Significance
The splicing of human pre-mRNAs is tightly controlled and regulated during the assembly of the spliceosome onto pre-mRNA introns. Recognition of regulatory RNA sequence motifs by splicing factors is an essential early step during spliceosome assembly. We combine single-pair FRET and NMR to show that the recognition of the 3′ splice site in pre-mRNA introns by the essential heterodimeric splicing factor U2 auxiliary factor (U2AF) involves conformational dynamics and population shifts of its RNA binding domains between open and closed conformations. Unexpectedly, the small subunit U2AF35 facilitates the recognition of weak splice sites by a population shift of the RNA binding domains of U2AF65 toward the open conformation. Notably, disease-linked mutations in U2AF65 do not affect RNA or U2AF35 binding.
Keywords: U2AF, dynamics, splicing, spFRET, NMR
Abstract
An essential early step in the assembly of human spliceosomes onto pre-mRNA involves the recognition of regulatory RNA cis elements in the 3′ splice site by the U2 auxiliary factor (U2AF). The large (U2AF65) and small (U2AF35) subunits of the U2AF heterodimer contact the polypyrimidine tract (Py-tract) and the AG-dinucleotide, respectively. The tandem RNA recognition motif domains (RRM1,2) of U2AF65 adopt closed/inactive and open/active conformations in the free form and when bound to bona fide Py-tract RNA ligands. To investigate the molecular mechanism and dynamics of 3′ splice site recognition by U2AF65 and the role of U2AF35 in the U2AF heterodimer, we have combined single-pair FRET and NMR experiments. In the absence of RNA, the RRM1,2 domain arrangement is highly dynamic on a submillisecond time scale, switching between closed and open conformations. The addition of Py-tract RNA ligands with increasing binding affinity (strength) gradually shifts the equilibrium toward an open conformation. Notably, the protein–RNA complex is rigid in the presence of a strong Py-tract but exhibits internal motion with weak Py-tracts. Surprisingly, the presence of U2AF35, whose UHM domain interacts with U2AF65 RRM1, increases the population of the open arrangement of U2AF65 RRM1,2 in the absence and presence of a weak Py-tract. These data indicate that the U2AF heterodimer promotes spliceosome assembly by a dynamic population shift toward the open conformation of U2AF65 to facilitate the recognition of weak Py-tracts at the 3′ splice site. The structure and RNA binding of the heterodimer was unaffected by cancer-linked myelodysplastic syndrome mutants.
During gene expression, the removal of introns is essential for translation of mature mRNA. The splicing process involves a large number of splicing factors for the correct recognition of introns (1). Whereas U1 snRNP contacts the 5′ splice site, recognition of the 3′ splice site involves binding of SF1/BBP to the branch point sequence (BPS) (2–5) and binding of the U2 auxiliary factor (U2AF) heterodimer to the poly-pyrimidine-tract (Py-tract) that precedes the AG dinucleotide at the intron/exon junction. Binding of U2AF to the 3′ splice site during the early steps of spliceosome assembly recruits the U2 snRNP (6–9). The strength, i.e., splicing efficiency, of a 3′ splice site requires recognition of the BPS, Py-tract, and the AG dinucleotide. However, of these three RNA elements, the Py-tract exhibits the largest degree of variability, and thus, weak to strong splice sites are primarily classified depending on the composition of the Py-tract (7, 10).
U2AF is a heterodimer consisting of a large (U2AF65) and a small (U2AF35) subunit. U2AF65 harbors two canonical RNA recognition motifs (RRM1,2) and an atypical C-terminal RRM domain, called the U2AF homology motif (UHM). U2AF35 has one RRM (which acts as an UHM), flanked N- and C-terminally by two CCCH-type zinc finger motifs, respectively (Fig. S1A) (9, 11, 12). The U2AF heterodimer is formed by recognition of a peptide motif, called the UHM Ligand Motif (ULM), in U2AF65 by the U2AF35 UHM domain (13, 14) and enhances RNA binding (15, 16). Recognition of the conserved AG dinucleotide at the 3′ splice site requires U2AF35 (15, 17–20). U2AF65 binds to the Py-tract sequence located at the 3′ splice site via its RRM domains. The Py-tract sequence is degenerate in eukaryotes (21, 22), and the efficiency of the 3′ splice site recognition largely depends on the Py-tract strength, i.e., the number of uridines present in the sequence (8, 9). U2AF65 is sufficient for splicing of introns harboring strong Py-tracts in vitro, whereas U2AF35 is required for splicing of introns with weak (low-affinity) Py-tracts and is essential in vivo (7, 10, 23–25). The substantial variability in Py-tracts and plasticity in intron recognition is exploited for alternative splicing, which in eukaryotes occurs in over 60% and in humans in the majority of multiexon genes (21, 26, 27).
Fig. S1.
Structural arrangement of U2AF65 and U2AF35 constructs and conformational dynamics of RRM1,2. (A) Schematic representation of (Left) U2AF65 and (Right) U2AF35 constructs with the respective domain organization. (B) Schematic representation of the domain arrangement of U2AF65 and U2AF35. The RRMs of U2AF65 contact the Py-tract, whereas the ULM is responsible for binding to U2AF35. U2AF35 contacts the AG dinucleotide. (C) From the previous NMR structures of the (Left) closed and (Right) open conformations of RRM1,2, accessible volume calculations of fluorophores at positions 187 and 326 were performed (32).
Recently, it was proposed that Py-tract RNA binding by U2AF65 employs a conformational selection mechanism, which involves a population shift of the arrangement of its tandem RRM domains from a closed to an open state (28). Moreover, based on a combined analysis of NMR and small angle X-ray scattering (SAXS) data, it has been shown that the unbound RRM1,2 protein adopts a range of closed and detached domain arrangements, which are not able to mediate high-affinity RNA binding (29). To understand the molecular mechanisms of these conformational changes and characterize the underlying conformational dynamics induced by RNA binding, we applied single-pair Förster resonance energy transfer (spFRET). The detailed investigation of the conformational dynamics on the single-molecule level revealed that the arrangement of the U2AF65 RRM1,2 tandem domains switches between an open and closed conformation. Increasing binding affinity to Py-tract RNA ligands correlates with a population shift toward the open state. Only strong Py-tracts promote the formation of a rigid protein–RNA complex, whereas complexes with weaker, i.e., lower-affinity, RNA ligands, still show significant conformational dynamics. Most notably, the presence of the small subunit, U2AF35, shifts the U2AF65 RRM1,2 domain arrangement toward an open, active conformation already in the absence of RNA, consistent with the known requirement of U2AF35 to enhance the recognition of weak Py-tracts by U2AF.
Results
The RRM1,2 Domains of U2AF65 Are Highly Dynamic.
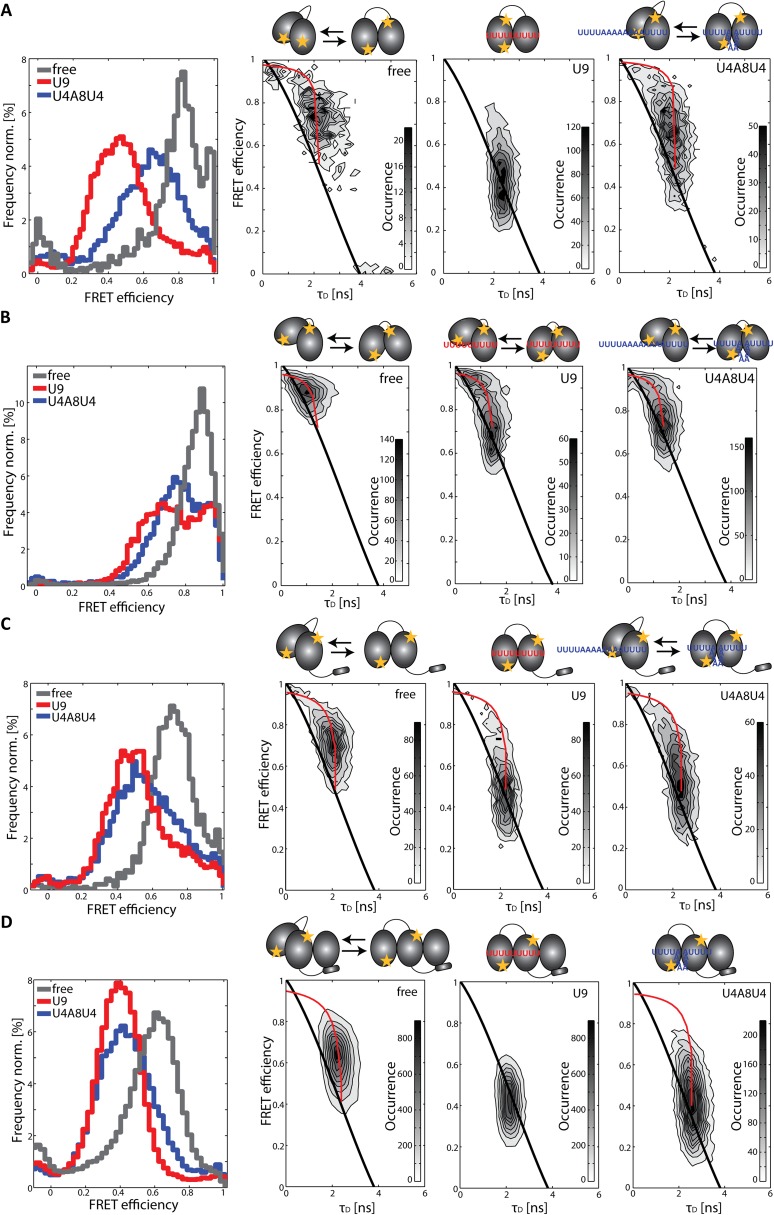
The two RNA recognition motifs 1 and 2 (RRM1,2), connected by a flexible linker, represent the minimal RNA binding region for U2AF65 (Fig. S1) (8, 28, 30). Molecular details of the RNA recognition of the individual RRM1 and RRM2 domains have been described previously (30). Recently, it was shown that U2AF65 RRM1,2 exists in a closed conformation in its free form and an open conformation when RNA is bound (28). An enhancement of the overall RNA binding affinity with increasing Py-tract strength correlates with a population shift toward the open conformation. NMR data demonstrate that this shift involves an increased contribution of RRM1 to RNA binding (28). To investigate the role of conformational dynamics in this population shift, we used spFRET to study the intrinsic dynamics of RRM1,2 in the absence and presence of RNA. For these experiments, one residue in each of the RNA recognition motifs RRM1 and RRM2 was mutated to a cysteine residue and labeled stochastically with a donor and acceptor dye pair (Fig. 1A and Fig. S1C). The cysteine positions were designed to be in close proximity when the RRM1,2 tandem domains adopt a closed conformation and to be at maximal distance when the domains adopt an open conformation (28). To ensure that the measurements are not subject to artifacts from the labeling, we analyzed the FRET efficiency and conformational change of the protein using different fluorescent labels and tested different labeling positions (Figs. S2 and S3). The constructs were measured in solution using a confocal microscope with multiparameter fluorescence detection and pulsed interleaved excitation (MFD-PIE) (31–34).
Fig. 1.
Conformation and dynamics of RRM1,2 in solution. (A) Closed (Left, PDB 2YH0) and open, RNA-bound (Right, PDB 2YH1) structure of U2AF65(RRM1,2) with labeling sites C187 in RRM1 and C326 in RRM2 shown as stars. (B) Histogram of spFRET efficiency as a function of donor lifetime of RRM1,2-Atto532-Alexa647 in the absence of RNA (9,160 molecules), in the presence of 5 µM U9 (10,015 molecules), or in the presence of 20 µM U4A8U4-RNA (12,083 molecules). Populations of static molecules are described by the polynomial static FRET line (Eq. S5; black line), whereas molecules undergoing conformational dynamics on the time scale of microseconds to milliseconds deviate from this line (dynamic FRET curve in Eq. S7; red line). The fully open and closed conformations were determined from lifetime fits of the data and correspond to the intersections of the dynamic FRET curve with the static FRET line (Fig. S2B). Representations of the open and closed conformations of the molecules are displayed schematically as a simple interpretation of the histograms.
