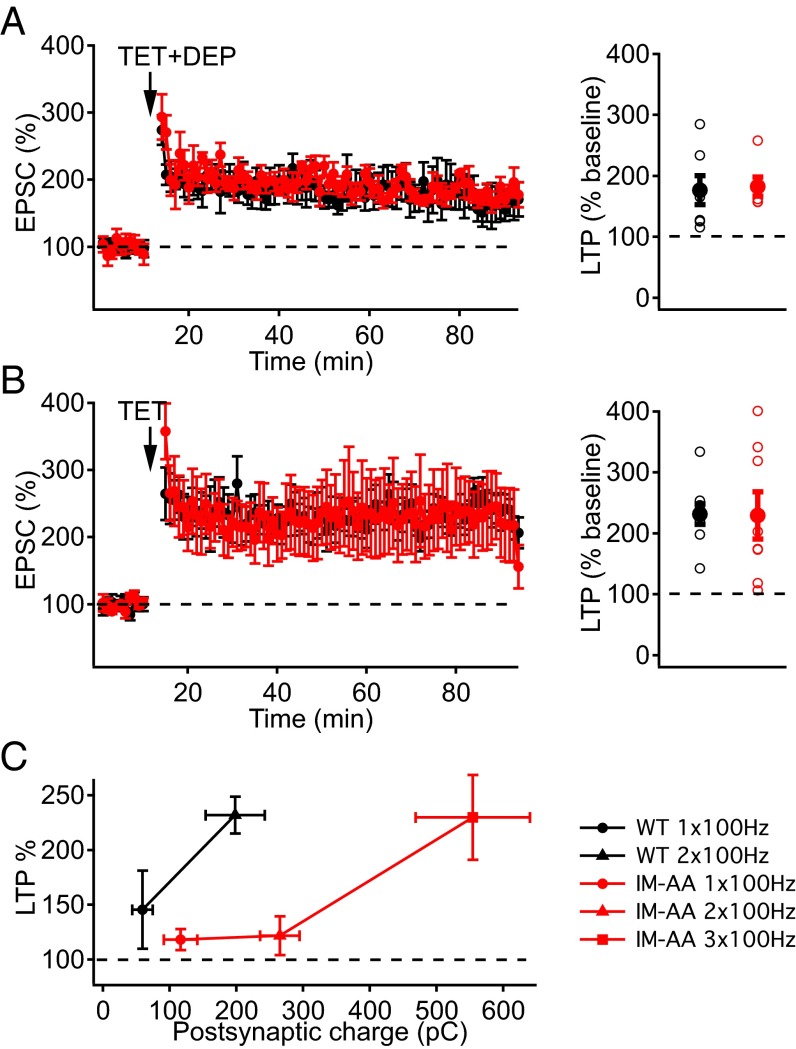
The response of NMDA receptors to glutamate is controlled by voltage-dependent Mg2+ block of the pore. To determine whether relief of Mg2+ blockade of NMDA receptors would enhance LTP at IM-AA synapses, we induced LTP while depolarizing postsynaptic CA1 pyramidal neurons to 0 mV during the tetanus. Under these conditions, there was no significant difference between the potentiated EPSCs of WT and IM-AA synapses (Fig. 2A). Furthermore, LTP comparable to WT could be elicited using a stronger, three-train stimulus paradigm in IM-AA synapses held at −60 mV (Fig. 2B). Taken together, these results demonstrate that the IM-AA mutation causes weakened LTP, which can be restored by stronger activation of NMDA receptors by using depolarization to reverse Mg2+ block (Fig. 2A) or by applying additional tetanic stimuli (Fig. 2B).
Fig. 2.
Induction of LTP in IM-AA SC-CA1 synapses by stronger stimulation and postsynaptic depolarization. (A) LTP induced by two 100-Hz trains at 0 mV in the presence of 1 μM ω-Ctx, 50 μM picrotoxin, and 10 μM CGP55845 hydrochloride from WT (black, n = 7) and IM-AA (red, n = 6) (Left). LTP values from individual cells (open symbols) and average LTP values (solid symbols) from WT (black) and IM-AA (red) are shown at Right. (B) LTP induced at −60 mV by two 100-Hz trains from WT (black, n = 8) and by three 100-Hz trains from IM-AA (red, n = 8) in the presence of 1 μM ω-Ctx, 50 μM picrotoxin, and 10 μM CGP55845 hydrochloride (Left). (Right) Summarized LTP values as in A. (C) Relationship between postsynaptic charge during 1x, 2x, 3x 100-Hz trains used to induce LTP and the normalized percentage of LTP from WT (black, n = 4–7) and IM-AA (red, n = 4–6).
