A 31-year-old woman (gravida 2, para 1, abortus 1), who had been attempting pregnancy, underwent ultrasonography in the first trimester; results were suspicious for molar pregnancy, showing echogenic and lobulated tissue inside an intrauterine gestational sac. A few days later, she presented with an apparent spontaneous abortion, passing blood and tissue. Repeat ultrasonography showed that the abnormal-appearing sac had been passed, and there was a small amount of echogenic debris remaining, consistent with blood. The adnexa were normal.
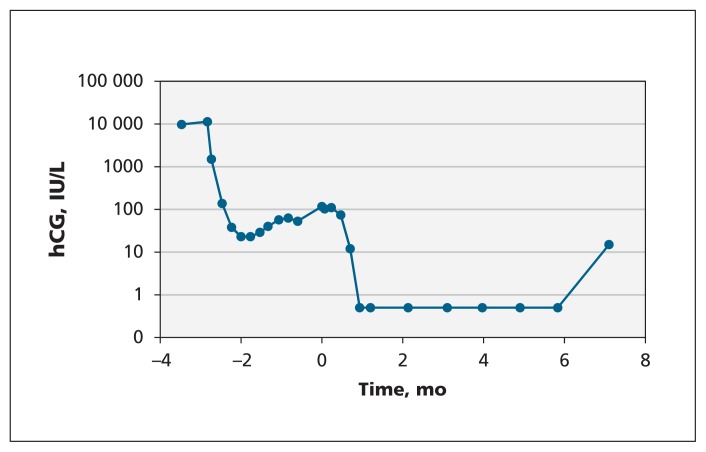
The gynecology service was consulted given that initial ultrasonography was suspicious for molar pregnancy. Because no tissue had been obtained for pathology, serum human chorionic gonadotropin (hCG) levels were monitored weekly. The patient’s serum hCG levels initially dropped from 10 000 to 23 IU/L within the first month; however, the level increased and plateaued at about 100 IU/L two months later (Figure 1).
Figure 1:
Serum human chorionic gonadotropin (hCG) levels of a 31-year-old woman after spontaneous abortion of a possible molar pregnancy. Month 0 indicates when she was referred to the gynecology service and medical biochemistry was consulted. Values from months 1 to 6 were below the assay detection limit (1 IU/L).
The gynecologic oncology service was consulted because of the persistent low-level increase in serum hCG levels. The possibility of persistent gestational trophoblastic disease was considered. Repeat ultrasonography was normal. Inspection of the patient’s uterus by hysteroscopy three months after the spontaneous abortion showed a normal endometrial cavity with no evidence of retained tissue. Results of endometrial biopsy were negative. Other investigations looking for metastatic disease, including chest radiography, pelvic magnetic resonance imaging (MRI), and computed tomography (CT) of the chest, abdomen and pelvis, all yielded negative results.
The medical biochemistry service was consulted for further investigations and to rule out the possibility of laboratory analytical error or assay interference. The patient’s serum was diluted at ratios of 1:20 and 1:40 for an immunoassay interference study, which showed a linear response and about 90% recovery on hCG value. Serum treated with a heterophilic blocking tube generated the same value as a neat specimen of 103 IU/L. A urine qualitative strip test for pregnancy was positive. Serum was sent to reference laboratories to test for hyperglycosylated hCG, which was undetectable (< 5.5 IU/L, the detection limit of the assay), and free β-hCG, which was low at 3.6 IU/L. Serum luteinizing hormone and follicle-stimulating hormone levels were normal at 4.1 and 6.4 IU/L, respectively. These results indicated that the observed increase of hCG levels were true values, supporting a diagnosis of quiescent gestational trophoblastic disease.
The patient’s serum level of hCG was followed weekly; by two weeks, the level started to drop and returned to normal in one month. Her serum hCG level remained below the assay detection limit (1 IU/L) for the next six months until she had another normal pregnancy, which was conceived about 10 months after the initial spontaneous abortion.
Discussion
Clinicians should consider several diagnoses when faced with the persistent presence of low-level serum hCG, as described in this case (Box 1).1–6 These include new pregnancy, retained products of conception, a false-positive hCG result, pituitary or menopausal hCG, nontrophoblastic cancer and gestational trophoblastic disease.