Fig. S2.
Conformation and conformational dynamics of RRM1,2-C187-C326. (A) Exemplary intensity recording of green and red photons after green and red excitation, respectively, over the course of a measurement. Right shows a zoom in of the measurement to display bursts of photons of individual molecules. (B) Exemplary biexponential fit to the donor lifetime histogram obtained from an MFD-PIE experiment of RRM1,2-C187-C326-Atto532/Alexa647. (C and D) For the following experiments, RRM1,2-C187-C326 was labeled with Atto532 and Atto647N. (C) FRET efficiency histogram of solution spFRET MFD experiments of RRM1,2 alone (gray; data from 31,659 molecules), in the presence of the strong Py-tract U9 (red; data from 38,481 molecules); and of the weak binding sequence U4A8U4 (blue; data from 18,057 molecules). (D) Two-dimensional histograms of spFRET efficiency versus donor lifetime from the same measurements as in C. Populations of static molecules are described by the polynomial static FRET line (Eq. S5; black line), whereas molecules undergoing conformational dynamics on the timescale of µs-ms deviate from this line (dynamic FRET curve in Eq. S7; red line). The fully open and closed conformations were determined from lifetime fits of the data and correspond to the intersections of the dynamic FRET curve with the static FRET line. (E) (Left) FRET efficiency histogram and (Right) 2D histogram of spFRET efficiency versus donor lifetime of RRM1,2-C187-C326-Atto532/Alexa647 in the absence (gray) and in the presence of A9 RNA (red) or A13ACAGG RNA (magenta). (F) (Left) FRET efficiency histogram and (Right) 2D histogram of spFRET efficiency versus donor lifetime of URRM1,2-C187-C326-Atto532/Alexa647/U2AF35(UHM) in the absence (gray) and in the presence of A9 RNA (red) or A13ACAGG RNA (magenta). (E and F) The static (black; Eq. S5) and dynamic (red; Eq. S7) FRET curves are shown.
Fig. S3.
Conformational dynamics of RRM1,2 constructs measured in solution on a confocal microscope equipped with MFD-PIE. First column shows FRET efficiency histograms, and the second through fourth columns show 2D histograms of FRET efficiency versus donor lifetime of (A) RRM1,2-C187-C318, (B) RRM1,2-Δ233–252-C187-C318, (C) URRM1,2-C187-C326, and (D) URRM1,2-C187-C326/U2AF35(UHM) alone (gray), in the presence of the strong Py-tract U9 (red), and in the presence of the weak binding sequence U4A8U4 (blue). Histograms of spFRET efficiency versus donor lifetime of the constructs are shown in the absence of RNA (second column), when bound to U9 (third column), and when bound to U4A8U4 (fourth column). Populations of static molecules in the 2D histograms are described by the polynomial static FRET line (Eq. S5; black line), whereas molecules undergoing conformational dynamics on the time scale of microseconds to milliseconds deviate from this line (dynamic FRET curve in Eq. S7; red line). The fully open and closed conformations were determined from lifetime fits of the data and correspond to the intersections of the dynamic FRET curve with the static FRET line. Schematic representations of the constructs transversing between the closed and the open conformation in the absence and presence of RNA are shown above the respective plots.
In the spFRET efficiency histogram, the free form of RRM1,2 is found to populate a high FRET state with a mean FRET efficiency of 0.78 (Figs. 1B and 2A and Table S1). By analyzing the lifetime information of the donor fluorophore, we could determine that this FRET efficiency is an average value obtained from a highly dynamic population of molecules (Fig. 1B, Left). In burst analysis experiments, an average FRET efficiency and donor lifetime is determined for each molecule. When molecules undergo conformational dynamics during their transit time through the confocal volume (of a few milliseconds), an average single-molecule FRET efficiency is measured. The presence of dynamics on the submillisecond time scale can be visualized by plotting the intensity-determined FRET efficiency versus donor lifetime. In the presence of dynamics, the intensity-determined FRET efficiency will be a species-averaged value (Eq. S6), whereas the donor lifetime is a lifetime-weighted average (Eq. S7). This yields a deviation from the relationship observed for a static FRET species (static FRET line; Eq. S5) (32, 35, 36). In Fig. 1B, Left, all molecules clearly deviate from the static FRET line, demonstrating that the conformation of RRM1,2 is highly dynamic in the absence of RNA. By combining the lifetime information for all the single-molecule in the spFRET experiments, we observed two donor lifetimes of 0.14 and 2.15 ns for the free form of RRM1,2, corresponding to FRET efficiencies of 0.96 and 0.43, respectively (Table S1, Fig. 1B, and Fig. S2B). Thus, we conclude that the protein undergoes conformational transitions between a closed conformation, an arrangement of the tandem domains where the donor–acceptor separation is ∼34–40 Å and an open conformation with a distance of 64–69 Å. Estimations of the spFRET histograms for the open and closed conformation are shown in Fig. 2D. Using accessible volume calculations (35), we estimated the average donor–acceptor separation from the published structures of 42 Å for the closed conformation (PDB 2YH0) (28) and a distance of 61 Å for the open conformation (PDB 2YH1). The measured FRET distances and accessible volume calculations are in excellent agreement, especially when considering that the tandem RRMs represent an ensemble of closed and detached conformations with respect to each other as indicated from the NMR and SAXS data (29).
Fig. 2.
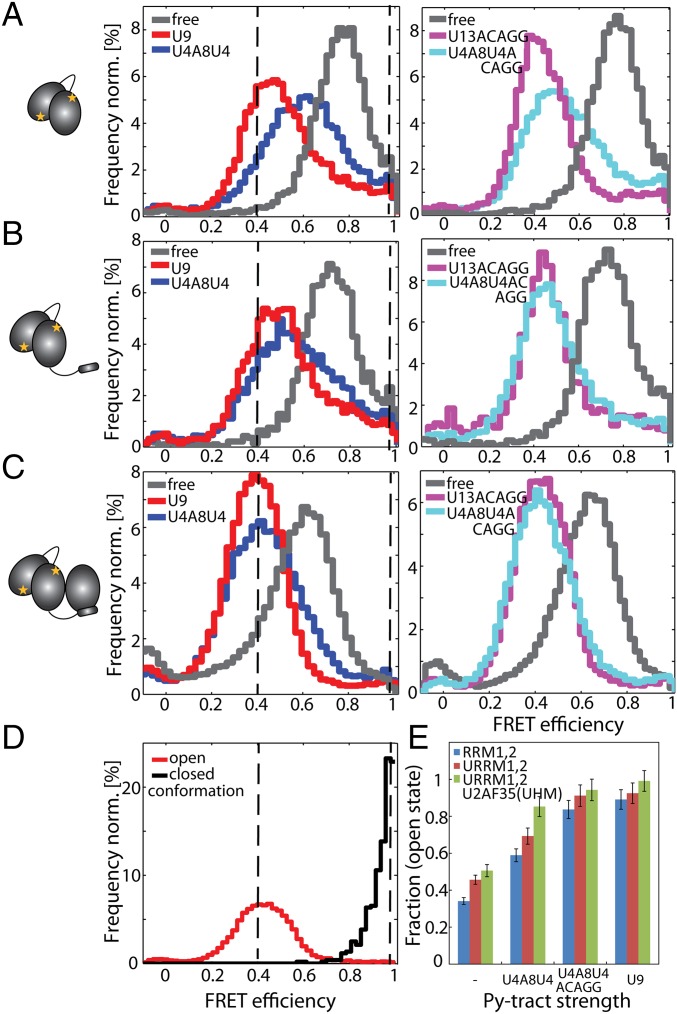
Comparison of the conformations of the U2AF65-C187-C326 constructs RRM1,2, URRM1,2, and URRM1,2/U2AF35(UHM). SpFRET experiments of the constructs were performed in solution using MFD-PIE. Schematic representations of the minimal (A) RRM1,2, (B) URRM1,2, and (C) URRM1,2/U2AF35(UHM) are shown on the left. SpFRET efficiency histograms of RRM1,2 (A), URRM1,2 (B), and URRM1,2/U2AF35(UHM) (C) are displayed in the free form (gray) or in the presence of U9 (red), U4A8U4 (blue), U13ACAGG (pink), and U4A8U4ACAGG (cyan). The dashed black lines serve as guides to the eye to show the peak FRET efficiency for the open and closed conformations as obtained from D. (D) Estimation of the open and the closed state of RRM1,2. The FRET efficiency histogram of RRM1,2 molecules with a donor lifetime τD < 1 ns (black) was selected to represent the closed conformation. The FRET efficiency histogram of U13ACAGG-bound URRM1,2/U2AF35(UHM) with a donor lifetime τD > 1 ns (red) was selected to represent the open conformation. (E) Quantitative description of the dynamic equilibrium in a bar diagram displaying the fraction of time the different U2AF65 constructs spend in the open conformation based on the spFRET experiments of the constructs in solution (RRM1,2, blue; URRM1,2, red; URRM1,2/U2AF35(UHM), green). Errors were determined by error propagation from the values of E1, E2, and (Table S1).
Table S1.
