Abstract
Interneurons of the cerebral cortex play a significant role in cortical information processing and are of clinical interest due to their involvement in neurological disorders. In the human neocortex, three subsets of interneurons can be identified based on the production of the calcium-binding proteins parvalbumin, calretinin or calbindin. A subset of interneurons in the mouse cortex expresses the serotonin 3A receptor (5-HT3AR). Previous work in humans has also demonstrated the presence of a subgroup of cortical neurons that produces the catecholaminergic enzyme tyrosine hydroxylase (TH). Many TH-producing cells in the rat cortex coexpress calretinin and are adjacent to blood vessels. However, little is known about the phenotype of these TH interneurons in humans. Here we immunohistochemically examined the coexpression of TH with parvalbumin, calretinin, calbindin or 5-HT3AR in human Brodmann’s areas 10 and 24, cortical regions with high densities of TH-containing neurons. Colocalization of TH with these calcium-binding proteins and with 5-HT3AR was not detected in either area. Cortical TH cells were rarely apposed to blood vessels, denoted by immunolabeling for the gliovascular marker aquaporin-4. Our results suggest that the TH-immunoreactive cells in the human cortex do not overlap with any known neurochemically-defined subsets of interneurons and provide further evidence of differences in the phenotype of these cells across species.
Keywords: Interneuron, Parvalbumin, Calretinin, Calbindin, 5-HT3A, Aquaporin-4
1. Introduction
Interneurons in the cerebral cortex are predominantly non-pyramidal, γ-aminobutyric acid (GABA)-producing cells that provide inhibitory input to the more numerous projection neurons, thereby regulating the cortical microcircuitry (DeFelipe, 2002; Markram et al., 2004). Cortical interneurons are clinically relevant; their dysfunction has been implicated in a variety of neurological disorders, including schizophrenia and epilepsy (Benes and Berretta, 2001; Chu and Anderson, 2015; Daskalakis et al., 2007; Di Cristo, 2007; Levitt et al., 2004; Lewis et al., 2012; Marin, 2012). Diverse subgroups of interneurons have been identified on the basis of their morphology, electrophysiological characteristics, embryonic origin, and molecular and neurochemical attributes (Ascoli et al., 2008; Chu and Anderson, 2015; Vitalis and Rossier, 2011; Wonders and Anderson, 2006). In the rodent neocortex, three non-overlapping subsets of interneurons have been defined based on the production of the calcium-binding proteins parvalbumin (PV) or calretinin (CR) or the peptide somatostatin (SST) (Gonchar and Burkhalter, 1997; Kubota et al., 1994; Xu et al., 2004). In the mouse, a subset of cortical interneurons can be identified by the expression of the ionotropic serotonergic receptor subtype, 5-hydroxytryptamine 3A (5-HT3AR), and this subgroup overlaps with the previously-identified CR subset (Lee et al., 2010; Rudy et al., 2011; Vucurovic et al., 2010). Interneurons in the primate neocortex, likewise, have been subdivided into three neurochemically-distinct groups that contain PV, CR, or another calcium-binding protein calbindin (CB) (Carder et al., 1996; Conde et al., 1994; DeFelipe, 1997; Gabbott et al., 1997; Glezer et al., 1993; Sherwood et al., 2007, 2010; Zaitsev et al., 2009). The presence of 5-HT3AR in human interneurons has not been reported.
A subpopulation of neurons in the neocortex of rodents (Asmus et al., 2008, 2011; Berger et al., 1985; Kosaka et al., 1987a, 1987b; Satoh and Suzuki, 1990; Wachter et al., 2014), non-human primates (Raghanti et al., 2009; Weihe et al., 2006), and humans (Benavides-Piccione and DeFelipe, 2003, 2007; Fukuda et al., 1999; Gaspar et al., 1987; Hornung et al., 1989; Ikemoto et al., 1999; Kuljis et al., 1989; Marui et al., 2003; Raghanti et al., 2009; Trottier et al., 1989), is immunoreactive (IR) for tyrosine hydroxylase (TH), the first enzyme in the catecholamine biosynthesis pathway. Cortical TH-IR cells are considered to be inhibitory interneurons because they are small, non-pyramidal cells, many of which coexpress GABA or its biosynthetic enzyme, glutamic acid decarboxylase (GAD). These cells are outside the classically-defined catecholaminergic cell groups that are present in subcortical brain regions (Hokfelt et al., 1984). Moreover, in all species examined, these TH-IR cells do not contain any subsequent catecholaminergic enzymes (Berger et al., 1985; Gaspar et al., 1987; Ikemoto et al., 1999; Satoh and Suzuki, 1990; Weihe et al., 2006), leaving their end-product in question. In rats TH-IR cells are present in all cortical laminae but are most abundant in layers II/III (Asmus et al., 2008; Berger et al., 1985; Kosaka et al., 1987a, 1987b). Coexpression of CR but not PV or SST was observed in many cortical TH-IR cells in normal rats (Asmus et al., 2008) and in rats treated intraventricularly with the catecholaminergic neurotoxin 6-hydryoxydopamine (6-OHDA) (Wachter et al., 2014). Furthermore, in rats many of the TH-IR somata are adjacent to cortical blood vessels that are outlined by aquaporin-4 (AQP4) (Asmus et al., 2011), which is a channel protein found in the astrocytic end-feet surrounding central nervous system (CNS) microvessels (Tait et al., 2008). In humans, TH-IR cells are found predominantly in layers V and VI, although more superficially-located TH-IR cells are observed in some areas (Benavides-Piccione and DeFelipe, 2003, 2007; Fukuda et al., 1999; Gaspar et al., 1987; Hornung et al., 1989; Ikemoto et al., 1999; Kuljis et al., 1989; Marui et al., 2003; Raghanti et al., 2009; Trottier et al., 1989). Neurons that produce TH are widely distributed throughout the human neocortex, with two of the highest densities of these cells being reported in the associative dorsolateral frontal cortex (Brodmann’s area (BA) 10) and in the limbic cingulate cortex (BA 24) (Benavides-Piccione and DeFelipe, 2007). In the human temporal cortex, approximately one-fourth of the TH-IR neurons coproduce neuronal nitric oxide synthase (nNOS), the biosynthetic enzyme for nitric oxide (Benavides-Piccione and DeFelipe, 2003). Colocalization of TH with other neurochemical markers and proximity of TH-IR cells to the cortical vasculature have not been reported in the human cortex.
The paucity of information regarding the neurochemical phenotype and possible function of cortical TH-IR neurons, especially in humans, is surprising given that a loss of these cells is seen in Parkinson’s disease (Fukuda et al., 1999) and dementia (Marui et al., 2003). Here, we tested the hypothesis that human cortical TH-IR somata in BA 10 and 24 overlap with one or more of the neurochemically-defined subgroups of interneurons that produce PV, CR, CB, or 5-HT3AR. The close association of TH-IR cell bodies and cortical microvessels was also examined in these areas using the gliovascular marker AQP4.
2. Materials and methods
2.1 Specimens, fixation and processing
Human cortical samples (BA 10 and 24) derived from the left hemisphere were provided by the Northwestern University Alzheimer’s Disease Center Brain Bank. The human subjects were non-geriatric adults, exhibited no evidence of cognitive changes before death, and all received a score of zero for the CERAD senile plaque grade (Mirra et al., 1991) and the Braak and Braak (1991) neurofibrillary tangle stage. Cortical samples were analyzed from a subset of seven brains, four female (age range 40–53) and three male (age range 54–56). The postmortem interval (PMI) prior to immersion-fixation did not exceed 17 hours (see Table 1 for complete demographic and PMI information). Because this research involved the use of postmortem specimens, the project was exempt from the need for approval of Centre College’s Institutional Review Board regarding human subjects.
