Significance
This study provides direct evidence supporting the model of reward–auditory cortex interaction as underlying musical pleasure: People who do not experience that pleasure have selectively reduced responses in that system. People who are especially sensitive to musical reward conversely seem to show an enhanced interaction. Our paper offers insights into the neurobiological basis of music-induced pleasure that could also provide the basis for thinking more broadly about other types of aesthetic rewards. Our results also provide an important step toward the understanding of how music may have acquired reward value through evolution.
Keywords: music, emotion, reward, anhedonia
Abstract
Although music is ubiquitous in human societies, there are some people for whom music holds no reward value despite normal perceptual ability and preserved reward-related responses in other domains. The study of these individuals with specific musical anhedonia may be crucial to understand better the neural correlates underlying musical reward. Previous neuroimaging studies have shown that musically induced pleasure may arise from the interaction between auditory cortical networks and mesolimbic reward networks. If such interaction is critical for music-induced pleasure to emerge, then those individuals who do not experience it should show alterations in the cortical-mesolimbic response. In the current study, we addressed this question using fMRI in three groups of 15 participants, each with different sensitivity to music reward. We demonstrate that the music anhedonic participants showed selective reduction of activity for music in the nucleus accumbens (NAcc), but normal activation levels for a monetary gambling task. Furthermore, this group also exhibited decreased functional connectivity between the right auditory cortex and ventral striatum (including the NAcc). In contrast, individuals with greater than average response to music showed enhanced connectivity between these structures. Thus, our results suggest that specific musical anhedonia may be associated with a reduction in the interplay between the auditory cortex and the subcortical reward network, indicating a pivotal role of this interaction for the enjoyment of music.
Without music, life would be a mistake.
Friedrich Nietzsche, Twilight of the Idols
This quote highlights the importance of music for the life of most people. Indeed, although music is not a primary reward (such as food or sex), it is reckoned as one of the most important sources of pleasure in life. Furthermore, music has been present in human societies since prehistory (1), and most of the current literature on music psychology has described it as a universal reward for human beings (2). Its ubiquity and antiquity prove the importance of music in our society (3).
Paradoxically, not everybody loves music: A small percentage of healthy individuals do not find music pleasurable, a phenomenon known as specific musical anhedonia (4). A detailed study on this population revealed that this phenomenon cannot be explained by perceptual problems [e.g., hearing impairment or specific impairment in perceptual capabilities, a condition known as amusia (5)] or by general anhedonia (lack of pleasure for all types of rewarding stimuli). When listening to music rated as pleasant by others, people with specific musical anhedonia showed a reduced emotional arousal as indexed by autonomic nervous system activity [in particular, skin conductance response (SCR) and heart rate measurements] compared with people having standard or high sensitivity to music. Notably, they showed normal responses to other types of reward [e.g., money (4)]. Therefore, individuals with specific musical anhedonia represent an ideal population in which to test models of music reward processing.
Previous studies have consistently reported that in addition to sensory and cognitive areas involved in the processing of musical information, such as auditory and frontal cortices (6–9), music recruits regions of the mesolimbic reward circuitry (9–17), especially the nucleus accumbens (NAcc). Most importantly, a recent study (17) has shown that the reward value of a novel piece of music was predicted by the functional connectivity between the NAcc and auditory cortices, as well as regions involved in valuation, such as the amygdala and orbitofrontal and ventromedial prefrontal regions. According to this model, it would be the functional link between the auditory perceptual/cognitive mechanisms, on the one hand, and the evaluative/reward mechanisms, on the other hand, that would be driving the experience of music reward. If this model is correct, then we would expect that reductions in the interactions within this network would lead to lack of experienced musical pleasure.
In the present study, we aim to unravel the brain mechanisms responsible for the specific impairment in music reward processing observed in people with specific musical anhedonia. To reach this goal, we selected 45 healthy subjects who differed in their sensitivity to music reward using a previously developed psychometric instrument, the Barcelona Musical Reward Questionnaire (BMRQ) (18), which is known to be a reliable indicator of interindividual variability in music reward sensitivity. Three groups of subjects (musical anhedonia and average or high sensitivity to musical reward) engaged in two separate experimental sessions. In the first session, SCRs were recorded while participants listened to excerpts of previously rated pleasant, neutral, and unpleasant music by an independent group of subjects to validate the group classification via an objective index of musical reward sensitivity. In the second session, subjects were scanned with fMRI while performing two different tasks: a music listening paradigm in which online pleasure ratings for 16 excerpts were given and a monetary gambling task used as a control. We hypothesized that specific musical anhedonia would be associated with reduced activity in the ventral striatum (VS; especially the NAcc) in response to pleasant music, and also a down-regulation in the interaction between auditory cortices and reward-related regions, compared with people with high and average sensitivity to music. Moreover, we hypothesized that the activation of these areas would be similar in the three groups in response to monetary rewards and punishments, demonstrating the specificity of this phenomenon for music reward processing. This study specifically addresses the neural mechanisms underpinning the phenomenon of specific musical anhedonia.
Results
Forty-five university students were divided into three groups of 15 subjects (eight females and seven males each) according to their sensitivity to music reward as assessed using the BMRQ: high sensitivity to music [hyperhedonic group (HHDN)], average sensitivity to music [hedonic group (HDN)], and low sensitivity to music [anhedonic group (ANH)]. All participants in this sample were nonmusicians and matched in other measures, such as age, general anhedonia, sensitivity to punishment and reward scale, and amusia score (Table 1).
Table 1.
Psychometric scores in the BMRQ, anhedonia, the SPSRQ and amusia of the three groups
| Demographic Information and Psychometric Scores | ANH | HDN | HHDN | P value |
| n | 15 | 15 | 15 | |
| Age, y | 21.9 (3.2) | 20.8 (3.4) | 21.3 (5.0) | 0.760 |
| BMRQ | ||||
| Overall | 54.1 (9.2) | 75.8 (4.0) | 89.4 (3.3) | <0.001 |
| Musical seeking | 8.0 (2.2) | 13.1 (1.5) | 16.0 (1.9) | <0.001 |
| Emotion evocation | 10.9 (2.9) | 15.8 (2.2) | 18.0 (1.4) | <0.001 |
| Mood regulation | 13.0 (3.1) | 16.3 (2.0) | 19.2 (1.0) | <0.001 |
| Sensory-motor | 11.7 (3.9) | 16.3 (2.4) | 18.4 (1.4) | <0.001 |
| Social reward | 10.5 (2.2) | 14.3 (1.9) | 17.0 (1.5) | <0.001 |
| Anhedonia | ||||
| PAS | 11.9 (4.3) | 10.2 (3.9) | 10.9 (3.9) | 0.531 |
| SPSRQ | ||||
| Sensitivity to punishment | 10.5 (4.0) | 8.2 (5.2) | 9.9 (5.7) | 0.439 |
| Sensitivity to reward | 9.9 (4.4) | 9.7 (4.3) | 9.5 (3.3) | 0.974 |
| Amusia | ||||
| MBEA | 85.7 (7.7) | 87.6 (7.2) | 87.4 (6.8) | 0.730 |
SDs are reported in parentheses. P value indicates the significance of the group effect in a one-way ANOVA.
SCR Data: Emotional Arousal.
In the first session, participants engaged in a music listening task, similar to the task used by Mas-Herrero et al. (4). They had to listen to 32 previously rated musical excerpts (10 pleasant, 10 neutral, 10 unpleasant, and 2 pleasant excerpts selected by the participant). We carried out this procedure for music selection to overcome the limitation that musical anhedonic participants have difficulties in providing self-selected pleasurable music. The fixed selection resulted from a preexperiment test in which an independent sample of 65 students with similar demographic characteristics rated 82 musical excerpts. All participants in this sample were nonmusicians, were matched by age to the main group [23% males, mean (M) = 22 y, SD = 3.6], and did not include any ANH participant (BMRQ scale, M = 81, SD = 7.0). From these data, we selected the 10 excerpts with the highest liking rate score across subjects (pleasant excerpts), the 10 with the lowest liking rate score (unpleasant excerpts), and another 10 with a mean liking rate just below the overall mean liking rate (neutral excerpts). While listening to music, participants were required to indicate online the degree of pleasure they were experiencing at any given time point (1 = unpleasant, 2 = neutral, 3 = low pleasure, 4 = high pleasure, 5 = chill) by pressing a corresponding key. After each excerpt, participants rated the degree of global pleasure (global liking rate), familiarity with the excerpt, emotional valence, arousal, and number and intensity of any experienced chills. For each participant, the four excerpts rated as most pleasant and the four rated as least pleasurable were selected to be used in the fMRI experiment, along with a fixed selection of four pleasant and four unpleasant excerpts (further details are provided in Materials and Methods).
Behaviorally, we confirmed significant differences on average in the global liking rates reported by the three groups on those 16 excerpts that were subsequently selected for the fMRI experiment [F(2,42) = 12.44, P < 0.001]. Post hoc comparisons using the Tukey honest significant difference (HSD) test revealed that ANH participants rated the excerpts as less pleasurable than the HDN (P = 0.044) and HHDN (P < 0.001) groups. The excerpts were also reported as less emotionally arousing by the ANH group [group effect, F(2,42) = 9.01, P < 0.001; post hoc Tukey HSD: ANH versus HDN, P = 0.071; ANH versus HHDN, P < 0.001]. In contrast, there were no differences between groups in the familiarity [F(2,42) = 1.60, P = 0.213] or mean valence [F(2,42) = 0.34, P = 0.712] rates.
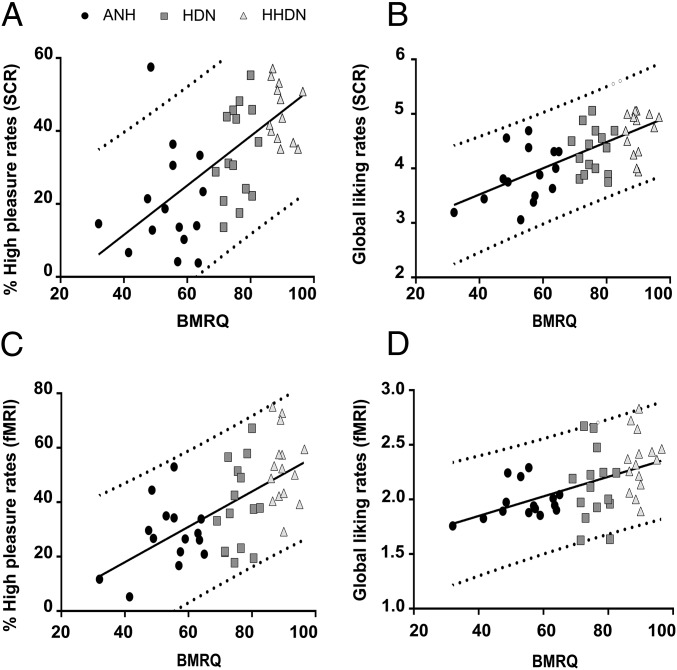
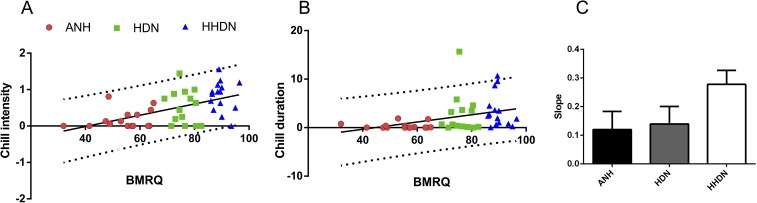
To study which variables affected pleasure ratings, the percentage of online responses associated with high pleasure (responses 4 and 5, corresponding to high pleasure rates and chills, respectively) was entered as a dependent variable in a stepwise regression with all of the psychometric scores available [BMRQ, Physical Anhedonia Scale (PAS), Sensitivity to Punishment and Sensitivity to Reward Questionnaire (SPSRQ), and Montreal Battery for Evaluation of Amusia (MBEA); details are provided in Materials and Methods] included as independent variables. Percentage of high pleasurable rates was predicted only by the overall BMRQ score [R2 = 0.40, F(1,43) = 29.59, P < 0.001; Fig. 1A]. Similarly, stepwise regression showed that global liking rates and duration and intensity of chills were only predicted by the BMRQ [global liking rate: R2 = 0.38, F(1,43) = 26.15, P < 0.001; Fig. 1B; duration of chills: R2 = 0.13, F(1,43) = 6.31, P = 0.016; intensity of the chills: R2 = 0.29, F(1,43) = 17.11, P < 0.001; Fig. S1 A and B].