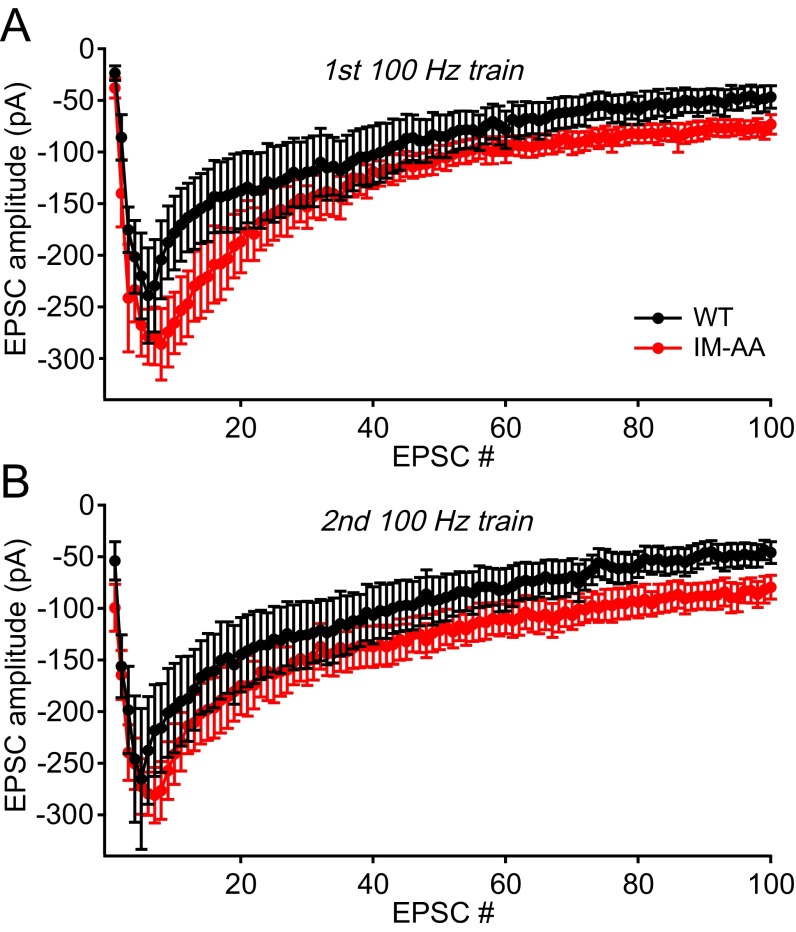
To better understand the basis for altered LTP induction, we directly recorded the EPSC during the trains of stimuli used to elicit LTP. Mean peak amplitudes of EPSCs during 100-Hz trains in IM-AA synapses were slightly increased relative to WT synapses, and the integral of EPSCs was greater (Fig. S1). These EPSCs reflect primarily the contribution of AMPA receptors, which are rapidly activated by released glutamate. To estimate the efficacy of postsynaptic depolarization to induce LTP more accurately, we integrated the EPSCs to yield a measure of total postsynaptic charge in response to one, two, or three 100-Hz trains, and we plotted the amount of LTP produced vs. postsynaptic charge (Fig. 2C). The IM-AA mutation dramatically weakened the coupling between the production of LTP and the integrated EPSC charge during the induction protocol in IM-AA SC-CA1 synapses, as illustrated by the rightward shift of the LTP vs. postsynaptic charge relationship, but it had no effect on the maximal LTP response.
Fig. S1.
Mean postsynaptic currents during 100-Hz trains in IM-AA synapses. (A) Average peak amplitude of evoked EPSCs during a 100-Hz train from WT (black, n = 7) and IM-AA (red, n = 6) in the presence of 1 μM ω-Ctx, 50 μM picrotoxin, and 10 μM CGP55845 hydrochloride. (B) Average peak amplitude of evoked EPSCs during a second 100-Hz train from WT (black, n = 7) and IM-AA (red, n = 6) in the presence of 1 μM ω-Ctx, 50 μM picrotoxin, and 10 μM CGP55845 hydrochloride.
IM-AA Mutation in CaV2.1 Channels Affects NMDA Receptor-Mediated Excitatory Synaptic Transmission in SC-CA1 Synapses.
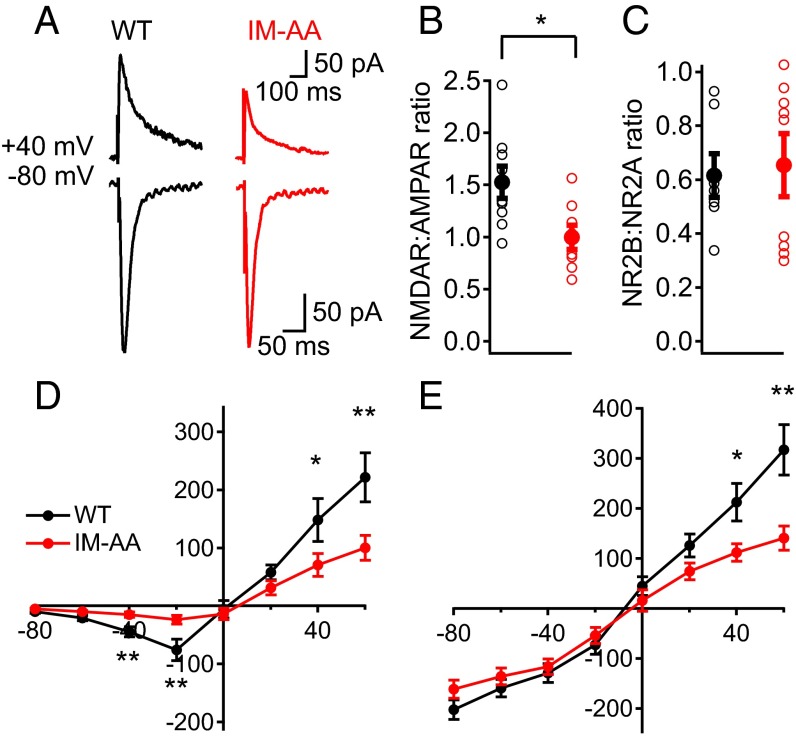
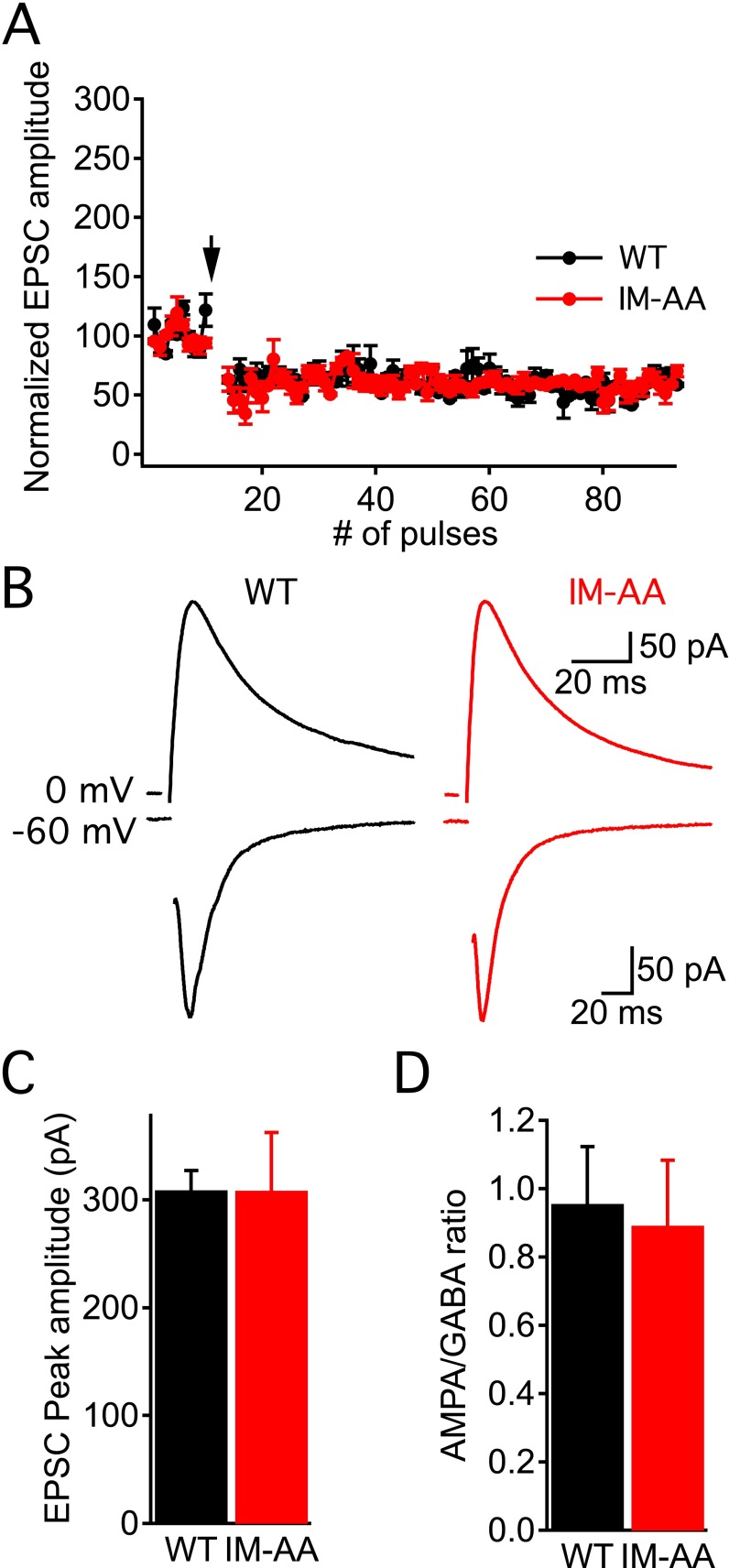
The ability of postsynaptic depolarization to rescue LTP induction suggested that a reduced contribution of NMDA receptors to the postsynaptic current might be responsible for reduced LTP. Therefore, we characterized the postsynaptic currents in hippocampal slices from WT and IM-AA mice in more detail. We recorded evoked AMPA receptor-mediated EPSCs at a holding potential, Vh, of −80 mV. We then added an AMPA receptor blocker (CNQX), recorded NMDA receptor-mediated EPSCs at Vh of +40 mV, and calculated the ratio of NMDA receptor-mediated to AMPA receptor-mediated EPSCs. We found that IM-AA synapses had significantly lower NMDA receptor/AMPA receptor ratio than WT in response to a single presynaptic stimulus (Fig. 3 A and B). The peak AMPA receptor-mediated amplitude was unchanged, indicating a selective reduction in the NMDA receptor-mediated component of the postsynaptic response.
