Significance
The Wnt pathway is implicated in multiple cancers, but to date no pharmacologically acceptable Wnt inhibitors have been introduced into the clinic. Analogs of the natural product prodigiosin are in early leukemia trials, but their mechanisms of action have not been established. We report that prodigiosin and its analog obatoclax block Wnt signaling at nanomolar concentrations by preventing the phosphorylation of Dishevelled. Cyclin D is an established target of Wnt signaling, and elevated cyclin D levels are characteristic of advanced breast cancer. In a Wnt-driven murine transgenic model of breast cancer, prodigiosin potently diminished cyclin D levels and blocked the growth of tumors. These results provide a rationale for the introduction of prodigiosin analogs into clinical trials of advanced breast cancer.
Keywords: prodigiosin, Wnt/beta-catenin signaling, breast cancer, LRP6, Dishevelled (DVL)
Abstract
Prodigiosin, a natural red pigment produced by numerous bacterial species, has exhibited promising anticancer activity; however, the molecular mechanisms of action of prodigiosin on malignant cells remain unclear. Aberrant activation of the Wnt/β-catenin signaling cascade is associated with numerous human cancers. In this study, we identified prodigiosin as a potent inhibitor of the Wnt/β-catenin pathway. Prodigiosin blocked Wnt/β-catenin signaling by targeting multiple sites of this pathway, including the low-density lipoprotein-receptor-related protein (LRP) 6, Dishevelled (DVL), and glycogen synthase kinase-3β (GSK3β). In breast cancer MDA-MB-231 and MDA-MB-468 cells, nanomolar concentrations of prodigiosin decreased phosphorylation of LRP6, DVL2, and GSK3β and suppressed β-catenin–stimulated Wnt target gene expression, including expression of cyclin D1. In MDA-MB-231 breast cancer xenografts and MMTV-Wnt1 transgenic mice, administration of prodigiosin slowed tumor progression and reduced the expression of phosphorylated LRP6, phosphorylated and unphosphorylated DVL2, Ser9 phosphorylated GSK3β, active β-catenin, and cyclin D1. Through its ability to inhibit Wnt/β-catenin signaling and reduce cyclin D1 levels, prodigiosin could have therapeutic activity in advanced breast cancers.
The Wnt/β-catenin signaling pathway plays critical roles in development and tissue homeostasis. Activation of the Wnt pathway by mutations, epigenetic changes, or cells in the tumor microenvironment contributes to the initiation and progression of various human cancers, including breast cancer (1–4). β-catenin is a key component of the Wnt signaling cascade and functions as a coactivator for transcription factors of the T-cell factor/lymphoid-enhancing factor (TCF/LEF) family. The protein level and activity of β-catenin are tightly controlled by a destruction complex composed of the scaffolding protein AXIN, the adenomatosis polyposis coli (APC) protein, and the enzyme glycogen synthase kinase-3β (GSK3β) (1). The destruction complex can phosphorylate β-catenin through GSK3β and promote β-catenin degradation by a ubiquitination-proteasome pathway. The canonical Wnt signaling cascade is initiated by the binding of secreted Wnt proteins to a receptor complex consisting of a member of the Frizzled (FZD) family and the low-density-lipoprotein receptor-related protein (LRP) 5 or 6. Subsequently, the adaptor protein Dishevelled (DVL) is phosphorylated, which increases the interaction between DVL and AXIN and inhibits GSK3β enzymatic activity within the destruction complex. This action promotes unphosphorylated β-catenin accumulation and translocation into the nucleus, where it interacts with TCF/LEF transcription factors to activate transcription of Wnt target genes, including cyclin D1 (CCND1), LEF1, and fibronectin (FN1) (5–7).
Prodigiosin, a red pigment produced by bacteria as a bioactive secondary metabolite, displays numerous biological activities, including antibacterial, antifungal, antiprotozoal, antimalarial, immunosuppressive, and anticancer properties (8–13). The prodigiosin analog obatoclax is currently in clinical trials in patients with cancer (14, 15). Prodigiosin has exhibited potent cytotoxic activity against human cancer cell lines with multidrug resistance phenotypes or defects in apoptotic pathways, with much less toxicity to normal cells (12, 13, 16). The specific mechanisms of prodigiosin action in malignant cells have been poorly elucidated, however. In breast cancer cells, pharmacologic inhibitors of GSK3β have been reported to prevent prodigiosin toxicity (17). Inhibitors of GSK3β promote β-catenin–dependent Wnt pathway activation. These results prompted us to assess the effects of prodigiosin on Wnt/β-catenin signaling. Our experiments demonstrate that prodigiosin is indeed a potent Wnt/β-catenin signaling antagonist that blocks the phosphorylation of LRP6 and DVL2, and activates GSK3β in vitro and in vivo.
Results
Inhibition of Wnt/β-Catenin Signaling by Prodigiosin.
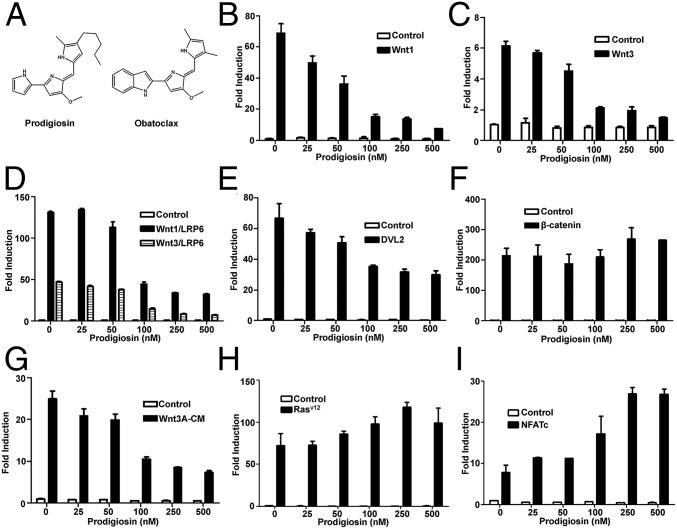
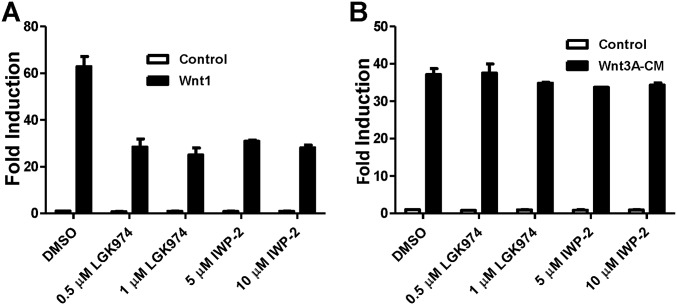
Using the cell-based TOPFlash reporter system (18), we performed an initial screen of known drugs and identified prodigiosin as an inhibitor of the Wnt/β-catenin signaling pathway. To further confirm the Wnt inhibitory effect of prodigiosin, HEK293T cells were transfected with a SuperTopFlash reporter plasmid together with Wnt1, Wnt3, Wnt1/LRP6, or Wnt3/LRP6, DVL2, or β-catenin expression plasmids (Fig. 1 B–F). Treatment with 25–500 nM prodigiosin dose-dependently blocked Wnt signaling activated by Wnt1 (Fig. 1B), Wnt3 (Fig. 1C), Wnt1/LRP6, Wnt3/LRP6 (Fig. 1D), and DVL2 (Fig. 1E). Wnt3A-conditioned medium (Wnt3A-CM) was also used to induce transcriptional activity of the SuperTopFlash reporter, and increased its activity by ∼25-fold. Prodigiosin treatment inhibited Wnt3A-CM–induced transcription in a dose-dependent manner (Fig. 1G). These results demonstrate that prodigiosin inhibits transcription of the SuperTopFlash reporter activated by either Wnt transfection or Wnt3A treatment, suggesting that prodigiosin does not inhibit Wnt ligand secretion. In contrast, the Porcupine (PORCN) inhibitors LGK974 and IWP-2, which block Wnt ligand secretion, depressed SuperTopFlash reporter activity induced by transfected Wnt1, but not that induced by Wnt3A-CM (Fig. S1).