Fig. 1.
Behavioral differences to music reward. Scatter plot of the proportion of responses associated with chills and high pleasurable ratings with overall scores of the BMRQ in the music task during the SCR (A) and fMRI (C) sessions. Scatter plot of the global liking rates for all excerpts listened to in the music task with overall scores of the BMRQ during the SCR (B) and fMRI (D) sessions (further details are provided in Materials and Methods). In both scatter plots, black circles represent ANH participants, dark gray squares represent HDN participants, and bright gray triangles represent HHDN participants. The solid black line represents the slope of the linear fit, and the dotted gray line represents the 95% confidence interval.
Fig. S1.
SCR experiment: behavioral correlates of sensitivity to music reward and physiological response to music reward. Scatter plot of the reported chill intensity (A) and chill duration (B) with overall score of the BMRQ in the music task. Red circles represent ANH participants, green squares represent HDN participants, and blue triangles represent HHDN participants. The solid black line represents the slope of the linear fit, and the dotted gray line represents the 95% confidence interval. (C) Mean slope for each group from the regression analysis performed with pleasure rating as an independent variable and the normalized SCR measure. Note the general increase in the mean slope when the groups are ordered as a function of increasing sensitivity to music (from ANH group to HHDN group). Error bars indicate SEM.
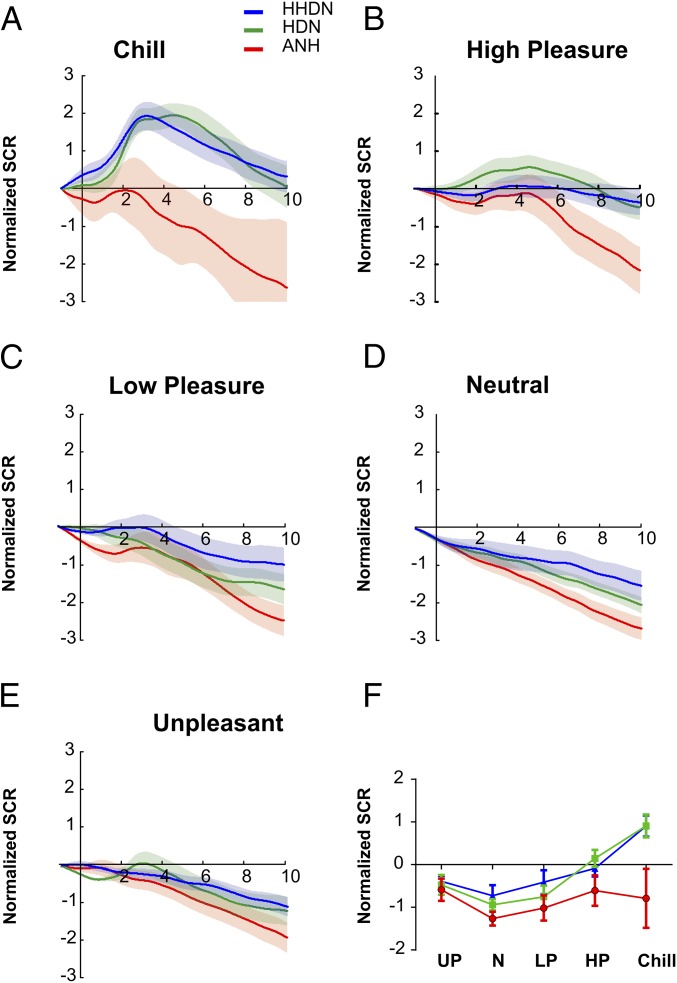
Fig. 2 presents SCR responses associated with the different online ratings experienced by the three groups for the 16 excerpts used as stimuli in the scanning session. Consistent with previous findings, SCR curves show that in all conditions, the ANH group has lower amplitude than the amplitudes of the other two groups. Indeed, those ANH participants reporting chills did not show an increase in the SCR (Fig. 2A). To test the relationship between the degree of pleasure experienced and SCR amplitude on a trial-by-trial basis, we carried out a regression analysis for each individual, using SCR amplitude as a dependent variable and pleasure rating as an independent measure. If SCR amplitude scales with the degree of pleasure rated by the participants, then the slope of this relationship should be positive and significantly different from 0. Such was the case for the HDN [t(14) = 2.28, P = 0.039] and HHDN [t(14) = 5.65, P < 0.001] groups; that is, higher online ratings were associated with larger SCR amplitude in these two groups (Fig. S1C), whereas ANH participants showed only a marginal relationship between the SCR and the behavioral rates of pleasure [t(14) = 1.91, P = 0.077]. Similarly, the stepwise regression analysis between the individual’s slope and all of the psychometric measures evaluated showed that the BMRQ was the only variable that significantly predicted each individual’s slope in the SCR analysis [R2 = 0.11, F(1,43) = 5.44, P = 0.024].
Fig. 2.
SCR to different degrees of musical pleasure. (A–E) Normalized SCR associated with five pleasure ratings (chill, high pleasure, low pleasure, neutral, and unpleasant) for the three groups in the music task. Note the lower amplitude of SCR in the ANH group compared with the other two groups. Solid lines indicate the averaged SCR with the corresponding SEM (shadow). The three groups are plotted separately: ANH, red line; HDN, green line; HHDN, blue line. The time unit is seconds. (F) Average of the normalized SCR in comparison to baseline levels while participants report different levels of pleasure in the music listening task (HP, high pleasure; LP, low pleasure; N, neutral; UP, unpleasant). Both HDN and HHDN groups showed a clear increase in SCR while increasing pleasure rates. The same color code for groups applies.
fMRI Data: Behavioral Correlates of Sensitivity to Music Reward.
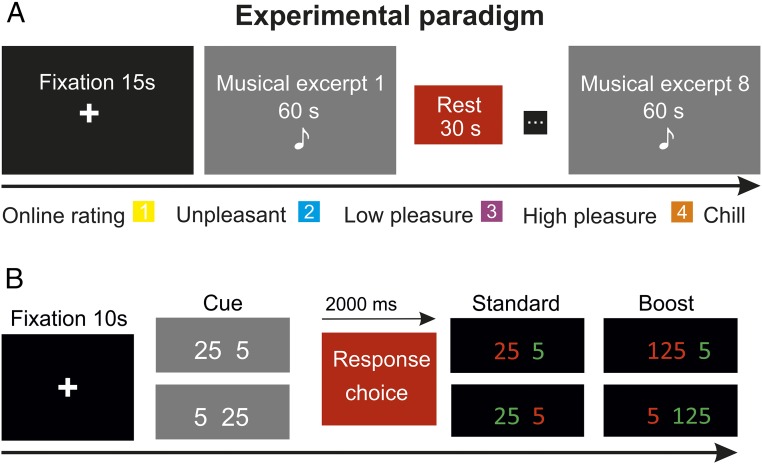
In the fMRI session, participants had to perform two experiments: a monetary gambling task and a music listening task, in which they had to provide online rates of the degree of pleasure they were experiencing (Fig. 3).
Fig. 3.
fMRI experimental paradigm. (A) Music task. Each of the two runs started with a fixation cross lasting 15 s. BOLD activity was collected while participants listened to 60-s excerpts of pleasant and unpleasant music (half matched to their own preferences and half fixed for all participants), with a rest period of 30 s between excerpts. While listening, participants had to rate their degree of pleasure from 1 (unpleasant) to 4 (chill). (B) Gambling task. Each of the two runs started with a fixation cross lasting 10 s. Trials started with the presentation of two numbers ([25 5] or [5 25]) for 2 s. Participants selected one of the two numbers, which then turned red (indicating a loss) or green (indicating a gain).
Behavioral effects paralleled the behavioral effects observed in the SCR session. Again, the frequency of online responses associated with high pleasure (high pleasure ratings and chills) for all excerpts during the scanning session was predicted only by the overall BMRQ score [R2 = 0.36, F(1,43) = 24.63, P < 0.001; Fig. 1C]. Similarly, the global online liking rate (calculated as the response value 1, 2, 3, or 4 multiplied by the duration of this response during each excerpt and averaged across excerpts) was only predicted by the BMRQ [R2 = 0.23, F(1,43) = 13.10, P < 0.001; Fig. 1D]. We found the same results in a stepwise regression considering as a dependent variable the global online liking rate provided for pleasant [R2 = 0.21, F(1,43) = 11.67, P < 0.001] and unpleasant [R2 = 0.12, F(1,43) = 5.74, P = 0.021] excerpts separately.
fMRI Results: Reduced Blood Oxygenated Level-Dependent Response in Specific Musical Anhedonia.