Fig. 3.
IM-AA mutation alters NMDAR-to-AMPAR ratio in SC-CA1 synapses. (A) Example of AMPAR-mediated EPSCs recorded at −80 mV in control solution, and, after AMPAR block by 10 μM CNQX, NMDAR-mediated EPSCs recorded at +40 mV from WT (black) and IM-AA (red). Stimulus artifacts were erased for clarity. (B) NMDAR-to-AMPAR ratio from individual cells (open symbols) and average values (solid symbols) from WT (black, n = 9) and IM-AA (red, n = 8) in the presence of 1 μM ω-Ctx, 50 μM picrotoxin, and 10 μM CGP55845 hydrochloride. (C) NR2B:NR2A ratio calculated after block by 10 μM ifenprodil from individual cells (open symbols) and average values (solid symbols) for WT (black, n = 9) and IM-AA (red, n = 11) as described in Materials and Methods. (D) I–V relationship of NMDAR-mediated currents in the presence of 20 μM CNQX, 1 μM ω-Ctx, 50 μM picrotoxin, and 10 μM CGP55845 hydrochloride from WT (black, n = 9) and IM-AA (red, n = 11). (E) I–V relationship of AMPAR-mediated currents in the presence of 50 μM APV, 1 μM ω-Ctx, and 50 μM picrotoxin from WT (black, n = 9) and IM-AA (red, n = 8). *P < 0.05; **P < 0.01.
NMDA receptor-mediated inward current was significantly smaller in IM-AA vs. WT synapses, although it peaked at the same postsynaptic membrane potential (−20 mV) in both (Fig. 3D). To determine whether alterations in NMDA receptor subunit composition contribute to the decreased NMDA receptor/AMPA receptor ratio in IM-AA synapses, we examined the effect of a NR2B subunit-specific blocker (ifenprodil) on NMDA receptor-mediated current (Fig. 3C). Interestingly, no difference in NR2B/NR2A ratio was found between WT and IM-AA synapses, suggesting that the ratio between NMDA receptor subunit types remains constant. We then measured current–voltage (I–V) curves for pharmacologically isolated AMPA receptor-mediated currents (Fig. 3E). AMPA receptor-mediated inward currents were similar between WT and IM-AA synapses (Fig. 3E). Taken together, these results show that the NMDA receptor-mediated postsynaptic response is reduced in mutant IM-AA SC-CA1 synapses, whereas inward current conducted by AMPA receptors was unchanged. These results are consistent with a selective reduction in the number of functional NMDA receptors in IM-AA synapses.
