Significance
Many plant species face increasing drought under climate change, making plant drought tolerance integral to predicting species and ecosystem responses. Many physiology traits interact to determine overall drought tolerance, but trait relationships have not been assessed for general patterns across global plant diversity. We analyzed stomatal, hydraulic, and mesophyll drought tolerance traits for 310 species from ecosystems worldwide. We evaluated the sequence of drought responses for plants under increasing water stress, and showed that coselection with environmental water stress drives most trait correlations across species, with functional coordination additionally important for some relationships. These results provide insight into how variation in multiple traits should be represented within plants and across species in models of plant responses to drought.
Keywords: drought tolerance, stem hydraulics, leaf hydraulics, stomatal closure, turgor loss point
Abstract
Climate change is expected to exacerbate drought for many plants, making drought tolerance a key driver of species and ecosystem responses. Plant drought tolerance is determined by multiple traits, but the relationships among traits, either within individual plants or across species, have not been evaluated for general patterns across plant diversity. We synthesized the published data for stomatal closure, wilting, declines in hydraulic conductivity in the leaves, stems, and roots, and plant mortality for 262 woody angiosperm and 48 gymnosperm species. We evaluated the correlations among the drought tolerance traits across species, and the general sequence of water potential thresholds for these traits within individual plants. The trait correlations across species provide a framework for predicting plant responses to a wide range of water stress from one or two sampled traits, increasing the ability to rapidly characterize drought tolerance across diverse species. Analyzing these correlations also identified correlations among the leaf and stem hydraulic traits and the wilting point, or turgor loss point, beyond those expected from shared ancestry or independent associations with water stress alone. Further, on average, the angiosperm species generally exhibited a sequence of drought tolerance traits that is expected to limit severe tissue damage during drought, such as wilting and substantial stem embolism. This synthesis of the relationships among the drought tolerance traits provides crucial, empirically supported insight into representing variation in multiple traits in models of plant and ecosystem responses to drought.
Plants worldwide are expected to face more frequent and severe droughts under climate change (1). Characterizing drought tolerance for diverse species is key to improved predictions of ecosystem responses to global change (2), and ecological and phylogenetic patterns have been established across many species for individual drought tolerance traits (3–7). However, plant drought tolerance is determined by multiple traits. The relationships among traits within plants and across species have not been evaluated for general patterns across global plant diversity. We synthesized the published data to elucidate global patterns in the relationships among stomatal, hydraulic, and leaf mesophyll drought tolerance traits. We evaluated the roles of functional coordination, covariance with water stress, and shared ancestry in driving trait correlations across species. Additionally, we focused on clarifying relationships among drought tolerance traits within plants of given species, i.e., determining the sequence of their water potential thresholds.
Classical drought tolerance traits quantify the water potentials that induce declines in key physiological processes, such as stomatal conductance, hydraulic conductivity, and cell turgor pressure. Previous studies have shown that these water potential thresholds are intercorrelated for small species sets (8–12). We tested these correlations for a large dataset to produce a framework for extrapolating plant responses to a wide range of water stress from one or two traits. Evaluating these correlations across a global dataset can provide additional insights into their drivers. Drought tolerance traits can be correlated across species because of (i) functional coordination, such as mechanistic linkages; (ii) concerted convergence (13), i.e., coselection by the environment, wherein traits are directionally but independently selected by water supply to optimize overall plant function; and/or (iii) shared ancestry. We compiled hypotheses from the literature for the drivers of each trait correlation, and evaluated these hypotheses by testing for greater coordination among traits than explained by water stress and relatedness. Water stress was measured as the minimum leaf water potential observed over the course of a year or during the dry season, at predawn (Ψmin, PD) and midday (Ψmin, MD). Ψmin, PD is taken when transpiration is at its minimum and the water potential of the plant is closest to equilibration with that of the soil, whereas Ψmin, MD is affected by any cuticular or stomatal transpiration and, thus, broadly captures the integrated effects of plant traits and the environment on the minimum water potential a plant reaches in natural conditions (14).
The sequence of water potential thresholds for drought tolerance traits within a plant is expected to strongly impact overall plant function under water stress (8, 15–17). Previous studies have compared values for some traits (e.g., refs. 9, 10, and 18), but have not included enough traits or species to characterize their overall sequence. We tested the degree to which plants exhibit a trait sequence that is expected to limit severe drought damage. Plants are expected to undergo stomatal closure at sufficiently high water potentials to prevent wilting and/or substantial (i.e., ≥50%) declines in stem hydraulic conductivity (6, 19, 20). Additionally, the vulnerability segmentation hypothesis predicts that plants limit stem embolism by exhibiting less negative thresholds for declines in hydraulic conductivity in the leaves and roots, thereby sequestering hydraulic damage in those organs (17). Plants that do not exhibit this trait sequence are expected to avoid drought damage by limiting water stress (i.e., maintaining a high Ψmin, MD relative to thresholds for damage) through deep roots, capacitance from stored water, drought deciduousness, or a preference for mesic environments (21, 22), or to experience significant damage at Ψmin, MD and survive through recovery processes (23).
We compiled species means from the published literature for 262 woody angiosperm and 48 gymnosperm species from 174 studies for the water potential thresholds for wilting, plant death, and declines in stomatal conductance (gs) and hydraulic conductivity (K) of leaves, stems, and roots (trait symbols and definitions in Table 1, references in SI Appendix, Table S1, and ranges in SI Appendix, Fig. S1). Controversy has recently arisen regarding measurements of stem and root hydraulic traits (24), in particular about whether nonsigmoidal vulnerability relationships (i.e., of K vs. Ψ) are caused by methodological artifacts that overestimate vulnerability. We tested the correlations across species by using all available data (SI Appendix, SI Methods), but confirmed our conclusions for the smaller dataset derived from sigmoidal relationships (n = 285) and present these results in the main text (Dataset S1). We evaluated the drivers of the correlations and the trait sequence for the subset of species for which all traits were measured at the same site during the same ≤6 mo sampling period, to minimize intraspecific variation (n = 238) (Dataset S2). Both analyses used hydraulic traits derived from sigmoidal relationships, and the sequence analyses focused on woody dicots, because there was insufficient data to test other curve shapes or plant functional types.