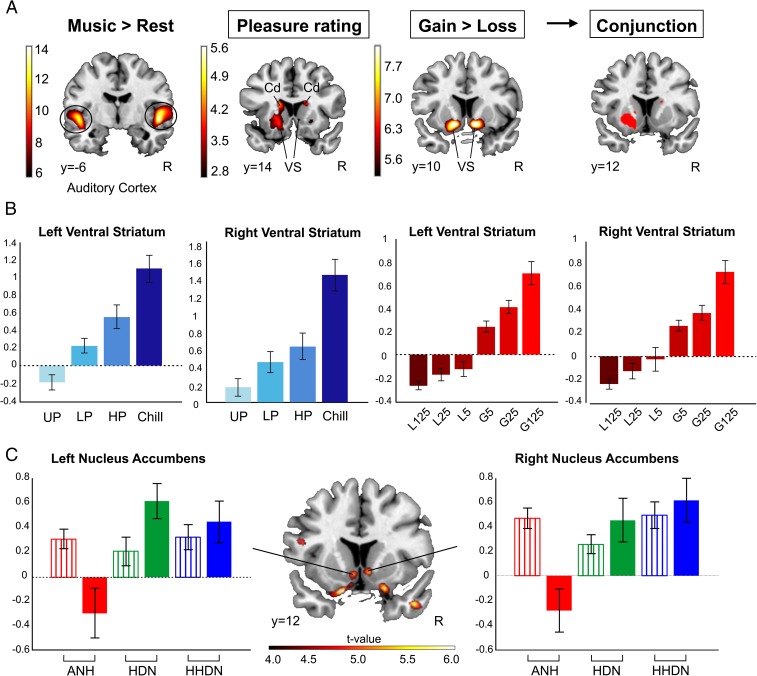
To examine the activation induced by music, we compared whole-brain fMRI activity when participants were listening to music against rest blocks. This contrast yielded significant blood oxygenated level-dependent (BOLD) signal change in the superior temporal gyrus (STG) of both hemispheres (right STG: x = 62, y = −25, z = 12; left STG: x = −47, y = −13, z = 0; P < 0.05, family-wise error (FWE)-corrected; Fig. 4A and Table S1) as expected. However, there were no significant changes in the activation of the STG in the music versus rest condition when performing a one-way ANOVA across the three groups, indicating that sensory/perceptual processing is similar regardless of sensitivity to music reward. In addition, we included the online pleasure ratings as a parametric effect to test the activation of brain areas specifically related to the degree of pleasure experienced by the participants. This contrast yielded increased hemodynamic activity in the left NAcc [left NAcc, x = −13, y = 12, z = −10; P < 0.05, small volume correction (SVC) for the NAcc defined in an unbiased manner using a neuroanatomical atlas (19, 20); Fig. 4A], suggesting that activity in this region was directly related to the pleasure experienced by participants. Fig. 4B shows the linear trend to increase of the beta value of the parametric regressor of pleasure rating split into the four online pleasure ratings for the peaks of the left and right VS when thresholding the parametric analysis at an uncorrected P < 0.005. Brain activity on monetary rewards and punishments was also determined by modulating reward magnitude and valence in the gambling task (further information provided in Materials and Methods). When including these variables as parametric regressors, activation was found bilaterally in the VS [right VS: x = 9, y = 9, z = −10; left VS: x = −13, y = 9, z = −10; P < 0.05, FWE corrected; Fig. 4 A and B and Table S2]. Conjunction analysis across music and gambling tasks confirmed that there was conjoint activation of the two types of rewards in the left NAcc (left NAcc: x = −13, y = 12, z = −10; P < 0.05, SVC; Fig. 4A).
Fig. 4.
Brain activation differences in specific musical anhedonia. (A) In red-yellow (from left to right), enhanced group-level fMRI signal for the music versus rest contrast in all subjects combined (Left; FWE-corrected, P < 0.05), the parametric effect of musical pleasure rating (Left Center; P < 0.005 uncorrected), and the parametric effect of monetary feedback in the gambling task (Right Center; FWE-corrected, P < 0.05). (Right) Map illustrates the conjunction analysis between both tasks (P < 0.005 uncorrected). Cd, caudate. (B) Bar graph represents contrast estimates (proportional to percentage of signal change; 90% confidence intervals are included; blue, parametric effect of pleasure rating; red, parametric effect of monetary feedback) for the peaks of the right and left VS in the whole-brain analysis. Note the linear increase in contrast estimates as the pleasure and monetary reward increase. L125, loss of 125 (Euro cents); L25, loss of 25; L5, loss of 5; G125, gain of 125; G25, gain of 25; G5, gain of 5. (C) Results for the interaction group × contrast (P < 0.005 uncorrected). The bar graph represents contrast estimates with SEM of the NAcc peak in the group × task contrast. Gambling task: striped bars; music task: solid bars; ANH: red; HDN: green; HHDN: blue. Neurological convention is used, with Montreal Neurological Institute (MNI) coordinates at the bottom left of each slice.
Table S1.
Whole-brain effects of music listening on fMRI signal: Music versus rest contrast thresholded at an FWE-corrected P < 0.05 threshold with 100 voxels of cluster extent
| Anatomical area | Cluster size | Coordinates | t value | ||
| Right STG | 1,449 | 62 | −25 | 12 | 19.76 |
| Right Heschl’s gyrus | 52 | −10 | 6 | 14.57 | |
| Left STG | 1,907 | −47 | −13 | 0 | 14.48 |
| Left lobule VI of cerebellar hemisphere | 829 | −28 | −66 | −22 | 11.95 |
| Right inferior frontal gyrus, pars opercularis | 258 | 49 | 18 | 27 | 8.67 |
Montreal Neurological Institute coordinates are used. Cluster size denotes number of voxels. For better location of the different regions, several peak voxels for each cluster are reported.
Table S2.
Whole-brain effects of monetary gains on fMRI signal: Parametric effect of feedback contrast thresholded at an FWE-corrected P < 0.05 threshold with 100 voxels of cluster extent
| Anatomical area | Cluster size | Coordinates | t value | ||
| Right VS | 827 | 9 | 9 | −10 | 9.06 |
| Right frontal superior orbital gyrus | 21 | 31 | −19 | 5.58 | |
| Right pallidum | 21 | 3 | −4 | 5.53 | |
| Left VS | 1,391 | −13 | 9 | −10 | 9.02 |
| Left frontal middle orbital gyrus | −25 | 34 | −19 | 6.29 | |
| Left insula | −35 | 0 | 9 | 6.23 | |
| Left inferior parietal lobe | 1,121 | −50 | −25 | 46 | 5.77 |
| Right midcingulate area | 3 | −10 | 49 | 5.75 | |
| Left midcingulate area | 0 | −4 | 43 | 5.54 | |
| Left superior frontal gyrus (BA 8) | 201 | −16 | 24 | 46 | 5.13 |
| Left middle frontal gyrus | −35 | 18 | 40 | 3.80 | |
| Right middle occipital gyrus | 824 | 34 | −97 | 0 | 5.11 |
| Right calcarine sulcus | 12 | −97 | 6 | 4.63 | |
| Right lobule VIII of cerebellar hemisphere | 729 | 18 | −62 | −50 | 5.03 |
| Left lobule VIII of cerebellar hemisphere | −22 | −50 | −50 | 4.95 | |
| Right precuneus | 443 | 6 | −50 | 65 | 4.96 |
| Left precuneus | −7 | −50 | 65 | 4.88 | |
| Right postcentral gyrus | 128 | 46 | −28 | 49 | 4.94 |
| Right supramarginal gyrus | 43 | −31 | 40 | 4.80 | |
| Left lobule IV, V of cerebellar hemisphere | 110 | −16 | −41 | −28 | 4.09 |
MNI coordinates are used. Cluster size denotes number of voxels. For better location of the different regions, several peak voxels for each cluster are reported.
Crucial to our hypothesis for the existence of dissociation between the activation induced by music and monetary gains in the ANH group was the interaction of group × task. Whole-brain analysis revealed a significant group × task interaction in the bilateral NAcc (right NAcc: x = 9, y = 12, z = −7; left NAcc: x = −7, y = 12, z = −7; P < 0.05, SVC; Fig. 4C), showing that this region presented a differential activation for music and monetary rewards across groups. Further analysis of contrast estimates at this region showed that striatal sensitivity to music-induced pleasure in ANH individuals was reduced in comparison to the other two groups, whereas the activation of monetary rewards was similar in all of the groups (Fig. 4C).
To decompose the effect of the group × task interaction, we conducted a region-of-interest (ROI) analysis in the NAcc using the same neuroanatomical mask used in our voxel-wise analysis with SVC. We computed the mean contrast estimates for the parametric effect of pleasure rating and monetary task within the left and right NAcc and then performed a repeated measures ANOVA with task and laterality as within factors. Only the main effect of group and the interaction of group × task were statistically significant (P = 0.01 and P = 0.03, respectively). Pairwise comparisons revealed that contrast estimates differed only when comparing the ANH group against the HHDN or HDN group [HDN vs. ANH: t(28) = 2.65, P = 0.01; HHDN vs.ANH: t(28) = 2.44, P = 0.02; HDN vs. HHDN: t(28) = −0.03; P = 0.98]. In contrast, no significant differences were found across groups for the contrast estimates of monetary feedback (all P > 0.2).
Further, when comparing the ANH group against the HHDN or HDN group in the whole-brain analysis of the parametric modulation of pleasure rating, we found enhanced activity in the bilateral NAcc (P < 0.05, SVC). However, the comparison between the HHDN group versus the HDN group and all pairwise comparisons of the parametric analysis of monetary reward yielded no suprathreshold voxels within the NAcc (SI Results).
Psychophysiological Interaction Results: Reduced Connectivity in Specific Musical Anhedonia.
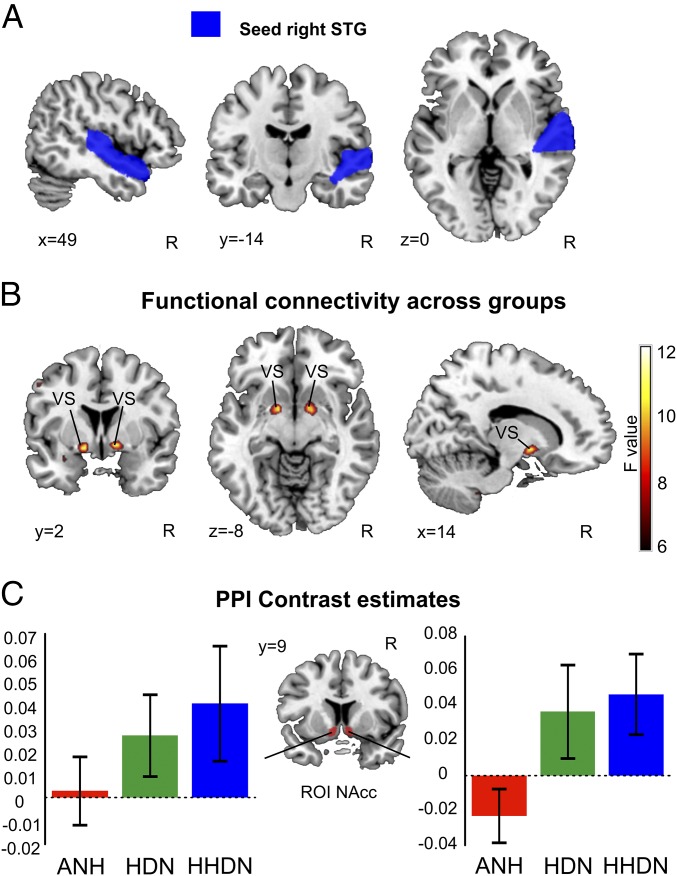
Critical to our hypothesis is to determine to what extent specific musical anhedonia may be explained by a reduced interaction between the cortical systems involved in perceptual and cognitive computations and the subcortical mechanisms for reward processing. To examine this connectivity, we performed a whole-brain “psychophysiological interaction” (PPI) analysis using the left and right STG [anterior and posterior, defined on the basis of a neuroanatomical atlas (19, 20)] as seed regions (Fig. 5A). To examine differences across groups, we specified a linear contrast in a one-way ANOVA. This analysis revealed that differences in connectivity across the three groups were confined to the NAcc during music listening (right NAcc: x = 12, y = 12, z = −7; P < 0.05, SVC; Fig. 5B; see Fig. S2 for whole-brain pairwise group comparisons). No significant connectivity was found between the left STG and other brain regions.
Fig. 5.