To probe the relationship between NMDA receptor currents and impaired LTP induction, we measured postsynaptic currents before and after application of the NMDA receptor blocker d-AP5 during 100-Hz trains of stimuli. The NMDA receptor current in response to a single stimulus does not contribute significantly to the total postsynaptic current recorded at −60 mV (Fig. 4A, example traces). However, the NMDA receptor current increased during repetitive stimuli (Fig. 4A), and at the end of the train it was actually larger in hippocampal slices from IM-AA mice (Fig. 4B). Thus, greater NMDA receptor current at the end of 100-Hz trains is coupled to less LTP in IM-AA synapses. Evidently, postsynaptic depolarization is less effective in activating the molecular pathways that induce LTP in the postsynaptic cell at IM-AA synapses, consistent with the rightward shift of the dependence of LTP on postsynaptic charge (Fig. 2C).
Fig. 4.
IM-AA mutation induces increases in NMDAR currents in response to 100-Hz tetanus in SC-CA1 synapses. (A) Example EPSCs recorded at −60 mV in response to a single stimulus in control solution from WT (Top, black) and IM-AA (Bottom, red) and after NMDAR block by 100 μM d-AP5 WT (Top, gray) and IM-AA (Bottom, pink) (Left). Stimulus artifacts were erased for clarity. Average EPSCs were recorded at −60 mV in response to 100-Hz tetanus in control solution from WT (Top, black) and IM-AA (Bottom, red) and after NMDAR block by 100 μM d-AP5 WT (Top, gray) and IM-AA (Bottom, pink) (Left). Example EPSCs were recorded at −60 mV in control solution from WT (Top, black) and IM-AA (Bottom, red) and after NMDAR block by 100 μM d-AP5 WT (Top, gray) and IM-AA (Bottom, pink) (Right). (B) Average peak amplitude of evoked NMDAR-mediated EPSCs during a 100-Hz train from WT (black, n = 4) and IM-AA (red, n = 4) in the presence of 1 μM ω-Ctx, 50 μM picrotoxin, and 10 μM CGP55845 hydrochloride.
In hippocampal synapses, LTP and long-term depression (LTD) balance each other in controlling synaptic strength (16). Low-frequency stimulation induces LTD, whereas high-frequency stimulation induces LTP via similar signaling pathways. Enhanced LTD could potentially reduce the sensitivity for induction of LTP, as we have observed in IM-AA synapses. Therefore, we tested whether low-frequency stimulation induces LTD similarly in WT and IM-AA synapses. We found no significant difference between WT and IM-AA synapses in LTD (Fig. S2A). Therefore, IM-AA synapses have a primary defect in LTP, which causes reduced sensitivity to induction of LTP by postsynaptic depolarization.
Fig. S2.
LTD and excitatory-to-inhibitory ratio is unaltered in IM-AA synapses. (A) LTD induced by 1-Hz stimulation for 15 min in the presence of 50 μM picrotoxin and 10 μM CGP55845 hydrochloride from WT (black, n = 2) and IM-AA (red, n = 2). (B) Example traces of AMPA receptor-mediated EPSCs at a holding potential, Vh, of −60 mV and GABA receptor-mediated IPSCs at Vh, of 0 mV in the presence of 20 μM CNQX and 100 μM d-AP5 from WT (black) and IM-AA (red). (C) Average peak amplitude of evoked AMPA receptor-mediated EPSCs from WT (black, n = 7) and IM-AA (red, n = 7). (D) Ratio of AMPA receptor-mediated EPSCs to GABA receptor-mediated IPSCs from WT (black, n = 7) and IM-AA (red, n = 7).
In neural circuits, the balance of excitation and inhibition is also important for information processing. To assess the effects of the IM-AA mutation on the overall balance of excitation vs. inhibition, we measured postsynaptic AMPA receptor EPSCs and compared them to GABAA receptor inhibitory postsynaptic currents (IPSCs) (Fig. S2 B–D). We found that the ratio of excitatory to inhibitory neurotransmission in the hippocampal slice was unchanged in IM-AA mice. Therefore, the effects of the IM-AA mutation are primarily on short-term synaptic plasticity and LTP, with no effect on LTD and the overall ratio of excitation to inhibition.
IM-AA Mice Display Impaired Spatial Learning and Memory.
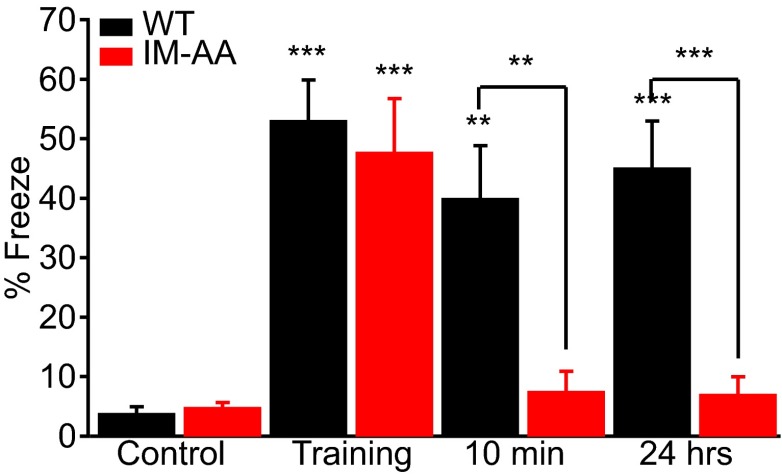
Because hippocampal plasticity is important for spatial learning, we examined the performance of WT and IM-AA mice in the context-dependent fear-conditioning test, a hippocampal-dependent cognitive task in which spatial memory is assessed from the fear-related freezing behavior of mice when returned to the spatial context of a previous fearful event (24). WT and IM-AA mice explored a cage with an electrified floor grid similarly when first introduced into it (Fig. 5, control). During the training phase, mice were subjected to a mild foot shock. WT and IM-AA mice exhibited comparable fear-related freezing behavior in response to the shock (Fig. 5, training). The mice were returned to their home cage. When they were brought back into the spatial context in which they had experienced the foot shock 10 min or 24 h earlier, IM-AA mice showed dramatically impaired freezing responses compared with WT (Fig. 5). These results reveal a nearly complete lack of spatial learning and memory for IM-AA mice in this behavioral test.
Fig. 5.