Table 1.
The symbol, definition, and functional significance of the drought tolerance traits and the environmental water supply and general plant water status variables
| Symbol | Definition | n | Significance |
| ΨW | Water potential | Potential energy of water; a thermodynamically explicit and scalable index of water status | |
| Ψleaf, Ψstem, Ψroot | ΨW of the leaf, stem, and root | Index of hydration and the demand for water of each organ | |
| πtlp | Bulk leaf turgor loss point, the Ψleaf where turgor potential = 0 | 285 | Point at which, on average, leaf cells lose turgor and the leaf wilts (7) |
| gS Ψ50 | Ψleaf at 50% loss of stomatal conductance | 49 | ΨW at 50% loss is a standard and, thus, comparable measure of drought tolerance across physiological processes (6) |
| gS Ψ95 | Ψleaf at 95% loss of stomatal conductance | 49 | Approximates the maximum leaf water stress a plant can tolerate while maintaining gas exchange and C uptake |
| Kleaf Ψ50 | Ψleaf at 50% loss of leaf conductivity | 117 | Hydraulic traits measure drought impacts on the water supply for transpiration, which limits gas exchange and C uptake (17). Leaf water supply is hypothesized to be the most direct hydraulic constraint on transpiration (8) |
| Kstem Ψ12 | Ψstem at 12% loss of stem conductivity | 208 | Early declines in stem water supply are expected to impact gas exchange and C uptake more directly than later declines (10) |
| Kstem Ψ50 | Ψstem at 50% loss of stem conductivity | 286 | Hypothesized to correspond closely to the maximum water stress plants tolerate in natural conditions (4) |
| Kstem Ψ88 | Ψstem at 88% loss of stem conductivity | 204 | Hypothesized to be the point of irreversible xylem damage (18) |
| Kroot Ψ50 | Ψroot at 50% loss of root conductivity | 44 | Roots are hypothesized to be the “weakest link” (least tolerant organ), limiting tolerance of the entire hydraulic system (45) |
| Plant Ψlethal | Ψleaf at plant death; here, the Ψleaf at which all leaves show tissue damage | 15 | Integrates physiological and metabolic drought responses and recovery and directly links drought to performance (11) |
| Ψmin, MD, Ψmin, PD | Seasonal minimum water potential (Ψmin), the most negative Ψleaf measured in the growing season at predawn (PD) or midday (MD) | 174 | Midday measurements quantify the strongest water stress the leaves experience in a typical year, whereas predawn measurements characterize the most negative soil water potential (13) |
n is the number of species compiled for each trait. All units are MPa.
Results and Discussion
Correlations Across Species in Drought Tolerance Traits.
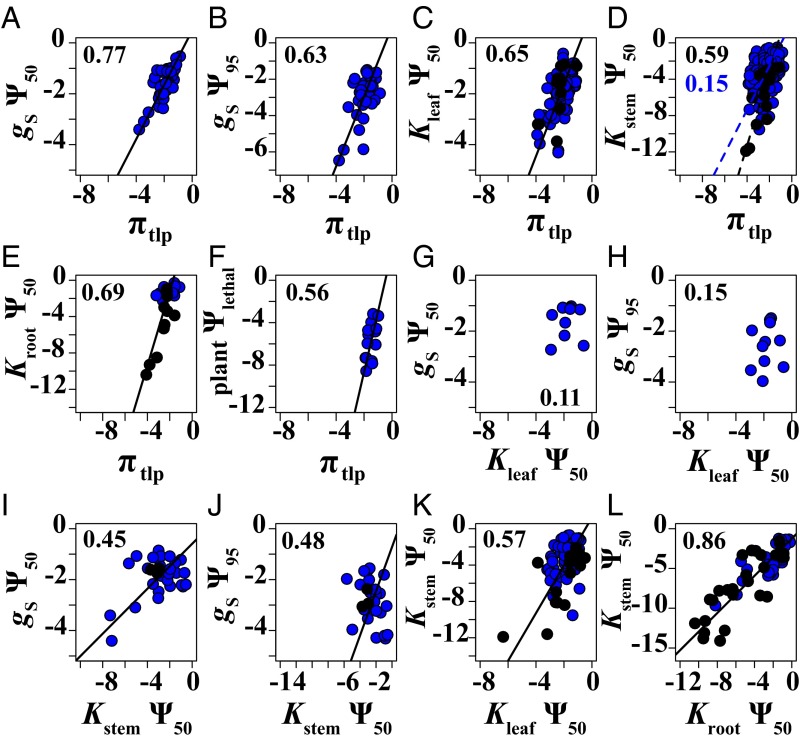
We found significant correlations among most of the drought tolerance traits, with r values ranging from 0.21 to 0.87 (Fig. 1 and SI Appendix, Table S2; n = 11–151). The nonsignificant correlations were between Kstem Ψ12 and gS Ψ50, and Kleaf Ψ50 and gS Ψ50, gS Ψ95 and Kstem Ψ88 (P > 0.1, n = 11–52). These correlations were robust to vulnerability curve shape, except that Kleaf Ψ50 and Kstem Ψ88 were correlated when including data for all curves (P = 0.03, n = 61; SI Appendix, Table S3). The stomatal and leaf hydraulic trait correlations represent particularly small species sets, indicating a need for more measurements of these traits. Nearly all traits were correlated with Ψmin, MD and Ψmin, PD, with r values ranging from 0.21 to 0.86 (SI Appendix, Figs. S2 and S3 and Table S2). Ψmin, PD and Kleaf Ψ50 were not significantly correlated (P = 0.07, n = 44), and there were insufficient data to test correlations between Ψmin, PD and the stomatal traits. Six of the 19 correlations with sufficient data to test (n ≥ 10 for each functional type) were significantly different between the angiosperms and gymnosperms. Kstem Ψ12 was significantly correlated with Kleaf Ψ50 and Ψmin, MD in the gymnosperms but not the angiosperms (SI Appendix, Table S4 and Fig. S2E), whereas the two functional types showed significantly different slopes for the correlations of Kstem Ψ50 with πtlp and Kstem Ψ12 (Fig. 1D), and of Ψmin, MD with Kstem Ψ50 and Kroot Ψ50 (SI Appendix, Fig. S2 F and H).