Donor lifetimes and fractions from MFD-PIE experiments of RRM1,2-C187-C318-Atto532/Alexa647 and RRM1,2-C187-C326-Atto532/Alexa647 constructs in the absence and presence of RNA
| Protein | RNA | τ1, ns | τ2, ns | E1 | E2 | D1, Å | D2, Å | A(τ1) | |
| RRM1,2 | — | 2.15 | 0.14 | 0.43 | 0.96 | 64.8 | 36 | 0.783 ± 0.004 | 0.34 ± 0.02 |
| C187-C326 | U9 | 2.11 | 0.10 | 0.44 | 0.97 | 64.3 | 34 | 0.50 ± 0.02 | 0.89 ± 0.06 |
| U4A8U4 | 2.28 | 0.13 | 0.40 | 0.97 | 66.3 | 35 | 0.632 ± 0.008 | 0.59 ± 0.04 | |
| U13ACAGG | 2.14 | — | 0.44 | — | 64.7 | — | 0.460 ± 0.008 | 0.96 ± 0.06 | |
| U4A8U4ACAGG | 2.21 | — | 0.42 | — | 65.5 | — | 0.51 ± 0.01 | 0.84 ± 0.05 | |
| URRM1,2 | — | 2.11 | 0.16 | 0.45 | 0.96 | 64.3 | 37 | 0.725 ± 0.005 | 0.46 ± 0.03 |
| C187-C326 | U9 | 2.12 | 0.18 | 0.44 | 0.95 | 64.4 | 37 | 0.48 ± 0.02 | 0.92 ± 0.06 |
| U4A8U4 | 2.35 | 0.21 | 0.38 | 0.95 | 67.2 | 39 | 0.55 ± 0.01 | 0.69 ± 0.05 | |
| U13ACAGG | 2.17 | 0.17 | 0.43 | 0.96 | 65.0 | 37 | 0.431 ± 0.007 | 1.00 ± 0.06 | |
| U4A8U4ACAGG | 2.29 | 0.27 | 0.40 | 0.93 | 66.5 | 40 | 0.44 ± 0.02 | 0.91 ± 0.06 | |
| URRM1,2/ U2AF35(UHM) | — | 2.47 | 0.22 | 0.35 | 0.94 | 68.7 | 39 | 0.642 ± 0.006 | 0.51 ± 0.04 |
| C187-C326 | U9 | 2.17 | 0.17 | 0.43 | 0.95 | 65.1 | 37 | 0.432 ± 0.005 | 0.99 ± 0.06 |
| U4A8U4 | 2.17 | 0.14 | 0.43 | 0.96 | 67.7 | 38 | 0.456 ± 0.007 | 0.85 ± 0.06 | |
| U13ACAGG | 2.39 | 0.21 | 0.37 | 0.95 | 67.7 | 38 | 0.417 ± 0.005 | 1.02 ± 0.06 | |
| U4A8U4ACAGG | 2.37 | — | 0.38 | — | 67.4 | — | 0.412 ± 0.005 | 0.94 ± 0.06 | |
| RRM1,2-Δ233–252 | — | 1.44 | 0.11 | 0.62 | 0.97 | 57.1 | 34 | 0.895 ± 0.004 | 0.22 ± 0.01 |
| C187-C318 | U9 | 1.44 ± 0.08 | 0.19 ± 0.08 | 0.62 ± 0.02 | 0.95 ± 0.02 | 57.1 ± 0.8 | 38 ± 4 | 0.71 ± 0.02 | 0.72 ± 0.04 |
| U4A8U4 | 1.47 | 0.10 | 0.61 | 0.97 | 57.4 | 34 | 0.770 ± 0.006 | 0.57 ± 0.03 | |
| RRM1,2 | — | 2.17 ± 0.08 | 0.3 ± 0.2 | 0.43 ± 0.02 | 0.91 ± 0.04 | 65.0 ± 0.9 | 42 ± 5 | 0.76 ± 0.02 | 0.33 ± 0.02 |
| C187-C318 | U9 | 2.03 | 0.2 | 0.46 | 0.95 | 63.5 | 38 | 0.48 ± 0.01 | 0.97 ± 0.06 |
| U4A8U4 | 2.15 | 0.2 | 0.43 | 0.96 | 64.8 | 37 | 0.66 ± 0.03 | 0.57 ± 0.04 | |
| URRM1,2/ U2AF35(UHM) | — | 2.72 | 0.3 | 0.28 | 0.93 | 72.4 | 40 | 0.38 ± 0.02 (0.46 ± 0.03) | 0.85 ± 0.08 |
| C187-C318 | U9 | 3.28 | 0.3 | 0.14 | 0.91 | 84.3 | 42 | 0.032 ± 0.003 (0.31 ± 0.05) | 1.1 ± 0.2 |
| U4A8U4 | 3.03 | 0.3 | 0.20 | 0.92 | 77.9 | 41 | 0.14 ± 0.01 (0.5 ± 0.2) | 1.1 ± 0.2 | |
| U13ACAGG | 3.03 | 0.4 | 0.20 | 0.89 | 78.0 | 43 | 0.036 ± 0.004 (0.30 ± 0.04) | 1.2 ± 0.2 |
FRET efficiencies and distances were calculated from the lifetime information. The donor lifetimes (τ1, τ2) were determined by reconvolution fitting of the data with the instrument response function and fitting with two exponentials. The FRET efficiencies E1 and E2 and distances D1 and D2 were calculated from the respective donor lifetimes with the lifetime of the donor-only population τD(0)=3.8 ns and the Förster radius R0 = 62 Å. SDs of the mean for each of the parameters are given as 0.08 ns for τ1 and σ2, 0.02 for E1 and E2, 0.8 Å for D1, and 4 Å for D2 from three independent measurements of RRM1,2-Δ233–252+U9 as shown in the respective slot in the table. gives the mean FRET efficiency determined by a Gaussian fit to the FRET efficiency distribution histogram. The 95% confidence interval is given as the error. A(τ1) is the fraction of time the molecules spend in the open conformation determined by a linear combination of the FRET efficiencies determined from the lifetime information [A(τ1)= ]. The error of A(τ1) was determined by error propagation of the errors of E1, E2, and . Dashes denote populations that are not present or present in such low quantities that the fluorescent lifetimes cannot be determined precisely. Hence, the distances were not calculated for these populations.
From the mean FRET efficiency and the FRET values obtained for the open and closed states from the donor lifetime data, we could estimate the fraction of time each molecule spends in the open conformation (Table S1). Free RRM1,2 molecules mainly populate the closed high FRET state (with a donor–acceptor separation of ∼35 Å) spending an average of just ∼1/3 of their time in the open conformation with a distance of ∼65 Å between the fluorophores.
A linker truncation mutant RRM1,2-Δ233–252, which was previously proposed to increase the population of the open domain arrangement (28), was investigated. This construct adopts a closed conformation with the same FRET efficiency as observed for RRM1,2, whereas the open conformation exhibits a slightly higher FRET efficiency (SI Text and Fig. S3B). Thus, truncation of the inner part of the linker region does not abolish the conformational dynamics of the unbound RRM1,2, whereas it promotes an open conformation that is not as extended as in the wild-type protein. At the same time, the mutant serves as an internal control to show that another different open conformation can be adopted and recognized by the lifetime analysis and supports that observation that the open conformation observed for all full-linker constructs in the absence and presence of RNA is the same.
U2AF65 Adopts an Open State When Binding to Strong Py-Tracts.
Next, we analyzed the conformational states of the minimal RRM1,2 in the presence of RNA substrates. For the strong Py-tract (U9), the apparent FRET efficiency shifts to an average value of around 0.5 (Fig. 1B and Table S1). The spFRET histogram represents a mixture of the same open and closed conformations as observed for the free protein (FRET efficiencies 0.97 and 0.44 for U9-bound RRM1,2) (Table S1). However, the equilibrium between the open and closed conformation of RRM1,2 is shifted significantly, with the complex spending around 90% of the time in the open conformation (Figs. 1B, Middle, and 2A). The population clearly lies on the static FRET curve indicating a stabilization of the open conformation. For a weaker Py-tract (U4A8U4), the apparent average FRET efficiency is 0.63 and the population deviates from the static FRET line (Figs. 1B, Right, and 2A). This indicates an increase in dynamics compared with the U9-bound U2AF65. Again, the spFRET data and fluorescence lifetime data reveal dynamic transitions between two states with FRET efficiencies of 0.40 (D = 66 Å) and 0.97 (D = 35 Å), i.e., the same two populations observed previously. The molecules populate the open conformation 59% of the time, whereas they were still found in the closed conformation quite frequently. Hence, the equilibrium between the open and the closed conformation of U2AF65 is influenced by the overall binding affinity for the Py-tract sequence. Py-tracts with higher affinity lead to a shift in the equilibrium by increasing the time the molecules spend in the open conformation. Consistently, purine-rich RNA ligands such as A9 and A13ACAGG that do not contain bona fide Py-tract sequences do not induce any shift in the FRET efficiency histogram or influence the conformational dynamics of RRM1,2 constructs with respect to the same protein in the absence of RNA (Fig. S2 E and F).
We investigated whether additional conformational changes are observable on a slower time scale with spFRET–total internal reflection fluorescence microscopy. Static RRM1,2-C187-C318-Atto532/Alexa647 molecules with FRET efficiencies corresponding to the conformations observed in solution-based measurements but no evidence for conformational transitions on time scales longer than 30 ms were found (Figs. S3 and S4).
Fig. S4.
Conformations of RRM1,2-C187-C318 and RRM1,2-Δ233–252-C187-C318 in lipid vesicles obtained from surface-based spFRET experiments. (A) Schematic representation (not to scale) of RRM1,2 encapsulated in a lipid vesicle, which is attached to the prism surface via biotin–streptavidin–biotin linkage. (B) Static intensity trace of free RRM1,2-Atto532-Atto647N. The acceptor fluorophore (red) photobleaches first, and thus, the intensity of the donor fluorophore (green) is increased. The FRET efficiency (black) of the single protein over time is displayed in the Bottom panel. (C) Framewise FRET efficiency histograms of static RRM1,2 molecules in the absence (gray) and in the presence (red) of the strong Py-tract U9 labeled with (Upper) Atto532-Alexa647 or (Lower) Atto532-Atto647N. (D) Framewise FRET efficiency histograms of static RRM1,2-Δ233–252 molecules in the absence (gray) and in the presence of the strong Py-tract U9 (red) labeled with (Upper) Atto532-Alexa647 or (Lower) Atto532-Atto647N.
Role of U2AF35 for Py-Tract Recognition by the U2AF Heterodimer.
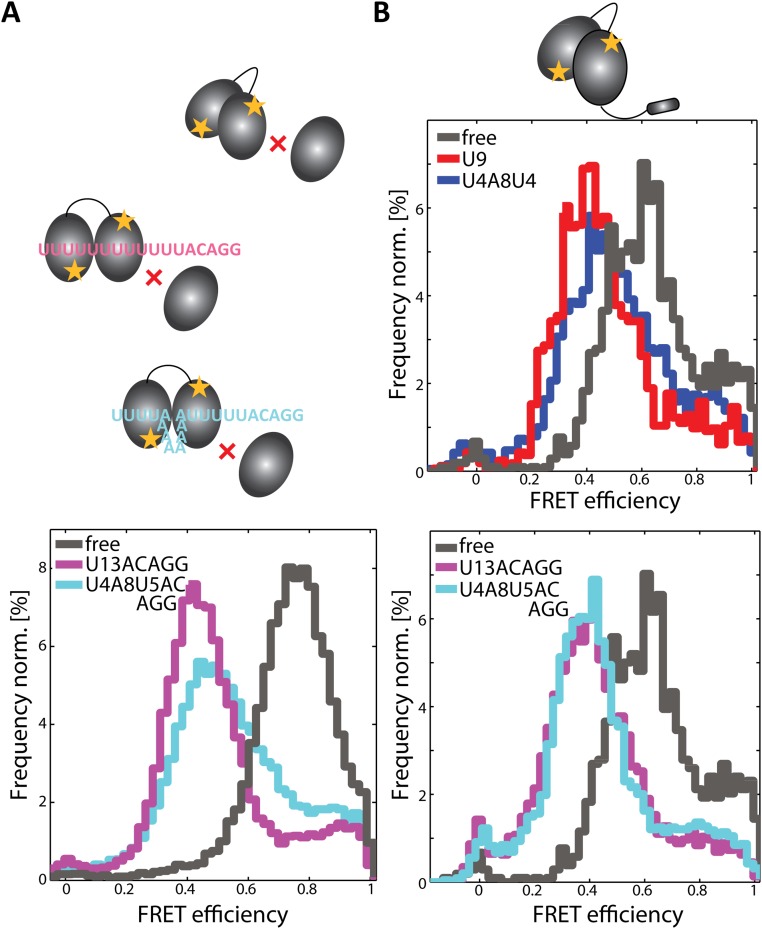
The ULM region of U2AF65 is necessary for binding to the U2AF35 subunit and thus formation of the U2AF heterodimer. Hence, before studying the conformation of U2AF65 in the presence of U2AF35, we first characterized an extended U2AF65 RRM1,2 protein that includes the ULM region (URRM1,2) in the absence and presence of RNA (Fig. 2B and Figs. S3C and S5B). We found a similar behavior as was observed for RRM1,2 without the additional ULM region. Dynamic transitions between the open and closed conformations were observed with the same FRET efficiencies of 0.44 and 0.95 as was observed for RRM1,2 alone. We observed an overall shift of the equilibrium toward the open conformation with URRM1,2 spending 46% of the time in the open conformation in the absence of RNA (compared with 34% for RRM1,2) (Fig. 2 A and B and Table S1). Binding of the strong Py-tract U9 and the weak Py-tract U4A8U4 to URRM1,2 induced a shift of the equilibrium toward the open state with the molecules now spending 92% and 69% of their time in the open conformation, respectively (Table S1). Isothermal titration experiments show that the addition of amino acids 88–147, preceding the RRM1 domain, increases the affinity of URRM1,2 sixfold compared with RRM1,2 alone (Table S2). We could show that this increased affinity is associated with faster binding rates (Table S2). An increasingly open conformation is thus accompanied by an increase in the binding kinetics.
Fig. S5.