Fig. 1.
Prodigiosin specifically inhibits the Wnt/β-catenin signaling pathway. (A) Structure of prodigiosin and obatoclax. (B–F) The SuperTopFlash reporter gene was transfected into HEK293T cells together with empty vector or expression plasmids encoding Wnt1 (B); Wnt3 (C); Wnt1 and LRP6, Wnt3 and LRP6 (D); DVL2 (E); and β-catenin (F). (G) The SuperTopFlash reporter gene was transfected into HEK293T cells, after which the cells were treated with control or Wnt3A conditioned medium (Wnt3A-CM). (H) HEK293T cells were transfected with an AP1-Luc reporter and empty vector or a constitutively active Rasv12 expression plasmid. (I) HEK293T cells were transfected with a NFAT-Luc reporter and empty vector or an expression plasmid for NFATc. The transfected cells were incubated with vehicle or prodigiosin (25–500 nM) for 24 h. The luciferase values were normalized to β-gal activities.
Fig. S1.
PORCN inhibitors inhibit Wnt1-induced (A), but not Wnt3A-CM–induced (B), transcriptional activity.
Prodigiosin did not suppress β-catenin–induced reporter activity (Fig. 1F), indicating that prodigiosin likely inhibits Wnt/β-catenin signaling upstream of β-catenin, such as at the level of the receptor complex (LRP6 phosphorylation) and the β-catenin destruction complex (DVL2 and GSK3β phosphorylation). In control experiments, Wnt inhibitory concentrations of prodigiosin did not influence the luciferase activity of an AP-1 reporter gene (Fig. 1H) or an NFAT reporter gene (Fig. 1I).
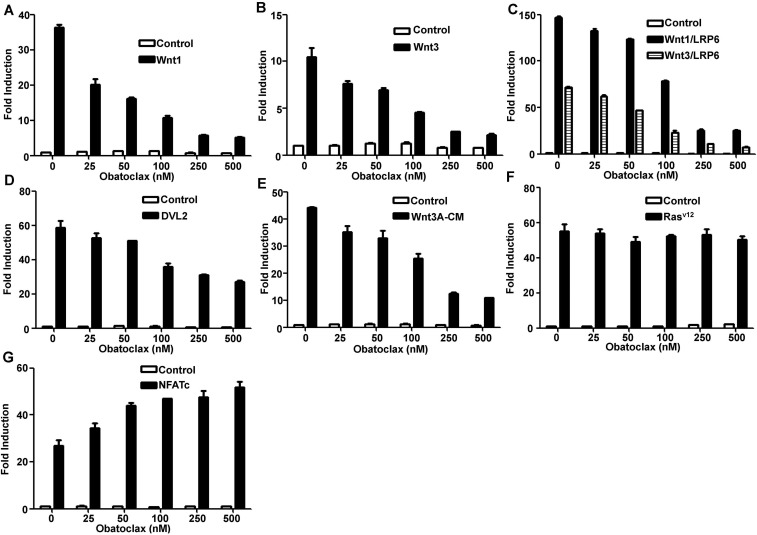
Obatoclax, a synthetic prodigiosin analog with an indole ring (Fig. 1A), has demonstrated promising anticancer activity in multiple clinical trials (14, 15). We examined the effect of obatoclax on Wnt/β-catenin signaling using a SuperTopFlash reporter assay. Similar to prodigiosin, obatoclax inhibited Wnt signaling activated by Wnt1 (SI Appendix, Fig. S2A), Wnt3 (Fig. S2B), Wnt1/LRP6 and Wnt3/LRP6 (Fig. S2C), and DVL2 (Fig. S2D). Obatoclax also suppressed Wnt3A-CM–induced transcriptional activity of the SuperTopFlash reporter in a dose-dependent manner (Fig. S2E). As expected, obatoclax did not inhibit the activity of the AP1-Luc and NFAT-Luc reporters (Fig. S2 F and G).
Fig. S2.
Obatoclax specifically inhibits the Wnt/β-catenin signaling pathway. (A–D) HEK293T cells were tranfected with the SuperTopFlash reporter gene together with empty vector or expression plasmids encoding Wnt1 (A); Wnt3 (B); Wnt1 and LRP6, Wnt3 and LRP6 (C); and DVL2 (D). (E) The SuperTopFlash reporter gene was transfected into HEK293T cells, and then cells were treated with control or Wnt3A-CM. (F) HEK293T cells were transfected with an AP1-Luc reporter and empty vector or a constitutively active Rasv12 expression plasmid. (G) HEK293T cells were transfected with a NFAT-Luc reporter and empty vector or an expression plasmid for NFATc. Transfection conditions were same as in Fig. 1, but using obatoclax as indicated.
Inhibition of the Wnt/β-Catenin Signaling Cascade in HEK293T Cells by Prodigiosin.
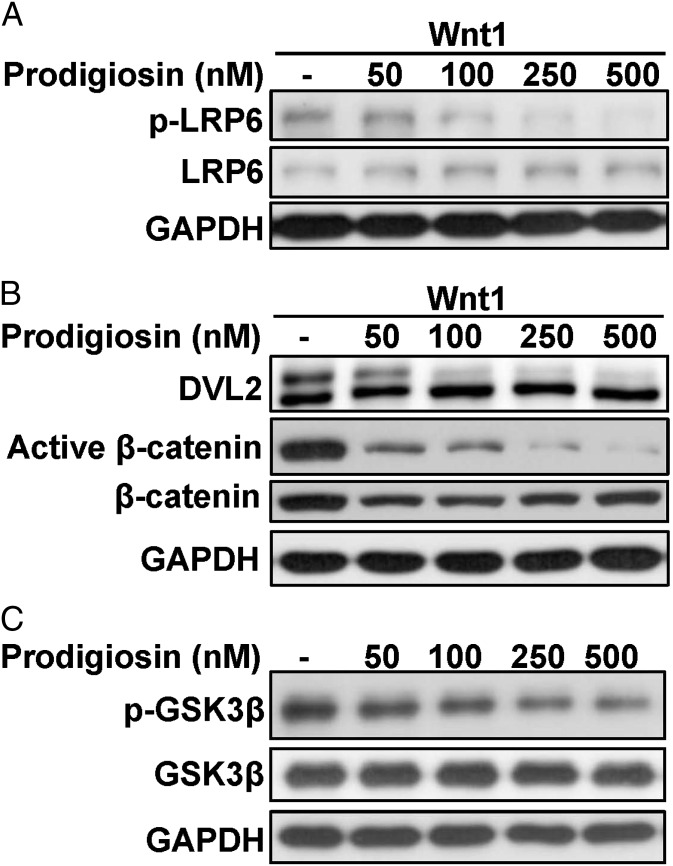
To further investigate the target of prodigiosin action in the Wnt pathway, HEK293T cells were transfected with a Wnt1 expression plasmid, and after 24 h were treated with increasing concentrations of prodigiosin. As expected, Wnt1 expression increased phosphorylation of LRP6 and DVL2 and activated β-catenin (Fig. 2 A and B). The phosphorylated DVL2 protein was visualized as a slower-migrating band on SDS/PAGE, whereas active β-catenin was identified by reduced phosphorylation of serine 37 (S37) and threonine 41 (T41), as recognized by a monoclonal antibody. Treatment with nanomolar concentrations of prodigiosin significantly reduced the levels of phosphorylated LRP6 and DVL2, active β-catenin, and total β-catenin (Fig. 2 A and B), indicating that prodigiosin may block Wnt/β-catenin signaling by targeting LRP6 and DVL.
Fig. 2.
