Abstract
Maintenance of tissue homeostasis is critical in tissues with high turnover such as the intestinal epithelium. The intestinal epithelium is under constant cellular assault due to its digestive functions and its function as a barrier to chemical and bacterial insults. The resulting high rate of cellular turnover necessitates highly controlled mechanisms of regeneration to maintain the integrity of the tissue over the lifetime of the organism. Transient increase in stem cell proliferation is a commonly used and elaborate mechanism to ensure fast and efficient repair of the gut. However, tissue repair is not limited to regulating ISC proliferation, as emerging evidence demonstrates that the Drosophila intestine uses multiple strategies to ensure proper tissue homeostasis that may also extend to other tissues.
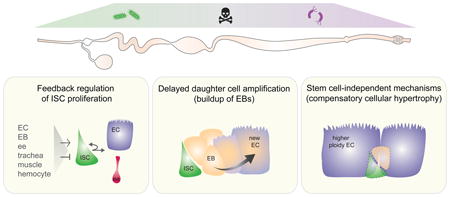
Graphical abstract
Strategies to maintain tissue HOMEOSTASIS in response to cell loss in the adult Drosophila intestine
Introduction
The adult Drosophila intestine can be divided into three regions based on morphology, function, and developmental origin: the foregut, the midgut, and the hindgut [1, 2] (Fig. 1A). Prior to 2006, the intestine was thought to be stable with little to no turnover. However, over the past ten years it has become increasingly clear that the gut is a highly dynamic tissue and that multiple mechanisms exist throughout the intestine to maintain tissue homeostasis in the face of cell turnover and damage. Here, we discuss various mechanisms used in the Drosophila adult foregut, midgut, and hindgut to maintain proper tissue homeostasis, with an emphasis on new insights gleaned in the past two to three years.
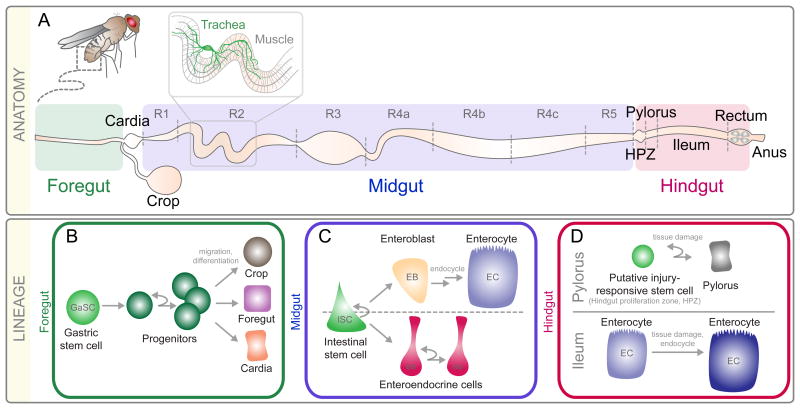
Figure 1. Anatomy and Lineage in the Drosophila Intestine.
A) The intestine is separated into three regions: foregut, midgut, and hindgut. The midgut is subdivided into seven regions, R1-R5. The hindgut is subdivided into the HPZ (hindgut proliferation zone), pylorus, ileum, and rectum. B-D) Schematic representation for lineages in the B) Foregut, C) Midgut, and D) Hindgut.
The Foregut
The foregut, a short narrow tube located at the most anterior part of the intestine, along with the crop, cardia, and anterior-most midgut act together to store food and regulate its passage into the midgut for further processing. In 2011, using a combination of lineage tracing and molecular marker localization, Singh et al. identified a band of multipotent progenitors, referred to as gastric stem cells (GaSCs), located at the foregut/midgut boundary capable of giving rise to new cells in the foregut, crop, and anterior midgut (Fig. 1B). Genetic analysis further revealed that wingless-signaling was required for progenitor maintenance, hedgehog-signaling was required for daughter differentiation, and jak-stat-signaling controlled progenitor proliferation [3] within this region. However, if and how GaSCs respond to injury has not been examined.
