Abstract
A US Food and Drug Administration (FDA) drug safety communication was released in March 2013, warning prescribers of the risk of QT prolongation associated with azithromycin. Overall azithromycin utilization and adherence to an inpatient QTc monitoring guideline during 8-month time periods before and after the warning were assessed to evaluate the impact of this warning on inpatient azithromycin utilization and QTc monitoring. Fifty-five patients were included in the prewarning time period and 50 were included in the postwarning period. A significant reduction in utilization in days of therapy per 1,000 patient days was observed (31.2 prewarning vs 17.5 postwarning, p < .001) in these groups. No changes in QTc monitoring among patients receiving azithromycin were identified. FDA warnings of severe, life-threatening toxicities can have a profound impact on utilization and prescribing of medications, however they may not necessarily change monitoring practices.
Keywords: azithromycin, FDA warning, QTc prolongation
In March 2013, the US Food and Drug Administration (FDA) released a drug safety communication warning patients and health care providers about the potential risk of QT prolongation associated with azithromycin, which may lead to life-threatening arrhythmias including torsades de pointes (TdP).1,2 Heightened concerns for an increased risk of QT prolongation and cardiovascular death with azithromycin initially surfaced with a study by Ray et al, which identified significant differences in the rates of cardiovascular death with azithromycin.3 In this study, patients who had received 5 days of azithromycin (N = 347,795) had a significantly higher risk of cardiovascular death compared to those who received no antibiotics, amoxicillin, and ciprofloxacin. Since that time, various additional studies have been published regarding the cardiovascular risk of azithromycin.4–7 The combination of the known proarrhythmic properties of azithromycin, the risk of cardiovascular death as identified in previous studies, and the resulting FDA warning encourages greater caution with the use of azithromycin and associated monitoring.
In terms of recommended monitoring for patients receiving QT-prolonging medications, there is no consensus on a gold standard strategy for QT monitoring. A scientific statement in 2010 encouraged providers in the hospital setting to monitor QT intervals using a consistent method before and after administration of potential QT-prolonging medications.8 Our medical center implemented a Drug-Induced QTc Prolongation Monitoring Guideline for Hospitalized Patients in May 2011. After the March 2013 FDA warning, these guidelines were updated to reflect the change of azithromycin categorization from a possible risk to a high-risk medication. For all high-risk medications, our guidelines recommend a baseline 12-lead electrocardiogram (ECG) and daily ECG monitoring thereafter if the baseline QTc is greater than or equal to 440 ms. In addition to modifications made to our monitoring guideline, a summary of the FDA warning was e-mailed to hospital staff by the Medication Safety Committee Chair one day after the communication.
The primary objective of our study was to determine how the available data on QT prolongation and cardiovascular death associated with azithromycin and the subsequent release of the FDA warning have influenced azithromycin utilization in number of days of therapy (DOT) normalized to 1,000 patient days (DOT/1,000 patient days). We also assessed the effect of the warning on compliance with our internal QTc prolongation monitoring guideline.
METHODS
This was a single-center, retrospective, observational study at an academic medical center. Patients aged 18 years and older, admitted before the FDA warning between July 1, 2012 through February 28, 2013, and after the FDA warning between April 1, 2013 through November 30, 2013, who were treated with at least 48 hours of azithromycin were included. The month of March was omitted to provide a lead-in period immediately following the warning, allowing time for providers to become aware of the warning and implement this knowledge into clinical practice. Patients were excluded if they received nonconsecutive doses of azithromycin (eg, one-time doses, prophylactic and anti-inflammatory dosing).
Patients were identified for inclusion using pharmacy utilization reports generated from the computerized order entry system, providing data on inpatient prescriptions. The following data points were collected: age, gender, presence of concomitant heart failure or renal or hepatic insufficiency at baseline, azithromycin indication, dose and duration of therapy, and availability of baseline and follow-up ECG data to assess for QTc interval prolongation. Hepatic insufficiency was defined as having an aspartate aminotransferase (AST), alanine transaminase (ALT), and/or alkaline phosphatase level greater than 3 times the upper limit of normal. Patients were also assessed if they had a historical diagnosis of viral hepatitis or had known liver dysfunction or disease. Renal insufficiency was defined by a baseline serum creatinine greater than 1.4 mg/dL and/or BUN greater than 20 mg/dL.
The number of DOT/1,000 patient days for the times before and after the FDA warning were evaluated using the Student's t test. For the difference in rate of baseline and follow-up ECGs consistent with the QTc monitoring guideline and baseline characteristic comparison, chi-square or Fisher's exact analysis was performed for categorical data and Student's t test was performed for continuous data.
RESULTS
A total of 620 patients were initially evaluated for inclusion in the study. Of these patients, 224 were excluded in the prewarning group and 291 were excluded in the postwarning group. The most common reason for exclusion was single doses (84%) followed by prophylactic or anti-inflammatory doses (16%). In total, 55 patients were included in the prewarning group and 50 patients in the postwarning group.