Table 1.
Demographics of cases used in this study.
| Case | Age (years) | Gender | PMI (hours) |
|---|---|---|---|
| 1 | 54 | M | 12 |
| 2 | 56 | M | 5 |
| 3 | 56 | M | 5 |
| 4 | 40 | F | 17 |
| 5 | 43 | F | 10 |
| 6 | 43 | F | 6 |
| 7 | 54 | F | 13 |
Brain samples were immersion-fixed in 10% buffered formalin for approximately one week, then transferred to 0.1 M phosphate buffered saline (PBS, pH 7.4) containing 0.1% sodium azide at 4º C. Prior to sectioning, specimens were cryoprotected in a series of sucrose solutions (10, 20, and 30%) until saturated. Brains were frozen in dry ice and sectioned at 40 μm using a freezing sliding microtome (Leica SM2000R, Buffalo Grove, IL). Sections were placed into individual microcentrifuge tubes containing a freezer storage solution (30% of each distilled water, ethylene glycol, and glycerol and 10% 0.244 M phosphate buffered saline) and numbered sequentially. Sections were stored at −20° C until further processing.
2.2 Immunohistochemistry
The protocol followed here was a modification of a previously-reported protocol (Raghanti et al., 2009). To examine the colocalization of TH with calcium-binding proteins, rabbit anti-TH (1:200, Pel-Freez Biologicals, Rogers, AR) was used in combination with one of the following antibodies: mouse anti-PV (1:500, Millipore, Billerica, MA), mouse anti-CR (1:1000, Millipore), or mouse anti-CB (1:8000, Swant, Bellinzona, Switzerland). To examine the colocalization of TH with 5-HT3AR, mouse anti-TH (1:200, ImmunoStar, Hudson, WI) was used in combination with rabbit anti-5-HT3AR (1:50, Sigma, St. Louis, MO). To determine the apposition of TH-IR cells and microvessels, mouse anti-TH (1:200, ImmunoStar) was used in combination with a rabbit antibody against the gliovascular marker AQP4 (1:100, Millipore).
The following secondary antibodies (Jackson ImmunoResearch, West Grove, PA) were used in combination: Alexa Fluor 488- or DyLight 488-conjugated donkey anti-rabbit (1:100) and Rhodamine Red-X-conjugated donkey anti-mouse (1:50). Control experiments confirmed that secondary antibodies bound only to the appropriate primary antibodies.
All incubations and wash steps were conducted with free-floating sections in a 24-well plate on an orbital shaker. The brain tissue sections were washed with PBS 10 times for 5 minutes each (10 X 5). All subsequent incubations were followed by 6 X 5 washes in PBS. For antigen retrieval, the sections were incubated in ImmunoSaver Antigen Retriever (1:200, Electron Microscopy Sciences, Hatfield, PA) in distilled water and heated for 30 minutes in a water bath at either 85° C (for TH/CB labeling) or 100° C (for TH/PV, TH/CR, TH/5-HT3AR, and TH/AQP4 labeling). The sections were allowed to cool at room temperature for 10–20 minutes and then were incubated for one hour at room temperature in dilution buffer (PBS containing 2% normal donkey serum, 0.3% Triton X-100, and 5% bovine serum albumin). The sections were incubated in the primary antibody cocktail in dilution buffer for either 24 hours (for TH/PV, TH/CR, TH/5-HT3AR and TH/AQP4 labeling) or 48 hours (for TH/CB labeling) at 4° C, followed by incubation in the secondary antibody cocktail in dilution buffer for 1 hour at room temperature in the dark. To reduce autofluorescence, the sections were placed in 70% ethanol for 5 minutes, incubated in Autofluorescence Eliminator Reagent (undiluted, Millipore) for 4 minutes at room temperature, and then washed in 70% ethanol 3 X 1. After rehydration in PBS (3 X 5), the sections were transferred to a slide and coverslipped with 1:1 PBS:glycerol.
To examine the laminar distribution of TH-IR cells, occasional sections were immunolabeled for TH as described above followed by incubation in dilution buffer containing the nuclear stain DAPI (1:5000, Molecular Probes, Eugene, OR) for three minutes before coverslipping. Because detailed density and laminar distribution data have been reported previously for TH, PV, CR and CB (Benavides-Piccione and DeFelipe 2003, 2007; Gabbot et al., 1997; Gonzalez-Albo et al., 2001; Hof et al., 1999; Raghanti et al., 2009; Sherwood et al., 2010), the laminar identity was not noted for every cell that was immunoreactive for one of these markers.
2.3 Data analysis and imaging
The double-labeled sections were analyzed and images captured and annotated using a Zeiss Axioskop 2 Plus fluorescence microscope equipped with an AxioCam Mrc5 CCD digital camera operated with AxioVision software (Carl Zeiss, Thornwood, NY).
To assess the colocalization of TH with PV, CR, CB, or 5-HT3AR, the number of TH-IR cell profiles in each tissue section from BA 10 and 24 were counted and examined for colocalization. Tissue sections were approximately 15 mm high X 15 mm wide when laid flat on the slide. Intact regions (i.e., no tears or wrinkles) that contained all neocortical layers and underlying white matter were examined. TH-IR cell profiles were counted by examining contiguous fields of view at 400X in a lawnmower pattern (one horizontal row running parallel to the brain surface was examined by moving left to right across the section, followed by examination of the adjacent underlying row moving right to left, and so on) beginning at the outer margin of the section (layers I and II) and continuing to the white matter subjacent to layer VI. This deep white matter was discernable due to its increased darkening by the autofluorescence eliminator. Within each field of view, the entire thickness of the section (40 μm) was scanned to identify TH-IR cell profiles. Free-floating immunohistochemical staining allowed antibody penetration through the section. When a TH-IR cell profile was observed, the filter set was switched to assess colocalization with the other marker.
Counts of cortical TH-IR cell profiles were taken across sections from a subset of the seven brains. At least one section, but typically two to five sections, of each of the two cortical areas (BA 10 and 24) from each brain was double-immunostained for TH and one of the other markers. Four brains were used to examine the colocalization of TH and PV in BA 10 (cases 1, 3, 6, 7) and BA 24 (cases 2, 3, 4, 6). To examine the colocalization of TH and CR, BA 10 of four brains was studied (cases 1, 5, 6, 7) and BA 24 of five brains was studied (cases 3, 4, 5, 6, 7). Four brains were used to examine the colabeling of TH and CB in BA 10 (cases 1, 2, 3, 7) and BA 24 (cases 2, 3, 5, 7). To examine the colocalization of TH and 5-HT3AR, BA 10 of three brains was examined (cases 2, 3, 5) and BA 24 of three brains was studied (cases 2, 5, 7). More than 150 TH-IR cell profiles were examined in sections from each of the two cortical areas (BA 10 and 24) for the TH/PV and TH/CR antibody combinations. More than 135 TH-IR cell profiles were examined in each of these two cortical areas for the TH/CB combination. Approximately 105 TH-IR cell profiles were examined in each of the two cortical areas for the TH/5-HT3AR combination.