The Midgut
Following the foregut is the midgut, a long tube where food undergoes digestion and absorption [2]. The midgut is surrounded by a complex network of epithelial tubules known as trachea, which deliver oxygen to the cells of the intestine [4]. In addition two layers of visceral muscle surround the midgut: an outer layer of longitudinal muscle and an inner layer of circular muscle [5]. The function of the visceral muscle is twofold; visceral muscle mediates intestinal peristalsis [6] and is an important source of signaling pathway ligands that regulate ISC proliferation [7-12].
The midgut epithelial layer itself consists of two differentiated cell types, polyploid absorptive enterocytes (ECs) and a much smaller population of hormone-producing enteroendocrine (ee) cells. Enteroendocrine cells secrete factors [13-15] that promote ISC proliferation [16, 17], influence cell fate choice [18, 19], regulate intestinal peristalsis and defecation [6, 20], and inhibit lipogenesis in enterocytes [21]. Interspersed among the enterocytes and ee cells, along the entire length of the midgut, are intestinal stem cells (ISCs) [22, 23]. Originally identified in 2006 by two labs, Drosophila ISCs are multipotent and give rise to either ECs or ee cells (Fig. 1C).
Until recently, the prevailing model argued that ECs and ee cells arose through a common progenitor, the enteroblast (EB), and that high Notch-signaling activation in EBs drove them to adopt a polyploid EC fate, whereas low Notch-signaling activation drove them to adopt an ee cell fate [24]. However, recent work has challenged this model [18, 19, 25, 26], suggesting that Notch-signaling may not be initially required for ee differentiation. Indeed, we have recently demonstrated that initial ee cell fate choice does not depend on Notch-signaling, but rather on asymmetric localization during ISC division of the neuroblast differentiation gene Prospero. Following ISC division, activation of Notch signaling in ISCs by ee cells is then required for ISCs to remain multipotent [27].
While all midgut ISCs use Notch-signaling to direct daughter differentiation and remain multipotent [22, 24], ISCs are not functionally equivalent along the length of the midgut. For example, the distinct types of absorptive and endocrine cells produced by ISCs and the effects of injury and mutants on ISC proliferation depends on the region a given ISC is located. [28-33]. Differences in the behavior of ISCs and their progeny also exist between males and females [34, 35]. Comparing ISC proliferation between male and female midguts, Hudry and colleagues found that male ISCs were less likely than female ISCs to proliferate during early development or in response to injury [35]. Significantly, knockdown of the sex determination pathway in female ISCs results in ISCs that behave like male ISCs whereas feminization of male ISC leads to increased proliferation under homeostatic conditions. Along these lines Regan et al. recently demonstrated that many of the hallmarks of aging previously described in the female intestine, such as increased proliferation [36] and decreased epithelial barrier function [37], are mostly absent or delayed in the male [34]. However in contrast to work of Hudry et al. [35], feminization of male enterocytes causes male ISCs to behave like their female counterparts suggesting that enterocytes may play an indirect role in the development of age-related intestinal hyperplasia.
The Hindgut
The remaining portion of the intestine, the hindgut, can be further subdivided into four morphologically distinct regions: the hindgut proliferation zone (HPZ), the pylorus, the ileum, and the rectum. Located at the most anterior region of the hindgut, the HPZ is made up a narrow band of diploid cells that proliferate and differentiate in response to wingless- and hedgehog-signaling. Clonal analysis by Takashima et al. [38] suggested that the HPZ contained a pool of ISCs and differentiating daughters that were continuously generated in order to replace the epithelial cells of the pylorus and ileum. However, more detailed clonal analysis by Fox et al. failed to identify any relationship between cells of the HPZ and pylorus or ileum in uninjured hindguts [39]. However, following killing of cells in the hindgut by genetic means, Fox et al. [39] observed incorporation of BrdU first into cells of the anterior HPZ followed by cells of the pylorus, suggesting that the HPZ might contain a population of injury-responsive facultative ISCs capable of repairing the pylorus. A definitive conclusion, however, awaits experiments involving more stringent lineage analysis following injury to the hindgut (Fig. 1D).