Conformational states of U2AF65-C187-C326 constructs obtained from solution MFD-PIE experiments. (A) (Upper) Schematic representation of RRM1,2-Atto532-Alexa647 in the presence of U2AF35(UHM) and elongated Py-tracts. (Lower) FRET efficiency histograms of RRM1,2 in the presence of U2AF35(UHM) (gray) and U13ACAGG (pink) or U4A8U5ACAGG (cyan). (B) FRET efficiency histograms of URRM1,2-Atto532-Atto647N. The protein was measured using solution-based MFD-PIE experiments in the absence of RNA (gray), bound to U9 (red), U4A8U4 (blue), U13ACAGG (pink), or U4A8U5ACAGG (cyan).
Table S2.
Association and dissociation of RRM1,2-C187-C326 constructs with U9-RNA
| Protein | Number of molecules | t/2, s | koff, 1/s | KD, µM | kon, 1/(s·µM) |
| RRM1,2 | 190/171 | 0.68 ± 0.05 | 1.02 ± 0.05 | 1.34 ± 0.03 | 0.76 ± 0.06 |
| URRM1,2 | 140/211 | 0.8 ± 0.1 | 0.9 ± 0.1 | 0.22 ± 0.01 | 4.1 ± 0.6 |
| URRM1,2/U2AF35(UHM) | 251 | 0.76 ± 0.06 | 0.92 ± 0.06 | 0.15 ± 0.01 | 6.1 ± 0.6 |
The dissociation times of U2AF65 constructs were determined from spFRET experiments on the surface with total internal reflection microscopy. The dwell time histogram of a given number of molecules was fit by an exponential decay and the dissociation rate extracted (koff). Affinity constants (KD) were obtained from isothermal titration calorimetry (ITC) and used for the estimation of the association rate (kon). Errors are SDs from two independent measurements, except for the heterodimer, where error propagation was used. ITC was performed using an ITC200 Microcal calorimeter (Microcal) at 25 °C. Three hundred microliters of 20 μM protein sample (20 mM sodium phosphate, pH 6.5, 50 mM NaCl) in the cell was titrated with 50 μL of 250 μM of U9 RNA in the same buffer. The data were further analyzed using Origin v5.0 from Microcal.
Next, we investigated the role of U2AF35 in Py-tract recognition at the 3′ splice site. The U2AF35 UHM domain mediates protein–protein interactions (13, 14) and folds upon binding to a UHM Ligand Motif (ULM) peptide sequence in U2AF65 (11) but does not directly contribute to the specific recognition of the AG-dinucleotide by U2AF35 (37). Instead, two conserved zinc finger domains flanking the UHM are expected to mediate the specific recognition at the 3′ splice site (20). To understand the contribution of the U2AF35 UHM domain to Py-tract recognition by U2AF65, we studied the RRM1,2 domain arrangements in the context of a minimal U2AF heterodimer, which has been used in previous studies (17, 19). The minimal U2AF heterodimer comprises the U2AF65 ULM and the RRM1,2 domains bound to the U2AF35 UHM domain (URRM1,2/U2AF35(UHM)). We measured the conformation of the RRM1,2 domains within the minimal heterodimer in the absence and presence of RNA. As before, we observed dynamics between the same open and closed conformations with the heterodimer spending even more time in the open conformation (51%) with respect to RRM1,2 (34%) and URRM1,2 (46%) (Table S1). Correspondingly, the affinity of the URRM1,2/U2AF35(UHM) heterodimer (KD = 150 nM) for the strong Py-tract U9 is higher than for RRM1,2 (KD = 1.34 µM) and URRM1,2 (KD = 220 nM) (Table S2). The shift of the equilibrium in the presence of U2AF35(UHM) is more strongly pronounced in the presence of weak Py-tract RNA, 99% in the open conformation for the strong U9 Py-tract versus 90% without U2AF35 and 85% for the weaker ligand (U4A8U4) versus 59% when the small subunit is absent (Table S1). The presence of U2AF35 has a large effect in shifting RRM1,2 to the open conformation when the weak Py track is bound (U4A8U4). U2AF35 also shifts RRM1,2 more to the open state when a strong Py-tract is bound, but this effect is less dramatic because the complex already exists predominantly in the open conformation. In either case, the likelihood of the heterodimer to be found in the open state is significantly increased compared with RRM1,2 or URRM1,2.
To explore potential contributions of additional RNA sequences present in the 3′ splice site on the RRM1,2 conformational equilibrium, we studied the interaction with an RNA comprising a strong Py-tract followed by an AG splice site, U13ACAGG. U2AF65 RRM1,2 shows the same open and closed conformations as in the presence of the shorter Py-tract U9 (Fig. 2A), consistent with the expectation that RRM1,2 specifically recognizes the Py-tract region of the RNA. The RRM1,2 proteins spend around 96% of the time in the open conformation. Upon binding to a weak Py-tract followed by the AG splice site, U4A8U4ACAGG, the equilibrium is shifted toward the open state (84%) compared with the binding of the weak Py-tract without the extended AG sequence, U4A8U4 (59%) (Fig. 2A and Table S1). Presumably, additional, nonspecific contacts involving the additional nucleotides following the weak Py-tract can induce a population shift toward the open state.
We also observe an increased stabilization of the open conformation upon binding of a weak Py-tract to URRM1,2 (Fig. 2B and Fig. S3C and Table S1). In the presence of U4A8U4ACAGG, URRM1,2 proteins spend 91% of their time in the open conformation compared with 69% for U4A8U4. This may correlate with the higher overall binding affinity of URRM1,2 for U4A8U4ACAGG than for U4A8U4. Additional contacts between U2AF65 and the additional nucleotides present downstream of the Py-tract thus lead to a higher probability of the RRM domains to be found in the open conformation. Additional chemical shift perturbations in the NMR data also revealed stabilizing RNA contacts for a few residues preceding the first β1-strand in RRM1 (Fig. S6A), consistent with a recent crystal structure of an extended RRM1,2 protein bound to a strong Py-tract (38). However, the NMR titration data also demonstrate that no additional contacts are observed for residues preceding this N-terminal region of RRM1.
Fig. S6.
Determination of the domain arrangements in the free U2AF65(URRM1,2) and the U2AF heterodimer URRM1,2/U2AF35(UHM) (HD) bound to RNA (U13ACAGG) from PRE measurements where the spin labels (SL) were incorporated at the individually engineered cysteines. (A) NMR-monitored amide chemical shift changes in U2AF65(URRM1,2) upon adding RNA, showing that the N-terminal region, residues 88–140, does not contribute to RNA binding. (B) Comparable domain arrangements of RRM1,2 in URRM1,2 to those in RRM1,2 with the SL at C318. (C) Interdomain arrangements of RRM1,2 in the U2AF heterodimer bound to RNA. PREs observed in U2AF65(URRM1,2) with the SLs incorporated into C155, C196, and C271 in U2AF65. PREs of U2AF65(RRM1,2) bound to RNA (U9) with the SL at C155 are shown in red (Top). Schematics depicting domain arrangements relative to the SL sites (Top). (D and E) Intersubunit arrangements of U2AF65/35 in the U2AF heterodimer bound to RNA. (D) PREs observed in U2AF65(URRM1,2) with the SLs incorporated at the positions C87, C102, C131, and C145 in U2AF35. PREs of U2AF65(URRM1,2) in the U2AF heterodimer in the absence of RNA with the SL at C145 are shown in red (fourth panel). Schematics depicting domain arrangements relative to the SL sites (fourth panel). (E) Comparable intersubunit PREs observed in U2AF35(UHM) with the SL incorporated into C187 in U2AF65 in the absence of RNA to those observed in the presence of RNA.
U2AF35 Enhances Py-Tract Recognition by a Dynamic Population Shift.
Surprisingly, in the minimal heterodimer, the population of the open conformation of RRM1,2 is substantially increased even in the absence of RNA, going from 34% for RRM1,2 and 46% for URRM1,2 to 51% in the U2AF heterodimer (Fig. 2 and Fig. S3 C and D). This indicates that the presence of the U2AF35 UHM domain enhances the open conformation of U2AF65 RRM1,2. Consistent with the increased fraction of open conformations in the unbound U2AF heterodimer, binding of a weak Py-tract U4A8U4ACAGG shows a dramatic population shift toward the open conformation similar to that measured in the presence of U9 (94% for U4A8U4ACAGG versus 99% for U9) (Fig. 2C and Table S1). A control experiment of RRM1,2 without the ULM binding domain in the presence of U2AF35(UHM) and U4A8U4ACAGG could not induce this stabilization of the open conformation (Fig. S5A). Thus, the presence of the U2AF35 UHM domain, when stably bound in the U2AF heterodimer, leads to a population shift of the RRM1,2 domains toward the open conformation. Thereby, the heterodimer promotes an open conformation that enables efficient binding even of weak Py-tract RNA ligands (Table S1 and Fig. 2 C and E).
An Interface in the U2AF Heterodimer Mediates the Population Shift.
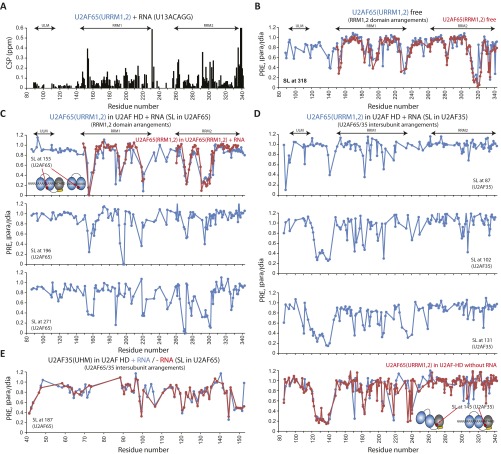
To characterize the structural mechanisms of how U2AF35 leads to a population shift toward the open conformation of RRM1,2, we studied the domain arrangements in the minimal U2AF heterodimer [URRM1,2/U2AF35(UHM)] using NMR spectroscopy. We determined local tumbling correlation times from 15N NMR relaxation data for the free U2AF heterodimer and when bound to a strong Py-tract AG RNA (U13ACAGG). These data indicate that, in the absence of RNA, the U2AF65 ULM, RRM1, and the U2AF35 UHM domains tumble together, whereas the U2AF65 RRM2 domain exhibits higher mobility (Fig. 3A). This suggests that the RRM2 may be partially detached from RRM1. In contrast, upon formation of the U2AF/RNA complex, all domains in both subunits tumble together, indicating the formation of a compact and rigid protein/RNA complex (Fig. 3 B and C).
Fig. 3.
NMR 15N-relaxation–based rotational correlation time of the U2AF heterodimer in the (A) absence and (B and C) presence of U13ACAGG (B) for URRM1,2 and (C) for U2AF35(UHM). Conformational changes of the domains in the U2AF heterodimer URRM1,2/U2AF35(UHM) upon binding of U2AF35(UHM) and RNA are shown as schematic diagrams. Two distinct rotational correlation times are shown in A. Missing data points denote proline, unassigned, or spectrally overlapped residues. (D) PREs (ratio of NMR signal intensities in the paramagnetic and diamagnetic state, Ipara/Idia) observed in URRM1,2 (red box) upon binding of U2AF65(UHM) (spin labeled at C145). (E) Stepwise schematic diagram depicting conformational changes of RRM1,2 and U2AF65/35 interfaces by introducing U2AF35(UHM), followed by RNA addition. (F) PRE-based model of the U2AF heterodimer URRM1,2/U2AF35(UHM) in the presence of RNA, with spin labels attached to multiple sites in U2AF to define the relative orientations/conformations of RRM1,2 (yellow circle) and U2AF65/35 (red circle). Selected key regions with intersubunit PREs in U2AF65/35 are highlighted (cyan circles) and connected to the corresponding spin label sites (red arrow).