Prodigiosin inhibits the Wnt/β-catenin signaling pathway in HEK293T cells. (A) HEK293T cells were transfected with Wnt1 expression plasmid. Then cells were treated with the indicated amounts of prodigiosin for 24 h. Phosphorylated LRP6 and total LRP6 were detected by immunoblotting. (B) Transfection conditions were as in A. HEK293T cells were treated with the indicated amounts of prodigiosin for 24 h. DVL2, active β-catenin, and total β-catenin were detected by immunoblotting. The phosphorylated DVL2 showed slower mobility following SDS/PAGE. (C) HEK293T cells were treated with the indicated amounts of prodigiosin for 24 h. Ser9 phosphorylated GSK3β and total GSK3β were detected by immunoblotting.
A previous study indicated that pharmacologic inhibitors of GSK3β could prevent prodigiosin toxicity (17). Thus, we evaluated the effect of prodigiosin on the phosphorylation state of GSK3β, a serine/threonine kinase involved in various cellular responses, including regulation of β-catenin stability. Phosphorylation of GSK3β at Ser9, which can be monitored using phosphor-specific antibodies, inhibits its kinase activity (19). Prodigiosin noticeably inhibited the phosphorylation of GSK3β at Ser9 in HEK293T cells (Fig. 2C), which is indicative of an increase in GSK3β activity.
Suppression of Wnt/β-Catenin Signaling and Cyclin D1 Levels in Breast Cancer Cells by Prodigiosin.
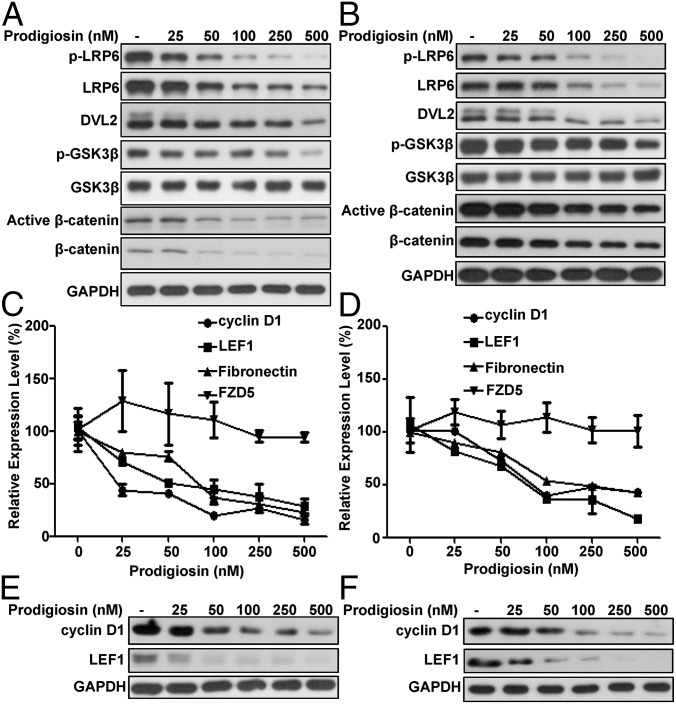
We studied the MDA-MB-231 and MDA-MB-468 cell lines to assess the effect of prodigiosin on Wnt/β-catenin signaling in breast cancer. In both cell lines, prodigiosin concentrations 25–500 nM decreased the levels of phosphorylated LRP6, total LRP6, phosphorylated and unphosphorylated DVL2, and Ser9 phosphorylated GSK3β (Fig. 3 A and B). Furthermore, both active β-catenin and total β-catenin levels were diminished after prodigiosin treatment (Fig. 3 A and B).
Fig. 3.
Prodigiosin inhibits the Wnt/β-catenin signaling pathway in breast cancer cells. MDA-MB-231 (A, C, and E) and MDA-MB-468 (B, D, and F) cells were treated with the indicated amounts of prodigiosin for 24 h. (A and B) Phosphorylated LRP6, total LRP6, DVL2, Ser9 phosphorylated GSK3β, total GSK3β, active β-catenin, and total β-catenin were detected by immunoblotting. The slower-migrating band (upper band) represents the phosphorylated DVL2. (C and D) Total RNA was extracted and then reverse-transcribed into cDNA. Prepared cDNA was then subjected to quantitative PCR analysis to detect the mRNA expression of cyclin D1, fibronectin, and LEF1. (E and F) Protein expression of cyclin D1 and LEF1 were detected by immunoblotting.
Activation of Wnt signaling increases the transcription of many genes, including cyclin D1, which is dysregulated in breast cancer. In MDA-MB-231 and MDA-MB-468 cells, prodigiosin at concentrations as low as 25 nM decreased cyclin D1 gene expression, and also depressed transcription of the Wnt-regulated genes LEF1 and fibronectin (Fig. 3 C and D); however, prodigiosin had no effect on the expression of FZD5, which is not a known target of Wnt/β-catenin signaling (Fig. 3 C and D). In agreement with the mRNA data, prodigiosin significantly suppressed the protein levels of cyclin D1 and LEF1 in breast cancer MDA-MB-231 and MDA-MB-468 cells (Fig. 3 E and F).
Effect of Prodigiosin on Viability, Proliferation, and Apoptosis in Breast Cancer Cells.
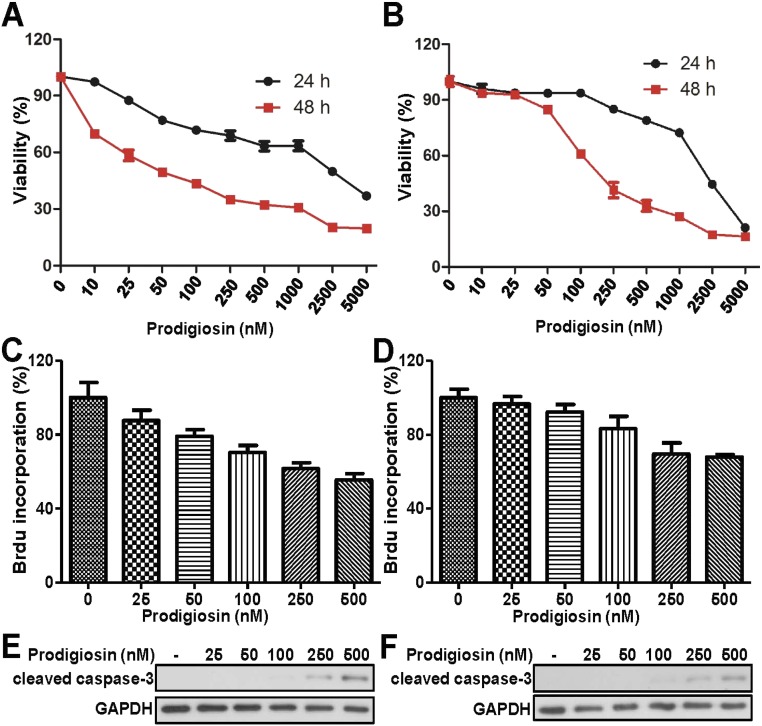
Consistent with previous experiments (16, 20), prodigiosin reduced the viability of breast cancer cells, with IC50 values at 48 h of 62.52 nM in MDA-MB-231 cells (Fig. S3A) and 261.2 nM in MDA-MB-468 cells (Fig. S3B). We investigated the effect of prodigiosin on the proliferation of breast cancer cells using the BrdU incorporation assay. Prodigiosin treatment resulted in a dose-dependent decrease in BrdU incorporation in MDA-MB-231 cells (Fig. S3C) and MDA-MB-468 cells (Fig. S3D). Cleaved caspase-3 is the active form of caspase-3, which is a well-known marker for apoptotic cell death. To test whether prodigiosin is able to induce apoptosis in breast cancer cells, we treated MDA-MB-231 and MDA-MB-468 cells with 25–500 nM prodigiosin for 24 h and then subjected cell extracts to Western blot analysis using an anti–cleaved caspase-3 antibody. Cleaved caspase-3 was detected in both cell lines (Fig. S3 E and F). These results indicate that prodigiosin can inhibit proliferation and induce apoptosis in breast cancer cells.