Feedback Regulation of Intestinal Cell Proliferation
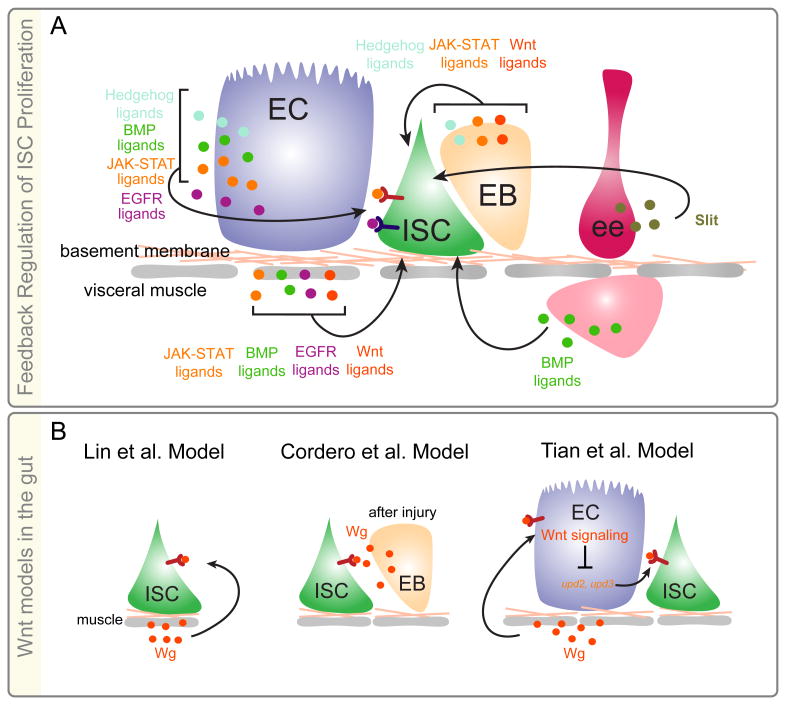
In the eight years since the identification of ISCs in the Drosophila midgut, a large body of literature has emerged demonstrating that midgut ISCs adjust their rates of proliferation in response to enterocyte turnover through a combination of positive and negative feedback loops initiated by enterocyte, enteroendocrine progenitor, visceral muscle, tracheal, and hemocyte derived signaling pathway ligands [7-12, 40-48]. These loops act in combination to link turnover of differentiated cells to both ISC division and enteroblast differentiation, thereby precisely and rapidly restoring tissue structure and function following loss of ECs and ee cells (Fig. 2A).
Figure 2. Feedback Regulation of Midgut Intestinal Stem Cell Proliferation.
A) Identity and sources of ligands controlling ISC proliferation. B) Contrasting models for Wnt/wg signaling in regulation of ISC proliferation.
Because of its role in colon cancer pathogenesis, the Wnt/wingless (wg) signaling pathway is of particular interest. Wnt signaling acts to maintain mammalian ISCs, regulate progenitor proliferation, and control differentiation of ISCs into secretory cells [49]. Furthermore, mutations in the tumor suppressor Adenomatous polyposis coli (Apc), a negative regulator of the Wnt signaling pathway, results in adenomas, which when combined with other mutations will progress to colon carcinoma [49]. In 2008 Lin and colleagues presented evidence that the wg ligand was expressed in visceral circular muscle, which is located directly underneath the ISCs [50]. Employing both loss-of and gain-of function approaches, [50] revealed that wg signaling in ISCs promoted Drosophila ISC proliferation, maintenance, and symmetric divisions, thus demonstrating partial conservation between fly and mammalian intestinal homeostasis (Fig. 2B). Consistent with these conserved roles, three labs found that removal of Apc function in Drosophila ISCs resulted in activation of wg signaling, increased ISC proliferation, and delay/disruption of EC differentiation [51-53]. However, in contrast to Lin et al. [50] both Cordero et al. [52] and Lee et al. [51] found that activation of wg signaling in ISCs did not result in an increase in ISC symmetric divisions. Furthermore, Cordero et al. [54] demonstrated that the wg signaling pathway was not required for Drosophila ISC maintenance but was required in ISCs to promote their division following midgut injury. Knockdown of the wg ligand in visceral muscle had no effect on the response of ISCs to injury arguing that wg expressed in muscle is not required for ISC function. Rather, midgut injury led to upregulation of wg ligand in ISCs and EBs and knockdown of wg in enteroblasts blocked injury induced proliferation. Together their data suggest that the wg signaling pathway acts as part of the positive feedback loop that regulates the response of ISCs to tissue turnover [54] (Fig. 2B).