Cortical neuron profiles were scored positively for TH, PV, CR, CB, or 5-HT3AR immunolabeling if 1) cell profiles were consistent with the patterns of cellular distribution and morphology described in the literature for each marker, and 2) at least one extension off of a clearly defined cell body could be observed (Asmus et al., 2011). Colocalization of TH with these proteins would have been confirmed by the precise overlap of cell body immunofluorescence in the same focal plane. Statistical analysis was not required because no colocalization was observed. Numerous previous reports, some of which included morphometric analysis, demonstrated detailed information regarding size, morphology, laminar distribution and density of human cortical TH-IR neurons (Benavides-Piccione and DeFelipe, 2003, 2007; Gaspar et al., 1987; Hornung et al., 1989; Ikemoto et al., 1999; Kuljis et al., 1989; Marui et al., 2003; Raghanti et al., 2009; Trottier et al., 1989), so it was not necessary to repeat the same analysis here.
To assess the proximity of TH-IR neurons with cortical microvessels, approximately 60 TH-IR cell profiles were examined in each of the two areas (BA 10 and 24) from two brains for the TH/AQP4 combination. BA 10 was examined from cases 2 and 5, and BA 24 was studied from cases 2 and 7. A TH-IR cell profile was noted as being apposed to a blood vessel if the somata profile was within 5 μm of the AQP4-IR astrocytic endfeet that surrounded the microvessel. Distance was measured using the scale bar function of the Axiovision software.
Photomicrographic images were cropped, lettered and merged using Adobe Photoshop CS3 software (Adobe Systems, Seattle, WA). No other photographic manipulation was performed.
3. Results
The morphology and laminar distribution of cell profiles that were individually immunolabeled for TH, PV, CR, or CB were similar to the characteristics of these cells reported previously in the human cerebral cortex (Benavides-Piccione and DeFelipe 2003, 2007; Gabbot et al., 1997; Gonzalez-Albo et al., 2001; Hof et al., 1999; Raghanti et al., 2009; Sherwood et al., 2010). The majority of cortical TH-IR cell profiles in BA 10 and 24 were small (15 μm or less), fusiform, and bipolar, with occasional multipolar cell profiles observed (Fig. 1A, 1B, 2B, 3B). Based on DAPI counterstaining and proximity to the underlying white matter, these profiles were found predominantly in cortical layers IV-VI. Occasional TH-IR profiles were seen in superficial layers. For schematic representations of TH-IR neurons in the human cortex, see Benavides-Piccione and DeFelipe (2007) and Raghanti et al. (2009).
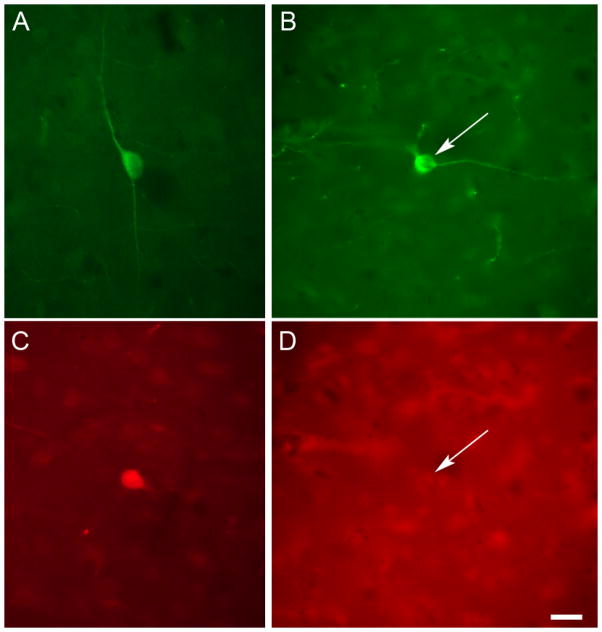
Fig. 1.
TH and PV did not colocalize in the human cerebral cortex. (A) Photomicrograph of a representative non-pyramidal TH-IR neuron profile in BA 10 of the human cerebral cortex, illustrating the small soma size and fusiform morphology. (C) Photomicrograph of a cortical PV-IR neuron profile in human BA 10. This section of cortex was immunolabeled for both PV and TH. This PV-IR cell was not colabeled for TH. (B, D) Photomicrographs of the same field of view in BA 24 showing a neuron profile (arrow) that was TH-IR (B) but not PV-IR (D). Scale bar = 20 μm.
Fig. 2.
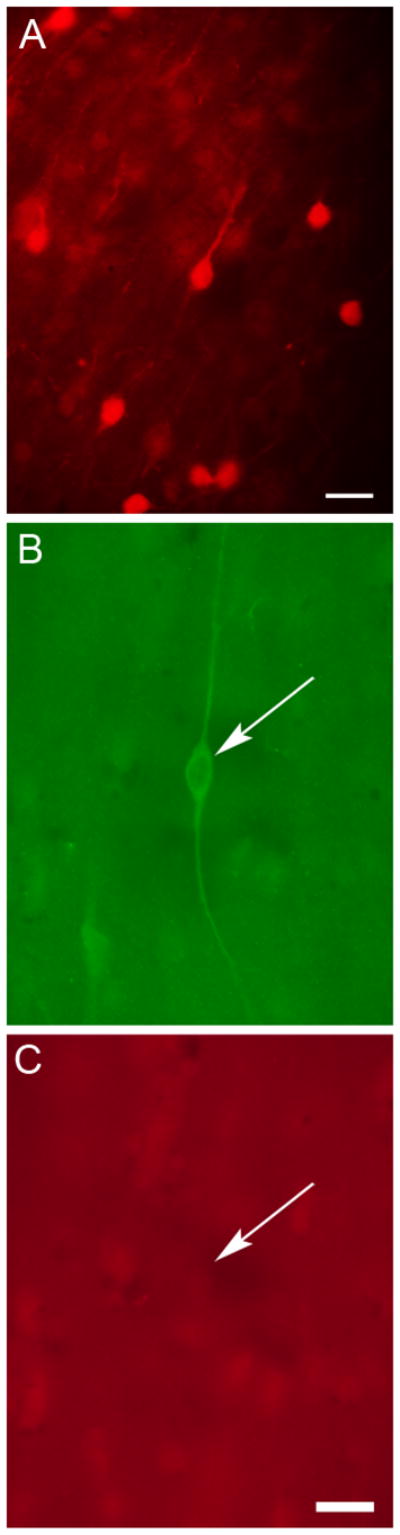
TH and CR did not colocalize in the human cerebral cortex. (A) Photomicrograph of cortical CR-IR neuron profiles in human BA 10. This section of cortex was immunolabeled for both CR and TH. These CR-IR cell profiles were not colabeled for TH. (B, C) Photomicrographs of the same field of view in BA 10 showing a neuron profile (arrow) that was TH-IR (B) but not CR-IR (C). Scale bars = 20 μm.
Fig. 3.
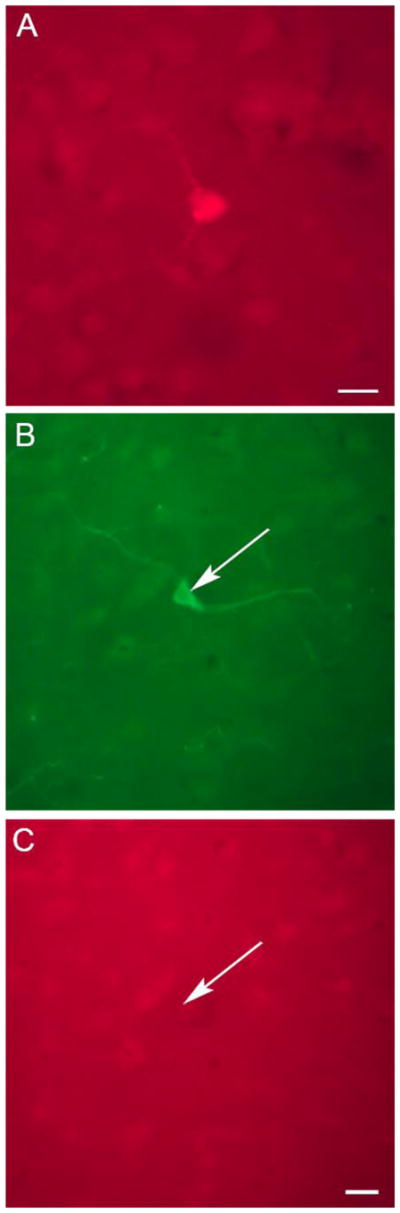
TH and CB did not colocalize in the human cerebral cortex. (A) Photomicrograph of a cortical CB-IR neuron profile in human BA 10. This section of cortex was immunolabeled for both CB and TH. This CB-IR cell profile was not colabeled for TH. Scale bar = 50 μm. (B, C) Photomicrographs of the same field of view in BA 24 showing a neuron profile (arrow) that was TH-IR (B) but not CB-IR (C). Scale bar = 20 μm.