Fig. S3.
Prodigiosin significantly reduces viability, inhibits proliferation, and induces apoptosis in breast cancer cells. (A and B) MDA-MB-231 (A) and MDA-MB-468 (B) cells were treated with the indicated amounts of prodigiosin for 24 and 48 h, respectively. Cell viability was assessed with an MTT assay. (C and D) MDA-MB-231 (C) and MDA-MB-468 (D) cells were treated with the indicated amounts of prodigiosin for 24 h. Cell proliferation was examined by a BrdU incorporation assay. (E and F) MDA-MB-231 (E) and MDA-MB-468 (F) cells were treated with the indicated amounts of prodigiosin for 24 h. Cleaved caspase-3 was detected by immunoblotting.
Effect of Prodigiosin on Breast Cancer Cell Migration and Invasion.
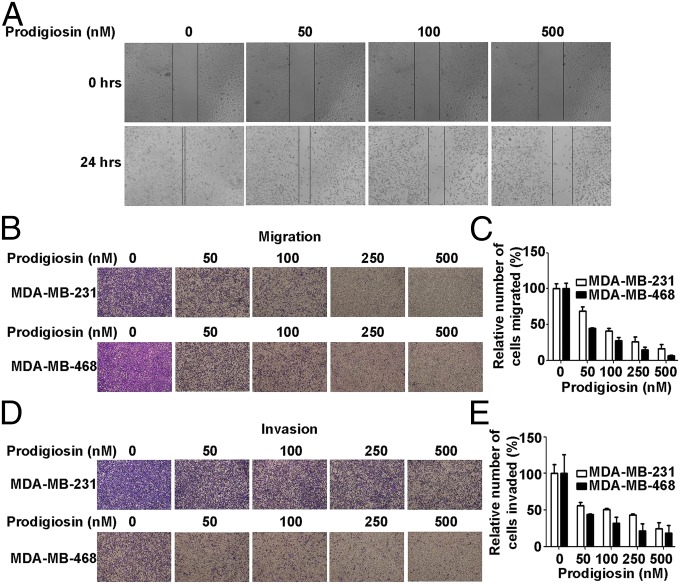
The Wnt pathway regulates migration and invasion of malignant cells, and thereby influences metastasis (21). The effect of prodigiosin on the migration of breast cancer cells was evaluated using an in vitro scratch assay and transwell assays. Prodigiosin suppressed the migration of breast cancer MDA-MB-231 and MDA-MB-468 cells in a dose-dependent manner (Fig. 4 A–C).
Fig. 4.
Prodigiosin suppresses breast cancer cell migration and invasion. (A) Prodigiosin inhibits migration of breast cancer cells. MDA-MB-231 cells were grown in 10% FBS-containing medium. The cell monolayers were scratched and incubated with the indicated concentrations of prodigiosin for 24 h and then photomicrographed. (B) Prodigiosin blocks migration of breast cancer cells. MDA-MB-231 (Upper) or MDA-MB-468 (Lower) cells were transferred to transwells with the indicated concentrations of prodigiosin for 6 h. The migrated cells were stained and photomicrographed. (C) Graphical representation of quantitative data shows the relative number of migrated cells shown in B. (D) Prodigiosin inhibits breast cancer cell invasion. MDA-MB-231 (Upper) and MDA-MB-468 (Lower) cells were placed in transwells coated with matrigel and treated with the indicated concentrations of prodigiosin for 24 h. The invaded cells were stained and photomicrographed. (E) Graphical representation of quantitative data shows the relative number of invaded cells shown in D.
To examine the effect of prodigiosin on invasion of breast cancer cells, we repeated the transwell assays using Matrigel-coated chambers. Prodigiosin treatment significantly reduced the numbers of penetrated MDA-MB-231 and MDA-MB-468 cells compared with the blank control (Fig. 4 D and E). These results indicate that prodigiosin exerts inhibitory effects on the migratory and invasive capacity of breast cancer cells in vitro.
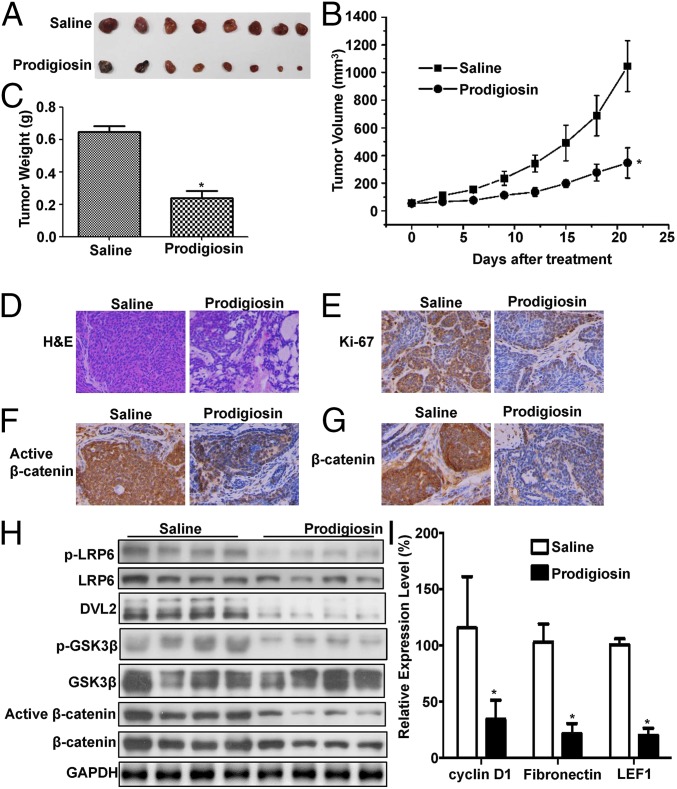
Inhibition of Tumor Growth in Vivo and Blockade of Wnt/β-Catenin Signaling in MDA-MB-231 Xenografts by Prodigiosin.
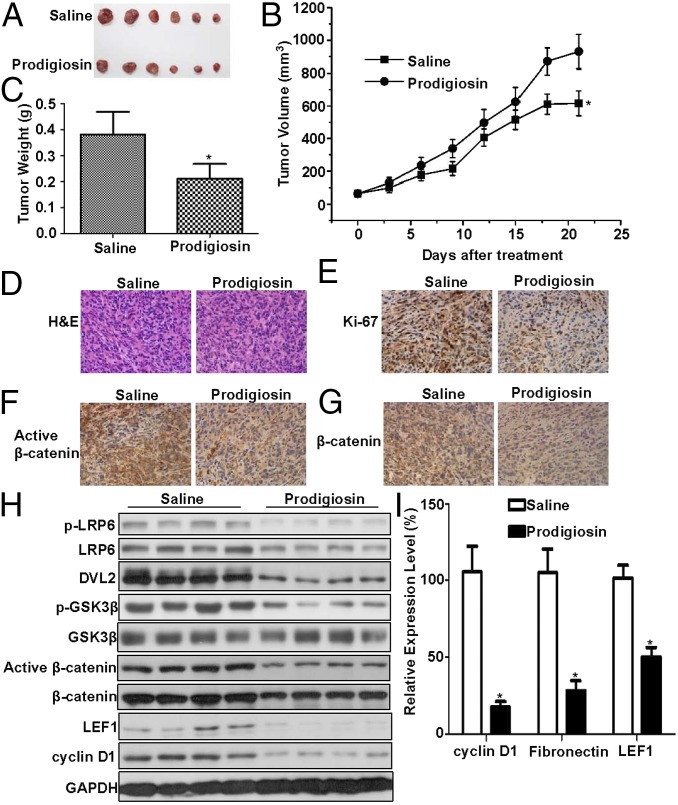
To determine whether prodigiosin could antagonize the Wnt signaling cascade in breast cancer cells in vivo, we injected MDA-MB-231 cells s.c. into nude mice. After the tumor volumes reached approximately 50 mm3, mice were treated by i.p. injection with vehicle or prodigiosin at 5 mg/kg twice weekly for 3 wk. Once the mice were killed, tumor volumes and weights were measured, the histological features of the tumors were evaluated, and the total RNAs and proteins in the xenografts were extracted. The prodigiosin treatment regimen significantly inhibited tumor growth, but did not eliminate the tumors (Fig. 5 A–C). The drug did not affect body weight. Histological analyses showed that treatment with prodigiosin decreased tumor cell density (Fig. 5D) and expression of the proliferation marker Ki-67 (Fig. 5E).