IM-AA mice have impaired spatial learning and memory in context-dependent fear conditioning. Mean freezing time (percent of total) in the conditioning chamber for WT (black, n = 10) and IM-AA mice (red, n = 10) before (control), immediately after (training), and at 10 min and 24 h after delivery of foot shock. **P < 0.01; ***P < 0.001.
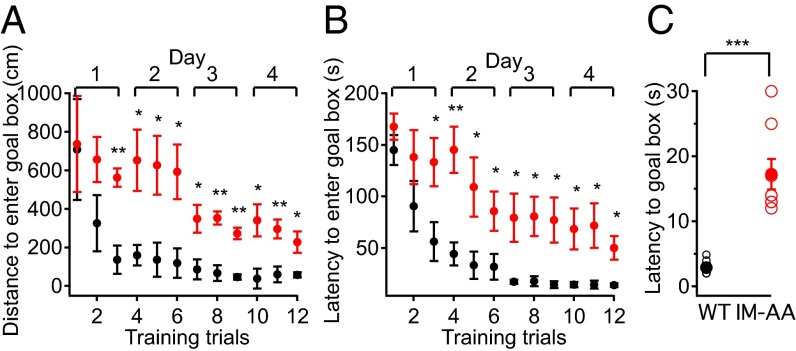
In context-dependent fear conditioning, mice must recognize fear as well as encode and remember the spatial context of the fearful event. Because WT and IM-AA mice exhibited similar freezing behavior in the training phase of the experiments, it is likely that the IM-AA mice can recognize fear but fail to encode or remember the spatial context of that fear. To further test their spatial learning and memory, we examined WT and IM-AA mice in the Barnes circular maze test, which does not involve fear (25). This test measures whether mice can learn and remember the location of a single escape hole with a dark refuge among 20 identical holes at the perimeter of a brightly lighted circular field. WT mice learned to locate the escape hole by the third training trial as indicated by the reduced distance they traveled to the vicinity of the hole (Fig. 6A). The amount of time required before entering the escape hole also decreased with each trial (Fig. 6A). The performance of IM-AA mice did improve during the training period, indicating that they were learning, but the decreases in distance traveled to the escape hole and in latency before entering the escape hole were markedly greater for WT than for IM-AA at each trial (Fig. 6 A and B). On the day following the last training trials, a probe test was conducted in which the refuge was removed and the latency before moving to the position of the former refuge for the first time was measured. In this probe test, the average latency was far higher for IMAA mice than for WT (Fig. 6C). Taken together, these data suggest that the IM-AA mutation resulted in greatly impaired spatial learning and memory.
Fig. 6.
IM-AA mice have impaired spatial learning and memory in the Barnes circular maze. (A) Average distance traveled to locate and enter the goal box in the Barnes maze test plotted against number of training trials for WT (black, n = 6) and IM-AA (red, n = 8) mice. (B) Average time spent to enter the goal box in the Barnes maze test plotted against number of training trials for WT (black, n = 6) and IM-AA (red, n = 8) mice. (C) Time required to first reach the former location of the goal box (goal box removed) 24 h after the last training session in the Barnes maze test from individual mice (open symbols) and average values (solid symbols) for WT (black, n = 6) and IM-AA (red, n = 8) mice. *P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
Our previous studies of IM-AA mice showed that regulation of presynaptic CaV2.1 channels by CaS proteins contributes substantially to short-term synaptic plasticity in hippocampal synapses (21). In those studies, we found that the IM-AA mutation does not alter the frequency or amplitude of miniature EPSCs caused by presynaptic release of single quanta of glutamate or the amplitudes of EPSCs recorded in response to increasing presynaptic stimulus intensities. We also found that the slow Ca2+ chelator EGTA had similar effects on evoked EPSCs in WT and IM-AA synapses. Together, these results indicated that the function of the presynaptic release machinery and its spatial relationship to presynaptic CaV2.1 channels and postsynaptic glutamate receptors were unaltered in IM-AA synapses. Here we tested whether this form of regulation of CaV2.1 channels also has a role in LTP and in spatial learning and memory. Our findings show that the IM-AA mutation impairs long-term synaptic plasticity as well as hippocampus-dependent spatial learning and memory in mice. These results forge a link between regulation of CaV2.1 channels, LTP, and spatial learning and memory.
Weakened LTP.
A key synaptic outcome of bursts of presynaptic action potentials is NMDA receptor-dependent LTP of the postsynaptic response at excitatory glutamatergic synapses in the hippocampus. Extensive studies have shown that LTP arises postsynaptically via a pathway involving Ca2+ entry via NMDA receptors, activation of Ca2+/calmodulin-dependent protein kinase II, phosphorylation of AMPA receptors, and increased trafficking of AMPA receptors into the postsynaptic membrane (16, 18, 19). Additional studies suggest involvement of presynaptic mechanisms as well (20). Surprisingly, we found that LTP was weakened in IM-AA synapses and required stronger postsynaptic depolarization to achieve the same level of LTP as WT synapses. Although the initial activation of NMDA receptors was reduced, the integral of NMDA receptor postsynaptic current during a train of stimuli was greater than WT. Our working hypothesis is that the postsynaptic molecular pathways leading to stable increases in synaptic strength downstream of the NMDA receptor are altered in IM-AA synapses. It seems unlikely that Ca2+ entry via CaV2.1 channels is directly involved in inducing LTP in our experiments because QX-314 present in our recording pipettes to block postsynaptic action potential generation via direct block of voltage-gated sodium channels also blocks CaV2.1 channels. Further work will be required to define the postsynaptic mechanisms involved in these changes in the strength of LTP.