Fig. 1.
Correlations among drought tolerance traits across species. Symbols follow Table 1. Blue points represent angiosperms, and black points represent gymnosperms. Solid black lines are standard major axis relationships that are significant across all species. Dashed lines are correlations that are significantly different between the gymnosperms (black lines) and angiosperms (blue lines). All significant correlations remained significant after correcting for multiple tests (46). The r values are shown on each graph, and P values and sample sizes are in SI Appendix, Table S2. All of the traits were significantly correlated (A–F and I–L), except for Kleaf Ψ50 and gS Ψ50 (G) and gS Ψ95 (H). For graphical clarity, correlations with Kstem Ψ12 and Ψ88 are not shown. All of the stem hydraulic traits showed the same correlations, except that Kstem Ψ12 was not significantly correlated with gS Ψ50 and Kleaf Ψ50 was not significantly correlated with Kstem Ψ88 (SI Appendix, Table S2). Kstem Ψ12 was significantly correlated with Kleaf Ψ50 in the gymnosperms but not the angiosperms, whereas the two functional types showed significantly different slopes for the correlations of Kstem Ψ50 with πtlp (D) and with Kstem Ψ12 (SI Appendix, Table S4). We did not compile variation in plant Ψlethal from the literature, because most published studies use different definitions for plant death, but instead show this correlation from the largest study of these traits (11) for comparison with the other correlations with πtlp (F).
Applying the Framework To Predict Drought Tolerance Traits.
These correlations provide a framework representative of many species for extrapolating plant responses to a wide range of water stress from a small number of measured traits. Extrapolating from the correlations with Kstem Ψ50, which has been measured for the most species (4), or πtlp, which can be assessed rapidly (25), provides a reasonable estimate for less commonly measured traits, until such data become available in the literature for more species (see Dataset S3 for estimating traits from these correlations). The correlations strongly support predicting Kleaf Ψ50 and, for the angiosperms, the stomatal traits from πtlp (r2 = 0.40–0.59), and πtlp enabled trait estimation with considerably smaller prediction intervals than Kstem Ψ50. πtlp also enabled estimation of Ψmin, MD with smaller prediction intervals than Kstem Ψ50 in both the angiosperms and gymnosperms. These “first pass” estimates lend expediency to assessing drought tolerance for many species, and potentially enable more detailed modeling of plant drought responses, given that few species have been studied relative to the worldwide diversity of plant species, and even these have only been assessed for a few traits.
Trait Correlations with Environmental Water Supply.
The significant correlations with Ψmin, MD support the selective pressure of plant water stress on all of the traits (SI Appendix, Fig. S2). Further, the correlations with Ψmin, PD supported the use of any of the traits but Kleaf Ψ50 to predict species distributions relative to soil water supply (SI Appendix, Fig. S3), although previous studies of smaller species sets have shown significant correlations between Kleaf Ψ50 and precipitation (5, 26), indicating a need to test this relationship across yet-larger species sets. Notably, Ψmin, MD was especially strongly correlated with gS Ψ50 and gS Ψ95 (r = 0.76–0.86), suggesting that these stomatal traits may be especially important influences on the maximum water stress the leaves experience (SI Appendix, Fig. S2 B and C), whereas Kroot Ψ50 had the strongest association with minimum soil water potential (r = 0.72) (SI Appendix, Fig. S3F and Table S2). Testing these hypotheses requires measuring more traits for the same species, and, especially, focusing on closely related species within clades that have diversified across habitats ranging widely in water availability.
Disentangling the Basis for Trait Correlations.
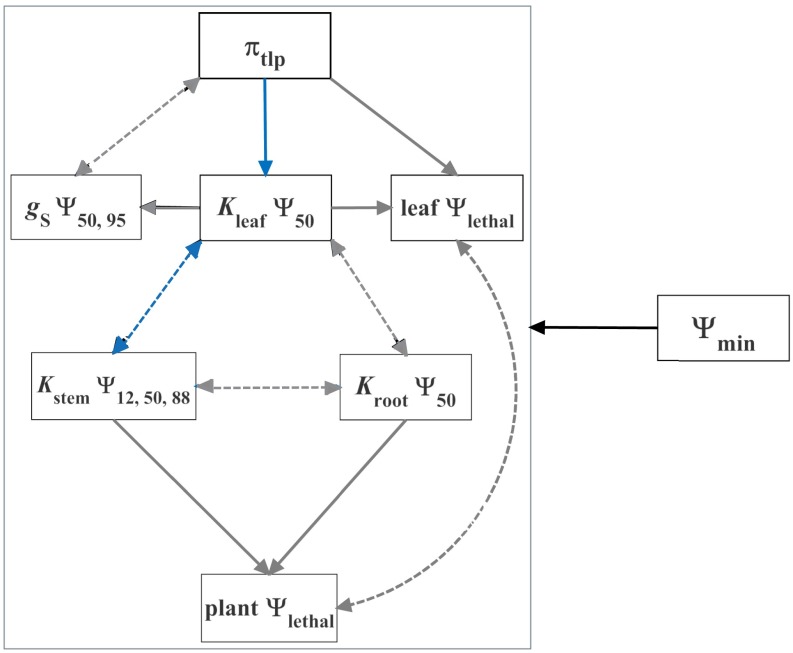
We found support for hypotheses from the literature (Fig. 2) that attributed drought tolerance trait correlations to functional coordination, concerted convergence (wherein water stress selects for each trait independently), and/or shared ancestry. Of the 14 correlations with sufficient data to test (n = 19–64), 4 correlations were improved beyond the correlation of each trait with Ψmin, MD alone by accounting for a trait predictor (29%), 1 by accounting for phylogeny (7%), and 1 by accounting for both (7%) (SI Appendix, Table S5). Thus, for a total of 43% of trait correlations, we could resolve linkages beyond simply a correlation arising from independent associations with water stress. As hypothesized, πtlp improved prediction of Kleaf Ψ50, and vice versa, whereas the stem hydraulic traits Kstem Ψ12 and Ψ88 were not correlated with πtlp after accounting for water stress. However, contrary to prediction, Kstem Ψ50 and Kleaf Ψ50 were more related than expected from correlations with water stress alone. Further, the πtlp improved prediction of KstemΨ50, and Kstem Ψ12 improved prediction of Kleaf Ψ50, but not vice versa.