Fig. 5.
Prodigiosin inhibits tumor growth in a breast cancer xenograft mouse model. Established MDA-MB-231 xenografts were treated with 5 mg/kg prodigiosin twice weekly for 3 wk. (A) Images of tumors from both the saline- and prodigiosin-treated groups. (B) Mean tumor volumes. (C) Mean tumor weight. (D) H&E staining. (E) Ki-67 antibody staining. (F) Active β-catenin antibody staining. (G) Total β-catenin antibody staining. (H) The expression levels of phosphorylated LRP6, total LRP6, DVL2, Ser9 phosphorylated GSK3β, total GSK3β, active β-catenin, total β-catenin, cyclin D1, and LEF1 in tumor samples were visualized after immunoblotting. The slower-migrating bands (upper bands) represent the phosphorylated DVL2. (I) The mRNA expression levels of cyclin D1, fibronectin, and LEF1 were quantitated by real-time PCR. *P < 0.05.
Immunohistochemical staining demonstrated that prodigiosin treatment decreased the expression of active β-catenin and total β-catenin in MDA-MB-231 xenografts (Fig. 5 F and G). Moreover, prodigiosin-treated tumors showed significantly decreased levels of phosphorylated LRP6, phosphorylated and unphosphorylated DVL2, Ser9 phosphorylated GSK3β, active and total β-catenin, LEF1, and cyclin D1 protein compared with control-treated tumors (Fig. 5H). These immunoblot results were confirmed by real-time PCR analyses, which showed a marked reduction in the mRNA expression of the Wnt target genes cyclin D1, LEF1, and fibronectin in prodigiosin-treated tumors (Fig. 5I).
Induction of Tumor Regression by Prodigiosin via Down-Regulation of Wnt/β-Catenin Signaling in MMTV-Wnt1 Transgenic Mice.
To investigate the in vivo efficacy of prodigiosin in Wnt-driven mammary tumors, we used MMTV-Wnt1 transgenic mice, which develop mammary hyperplasia early in development, followed by the appearance of solitary tumors (22). The mammary glands of the mice were palpated twice weekly, and the tumor appearance was recorded. Once the developing tumors reached approximately 50 mm3 in diameter, treatment was initiated with i.p. injection of either the vehicle or prodigiosin (5 mg/kg) twice weekly. After a 3-wk treatment regimen, the mice were killed and tumors were removed for further analysis. Prodigiosin treatment caused tumor regression (Fig. 6 A–C), concomitant with reduced tumor cell density (Fig. 6D) and proliferation as indicated by Ki-67 staining (Fig. 6E).
Fig. 6.
Prodigiosin induces tumor regression in MMTV-Wnt1 transgenic mice. MMTV-Wnt1 transgenic mice were treated with either vehicle or prodigiosin (5 mg/kg) twice weekly for 3 wk. (A) Images of tumors from the saline- and prodigiosin-treated groups. (B) Mean tumor volume. (C) Mean tumor weight. (D) H&E staining. (E) Ki-67 antibody staining. (F) Active β-catenin antibody staining. (G) Total β-catenin antibody staining. (H) Expression levels of phosphorylated LRP6, total LRP6, DVL2, Ser9 phosphorylated GSK3β, total GSK3β, active β-catenin, and β-catenin in tumor samples were visualized after immunoblotting. The slower-migrating bands (upper bands) represent the phosphorylated DVL2. (I) mRNA expression levels of cyclin D1, fibronectin, and LEF1 were quantitated by real-time PCR. *P < 0.05.
To determine the effect of prodigiosin on Wnt/β-catenin signaling in Wnt1-driven tumors, we examined the tumors from control and treated groups by immunohistochemical staining, immunoblot analyses, and real-time PCR. The prodigiosin-treated tumors showed reduced expression of active and total β-catenin (Fig. 6 F and G). Importantly, administration of prodigiosin noticeably decreased the levels of phosphorylated LRP6, phosphorylated and unphosphorylated DVL2, Ser9 phosphorylated GSK3β, and active and total β-catenin (Fig. 6H). These results indicate that prodigiosin blocks growth of Wnt ligand-driven mammary tumors through targeting multiple sites of the Wnt signaling cascade. Inhibition of Wnt signaling by prodigiosin was accompanied by a treatment-induced decrease in mRNA expression of Wnt target genes cyclin D1, LEF1, and fibronectin (Fig. 6I).
Discussion
These results document the ability of prodigiosin to inhibit Wnt signaling in breast cancer cells in tissue culture and in vivo. The Wnt antagonism occurs at the same concentrations that inhibit breast cancer cell growth, migration, and invasion. Prodigiosin acts proximally in the Wnt signaling cascade by inhibiting the phosphorylation of LRP6, DVL2, and GSK3β, thereby preventing the activation of β-catenin.
The various prodigiosins are characterized by a common 4-methoxy-2,2-bipyrrole ring system that can exist in cis and trans forms (23). The planar nitrogens in prodigiosin and its analogs can chelate copper and zinc, and also alter cellular pH (23–25). In cultured cell lines, prodigiosin can exert effects similar to those of the polyether ionophores salinomycin and nigericin, which inhibit the activity of vacuolar H+-adenosine triphosphatase (V-ATPase) (26). Inhibition of V-ATPase interferes with vesicle acidification, which is necessary for endocytosis and activation of the FZD/LRP6 complex that mediates Wnt signaling (27, 28). We previously showed that both salinomycin and nigericin inhibit Wnt signaling proximal to β-catenin, possibly by interference with V-ATPase (18); however, salinomycin and nigericin are complex natural products that are difficult to isolate and synthesize. In contrast, numerous prodigiosin analogs have been prepared, and one prodigiosin analog (obatoclax) is currently in clinical trials for treating hematologic malignancies (14, 15). It will be interesting to determine whether patients treated with prodigiosin congeners have alterations in the Wnt signaling cascade.
Wnt/β-catenin signaling plays an important role in the development and progression of breast cancer (29). Wnt ligands and FZD receptors are expressed in human breast cancer cell lines and primary tumors (30–33). Multiple negative regulators of Wnt/β-catenin signaling, such as SFRP1, SFRP2, SFRP5, WIF1, DKK1, and DKK3, are down-regulated in many breast tumors (34–39). Although Wnt/β-catenin pathway mutations are rarely detected in breast tumors, elevated levels of nuclear and/or cytoplasmic β-catenin are frequently found and are associated with poor prognosis (40). Furthermore, DVL1 is up-regulated in 50% of human breast cancers, and phosphorylated DVL proteins have been detected in many breast tumor cell lines (41, 42).
The anticancer effect of prodigiosin has attracted much attention owing to its potent anticancer activity in various human cancer cells with low cytotoxicity in normal cells. Given the importance of Wnt/β-catenin signaling in the development of human cancers (1–4), the Wnt inhibitory function of prodigiosin may be attributed to its selective cytotoxicity toward cancer cells. A previous study noted that prodigiosin-induced apoptosis could be blocked by GSK3β pharmacologic inactivation, suggesting that prodigiosin may mediate GSK3β activation (17). In this study, we have demonstrated that prodigiosin can reduce GSK3β phosphorylation at Ser9, leading to the increased activity of GSK3β. This result may explain, at least in part, the inhibitory effect of prodigiosin on Wnt//β-catenin signaling. The antiapoptosis gene survivin has been reported to be a target of the Wnt/β-catenin pathway (43). Ho et al. (44) reported that prodigiosin could down-regulate the expression of survivin gene, and this down-regulation is regulated mainly at the level of transcription. Our results support the idea that prodigiosin may suppress survivin expression by blocking Wnt/β-catenin signaling. Further studies are needed to clarify this possibility.