Functional connectivity between the right STG and VS. (A) Right STG seed used in the PPI analysis is depicted in blue. (B) Group differences in coupling (red-yellow) of the right STG with the bilateral VS in the context of the music versus rest condition for one-way ANOVA of PPI regressor (P < 0.005 uncorrected) (C) Bar graphs indicate contrast estimates with SEM of functional connectivity within the NAcc ROI: ANH, red; HDN, green; HHDN, blue. Neurological convention is used, with MNI coordinates.
Fig. S2.
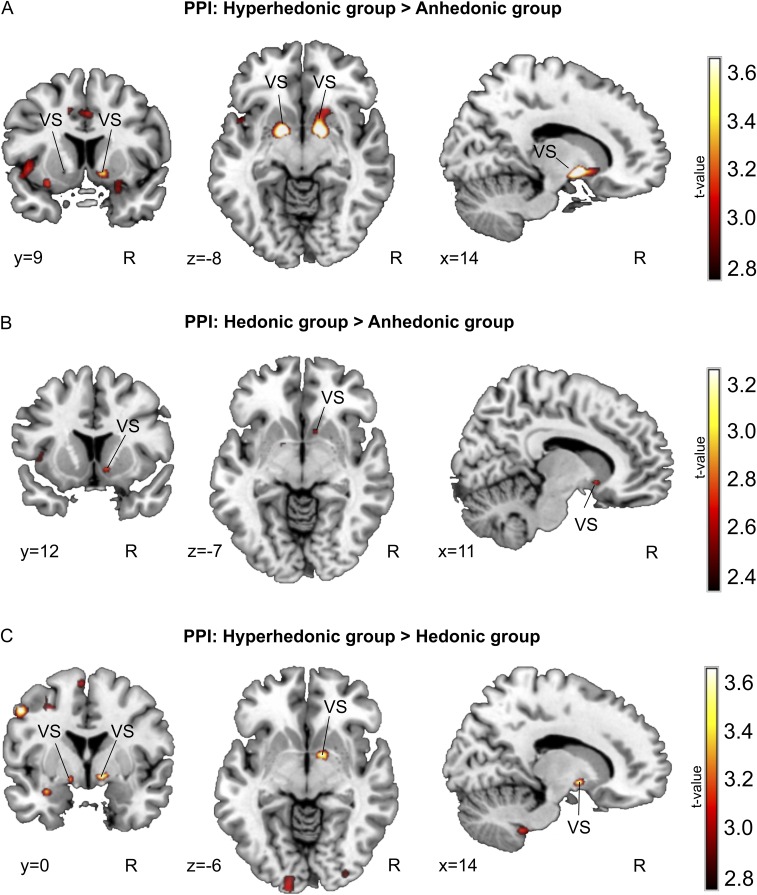
Functional connectivity results. (A) Higher coupling between the right STG (seed region; Fig. 5A) and the VS in the context of music versus rest in the HHN group compared with the ANH group (P < 0.005 uncorrected.). (B) Higher coupling between the right STG and the VS in the context of music versus rest in the HDN group compared with the ANH group. A lower statistical level (P < 0.01 uncorrected) is used for visual display. (C) Higher coupling between the right STG and the VS in the context of music versus rest in the HHDN group compared with the HDN group (P < 0.005 uncorrected). (Neurological convention is used, with Montreal Neurological Institute (MNI) coordinates at the bottom left of each slice.)
To determine what differences were driving this effect, we conducted an ROI analysis focusing on the NAcc using the same neuroanatomical mask as for fMRI activation (Fig. 5C). In a one-way ANOVA, we found a main effect of group (P = 0.05). Pairwise comparisons indicated a lower functional connectivity of the right STG and the NAcc in ANH participants compared with the HHDN group (HHDN vs. ANH, P = 0.02; HDN vs. ANH, P = 0.10; HHDN vs. HDN, P = 0.39).
Further, we found a down-regulated interaction between the right STG and right NAcc when comparing the ANH group with either the HHDN or HDN group using a t test in the whole-brain PPI with SVC for the NAcc, but this down-regulated interaction was not the case for the comparison between the HHDN group versus the HDN group (SI Results). In addition to the difference between the ANH group and the HHDN and HDN participants, we found an enhanced interaction between the right STG and VS in the comparison between the HHDN and HDN groups when using SVC for the VS defined using NeuroSynth, a platform for large-scale automated metaanalysis of fMRI data (21) and reward as the search term (SI Results). Future research should confirm this three-way distinction in functional connectivity across groups.
Taken together, these findings are consistent with the idea that specific musical anhedonia is associated with down-regulation of functional connectivity between the auditory cortex and NAcc.
SI Results
Decomposition of the Interaction Group × Task of the fMRI Data: Pairwise Comparisons.
We found a significant group × task interaction in the bilateral NAcc [right NAcc: x = 9, y = 12, z = −7; left NAcc: x = −7, y = 12, z = −7; P < 0.05, SVC for the NAcc defined in an unbiased manner using a neuroanatomical atlas (19, 20)]. The decomposition of this interaction focused on the NAcc revealed that the effects were driven by the ANH group in the music task: The NAcc was only engaged when comparing the parametric regressor of pleasure rating between the HHDN and ANH groups (right NAcc: x = 9, y = 9, z = −4; left NAcc: x = −4, y = 9, z = −7; P < 0.05, SVC) and between the HDN and ANH groups (right NAcc: x = 6, y = 12, z = −7; left NAcc: x = −7, y = 12, z = −7; P < 0.05, SVC). No suprathreshold voxels were found between the HHDN and HDN groups in the music task and all pairwise comparisons of the parametric analysis of monetary reward.
Changes in Whole-Brain PPI Using SVC for the NAcc Based on a Neuroanatomical Mask (Materials and Methods): Pairwise Comparisons.
In addition to the group effect, the comparison between the HHDN and ANH groups indicated enhanced coupling between the right STG and the right NAcc in the HHDN group compared with the ANH group (right NAcc: x = 9, y = 6, z = −7; P < 0.05, SVC). In addition, we found higher coupling in the right NAcc in the HDN versus ANH contrast (right NAcc: x = 12, y = 12, z = −7; P < 0.05, SVC). When comparing the HHDN and HDN groups, no suprathreshold voxels were found within the NAcc.
Changes in Whole-Brain PPI Using SVC for the VS Defined Using Neurosynth: Pairwise Comparisons.
We found enhanced coupling between the right STG and the VS bilaterally in the HHDN group compared with the ANH group (right VS: x = 12, y = 3, z = −7; left VS: x = −13, y = 3, z = −10; P < 0.05, SVC). In addition, we found higher coupling in the right VS in the HDN versus ANH contrast [right VS: x = 12, y = 12, z = −7; P < 0.005 uncorrected (P = 0.1, SVC)]. When comparing the HHDN group versus the HDN group, we also found differences in the VS bilaterally (right VS: x = 13, y = 3, z = −7; left VS: x = −13, y = 3, z = −10; P < 0.05, SVC).
Discussion
In the present study, we provided compelling evidence that activity in the VS (including the NAcc) and functional connectivity between this region and the right STG are crucial in giving value and experiencing pleasure from music. This finding was revealed by studying people with specific insensitivity to musical reward (specific musical anhedonia, ANH) along with people with standard (HDN) and high (HHDN) sensitivity to music. Our main results suggest two brain mechanisms associated with specific musical anhedonia, a phenomenon that has broader theoretical implications for music reward processing. First, the NAcc activity of the ANH group was significantly reduced during music listening but not when participants were winning or losing money in a gambling task. Second, the functional connectivity between the right STG and VS (including the NAcc) is down-regulated in ANH participants and up-regulated in HHDN participants. In addition, activity in temporal lobe auditory cortices was not changed in the ANH group, consistent with their intact auditory capacities. Taken together, these findings are in accordance with previous work (4), where ANH participants reported fewer chills and of lower SCR magnitude than people with average (HDN) or higher (HHDN) sensitivity to music, thus indicating a low emotional arousal in response to music that cannot be explained by perceptual problems or generalized anhedonia.
It is well-established that the VS, and especially the NAcc, plays a prominent role in music reward processing (10–12, 16, 17), as it does in processing of other, more biologically basic rewards (22, 23). Indeed, in a prior study, it was shown that the degree of activity in the right NAcc was the best predictor of the bid amount participants were willing to spend to purchase previously unheard music (17). Moreover, the NAcc is consistently recruited as a function of increasing music-induced pleasure (24) and reaches its maximum activity during peak pleasure (chills) (12). In the current study, we observed a reduced activation in the NAcc in participants who subjectively reported music listening as not being a pleasurable experience. This finding is consistent with the aforementioned studies and with previous work showing that the trait of anhedonia is associated with a reduction in the activation of the NAcc (25).
Most importantly for purposes of testing our model, we found reduced functional connectivity between the right STG and VS (including the NAcc) in ANH participants compared with the other groups. This reduction fits in well with previous neuroimaging studies showing that music reward value increases with enhanced functional connectivity between the NAcc and a high-order temporofrontal cortical network involved in perceptual analysis, emotional processing, and valuation (17). However, it is important to note that current results show that the interaction between the VS and right STG is directly related to the ability to experience pleasure from music and, in consequence, predicts individual differences in general music reward processing.
In line with this idea, the unique ability of humans to appreciate aesthetic rewards such as music relies on higher order perceptual/cognitive analysis and encompasses learning, experience, and cultural factors that would be expected to involve cortical systems (26–28). Our results also support the notion that to derive pleasure from music, the cortical and subcortical systems must act in concert; in particular, we found the interplay between the right STG and VS to be crucial. Interestingly, no significant differences were found between the left STG and other brain areas, supporting the notion of an asymmetry favoring the right auditory cortex in music processing. Indeed, the right auditory cortex and related networks have been consistently implicated in various processes relevant for music perception, including pitch representation (29–31), tonal pattern processing (32, 33), tonal working memory (34, 35), tonal learning (36, 37), and musical imagery (38). These cognitive processes would be important not just in decoding musical patterns but also in generating the expectancies that we believe are critical to generating musical pleasure (39). Thus, the finding that the NAcc preferentially interacts with the right rather than left auditory cortex would make sense, given the more specialized tonal processing mechanisms of the right auditory cortical network. Moreover, the role of the auditory cortex as a central hub of an affective-emotional network has also been highlighted by a previous study comparing music-evoked fear and joy (40). When listening to joyful music, the auditory cortex showed enhanced functional connectivity with the cingulate and insular cortices, which are regions implicated in autonomic regulation and production of subjective feelings. Conversely, effective connectivity modulations between the auditory cortex and the amygdala have been involved in the processing of aversive sounds (41). Taken together, these findings implicate cortical–subcortical interactions in relating auditory features to affective value, a conclusion that fits well with our findings that reductions in these interactions are associated with lack of affective response to music.