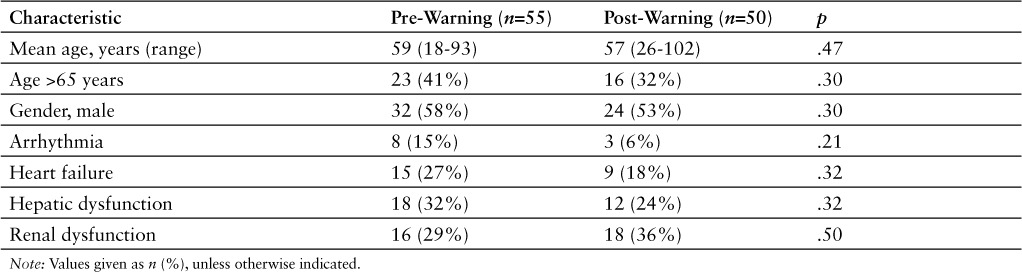
There were no significant differences in demographic or clinical characteristics between the 2 groups (Table 1). Azithromycin utilization in DOT/1,000 patient days decreased from 31.2 in the prewarning group to 17.5 in the postwarning group (p < .001). Most patients (94%) in the prewarning (n = 47) and postwarning (n = 52) groups received azithromycin for pneumonia or chronic obstructive pulmonary disorder exacerbation at treatment dosing. The average duration of azithromycin therapy while participants were inpatients was 5.3 days and 3.4 days in the prewarning and postwarning groups, respectively (p = .003).
Table 1.
Baseline characteristics
A baseline ECG was obtained in 89% (n = 49) of patients in the prewarning group and in 86% (n = 43) of patients in the postwarning group (p = .77). Follow-up ECGs were ordered 15.8% (n = 9) of the time in the prewarning group and 16% (n = 8) in the postwarning group (p = 1.0). However, 69% (n = 34) versus 58% (n = 25) of the patients that had a baseline ECG in the prewarning versus post-warning groups, respectively, met guideline criteria for daily ECGs. Only 32% (n = 11) of patients in the prewarning group and 20% (n = 5) in the postwarning groups with baseline ECGs that met criteria for follow-up ECGs daily had a documented follow-up ECG. The average baseline QTc interval in the prewarning group was 457.9 ms and postwarning group was 446.5 ms (p = .18).
DISCUSSION
We observed a significant reduction in the utilization of azithromycin following the FDA warning regarding the increased risk of QT prolongation. However, no significant changes in monitoring practices were observed, suggesting that the warning encouraged physicians to choose alternative therapy rather than implementing heightened QTc interval monitoring.
Several studies have evaluated the impact of FDA drug safety communications, advisories, and warnings on the use of other medications, but few have evaluated the impact on antimicrobials.9–11 One study evaluating the impact of the FDA health advisory regarding the risk of hepatotoxicity with telithromycin released in 2007 assessed utilization by pharmacy claims data from an insurer with 1.8 million members.10 Telithromycin utilization fell by 80% following the FDA advisory, from a peak of 940 claims per million members to 186 claims per million members. In the present study, we implemented an analysis using an accepted metric for assessing antimicrobial utilization (DOT/1,000 patient days) and observed an overall 46% reduction in utilization. Both FDA communications for telithromycin and azithromycin alert prescribers to a severe, potentially life-threatening reaction that resulted in labelling changes. The impact of such warnings can change prescribing patterns and overall usage of the agent in question based on the data from our study and the telithromycin utilization study.
We observed no significant change in QTc interval monitoring with baseline or follow-up ECG monitoring among patients receiving azithromycin. Similarly, a recent study that evaluated the impact of an FDA warning on the utilization and monitoring of hepatic function among patients receiving dronedarone also found no significant changes in monitoring practices following the warning.11 In our study, more than 80% of patients in both the pre- and postwarning groups had a baseline ECG. This may be due to clinicians already being aware of the potential risk of QT prolongation, however prescribers may have obtained a baseline ECG for a variety of reasons. The percentage of patients with repeat ECGs, particularly among those with a baseline QTc greater than 440 ms for whom our guidelines recommended daily ECGs, was low in both groups. Although our guideline was vetted through several physician groups at our institution, adherence to recommended monitoring ultimately depends on providers' discretion. Additionally, continuous ECG monitoring is an alternate method that is recommended by our institution's guideline. Other than intensive care and cardiac units, most wards were not equipped to provide this during the observed study periods. Consequently, we chose not to include continuous ECG monitoring, which may have contributed to the low percentages of repeat ECGs overall. Lastly, the low percentage of repeat ECGs may also be attributable to the short duration of azithromycin for atypical bacterial coverage; the average duration of therapy during the inpatient stay was 5.3 and 3.4 days in the prewarning and postwarning group, respectively. Even though daily ECGs may have been recommended, physicians may have elected not to repeat given the short duration.