While Cordero et al. [54] has reported that wg signaling is not required for ISC proliferation in uninjured intestines, Tian and colleagues have recently demonstrated that loss of wg pathway activation in enterocytes results in a non-autonomous increase in ISC proliferation [55]. In their model, wingless signaling inhibits the expression of upd2 and upd3 in ECs, two of three ligands involved in activation of the jak-stat signaling pathway, a major effector of injury-induced ISC proliferation [56]. In ECs that are fated to die, for example secondary to injury, wingless signaling becomes inactivated through a mechanism that is currently unclear. As a result ECs secrete upd2 and upd3 which acts on ISCs directly to increase their proliferation (Fig. 2B).
Given the similarities between Drosophila and vertebrate intestinal homeostasis, it is surprising that the autonomous requirement for Wnt/wg signaling in ISC function is not conserved. However, the dual role for wg-signaling in injury-induced repair of the Drosophila intestine, while unexpected, raises the intriguing possibility that a non-autonomous requirement for mammalian Wnt signaling in ISC maintenance and proliferation may exist and therefore warrants consideration for further study.
Transient Amplification Through Delayed Daughter Cell Amplification
Increase in ISC proliferation, following extensive cell loss, is an exquisite and effective strategy to quickly restore cell number. However, in the past few years, evidence has emerged that the Drosophila intestine uses other mechanisms to ensure tissue homeostasis is achieved following tissue injury.
Previous models describing tissue homeostasis in the midgut suggests that enteroblasts are normally produced only following cell loss, where they will then quickly differentiate into ECs, ensuring that proper cell number is restored. However, recently published data has challenged this notion [57]. Using a combination of lineage analysis tools, Antonello et al. [57] demonstrate that enteroblasts are produced by ISCs even in the absence of local turnover. Under these conditions, EBs pause their differentiation as long as demand for new cells is lacking. Following local cell turnover, “paused” EBs will then resume differentiation into new ECs. Mechanistically how might this work? Antonello et al. [57] demonstrate that paused EBs express the escargot/Snail2 transcription factor, a gene recently shown to repress midgut progenitor differentiation [58, 59]. Loss of differentiated cells results in the expression of the microRNA mir-8 in the EB, possibly due to changes in mechanical tension sensed by the EB, which directly represses esg, resulting in resumption of the enterocyte differentiation program. Thus, by coupling delayed differentiation of enteroblasts to EC turnover, the time normally required to generate new cells by an ISC division becomes significantly reduced.
In addition to buildup of differentiation-delayed EBs, division of enteroblasts could also serve as a source of new cells that could rapidly contribute to tissue homeostasis. Previous data suggests that EBs normally do not divide [22]. In contrast to this model, Kohlmaier et al. recently found in injured midguts the presence of a small population of low Notch reporter positive dividing cells, leading them to argue that EBs do divide [60].
However, given the recent finding that ISCs become Notch reporter positive following ee cell production [27], a definitive conclusion regarding the capability of EBs to divide will require the identification of specific EB markers.
Intestinal Maintenance Without Stem Cells
As discussed above, substantial doubt exists regarding the presence of hindgut stem cells [39]. In the absence of stem cells, how might tissue homeostasis be achieved? Recent investigations by two groups into the response of loss of cells from post-mitotic tissue in either the Drosophila ovary [61], epidermis or hindgut [62] revealed that nearby cells compensate for loss of cells, not by dividing, but rather by re-entering the endocycle (Fig. 1D). Re-entry into the endocycle leads to both increased cell and nuclear size, resulting in retention of wildtype tissue area. This process termed “compensatory cellular hypertrophy” by Tamori et al. [61] requires the insulin/IGF-like signaling pathway in the ovary and the hippo-signaling pathway in the epidermis [62]. The contribution of these pathways and their requirement for cellular hypertrophy during hindgut repair remains to be determined.
