Abstract
Lipid microdomains are ordered regions on the plasma membrane of cells, rich in cholesterol and sphingolipids, ranging in size from 10 to 200 nm in diameter. These lipid-ordered domains may serve as platforms to facilitate colocalization of intracellular signaling proteins during agonist-induced signal transduction. It is hypothesized that fish oil will disrupt the lipid microdomains, increasing spatial distribution of these lipid-ordered domains and lateral mobility of the prostaglandin (PG) F2α (FP) receptors in bovine luteal cells. The objectives of this study were to examine the effects of fish oil on (1) the spatial distribution of lipid microdomains, (2) lateral mobility of FP receptors, and (3) lateral mobility of FP receptors in the presence of PGF2α on the plasma membrane of bovine luteal cells in vitro. Bovine ovaries were obtained from a local abattoir and corpora lutea were digested using collagenase. In experiment 1, lipid microdomains were labeled using cholera toxin subunit B Alexa Fluor 555. Domains were detected as distinct patches on the plasma membrane of mixed luteal cells. Fish oil treatment decreased fluorescent intensity in a dose-dependent manner (P < 0.01). In experiment 2, single particle tracking was used to examine the effects of fish oil treatment on lateral mobility of FP receptors. Fish oil treatment increased microdiffusion and macrodiffusion coefficients of FP receptors as compared to control cells (P < 0.05). In addition, compartment diameters of domains were larger, and residence times were reduced for receptors in fish oil–treated cells (P < 0.05). In experiment 3, single particle tracking was used to determine the effects of PGF2α on lateral mobility of FP receptors and influence of fish oil treatment. Lateral mobility of receptors was decreased within 5 min following the addition of ligand for control cells (P < 0.05). However, lateral mobility of receptors was unaffected by addition of ligand for fish oil–treated cells (P > 0.10). The data presented provide strong evidence that fish oil causes a disruption in lipid microdomains and affects lateral mobility of FP receptors in the absence and presence of PGF2α.
Keywords: Microdomain, Fish oil, Prostaglandin F2α FP receptor, Single particle tracking, Corpus luteum
1. Introduction
Prostaglandin (PG) F2α is the endogenous luteolysin in domestic ruminants [1–4]. It is secreted in a series of pulses late in the estrous cycle from the uterus, causing regression of the corpus luteum (CL) [5–7]. In nonpregnant cows, PGF2α binds to the PGF2α (FP) receptor which is a seven-helix, G-protein–coupled, membrane-bound receptor located on luteal cells [8–10]. The binding of PGF2α to its receptor initiates the phosphatidylinositol-phospholipase C intracellular signaling pathway that leads to the inhibition of progesterone synthesis and induction of apoptosis within the CL [11]. However, the interactions of ligand-bound receptors and –associated heterotrimeric G-proteins that lead to activation of phospholipase C in bovine luteal cells are largely unknown.
The plasma membrane of cells is composed of a lipid bilayer containing cholesterol, sphingolipids, and glycerophospholipids [12–14]. The lipids of the bilayer are not homogenous but rather segregated into microdomains [15,16]. Lipid microdomains are regions on the plasma membrane of cells rich in cholesterol and sphingolipids, ranging in size from 10 to 200 nm in diameter [17,18]. Membrane-bound receptors, including G-protein–coupled receptors, have been reported to be associated with lipid microdomains in both ligand-bound and -unbound states [19]. Moreover, heterotrimeric G-protein alpha subunits have been reported to reside in lipid microdomains as reviewed in depth by Chini et al, [20]. The cellular functions of these domains are still being resolved but may allow for the colocalization of receptors with its associated heterotrimeric G-protein, leading to the activation of downstream signaling. Thus, disruption of lipid-lipid, lipid-protein, or protein-protein structure may cause alteration in downstream signaling.
Degree of unsaturation present in long-chain fatty acids determines fluidity of biological membranes. It has been reported in bovine platelet cells that cis-unsaturated fatty acids greatly increase fluidity [21]. In addition, inclusion of marine fish oil into the diet has been reported to alter lipid dynamics within the plasma membrane of many cell types. Studies have shown that fish oil supplementation alters membrane fluidity in ovine oocytes [22], murine macrophages [23], and primate erythrocytes [24] which may influence lateral mobility of membrane-bound receptors. Other studies have shown that omega-3 fatty acids, predominantly eicosapentaenoic and docosahexaenoic acid, both present in triglycerides found in fish oil, incorporate into plasma membranes of T-cells which have an effect on the fatty acid composition and morphology of the lipid microdomains [25,26]. This suggests that the incorporation of omega-3 fatty acids from fish oil could also affect the composition of lipid microdomains of bovine luteal cells which may lead to the alteration of lateral mobility of membrane-bound receptors. It is hypothesized that fish oil will alter lipid microdomains leading to an increase in spatial distribution of these lipid-ordered domains resulting in an increase lateral mobility of FP receptors in bovine luteal cells. The objectives of this study were to determine the effects of fish oil on (1) the spatial distribution of lipid microdomains, (2) lateral mobility of FP receptors, and (3) lateral mobility of FP receptors in the presence of PGF2α on the plasma membrane in bovine luteal cells in vitro.