An interesting analogy for the requirement of an intact coupling between the STG and the reward network for typical affective processing of auditory stimuli comes from studies of children with autism spectrum disorder, whose inability to experience the human voice as pleasurable may be explained by a reduced coupling between the bilateral posterior superior temporal sulcus and distributed nodes of the reward system, including the NAcc (42). These cortical–subcortical impoverished interactions could be crucial for the correct attainment of language learning milestones, probably because of the reduced capacity in these children to experience auditory processing and language learning experiences as rewarding (43). On the other hand, enhancements to this system may be observed in musicians. Musical training is known to be associated with functional and anatomical enhancements of the superior temporal cortex (30, 44, 45), but it also has been shown to modulate striatocortical connectivity during music listening (46). Also relevant is that individual differences in structural connectivity in the tracts connecting the posterior STG with the anterior insula and medial prefrontal cortex have recently been associated with individual differences in music reward sensitivity (47). In future studies, it would be interesting to examine whether similar structural differences can be found in our sample in which we included individuals lacking reward responses to music specifically but with preserved capacities to experience pleasure from other reinforcers. The above-mentioned studies all point to individual differences in the links between cortical and subcortical systems as relevant for understanding differences in reward-related processing, a conclusion that is consonant with our claim that individuals with specific musical anhedonia lack the relevant functional relationship between auditory processing regions and reward-related structures.
Finally, our results support the idea that people might present distinct sensitivity to reward for different stimuli and, concretely, the existence of specific anhedonias and hyperhedonias. Indeed, a recent metaanalysis showed that different types of rewarding stimuli (such as food, sex, and money) specifically recruited brain areas associated with the sensory modality and/or associative areas involved in processing these stimuli (48). Moreover, previous studies of lesions have reported single cases of patients with specific loss of pleasure for music after lesions, not only in reward-related areas but also in temporal and parietal cortices (49–51). All these findings give support to the hypothesis that to assign reward value, the recruitment of cortical structures related to the perceptual, integrative, and cognitive aspects of these complex reinforcing stimuli is essential. Therefore, our concept is that there would be different ways to access the core reward circuit, which would depend on the modality and nature of the reinforcer (52). Following this rationale, a reduced connectivity between these regions and the reward network, as is the case of reduced connectivity between the NAcc and STG, would result in selective anhedonia for these reinforcers, and, conversely, an increased connectivity would yield increased hedonic experience as is the case for the HHDN group.
In conclusion, we showed that a reduced interaction between the auditory cortex and the mesolimbic reward network may point to the top-down mechanism that is impaired in people unable to derive pleasure from music but who show otherwise normal perceptual and reward processing. This finding may pave the way for the detailed study of the neural substrates underlying other domain-specific anhedonias and, from an evolutionary perspective, help us to understand how music acquired reward value.
Materials and Methods
Participants.
Forty-five university students participated in the experiment and were divided into three groups of 15 subjects (eight females and seven males). Participants were selected using the BMRQ, a psychometric instrument known to be a reliable indicator of interindividual variability in music-induced reward (18). In the first round of selection, the BMRQ was delivered to a population of 2,600 university students from Barcelona (33.8% males, M = 18.3, SD = 6.9) in their classrooms or by email in reply to an advertisement. In the second round of selection, 111 right-handed individuals with no formal musical training were selected and asked to complete a second BMRQ in the laboratory to ensure consistency across measures. Participants were also assessed in their (i) global sensitivity to reward and punishment using the SPSRQ (53); (ii) hedonia trait using the PAS (excluding those items referring to musical rewarding experiences to assess the hedonic impact of other activities or stimulus outside the music domain) (54); and (iii) amusia score using the MBEA (5). Upon completion of this round, we selected 45 participants who scored within the normal range on these scales and presented normal SCR levels. We classified participants in three groups of 15 people according to their overall BMRQ scores: those individuals scoring equal to or lower than 65 were included in the ANH group, those individuals scoring higher than 65 but lower than 87 were included in the HDN group, and those individuals scoring higher than 87 were included in the HHDN group [adapted from Mas-Herrero et al. (4)]. The three groups were matched in these three measures (PAS, MBEA, and SPSRQ) but differentiated in the BMRQ [overall scores averaged between the BMRQ overall score available from the first round of participant selection and the score from the BMRQ completed in the laboratory during the SCR session (Table 1)]. There was a high reliability in the two BMRQ tests [correlation r(43) = 0.94, P < 0.001]. All participants were healthy and free from any neurological or psychological disorders and gave written informed consent before participating in the study. All procedures were approved by the Ethics Committee of the Hospital Universitari de Bellvitge, Barcelona (PR181/13).
Stimuli Selection.
Participants were instructed to provide two pieces of instrumental music that elicited intensely pleasant emotional responses for them. Musical anhedonic individuals, by definition, experience low emotional responses to music, and thus had difficulties in providing such intensely pleasurable music. To guarantee that all participants were exposed to music that had a “global” emotional impact, we created a musical list of 82 excerpts that was assessed by an independent group of students with similar demographics (n = 65). The first inclusion criterion was that the selected music could not include any lyrics to avoid language-related activations in the fMRI experiment. The second inclusion criterion was that music should have similar familiarity levels between groups. To this aim, and because most students are exposed to classical music since their early years of education in the Spanish syllabus, we restricted the selection to the classical genre. We also included some excerpts that could be considered less pleasant or even unpleasant by the participants to have a continuous representation of all pleasure ratings and minimize the probability that those excerpts associated with higher degrees of pleasure would be overrepresented. Forty potentially pleasant and two potentially unpleasant excerpts (∼50% of the musical stimuli) were selected from a sample of 200 chill-inducing music selections adapted from a study by Salimpoor et al. (55).The rest of the stimuli were selected by using the music recommendation program, Spotify radio (https://www.spotify.com). This program uses “collaborative filtering” to match new recommendations based on popular choices of other individuals who have similar music preferences. Specifically, we created a playlist with the 40 pleasant excerpts and started a radio from this playlist. Spotify recommendations of similar music were saved into a new playlist. The same procedure was followed to select new unpleasant music. Because there were only two unpleasant musical pieces in the initial playlist, we also added some music composed before the 16th century or atonal compositions, including compositions from the Second Viennese School. These compositions do not follow the rules of traditional Western music, and even though these pieces might be considered highly pleasant for some professional musicians, we would expect them to elicit a lower rewarding experience in our sample of nonmusician participants. The new music stimuli included 30 potentially unpleasant and 10 potentially pleasant songs. These 82 excerpts (Table S3) were tested in a preexperiment session with a sample of 65 university students who provided liking rates for each excerpt (from 1 = unpleasant to 7 = extremely pleasurable). Musical excerpts were then sorted according to the mean liking rate. The 10 excerpts that showed the highest mean liking rate were selected as pleasant, and the 10 excerpts that showed the lowest mean liking rate were selected as unpleasant. Furthermore, the 10 excerpts with a mean liking rate just below the overall mean liking rate (4.5) were included in a neutral category. Hence, the final fixed selection for the music stimuli in the SCR session included 30 musical excerpts (10 pleasant, 10 neutral, and 10 unpleasant; Table S4). Musical excerpts were cut down to 1-min clips using Audacity software (version 2.0.2) and normalized to a maximum peak amplitude of −1.0 dB. The 1-min selection was made so as to ensure that at least one entire musical phrase was presented in the excerpt. All excerpts were saved in mp3 format at a 296-bit rate. For the two self-selected excerpts, participants were asked to select the minute themselves to ensure that the most subjectively pleasurable minutes were used.
Table S3.
First selection of 82 potentially pleasant and unpleasant excerpts
| Artist | Title | Type | Source | Mean LR |
| Vivaldi | The Four Seasons “Spring,” Mov.1 | PPM | 1 | 6.30 |
| Beethoven | Für Elise | PPM | 2 | 6.26 |
| Pachelbel | Canon in D | PPM | 1 | 6.11 |
| Vivaldi | The Four Seasons “Winter,” Mov.1 | PPM | 1 | 6.06 |
| Tchaikovsky | Dance of the Sugar Plum Fairy | PPM | 1 | 5.90 |
| Tchaikovsky | Swan Lake, Op. 20, Scene Finale | PPM | 1 | 5.86 |
| Beethoven | Symphony No. 9, Op. 125, Mov. 2 | PPM | 1 | 5.77 |
| Dvorak | New World Symphony No. 9, Mov. 4 | PPM | 1 | 5.77 |
| Beethoven | Moonlight Sonata | PPM | 2 | 5.43 |
| Holst | The Planets—Jupiter, the Bringer of Jollity | PPM | 1 | 5.40 |
| Dvorak | New World Symphony No. 9, Mov. 2 | PPM | 1 | 5.31 |
| Vivaldi | Concerto Ripieno in C Major, Mov. 3 | PUM | 2 | 5.26 |
| Debussy | Clair de Lune | PPM | 1 | 5.20 |
| Vivaldi | The Four Seasons “Summer,” Mov. 3 | PPM | 1 | 5.17 |
| Beethoven | Violin Sonata No. 5 “Spring,” Mov. 1 | PPM | 1 | 5.14 |
| Rachmaninoff | Piano Concerto in C Minor No. 2, Mov. 2 | PPM | 1 | 5.09 |
| Rimski-Korsakov | Scheherazade “The Kalender Prince” | PPM | 1 | 5.07 |
| Dvorak | Symphony No. 8, Mov. 4 | PPM | 1 | 5.00 |
| Beethoven | Piano Sonata No. 8 in C Minor, Mov. 3 | PPM | 1 | 5.00 |
| Haendel | Organ Concerto, Op. 7, No. 5 in G Minor | PUM | 2 | 4.97 |
| Elgar | Cello Concerto in E Minor, Op. 85, Mov. 1 | PPM | 1 | 4.97 |
| Chopin | Prelude, Op. 28, No. 4 in E Minor | PPM | 1 | 4.94 |
| Corelli | Concerto Grosso in G Minor, Op. 6, No. 8 “Christmas” | PUM | 2 | 4.94 |
| Bach | Cantata, BWV 208 | PUM | 2 | 4.90 |
| Ravel | String Quartet in F Major, Mov. 2 | PPM | 1 | 4.87 |