A similar phenomenon might also occur in the midgut. Following ablation of progenitors by genetic means, ISCs do not regenerate [63]. However, Jiang et al. [56] found that the intestine contained larger enterocytes with higher ploidy able to partially compensate for the reduced number of ECs secondary to progenitor dysfunction. This result suggests mechanisms are present in the intestine to preserve tissue size in the absence of progenitors.
Conclusion
Since the initial identification of intestinal stem cells in the Drosophila midgut ten years ago it has become increasingly clear that the adult fly intestine is a complex and dynamic organ. Tissue homeostasis is achieved in large part through an intricate network of injury-induced signaling pathways that feedback on ISCs to help match output to demand. Differences in stem cells exist not only between the three regions of the intestine, but between regions of the midgut and between males and females. In addition to proliferative homeostasis, more recent work demonstrates that the intestine uses other mechanisms to maintain tissue homeostasis. These include progenitor accumulation, increased cell size, and possibly division of stem cell daughters (See Graphic Abstract)
Acknowledgments
Supported by NIH Grant R01 DK082456-05 and ACS Grant CSM-12499 to Benjamin Ohlstein.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zeng X, Chauhan C, Hou SX. Stem cells in the Drosophila digestive system. Adv Exp Med Biol. 2013;786:63–78. doi: 10.1007/978-94-007-6621-1_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skaer H. The Alimentary Canal. In: Arias MBaAM., editor. The Development of Drosophila melanogaster. Cold Spring Harbor Laboratory Press; 1993. pp. 941–1012. [Google Scholar]
- 3.Singh SR, et al. The adult Drosophila gastric and stomach organs are maintained by a multipotent stem cell pool at the foregut/midgut junction in the cardia (proventriculus) Cell Cycle. 2011;10(7):1109–20. doi: 10.4161/cc.10.7.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Linneweber GA, et al. Neuronal control of metabolism through nutrient-dependent modulation of tracheal branching. Cell. 2014;156(1-2):69–83. doi: 10.1016/j.cell.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klapper R. The longitudinal visceral musculature of Drosophila melanogaster persists through metamorphosis. Mech Dev. 2000;95(1-2):47–54. doi: 10.1016/s0925-4773(00)00328-2. [DOI] [PubMed] [Google Scholar]
- 6.LaJeunesse DR, et al. Peristalsis in the junction region of the Drosophila larval midgut is modulated by DH31 expressing enteroendocrine cells. BMC Physiol. 2010;10:14. doi: 10.1186/1472-6793-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pasco MY, Loudhaief R, Gallet A. The cellular homeostasis of the gut: what the Drosophila model points out. Histol Histopathol. 2015;30(3):277–92. doi: 10.14670/HH-30.277. [DOI] [PubMed] [Google Scholar]
- 8.Kux K, Pitsouli C. Tissue communication in regenerative inflammatory signaling: lessons from the fly gut. Front Cell Infect Microbiol. 2014;4:49. doi: 10.3389/fcimb.2014.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L, Karpac J, Jasper H. Promoting longevity by maintaining metabolic and proliferative homeostasis. J Exp Biol. 2014;217(Pt 1):109–18. doi: 10.1242/jeb.089920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lemaitre B, Miguel-Aliaga I. The digestive tract of Drosophila melanogaster. Annu Rev Genet. 2013;47:377–404. doi: 10.1146/annurev-genet-111212-133343. [DOI] [PubMed] [Google Scholar]
- 11.Lucchetta EM, Ohlstein B. The Drosophila midgut: a model for stem cell driven tissue regeneration. Wiley Interdiscip Rev Dev Biol. 2012;1(5):781–8. doi: 10.1002/wdev.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naszai M, Carroll LR, Cordero JB. Intestinal stem cell proliferation and epithelial homeostasis in the adult Drosophila midgut. Insect Biochem Mol Biol. 2015;67:9–14. doi: 10.1016/j.ibmb.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 13.Veenstra JA, Ida T. More Drosophila enteroendocrine peptides: Orcokinin B and the CCHamides 1 and 2. Cell Tissue Res. 2014;357(3):607–21. doi: 10.1007/s00441-014-1880-2. [DOI] [PubMed] [Google Scholar]