As reported previously, most cell profiles immunolabeled for PV appeared to be relatively large and round and were scattered throughout both superficial and deep layers of the cortex (Fig. 1C) (Gonzalez-Albo et al., 2001; Hof et al., 1999; Sherwood et al., 2010). After double-labeling brain sections from BA 10 and 24 from at least 4 individuals for TH and PV, we examined a total of more than 150 TH-IR cell profiles in each area. No TH-IR profiles were labeled for PV (Fig. 1B, 1D).
Cortical CR-IR cell profiles were typically small and bipolar and were distributed predominantly in superficial cortical layers (Fig. 2A), as reported previously (Gabbot et al., 1997; Gonzalez-Albo et al., 2001; Hof et al., 1999; Sherwood et al., 2010). At least 150 total TH-IR cell profiles were examined in sections from BA 10 and 24 from at least 4 brains that were double-labeled for TH and CR. No TH-IR profiles were colabeled for CR (Fig. 2B, 2C).
Similar to previous reports, cell profiles immunolabeled for CB were most commonly multipolar and were prevalent in superficial layers of the cortex (Fig. 3A) (Gonzalez-Albo et al., 2001; Hof et al., 1999; Sherwood et al., 2010). After double-labeling for TH and CB in 4 different brains, none of the 179 TH-IR cell profiles that were examined in BA 10 colocalized with CB, and none of the 137 TH-IR profiles in BA 24 colabeled with CB (Fig. 3B, 3C).
Cells immunoreactive for 5-HT3AR have not been reported in the human cortex. Sections from BA 10 and 24 from 3 brains were examined for 5-HT3AR immunoreactivity. Cell profiles immunolabeled for 5-HT3AR were typically small, multipolar and distributed throughout superficial and deep cortical layers (Fig. 4). Numerous 5-HT3AR-IR fibers could be seen. After co-labeling for TH and 5-HT3AR, only 1 of the 111 TH-IR cell profiles in BA 10 coproduced 5-HT3AR, and none of the 105 TH-IR cell profiles in BA 24 were colabeled.
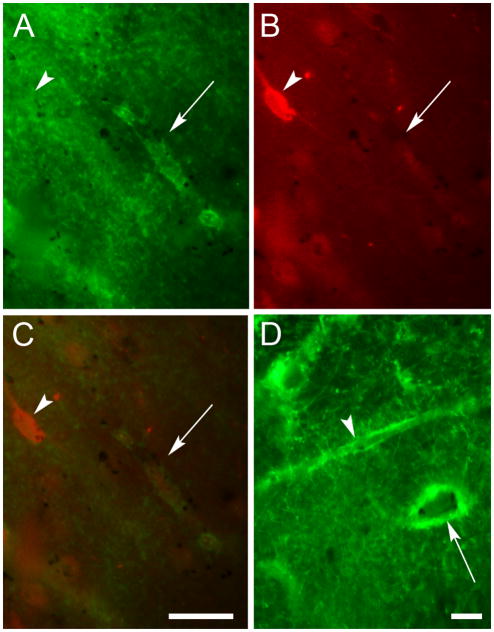
Fig. 4.
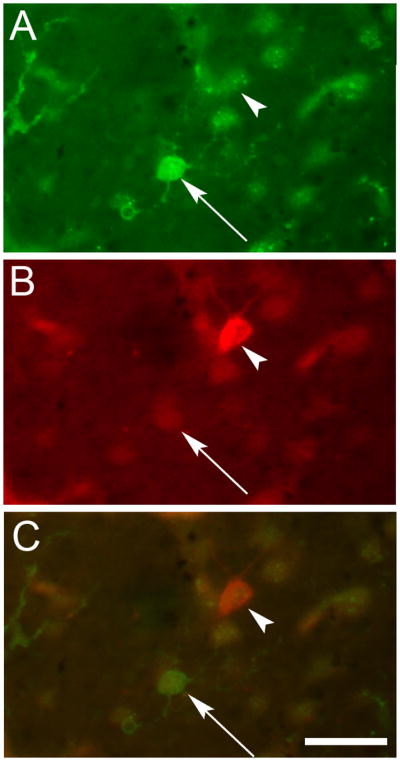
TH and 5-HT3AR did not colocalize in the human cerebral cortex. Photomicrographs of the same field of view in BA 10 showing (A) a neuron profile (arrow) and fibers (left side of image) that were 5-HT3AR-IR but (B) not TH-IR. The arrowhead indicates a TH-IR neuron profile. Panel C is an overlay of the two images showing that these markers were not colocalized. Scale bar = 20 μm.
To examine the proximity of TH-IR cell profiles with the cortical microvasculature, sections from BA 10 and 24 from 2 brains were co-labeled for TH and AQP4. Numerous microvessels outlined by AQP4 immunoreactivity were observed throughout the cortical layers (Fig. 5). The margins of each vessel were clearly delineated by the AQP4-IR astrocytic endfeet that closely enveloped the cortical microvasculature, revealing vessel lumens that were running in various longitudinal and cross-sectional planes. In BA 10 only 1 out of 60 TH-IR cell profiles was apposed to a cortical blood vessel outlined by AQP4 staining. In BA 24 only 4 out of 70 TH-IR cell profiles were in contact with microvessels. The majority of TH-IR cell profiles had no apparent association with the cortical microvasculature.
Fig. 5.
TH-IR neuron profiles were rarely in contact with blood vessels in the human cerebral cortex. (A–C) Photomicrographs of the same field of view in BA 24 showing a neuron profile that was TH-IR (B, arrowhead) but that was not apposed to blood vessels outlined by AQP4-IR astrocytic endfeet (A, arrow). Panel C is an overlay of the fields of view in A and B showing that these markers were not in close proximity. Panel D is a photomicrograph of blood vessels in BA 10 that were highlighted by AQP4 immunoreactivity and that were cut longitudinally (arrowhead) and in cross-section (arrow). No TH-IR neuron profiles were present in this field of view. Scale bar for A–C = 20 μm; scale bar for D = 20 μm.
4. Discussion
Our results demonstrate that TH-IR neurons in BA 10 and 24 of the human neocortex do not produce immunohistochemically-detectable levels of PV, CR, CB, or 5-HT3AR and that these TH-IR cells are not observed in close proximity to cortical microvessels. The expression of PV, CR, and CB has been used to demarcate three non-overlapping subsets of cortical interneurons in both non-human and human primates (Hof et al., 1999; Sherwood et al., 2007). The absence of these calcium-binding proteins in human cortical TH-IR neurons prevents the categorization of these cells into one of the established neurochemically-defined subgroups of interneurons. Because the majority of human cortical GABAergic interneurons contains one of these calcium-binding proteins (Sherwood et al., 2007), we hypothesized that cortical TH-IR cells would overlap with at least one of these subsets. Our data did not support this hypothesis.