In addition to potential postsynaptic mechanisms acting directly on coupling of NMDA receptor activation to LTP, it is possible that the alteration of presynaptic plasticity caused by the impaired regulation of CaV2.1 channels by CaS proteins that we have observed could reduce the strength of LTP by homeostatic mechanisms. In IM-AA synapses, the initial facilitation of EPSCs in response to a train of action potentials is reduced in amplitude, but facilitation eventually reaches a comparable level to WT and is sustained longer because the rapid phase of synaptic depression is slowed (21). Overall, the integral of EPSCs is actually increased during a train of stimuli (21). This persistent increase in EPSC amplitude during trains of stimuli in vivo may engage homeostatic regulatory mechanisms that weaken LTP, as we have reported here. Such homeostatic regulation might involve inhibition of NMDA receptors in the postsynaptic membrane or internalization of NMDA receptors in intracellular vesicles under basal conditions. It will be interesting to determine the time course of weakening of LTP during neural development to determine whether homeostatic compensatory mechanisms are involved and at what age they are engaged. It will also be important to probe the downstream signaling mechanisms that connect NMDA receptor activation to LTP to find the step(s) in that process that is subject to homeostatic regulation by short-term synaptic plasticity.
Importantly, weakened LTP was observed during stimulations at θ-frequency, which is thought to represent a natural pattern of stimulation during spatial exploration in vivo (22, 23, 26). Increased firing of action potentials by CA3 neurons is thought to induce LTP in CA1 neurons, which contributes to formation and stability of place cells whose firing denotes location during movement (27–31). Because LTP would contribute to increased excitability of hippocampal place cells during spatial learning (27–31), these deficits in LTP we have observed would be expected to cause deficits in spatial learning and memory in IM-AA mice.
Impaired Spatial Learning and Memory.
In IM-AA mice, impaired short-term facilitation and weakened hippocampal LTP were accompanied by deficits in two well-established tests of hippocampal-dependent spatial learning and memory. IM-AA mice failed to learn the spatial context of a fear-provoking foot shock or remember the location of a dark refuge in the Barnes circular maze. Together, these results document a major loss of spatial learning and memory in IM-AA mice. As both synaptic facilitation and LTP are thought to be required for development of place cells that encode spatial learning and memory (32), it is not surprising that these changes in synaptic plasticity are accompanied by failure of spatial learning and memory. In all, our data show that activity-dependent regulation of presynaptic CaV2.1 channels by CaS proteins contributes significantly to short-term synaptic plasticity in presynaptic terminals in vivo and reveal unexpectedly that this key regulatory process is also required for normal LTP in postsynaptic hippocampal neurons and for hippocampus-dependent spatial learning and memory. These results open unexplored avenues of inquiry into the relationship between synaptic plasticity at the molecular and cellular levels and spatial learning and memory at the levels of neural circuits and behavior.
Possible Relationship Among Short-Term Synaptic Plasticity, LTP, and Spatial Learning.
Our results do not yet reveal the mechanistic connections among short-term synaptic plasticity, LTP, and spatial learning and memory. Exploratory behavior in rodents generates θ-rhythm in the hippocampus (33). Points of demarcation or reward in the navigation pathway are denoted by formation of sharp-wave ripples superimposed on θ-rhythm, which are replayed to consolidate spatial memory retention (32–34). Formation of sharp-wave ripples depends on coordinated firing of CA3 neurons, orchestrated by parvalbumin-expressing fast-spiking interneurons (35). Place cells fire when an animal reaches a specific position in space, and firing of place cells in turn can induce LTP in vitro (32, 36, 37). How might the changes in short-term and long-term synaptic plasticity that we observe in IM-AA mice alter sharp-wave ripples, place cell formation and stability, and ultimately spatial learning and memory? One possibility is that short-term synaptic plasticity on the millisecond time scale may contribute to formation of sharp-wave ripples, which are generated on a similar time scale. On the other hand, LTP on the time scale of hundreds of seconds and longer may contribute to place cell formation and stability. Thus, the deficits in short-term synaptic plasticity and LTP caused by loss of regulation of CaV2.1 channels in IM-AA mice may both contribute significantly to the impairment of spatial learning and memory. Future experiments may reveal the functional relationships among impairments of short-term neuroplasticity, LTP, and spatial learning and memory in IM-AA mice through correlation of synaptic deficits with changes in neural circuit functions assessed in recordings of sharp-wave ripples and place cells.
Materials and Methods
Animals.
IM-AA mice with a point mutation in the IQ-like motif of CaV2.1 (IM>>AA), (ATCATG to GCCGCT) were generated by Ingenious Targeting Laboratory. The mutation (within exon 40) was generated by PCR mutagenesis and confirmed by sequencing. Traditional blastocyst injection of ES cells expressing the targeting vector resulted in chimeric mice. These chimeric mice were mated first to generate heterozygotes, which were then backcrossed for 10 generations with C57BL/6J to generate homozygous IM-AA mutant mice in a pure genetic background. All experiments were performed according to the guidelines for the care and use of animals approved by the Animal Care and Use Committee at the University of Washington.
Electrophysiology in Hippocampal Slices.
WT and IM-AA mice 16–21 d old were anesthetized with isoflurane. Brains were rapidly removed and placed in ice-cold, high sucrose cutting solution containing (in mM): 75 sucrose, 25 NaHCO3, 25 glucose, 2.5 KCl, 1.25 NaH2PO4, 87 NaCl, 7 MgCl2, and 0.5 CaCl2. Acute transverse hippocampal slices (400 μm) were cut on a 1000 Plus Vibratome in the high sucrose cutting solution, and transferred immediately to an incubation chamber containing artificial cerebrospinal fluid (ACSF) (in mM): 125 NaCl, 3 KCl, 2 CaCl2, 2 MgCl2, 125 NaH2PO4, 26 NaHCO3, and 10 glucose, saturated with 95% (vol/vol) O2 and 5% (vol/vol) CO2. The slices were allowed to recover at 37 °C for 45 min and then maintained at room temperature for at least 30 min before recording.