Fig. 2.
Testing hypotheses for the drivers of the correlations among the drought tolerance traits. Most of the trait correlations are predicted to be driven by concerted convergence, wherein the selective pressure of water stress (Ψmin, MD or Ψmin, PD) acts independently on each trait to optimize overall plant function during drought (10, 17, 28). These hypotheses are indicated with dashed lines. Additionally, πtlp was hypothesized to influence Kleaf Ψ50 mechanistically (20). Kleaf Ψ50, in turn, would influence gS Ψ50 and Ψ95 and the threshold Ψleaf for leaf death (leaf Ψlethal) (30, 31), and the stem and root hydraulic traits would influence the plant mortality threshold (plant Ψlethal) (19). These hypotheses are indicated with solid lines. As predicted, πtlp and Kleaf Ψ50 were more correlated than expected from water stress and relatedness alone. Functionally coordinated traits are indicated with blue lines. Other correlations were best explained by the independent relationship of each trait with water stress. Concerted convergence is indicated with black lines. Conversely, Kstem Ψ50 was also more strongly correlated with Kleaf Ψ50 and, when characterizing water stress with Ψmin, MD, with πtlp than expected from concerted convergence, consistent with strong functional coordination within the hydraulic system across organs (SI Appendix, Tables S5 and S6). The remaining hypotheses had insufficient data to test (indicated with gray lines).
It is well recognized that Ψmin, MD can be affected by plant traits in addition to soil dryness (14), so we verified these findings for Ψmin, PD (n = 18–40; SI Appendix, Table S6). The water stress variables were strongly correlated (r2 = 0.85, P < 0.001, n = 71). The coordination analyses showed largely similar results, with the exceptions that Kleaf Ψ50 and Kstem Ψ12 were both more strongly related than expected from associations with Ψmin, PD, whereas Kstem Ψ50 and πtlp were not correlated after accounting for Ψmin, PD.
Several mechanisms could potentially drive the observed trait coordination. The coordination between Kleaf Ψ50 and πtlp supports the hypothesized mechanistic effect of turgor loss in the mesophyll on declines in Kleaf via the extraxylary pathway (20). As a leaf dries, and the mesophyll cells lose turgor, the cells shrink, and aquaporin activity and abscisic acid levels can shift rapidly, affecting water transport (20). The extraxylary pathway accounts for a significant proportion of overall leaf hydraulic resistance (∼25–70%) (27), and the vulnerability of this pathway strongly impacts Kleaf Ψ50 (20). Indeed, species with more negative πtlp values undergo less cell shrinkage under dehydration and have slower declines in Kleaf with leaf water potential (20). The coordination between Kleaf Ψ50 and Kstem Ψ50, and potentially, Kstem Ψ12, might arise because hydraulic function in these organs is closely linked. At a given transpiration rate, Kstem influences Ψleaf, and Kleaf impacts the gradient between Ψleaf and Ψstem (17, 27). Further, many other extrinsic factors beyond Ψmin (e.g., vapor pressure deficit, light exposure) may directionally select for stem and leaf hydraulic traits, producing correlations among these traits within habitats with similar soil water supply. Conversely, independent linkages with Kleaf Ψ50 may partly drive the correlation between Kstem Ψ50 and πtlp. Sampling these traits across a wider range of species and environments has the potential to resolve the coordination between πtlp and Kstem Ψ50 after accounting for their linkages with Kleaf Ψ50 and water stress.
Linkages Between the Stomatal and Hydraulic Traits.
The correlations of stomatal and hydraulic traits can provide insight into their functional linkages. Whereas the drivers of stomatal closure are not fully resolved, the hydromechanical model predicts that guard cells regulate their aperture in response to the water status at the stomatal evaporation site; this water status, in turn, is influenced by the hydraulic conductivity of the stems, leaves, and roots (8, 28, 29). Further, declines in stomatal conductance have been hypothesized to respond more directly to Kleaf than Kstem (30, 31). Our analyses instead showed that across species, the stomatal traits were significantly correlated with stem but not leaf vulnerability. The statistical independence of gS Ψ50 and Ψ95 and Kleaf Ψ50 is consistent with previous studies, showing wide species variation in the safety margins between stomatal closure and leaf hydraulic dysfunction (32), wherein species vary between “isohydry,” which maintains high Ψleaf and Kleaf via early stomatal closure, and “anisohydry,” which maintains gas exchange to low Ψleaf at the expense of hydraulic function. The correlation between the stomatal traits and Kstem Ψ50 and Ψ88 corroborates a previous metaanalysis of species from ecosystems worldwide (6), but contradicts two studies within specific ecosystems (10, 33). Thus, the coordination of stomatal sensitivity with stem vulnerability across species appears to be related to their independent roles in drought tolerance rather than to coordinated function, with stomatal responses affecting carbon uptake during mild and moderate drought, and vulnerability affecting the ability of stems to survive strong drought (2, 15).
Sequence of Drought Response Traits.