Although the presence of 5-HT3AR-IR cells has not been reported previously in the human cortex, the expression of this receptor subtype has been used to identify a distinct subset of interneurons in the mouse (Lee et al., 2010; Rudy et al., 2011; Vucurovic et al., 2010). These reports prompted us to examine the possible coexpression of TH and 5-HT3AR in the human cortex. Our results suggest that 5-HT3AR-IR cells are present in BA 10 and 24 of the human cortex. Further work is necessary to determine whether the expression of this receptor subtype delineates a distinct subset of interneurons in the human cortex. Only 1 of the 216 TH-IR cells in BA 10 and 24 colocalized with 5-HT3aR immunoreactivity, suggesting that the cortical TH-IR neurons in these areas do not produce 5-HT3aR. The expression of this receptor subtype in the TH-containing cells in other cortical areas warrants further study.
Previous work demonstrated that many of the TH-IR somata in the rat neocortex are apposed to cortical blood vessels, which are outlined by the gliovascular marker AQP4 (Asmus et al., 2011). Intrinsic cortical neurons that are closely associated with microvessels have been hypothesized to regulate local blood flow (Cauli et al., 2004; Cauli and Hamel, 2010; Chedotal et al., 1994; Fukuyama et al., 1996). We therefore examined the association of TH-IR neurons with microvessels outlined by AQP4 in the human cortex, but found no appreciable evidence that these cells were in apposition to the cortical vasculature.
The results reported here provide further evidence of species differences between cortical TH-IR cells in humans and rodents. Whereas no coexpression of TH with PV, CR or CB is observed in the human cortex, many cortical TH-IR cells coexpress CR in untreated (Asmus et al, 2008) and 6-OHDA-treated (Wachter et al., 2014) rats. Furthermore, the laminar distribution and density of TH-IR cells differs between humans and rodents. In humans many of these cells are present in deep cortical layers (Benavides-Piccione and DeFelipe, 2003, 2007; Fukuda et al., 1999; Gaspar et al., 1987; Hornung et al., 1989; Ikemoto et al., 1999; Kuljis et al., 1989; Marui et al., 2003; Raghanti et al., 2009; Trottier et al., 1989), while in rats most TH-IR cells are superficially located (Asmus et al., 2008; Berger et al., 1985; Kosaka et al., 1987a, 1987b). The density of TH-IR neurons appears to be dramatically higher in the human cortex compared to that of rodents (Benavides-Piccione and DeFelipe, 2007). Although the two- to three-week old rat neocortex contains relatively high numbers of TH-IR neurons, the number of cells immunolabeled for TH in the adult rat cortex is significantly lower (Asmus et al., 2008). This decrease in the observable number of TH-IR cells in the rat cortex was attributed not to cell death but to a change in the cells’ neurochemical phenotype (Asmus et al., 2008, 2011). Interestingly, the number of cortical TH-IR cells increases dramatically in rats given intraventricular 6-OHDA (Wachter et al., 2014). Wachter and colleagues (2014) contend that this increase is due to a phenotypic transition in existing rather than newly-generated cells, suggesting plasticity in the number of cells that are capable of TH production in the rodent cortex. Approximately one-fourth of human cortical TH-IR cells coexpress nNOS (Benavides-Piccione and DeFelipe, 2003), whereas no colocalization of TH with nNOS was observed in the rat cortex (Asmus et al., 2011). Finally, although no association of TH-IR cells with microvessels was noted in the current study of human cortex, many TH-IR cells were reported to be closely apposed to blood vessels in the rat cortex, suggesting that at least some of these cells may play a role in the regulation of microcirculation in this species (Asmus et al. 2011).
Cortical interneurons in rodents display a distinct neurochemical profile depending on their site of origin. In the embryonic rodent cortex, projection neurons originate locally in the ventricular zone. Most interneurons, in contrast, are generated in subcortical forebrain sites, including the medial and caudal ganglionic eminences (MGE and CGE, respectively) and the preoptic area, and these cells then migrate into the developing cortex (Chu and Anderson, 2015; Gelman et al., 2009; Wonders and Anderson, 2006). A small subset of rodent interneurons appears to arise in the cortical subventricular zone (Inta et al., 2008). Adult rodent interneurons that express PV and SST migrate developmentally from the MGE, whereas the majority of CR-expressing interneurons are generated in the CGE (Butt et al., 2005; Fogarty et al., 2007; Nery et al., 2002; Wichterle et al., 2001; Wonders et al., 2008; Xu et al., 2004). The partial colocalization of TH with CR in rat cortical interneurons indirectly suggests that at least some of these TH-IR cells arise in the CGE in this species (Asmus et al., 2008). Further work is necessary to compare the embryonic origin of human cortical interneurons with that of rodents. Earlier work showed that cortical interneurons in humans migrate from the ganglionic eminence at early developmental stages, whereas those generated later in development, predominantly CR-expressing cells, arise locally in the developing neocortex (Letinic et al., 2002; Petanjek et al., 2009; Rakic, 2009; Zecevic et al., 2011). In contrast, more recent work demonstrated that the majority of human cortical interneurons originate in the ganglionic eminence, as seen in rodents, and that far fewer interneurons originate in the wall of the embryonic cortex than previously reported (Hansen et al., 2013; Ma et al., 2013).
The catecholaminergic end-product of TH-IR neurons in the human, as well as in the rodent, cortex is not known. These cells do not contain immunohistochemically-detectable levels of any subsequent catecholaminergic enzymes, including aromatic amino acid decarboxylase, nor do they contain the vesicular transporter required for catecholamine storage (Gaspar et al., 1987; Ikemoto et al., 1999; Weihe et al., 2006). If cortical TH-IR cells contain active TH but lack other catecholaminergic enzymes and uptake proteins, they may be classified as “postclassical” monoenzymatic catecholaminergic cells that synthesize L-DOPA as an end-product (i.e., dopaergic) (Ugrumov, 2009; Ugrumov et al., 2014; Weihe et al., 2006). Dopaergic neurons in the developing and adult rat hypothalamus, for example, supply L-DOPA to neighboring monoenzymatic AADC-producing cells, which then synthesize dopamine (Ugrumov et al., 2004, 2014). Results from Misu and coworkers (2003), on the other hand, suggest that L-DOPA exocytosis shares properties of neurotransmitter release and therefore L-DOPA may function as a transmitter.
Because little is known about the function of cortical TH-IR neurons, the role that these cells play in local circuitry is unclear. In humans these cells are predominantly bipolar and bitufted and a minority are multipolar (Benavides-Piccione and DeFelipe, 2003, 2007; Gaspar et al., 1987; Hornung et al., 1989; Ikemoto et al., 1999; Kuljis et al., 1989; Marui et al., 2003; Raghanti et al., 2009; Trottier et al., 1989). Based solely on their morphology, Benavides-Piccione and DeFelipe (2007) suggested that at least some cortical TH-IR cells may overlap with the Martinotti cell population, and that it is unlikely that TH-IR neurons are double bouquet cells, chandelier cells or basket cells. Half of the cortical TH-IR cells in humans were reported to contain GABA (Trottier et al., 1989). Although the technical limitation of GABA detection may explain these results, it is also possible that the GABA-negative, TH-IR neurons are excitatory neurons, which make up a small subpopulation of cortical interneurons (Markram et al., 2004).