The relative arrangement of the domains in the U2AF heterodimer/RNA complex was determined by paramagnetic relaxation enhancement (PRE) data with single nitroxyl spin labels attached to specific sites in U2AF65 RRM1, RRM2, and U2AF35 UHM (Fig. 3 D and F and Fig. S6). Notably, SL155 shows the same PRE effects for RRM1,2 in the context of the heterodimer bound to the extended strong Py-tract as observed for RRM1,2 alone bound to a strong Py-tract (Fig. S6C). This is fully consistent with the FRET data, which indicate that the same open conformation is adopted upon binding to strong Py-tracts. A number of spin labels attached to RRM1 or UHM show strong intermolecular PRE effects between these two domains (Fig. 3 and Fig. S6). These data demonstrate that the U2AF35 UHM domain shares a binding interface with the U2AF65 RRM1 domain in addition to the known recognition of the U2AF65 ULM peptide on the helical face of the UHM domain. A structural model for the domain arrangements in the U2AF heterodimer when bound to a strong 3′ splice site Py-tract AG RNA is shown in Fig. 3F. Notably, the same interaction is already present in the absence of RNA (Fig. S6D). Thus, the RRM1/UHM domain interaction destabilizes the closed conformation of the U2AF65 RRM1,2 tandem domains and thereby promotes the population shift toward the open domain arrangement and thus contributes to an increased RNA affinity even for weak Py-tracts.
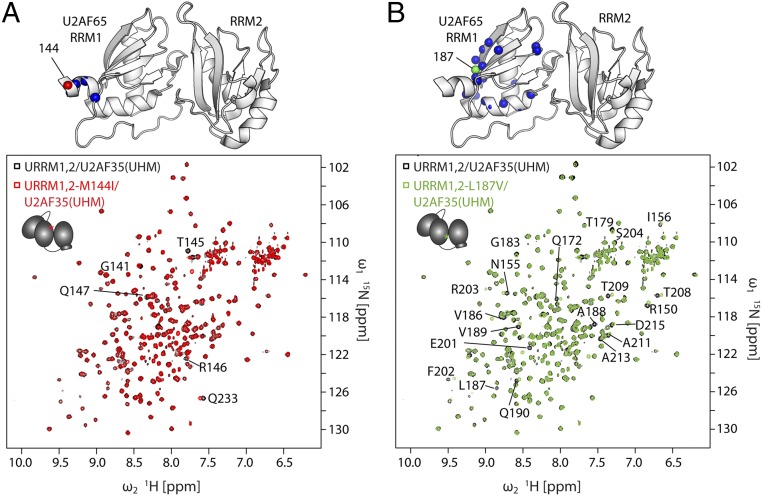
Disease-Linked Mutations in U2AF65 Do Not Affect the Structure and RNA Binding of the U2AF Heterodimer.
Yoshida et al. (39) described point mutations in several components of the splicing machinery leading to myelodysplastic syndromes. Here we investigated the effect of the confirmed somatic mutations L187V and M144I. To analyze whether these mutations could affect the structure of U2AF65 or the conformation of the heterodimer [by modulating the interface with U2AF35(UHM)], we compared NMR spectra of 15N-labeled U2AF65 in the wild-type heterodimer with URRM1,2-M144I/U2AF35(UHM) and URRM1,2-L187V/U2AF35(UHM). Analysis of the chemical shift differences shows mainly local effects in the vicinity of the mutation (Fig. 4). This demonstrates that the structure of U2AF65 RRM1,2 and the formation of the U2AF heterodimer is not affected by these disease mutations. We then tested if the mutations could affect RNA binding by the U2AF heterodimer by using isothermal titration calorimetry (ITC) (Fig. S7 A and B) and fluorescence correlation spectroscopy (FCS) (Fig. S7 C and D). We measured the binding affinities of both mutants for the strong and weak Py-tract RNAs and compared them to the wild-type heterodimer (Table S3). All three constructs showed similar RNA binding affinities with an approximately sevenfold higher RNA binding affinity in the presence of U2AF35(UHM). This demonstrates that U2AF35 still contributes to the overall RNA binding in the mutant heterodimers. We did, however, observe a more aggregation-prone behavior of the two point mutants compared with the wild type in FCS experiments. The point mutations in URRM1,2 leading to myelodysplastic syndromes, therefore, do not directly influence the binding affinity of the RNA or binding of the small subunit U2AF35. This suggests that these mutations potentially affect interactions with other factors.
Fig. 4.
NMR spectra of U2AF heterodimers formed with U2AF35(UHM) and URRM1,2 harboring the somatic point mutations (A) M144I and (B) L187V. (Top) Notable chemical shift changes for amides in U2AF65 observed for the heterodimers harboring the mutations are indicated as red spheres on RNA-bound RRM1,2 (PDB 5EV1). (Bottom) The 1H,15N-HSQC spectra of URRM1,2/U2AF35(UHM) (black), URRM1,2-M144I/U2AF35(UHM) (red), and URRM1,2-L187V/U2AF35(UHM) (green).
Fig. S7.
Effects of the somatic point mutations M144I and L187V on the binding affinity of URRM1,2 and URRM1,2/U2AF35(UHM). Binding affinity of (Left) URRM1,2/U2AF35(UHM), (Middle) URRM1,2-M144I/U2AF35(UHM), and (Right) URRM1,2-L187V/U2AF35(UHM) for (A) U13ACAGG or (B) U4A8U4ACAGG determined by isothermal titration calorimetry. (C) Binding affinity of U13-Atto488 to (Left) URRM1,2-C187-C326, (Middle) URRM1,2-M144I, and (Right) URRM1,2-L187V determined by FCS using 500 pM U13-Atto488 and increasing URRM1,2 concentrations. (D) Binding affinity of U13-Atto488 to (Left) URRM1,2/U2AF35(UHM), (Middle) URRM1,2-M144I/U2AF35(UHM), and (Right) URRM1,2-L187V/U2AF35(UHM) determined by FCS using 500 pM U13-Atto488 for increasing concentrations of URRM1,2, and 1.3 µM of U2AF35(UHM).
Table S3.
Comparison of the RNA binding affinities of the somatic point mutations U2AF65-M144I and U2AF65-L187V to the wild-type protein in URRM1,2 and URRM1,2/U2AF35(UHM)
| Protein | RNA | KD, nM |
| URRM1,2 WT/U2AF35(UHM) | U13ACAGG | 124 ± 42 |
| URRM1,2-M144I/U2AF35(UHM) | U13ACAGG | 186 ± 58 |
| URRM1,2-L187V/U2AF35(UHM) | U13ACAGG | 209 ± 60 |
| URRM1,2 WT/U2AF35(UHM) | U4A8U4ACAGG | 1960 ± 220 |
| URRM1,2-M144I/U2AF35(UHM) | U4A8U4ACAGG | 2230 ± 70 |
| URRM1,2-L187V/U2AF35(UHM) | U4A8U4ACAGG | 1970 ± 120 |
| URRM1,2-C187-C326 | U13-Atto488 | 307 ± 115 |
| URRM1,2-M144I | U13-Atto488 | 174 ± 84 |
| URRM1,2-L187V | U13-Atto488 | 321 ± 169 |
| URRM1,2-C187-C326/U2AF35(UHM) | U13-Atto488 | 55 ± 11 |
| URRM1,2-M144I/U2AF35(UHM) | U13-Atto488 | 24 ± 8 |
| URRM1,2-L187V/U2AF35(UHM) | U13-Atto488 | 35 ± 13 |
Binding affinity constants (KD) for U13ACAGG and U4A8U4ACAGG were obtained from ITC. ITC was performed using an ITC200 Microcal calorimeter (Microcal) at 25 °C. Two hundred microliters of 20 μM protein sample (20 mM sodium phosphate, pH 6.5, 50 mM NaCl, 5 mM β-mercaptoethanol) in the cell was titrated with 20 μL of 200 μM of RNA in the same buffer. The data were analyzed using MicroCal PEAQ-ITC. Binding affinities for U13-Atto488 were determined by FCS. The diffusion coefficient of 500 pM U13-Atto488 was held constant while the amplitude was fitted to determine the fraction of RNA–protein complex compared with the total number of labeled RNA in experiments with increasing concentration of the URRM1,2 constructs in the absence or presence of 1.3 µM U2AF35(UHM). A Michaelis–Menten fit was used to determine the affinity constant and the 95% confidence interval is given as the error (Fig. S7).
Discussion
RNA binding of U2AF65 is associated with an open domain configuration, whereas unbound U2AF65 represents an ensemble of closed conformations (28, 29). When analyzing its conformational dynamics, we found that U2AF65 can adopt different conformational states, dynamically switching between a closed conformation with a FRET efficiency of ∼0.96 and an open state with a FRET efficiency of ∼0.43. The free form of U2AF65 is highly dynamic on the submillisecond time scale spending ∼1/3 of the time in the open conformation (Fig. 2E). This is fully consistent with the NMR spectra, which show a single set of signals, indicating conformational averaging that is fast on the NMR chemical shift time scale (i.e., less than milliseconds). We do not observe conformational dynamics on time scales longer than 30 ms. Agrawal et al. (38) described conformational dynamics of U2AF65 on the time scale of 5–30 s. Although the different types of spFRET measurements reveal different dynamic time scales, they consistently show more dynamics in the absence of RNA, and the open conformation of U2AF65 is stabilized in the presence of a strong Py-tract.
High-affinity Py-tracts strongly stabilize the open, active conformation of the complex. This effect is reduced for weak Py-tract sequences. We found a correlation between the dynamic equilibrium between the open and closed conformation and the binding affinity of the complexes (Fig. 2E). An increase in the population of the open state corresponds to a higher probability for prespliceosomal complex formation (28) because the open conformation is the active form of U2AF65 required for spliceosome assembly.
Alternative splicing occurs in 90% of all human genes and is extremely important for the high level of phenotypic complexity in mammals (26, 27). Py-tracts of higher eukaryotes contain only 50% uridines, whereas 30% of the nucleotides are cytosines and 10% adenines and guanines, each (40). Moreover, although the binding site in U2AF65 and the consensus length of Py-tracts corresponds to eight pyrimidines (28), Py-tracts found in natural 3′ splice site sequences can span 30 or more nucleotides. U2AF65 therefore has to be able to recognize a variety of Py-tract sequences and lengths (6), while at the same time being able to specifically and unambiguously identify bona fide splice sites. The splicing efficiency depends proportionally on the number of consecutive uridines in the sequence (7, 22). We observed a similar trend when analyzing the dynamics of U2AF65 between its open and its closed conformation. The equilibrium of U2AF65 shifts toward the open conformation with increasing Py-tract strength. The presence of additional residues flanking RRM1 in URRM1,2 and the binding of U2AF35 also shift the equilibrium of the complex toward the open conformation (Fig. 2E). The additional amino acids present in URRM1,2 compared with RRM1,2 enhance the RNA binding affinity (Table S2), consistent with a recent study that indicates that N- and C-terminally flanked regions in U2AF65 RRM1,2 mediate additional RNA contacts (38).
An important finding of our study is that the open conformation is further stabilized by binding of the small subunit U2AF35 (Fig. 2E). Our NMR data show that this involves an interaction between the U2AF35 UHM domain and U2AF65 RRM1 (Fig. 3). The shift in equilibrium is already induced in the absence of RNA and thus greatly enhances the overall affinity for weak Py-tracts. This is consistent with the requirement of U2AF35 for splicing of introns with weak 3′ splice sites and for viability in eukaryotes (18, 41, 42), whereas AG-independent introns exhibit strong Py-tract sequences, for which U2AF35 is not necessarily required (7, 23, 25). However, the molecular details for the recognition of weak 3′ splice sites remain poorly understood. Recent reports indicate that the zinc fingers flanking the U2AF35(UHM) domain mediate direct recognition of the 3′ splice site AG dinucleotide (20), consistent with the fact that the U2AF35(UHM) does not contribute to RNA binding in the context of a minimal heterodimer [U2AF65(RRM1,2)/U2AF35(UHM)] lacking the zinc finger domains (37). Nevertheless, our data demonstrate that the small subunit U2AF35(UHM) allosterically enhances the interaction of U2AF65 with weak Py-tract RNA sequences by inducing a population shift toward the open conformation already in the absence of RNA. This unexpected finding also implies an indirect role for the UHM domain for 3′ splice site recognition, which will enhance the recognition of weak Py-tracts by stabilization the open conformation of U2AF65 RRM1,2 (Fig. 5).