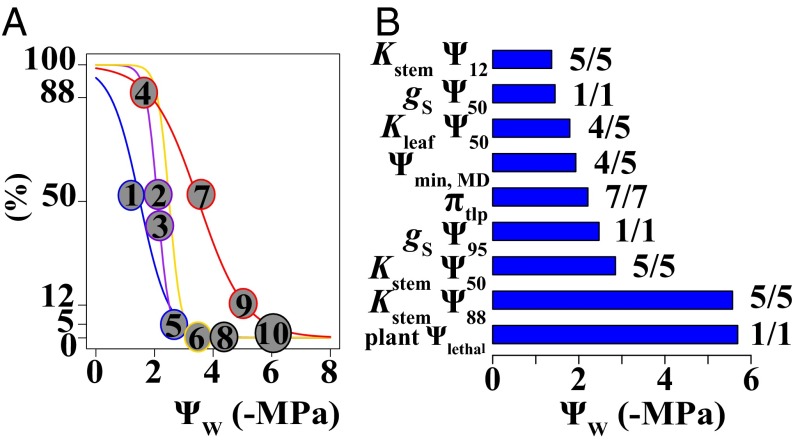
On average, the woody dicots exhibited a typical trait sequence that is expected to limit severe tissue damage during drought, such as wilting and substantial stem embolism (Fig. 3). The 12% declines in stem conductivity (Kstem Ψ12) occurred at the least negative water potentials, followed sequentially by Kleaf Ψ50, wilting (πtlp), and 50% and 88% declines in stem conductivity (Kstem Ψ50 and Ψ88) (Fig. 3B). The positions of these traits in the sequence were clearly resolved by mixed effects models, which showed significant differences between all of these traits (SI Appendix, Table S7. Wilting (πtlp) occurred after gS Ψ50, as predicted, but before gS Ψ95, contrary to the expectation that plants would undergo stomatal closure at sufficiently high water potentials to prevent wilting. Placing Ψmin, MD in this sequence indicated the drought responses that plants experience under seasonal water stress in natural conditions. Ψmin, MD occurred at similar water potentials as Kleaf Ψ50, and significantly before wilting and Kstem Ψ50, but after Kstem Ψ12 (SI Appendix, Table S7). The water potential at plant death (plant Ψlethal) was the most negative trait. There were insufficient data to compare gS Ψ50 and gS Ψ95 to traits besides πtlp, or to place Kroot Ψ50 in the sequence.
Fig. 3.
The hypothesized (A) and observed (B) sequence of water potential values for the drought tolerance traits within individual plants. A shows the relationship between organ water potential (ΨW) and the percent decline in stomatal conductance (gS, blue), hydraulic conductivity in the leaves, roots and stems (Kleaf and Kroot, purple; Kstem, red), and turgor pressure (ΨP, yellow). The numbered circles show the order in which given declines in function will occur if plants generally follow a trait sequence that is expected to limit tissue damage during drought. In this sequence, 50% declines in stomatal conductance (gS Ψ50, #1) are expected to occur at the least negative water potentials to slow transpiration (37), followed by moderate (50%) declines in Kleaf and Kroot (Kleaf and Kroot Ψ50) and minor (12%) declines in Kstem (Kstem Ψ12), if leaf and root dysfunction protects the stem from embolism, as predicted by vulnerability segmentation (17). (These traits are labeled #2–4 but shown in the same position, because their order is not hypothesized). Stomatal closure, or gS Ψ95 (#5), would occur before potentially major damage, including loss of turgor pressure in the leaf cells, or wilting (πtlp, #6), and 50% declines in Kstem (Kstem Ψ50, #7) (6, 10). Kstem Ψ50 is hypothesized to limit the water stress that plants tolerate, and thus, we expected the most negative Ψleaf values plants reach under natural growing conditions (Ψmin, MD, #8) to be near Kstem Ψ50 (4). Eighty-eight percent declines in Kstem (Kstem Ψ88, #9) have been hypothesized to induce irreversible xylem damage and, thus, to occur somewhat before plant death (plant Ψlethal, #10) (19), which we estimated as the Ψleaf at which all leaves showed tissue damage (11). The sequence is determined from pairwise comparisons between all of the traits (SI Appendix, Table S7), but, for clarity, B shows the mean of each trait from its pairwise comparison with the trait immediately after (i.e., more negative than) it in the sequence. The traits generally followed this sequence, with the order of Kstem Ψ12 > Kleaf Ψ50 & Ψmin, MD > πtlp > Kstem Ψ50 > Kstem Ψ88 supporting the hypothesized sequence, with the exception that Kleaf Ψ50 and Ψmin, MD were not significantly different. πtlp occurred after gS Ψ50, as hypothesized, but before gS Ψ95, contrary to prediction. There were insufficient data to test Kroot Ψ50, or to compare the stomatal traits to any other trait. For each trait, the number to the left is the number of other traits it was significantly different from, and the number to the right is the total number of trait comparisons with sufficient data to test. Notably, the sequence is shown with respect to organ-specific water potentials; in the transpiring plant, the high resistance of the hydraulic pathway produces a gradient of increasingly negative water potentials from the root to the leaf. Thus, the stem may undergo less embolism than suggested by this sequence.
Phenology significantly affected one comparison (SI Appendix, Table S8). Kleaf Ψ50 occurred after Ψmin, MD in evergreen but not deciduous species, consistent with previous studies of smaller species sets showing that deciduous species undergo greater leaf hydraulic dysfunction to maximize carbon uptake, because the leaves are replaced annually (16). More studies are needed to characterize the variation in the sequence across leaf functional types within ecosystems and across ecosystems relative to water supply.
We applied additional statistics to confirm that the mean trait differences are robust to measurement uncertainty, and to evaluate the degree to which plants conform to the average trait sequence. We compared the 95% confidence intervals around mean trait values for each species for all traits for which SEs were provided (i.e., gS Ψ50, Kleaf Ψ50, Kstem Ψ50, πtlp, and Ψmin, MD). Across all comparisons, 42–82% of the species significantly supported the findings for the mean trait differences shown in the general sequence (SI Appendix, Figs. S4–S6), confirming that these results were largely robust to measurement uncertainty. Vulnerability segmentation was strongly supported, with Kstem Ψ50 significantly more negative than Kleaf Ψ50 for 82% of the species, and no species significantly showing the opposite pattern (SI Appendix, Fig. S4). Plants showed the most variation in the order of πtlp and Kstem Ψ50, with the finding that πtlp occurs at a less negative water potential significantly supported by 33% of the species and opposed by 21% (SI Appendix, Fig. S5). Notably, the low sample size at the ends of the stomatal response and hydraulic vulnerability curves and the nonlinear curve shapes suggest that gS Ψ95, Kstem Ψ12, and Kstem Ψ88 will tend to have much larger errors. Further, these traits are typically estimated from nonlinear regressions with organ water potential as the independent variable and extrapolated as x values from the regression at given y values. This convention precludes estimating SEs for these traits. Thus, strongly resolving the certainty of the position of these traits in the sequence will require the further development of statistical and computational methods to estimate these uncertainties (34).