Box 1: Differential diagnosis for a persistent low-level increase of serum human chorionic gonadotropin levels1–6.
| Possible causes | Investigations/results |
|---|---|
| Pregnancy, ectopic pregnancy, heterotopic pregnancy |
|
| Retained products of conception |
|
| Phantom hCG1 | Interference confirmed if
|
| Pituitary hCG production2,3 |
|
| Gestational trophoblastic disease | |
| Quiescent gestational trophoblastic disease4–6 |
|
| Other gestational trophoblastic diseases4 |
|
| Nontrophoblastic cancer2 |
|
Note: hCG = human chorionic gonadotropin.
Our patient was diagnosed with quiescent gestational trophoblastic disease, a disease entity identified in 2001–2003, which is characterized by persistent low-level hCG elevation and usually has a benign prognosis.4–6
Differential diagnosis of low-level hCG elevation
New pregnancy
Serum hCG is the most sensitive marker for pregnancy. If a patient has actively been trying to get pregnant, the presence of low-level serum hCG could be consistent with a new viable pregnancy, an ectopic pregnancy or a recurrent molar pregnancy. However, in the case of our patient, the serum hCG pattern did not support a new viable pregnancy. Her sexual history did not support a new conception, and thus pregnancy was thought to be an unlikely cause of her rise in serum hCG levels.
Retained products of conception
Retained products of conception can cause mild persistent hCG, but the levels should not increase as seen with our patient (from 23 to about 100 IU/L). Persistent heterotopic pregnancy is rare but should be considered (Box 1). In the case of our patient, repeat ultrasonography, endometrial biopsy and hysteroscopy were all negative for retained products of conception.
Phantom hCG
False-positive serum hCG, so-called phantom hCG, due to heterophilic antibody interference on the hCG immunoassay, is another possible cause of low-level increase in serum hCG level. Heterophilic antibodies are human antibodies that have the capability to bind to other species’ immunoglobulins; among them, the most important ones noted in laboratory and clinical medicine, are human anti-animal antibodies and human anti-mouse antibodies. Most heterophilic antibodies are common, naturally occurring antibodies with low affinities. However, human anti-animal antibodies and human anti-mouse antibodies are highaffinity and high-specificity antibodies, which are encountered in individuals who have had prior sensitization to an animal species, through occupational animal exposure, animal products in diet or therapeutic intervention. These two antibodies may bind to reagent antibodies used in the immunoassay (e.g., mouse monoclonal antibody) to bridge the capture antibody and the tracer antibody in the immunometric reaction of the hCG assay, and lead to a false-positive result.7
A 10-year report of the USA hCG Reference Service identified 83 of 565 recorded consulting cases to be false positives.2 In 62 of these 83 cases, patients underwent unnecessary chemotherapy or hysterectomy, which led to lawsuits. 2 The American College of Obstetricians and Gynecologists recommends three procedures to rule out the presence of heterophilic antibodies or other interfering substances:1
A urine test (either quantitative or qualitative). Interference is confirmed if the urine pregnancy test is negative and the serum hCG value is at least 50 IU/L.
Serial dilutions to check for linearity and recovery. Lack of linearity and recovery confirms interference because the interfering antibody or substance usually presents a different dynamic reaction to reagent antibodies compared with the analyte in an immunoassay.
Pretreatment of serum to remove heterophilic antibodies (e.g., with a heterophilic blocking tube). If the result becomes negative after removal of the heterophilic antibody, interference is present.
We performed these procedures and concluded that there was neither evidence of assay interference nor any other laboratory error in this case. Additionally, the serum can be tested with a different commercial hCG immunoassay to see if this results in a substantially discrepant level.
Pituitary or menopausal hCG
Pituitary hCG is produced in the anterior pituitary gland, along with luteinizing hormone and follicle-stimulating hormone, when production of gonadotropin-releasing hormone becomes unrestricted owing to the absence of sex steroid feedback to the hypothalamus. Pituitary hCG usually increases mildly (≤ 39 IU/L) along with rising follicle-stimulating hormone (> 30 IU/L) during the peri- and postmenopausal period.2,3 Because our patient was 31 years of age, without premature ovarian failure and with normal follicle-stimulating hormone and luteinizing hormone levels, we excluded pituitary hCG as a diagnosis.