The abundance of TH-IR neurons in the human cortex compared to species such as rodents has given rise to the suggestion that these cells play a greater variety of roles in the circuitry of the human cortex (Benavides-Piccione and DeFelipe, 2007). However, the significance of cortical TH-producing cells cannot be extrapolated from density alone; some non-human primates, for example, contain higher densities of cortical TH-IR neurons compared to humans, while neurons that produce TH are absent in the cortex of great apes (Raghanti et al., 2009).
5. Conclusions
Here we report that TH-IR neurons in BA 10 and 24 of the human cerebral cortex do not coexpress PV, CR or CB, calcium-binding proteins that are present in three distinct subgroups of interneurons, nor do these TH-IR cells coproduce 5-HT3AR. Human cortical TH-IR cells, therefore, represent an enigmatic subset of cells that cannot be readily categorized into any of the neurochemically-identified subsets of interneurons. In contrast to the rat cortex, where most TH-IR cells are superficially located and many coproduce CR, the human cortex contains TH-IR neurons that are present in deep layers and that do not coexpress any calcium-binding proteins. Furthermore, many TH-IR somata in the rat cortex are apposed to microvessels, whereas human cortical TH-IR cells are not associated with the cerebral vasculature. These data highlight differences in the characteristics of cortical TH-IR cells between humans and rodents. Future work is warranted in order to gain a more complete understanding of the neurochemical phenotype and function of TH-IR neurons in the human cortex. Cortical interneuron abnormalities, in general, have been linked to a variety of neurological disorders, ranging from schizophrenia and bipolar disorders to epilepsy (Benes and Berretta, 2001; Daskalakis et al., 2007; Di Cristo, 2007; Levitt et al., 2004; Lewis et al., 2012; Marin, 2012). A decrease in the number of cortical TH-IR cells, in particular, was reported in Parkinson’s disease (Fukuda et al., 1999) and dementia (Marui et al., 2003).
Highlights.
TH-IR neurons in human BA 10 and 24 did not coexpress PV, CR, CB or 5-HT3AR
5-HT3AR-IR neurons were present in the human cortex
TH-IR somata were rarely adjacent to cortical blood vessels
Human cortical TH cells do not overlap with chemically-defined interneuron subsets
Acknowledgments
Brain materials were obtained from the Northwestern University Alzheimer’s Disease Center Brain Bank (supported by an Alzheimer’s Disease Core Center grant, P30, AG13854, from the National Institute on Aging to Northwestern University, Chicago, Illinois). Funding was provided by the National Science Foundation (NSF BCS-0921079 to M.A.R), the National Institutes of Health (NIH 1R15 NS066245-01 to SEA), and through institutional support from the Dowling and Stodghill Professorships (SEA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ascoli GA, Alonso-Nanclares L, Anderson SA, Barrionuevo G, Benavides-Piccione R, Burkhalter A, Buzsaki G, Cauli B, Defelipe J, Fairen A, Feldmeyer D, Fishell G, Fregnac Y, Freund TF, Gardner D, Gardner EP, Goldberg JH, Helmstaedter M, Hestrin S, Karube F, Kisvarday ZF, Lambolez B, Lewis DA, Marin O, Markram H, Munoz A, Packer A, Petersen CC, Rockland KS, Rossier J, Rudy B, Somogyi P, Staiger JF, Tamas G, Thomson AM, Toledo-Rodriguez M, Wang Y, West DC, Yuste R. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci. 2008;9:557–68. doi: 10.1038/nrn2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmus SE, Anderson EK, Ball MW, Barnes BA, Bohnen AM, Brown AM, Hartley LJ, Lally MC, Lundblad TM, Martin JB, Moss BD, Phelps KD, Phillips LR, Quilligan CG, Steed PR, Terrell SL, Warner AE. Neurochemical characterization of tyrosine hydroxylase-immunoreactive interneurons in the developing rat cerebral cortex. Brain Res. 2008;1222:95–105. doi: 10.1016/j.brainres.2008.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmus SE, Cocanougher BT, Allen DL, Boone JB, Brooks EA, Hawkins SM, Hench LA, Ijaz T, Mayfield MN. Increasing proportions of tyrosine hydroxylase-immunoreactive interneurons colocalize with choline acetyltransferase or vasoactive intestinal peptide in the developing rat cerebral cortex. Brain Res. 2011;1383:108–119. doi: 10.1016/j.brainres.2011.01.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavides-Piccione R, DeFelipe J. Different populations of tyrosine-hydroxylase-immunoreactive neurons defined by differential expression of nitric oxide synthase in the human temporal cortex. Cereb Cortex. 2003;13:297–307. doi: 10.1093/cercor/13.3.297. [DOI] [PubMed] [Google Scholar]
- Benavides-Piccione R, DeFelipe J. Distribution of neurons expressing tyrosine hydroxylase in the human cerebral cortex. J Anat. 2007;211:212–22. doi: 10.1111/j.1469-7580.2007.00760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25:1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- Berger B, Verney C, Gaspar P, Febvret A. Transient expression of tyrosine hydroxylase immunoreactivity in some neurons of the rat neocortex during postnatal development. Brain Res. 1985;355:141–4. doi: 10.1016/0165-3806(85)90013-6. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Butt SJ, Fuccillo M, Nery S, Noctor S, Kriegstein A, Corbin JG, Fishell G. The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron. 2005;48:591–604. doi: 10.1016/j.neuron.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Carder RK, Leclerc SS, Hendry SH. Regulation of calcium-binding protein immunoreactivity in GABA neurons of macaque primary visual cortex. Cereb Cortex. 1996;6:271–87. doi: 10.1093/cercor/6.2.271. [DOI] [PubMed] [Google Scholar]
- Cauli B, Hamel E. Revisiting the role of neurons in neurovascular coupling. Front Neuroenergetics. 2010;2:9. doi: 10.3389/fnene.2010.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauli B, Tong XK, Rancillac A, Serluca N, Lambolez B, Rossier J, Hamel E. Cortical GABA interneurons in neurovascular coupling: relays for subcortical vasoactive pathways. J Neurosci. 2004;24:8940–9. doi: 10.1523/JNEUROSCI.3065-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chedotal A, Cozzari C, Faure MP, Hartman BK, Hamel E. Distinct choline acetyltransferase (ChAT) and vasoactive intestinal polypeptide (VIP) bipolar neurons project to local blood vessels in the rat cerebral cortex. Brain Res. 1994;646:181–93. doi: 10.1016/0006-8993(94)90076-0. [DOI] [PubMed] [Google Scholar]
- Chu J, Anderson SA. Development of cortical interneurons. Neuropsychopharmacology. 2015;40:16–23. doi: 10.1038/npp.2014.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde F, Lund JS, Jacobowitz DM, Baimbridge KG, Lewis DA. Local circuit neurons immunoreactive for calretinin, calbindin D-28k or parvalbumin in monkey prefrontal cortex: distribution and morphology. J Comp Neurol. 1994;341:95–116. doi: 10.1002/cne.903410109. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Fitzgerald PB, Christensen BK. The role of cortical inhibition in the pathophysiology and treatment of schizophrenia. Brain Res Rev. 2007;56:427–42. doi: 10.1016/j.brainresrev.2007.09.006. [DOI] [PubMed] [Google Scholar]