Fig. 5.
Summary of the conformational changes observed in U2AF65 RRM1,2 and the U2AF heterodimer upon binding to strong and weak Py-tract RNAs.
It is important to note that the population shifts involving the RNA binding domains of U2AF for 3′ splice site recognition are not affected by the presence of SF1 and its binding to the BPS sequence. Comparison of NMR spectra of U2AF65, the U2AF heterodimer, and U2AF65-SF1 complexes demonstrates that the conformations and conformational arrangements coupled with RNA binding of U2AF are not affected by the presence of SF1 (43, 44). Thus, although 3′ splice site recognition involves cooperative binding of SF1, U2AF65, and U2AF35, the fine-tuning and regulation of the assembly depends on the variability in the Py-tract and its recognition by population shifts involving the RNA binding domains of the U2AF heterodimer. It will be interesting to analyze how additional factors, such as hnRNP A1 (45) or phosphorylation of SF1 (44, 46, 47), influence 3′ splice recognition by U2AF.
In summary, we provide insight into the underlying molecular mechanism of U2AF binding to 3′ splice sites. An emerging paradigm resulting from the current and our previous study (28) is that the high splicing efficiency is introduced by a stabilization of the open conformation of U2AF65. Because the open conformation is significantly populated already in the absence of RNA, protein–RNA complex formation uses a conformational selection mechanism. Py-tracts with varying strength correlate with splicing efficiencies and are reflected in the equilibrium between the two conformational states (this work and ref. 28). Nevertheless, depending on the Py-tract strength, an initial RNA binding to RRM2 in the closed state with a subsequent conformational change may contribute to complex formation. This would allow fly-casting of the RRM1 domain to scan the RNA and identify Py-tracts in the context of introns with extended Py-tract regions (28). Most importantly, our data imply that the population shift induced by the U2AF35 UHM domain, combined with direct recognition of the AG-dinucleotide by the U2AF35 zinc fingers, contributes to the fidelity of 3′ splice site recognition by U2AF as an essential early step in spliceosome assembly.
Methods
Plasmids and Constructs.
The human RRM1,2 (U2AF65-148-342) minimal RNA binding mutant, RRM1,2-Δ233–252, URRM1,2 (U2AF65-88-342), and U2AF35(UHM) [U2AF35(38-152)] were cloned by PCR amplification (Fig. S1A). The constructs were inserted into a pETM11 (U2AF65) or pET9d (U2AF35) vector containing an N-terminal 6xHis-tag (28). Double cysteines were introduced into U2AF65 constructs by site-directed mutagenesis at positions C187/C318 or C187/C326.
Expression and Purification of Recombinant Proteins.
U2AF65 mutants were expressed in Escherichia coli BL21(DE3) and purified as described in Mackereth et al. (28). For the U2AF heterodimer, URRM1,2 and U2AF35(38-152) were expressed and purified separately, incubated at equimolar ratios, and purified as a complex by size-exclusion chromatography as described in Kellenberger et al. (11).
Single-Pair FRET Measurements.
Single-pair FRET measurements were performed on a custom-build confocal microscope using multiparameter fluorescence detection with pulsed interleaved excitation (SI Text) (32). Proteins were site-specifically labeled at cysteines and diluted to concentrations of 20–50 pM in 20 mM potassium phosphate (pH 6.5), 50 mM NaCl. Measurements were performed with proteins in their free form or mixed with 5 µM of U9, U13ACAGG, A9, or A13ACAGG or 20 µM of U4A8U4, U4A8U4ACAGG, or U4A8U5ACAGG.
NMR Spectroscopy.
All NMR samples contained protein concentrations of 0.1–0.5 mM in 20 mM sodium phosphate (pH 6.5), 50 mM NaCl, 2 mM DTT, and 5–10% (vol/vol) 2H2O. Spectra were recorded at 295 K and analyzed using Sparky 3 in combination with previously published assignments (28).
SI Text
Truncation of the Linker Region Between RRM1 and RRM2 Does Not Inhibit the Conformational Changes and Dynamics of U2AF65.
We investigated the effect of the flexible linker between the two RNA binding motifs using a linker truncation mutant that was previously proposed to increase the population of the open domain arrangement (28). Amino acids 233–252 in the linker connecting both domains (residues 227–261) were deleted (RRM1,2-Δ233–252), leaving only 15 amino acids from the outer edges of the linker (Fig. S1A). A similar linker truncation of residues 238–257 has been used in a crystallographic study of the U2AF65 RNA interaction (30). Even though a large fraction of the linker region was missing, the protein still switched dynamically between an open and a closed form (Fig. S3B). Again, by combining the lifetime information for all of the individual molecules measured, we could accurately determine the donor lifetimes for the closed and open conformations, which lie at 0.1 and 1.4 ns, respectively (Table S1). This corresponds to FRET efficiencies of 0.62 (D = 59 Å) and 0.97 (D = 36 Å) for the fully open and the fully closed states, respectively. The open conformation of RRM1,2-Δ233–252 clearly deviates from the open conformation of the RRM1,2 with the full linker by ∼8 Å (i.e., 67 Å). Hence, the full-length linker is necessary for the protein to adopt the native open state that is found in the U9 bound form. On the other hand, for the fully closed conformation, we observed the same FRET efficiency as for RRM1,2 (∼0.96), which may suggest that the same closed state can be adopted also with the shortened linker.
In the presence of U9 or U4A8U4 RNA, dynamics between the same open and closed conformations was also observed. As for RRM1,2 with the wild-type linker, we found a shift of the equilibrium towards the open state from the highly dynamic free form of RRM1,2-Δ233–252 (22% open) via the weak U4A8U4-bound form (57% open) to the U9-bound conformation (72% open) (Table S1 and Fig. S3B). The fraction of molecules remaining in the closed population in the presence of RNA might be attributable to proteins that have not bound an RNA. This suggests that the native open domain arrangement cannot be adopted with the linker deletion. Alternatively, the shortened linker may restrict the mobility of RRM1, which has an intrinsically lower RNA binding affinity (28) to search for its RNA binding site, when RRM2 has already bound to the Py-tract.
SI Methods
Protein Labeling.
Attachment of fluorophores to U2AF65(RRM1,2) constructs was performed using sulfhydryl–maleimide coupling. RRM1,2-C187-C318 and RRM1,2-C187-C326 were reduced in 20 mM potassium phosphate (pH 6.5) and 50 mM NaCl containing 1 mM DTT. After removal of the reducing agent, the labeling reaction was performed by the addition of twofold to threefold molar excess of fluorophore–maleimide conjugates (Atto532-Maleimide, Atto647N-Maleimide, Alexa647-Maleimide, Cy5-Maleimide, and Atto565-Maleimide; AttoTec and Life Technologies) in 20 mM potassium phosphate (pH 6.8), 50 mM NaCl, 50 µM TCEP, deprived of oxygen at room temperature for 2 h. The attachment of fluorophores to the protein was confirmed on a denaturing polyacrylamide gel using UV excitation. Unbound fluorophores were removed by a size-dependent filtration with 20 mM potassium phosphate (pH 6.5) and 50 mM NaCl. FCS measurements validated the labeling and removal of unbound fluorophores.
Solution-Based Single-Pair FRET Measurements.
spFRET measurements were performed on a custom-build confocal microscope (32). In solution-based spFRET measurements, the FRET efficiency of a single molecule is determined as it diffuses through the focus of the laser, which occurs on the time scale of a couple of milliseconds. The concentration of labeled sample is in the range of 10s of pM to ensure that only one molecule is in the focus of the microscope at a time (one to five fluorescent bursts per second).
The fluorophores were excited using pulsed interleaved excitation (PIE) (31) with a 532-nm (PicoTA 530; Picoquant) and a 640-nm subnanosecond pulsed laser (LDH-d-C640; Picoquant) synchronized at a repetition rate of 26.66 MHz with a delay of 18 ns and a laser power of 100 µW each (measured at the entrance point of the beam into the objective). Fluorescence from 20 to 50 pM fluorescently labeled U2AF in the absence and presence of 5 µM U9, U13ACAGG, A9, or A13ACAGG or 20 µM U4A8U4, U4A8U4ACAGG, or U4A8U5ACAGG in 20 mM potassium phosphate (pH 6.5) and 50 mM NaCl was collected by a 60× water immersion objective (Plan Apo IR 60×/1.27 WI; Nikon) and focused onto a pinhole with diameter d = 75 µm. Multiparameter fluorescence detection (MFD) was used where the fluorescence is first separated according to polarization using a polarizing beam-splitter (PBS3; Thorlabs). The green fluorescence was then reflected and the red fluorescence was transmitted by a dichroic mirror (640DCXR; AHF Analysentechnik). After passing the emission filters (Brightline HQ582/75 and HQ700/75 for green and red photons, respectively; AHF Analysentechnik) the signals were detected on four single-photon-counting avalanche photodiodes (Perkin–Elmer). The data were recorded using synchronized single-photon-counting cards (SPC-154; Becker and Hickl), and data analysis was performed with a custom-written MATLAB program (The MathWorks).
For the fluorophore combinations Atto565/Atto647N and Atto565/Alexa647, a similar confocal microscope was used with the laser excitation lines 480 nm (LDH-D-C-485), 565 nm (LDH-D-TA-560), and 641 nm (LDH-D-C-640) (Picoquant). Fluorescence was separated by a polychroic mirror (zt405/488/561/633) and filtered by the emission filters for red (ET670/30) and green (ET607/36) (AHF Analysentechnik) detection.
Analysis of Fluorescence Parameters from Solution-Based spFRET Measurements.
Using MFD-PIE, multiple parameters of each molecule, such as its FRET efficiency, stoichiometry, fluorescence lifetime, and fluorescence anisotropy, can be obtained. Photons arising from individual molecules were selected by an all-photon or a dual-channel burst search algorithm. From the photons originating from a single molecule, each of the above parameters was calculated (48, 49).
Photons were considered to belong to a burst when the local count rate obtained by a sliding time window exceeded a certain threshold. Bursts with a minimum number of 100 photons and a count rate of at least 10 photons in a time window of 500 µs after green and red excitation were considered for further analysis. One advantage of using PIE is that dual-labeled molecules can be directly selected by the burst search algorithm (dual-channel burst search). Additionally, we used the stoichiometry information to further refine the selection of dual-labeled molecules. The stoichiometry is defined as in ref. 34:
| [S1] |
where FDD and FDA are the fluorescence intensity of the donor and acceptor channels after donor excitation, respectively, and FAA is the background corrected fluorescence intensity of the acceptor channel after acceptor excitation. Via this ratio, the stoichiometry provides information regarding the labeling of the molecules. The stoichiometry value can also be normalized to allow better comparison between experiments:
| [S2] |
where the additional factors correct for direct excitation (α), cross-talk (β), and differences in detection efficiency and quantum yield (γ). Dual-labeled molecules were selected for analysis using a normalized stoichiometry value between 0.3 and 0.8. To remove molecules that underwent photobleaching during a burst, we compared the mean time of burst detection using green excitation with that for red excitation (−0.1 ms < TDD + DA − TAA < 0.1 ms). FRET efficiencies were calculated following Eq. S3 and corrected for background (BGDD = 1.1 kHz, BGDA = 0.4 kHz, and BGAA = 0.5 kHz), cross-talk (∼0.03), direct excitation (∼0.1), and differences in detection efficiency (γ = 0.85 for Atto647N and γ = 0.45 for Alexa647 as acceptor fluorophore):
| [S3] |
The exact correction factors were determined for each measurement individually by analysis of the donor- and acceptor-only populations and background measurements with water and buffer (32). Around 30% of all molecules fulfilled all of the above mentioned selection criteria. The fluorescence lifetime and anisotropy were calculated as discussed in Schaffer et al. (50). These two parameters were used to obtain information about the behavior of the fluorophores attached to the protein of interest. We can thus correct for fluorophore quenching of the acceptor molecule using the correction factor γ. To validate the assumption that the orientation factor κ2 can be estimated as 2/3 for the donor and acceptor fluorophores bound to the protein, we used the rotational correlation time of the fluorophores obtained from the anisotropy information.