Cancer stem cells (CSCs) play crucial roles in the recurrence, metastasis, and drug resistance of breast cancer. As few as 100 human breast CSCs with the cell surface markers CD44+CD24−/low can produce tumors when xenotransplanted into NOD/SCID mice (45). The Wnt/β-catenin pathway is essential for self-renewal and migration of breast CSCs (46). The Wnt/β-catenin signaling inhibitors salinomycin and niclosamide exert selective inhibitory effects on breast CSCs compared with other cell types (18, 47–49). As shown here, prodigiosin also exhibits a potent inhibitory effect on Wnt/β-catenin signaling in breast cancer cells through targeting LRP6, DVL2, and GSK3β, leading to the inhibition of β-catenin activity and down-regulation of Wnt target genes, especially cyclin D1. Consistent with the results obtained in vitro, prodigiosin strongly depressed Wnt/β-catenin signaling and cyclin D1 expression in MDA-MB-231 breast cancer xenografts and MMTV-Wnt1 transgenic mice. Our results also demonstrate that prodigiosin is highly efficacious at well-tolerated doses in the transgenic breast cancer disease model driven by aberrant Wnt1 signaling.
Overexpression of cyclin D1 occurs in more than 50% of breast cancers (50). The cyclin D-dependent kinases 4 and 6 (CDK4/6) are frequently dysregulated in advanced breast cancer and are targets for new therapies. The CDK4/6 inhibitor pablociclib was recently shown to prolong progression-free survival when combined with letrozole in estrogen receptor-positive metastatic breast cancer (51); however, tumor resistance to pablociclib eventually develops. Increased activity of the Wnt pathway is a common property of drug resistant cancers. Thus, it is conceivable that the addition of prodigiosin or an analog to a CDK4 inhibitor and antiestrogen regimen could improve the treatment of advanced breast cancer.
Materials and Methods
Reagents and Plasmids.
Prodigiosin was purchased from Santa Cruz Biotechnology. LGK974, IWP-2, and obatoclax were obtained from Selleck. The SuperTopFlash reporter plasmid was gift from Karl Willert, University of California, San Diego. The reporter plasmids AP1-Luc and NFAT-Luc and the expression plasmids encoding Wnt1, Wnt3, LRP6, DVL2, β-catenin, NFATc, H-rasv12, and β-gal have been described previously (18, 52).
Luciferase Reporter Gene Assay.
HEK293T cells were transfected in 24-well plates with 0.25 μg of reporter plasmid, 50 ng of control plasmid pCMXβgal, and 50–200 ng of the indicated expression plasmids using Lipofectamine 2000 according to the manufacturer’s instructions. After transfection for 24 h, the cells were treated with the indicated concentrations of compound. Wnt3A conditioned medium (Wnt3A-CM) and control conditioned medium were prepared as described previously (53). Luciferase assays were carried out using a luciferase assay kit (Promega), and the luciferase values were normalized according to the β-gal activity.
Transwell Assays.
As described previously (54), 2 × 105 cells were seeded in 24 transwell chambers with 8-μm pore membrane in serum-free medium in the absence and presence of 50, 100, 250, 500 nM prodigiosin. The lower chamber of the transwell chamber contained medium with 20% FBS. After incubation at 37 °C for 6 h, the unmigrated cells on the upper side of membrane were removed by a cotton swab, and the migrated cells were stained with crystal violet and then photomicrographed. For invasion assays, the transwell chambers with 8-μm pore membranes were coated with Matrigel. For quantitative analysis, the stained cells were eluted in 33% acetic acid, and absorbance was detected at 570 nm.
Animal Model Study.
All animal experiments were performed according to the protocols approved by the Administrative Committee on Animal Research of Shenzhen University. For the breast cancer xenograft mouse model, female BALB/c nude mice were purchased from Guangdong Medical Lab Animal Center, and MDA-MB-231 cells were implanted s.c. into the right flank of nude mice at a dosage of 1 ×107 cells per mouse. Following implantation, tumor growth was closely observed and measured every 3 d. For the Wnt1-driven mouse mammary tumor model, MMTV-Wnt1 transgenic mice on an FVB background [FVB.Cg-Tg(Wnt1)1Hev/J] were obtained from Jackson Laboratories. The mice were palpated twice a week to detect mammary tumor formation. When the tumors reached ∼50 mm3, the mice were divided at random into two groups and treated with the vehicle (0.8% DMSO/12% Cremophor/8% ethanol in normal saline) or 5 mg/kg prodigiosin in vehicle twice weekly by i.p. injection. Subsequently, tumor volumes were measured with a caliper and calculated using the following formula: 0.528 × (length/2) × (width/2)2. After treatment for 3 wk, mice were killed, and tumors were excised and weighed.
Histological Analyses.
Tumors were fixed in formalin, embedded with paraffin, and sectioned. Hematoxylin and eosin staining and immunohistochemistry analysis were performed as described previously (55). The following primary antibodies were used for immunohistochemistry analyses: anti–Ki-67 (Biolegend), anti–nonphospho (active) β-catenin (Cell Signaling Technology), and anti–β-catenin (Santa Cruz Biotechnology).
Statistical Analyses.
Statistical analyses were carried out using the unpaired Student t test or two-way ANOVA GraphPad Prism 5.0. Differences at P < 0.05 were considered statistically significant. Results are presented as mean ± SD.
Other procedures, including cell culture and transfection, immunoblot analyses, real-time PCR analyses, cell viability assays, cell proliferation assays, and scratch assays, are described in SI Materials and Methods.
SI Materials and Methods
Cell Culture and Transfection.
HEK293T cells, mouse fibroblast L-cells, and L-cells stably transfected with Wnt3A (l-Wnt3A) were maintained in DMEM supplemented with 10% FBS, 100 U/mL penicillin, and 100 mg/mL streptomycin in 5% CO2 at 37 °C. MDA-MB-231 and MDA-MB-468 cells were grown in Leibovitz’s L-15 medium supplemented with 10% FBS, 100 U/mL penicillin, and 100 mg/mL streptomycin at 37 °C without CO2. Transfection of plasmids was performed with Lipofectamine 2000 transfection reagent (Invitrogen) according to the manufacturer’s protocol.
Immunoblot Analyses.
Cells or tumor tissues were harvested and sonicated in lysis buffer (20 mM Tris·HCl pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerol phosphate, 1 mM sodium orthovanadate, 2 μg/mL leupeptin, and 1 mM PMSF). Equal amount of proteins were resolved by SDS/PAGE, followed by immunoblotting with anti-phospho LRP6 (Ser1490) (Cell Signaling Technology), anti-LRP6 (Cell Signaling Technology), anti-DVL2 (Cell Signaling Technology), anti-nonphospho (active) β-catenin (Millipore), anti–β-catenin (Santa Cruz Biotechnology), anti-phospho GSK3β (Ser9) (Cell Signaling Technology), anti-GSK3β (Cell Signaling Technology), anti-cyclin D1 (Santa Cruz Biotechnology), anti-LEF1 (Cell Signaling Technology), anti-cleaved caspase-3 (Cell Signaling Technology), and anti-GAPDH (Abmart).
Real-time PCR Analyses.