- 14.Park JH, et al. A subset of enteroendocrine cells is activated by amino acids in the Drosophila midgut. FEBS Lett. 2016;590(4):493–500. doi: 10.1002/1873-3468.12073. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, et al. Isoform-specific expression of the neuropeptide orcokinin in Drosophila melanogaster. Peptides. 2015;68:50–7. doi: 10.1016/j.peptides.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Amcheslavsky A, et al. Enteroendocrine cells support intestinal stem-cell-mediated homeostasis in Drosophila. Cell Rep. 2014;9(1):32–9. doi: 10.1016/j.celrep.2014.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scopelliti A, et al. Local control of intestinal stem cell homeostasis by enteroendocrine cells in the adult Drosophila midgut. Curr Biol. 2014;24(11):1199–211. doi: 10.1016/j.cub.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biteau B, Jasper H. Slit/Robo signaling regulates cell fate decisions in the intestinal stem cell lineage of Drosophila. Cell Rep. 2014;7(6):1867–75. doi: 10.1016/j.celrep.2014.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeng X, et al. Genome-wide RNAi screen identifies networks involved in intestinal stem cell regulation in Drosophila. Cell Rep. 2015;10(7):1226–38. doi: 10.1016/j.celrep.2015.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Du EJ, et al. TrpA1 Regulates Defecation of Food-Borne Pathogens under the Control of the Duox Pathway. PLoS Genet. 2016;12(1):e1005773. doi: 10.1371/journal.pgen.1005773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song W, Veenstra JA, Perrimon N. Control of lipid metabolism by tachykinin in Drosophila. Cell Rep. 2014;9(1):40–7. doi: 10.1016/j.celrep.2014.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439(7075):470–4. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- 23.Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439(7075):475–9. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- 24.Ohlstein B, Spradling A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science. 2007;315(5814):988–92. doi: 10.1126/science.1136606. [DOI] [PubMed] [Google Scholar]
- 25.Zeng X, Hou SX. Enteroendocrine cells are generated from stem cells through a distinct progenitor in the adult Drosophila posterior midgut. Development. 2015;142(4):644–53. doi: 10.1242/dev.113357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beehler-Evans R, Micchelli CA. Generation of enteroendocrine cell diversity in midgut stem cell lineages. Development. 2015;142(4):654–64. doi: 10.1242/dev.114959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27**.Guo Z, Ohlstein B. Stem cell regulation. Bidirectional Notch signaling regulates Drosophila intestinal stem cell multipotency. Science. 2015;350(6263) doi: 10.1126/science.aab0988. This study demonstrates that distinct mechanisms are used by multipotent intestinal stem cells to give rise to enteroendocrine cells or enterocytes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marianes A, Spradling AC. Physiological and stem cell compartmentalization within the Drosophila midgut. Elife. 2013;2:e00886. doi: 10.7554/eLife.00886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buchon N, et al. Morphological and molecular characterization of adult midgut compartmentalization in Drosophila. Cell Rep. 2013;3(5):1725–38. doi: 10.1016/j.celrep.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Dutta D, et al. Regional Cell Specific RNA Expression Profiling of FACS Isolated Drosophila Intestinal Cell Populations. Curr Protoc Stem Cell Biol. 2015;34:2F 2 1–2F 2 14. doi: 10.1002/9780470151808.sc02f02s34. [DOI] [PubMed] [Google Scholar]