| Beethoven | Symphony No. 5, Op. 67, Mov. 2 | PPM | 1 | 4.87 |
| Mozart | Symphony No. 29 in A Major, Mov. 2 | PUM | 2 | 4.83 |
| Holst | First Suite in E Flat Major, Op. 28, No. 1 | PPM | 1 | 4.83 |
| Beethoven | Symphony No. 7 in A Major, Op. 92, Mov. 2 | PUM | 2 | 4.80 |
| Haendel | II Concerto Grosso, Op. 6, No. 4 in A Minor | PUM | 2 | 4.80 |
| Kreisler | Praeludium and Allegro | PPM | 1 | 4.77 |
| Bach | Sonata No. 4 in C Minor, BWV 1017, Mov. 1 | PPM | 2 | 4.77 |
| Haendel | Organ Concerto, Op. 7, No. 4 in D Minor | PUM | 2 | 4.77 |
| Haydn | Symphony No. 38 in C Major, Mov. 3 | PUM | 2 | 4.77 |
| Beethoven | Symphony No. 2 in D Major, Op. 36, Mov. 1 | PPM | 2 | 4.74 |
| Tchaikovsky | Violin Concerto in D Major, Op. 35, Mov. 1 | PPM | 1 | 4.74 |
| Tchaikovsky | Symphony No. 4, Mov. 1 | PPM | 1 | 4.71 |
| Haendel | Organ Concerto, Op. 4, No. 2 in B Flat Major | PUM | 2 | 4.70 |
| Stravinsky | Firebird Suite, Finale | PPM | 1 | 4.70 |
| Haydn | Symphony No. 38 in C Major, Mov. 3 | PUM | 2 | 4.67 |
| Chopin | Mazurka in A Minor, Op. 17, No. 4 | PPM | 1 | 4.67 |
| Fauré | Violin Sonata in A Major, Mov. 1 | PPM | 1 | 4.66 |
| Beethoven | Symphony No. 4 in B Flat Major, Op. 60, Mov. 2 | PUM | 2 | 4.63 |
| Holst | The Planets—Venus, the Bringer of Peace | PPM | 1 | 4.57 |
| Mahler | Symphony No. 2 “Résurrection,” Mov. 1 | PPM | 1 | 4.57 |
| Mozart | Requiem Lacrimosa | PPM | 1 | 4.57 |
| Mozart | Symphony No. 25 in G Minor, Mov. 2 | PUM | 2 | 4.57 |
| Gibbons | Fantasies a6 | PUM | 2 | 4.53 |
| Mahler | Symphony No. 1 “Titan,” Mov. 4 | PPM | 1 | 4.33 |
| Bach | Chorals for Organ, BWV 714–740 | PPM | 2 | 4.33 |
| Brahms | String Quartet No. 1, Mov. 2 | PPM | 1 | 4.30 |
| Chopin | Nocturne in G Minor, Op. 37, No. 1 | PPM | 1 | 4.30 |
| Saint-Saëns | Symphony No. 3, Mov. 1 | PPM | 1 | 4.29 |
| Schubert | Minuet and Finale for Wind Octet in F Major D.72, I Menuetto Two Trios | PUM | 2 | 4.26 |
| Walton | Violin Concerto, Mov. 1 | PPM | 1 | 4.23 |
| Bach | Highlights for Trumpet and Organ, BWV 972, Mov. 2 | PPM | 2 | 4.23 |
| Rameau | Suite La triomphante, Mov. 2 | PPM | 2 | 4.20 |
| Rameau | Pieces De Clavecin Suite in D Minor-Major, Mov. 10 | PPM | 2 | 4.07 |
| Pärt | Tabula rasa IV (clip 1) | PUM | 2 | 4.07 |
| Pärt | Tabula rasa IV (clip 3) | PUM | 2 | 4.06 |
| Barber | Adagio for Strings | PPM | 1 | 4.00 |
| Tchaikovsky | Symphony No. 5, Mov. 1 | PPM | 1 | 3.97 |
| Pärt | Tabula rasa I (clip 1) | PUM | 2 | 3.97 |
| Haendel | Organ Concerto No. 13 “Cuckoo and Nightingale”: 1. Larghetto | PUM | 2 | 3.94 |
| Vivaldi | Concerto in C Major for Sopranino Recorder and Strings | PPM | 2 | 3.91 |
| Von Bingen | Salve Regina (Harp) | PUM | 2 | 3.89 |
| Ravel | Oiseaux Tristes | PPM | 1 | 3.89 |
| Schönberg | String Quartet No. 1 in D Minor, Op. 7, Mov. 3 | PUM | 2 | 3.87 |
| Bach | Choral Der Gott | PPM | 2 | 3.86 |
| Desprez | Ile fantazies de Joskin | PUM | 2 | 3.83 |
| Rachmaninoff | Morceaux de Fantaisie, Op. 3, No. 2, Prelude in C Sharp Minor | PPM | 1 | 3.83 |
| Shostakovich | Symphony No. 4, Mov. 3 | PPM | 1 | 3.69 |
| Desprez | The Battle | PUM | 2 | 3.66 |
| Liszt | Danse Macabre | PUM | 1 | 3.53 |
| Pärt | Tabula rasa I (clip 3) | PUM | 2 | 3.53 |
| Gibbons | Fantazia of Four Parts | PUM | 2 | 3.37 |
| Schönberg | String Quartet No.1 in D Minor, Op. 7, Mov. 3 | PUM | 2 | 3.23 |
| Schönberg | String Quartet, Op. 30, No. 3, Mov. 2 | PUM | 2 | 2.89 |
| Berg | String Quartet Op. 31, Mov.1 | PUM | 2 | 2.71 |
| Webern | Symphony, Op. 21, Mov. 1 | PUM | 2 | 2.53 |
| Penderecki | Threnody for the Victims of Hiroshima | PUM | 1 | 2.27 |
| Webern | Variations for Piano, Op. 0.7, Mov. 3 | PUM | 2 | 2.11 |
BWV, Bach-Werke-Verzeichnis; LR, liking rate; Mov., movement; No., number; Op., opus; PPM, potentially pleasant music; PUM, potentially unpleasant music. Source: 1, adopted from Salimpoor et al. (55); 2, Spotify radio recommendations (further details are provided in main text).
Table S4.
Music selection for the experimental paradigm in the SCR session and fixed music selection for the fMRI experiment
| Artist | Title | Type |
| SCR experiment | ||
| Vivaldi | The Four Seasons “Spring” Mov. 1 | PM |
| Beethoven | Für Elise | PM |
| Pachelbel | Canon In D | PM |
| Vivaldi | The Four Seasons “Winter” Mov. 1 | PM |
| Tchaikovsky | Dance of the Sugar Plum Fairy | PM |
| Tchaikovsky | Swan Lake, Op. 20, Scene Finale | PM |
| Beethoven | Symphony No. 9, Op. 125, Mov. 2 | PM |
| Dvorak | New World Symphony No. 9, Mov. 4 | PM |
| Beethoven | Moonlight Sonata | PM |
| Holst | The Planets—Jupiter, the Bringer of Jollity | PM |
| Mahler | Symphony No. 1 “Titan,” Mov. 4 | NM |
| Bach | Chorals for Organ, BWV 714–740 | NM |
| Brahms | String Quartet No. 1, Mov. 2 | NM |
| Chopin | Nocturne in G Minor, Op. 37, No. 1 | NM |
| Saint-Saëns | Symphony No. 3, Mov. 1 | NM |
| Schubert | Minuet and Finale for Wind Octet in F Major D.72, I Menuetto Two Trios | NM |
| Walton | Violin Concerto Mov. 1 | NM |
| Bach | Highlights for Trumpet and Organ, BWV 972, Mov. 2 | NM |
| Rameau | Suite La triomphante, Mov. 2 | NM |
| Rameau | Pieces De Clavecin Suite in D Minor-Major, Mov. 10 | NM |
| Desprez | The Battle | UM |
| Liszt | Danse Macabre | UM |
| Pärt | Tabula rasa I (clip 3) | UM |
| Gibbons | Fantazia of Four Parts | UM |
| Schönberg | String Quartet No. 1 in D Minor, Op. 7, Mov. 3 | UM |
| Schönberg | String Quartet, Op. 30, No. 3, Mov. 2 | UM |
| Berg | String Quartet, Op. 31, Mov. 1 | UM |
| Webern | Symphony, Op. 21, Mov. 1 | UM |
| Penderecki | Threnody for the Victims of Hiroshima | UM |
| Webern | Variations for Piano, Op. 0.7, Mov. 3 | UM |
| fMRI experiment | ||
| Vivaldi | The Four Seasons “Spring,” Mov. 1 | PM |
| Beethoven | Für Elise | PM |
| Pachelbel | Canon in D | PM |
| Vivaldi | The Four Seasons “Winter,” Mov. 1 | PM |
| Berg | String Quartet, Op. 31, Mov. 1 | UM |
| Webern | Symphony, Op. 21, Mov. 1 | UM |
| Penderecki | Threnody for the Victims of Hiroshima | UM |
| Webern | Variations for Piano, Op. 0.7, Mov. 3 | UM |
NM, neutral music; PM, pleasant music; UM, unpleasant music.
Music Task Design (SCR Experiment).
Participants listened to all musical pieces in randomized order. Two blocks (15 excerpts lasting 1 min each, five of each type, and one self-selected) with a break in between were presented using Presentation software. While listening to music, the participants had to rate, in real time, the degree of pleasure they were experiencing by pressing one of five different buttons on a keyboard [1 = unpleasant, 2 = neutral, 3 = low pleasure, 4 = high pleasure, 5 = chill; adapted from Mas-Herrero et al. (4) and Salimpoor et al. (55)], and they had to hold down the button as long as they were experiencing the respective degree of pleasure. At the end of each excerpt, the participants were asked to rate the overall degree of pleasure (from 1 = disliking to 7 = extremely pleasurable) and their familiarity with the excerpt (from 1 = unfamiliar to 5 = highly familiar), as well as the emotional valence (from 1 = sad to 9 = happy) and arousal (from 1 = not at all arousing to 5 = highly arousing) they felt in response to the musical excerpt. For these last two subjective rates, a self-assessment manikin was displayed for visual support (56). Finally, participants were asked to report the number and the intensity of chills they experienced (from 1 to 3). In addition to the experimenter’s instructions, written instructions were provided on the screen at the beginning of the task.
SCR Procedure.
SCR was recorded during the task with two Ag-AgCl electrodes using a Brainvision Brainamp device. The electrodes were attached to the forefinger and the middle finger of the left hand and placed on the first phalange. The level of SCR was determined by measuring the mean SCR amplitude after stimulus or response onset with respect to baseline (−1,000 ms). SCR amplitude was determined in the 0- to 10-s window after participants pressed a button to indicate a change in pleasure levels. Previous studies have shown that SCR during this time window is modulated according to the degree of pleasure experienced (57, 58).
SCR Statistical Analysis.
As highlighted in a previous study by our group (4), most of the ANH participants did not report chills and some of the HDN and HHDN participants did not report neutral rates. In accordance with the analysis reported in that study, the relationship between pleasure ratings and SCR amplitude was assessed by a linear regression model for each subject using SCR amplitude as the dependent measure and rating as the independent variable. The SCR amplitude for each trial was determined separately for each subject. Using these values, a linear model could then be fitted for each subject:
We then determined whether the mean value of the slope (β) was different from 0 for each group using a one-sample t test.
Lastly, stepwise linear regression analysis was used to assess the relationship of each dependent behavioral variable and the individual’s slopes with the psychometric independent variables (BMRQ, SPSRQ, PAS, and MBEA). The entry criterion was P < 0.05, and the exit criterion was P > 0.10. Tests for multicollinearity indicated that a very low level of multicollinearity was present in the analysis (variance inflation factors < 1.09 and tolerances > 0.9).
Music Task Design (fMRI Experiment).
A total of 16 excerpts, lasting 1 min each, were presented in two runs encompassing eight excerpts each. Half of the excerpts were fixed for all participants (Table S4), whereas the other half were selected for each individual based on the liking rates provided in the SCR experiment. The fixed excerpts consisted of the four excerpts (from the 30 stimuli used in the SCR experiment) that obtained the highest mean liking rate in the preexperiment screening (pleasant music) and the four excerpts with the lowest rating (unpleasant music). In addition, for each individual, those four excerpts with the highest liking rate, number, intensity, and duration of chills in the SCR experiment were included as pleasant music and those four with the lowest liking rate, number, intensity, and duration of chills were selected as unpleasant music (a detailed list of the excerpts selected for each group is provided in Table S5). One day before the fMRI study, participants were presented with the musical stimuli from the SCR experiment to ensure that all participants were similarly familiar with the stimulus material. In the scanning session, individuals listened to 1-min musical excerpts while rating the degree of pleasure they were experiencing to the music in real time (1 = unpleasant, 2 = low pleasure, 3 = high pleasure) using three separate buttons on a magnetic resonance-compatible, four-button input device. They were required to hold down the appropriate button as long as they were experiencing the respective degree of pleasure and to press a fourth button when they were experiencing a chill. Individuals were always holding down one button to ensure that the neural activity involved in button pressing, and anticipation of button presses was equally distributed. Four excerpts of pleasant music, lasting 1 min each, and four excerpts of unpleasant music, lasting 1 min each, were played in pseudorandomized order such that no more than two excerpts of the same type were presented consecutively. Between excerpts, a 30-s rest period was included to reduce carry-over effects. To allow for equilibration, each run started with a fixation cross lasting for 15 s. All participants were given instructions before entering the scanner to familiarize them with the music task.