The relation of the intensity-weighted donor fluorescence lifetime and the species-weighted FRET efficiency contains information about the conformational dynamics of individual molecules on the time scale of the diffusion through the focal volume. In the absence of dynamics, the FRET efficiency is related to the donor lifetime by
| [S4] |
and should yield the same value as the intensity-determined FRET efficiency (Eq. S3). The static FRET relationship can be improved to account for dynamics of the linkers attaching the fluorophores to the molecule of interest using an empirically determined third-order polynomial (following ref. 32):
| [S5] |
For the data presented here, the static FRET curve is given by τD = 3.8 ns and a = −0.0421, b = 0.508, c = 0.2691, and d = 0.03589 for the donor–acceptor pair Atto532–Atto647N and a = −0.0178, b = 0.6226, c = 0.2188, and d = 0.0312 for Atto532–Alexa647 (32, 51). Whenever molecules showed dynamic conversions during their transit time in the confocal volume, the intensity-determined FRET efficiency will be a species-weighted value:
| [S6] |
where xi describes the fraction of time during the burst that the molecule was in the ith state. Because only a single lifetime can be calculated from a burst, the determined donor lifetime will be an intensity-weighted lifetime, and the calculated FRET efficiency is given by the dynamic FRET curve:
| [S7] |
where τ1 and τ2 are the respective donor lifetimes of both FRET states.
To determine the FRET efficiencies of the closed and open conformations, we used the cumulative fluorescence lifetime information for all bursts. The lifetime histogram was then fit to a biexponential decay function (Fig. S2B). From the fluorescence lifetimes, the FRET efficiencies and thus distances could be calculated. To visualize the closed and open conformation, we used MFD-PIE experiments of two constructs filtered by the donor lifetime to exclude any potential fluctuations. For the closed conformation, we used an MFD-PIE experiment of RRM1,2 molecules with donor lifetimes τD < 0.1 ns, and to display the open state, the spFRET histogram of URRM1,2/U2AF35(UHM) complexes in the presence of U13ACAGG with τD > 0.1 ns was used.
Single-Pair FRET Measurements of Immobilized Molecules.
Surface-based single-pair FRET measurements were performed on a custom-build prism-type total internal reflection microscope. Preparation of the prism was performed as described in Schluesche et al. (52). Surface passivation was achieved by incubating the silanized (3-aminopropyl-triethoxysilane; Sigma-Aldrich) prisms with a solution of 45% polyethylene glycol (mPEG-SVA, MW 5000) and 3% biotin-PEG (biotin-PEG-SVA, MW 5000) (Laysan Bio Inc.) in 0.1 M sodium bicarbonate (pH 9.0). The quartz prism was used to serve as part of the sample chamber as well as to create an angle of total internal reflection. Excitation of the fluorophores was achieved by illumination of the prism by a diode-pumped solid state laser (Cobolt Samba 100, 532 nm) and a helium–neon laser (Polytec Laser 633 nm). An acousto-optical tunable filter (AOTFnC.400–650-PV-TN; Pegasus Optik) combined with a single-mode polarization maintaining fiber was used for switching and intensity regulation of the lasers. Fluorescence of the sample was collected by a water immersion objective (CFI Plan Apochromat 60xWI, NA 1.2; Nikon) and separated by a dichroic mirror (630DCXR; AHF Analysentechnik). After passing the emission filters HQ550/88 or HQ715/150 (AHF Analysentechnik) for green and red detection, respectively, the fluorescence of the donor and acceptor fluorophores was detected on two different regions of an EMCCD camera (Andor iXON; Andor Technology). Measurements were performed either by directly immobilizing RNA or by encapsulation of the protein complex in lipid vesicles.
Determination of Dissociation Rates of U2AF65 from Immobilized RNA.
We used spFRET TIRF experiments with immobilized biotinylated RNA to determine the lifetime of the RNA–U2AF65 complex. To avoid surface effects on the protein, we used a biotinylated RNA, which was extended by a double-stranded RNA to introduce a separation of the protein from the surface. The U2AF65-binding RNA strand (biotin-5′-UGACCCAAGGGCAGUUUUUUUUU-3′) was annealed to the partly complementary strand (5′-CUGCCCUUGGGUCA-3′) at a concentration of 100 µM in annealing buffer (10 mM Tris, 100 mM NaCl, 1 mM EDTA, pH 7.5) by denaturation at 62 °C and cooling at −0.6 °C/min. Before immobilization, the biotinylated RNA was incubated with the U2AF65 mutant in a 1:1 ratio at a concentration of 4 µM. It was then diluted to a final measurement concentration of 50 pM and flown through the sample chamber. The laser power was chosen such that the bleaching rate of the fluorophores was significantly lower than the time for dissociation. The half-life of the complexes was determined by detecting the residence time of dual-labeled U2AF65 on the surface. The residence time was described by a single-exponential fit, which returned the dissociation rate of U2AF65. From this value, the association rate was determined for mutants with known affinity constants.
Conformational Dynamics of Immobilized Molecules.
To analyze conformational dynamics on the time scale of seconds, U2AF65 was encapsulated into lipid vesicles. Lipid vesicles made from DOPC and 3% biotinylated PE were prepared as described in Sikor et al. (53). Lipids were hydrated with 20 mM potassium phosphate (pH 6.5), 50 mM NaCl containing 400 nM of the respective U2AF65 mutant and 5 µM U9 or 20 µM U4A8U4 RNA. Liposomes with a diameter of 200 nm were prepared by extrusion. The biotinylated vesicles or RNA complexes were immobilized via biotin–streptavidin–biotin linkage on the surface of the quartz prisms (53).
Signal from single molecules was only analyzed when single bleaching steps were observed. Individual proteins were manually selected and evaluated for dynamics. No significant population of dynamic molecules were observed on the time scale of 30 ms or longer.
Accessible Volume Calculations.
The accessible volumes of the fluorophores were calculated on the structures of RRM1,2 (PDB: 2YH0) and U8-bound RRM1,2 (PDB: 2YH1). We used the FRET positioning software described by Kalinin et al. (35) to determine the accessible volumes of the fluorophores with linker length (20 Å), linker width (4.5 Å), and radius of the fluorophore (3.5 Å).
NMR Spectroscopy.
All of the final NMR samples contained protein concentrations of 0.1–0.5 mM in 20 mM sodium phosphate (pH 6.5), 50 mM NaCl, 2 mM DTT, and 5–10% (vol/vol) 2H2O. Spectra were recorded at 295 K using AV III 600 MHz, AV III 750 MHz, or AV III 800 MHz Bruker spectrometers, equipped with cryogenic triple resonance gradient probes. All of the backbone assignments were obtained using typical triple resonance experiments (HNCA, HNCACB). Spectra were analyzed using Sparky 3 (T. D. Goddard and D. G. Kneller, University of California), in combination with the previously published assignments (28). The 15N-relaxation T1/T2 curves were analyzed and fitted using NMRview v.4 (54) and subsequently converted to the rotational correlation time by
| [S8] |
with η for solvent viscosity, r for effective radius of protein, κ for Boltzmann constant, T for temperature, and νN and 15N resonance frequency). PRE measurements were performed on the U2AF heterodimer with and without RNA (U13ACAGG) by incorporating free radical 3-(2-Iodoacetamido)-PROXYL spin labels to the individually engineered cysteines (C155, C196, C187, and C271 in U2AF65 and C87, C102, C131, and C145 in U2AF35). To obtain the ratio of the peak intensities, , 1H,15N-HSQCs were recorded before (paramagnetic) and after (diamagnetic) adding ascorbic acid, following the protocols described previously (55, 56).
Acknowledgments
We thank W. Kügel for providing the burst analysis software, and W. Schrimpf and J. Valcarcel for valuable discussions. We are grateful to Y. Zhang for constructs used in preliminary experiments and G. Demiraslan for technical assistance. We gratefully acknowledge the financial support of the Deutsche Forschungsgemeinschaft through Grants SFB1035 (Projects A11, B03) and GRK1721, and support from the Ludwig-Maximilians-Universität through the Center for NanoScience and the BioImaging Network. T.M. was supported by the Bavarian Ministry of Sciences, Research and the Arts (Bavarian Molecular Biosystems Research Network), the German Research Foundation (Emmy Noether Program MA 5703/1-1), and the Austrian Science Fund (FWF; Grants P28854 and DK-MCD W1226). L.R.W. acknowledges a Long-Term EMBO postdoctoral fellowship (Grant ALTF 1520-2011).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. G.V. is a Guest Editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1605873113/-/DCSupplemental.