The sequence provides several key insights into plant responses to drought. First, the occurrence of Kstem Ψ50 at lower water potentials than Ψmin, MD is generally consistent with the “high embolism resistance” paradigm, wherein plants are predicted to prevent substantial (i.e., 50%) declines in Kstem over the course of typical variation in water supply, and contrary to the “high embolism repair” paradigm, which expects plants to typically reach such declines and maintain function through recovery mechanisms (15, 23, 35). However, Ψmin, MD was more negative than Kstem Ψ50 for nearly one-fifth of the species (SI Appendix, Fig. S4), consistent with a previous metaanalysis of data for stem hydraulic dysfunction that were also included in this study (4). These species may experience substantial embolism during drought and depend strongly on recovery mechanisms to survive, such as refilling embolisms from stored water and/or growing new xylem in branching patterns that circumvent embolized conduits (36). However, when inferring Kstem responses to drought, it is important to note that, during transpiration, the leaf experiences more negative water potentials than the stem, given the high resistance of the leaf hydraulic pathway (27). This water potential difference protects the stem and, especially, the roots from extreme tension that would drive embolism during dehydration; thus, for a plant experiencing a Ψleaf equal to Ψmin, MD, the actual Ψstem should be less negative. Therefore, these species could potentially experience less severe embolism than expected from this sequence of organ-scale water potential thresholds. Under drought, the water potentials across organs are expected to be highly variable, depending on hydraulic conductivity and influx from water storage. Thus, either in situ psychrometer measurements or a modeling approach is needed to quantify the impact of the trait sequence on the actual organ water potentials and conductivities that the plant experiences at a given soil water potential and transpiration rate.
The strong support for vulnerability segmentation and for leaf hydraulic decline under mild drought indicates that hydraulic redundancy (i.e., excess hydraulic capacity) and/or the capacity for hydraulic recovery in the leaf is crucial to drought tolerance for many plants (12, 16, 37). These findings point to the importance of elucidating the leaf traits that determine this capacity (20). Although contrary to our hypotheses, the occurrence of gS Ψ95 at more negative water potentials than πtlp is consistent with previous findings that the guard cells that control stomatal aperture (38) are largely isolated from bulk leaf turgor (28). Notably, many species are known to adjust πtlp under water stress to improve drought tolerance (39), but only a few species were assessed for drought response traits during the dry season. Although moderate plastic shifts would tend to be toward the direction of greater tolerance and, thus, unlikely to affect the sequence of traits, further studies are needed to evaluate the degree to which plasticity in πtlp, or in other traits, impacts this sequence. Greater sampling is also required to characterize the role of stomatal closure in preventing damage to the hydraulic system.
Future Directions To Improve the Predictive Capacity of Drought Tolerance Traits.
This synthesis provides insight into the roles of trait coordination, coselection with water stress, and shared ancestry in the correlations of stomatal, hydraulic, and mesophyll drought tolerance traits, as well as the average trait sequence within plants.
This perspective also points to key developments needed to improve the predictive capacity of trait-based approaches for plant drought tolerance. More measurements are needed for the stomatal and root hydraulic traits, especially because these traits were the strongest correlates of environmental water stress. More data are also needed for gymnosperms, which have a lower capacity for recovery and may thus depend more strongly on the trait sequence (4, 40). Further, 70% of the species were represented in more than one comparison in the sequence analysis, but most of this overlap is accounted for by Ψmin, MD, with only 30% of species assessed for more than two plant traits. It is thus critical that the general sequence be verified by sampling more traits within given species, with this sequence serving as a “first-pass” approximation until such data are more widely available. In addition, many physiological processes contribute to growth and survival during drought. Capacitance, embolism recovery, and metabolic synthesis of abscisic acid and nonstructural carbon reserves have all been predicted to influence drought survival, but the roles of these traits and their interactions with the classical drought tolerance traits, or their influence on plant Ψlethal, are not well understood (15, 23). Indeed, measurements of plant Ψlethal are sparse in the literature, and most studies use different definitions for plant death (11, 41). These values correlate with πtlp (11), as shown here, and with leaf and stem hydraulic traits across small species sets (n ≤ 5) (19, 37, 41), and it is increasingly critical for further studies to determine how these traits interact to influence plant mortality during drought.
Methods
To compile the drought tolerance trait dataset, we drew on references from several recent metaanalyses of variation in individual drought tolerance traits (4, 6, 7, 26) and conducted Web of Science and Google Scholar searches by using the keywords “turgor loss point,” “wilting point,” “stomatal closure,” “stomatal conductance,” “lethal leaf water potential,” and “hydraulic vulnerability” or “cavitation” paired with “leaf,” “stem,” or “root.” These studies measured traits with standard methods (detailed in the SI Appendix, SI Methods). To minimize ontogenetic and methodological variation, we included only studies that met the following criteria. For all traits, we included only studies that sampled (i) mature plant organs from (ii) sapling or adult plants, and not seedlings, growing in (iii) natural ecosystems or urban conditions for wild species, or typical agricultural conditions for crop species. For πtlp values, we selected only studies that measured (iv) leaves that were rehydrated ≥6 h before measurement, unless the study reported no significant effect of a shorter rehydration time. We included gS Ψ50 and Ψ95 values only from studies that (v) measured ΨL and gS for leaves collected at the same time and (vi) included ΨL values that were less negative than −1.5 MPa to capture early declines in gS.
We evaluated the correlations among traits with standard major axis regressions by using the smatr package for R software (version 3.3.0) (42). We present the correlations for untransformed data and confirmed these findings for log-transformed values. We identified the drivers of the trait correlations by fitting regression models predicting each trait as a function of (i) Ψmin, MD or Ψmin, PD, and (ii) Ψmin, MD or Ψmin, PD and one trait variable. To account for relatedness, we constructed a phylogeny with Phylocom (43) and fitted phylogenetic least-squares regression relationships with the caper package (44). We used Aikake Information Criteria corrected for small sample sizes (AICc) to evaluate model support, with AICcnested – AICcfull ≥ 2 supporting the full model. We tested the trait sequence by fitting a mixed-effects model to the trait differences to calculate the mean trait difference while accounting for study effects. We constructed 95% confidence intervals (CI) for the mean differences with 1,000 nonparametric bootstraps to correct for nonnormality. To confirm these results were robust to measurement uncertainty, we constructed 95% CI around the mean trait values for the species with available data (n = 182) (SI Appendix, SI Methods).