- 31.Dutta D, et al. Regional Cell-Specific Transcriptome Mapping Reveals Regulatory Complexity in the Adult Drosophila Midgut. Cell Rep. 2015;12(2):346–58. doi: 10.1016/j.celrep.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 32.Driver I, Ohlstein B. Specification of regional intestinal stem cell identity during Drosophila metamorphosis. Development. 2014;141(9):1848–56. doi: 10.1242/dev.104018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo Z, Driver I, Ohlstein B. Injury-induced BMP signaling negatively regulates Drosophila midgut homeostasis. J Cell Biol. 2013;201(6):945–61. doi: 10.1083/jcb.201302049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34**.Regan JC, et al. Sex difference in pathology of the ageing gut mediates the greater response of female lifespan to dietary restriction. Elife. 2016;5 doi: 10.7554/eLife.10956. This study identifies differences in proliferation rates of male and female intestinal stem cells in response to injury. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35**.Hudry B, Khadayate S, Miguel-Aliaga I. The sexual identity of adult intestinal stem cells controls organ size and plasticity. Nature. 2016;530(7590):344–8. doi: 10.1038/nature16953. This study identifies differences in proliferation rates of male and female intestinal stem cells in response to injury. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Biteau B, et al. Lifespan extension by preserving proliferative homeostasis in Drosophila. PLoS Genet. 2010;6(10):e1001159. doi: 10.1371/journal.pgen.1001159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rera M, et al. Modulation of longevity and tissue homeostasis by the Drosophila PGC-1 homolog. Cell Metab. 2011;14(5):623–34. doi: 10.1016/j.cmet.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takashima S, et al. The behaviour of Drosophila adult hindgut stem cells is controlled by Wnt and Hh signalling. Nature. 2008;454(7204):651–5. doi: 10.1038/nature07156. [DOI] [PubMed] [Google Scholar]
- 39*.Fox DT, Spradling AC. The Drosophila hindgut lacks constitutively active adult stem cells but proliferates in response to tissue damage. Cell Stem Cell. 2009;5(3):290–7. doi: 10.1016/j.stem.2009.06.003. This study providence evidence that the anterior hindgut is maintained by facultative inury responsive stem cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Q, et al. The conserved misshapen-warts-Yorkie pathway acts in enteroblasts to regulate intestinal stem cells in Drosophila. Dev Cell. 2014;31(3):291–304. doi: 10.1016/j.devcel.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deng H, Gerencser AA, Jasper H. Signal integration by Ca(2+) regulates intestinal stem-cell activity. Nature. 2015;528(7581):212–7. doi: 10.1038/nature16170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ayyaz A, Li H, Jasper H. Haemocytes control stem cell activity in the Drosophila intestine. Nat Cell Biol. 2015;17(6):736–48. doi: 10.1038/ncb3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patel PH, Dutta D, Edgar BA. Niche appropriation by Drosophila intestinal stem cell tumours. Nat Cell Biol. 2015;17(9):1182–92. doi: 10.1038/ncb3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tian A, et al. Injury-stimulated Hedgehog signaling promotes regenerative proliferation of Drosophila intestinal stem cells. J Cell Biol. 2015;208(6):807–19. doi: 10.1083/jcb.201409025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Z, et al. Debra-mediated Ci degradation controls tissue homeostasis in Drosophila adult midgut. Stem Cell Reports. 2014;2(2):135–44. doi: 10.1016/j.stemcr.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ren W, et al. Windpipe controls Drosophila intestinal homeostasis by regulating JAK/STAT pathway via promoting receptor endocytosis and lysosomal degradation. PLoS Genet. 2015;11(4):e1005180. doi: 10.1371/journal.pgen.1005180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang H, Edgar BA. EGFR signaling regulates the proliferation of Drosophila adult midgut progenitors. Development. 2009;136(3):483–93. doi: 10.1242/dev.026955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhai Z, et al. Accumulation of differentiating intestinal stem cell progenies drives tumorigenesis. Nat Commun. 2015;6:10219. doi: 10.1038/ncomms10219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Novellasdemunt L, Antas P, Li VS. Targeting Wnt signaling in colorectal cancer. A Review in the Theme: Cell Signaling: Proteins, Pathways and Mechanisms. Am J Physiol Cell Physiol. 2015;309(8):C511–21. doi: 10.1152/ajpcell.00117.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin G, Xu N, Xi R. Paracrine Wingless signalling controls self-renewal of Drosophila intestinal stem cells. Nature. 2008;455(7216):1119–23. doi: 10.1038/nature07329. [DOI] [PubMed] [Google Scholar]