Slices were transferred to a submerged recording chamber mounted on a Nikon microscope (E600FN) equipped for infrared differential interference contrast microscopy and were perfused with ACSF at a rate of 1.5 mL/min at room temperature. All experiments unless specified otherwise were performed in the presence of the GABAA blocker picrotoxin (50 μM), GABAB blocker CGP55845 hydrochloride (10 μM), and CaV2.2 blocker ω-conotoxin (ω-Ctx) GVIA. In addition, a cut was made between CA1 and CA3 to prevent the propagation of epileptiform activity. Evoked postsynaptic responses were recorded from CA1 pyramidal cells, which were visualized by infrared differential interference contrast. EPSCs were induced by stimulating Schaffer collaterals in stratum radiatum by a concentric bipolar stimulating electrode (FHC). Whole-cell recording pipettes (4–6 MΩ) were filled with a solution containing (in mM): 145 Cs-gluconate, 2 MgCl2, 10 Hepes, 0.5 EGTA, 2 Tris-ATP, 0.2 Na2GTP, and 5 QX-314. Data were collected with a MultiClamp 700A amplifier (Axon Instruments), filtered at 2 kHz and digitized at 10 kHz. Multiple step depolarizations were given at the beginning of every experiment to induce block of Na2+ and Ca2+ currents in the CA1 pyramidal cells by QX-314 (21).
LTP was induced in both WT and IM-AA in SC-CA1 synapses by eight trains of θ-burst stimulation (10 bursts delivered at 5 Hz, each burst consisting of 10 pulses at 100 Hz), while CA1 neurons were depolarized to −40 mV. LTP was also induced by two or three trains of high-frequency stimulation (100 Hz, 1 s) separated by 20 s, while cells were held at −60 mV or depolarized to 0 mV. The induction protocols were applied within 5 min of achieving whole-cell configuration, to prevent “wash-out” of LTP. The magnitude of LTP was calculated based on the EPSC values 30 min after the end of the induction protocol. Data were analyzed using Clampfit (Axon) and Igor Pro software (Wavemetrics). Postsynaptic charge during the 100-Hz stimulation was calculated by integrating the area under the EPSCs. LTD was induced by low frequency (1 Hz, 15 min). All data are presented as means ± SEMs. Statistical significance was calculated using a Student’s t test.
Context-Dependent Fear Conditioning.
Two-month-old male mice were placed in a conditioning chamber (Coulbourn Instruments) and allowed to explore. After 2 min, a footshock of 0.7 mA was delivered for 2 s. Animals were then allowed to recover for 1 min and then returned to their home cages. At increasing time intervals after training, animals were returned to the conditioning chamber (10 min, 24 h). The formation of memory for fear conditioning was measured by testing freezing behavior upon return to the conditioning chamber. Freezing behavior was defined as cessation of all but respiratory movement and was assessed by means of a time-sampling procedure. The scoring of freezing behavior was quantified using a computerized beam-grid system (TruScan, Coulbourn Instruments).
Barnes Circular Maze.
The Barnes circular maze test was conducted on a white circular surface, 92 cm in diameter, with 20 holes equally spaced around the perimeter. A black Plexiglas escape box (15 × 7 × 7 cm) containing paper cage bedding on its floor was located under one of the holes. The location of the target was consistent for a given mouse and two 500-lux (1 lux = 1 lumen per sq. m.) lights were turned on during trials. The platform and the escape box were cleaned thoroughly with 70% ethanol and paper towels between each trial to remove olfactory cues. Training lasted for 4 successive days and three trials 15 min apart were performed each day. At the beginning of each trial, a test mouse was placed in the center of the maze, held there for 10 s, and then given 3 min to locate the target hole. If the mouse failed to find the target hole it was gently guided to the escape box. When the mouse entered the escape box the light was turned off and the mouse remained undisturbed for 1 min in the escape box. On the fifth day, a probe trial was conducted after the last training session without the escape box to confirm that this spatial task was performed based on navigation cues. Time latency to reach the target hole and the distance traveled to reach the target hole were recorded and analyzed by Noldus analysis software.
Acknowledgments
We thank Dr. Jane Sullivan (Department of Physiology and Biophysics, University of Washington) for valuable discussions and comments on the manuscript. This work was supported by NIH Grant R01 NS022625 (to W.A.C.) and by a postdoctoral fellowship from the Swedish Society for Medical Research (to E.N.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1616206113/-/DCSupplemental.
References
- 1.Mayford M, Siegelbaum SA, Kandel ER. Synapses and memory storage. Cold Spring Harb Perspect Biol. 2012;4(6):4. doi: 10.1101/cshperspect.a005751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- 3.Fioravante D, Regehr WG. Short-term forms of presynaptic plasticity. Curr Opin Neurobiol. 2011;21(2):269–274. doi: 10.1016/j.conb.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catterall WA, Few AP. Calcium channel regulation and presynaptic plasticity. Neuron. 2008;59(6):882–901. doi: 10.1016/j.neuron.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Neher E, Sakaba T. Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron. 2008;59(6):861–872. doi: 10.1016/j.neuron.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 6.Lee A, et al. Ca2+/calmodulin binds to and modulates P/Q-type calcium channels. Nature. 1999;399(6732):155–159. doi: 10.1038/20194. [DOI] [PubMed] [Google Scholar]
- 7.Lee A, Scheuer T, Catterall WA. Ca2+/calmodulin-dependent facilitation and inactivation of P/Q-type Ca2+ channels. J Neurosci. 2000;20(18):6830–6838. doi: 10.1523/JNEUROSCI.20-18-06830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeMaria CD, Soong TW, Alseikhan BA, Alvania RS, Yue DT. Calmodulin bifurcates the local Ca2+ signal that modulates P/Q-type Ca2+ channels. Nature. 2001;411(6836):484–489. doi: 10.1038/35078091. [DOI] [PubMed] [Google Scholar]
- 9.Tsujimoto T, Jeromin A, Saitoh N, Roder JC, Takahashi T. Neuronal calcium sensor 1 and activity-dependent facilitation of P/Q-type calcium currents at presynaptic nerve terminals. Science. 2002;295(5563):2276–2279. doi: 10.1126/science.1068278. [DOI] [PubMed] [Google Scholar]