Gestational trophoblastic disease
Gestational trophoblastic disease is a general term for a group of rare tumours involving abnormal growth of trophoblasts in the uterus. Levels of hCG are substantially elevated during the active state of gestational trophoblastic disease (e.g., partial and complete moles of gestational trophoblastic disease have an average hCG serum level of 49 000 and up to 100 000 IU/L, respectively).4 Choriocarcinomas may have even higher levels of hCG production (e.g., > 600 000 IU/L).4
When gestational trophoblastic disease is present, the secretion of β subunits of hCG increases. Moreover, invasive cytotrophoblast cells secrete hyperglycosylated hCG. Studies suggest that free β-hCG and hyperglycosylated hCG are effective biomarkers for detecting hCG-producing tumours and invasive gestational trophoblastic disease.8,9 The USA hCG Reference Service recommends using the cutoff of free β-hCG greater than 40% of total hCG to identify cancers and cases of placental site trophoblastic disease.2 Serum hyperglycosylated hCG greater than 30% of total hCG is suggested to distinguish aggressive gestational trophoblastic disease and minimally invasive gestational trophoblastic disease, which is generally chemorefractory, with a very slow hCG doubling rate of about two to six weeks.2
Quiescent gestational trophoblastic disease
In the 10-year report of the USA hCG Reference Service, 168 of the 565 consulting cases had no demonstrated serum hyperglycosylated hCG. In these 168 cases, three-month to two-year plateaus of stable hCG levels were seen, with levels ranging from 1.6 to 212 IU/L. Dilation, curettage and histology on some of these cases found only highly differentiated syncytiotrophoblast tissue.2 This group of benign disorders is collectively named quiescent gestational trophoblastic disease and is characterized by the following criteria: history of gestational trophoblastic disease, persistent low-level stable hCG elevation of less than 212 IU/L and undetectable hyperglycosylated hCG.4–6
Our patient’s history of spontaneous abortion and suspicion of molar pregnancy on ultrasonography followed by persistent low-level hCG elevation, undetectable hyperglycosylated hCG and low free β-hCG pointed to a diagnosis of quiescent gestational trophoblastic disease.
Quiescent gestational trophoblastic disease usually occurs in women who have had a therapeutic or spontaneous abortion of a molar pregnancy. Because the mass is extremely small, it cannot be seen on imaging (ultrasonography, MRI or CT) or hysteroscopy. It usually does not grow or invade, and does not respond to chemotherapy.
Nontrophoblastic cancer
Persistent low levels of hCG may be caused by nontrophoblastic cancers originating in the ovary, bladder, colon, brain, liver, breast, pancreas, kidney, lung and prostate. In these cancers, levels of serum free β-hCG are usually higher than 40% (mean 73% ± 21%) of total hCG, and urine β-core fragment levels are also elevated (mean 87% ± 35%).2 Magnetic resonance imaging of the head and abdomen, and CT of the chest, abdomen and pelvis are typically included in the workup, as was done with our patient.
Management of quiescent gestational trophoblastic disease
There is no specific treatment required for quiescent gestational trophoblastic disease, because the tissue usually dies out or is aborted by itself without medical interference, and the hCG production will return to normal. About 85% of cases resolve over three to six months with the spontaneous disappearance of hCG; however, the remaining 15% lead to recurrent gestational trophoblastic disease requiring chemotherapy.2
Because of the possibility of recurrent gestational trophoblastic disease, the Society of Obstetricians and Gynaecologists of Canada recommends that hCG levels be followed to normal levels for six months with no new conceptions after evacuation of a molar pregnancy. 10 The International Society for the Study of Trophoblastic Disease 2001 recommendations on the management of quiescent gestational trophoblastic disease suggest excluding false-positive hCG levels, thoroughly investigating the extent of disease, avoiding immediate chemotherapy or surgery, and monitoring with serial hCG while avoiding pregnancy for a certain time.11 Treatment should be initiated promptly only when there is a substantial rise in hCG or presence of overt clinical disease.11
This policy of clinical surveillance is supported by a recent retrospective study involving a cohort of 13 960 patients with hydatidiform moles, registered from 1993 to 2008.12 In this cohort, 76 patients had persistently elevated, but declining, hCG levels six months after evacuation. Most (n = 66) of these patients (hCG range 5–887 [median 13] IU/L) continued under surveillance; in 65, hCG values spontaneously normalized, and one patient had persistent hCG elevation because of chronic renal failure, but remained healthy. The remaining 10 patients (hCG range 6–6438 [median 157] IU/L) received chemotherapy; hCG levels returned to normal in eight of these individuals and remained slightly high (6–11 IU/L) in two patients who were asymptomatic. There was no significant difference in outcomes between individuals in the surveillance and chemotherapy groups.12
Therefore, we suggest that patients with quiescent gestational trophoblastic disease have their hCG levels followed weekly until negative, then monthly for 6 to 12 months. Patients can be reassured that this is a mostly benign disease with good outcomes and good prognosis for future pregnancies.