- 51*.Lee WC, et al. Adenomatous polyposis coli regulates Drosophila intestinal stem cell proliferation. Development. 2009;136(13):2255–64. doi: 10.1242/dev.035196. This study demonstrates that the role for the Drosophila Adenomatous polyposis coli gene is conserved between mammalian and fly intestinal stem cells. [DOI] [PubMed] [Google Scholar]
- 52*.Cordero J, Vidal M, Sansom O. APC as a master regulator of intestinal homeostasis and transformation: from flies to vertebrates. Cell Cycle. 2009;8(18):2926–31. This study demonstrates that the role for the Drosophila Adenomatous polyposis coli gene is conserved between mammalian and fly intestinal stem cells. [PubMed] [Google Scholar]
- 53.Wang C, et al. APC loss-induced intestinal tumorigenesis in Drosophila: Roles of Ras in Wnt signaling activation and tumor progression. Dev Biol. 2013;378(2):122–40. doi: 10.1016/j.ydbio.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 54**.Cordero JB, et al. Inducible progenitor-derived Wingless regulates adult midgut regeneration in Drosophila. EMBO J. 2012;31(19):3901–17. doi: 10.1038/emboj.2012.248. This study demonstrates that wingless/Wnt signaling is an injury induced signal required for intestinal stem cell proliferation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55**.Tian A, et al. Regulation of Stem Cell Proliferation and Cell Fate Specification by Wingless/Wnt Signaling Gradients Enriched at Adult Intestinal Compartment Boundaries. PLoS Genet. 2016;12(2):e1005822. doi: 10.1371/journal.pgen.1005822. This study demonstrates that wingless/Wnt signaling acts in enterocytes to inhibit production of injury induced ligands that non-autonomously act to drive intestinal stem cell proliferation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jiang H, et al. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137(7):1343–55. doi: 10.1016/j.cell.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57**.Antonello ZA, et al. Robust intestinal homeostasis relies on cellular plasticity in enteroblasts mediated by miR-8-Escargot switch. EMBO J. 2015;34(15):2025–41. doi: 10.15252/embj.201591517. This study demonstrates that the microRNA mir-8 regulates the differentiation of enteroblasts into more differentiated enterocytes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Loza-Coll MA, et al. Regulation of Drosophila intestinal stem cell maintenance and differentiation by the transcription factor Escargot. EMBO J. 2014;33(24):2983–96. doi: 10.15252/embj.201489050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Korzelius J, et al. Escargot maintains stemness and suppresses differentiation in Drosophila intestinal stem cells. EMBO J. 2014;33(24):2967–82. doi: 10.15252/embj.201489072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kohlmaier A, et al. Src kinase function controls progenitor cell pools during regeneration and tumor onset in the Drosophila intestine. Oncogene. 2015;34(18):2371–84. doi: 10.1038/onc.2014.163. [DOI] [PubMed] [Google Scholar]
- 61**.Tamori Y, Deng WM. Tissue repair through cell competition and compensatory cellular hypertrophy in postmitotic epithelia. Dev Cell. 2013;25(4):350–63. doi: 10.1016/j.devcel.2013.04.013. This study demonstrates that Drosophila ovarian follicle cells undergo hypertrophy to compensate for the loss of their neighbors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62**.Losick VP, Fox DT, Spradling AC. Polyploidization and cell fusion contribute to wound healing in the adult Drosophila epithelium. Curr Biol. 2013;23(22):2224–32. doi: 10.1016/j.cub.2013.09.029. This study demonstrates that the cells of the hindgut and cuticle undergo hypertrophy to compensate for the loss of their neighbors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lu Y, Li Z. No intestinal stem cell regeneration after complete progenitor ablation in Drosophila adult midgut. J Genet Genomics. 2015;42(2):83–6. doi: 10.1016/j.jgg.2014.10.002. [DOI] [PubMed] [Google Scholar]