- DeFelipe J. Types of neurons, synaptic connections and chemical characteristics of cells immunoreactive for calbindin-D28K, parvalbumin and calretinin in the neocortex. J Chem Neuroanat. 1997;14:1–19. doi: 10.1016/s0891-0618(97)10013-8. [DOI] [PubMed] [Google Scholar]
- DeFelipe J. Cortical interneurons: from Cajal to 2001. Prog Brain Res. 2002;136:215–38. doi: 10.1016/s0079-6123(02)36019-9. [DOI] [PubMed] [Google Scholar]
- Di Cristo G. Development of cortical GABAergic circuits and its implications for neurodevelopmental disorders. Clin Genet. 2007;72:1–8. doi: 10.1111/j.1399-0004.2007.00822.x. [DOI] [PubMed] [Google Scholar]
- Fogarty M, Grist M, Gelman D, Marin O, Pachnis V, Kessaris N. Spatial genetic patterning of the embryonic neuroepithelium generates GABAergic interneuron diversity in the adult cortex. J Neurosci. 2007;27:10935–46. doi: 10.1523/JNEUROSCI.1629-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda T, Takahashi J, Tanaka J. Tyrosine hydroxylase-immunoreactive neurons are decreased in number in the cerebral cortex of Parkinson’s disease. Neuropathology. 1999;19:10–13. doi: 10.1046/j.1440-1789.1999.00196.x. [DOI] [PubMed] [Google Scholar]
- Fukuyama H, Ouchi Y, Matsuzaki S, Ogawa M, Yamauchi H, Nagahama Y, Kimura J, Yonekura Y, Shibasaki H, Tsukada H. Focal cortical blood flow activation is regulated by intrinsic cortical cholinergic neurons. Neuroimage. 1996;3:195–201. doi: 10.1006/nimg.1996.0021. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Jays PR, Bacon SJ. Calretinin neurons in human medial prefrontal cortex (areas 24a,b,c, 32′, and 25) J Comp Neurol. 1997;381:389–410. doi: 10.1002/(sici)1096-9861(19970519)381:4<389::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Berger B, Febvret A, Vigny A, Krieger-Poulet M, Borri-Voltattorni C. Tyrosine hydroxylase-immunoreactive neurons in the human cerebral cortex: a novel catecholaminergic group? Neurosci Lett. 1987;80:257–62. doi: 10.1016/0304-3940(87)90464-2. [DOI] [PubMed] [Google Scholar]
- Gelman DM, Martini FJ, Nobrega-Pereira S, Pierani A, Kessaris N, Marin O. The embryonic preoptic area is a novel source of cortical GABAergic interneurons. J Neurosci. 2009;29:9380–9389. doi: 10.1523/JNEUROSCI.0604-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glezer II, Hof PR, Leranth C, Morgane PJ. Calcium-binding protein-containing neuronal populations in mammalian visual cortex: a comparative study in whales, insectivores, bats, rodents, and primates. Cereb Cortex. 1993;3:249–72. doi: 10.1093/cercor/3.3.249. [DOI] [PubMed] [Google Scholar]
- Gonchar Y, Burkhalter A. Three distinct families of GABAergic neurons in rat visual cortex. Cereb Cortex. 1997;7:347–58. doi: 10.1093/cercor/7.4.347. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Albo MC, Elston GN, DeFelipe J. The human temporal cortex: characterization of neurons expressing nitric oxide synthase, neuropeptides and calcium-binding proteins, and their glutamate receptor subunit profiles. Cereb Cortex. 2001;11:1170–1181. doi: 10.1093/cercor/11.12.1170. [DOI] [PubMed] [Google Scholar]
- Hansen DV, Lui JH, Flandin P, Yoshikawa K, Rubenstein JL, Alvarez-Buylla A, Kriegstein AR. Non-epithelial stem cells and cortical interneuron production in the human ganglionic eminences. Nat Neurosci. 2013;16:1576–1587. doi: 10.1038/nn.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hof PR, Glezer II, Conde F, Flagg RA, Rubin MB, Nimchinsky EA, Vogt Weisenhorn DM. Cellular distribution of the calcium-binding proteins parvalbumin, calbindin, and calretinin in the neocortex of mammals: phylogenetic and developmental patterns. J Chem Neuroanat. 1999;16:77–116. doi: 10.1016/s0891-0618(98)00065-9. [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Martensson R, Bjorklund A, Kleinau S, Goldstein M. Distributional maps of tyrosine-hydroxylase-immunoreactive neurons in the rat brain. In: Bjorklund A, Hokfelt T, editors. Handbook of Chemical Neuroanatomy, Vol. 2, Classical Transmitters in the CNS, Part I. Elsevier; Amsterdam: 1984. pp. 277–379. [Google Scholar]
- Hornung JP, Tork I, De Tribolet N. Morphology of tyrosine hydroxylase-immunoreactive neurons in the human cerebral cortex. Exp Brain Res. 1989;76:12–20. doi: 10.1007/BF00253618. [DOI] [PubMed] [Google Scholar]
- Ikemoto K, Kitahama K, Nishimura A, Jouvet A, Nishi K, Arai R, Jouvet M, Nagatsu I. Tyrosine hydroxylase and aromatic L-amino acid decarboxylase do not coexist in neurons in the human anterior cingulate cortex. Neurosci Lett. 1999;269:37–40. doi: 10.1016/s0304-3940(99)00409-7. [DOI] [PubMed] [Google Scholar]
- Inta D, Alfonso J, von Engelhardt J, Kreuzberg MM, Meyer AH, van Hooft JA, Monyer H. Neurogenesis and widespread forebrain migration of distinct GABAergic neurons from the postnatal subventricular zone. Proc Natl Acad Sci USA. 2008;105:20994–20999. doi: 10.1073/pnas.0807059105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka T, Hama K, Nagatsu I. Tyrosine hydroxylase-immunoreactive intrinsic neurons in the rat cerebral cortex. Exp Brain Res. 1987a;68:393–405. doi: 10.1007/BF00248804. [DOI] [PubMed] [Google Scholar]
- Kosaka T, Kosaka K, Hataguchi Y, Nagatsu I, Wu JY, Ottersen OP, Storm-Mathisen J, Hama K. Catecholaminergic neurons containing GABA-like and/or glutamic acid decarboxylase-like immunoreactivities in various brain regions of the rat. Exp Brain Res. 1987b;66:191–210. doi: 10.1007/BF00236215. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Hattori R, Yui Y. Three distinct subpopulations of GABAergic neurons in rat frontal agranular cortex. Brain Res. 1994;649:159–73. doi: 10.1016/0006-8993(94)91060-x. [DOI] [PubMed] [Google Scholar]
- Kuljis RO, Martin-Vasallo P, Peress NS. Lewy bodies in tyrosine hydroxylase-synthesizing neurons of the human cerebral cortex. Neurosci Lett. 1989;106:49–54. doi: 10.1016/0304-3940(89)90200-0. [DOI] [PubMed] [Google Scholar]
- Lee S, Hjerling-Leffler J, Zagha E, Fishell G, Rudy B. The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors. J Neurosci. 2010;30:16796–16808. doi: 10.1523/JNEUROSCI.1869-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letinic K, Zoncu R, Rakic P. Origin of GABAergic neurons in the human neocortex. Nature. 2002;417:645–9. doi: 10.1038/nature00779. [DOI] [PubMed] [Google Scholar]
- Levitt P, Eagleson KL, Powell EM. Regulation of neocortical interneuron development and the implications for neurodevelopmental disorders. Trends Neurosci. 2004;27:400–6. doi: 10.1016/j.tins.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Curley AA, Glausier JR, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35:57–67. doi: 10.1016/j.tins.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T, Wang C, Wang L, Zhou X, Tian M, Zhang Q, Zhang Y, Li J, Liu Z, Cai Y, Liu F, You Y, Chen C, Campbell K, Song H, Ma L, Rubenstein JL, Yang Z. Subcortical origins of human and monkey neocortical interneurons. Nat Neurosci. 2013;16:1588–97. doi: 10.1038/nn.3536. [DOI] [PubMed] [Google Scholar]