Table S5.
Distribution of excerpts for the music task in the fMRI experiment based on individual selection
 |
AND, red; HDN, green; HHDN, blue; pleasant (P), pale; unpleasant (U), dark.
Gambling Task Design (fMRI Session).
Stimuli were presented using Presentation software. Each trial of this task started with the presentation of two numbers ([25 5] or [5 25]) for 2 s (59). Participants were instructed to bet on one of the two numbers by pressing the spatially corresponding button with their right hand. The left-hand button-pad was not used in this task. After the response, one of the numbers turned red and the other turned green. If the number selected by the participant turned green, the participant gained the corresponding amount of money in Euro cents. The number turning red indicated a loss. To take into account unexpected gains or losses, two more conditions were created (boost gain and boost loss). In these trials, instead of winning or losing 5 or 25 cents, participants gained or lost 125 cents. Thirty gain trials, 30 loss trials, 15 boost gain, and 15 boost loss trials were presented in each of the two runs of the task. Additionally, 25 trials of a 3-s-long fixation cross were presented per run. The intertrial time varied between 0 and 2 s. After each run, the amount of money gained or lost was presented in the middle of the screen to the participant. As in the music task, each run started with a fixation cross lasting for 10 s to allow for equilibration. All participants completed a training block before entering the scanner to familiarize them with the gambling task. Unknown to the participants, the characteristics of the trial and its result (gain or loss) were decided by the computer program before the start of the experiment. Participants started the gambling task with 10 Euros and were instructed to earn as much money as possible. The amount of money won by a participant was paid to him/her at the end of the scanning session. The gambling task was counterbalanced for order with the music task across participants.
fMRI Acquisition.
MRI data were collected on a 3-T scanner (GE Discovery MR750w) using an eight-channel, phased-array coil (GE Healthcare). The session started with the acquisition of high-resolution, whole-brain structural images (BRAVO; repetition time (TR) = 11.668 ms, echo time (TE) = 4.79 ms, inversion time = 450 ms, flip angle = 12°, slice thickness = 1 mm, matrix size = 512 × 512) to allow precise coregistration with functional data. After this acquisition, whole-brain volumes of echo-planar imaging (EPI) images sensitive to BOLD contrast (gradient echo EPI: TR = 2,500 ms, TE = 30 ms, flip angle = 90°, slice thickness = 3.1 mm, 43 axial slices angled +30° to the plane intersecting the anterior commissure–posterior commissure (ACPC) line, matrix size = 64 × 64, fat saturation band placed in the frontal sinus) were acquired for the two runs of the 288 sequential images of the music task. The same protocol was applied to acquire the two runs of the 216 sequential images of the gambling task.
Preprocessing and Statistical Analysis.
Data were preprocessed using Statistical Parametric Mapping software (SPM8; Wellcome Trust Centre for Neuroimaging, University College London; www.fil.ion.ucl.ac.uk/spm/). Functional runs were first slice timing-corrected (interleaved order bottom-up, sinc interpolation, reference slice 43) and realigned, and a mean image of all EPIs was created. The T1 image was coregistered to the mean EPI image and segmented into gray matter (GM) by means of the default segmentation options in SPM8 [unified segmentation algorithm (60)]. After an initial 12-parameter affine transformation of the GM tissue probability map to the GM Montreal Neurological Institute template included in SPM8 (fourth degree B-spline interpolation), the resulting normalization parameters were applied to the whole functional set (voxel size = 3.1 mm). Finally, functional EPI volumes were smoothed with an 8-mm FWHM kernel. For the music and gambling tasks, the statistical analysis was performed according to the general linear model as implemented in SPM8. “Pleasant” and “unpleasant” conditions were modeled time-locked to 5 s after the onset of each excerpt. The “rest” condition was modeled in a separate regressor with 30-s duration. Music-related “responses” were modeled as events time-locked to the moment in which a participant pressed the button to provide the pleasure rating. Consecutive responses of the same pleasure rate were excluded from this regressor. In addition, those chills occurring two events before the current chill were excluded if the difference in latency was less than 5 s. For the responses condition, a first-order parametric regressor modeled the pleasure rate (range: 1–4). Separate regressors to model the first 5 s of each excerpt and the excluded responses were also specified in the design matrix. For the gambling task, trial onsets were modeled time-locked to the moment in which the cue ([25 5] or [5 25]) appeared on screen. For the “feedback” condition, a first-order parametric regressor modeled the reward magnitude and valence, including six levels to represent gain and losses for both standard and boost trials (−125, −25, −5, 125, 25, and 25). To model the moment at which participants pressed the button, a “response” condition was included along with a first-order parametric regressor modulating whether participants used the left or right button.
For both tasks, remaining motion effects were minimized by including 24 confounding factors from head movement. All regressors were subsequently convolved with the canonical hemodynamic response function. After model estimation, the contrast for the parametric modulator in the music task (responses modulated by pleasure rate) was calculated for each subject and introduced into a second-level random effects (RFX) analysis by using a one-sample t test to calculate group effects. The same was done in the gambling task by calculating the contrast for the parametric modulator of feedback. In the music task, we confirmed activation in auditory areas using the contrast music versus rest. To analyze the effect of group on each task, we used a flexible factorial design that included the interaction of group × task and specified a linear contrast with increasing levels of sensitivity to music. Results are reported at an FWE-corrected P < 0.05 with 100 voxels of cluster extent or at an FWE-corrected P < 0.05 using SVC for a mask of the bilateral NAcc based on a neuroanatomical atlas (19, 20) (in this case, whole-brain analysis was thresholded at an uncorrected P < 0.005).
Because we predicted specific musical anhedonic participants would show reduced activation especially in the NAcc, we performed an ROI analysis with a NAcc ROI based on the aforementioned neuroanatomical atlas (19, 20) and computed the mean contrast estimate for the parametric modulator of pleasure ratings in the music task and monetary feedback in the gambling task.
Functional Connectivity MRI and Statistical Analysis.
Having identified reduced activity in the NAcc for the ANH group in response to music, we tested whether there would be differences in functional connectivity between the auditory cortex and other brain regions (presumably the NAcc). This hypothesis was tested by using a targeted PPI analysis using the auditory cortex as a seed ROI. We constrained our search to the STG anatomically defined using a probabilistic neuroanatomical adult atlas developed by Hammers et al. (19) and Hammers and coworkers (20). The anterior and posterior parts of the STG were merged to generate the mask, one for the left hemisphere and the other for the right hemisphere. The seed ROI was defined individually around the single subject peak value of the right and left STG for the parametric modulator of pleasure rating. Individual time courses from this ROI were extracted for the music listening task (music vs. rest contrast). Next, an extended model was built, including the derived PPI term, the time course of the STG, and the time course of the music listening task within the standard PPI approach (61). The computed first-level PPI results were taken to a second-level RFX analysis by using a one-way ANOVA, and a linear contrast was specified. Similarly, independent t tests were specified for pairwise comparisons. Results are reported at an FWE-corrected P < 0.05 using SVC for a mask of the bilateral NAcc based on a neuroanatomical atlas (19, 20) (in this case, whole-brain analysis was thresholded at an uncorrected P < 0.005).
In the ROI analysis, we used the same neuroanatomical NAcc mask as for SVC and applied this mask to the contrast estimated for the PPI regressor.
Acknowledgments
R.J.Z. is supported by funds from the Canadian Institutes of Health Research and the Canada Fund for Innovation. A.R.-F. has been supported by a grant from the Generalitat de Catalunya for supporting research groups (Grant 2014SGR1413). J.M.-P. has been supported by grants from the Spanish Ministry of Economy (Grants PSI2012-37472 and PSI2015-69664-P). N.M.-M. was supported by predoctoral Grant 2013-FI_B2 00133 from the Catalan Government. E.M.-H. was supported by the Formación de Personal Investigador Program BES-2010-032702 and a Jeanne Timmins Costello Fellowship from the Montreal Neurological Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1611211113/-/DCSupplemental.