Definitive data showing that QT monitoring reduces rates of patients developing QT prolongation while receiving QT prolonging agents is lacking, but the implementation of an alerting system to identify patients at risk and to recommend more intense monitoring has been found to be associated with a reduction in the risk and in the development of QTc prolongation.12 Through the use of a computerized medication order entry clinical decision support system that triggered alerts at the point of order entry when patients with elevated QTc prolongation risk (based on a validated risk score) were prescribed medications associated with QTc prolongation, there was a lower proportion of patients developing QTc prolongation (9.7% vs 16.9%) and a significant reduction in the odds ratio (OR, 0.65; 95% CI, 0.56–0.89) for QTc prolongation among cardiac critical care patients. This study highlights the potential utility of computerized medication order entry systems to generate alerts that identify high-risk patients and provide a potential strategy for hospitals to better implement FDA warnings and associated recommendations in monitoring for severe adverse drug events.
There were several limitations with the present study. Because it was a single-center study, the external applicability may be limited to centers where timely communications of FDA warnings are part of standard practice, QTc monitoring guidelines are available and enforced, and where antimicrobial stewardship presence may influence dissemination of warning information and prescribing practices. Additionally, other factors could have contributed to the decrease in utilization observed in the postwarning time period. For example, in addition to modifying the QTc monitoring guideline, we developed a community-acquired pneumonia treatment pathway in August 2013 that advised referral to the QTc monitoring guideline if baseline QTc was greater than 440 ms. The availability of this pathway could have further influenced antibiotic selection. Last, the small sample size may have resulted in the study being underpowered to detect a difference in monitoring practices in the 2 time periods. Additionally, we did not evaluate clinical outcomes or occurrence of TdP.
FDA drug safety communications can have a significant impact on prescribing patterns and utilization of medications, however they do not necessarily influence monitoring practices. In relation to the effect on antimicrobial utilization specifically, our study contributes to a limited body of literature illustrating how FDA communications regarding severe toxicities associated with antimicrobials can result in significant reductions in utilization.
ACKNOWLEDGMENTS
The authors declare no conflicts of interest.
REFERENCES
- 1. Azithromycin [package insert]. New York: Pfizer Inc.; 2013. [Google Scholar]
- 2. U.S. Food and Drug Administration. . FDA Drug Safety Communication: Azithromycin (Zithromax or Zmax) and the risk of potentially fatal heart rhythms (March 2013). www.fda.gov/Drugs/DrugSafety/ucm341822.htm. Accessed November 2, 2013.
- 3. Ray WA, Murray KT, Hall K. et al. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012; 366: 1881– 1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Svanstrom H, Pasternak B, Hviid A. Use of azithromycin and death from cardiovascular causes. N Engl J Med. 2013; 368: 1704– 1712. [DOI] [PubMed] [Google Scholar]
- 5. Chou HW, Wang JL, Chang CH. et al. Risks of cardiac arrhythmia and mortality among patients using newgeneration macrolides, fluoroquinolones, and beta-lactams/beta-lactamase inhibitors: A Taiwanese nationwide study. Clin Infect Dis. 2015; 60( 4): 566– 577. [DOI] [PubMed] [Google Scholar]
- 6. Rao G, Mann J, Shoaibi A. et al. Azithromycin and levofloxacin use and increased risk of cardiac arrhythmia and death. Ann Fam Med. 2014; 12( 2): 121– 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mortensen EM, Halm EA, Pugh MJ. et al. Association of azithromycin with mortality and cardiovascular events among older patients hospitalized with pneumonia. JAMA. 2014; 311( 21): 2199– 2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Drew BJ, Ackerman MJ, Funk M. et al; on behalf of the American Heart Association Acute Cardiac Care Committee of the Council on Clinical Cardiology, the Council on Cardiovascular Nursing, and the American College of Cardiology Foundation Prevention of torsade de pointes in hospital settings: A scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation. 2010; 121: 1047– 1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dusetzina SB, Higashi AS, Dorsey R. et al. Impact of FDA drug risk communications on healthcare utilization and health behaviors: A systematic review. Med Care. 2012: 56( 6): 466– 478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gleason PP, Walters A, Heaton AH, Schafer JA. Telithromycin: The perils of hasty adoption and persistence of off-label prescribing. J Managed Care Pharm. 2007; 13( 5): 420– 425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arif SA, Drury R, Ader P. Impact of Food and Drug Administration hepatotoxicity warning on prescribing and monitoring of dronedarone in a tertiary teaching hospital [published online ahead of print September 18, 2015]. Int J Pharm Pract. [DOI] [PubMed] [Google Scholar]
- 12. Tisdale JE, Jaynes HA, Kingery JR. et al. Effectiveness of a clinical decision support system for reducing the risk of QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes. 2014; 7: 381– 390. [DOI] [PMC free article] [PubMed] [Google Scholar]