- 10.Lee A, et al. Differential modulation of Cav2.1 channels by calmodulin and Ca2+-binding protein 1. Nat Neurosci. 2002;5(3):210–217. doi: 10.1038/nn805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lautermilch NJ, Few AP, Scheuer T, Catterall WA. Modulation of CaV2.1 channels by the neuronal calcium-binding protein visinin-like protein-2. J Neurosci. 2005;25(30):7062–7070. doi: 10.1523/JNEUROSCI.0447-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan J, et al. Modulation of CaV2.1 channels by neuronal calcium sensor-1 induces short-term synaptic facilitation. Mol Cell Neurosci. 2014;63:124–131. doi: 10.1016/j.mcn.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mochida S, Few AP, Scheuer T, Catterall WA. Regulation of presynaptic CaV2.1 channels by Ca2+ sensor proteins mediates short-term synaptic plasticity. Neuron. 2008;57(2):210–216. doi: 10.1016/j.neuron.2007.11.036. [DOI] [PubMed] [Google Scholar]
- 14.Leal K, Mochida S, Scheuer T, Catterall WA. Fine-tuning synaptic plasticity by modulation of CaV2.1 channels with Ca2+ sensor proteins. Proc Natl Acad Sci USA. 2012;109(42):17069–17074. doi: 10.1073/pnas.1215172109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inchauspe CG, Martini FJ, Forsythe ID, Uchitel OD. Functional compensation of P/Q by N-type channels blocks short-term plasticity at the calyx of Held presynaptic terminal. J Neurosci. 2004;24(46):10379–10383. doi: 10.1523/JNEUROSCI.2104-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lüscher C, Nicoll RA, Malenka RC, Muller D. Synaptic plasticity and dynamic modulation of the postsynaptic membrane. Nat Neurosci. 2000;3(6):545–550. doi: 10.1038/75714. [DOI] [PubMed] [Google Scholar]
- 17.Collingridge GL, Kehl SJ, McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol. 1983;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malenka RC, Kauer JA, Zucker RS, Nicoll RA. Postsynaptic calcium is sufficient for potentiation of hippocampal synaptic transmission. Science. 1988;242(4875):81–84. doi: 10.1126/science.2845577. [DOI] [PubMed] [Google Scholar]
- 19.Huganir RL, Nicoll RA. AMPARs and synaptic plasticity: The last 25 years. Neuron. 2013;80(3):704–717. doi: 10.1016/j.neuron.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang YY, et al. Genetic evidence for a protein-kinase-A-mediated presynaptic component in NMDA-receptor-dependent forms of long-term synaptic potentiation. Proc Natl Acad Sci USA. 2005;102(26):9365–9370. doi: 10.1073/pnas.0503777102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nanou E, Sullivan JM, Scheuer T, Catterall WA. Calcium sensor regulation of the CaV2.1 Ca2+ channel contributes to short-term synaptic plasticity in hippocampal neurons. Proc Natl Acad Sci USA. 2016;113(4):1062–1067. doi: 10.1073/pnas.1524636113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larson J, Wong D, Lynch G. Patterned stimulation at the theta frequency is optimal for the induction of hippocampal long-term potentiation. Brain Res. 1986;368(2):347–350. doi: 10.1016/0006-8993(86)90579-2. [DOI] [PubMed] [Google Scholar]
- 23.Capocchi G, Zampolini M, Larson J. Theta burst stimulation is optimal for induction of LTP at both apical and basal dendritic synapses on hippocampal CA1 neurons. Brain Res. 1992;591(2):332–336. doi: 10.1016/0006-8993(92)91715-q. [DOI] [PubMed] [Google Scholar]
- 24.Crawley JN. Behavioral phenotyping of transgenic and knockout mice: Experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Res. 1999;835(1):18–26. doi: 10.1016/s0006-8993(98)01258-x. [DOI] [PubMed] [Google Scholar]
- 25.Barnes CA. Memory deficits associated with senescence: A neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979;93(1):74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- 26.Staubli U, Lynch G. Stable hippocampal long-term potentiation elicited by ‘theta’ pattern stimulation. Brain Res. 1987;435(1-2):227–234. doi: 10.1016/0006-8993(87)91605-2. [DOI] [PubMed] [Google Scholar]
- 27.O’Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34(1):171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- 28.O’Keefe J. Place units in the hippocampus of the freely moving rat. Exp Neurol. 1976;51(1):78–109. doi: 10.1016/0014-4886(76)90055-8. [DOI] [PubMed] [Google Scholar]
- 29.O’Mara SM. Spatially selective firing properties of hippocampal formation neurons in rodents and primates. Prog Neurobiol. 1995;45(3):253–274. doi: 10.1016/0301-0082(94)00050-r. [DOI] [PubMed] [Google Scholar]
- 30.Wiener SI. Spatial, behavioral and sensory correlates of hippocampal CA1 complex spike cell activity: Implications for information processing functions. Prog Neurobiol. 1996;49(4):335–361. doi: 10.1016/0301-0082(96)00019-6. [DOI] [PubMed] [Google Scholar]
- 31.Mizumori SJ, Ragozzino KE, Cooper BG, Leutgeb S. Hippocampal representational organization and spatial context. Hippocampus. 1999;9(4):444–451. doi: 10.1002/(SICI)1098-1063(1999)9:4<444::AID-HIPO10>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 32.Buzsáki G, Moser EI. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat Neurosci. 2013;16(2):130–138. doi: 10.1038/nn.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mehta MR. From synaptic plasticity to spatial maps and sequence learning. Hippocampus. 2015;25(6):756–762. doi: 10.1002/hipo.22472. [DOI] [PubMed] [Google Scholar]
- 34.Buzsáki G. Hippocampal sharp wave-ripple: A cognitive biomarker for episodic memory and planning. Hippocampus. 2015;25(10):1073–1188. doi: 10.1002/hipo.22488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schlingloff D, Káli S, Freund TF, Hájos N, Gulyás AI. Mechanisms of sharp wave initiation and ripple generation. J Neurosci. 2014;34(34):11385–11398. doi: 10.1523/JNEUROSCI.0867-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Isaac JT, Buchanan KA, Muller RU, Mellor JR. Hippocampal place cell firing patterns can induce long-term synaptic plasticity in vitro. J Neurosci. 2009;29(21):6840–6850. doi: 10.1523/JNEUROSCI.0731-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dobrunz LE, Stevens CF. Response of hippocampal synapses to natural stimulation patterns. Neuron. 1999;22(1):157–166. doi: 10.1016/s0896-6273(00)80687-x. [DOI] [PubMed] [Google Scholar]