References
- 1.Will CL, Lührmann R. Spliceosome structure and function. Cold Spring Harb Perspect Biol. 2011;3(7):a003707. doi: 10.1101/cshperspect.a003707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berglund JA, Abovich N, Rosbash M. A cooperative interaction between U2AF65 and mBBP/SF1 facilitates branchpoint region recognition. Genes Dev. 1998;12(6):858–867. doi: 10.1101/gad.12.6.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berglund JA, Chua K, Abovich N, Reed R, Rosbash M. The splicing factor BBP interacts specifically with the pre-mRNA branchpoint sequence UACUAAC. Cell. 1997;89(5):781–787. doi: 10.1016/s0092-8674(00)80261-5. [DOI] [PubMed] [Google Scholar]
- 4.Liu Z, et al. Structural basis for recognition of the intron branch site RNA by splicing factor 1. Science. 2001;294(5544):1098–1102. doi: 10.1126/science.1064719. [DOI] [PubMed] [Google Scholar]
- 5.Rain JC, Rafi Z, Rhani Z, Legrain P, Krämer A. Conservation of functional domains involved in RNA binding and protein-protein interactions in human and Saccharomyces cerevisiae pre-mRNA splicing factor SF1. RNA. 1998;4(5):551–565. doi: 10.1017/s1355838298980335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banerjee H, Rahn A, Davis W, Singh R. Sex lethal and U2 small nuclear ribonucleoprotein auxiliary factor (U2AF65) recognize polypyrimidine tracts using multiple modes of binding. RNA. 2003;9(1):88–99. doi: 10.1261/rna.2131603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reed R. The organization of 3′ splice-site sequences in mammalian introns. Genes Dev. 1989;3(12B):2113–2123. doi: 10.1101/gad.3.12b.2113. [DOI] [PubMed] [Google Scholar]
- 8.Singh R, Valcárcel J, Green MR. Distinct binding specificities and functions of higher eukaryotic polypyrimidine tract-binding proteins. Science. 1995;268(5214):1173–1176. doi: 10.1126/science.7761834. [DOI] [PubMed] [Google Scholar]
- 9.Zamore PD, Patton JG, Green MR. Cloning and domain structure of the mammalian splicing factor U2AF. Nature. 1992;355(6361):609–614. doi: 10.1038/355609a0. [DOI] [PubMed] [Google Scholar]
- 10.Moore MJ. Intron recognition comes of AGe. Nat Struct Biol. 2000;7(1):14–16. doi: 10.1038/71207. [DOI] [PubMed] [Google Scholar]
- 11.Kellenberger E, Stier G, Sattler M. Induced folding of the U2AF35 RRM upon binding to U2AF65. FEBS Lett. 2002;528(1-3):171–176. doi: 10.1016/s0014-5793(02)03294-5. [DOI] [PubMed] [Google Scholar]
- 12.Mollet I, Barbosa-Morais NL, Andrade J, Carmo-Fonseca M. Diversity of human U2AF splicing factors. FEBS J. 2006;273(21):4807–4816. doi: 10.1111/j.1742-4658.2006.05502.x. [DOI] [PubMed] [Google Scholar]
- 13.Kielkopf CL, Rodionova NA, Green MR, Burley SK. A novel peptide recognition mode revealed by the X-ray structure of a core U2AF35/U2AF65 heterodimer. Cell. 2001;106(5):595–605. doi: 10.1016/s0092-8674(01)00480-9. [DOI] [PubMed] [Google Scholar]
- 14.Corsini L, et al. U2AF-homology motif interactions are required for alternative splicing regulation by SPF45. Nat Struct Mol Biol. 2007;14(7):620–629. doi: 10.1038/nsmb1260. [DOI] [PubMed] [Google Scholar]
- 15.Zuo P, Maniatis T. The splicing factor U2AF35 mediates critical protein-protein interactions in constitutive and enhancer-dependent splicing. Genes Dev. 1996;10(11):1356–1368. doi: 10.1101/gad.10.11.1356. [DOI] [PubMed] [Google Scholar]
- 16.Rudner DZ, Kanaar R, Breger KS, Rio DC. Interaction between subunits of heterodimeric splicing factor U2AF is essential in vivo. Mol Cell Biol. 1998;18(4):1765–1773. doi: 10.1128/mcb.18.4.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu S, Romfo CM, Nilsen TW, Green MR. Functional recognition of the 3′ splice site AG by the splicing factor U2AF35. Nature. 1999;402(6763):832–835. doi: 10.1038/45590. [DOI] [PubMed] [Google Scholar]
- 18.Zorio DA, Blumenthal T. U2AF35 is encoded by an essential gene clustered in an operon with RRM/cyclophilin in Caenorhabditis elegans. RNA. 1999;5(4):487–494. doi: 10.1017/s1355838299982225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merendino L, Guth S, Bilbao D, Martínez C, Valcárcel J. Inhibition of msl-2 splicing by Sex-lethal reveals interaction between U2AF35 and the 3′ splice site AG. Nature. 1999;402(6763):838–841. doi: 10.1038/45602. [DOI] [PubMed] [Google Scholar]
- 20.Yoshida H, et al. A novel 3′ splice site recognition by the two zinc fingers in the U2AF small subunit. Genes Dev. 2015;29(15):1649–1660. doi: 10.1101/gad.267104.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson JM, et al. Genome-wide survey of human alternative pre-mRNA splicing with exon junction microarrays. Science. 2003;302(5653):2141–2144. doi: 10.1126/science.1090100. [DOI] [PubMed] [Google Scholar]
- 22.Coolidge CJ, Seely RJ, Patton JG. Functional analysis of the polypyrimidine tract in pre-mRNA splicing. Nucleic Acids Res. 1997;25(4):888–896. doi: 10.1093/nar/25.4.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guth S, Martínez C, Gaur RK, Valcárcel J. Evidence for substrate-specific requirement of the splicing factor U2AF(35) and for its function after polypyrimidine tract recognition by U2AF(65) Mol Cell Biol. 1999;19(12):8263–8271. doi: 10.1128/mcb.19.12.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banerjee H, et al. The conserved RNA recognition motif 3 of U2 snRNA auxiliary factor (U2AF 65) is essential in vivo but dispensable for activity in vitro. RNA. 2004;10(2):240–253. doi: 10.1261/rna.5153204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pacheco TR, Coelho MB, Desterro JM, Mollet I, Carmo-Fonseca M. In vivo requirement of the small subunit of U2AF for recognition of a weak 3′ splice site. Mol Cell Biol. 2006;26(21):8183–8190. doi: 10.1128/MCB.00350-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang ET, et al. Alternative isoform regulation in human tissue transcriptomes. Nature. 2008;456(7221):470–476. doi: 10.1038/nature07509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim E, Magen A, Ast G. Different levels of alternative splicing among eukaryotes. Nucleic Acids Res. 2007;35(1):125–131. doi: 10.1093/nar/gkl924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mackereth CD, et al. Multi-domain conformational selection underlies pre-mRNA splicing regulation by U2AF. Nature. 2011;475(7356):408–411. doi: 10.1038/nature10171. [DOI] [PubMed] [Google Scholar]
- 29.Huang JR, et al. Transient electrostatic interactions dominate the conformational equilibrium sampled by multidomain splicing factor U2AF65: A combined NMR and SAXS study. J Am Chem Soc. 2014;136(19):7068–7076. doi: 10.1021/ja502030n. [DOI] [PubMed] [Google Scholar]
- 30.Sickmier EA, et al. Structural basis for polypyrimidine tract recognition by the essential pre-mRNA splicing factor U2AF65. Mol Cell. 2006;23(1):49–59. doi: 10.1016/j.molcel.2006.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Müller BK, Zaychikov E, Bräuchle C, Lamb DC. Pulsed interleaved excitation. Biophys J. 2005;89(5):3508–3522. doi: 10.1529/biophysj.105.064766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kudryavtsev V, et al. Combining MFD and PIE for accurate single-pair Förster resonance energy transfer measurements. ChemPhysChem. 2012;13(4):1060–1078. doi: 10.1002/cphc.201100822. [DOI] [PubMed] [Google Scholar]
- 33.Lee NK, et al. Accurate FRET measurements within single diffusing biomolecules using alternating-laser excitation. Biophys J. 2005;88(4):2939–2953. doi: 10.1529/biophysj.104.054114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kapanidis AN, et al. Fluorescence-aided molecule sorting: Analysis of structure and interactions by alternating-laser excitation of single molecules. Proc Natl Acad Sci USA. 2004;101(24):8936–8941. doi: 10.1073/pnas.0401690101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalinin S, Valeri A, Antonik M, Felekyan S, Seidel CAM. Detection of structural dynamics by FRET: A photon distribution and fluorescence lifetime analysis of systems with multiple states. J Phys Chem B. 2010;114(23):7983–7995. doi: 10.1021/jp102156t. [DOI] [PubMed] [Google Scholar]
- 36.Antonik M, Felekyan S, Gaiduk A, Seidel CA. Separating structural heterogeneities from stochastic variations in fluorescence resonance energy transfer distributions via photon distribution analysis. J Phys Chem B. 2006;110(13):6970–6978. doi: 10.1021/jp057257+. [DOI] [PubMed] [Google Scholar]
- 37.Soares LM, Zanier K, Mackereth C, Sattler M, Valcárcel J. Intron removal requires proofreading of U2AF/3′ splice site recognition by DEK. Science. 2006;312(5782):1961–1965. doi: 10.1126/science.1128659. [DOI] [PubMed] [Google Scholar]
- 38.Agrawal AA, et al. An extended U2AF(65)-RNA-binding domain recognizes the 3′ splice site signal. Nat Commun. 2016;7:10950. doi: 10.1038/ncomms10950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshida K, et al. Frequent pathway mutations of splicing machinery in myelodysplasia. Nature. 2011;478(7367):64–69. doi: 10.1038/nature10496. [DOI] [PubMed] [Google Scholar]
- 40.Senapathy P, Shapiro MB, Harris NL. Splice junctions, branch point sites, and exons: sequence statistics, identification, and applications to genome project. Methods Enzymol. 1990;183:252–278. doi: 10.1016/0076-6879(90)83018-5. [DOI] [PubMed] [Google Scholar]
- 41.Kanaar R, Roche SE, Beall EL, Green MR, Rio DC. The conserved pre-mRNA splicing factor U2AF from Drosophila: Requirement for viability. Science. 1993;262(5133):569–573. doi: 10.1126/science.7692602. [DOI] [PubMed] [Google Scholar]
- 42.Rudner DZ, Kanaar R, Breger KS, Rio DC. Mutations in the small subunit of the Drosophila U2AF splicing factor cause lethality and developmental defects. Proc Natl Acad Sci USA. 1996;93(19):10333–10337. doi: 10.1073/pnas.93.19.10333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mackereth CD, Simon B, Sattler M. Extending the size of protein-RNA complexes studied by nuclear magnetic resonance spectroscopy. ChemBioChem. 2005;6(9):1578–1584. doi: 10.1002/cbic.200500106. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, et al. Structure, phosphorylation and U2AF65 binding of the N-terminal domain of splicing factor 1 during 3′-splice site recognition. Nucleic Acids Res. 2013;41(2):1343–1354. doi: 10.1093/nar/gks1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tavanez JP, Madl T, Kooshapur H, Sattler M, Valcárcel J. hnRNP A1 proofreads 3′ splice site recognition by U2AF. Mol Cell. 2012;45(3):314–329. doi: 10.1016/j.molcel.2011.11.033. [DOI] [PubMed] [Google Scholar]
- 46.Manceau V, et al. Major phosphorylation of SF1 on adjacent Ser-Pro motifs enhances interaction with U2AF65. FEBS J. 2006;273(3):577–587. doi: 10.1111/j.1742-4658.2005.05091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang W, et al. Structure of phosphorylated SF1 bound to U2AF65 in an essential splicing factor complex. Structure. 2013;21(2):197–208. doi: 10.1016/j.str.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eggeling C, et al. Data registration and selective single-molecule analysis using multi-parameter fluorescence detection. J Biotechnol. 2001;86(3):163–180. doi: 10.1016/s0168-1656(00)00412-0. [DOI] [PubMed] [Google Scholar]
- 49.Nir E, et al. Shot-noise limited single-molecule FRET histograms: comparison between theory and experiments. J Phys Chem B. 2006;110(44):22103–22124. doi: 10.1021/jp063483n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schaffer J, et al. Identification of single molecules in aqueous solution by time-resolved fluorescence anisotropy. J Phys Chem A. 1999;103(3):331–336. [Google Scholar]
- 51.Gansen A, et al. Nucleosome disassembly intermediates characterized by single-molecule FRET. Proc Natl Acad Sci USA. 2009;106(36):15308–15313. doi: 10.1073/pnas.0903005106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schluesche P, Stelzer G, Piaia E, Lamb DC, Meisterernst M. NC2 mobilizes TBP on core promoter TATA boxes. Nat Struct Mol Biol. 2007;14(12):1196–1201. doi: 10.1038/nsmb1328. [DOI] [PubMed] [Google Scholar]
- 53.Sikor M, Mapa K, von Voithenberg LV, Mokranjac D, Lamb DC. Real-time observation of the conformational dynamics of mitochondrial Hsp70 by spFRET. EMBO J. 2013;32(11):1639–1649. doi: 10.1038/emboj.2013.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johnson BA, Blevins RA. NMRView: A computer program for the visualization and analysis of NMR data. J Biomol NMR. 1994;4(5):603–614. doi: 10.1007/BF00404272. [DOI] [PubMed] [Google Scholar]
- 55.Battiste JL, Wagner G. Utilization of site-directed spin labeling and high-resolution heteronuclear nuclear magnetic resonance for global fold determination of large proteins with limited nuclear overhauser effect data. Biochemistry. 2000;39(18):5355–5365. doi: 10.1021/bi000060h. [DOI] [PubMed] [Google Scholar]
- 56.Simon B, Madl T, Mackereth CD, Nilges M, Sattler M. An efficient protocol for NMR-spectroscopy-based structure determination of protein complexes in solution. Angew Chem Int Ed Engl. 2010;49(11):1967–1970. doi: 10.1002/anie.200906147. [DOI] [PubMed] [Google Scholar]