- Marin O. Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci. 2012;13:107–20. doi: 10.1038/nrn3155. [DOI] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Marui W, Iseki E, Kato M, Kosaka K. Degeneration of tyrosine hydroxylase-immunoreactive neurons in the cerebral cortex and hippocampus of patients with dementia with Lewy bodies. Neurosci Lett. 2003;340:185–8. doi: 10.1016/s0304-3940(03)00101-0. [DOI] [PubMed] [Google Scholar]
- Mirra S, Heyman A, McKeel S, Sumi S, Crain B, Brownlee L, Vogel F, Hughes J, van Belle G, Berg L. The consortium to establish a registry for Alzheimer’s disease (CERAD) Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Misu Y, Kitahama K, Goshima Y. L-3,4-Dihydroxyphenylalanine as a neurotransmitter candidate in the central nervous system. Pharmacol Ther. 2003;97:117–37. doi: 10.1016/s0163-7258(02)00325-x. [DOI] [PubMed] [Google Scholar]
- Nery S, Fishell G, Corbin JG. The caudal ganglionic eminence is a source of distinct cortical and subcortical cell populations. Nat Neurosci. 2002;5:1279–87. doi: 10.1038/nn971. [DOI] [PubMed] [Google Scholar]
- Petanjek Z, Kostovic I, Esclapez M. Primate-specific origins and migration of cortical GABAergic neurons. Front Neuroanat. 2009;3:1–12. doi: 10.3389/neuro.05.026.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghanti MA, Spocter MA, Stimpson CD, Erwin JM, Bonar CJ, Allman JM, Hof PR, Sherwood CC. Species-specific distributions of tyrosine hydroxylase-immunoreactive neurons in the prefrontal cortex of anthropoid primates. Neuroscience. 2009;158:1551–9. doi: 10.1016/j.neuroscience.2008.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Evolution of the neocortex: a perspective from developmental biology. Nat Rev Neurosci. 2009;10:724–735. doi: 10.1038/nrn2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B, Fishell G, Lee S, Hjerling-Leffler J. Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Develop Neurobiol. 2011;71:45–61. doi: 10.1002/dneu.20853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh J, Suzuki K. Tyrosine hydroxylase-immunoreactive neurons in the mouse cerebral cortex during the postnatal period. Brain Res Dev Brain Res. 1990;53:1–5. doi: 10.1016/0165-3806(90)90119-j. [DOI] [PubMed] [Google Scholar]
- Sherwood CC, Raghanti MA, Stimpson CD, Bonar CJ, de Sousa AA, Preuss TM, Hof PR. Scaling of inhibitory interneurons in areas v1 and v2 of anthropoid primates as revealed by calcium-binding protein immunohistochemistry. Brain Behav Evol. 2007;69:176–95. doi: 10.1159/000096986. [DOI] [PubMed] [Google Scholar]
- Sherwood CC, Raghanti MA, Stimpson CD, Spocter MA, Uddin M, Boddy AM, Wildman DE, Bonar CJ, Lewandowski AH, Phillips KA, Erwin JM, Hof PR. Inhibitory interneurons of the human prefrontal cortex display conserved evolution of the phenotype and related genes. Proc Biol Sci. 2010;277:1011–20. doi: 10.1098/rspb.2009.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait MJ, Saadoun S, Bell BA, Papadopoulos MC. Water movements in the brain: role of aquaporins. Trends Neurosci. 2008;31:37–43. doi: 10.1016/j.tins.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Trottier S, Geffard M, Evrard B. Co-localization of tyrosine hydroxylase and GABA immunoreactivities in human cortical neurons. Neurosci Lett. 1989;106:76–82. doi: 10.1016/0304-3940(89)90205-x. [DOI] [PubMed] [Google Scholar]
- Ugrumov M, Taxi J, Pronina T, Kurina A, Sorokin A, Sapronova A, Calas A. Neurons expressing individual enzymes of dopamine synthesis in the mediobasal hypothalamus of adult rats: functional significance and topographic interrelations. Neuroscience. 2014;277:45–54. doi: 10.1016/j.neuroscience.2014.06.051. [DOI] [PubMed] [Google Scholar]
- Ugrumov MV. Non-dopaminergic neurons partly expressing dopaminergic phenotype: distribution in the brain, development and functional significance. J Chem Neuroanat. 2009;38:241–56. doi: 10.1016/j.jchemneu.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Ugrumov MV, Melnikova VI, Lavrentyeva AV, Kudrin VS, Rayevsky KS. Dopamine synthesis by non-dopaminergic neurons expressing individual complementary enzymes of the dopamine synthetic pathway in the arcuate nucleus of fetal rats. Neuroscience. 2004;124:629–35. doi: 10.1016/j.neuroscience.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Vucurovic K, Gallopin T, Ferezou I, Rancillac A, Chameau P, van Hooft JA, Geoffroy H, Monyer H, Rossier J, Vitalis T. Serotonin 3A receptor subtype as an early and protracted marker of cortical interneuron subpopulations. Cereb Cortex. 2010;20:2333–2347. doi: 10.1093/cercor/bhp310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitalis T, Rossier J. New insights into cortical interneuron development and classification: contribution of developmental studies. Develop Neurobiol. 2011;71:34–44. doi: 10.1002/dneu.20810. [DOI] [PubMed] [Google Scholar]
- Wachter B, Caradonna S, Gittinger K, Schlager A, Kuppers E. Intraventricular injection of 6-hydroxydopamine results in an increased number of tyrosine hydroxylase immune-positive cells in the rat cortex. Neuroscience. 2014;280:99–110. doi: 10.1016/j.neuroscience.2014.09.010. [DOI] [PubMed] [Google Scholar]
- Weihe E, Depboylu C, Schutz B, Schafer MK, Eiden LE. Three types of tyrosine hydroxylase-positive CNS neurons distinguished by dopa decarboxylase and VMAT2 co-expression. Cell Mol Neurobiol. 2006;26:659–78. doi: 10.1007/s10571-006-9053-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichterle H, Turnbull DH, Nery S, Fishell G, Alvarez-Buylla A. In utero fate mapping reveals distinct migratory pathways and fates of neurons born in the mammalian basal forebrain. Development. 2001;128:3759–71. doi: 10.1242/dev.128.19.3759. [DOI] [PubMed] [Google Scholar]
- Wonders CP, Anderson SA. The origin and specification of cortical interneurons. Nat Rev Neurosci. 2006;7:687–96. doi: 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]
- Wonders CP, Taylor L, Welagen J, Mbata IC, Xiang JZ, Anderson SA. A spatial bias for the origins of interneuron subgroups within the medial ganglionic eminence. Dev Biol. 2008;314:127–36. doi: 10.1016/j.ydbio.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Cobos I, De La Cruz E, Rubenstein JL, Anderson SA. Origins of cortical interneuron subtypes. J Neurosci. 2004;24:2612–22. doi: 10.1523/JNEUROSCI.5667-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaitsev AV, Povysheva NV, Gonzalez-Burgos G, Rotaru D, Fish KN, Krimer LS, Lewis DA. Interneuron diversity in layers 2–3 of monkey prefrontal cortex. Cereb Cortex. 2009;19:1597–615. doi: 10.1093/cercor/bhn198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecevic N, Hu F, Jakovcevski I. Interneurons in the developing human neocortex. Develop Neurobiol. 2011;71:18–33. doi: 10.1002/dneu.20812. [DOI] [PMC free article] [PubMed] [Google Scholar]