Supplementary Material
Acknowledgments
We thank Sylvain Delzon for insightful and helpful discussion and Sylvain Delzon, Tim Brodribb, Chris Blackman, and Hervé Cochard for contributing valuable stem hydraulic data. This work was funded by National Science Foundation Awards 1108534 and 1457279, the UCLA Department of Ecology and Evolutionary Biology, the UCLA Dissertation Year Fellowship, and the Charles E. and Sue K. Young Graduate Fellowship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1604088113/-/DCSupplemental.
References
- 1.Sheffield J, Wood EF. Characteristics of global and regional drought, 1950–2000: Analysis of soil moisture data from off-line simulation of the terrestrial hydrologic cycle. J Geophys Res. 2007;112(D17):115. [Google Scholar]
- 2.Anderegg WR, et al. Meta-analysis reveals that hydraulic traits explain cross-species patterns of drought-induced tree mortality across the globe. Proc Natl Acad Sci USA. 2016;113(18):5024–5029. doi: 10.1073/pnas.1525678113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maherali H, Pockman WT, Jackson RB. Adaptive variation in the vulnerability of woody plants to xylem cavitation. Ecology. 2004;85(8):2184–2199. [Google Scholar]
- 4.Choat B, et al. Global convergence in the vulnerability of forests to drought. Nature. 2012;491(7426):752–755. doi: 10.1038/nature11688. [DOI] [PubMed] [Google Scholar]
- 5.Blackman CJ, Brodribb TJ, Jordan GJ. Leaf hydraulic vulnerability influences species’ bioclimatic limits in a diverse group of woody angiosperms. Oecologia. 2012;168(1):1–10. doi: 10.1007/s00442-011-2064-3. [DOI] [PubMed] [Google Scholar]
- 6.Klein T. The variability of stomatal sensitivity to leaf water potential across tree species indicates a continuum between isohydric and anisohydric behaviours. Funct Ecol. 2014;28(6):1313–1320. [Google Scholar]
- 7.Bartlett MK, Scoffoni C, Sack L. The determinants of leaf turgor loss point and prediction of drought tolerance of species and biomes: A global meta-analysis. Ecol Lett. 2012;15(5):393–405. doi: 10.1111/j.1461-0248.2012.01751.x. [DOI] [PubMed] [Google Scholar]
- 8.Brodribb TJ, Holbrook NM. Stomatal closure during leaf dehydration, correlation with other leaf physiological traits. Plant Physiol. 2003;132(4):2166–2173. doi: 10.1104/pp.103.023879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bucci SJ, et al. Hydraulic differences along the water transport system of South American Nothofagus species: Do leaves protect the stem functionality? Tree Physiol. 2012;32(7):880–893. doi: 10.1093/treephys/tps054. [DOI] [PubMed] [Google Scholar]
- 10.Brodribb T, Holbrook NM, Edwards EJ, Gutierrez MV. Relations between stomatal closure, leaf turgor and xylem vulnerability in eight tropical dry forest trees. Plant Cell Environ. 2003;26:443–450. [Google Scholar]
- 11.Baltzer JL, Davies SJ, Bunyavejchewin S, Noor NSM. The role of desiccation tolerance in determining tree species distributions along the Malay–Thai Peninsula. Funct Ecol. 2008;22(2):221–231. [Google Scholar]
- 12.Scoffoni C, McKown AD, Rawls M, Sack L. Dynamics of leaf hydraulic conductance with water status: Quantification and analysis of species differences under steady state. J Exp Bot. 2012;63(2):643–658. doi: 10.1093/jxb/err270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patterson TB, Givnish TJ. Phylogeny, concerted convergence, and phylogenetic niche conservatism in the core Liliales: Insights from rbcL and ndhF sequence data. Evolution. 2002;56(2):233–252. doi: 10.1111/j.0014-3820.2002.tb01334.x. [DOI] [PubMed] [Google Scholar]
- 14.Bhaskar R, Ackerly DD. Ecological relevance of minimum seasonal water potentials. Physiol Plant. 2006;127(3):353–359. [Google Scholar]
- 15.Delzon S, Cochard H. Recent advances in tree hydraulics highlight the ecological significance of the hydraulic safety margin. New Phytol. 2014;203(2):355–358. doi: 10.1111/nph.12798. [DOI] [PubMed] [Google Scholar]
- 16.Johnson DM, McCulloh KA, Meinzer FC, Woodruff DR, Eissenstat DM. Hydraulic patterns and safety margins, from stem to stomata, in three eastern U.S. tree species. Tree Physiol. 2011;31(6):659–668. doi: 10.1093/treephys/tpr050. [DOI] [PubMed] [Google Scholar]
- 17.Tyree MT, Ewers FW. The hydraulic architecture of trees and other woody plants. New Phytol. 1991;119(3):345–360. [Google Scholar]
- 18.Guyot G, Scoffoni C, Sack L. Combined impacts of irradiance and dehydration on leaf hydraulic conductance: Insights into vulnerability and stomatal control. Plant Cell Environ. 2012;35(5):857–871. doi: 10.1111/j.1365-3040.2011.02458.x. [DOI] [PubMed] [Google Scholar]
- 19.Urli M, et al. Xylem embolism threshold for catastrophic hydraulic failure in angiosperm trees. Tree Physiol. 2013;33(7):672–683. doi: 10.1093/treephys/tpt030. [DOI] [PubMed] [Google Scholar]
- 20.Scoffoni C, Vuong C, Diep S, Cochard H, Sack L. Leaf shrinkage with dehydration: Coordination with hydraulic vulnerability and drought tolerance. Plant Physiol. 2014;164(4):1772–1788. doi: 10.1104/pp.113.221424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bucci SJ, Goldstein G, Scholz FG, Meinzer FC. Physiological significance of hydraulic segmentation, nocturnal transpiration and capacitance in tropical trees: Paradigms revisited. In: Goldstein G, Santiago LS, editors. Tropical Tree Physiology. Springer International; Cham, Switzerland: 2016. pp. 205–225. [Google Scholar]