Total RNA was extracted using RNAiso Plus (TaKaRa) and was reverse-transcribed into cDNA using the Primescript RT Reagent Kit (TaKaRa) according to the manufacturer’s instructions. Prepared cDNA was then subjected to quantitative PCR analysis using FastStart Universal SYBR Green Master (Roche). The primers were as follows: cyclin D1 (human): sense, 5′-AATGACCCCGCACGATTTC-3′; antisense, 5′-TCAGGTTCAGGCCTTGCAC-3′; cyclin D1 (mouse): sense, 5′-GCGTACCCTGACACCAATCTC-3′; antisense, 5′-CTCCTCTTCGCACTTCTGCTC-3′; fibronectin (human): sense, 5′-ACCTACGGATGACTCGTGCTTT-3′; antisense, 5′-TTCAGACATTCGTTCCCACTCA-3′; fibronectin (mouse): sense, 5′-ATGTGGACCCCTCCTGATAGT-3′; antisense, 5′-GCCCAGTGATTTCAGCAAAGG-3′; LEF1 (human): sense, 5′-AGGAACATCCCCACACTGAC-3′; antisense, 5′-AGGTCTTTTTGGCTCCTGCT-3′; LEF1 (mouse): sense, 5′-TGTTTATCCCATCACGGGTGG-3′; antisense, 5′-CATGGAAGTGTCGCCTGACAG-3′; GAPDH (human): sense, 5′-CCAGAACATCATCCCTGCCTCTACT-3′; antisense, 5′-GGTTTTTCTAGACGGCAGGTCAGGT-3′; and GAPDH (mouse): sense, 5′-TGGATTTGGACGCATTGGTC-3′; antisense, 5′-TTTGCACTGGTACGTGTTGAT-3′.
Cell Viability Assays.
MDA-MB-231 and MDA-MB-468 cells were plated onto 96-well plates at a density of 1 × 104 cells/well and grown overnight. The cells were then treated with DMSO at concentrations of 10, 25, 50, 100, 250, 500, 1,000, 2,500, and 5,000 nM for 24 h and with prodigiosin at the same concentrations for 48 h. The cells were then cultured with fresh medium containing MTT (5 mg/mL), followed by incubation for another 4 h. The formazan crystals were dissolved in DMSO and the absorbance of the formazan solution was measured at 570 nm.
Cell Proliferation Assays.
MDA-MB-231 and MDA-MB-468 cells were plated onto 96-well plates at a density of 1 × 104 cells/well and grown overnight. Then the cells were treated with DMSO or prodigiosin at concentrations of 25, 50, 100, 250, and 500 nM for 24 h. The BrdU cell proliferation assay was performed using the Cell Proliferation ELISA BrdU Chemiluminescent Kit (Roche).
Scratch Assays.
MDA-MB-231 cells were grown in monolayer and scratched in the center of the monolayer by a pipette tip. The cell monolayers were then incubated with DMSO or prodigiosin at concentrations of 50, 100, and 500 nM for 24 h and then photomicrographed.
Acknowledgments
This work was supported by the National Nature Science Foundation of China (Grants 81372342 and 31501143), the Nature Science Foundation of Guangdong Province (Grant 2014A030310168), the Shenzhen Peacock Innovation Team Project (Grant KQTD20140630100658078), the Key Laboratory Project of Shenzhen (Grant ZDSY20130329101130496), and the Shenzhen Basic Research Program (Grants JCYJ20150525092941006 and JCYJ20150525092941030).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1616336113/-/DCSupplemental.
References
- 1.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149(6):1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 2.Kypta RM, Waxman J. Wnt/β-catenin signalling in prostate cancer. Nat Rev Urol. 2012;9(8):418–428. doi: 10.1038/nrurol.2012.116. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, van der Zee M, Fodde R, Blok LJ. Wnt/Β-catenin and sex hormone signaling in endometrial homeostasis and cancer. Oncotarget. 2010;1(7):674–684. doi: 10.18632/oncotarget.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8(5):387–398. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- 5.Zeng X, et al. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature. 2005;438(7069):873–877. doi: 10.1038/nature04185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tamai K, et al. A mechanism for Wnt coreceptor activation. Mol Cell. 2004;13(1):149–156. doi: 10.1016/s1097-2765(03)00484-2. [DOI] [PubMed] [Google Scholar]
- 7.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev Cell. 2009;17(1):9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castro AJ. Antimalarial activity of prodigiosin. Nature. 1967;213(5079):903–904. doi: 10.1038/213903a0. [DOI] [PubMed] [Google Scholar]
- 9.Berg G. Diversity of antifungal and plant-associated Serratia plymuthica strains. J Appl Microbiol. 2000;88(6):952–960. doi: 10.1046/j.1365-2672.2000.01064.x. [DOI] [PubMed] [Google Scholar]
- 10.Magae J, Miller MW, Nagai K, Shearer GM. Effect of metacycloprodigiosin, an inhibitor of killer T cells on murine skin and heart transplants. J Antibiot (Tokyo) 1996;49(1):86–90. doi: 10.7164/antibiotics.49.86. [DOI] [PubMed] [Google Scholar]
- 11.Kataoka T, et al. Prodigiosin 25-C uncouples vacuolar type H(+)-ATPase, inhibits vacuolar acidification, and affects glycoprotein processing. FEBS Lett. 1995;359(1):53–59. doi: 10.1016/0014-5793(94)01446-8. [DOI] [PubMed] [Google Scholar]
- 12.Williamson NR, Fineran PC, Leeper FJ, Salmond GP. The biosynthesis and regulation of bacterial prodiginines. Nat Rev Microbiol. 2006;4(12):887–899. doi: 10.1038/nrmicro1531. [DOI] [PubMed] [Google Scholar]
- 13.Williamson NR, et al. Anticancer and immunosuppressive properties of bacterial prodiginines. Future Microbiol. 2007;2(6):605–618. doi: 10.2217/17460913.2.6.605. [DOI] [PubMed] [Google Scholar]
- 14.Schimmer AD, et al. A phase I study of the pan bcl-2 family inhibitor obatoclax mesylate in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14(24):8295–8301. doi: 10.1158/1078-0432.CCR-08-0999. [DOI] [PubMed] [Google Scholar]
- 15.Schimmer AD, et al. A multicenter phase I/II study of obatoclax mesylate administered as a 3- or 24-hour infusion in older patients with previously untreated acute myeloid leukemia. PLoS One. 2014;9(10):e108694. doi: 10.1371/journal.pone.0108694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soto-Cerrato V, Llagostera E, Montaner B, Scheffer GL, Perez-Tomas R. Mitochondria-mediated apoptosis operating irrespective of multidrug resistance in breast cancer cells by the anticancer agent prodigiosin. Biochem Pharmacol. 2004;68(7):1345–1352. doi: 10.1016/j.bcp.2004.05.056. [DOI] [PubMed] [Google Scholar]
- 17.Soto-Cerrato V, Viñals F, Lambert JR, Kelly JA, Pérez-Tomás R. Prodigiosin induces the proapoptotic gene NAG-1 via glycogen synthase kinase-3beta activity in human breast cancer cells. Mol Cancer Ther. 2007;6(1):362–369. doi: 10.1158/1535-7163.MCT-06-0266. [DOI] [PubMed] [Google Scholar]
- 18.Lu D, et al. Salinomycin inhibits Wnt signaling and selectively induces apoptosis in chronic lymphocytic leukemia cells. Proc Natl Acad Sci USA. 2011;108(32):13253–13257. doi: 10.1073/pnas.1110431108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378(6559):785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 20.Soto-Cerrato V, Viñals F, Lambert JR, Pérez-Tomás R. The anticancer agent prodigiosin induces p21WAF1/CIP1 expression via transforming growth factor-beta receptor pathway. Biochem Pharmacol. 2007;74(9):1340–1349. doi: 10.1016/j.bcp.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 21.Valkenburg KC, Steensma MR, Williams BO, Zhong Z. Skeletal metastasis: Treatments, mouse models, and the Wnt signaling. Chin J Cancer. 2013;32(7):380–396. doi: 10.5732/cjc.012.10218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsukamoto AS, Grosschedl R, Guzman RC, Parslow T, Varmus HE. Expression of the int-1 gene in transgenic mice is associated with mammary gland hyperplasia and adenocarcinomas in male and female mice. Cell. 1988;55(4):619–625. doi: 10.1016/0092-8674(88)90220-6. [DOI] [PubMed] [Google Scholar]