References
- 1.Conard NJ, Malina M, Münzel SC. New flutes document the earliest musical tradition in southwestern Germany. Nature. 2009;460(7256):737–740. doi: 10.1038/nature08169. [DOI] [PubMed] [Google Scholar]
- 2.Trehub SE, Becker J, Morley I. Cross-cultural perspectives on music and musicality. Philos Trans R Soc Lond B Biol Sci. 2015;370(1664):20140096. doi: 10.1098/rstb.2014.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huron D. Is music an evolutionary adaptation? Ann N Y Acad Sci. 2001;930:43–61. doi: 10.1111/j.1749-6632.2001.tb05724.x. [DOI] [PubMed] [Google Scholar]
- 4.Mas-Herrero E, Zatorre RJ, Rodriguez-Fornells A, Marco-Pallarés J. Dissociation between musical and monetary reward responses in specific musical anhedonia. Curr Biol. 2014;24(6):699–704. doi: 10.1016/j.cub.2014.01.068. [DOI] [PubMed] [Google Scholar]
- 5.Peretz I, Champod AS, Hyde K. Varieties of musical disorders. The Montreal Battery of Evaluation of Amusia. Ann N Y Acad Sci. 2003;999:58–75. doi: 10.1196/annals.1284.006. [DOI] [PubMed] [Google Scholar]
- 6.Zatorre RJ, Zarate JM. Cortical processing of music. In: Poeppel D, Overath T, Popper AN, Fay RR, editors. The Human Auditory Cortex. Springer; New York: 2012. pp. 261–294. [Google Scholar]
- 7.Koelsch S. Toward a neural basis of music perception - a review and updated model. Front Psychol. 2011;2:110. doi: 10.3389/fpsyg.2011.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zatorre RJ, Chen JL, Penhune VB. When the brain plays music: Auditory-motor interactions in music perception and production. Nat Rev Neurosci. 2007;8(7):547–558. doi: 10.1038/nrn2152. [DOI] [PubMed] [Google Scholar]
- 9.Koelsch S. Brain correlates of music-evoked emotions. Nat Rev Neurosci. 2014;15(3):170–180. doi: 10.1038/nrn3666. [DOI] [PubMed] [Google Scholar]
- 10.Koelsch S, Fritz T, V Cramon DY, Müller K, Friederici AD. Investigating emotion with music: An fMRI study. Hum Brain Mapp. 2006;27(3):239–250. doi: 10.1002/hbm.20180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menon V, Levitin DJ. The rewards of music listening: Response and physiological connectivity of the mesolimbic system. Neuroimage. 2005;28(1):175–184. doi: 10.1016/j.neuroimage.2005.05.053. [DOI] [PubMed] [Google Scholar]
- 12.Blood AJ, Zatorre RJ. Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proc Natl Acad Sci USA. 2001;98(20):11818–11823. doi: 10.1073/pnas.191355898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salimpoor VN, Benovoy M, Larcher K, Dagher A, Zatorre RJ. Anatomically distinct dopamine release during anticipation and experience of peak emotion to music. Nat Neurosci. 2011;14(2):257–262. doi: 10.1038/nn.2726. [DOI] [PubMed] [Google Scholar]
- 14.Mitterschiffthaler MT, Fu CHY, Dalton JA, Andrew CM, Williams SCR. A functional MRI study of happy and sad affective states induced by classical music. Hum Brain Mapp. 2007;28(11):1150–1162. doi: 10.1002/hbm.20337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trost W, Ethofer T, Zentner M, Vuilleumier P. Mapping aesthetic musical emotions in the brain. Cereb Cortex. 2012;22(12):2769–2783. doi: 10.1093/cercor/bhr353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown S, Martinez MJ, Parsons LM. Passive music listening spontaneously engages limbic and paralimbic systems. Neuroreport. 2004;15(13):2033–2037. doi: 10.1097/00001756-200409150-00008. [DOI] [PubMed] [Google Scholar]
- 17.Salimpoor VN, et al. Interactions between the nucleus accumbens and auditory cortices predict music reward value. Science. 2013;340(6129):216–219. doi: 10.1126/science.1231059. [DOI] [PubMed] [Google Scholar]
- 18.Mas-Herrero E, Marco-Pallares J, Lorenzo-Seva U, Zatorre RJ, Rodriguez-Fornells A. Individual differences in music reward experiences. Music Percept. 2013;31(2):118–138. [Google Scholar]
- 19.Hammers A, et al. Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Hum Brain Mapp. 2003;19(4):224–247. doi: 10.1002/hbm.10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gousias IS, et al. Automatic segmentation of brain MRIs of 2-year-olds into 83 regions of interest. Neuroimage. 2008;40(2):672–684. doi: 10.1016/j.neuroimage.2007.11.034. [DOI] [PubMed] [Google Scholar]
- 21.Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011;8(8):665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M. Changes in brain activity related to eating chocolate: From pleasure to aversion. Brain. 2001;124(Pt 9):1720–1733. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- 23.Aharon I, et al. Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron. 2001;32(3):537–551. doi: 10.1016/s0896-6273(01)00491-3. [DOI] [PubMed] [Google Scholar]
- 24.Mueller K, et al. Investigating the dynamics of the brain response to music: A central role of the ventral striatum/nucleus accumbens. Neuroimage. 2015;116:68–79. doi: 10.1016/j.neuroimage.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 25.Keller J, et al. Trait anhedonia is associated with reduced reactivity and connectivity of mesolimbic and paralimbic reward pathways. J Psychiatr Res. 2013;47(10):1319–1328. doi: 10.1016/j.jpsychires.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 26.Zatorre RJ, Salimpoor VN. From perception to pleasure: Music and its neural substrates. Proc Natl Acad Sci USA. 2013;110(Suppl 2):10430–10437. doi: 10.1073/pnas.1301228110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brattico E, Pearce M. The neuroaesthetics of music. Psychol Aesthet Creat Arts. 2013;7(1):48–61. [Google Scholar]
- 28.Chatterjee A, Vartanian O. Neuroscience of aesthetics. Ann N Y Acad Sci. 2016;1369(1):172–194. doi: 10.1111/nyas.13035. [DOI] [PubMed] [Google Scholar]
- 29.Johnsrude IS, Penhune VB, Zatorre RJ. Functional specificity in the right human auditory cortex for perceiving pitch direction. Brain. 2000;123(Pt 1):155–163. doi: 10.1093/brain/123.1.155. [DOI] [PubMed] [Google Scholar]
- 30.Schneider P, et al. Morphology of Heschl’s gyrus reflects enhanced activation in the auditory cortex of musicians. Nat Neurosci. 2002;5(7):688–694. doi: 10.1038/nn871. [DOI] [PubMed] [Google Scholar]
- 31.Coffey EBJ, Herholz SC, Chepesiuk AMP, Baillet S, Zatorre RJ. Cortical contributions to the auditory frequency-following response revealed by MEG. Nat Commun. 2016;7:11070. doi: 10.1038/ncomms11070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patterson RD, Uppenkamp S, Johnsrude IS, Griffiths TD. The processing of temporal pitch and melody information in auditory cortex. Neuron. 2002;36(4):767–776. doi: 10.1016/s0896-6273(02)01060-7. [DOI] [PubMed] [Google Scholar]
- 33.Foster NEV, Zatorre RJ. Cortical structure predicts success in performing musical transformation judgments. Neuroimage. 2010;53(1):26–36. doi: 10.1016/j.neuroimage.2010.06.042. [DOI] [PubMed] [Google Scholar]
- 34.Albouy P, et al. Impaired pitch perception and memory in congenital amusia: The deficit starts in the auditory cortex. Brain. 2013;136(Pt 5):1639–1661. doi: 10.1093/brain/awt082. [DOI] [PubMed] [Google Scholar]
- 35.Grimault S, et al. Brain activity is related to individual differences in the number of items stored in auditory short-term memory for pitch: Evidence from magnetoencephalography. Neuroimage. 2014;94:96–106. doi: 10.1016/j.neuroimage.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 36.Lappe C, Herholz SC, Trainor LJ, Pantev C. Cortical plasticity induced by short-term unimodal and multimodal musical training. J Neurosci. 2008;28(39):9632–9639. doi: 10.1523/JNEUROSCI.2254-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herholz SC, Coffey EBJ, Pantev C, Zatorre RJ. Dissociation of neural networks for predisposition and for training-related plasticity in auditory-motor learning. Cereb Cortex. 2016;26(7):3125–3134. doi: 10.1093/cercor/bhv138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herholz SC, Halpern AR, Zatorre RJ. Neuronal correlates of perception, imagery, and memory for familiar tunes. J Cogn Neurosci. 2012;24(6):1382–1397. doi: 10.1162/jocn_a_00216. [DOI] [PubMed] [Google Scholar]
- 39.Salimpoor VN, Zald DH, Zatorre RJ, Dagher A, McIntosh AR. Predictions and the brain: How musical sounds become rewarding. Trends Cogn Sci. 2015;19(2):86–91. doi: 10.1016/j.tics.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 40.Koelsch S, et al. The roles of superficial amygdala and auditory cortex in music-evoked fear and joy. Neuroimage. 2013;81:49–60. doi: 10.1016/j.neuroimage.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 41.Kumar S, von Kriegstein K, Friston K, Griffiths TD. Features versus feelings: Dissociable representations of the acoustic features and valence of aversive sounds. J Neurosci. 2012;32(41):14184–14192. doi: 10.1523/JNEUROSCI.1759-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abrams DA, et al. Underconnectivity between voice-selective cortex and reward circuitry in children with autism. Proc Natl Acad Sci USA. 2013;110(29):12060–12065. doi: 10.1073/pnas.1302982110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ripollés P, et al. The role of reward in word learning and its implications for language acquisition. Curr Biol. 2014;24(21):2606–2611. doi: 10.1016/j.cub.2014.09.044. [DOI] [PubMed] [Google Scholar]
- 44.Bermudez P, Lerch JP, Evans AC, Zatorre RJ. Neuroanatomical correlates of musicianship as revealed by cortical thickness and voxel-based morphometry. Cereb Cortex. 2009;19(7):1583–1596. doi: 10.1093/cercor/bhn196. [DOI] [PubMed] [Google Scholar]
- 45.Gaser C, Schlaug G. Brain structures differ between musicians and non-musicians. J Neurosci. 2003;23(27):9240–9245. doi: 10.1523/JNEUROSCI.23-27-09240.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alluri V, et al. Musical expertise modulates functional connectivity of limbic regions during continuous music listening. Psychomusicology. 2015;25(4):443–454. [Google Scholar]
- 47.Sachs ME, Ellis RJ, Schlaug G, Loui P. Brain connectivity reflects human aesthetic responses to music. Soc Cogn Affect Neurosci. 2016;11(6):884–891. doi: 10.1093/scan/nsw009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sescousse G, Caldú X, Segura B, Dreher J-C. Processing of primary and secondary rewards: A quantitative meta-analysis and review of human functional neuroimaging studies. Neurosci Biobehav Rev. 2013;37(4):681–696. doi: 10.1016/j.neubiorev.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 49.Mazzoni M, et al. A case of music imperception. J Neurol Neurosurg Psychiatry. 1993;56(3):322. doi: 10.1136/jnnp.56.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Satoh M, Nakase T, Nagata K, Tomimoto H. Musical anhedonia: Selective loss of emotional experience in listening to music. Neurocase. 2011;17(5):410–417. doi: 10.1080/13554794.2010.532139. [DOI] [PubMed] [Google Scholar]
- 51.Griffiths TD, Warren JD, Dean JL, Howard D. “When the feeling’s gone”: A selective loss of musical emotion. J Neurol Neurosurg Psychiatry. 2004;75(2):344–345. [PMC free article] [PubMed] [Google Scholar]
- 52.Marco-Pallarés J, Mas-Herrero E. The Quartet does not play alone: Comment on “The quartet theory of human emotions: An integrative and neurofunctional model” by S. Koelsch et al. Phys Life Rev. 2015;13:71–72. doi: 10.1016/j.plrev.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 53.Torrubia R, Ávila C, Moltó J, Caseras X. The Sensitivity to Punishment and Sensitivity to Reward Questionnaire (SPSRQ) as a measure of Gray’s anxiety and impulsivity dimensions. Pers Individ Dif. 2001;31(6):837–862. [Google Scholar]
- 54.Chapman LJ, Chapman JP, Raulin ML. Scales for physical and social anhedonia. J Abnorm Psychol. 1976;85(4):374–382. doi: 10.1037//0021-843x.85.4.374. [DOI] [PubMed] [Google Scholar]
- 55.Salimpoor VN, Benovoy M, Longo G, Cooperstock JR, Zatorre RJ. The rewarding aspects of music listening are related to degree of emotional arousal. PLoS One. 2009;4(10):e7487. doi: 10.1371/journal.pone.0007487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bradley MM, Lang PJ. Measuring emotion: The self-assessment manikin and the semantic differential. J Behav Ther Exp Psychiatry. 1994;25(1):49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 57.Ben-Shakhar G. Standardization within individuals: A simple method to neutralize individual differences in skin conductance. Psychophysiology. 1985;22(3):292–299. doi: 10.1111/j.1469-8986.1985.tb01603.x. [DOI] [PubMed] [Google Scholar]
- 58.Grewe O, Nagel F, Kopiez R, Altenmüller E. How does music arouse “chills”? Investigating strong emotions, combining psychological, physiological, and psychoacoustical methods. Ann N Y Acad Sci. 2005;1060(1):446–449. doi: 10.1196/annals.1360.041. [DOI] [PubMed] [Google Scholar]
- 59.Camara E, Rodriguez-Fornells A, Münte TF. Microstructural brain differences predict functional hemodynamic responses in a reward processing task. J Neurosci. 2010;30(34):11398–11402. doi: 10.1523/JNEUROSCI.0111-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 61.Friston KJ, et al. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6(3):218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]