- 22.Machado JL, Tyree MT. Patterns of hydraulic architecture and water relations of two tropical canopy trees with contrasting leaf phenologies: Ochroma pyramidale and Pseudobombax septenatum. Tree Physiol. 1994;14(3):219–240. doi: 10.1093/treephys/14.3.219. [DOI] [PubMed] [Google Scholar]
- 23.Klein T, Yakir D, Buchmann N, Grünzweig JM. Towards an advanced assessment of the hydrological vulnerability of forests to climate change-induced drought. New Phytol. 2014;201(3):712–716. doi: 10.1111/nph.12548. [DOI] [PubMed] [Google Scholar]
- 24.Sperry JS, Christman MA, Torres-Ruiz JM, Taneda H, Smith DD. Vulnerability curves by centrifugation: Is there an open vessel artefact, and are ‘r’ shaped curves necessarily invalid? Plant Cell Environ. 2012;35(3):601–610. doi: 10.1111/j.1365-3040.2011.02439.x. [DOI] [PubMed] [Google Scholar]
- 25.Bartlett MK, et al. Rapid determination of comparative drought tolerance traits: Using an osmometer to predict turgor loss point. Methods Ecol Evol. 2012;3(5):880–888. [Google Scholar]
- 26.Nardini A, Luglio J. Leaf hydraulic capacity and drought vulnerability: Possible trade-offs and correlations with climate across three major biomes. Funct Ecol. 2014;28(4):810–818. [Google Scholar]
- 27.Sack L, Holbrook NM. Leaf hydraulics. Annu Rev Plant Biol. 2006;57:361–381. doi: 10.1146/annurev.arplant.56.032604.144141. [DOI] [PubMed] [Google Scholar]
- 28.Buckley TN. The control of stomata by water balance. New Phytol. 2005;168(2):275–292. doi: 10.1111/j.1469-8137.2005.01543.x. [DOI] [PubMed] [Google Scholar]
- 29.Salleo S, Nardini A, Pitt F, Lo Gullo MA. Xylem cavitation and hydraulic control of stomatal conductance in laurel (Laurus nobilis L.) Plant Cell Environ. 2000;23:71–79. [Google Scholar]
- 30.Brodribb TJ, Holbrook NM. Stomatal protection against hydraulic failure: A comparison of coexisting ferns and angiosperms. New Phytol. 2004;162(3):663–670. doi: 10.1111/j.1469-8137.2004.01060.x. [DOI] [PubMed] [Google Scholar]
- 31.Lo Gullo MA, Nardini A, Trifilo P, Salleo S. Changes in leaf hydraulics and stomatal conductance following drought stress and irrigation in Ceratonia siliqua (Carob tree) Physiol Plant. 2003;117:186–194. [Google Scholar]
- 32.Johnson DM, Woodruff DR, McCulloh KA, Meinzer FC. Leaf hydraulic conductance, measured in situ, declines and recovers daily: Leaf hydraulics, water potential and stomatal conductance in four temperate and three tropical tree species. Tree Physiol. 2009;29(7):879–887. doi: 10.1093/treephys/tpp031. [DOI] [PubMed] [Google Scholar]
- 33.Skelton RP, West AG, Dawson TE. Predicting plant vulnerability to drought in biodiverse regions using functional traits. Proc Natl Acad Sci USA. 2015;112(18):5744–5749. doi: 10.1073/pnas.1503376112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogle K, Barber JJ, Willson C, Thompson B. Hierarchical statistical modeling of xylem vulnerability to cavitation. New Phytol. 2009;182(2):541–554. doi: 10.1111/j.1469-8137.2008.02760.x. [DOI] [PubMed] [Google Scholar]
- 35.Cochard H, Delzon S. Hydraulic failure and repair are not routine in trees. Ann Sci. 2013;70(7):659–661. [Google Scholar]
- 36.Brodersen CR, McElrone AJ. Maintenance of xylem network transport capacity: A review of embolism repair in vascular plants. Front Plant Sci. 2013;4:108. doi: 10.3389/fpls.2013.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blackman CJ, Brodribb TJ, Jordan GJ. Leaf hydraulics and drought stress: Response, recovery and survivorship in four woody temperate plant species. Plant Cell Environ. 2009;32(11):1584–1595. doi: 10.1111/j.1365-3040.2009.02023.x. [DOI] [PubMed] [Google Scholar]
- 38.Buckley TN, Mott KA. Dynamics of stomatal water relations during the humidity response: Implications of two hypothetical mechanisms. Plant Cell Environ. 2002;25:407–419. [Google Scholar]
- 39.Bartlett MK, et al. Global analysis of plasticity in turgor loss point, a key drought tolerance trait. Ecol Lett. 2014;17(12):1580–1590. doi: 10.1111/ele.12374. [DOI] [PubMed] [Google Scholar]
- 40.Choat B, Brodersen CR, McElrone AJ. Synchrotron X-ray microtomography of xylem embolism in Sequoia sempervirens saplings during cycles of drought and recovery. New Phytol. 2015;205(3):1095–1105. doi: 10.1111/nph.13110. [DOI] [PubMed] [Google Scholar]
- 41.Li S, et al. Leaf gas exchange performance and the lethal water potential of five European species during drought. Tree Physiol. 2015;36(2):179–192. doi: 10.1093/treephys/tpv117. [DOI] [PubMed] [Google Scholar]
- 42.Warton DI, Duursma RA, Falster DS, Taskinen S. smatr 3- an R package for estimation and inference about allometric lines. Methods Ecol Evol. 2012;3(2):257–259. [Google Scholar]
- 43.Webb CO, Ackerly DD, Kembel SW. Phylocom: Software for the analysis of phylogenetic community structure and trait evolution. Bioinformatics. 2008;24(18):2098–2100. doi: 10.1093/bioinformatics/btn358. [DOI] [PubMed] [Google Scholar]
- 44.Orme D, et al. 2013. caper: Comparative Analyses of Phylogenetics and Evolution in R.), R package version 0.5.2.
- 45.Jackson RB, Sperry JS, Dawson TE. Root water uptake and transport: Using physiological processes in global predictions. Trends Plant Sci. 2000;5(11):482–488. doi: 10.1016/s1360-1385(00)01766-0. [DOI] [PubMed] [Google Scholar]
- 46.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57(1):289–300. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.