- 23.Manderville RA. Synthesis, proton-affinity and anti-cancer properties of the prodigiosin-group natural products. Curr Med Chem Anticancer Agents. 2001;1(2):195–218. doi: 10.2174/1568011013354688. [DOI] [PubMed] [Google Scholar]
- 24.Park G, et al. Zinc and copper complexes of prodigiosin: Implications for copper-mediated double-strand DNA cleavage. Org Lett. 2003;5(2):113–116. doi: 10.1021/ol027165s. [DOI] [PubMed] [Google Scholar]
- 25.Chang TM, Sinharay S, Astashkin AV, Tomat E. Prodigiosin analogue designed for metal coordination: Stable zinc and copper pyrrolyldipyrrins. Inorg Chem. 2014;53(14):7518–7526. doi: 10.1021/ic5008439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castillo-Avila W, Abal M, Robine S, Pérez-Tomás R. Non-apoptotic concentrations of prodigiosin (H+/Cl− symporter) inhibit the acidification of lysosomes and induce cell cycle blockage in colon cancer cells. Life Sci. 2005;78(2):121–127. doi: 10.1016/j.lfs.2005.04.059. [DOI] [PubMed] [Google Scholar]
- 27.Cruciat CM, et al. Requirement of prorenin receptor and vacuolar H+-ATPase-mediated acidification for Wnt signaling. Science. 2010;327(5964):459–463. doi: 10.1126/science.1179802. [DOI] [PubMed] [Google Scholar]
- 28.Sun-Wada GH, Wada Y. Role of vacuolar-type proton ATPase in signal transduction. Biochim Biophys Acta. 2015;1847(10):1166–1172. doi: 10.1016/j.bbabio.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 29.DiMeo TA, et al. A novel lung metastasis signature links Wnt signaling with cancer cell self-renewal and epithelial-mesenchymal transition in basal-like breast cancer. Cancer Res. 2009;69(13):5364–5373. doi: 10.1158/0008-5472.CAN-08-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Howe LR, Brown AM. Wnt signaling and breast cancer. Cancer Biol Ther. 2004;3(1):36–41. doi: 10.4161/cbt.3.1.561. [DOI] [PubMed] [Google Scholar]
- 31.Milovanovic T, et al. Expression of Wnt genes and frizzled 1 and 2 receptors in normal breast epithelium and infiltrating breast carcinoma. Int J Oncol. 2004;25(5):1337–1342. [PubMed] [Google Scholar]
- 32.Ayyanan A, et al. Increased Wnt signaling triggers oncogenic conversion of human breast epithelial cells by a Notch-dependent mechanism. Proc Natl Acad Sci USA. 2006;103(10):3799–3804. doi: 10.1073/pnas.0600065103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benhaj K, Akcali KC, Ozturk M. Redundant expression of canonical Wnt ligands in human breast cancer cell lines. Oncol Rep. 2006;15(3):701–707. [PubMed] [Google Scholar]
- 34.Veeck J, et al. Aberrant methylation of the Wnt antagonist SFRP1 in breast cancer is associated with unfavourable prognosis. Oncogene. 2006;25(24):3479–3488. doi: 10.1038/sj.onc.1209386. [DOI] [PubMed] [Google Scholar]
- 35.Lo PK, et al. Epigenetic suppression of secreted frizzled related protein 1 (SFRP1) expression in human breast cancer. Cancer Biol Ther. 2006;5(3):281–286. doi: 10.4161/cbt.5.3.2384. [DOI] [PubMed] [Google Scholar]
- 36.Veeck J, et al. Epigenetic inactivation of the secreted frizzled-related protein-5 (SFRP5) gene in human breast cancer is associated with unfavorable prognosis. Carcinogenesis. 2008;29(5):991–998. doi: 10.1093/carcin/bgn076. [DOI] [PubMed] [Google Scholar]
- 37.Ai L, et al. Inactivation of Wnt inhibitory factor-1 (WIF1) expression by epigenetic silencing is a common event in breast cancer. Carcinogenesis. 2006;27(7):1341–1348. doi: 10.1093/carcin/bgi379. [DOI] [PubMed] [Google Scholar]
- 38.Xiang T, et al. Epigenetic silencing of the WNT antagonist Dickkopf 3 disrupts normal Wnt/β-catenin signalling and apoptosis regulation in breast cancer cells. J Cell Mol Med. 2013;17(10):1236–1246. doi: 10.1111/jcmm.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suzuki H, et al. Frequent epigenetic inactivation of Wnt antagonist genes in breast cancer. Br J Cancer. 2008;98(6):1147–1156. doi: 10.1038/sj.bjc.6604259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin SY, et al. Beta-catenin, a novel prognostic marker for breast cancer: Its roles in cyclin D1 expression and cancer progression. Proc Natl Acad Sci USA. 2000;97(8):4262–4266. doi: 10.1073/pnas.060025397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagahata T, et al. Amplification, up-regulation and over-expression of DVL-1, the human counterpart of the Drosophila disheveled gene, in primary breast cancers. Cancer Sci. 2003;94(6):515–518. doi: 10.1111/j.1349-7006.2003.tb01475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schlange T, Matsuda Y, Lienhard S, Huber A, Hynes NE. Autocrine WNT signaling contributes to breast cancer cell proliferation via the canonical WNT pathway and EGFR transactivation. Breast Cancer Res. 2007;9(5):R63. doi: 10.1186/bcr1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang T, et al. Evidence that APC regulates survivin expression: A possible mechanism contributing to the stem cell origin of colon cancer. Cancer Res. 2001;61(24):8664–8667. [PubMed] [Google Scholar]
- 44.Ho TF, et al. Prodigiosin down-regulates survivin to facilitate paclitaxel sensitization in human breast carcinoma cell lines. Toxicol Appl Pharmacol. 2009;235(2):253–260. doi: 10.1016/j.taap.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 45.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100(7):3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434(7035):843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 47.Curtin JC, Lorenzi MV. Drug discovery approaches to target Wnt signaling in cancer stem cells. Oncotarget. 2010;1(7):552–566. doi: 10.18632/oncotarget.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gupta PB, et al. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138(4):645–659. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang YC, et al. Drug screening identifies niclosamide as an inhibitor of breast cancer stem-like cells. PLoS One. 2013;8(9):e74538. doi: 10.1371/journal.pone.0074538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arnold A, Papanikolaou A. Cyclin D1 in breast cancer pathogenesis. J Clin Oncol. 2005;23(18):4215–4224. doi: 10.1200/JCO.2005.05.064. [DOI] [PubMed] [Google Scholar]
- 51.VanArsdale T, Boshoff C, Arndt KT, Abraham RT. Molecular pathways: Targeting the cyclin D-CDK4/6 axis for cancer treatment. Clin Cancer Res. 2015;21(13):2905–2910. doi: 10.1158/1078-0432.CCR-14-0816. [DOI] [PubMed] [Google Scholar]
- 52.Lu D, et al. Activation of the Wnt signaling pathway in chronic lymphocytic leukemia. Proc Natl Acad Sci USA. 2004;101(9):3118–3123. doi: 10.1073/pnas.0308648100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Willert K, Shibamoto S, Nusse R. Wnt-induced dephosphorylation of axin releases beta-catenin from the axin complex. Genes Dev. 1999;13(14):1768–1773. doi: 10.1101/gad.13.14.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Z, et al. Interplay of mevalonate and Hippo pathways regulates RHAMM transcription via YAP to modulate breast cancer cell motility. Proc Natl Acad Sci USA. 2014;111(1):E89–E98. doi: 10.1073/pnas.1319190110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hong B, et al. Prodigiosin rescues deficient p53 signaling and antitumor effects via up-regulating p73 and disrupting its interaction with mutant p53. Cancer Res. 2014;74(4):1153–1165. doi: 10.1158/0008-5472.CAN-13-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]