The section Cases presents brief case reports that convey clear, practical lessons. Preference is given to common presentations of important rare conditions, and important unusual presentations of common problems. Articles start with a case presentation (500 words maximum), and a discussion of the underlying condition follows (1000 words maximum). Visual elements (e.g., tables of the differential diagnosis, clinical features or diagnostic approach) are encouraged. Consent from patients for publication of their story is a necessity. See information for authors at www.cmaj.ca.
Key points
Pregnancy, ectopic pregnancy, retained products of conception, false-positive human chorionic gonadotropin (hCG) test results, pituitary production of hCG, gestational trophoblastic disease and nontrophoblastic cancer are all possible causes of increases in serum hCG levels.
A false-positive result of serum hCG, if undetected, may lead to unnecessary chemotherapy or hysterectomy.
Quiescent gestational trophoblastic disease is a benign condition characterized by a low-level increase of serum hCG, undetectable hyperglycosylated hCG and low free β-hCG.
Levels of hCG should be monitored in patients with quiescent gestational trophoblastic disease; in most cases, the levels will gradually decrease to normal.
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
The authors have obtained patient consent.
Contributors: Yu Chen and Sheri-Lee Samson contributed to the conception of the project. Jianing Chen drafted the manuscript, which Sheri-Lee Samson, James Bentley and Yu Chen revised. All of the authors approved the final version to be published and agreed to act as guarantors of the work.
References
- 1.Committee on Gynecologic Practice, The American College of Obstetricians and Gynecologists. Committee opinion: number 278, November 2002. Avoiding inappropriate clinical decisions based on false-positive human chorionic gonadotropin test results. Obstet Gynecol 2002;100:1057–9. [DOI] [PubMed] [Google Scholar]
- 2.Cole LA, Laidler LL, Muller CY. USA hCG reference service, 10-year report. Clin Biochem 2010;43:1013–22. [DOI] [PubMed] [Google Scholar]
- 3.Gronowski AM, Fantz CR, Parvin CA, et al. Use of serum FSH to identify perimenopausal women with pituitary hCG. Clin Chem 2008;54:652–6. [DOI] [PubMed] [Google Scholar]
- 4.Kohorn EI. Persistent low-level “real” human chorionic gonadotropin: a clinical challenge and a therapeutic dilemma. Gynecol Oncol 2002;85:315–20. [DOI] [PubMed] [Google Scholar]
- 5.Khanlian SA, Smith HO, Cole LA. Persistent low levels of human chorionic gonadotropin: a premalignant gestational trophoblastic disease. Am J Obstet Gynecol 2003;188:1254–9. [DOI] [PubMed] [Google Scholar]
- 6.Hancock BW, Tidy JA. Clinical management of persistent low level hCG elevation. Trophobl Dis Upd 2003;4:5–6. [Google Scholar]
- 7.Klee GG. Interferences in hormone immunoassays. Clin Lab Med 2004;24:1–18. [DOI] [PubMed] [Google Scholar]
- 8.Cole LA, Khanlian SA, Muller CY, et al. Gestational trophoblastic diseases: 3. Human chorionic gonadotropin-free beta-subunit, a reliable marker of placental site trophoblastic tumors. Gynecol Oncol 2006;102:160–4. [DOI] [PubMed] [Google Scholar]
- 9.Cole LA, Muller CY. Hyperglycosylated hCG in the management of quiescent and chemorefractory gestational trophoblastic diseases. Gynecol Oncol 2010;116:3–9. [DOI] [PubMed] [Google Scholar]
- 10.Gerulath AH, Ehlen TG, Bessette P, et al. ; Society of Obstetricians and Gynaecologists of Canada; Gynaecologic Oncologists of Canada; Society of Canadian Colposcopists. Gestational trophoblastic disease. J Obstet Gynaecol Can 2002;24: 434–46. [PubMed] [Google Scholar]
- 11.Lurain JR. Gestational trophoblastic disease I: epidemiology, pathology, clinical presentation and diagnosis of gestational trophoblastic disease, and management of hydatidiform mole. Am J Obstet Gynecol 2010;203:531–9. [DOI] [PubMed] [Google Scholar]
- 12.Agarwal R, Teoh S, Short D, et al. Chemotherapy and human chorionic gonadotropin concentrations 6 months after uterine evacuation of molar pregnancy: a retrospective cohort study. Lancet 2012;379:130–5. [DOI] [PMC free article] [PubMed] [Google Scholar]