2. Methods
2.1. Tissue collection, cell preparation, and cell culture
Bovine ovaries containing a CL were collected at a local abattoir and transported to the laboratory at the University of Northern Colorado in 1× sterile PBS. Age of the CL was determined by gross morphological differences as previously described by Miyamoto et al [27]. Mature CLs were used for all experiments in the present study. The ovaries were then immersed in 70% ethanol to destroy any microorganisms that may be present from time of collection.
Using sterile techniques under a laminar flow hood, the CL was removed from the ovary and placed into a 60-mm2 Petri dish containing ice-cold Ca+2/Mg+2-free Hank’s-balanced salt solution (HBSS, pH 7.34). The CL was dissected free of connective tissue and cut into approximately 1-mm3 fragments. Approximately 1 g of tissue was placed into T-25 culture flasks containing 5-mL dissociation medium (HBSS supplemented with 2000 units of collagenase type 1 and 0.1% BSA) and incubated in a water bath at 37°C with agitation for 45 min. Following incubation, the supernatant was removed and transferred to a sterile 15-mL culture tube. Cells were then washed 3× with sterile PBS, resuspended in 10 mL of culture medium (Ham’s F12 supplemented with fetal bovine serum [5%], insulin [5 µg/mL], transferrin [5 µg/mL], sodium selenate [5 ng/mL], 100 unit per mL penicillin, 0.1-mg/mL streptomycin, and 0.25-mg/mL amphotericin B [pH 7.34]), and placed on ice. Fresh dissociation medium was added to the remaining undigested tissue and incubated with agitation for an additional 45 min. The remaining cells were collected, washed 2× with sterile PBS, and combined with the previous sample. After the final wash, cells were resuspended in 10 mL of culture medium.
Viability of cells was determined using trypan blue and cell concentration was estimated using a hemocytometer. Only preparations with a cell population of greater than 85% viability were used for each experiment. Cell cultures were maintained at 37°C in an atmosphere of 95% humidified air and 5% CO2.
2.2. Fish oil preparation for in vitro culture
A commercial fish oil was used for all the experiments (Pharmavite, Mission Hills, CA, USA). Lipids in fish oil were prebound to BSA before the addition to culture. In brief, fish oil was added to culture medium containing 33-mg/mL fatty acid free BSA at the appropriate dose for each experiment as described by Mattos et al [28] Control medium was prepared using the same conditions as fish oil treatment, without the addition of fish oil.
2.2.1. Experiment 1: effects of fish oil on spatial distribution of lipid microdomains
Mixed luteal cell cultures were prepared from 4 CL and plated in 35-mmglass bottom culture dishes at 5 × 104 cells/dish (n = 15 dishes/CL). Cells were incubated overnight at 37°C in an atmosphere of 95% humidified air and 5% CO2 to allow adhesion to glass cover slips. Culture medium was removed and cells were treated with 0, 0.0003, 0.003, 0.03, or 0.3% (vol/vol) fish oil (n = 3 dishes/treatment) for 72 h to allow incorporation of fatty acids into membranes. Additional dishes (n = 6 dishes/CL) were incubated in 0% (vol/vol) fish oil to serve as positive and negative controls.
2.2.2. Lipid microdomain labeling and visualization
Lipid microdomains were labeled using a commercially available kit per manufacture’s protocol (Vybrant Lipid Raft Labeling kit; Invitrogen, Carlsbad, CA, USA). In brief, cells were washed 3× with 1-mL PBS. After the final wash, 1-mL ice-cold culture medium was added to each dish containing 1-µg cholera toxin subunit B Alexa Fluor 555 conjugate. Dishes were incubated at 4°C in the dark for 10 min. Cells were then washed 3× with PBS and 1-mL ice-cold culture medium containing anticholera toxin subunit B antibody (1:200 dilution) was added. Dishes were incubated at 4°C for 15 min in the dark and subsequently washed 3× with PBS. After the finalwash,1-mL PBS was added to dishes and cells were immediately visualized. As a positive control, 3 dishes were treated with 10-mM beta-methylcyclodextrin (β-MCD) for 1 h under control conditions to deplete membrane cholesterol and disruption of lipid microdomains. As a negative control, 3 dishes were treated with only anticholera toxin subunit B antibody.
An Olympus IX81 motorized inverted microscope (Tokyo, Japan), equipped with a 60× oil objective and 0.8× digital zoom was used to visualize lipid microdomains. Approximately 10 cells were randomly selected from each dish, and 1-µm slice z-stacked images were generated from bottom to top of each cell. A 3-dimensional image of each cell was created using Fluoview version 4.3 software. Images were converted to 8-bit/channel TIFF format and were processed utilizing ImageJ (National Institutes of Health, Stapleton, NY, USA) analysis software. Mean cell fluorescent intensity was determined by outlining the cell boundary and subtracting background fluorescence.
2.2.3. Experiment 2: effects of fish oil on lateral mobility of FP receptors on bovine luteal cells
Mixed luteal cell cultures were prepared from 9 CLs and cultured in 35-mm round bottom glass dishes at 5 × 104 cells/dish. Cells were cultured in control medium or medium supplemented with 0.03% (vol/vol) fish oil for 72 h. Following 72-h incubation at 37°C, cells were then prepared for single particle tracking.
Biotin was added to FP receptor polyclonal antibody (101802; Cayman Chemical Company, Ann Arbor, MI, USA) using a commercially available kit per manufacture’s protocol (DSB-X Biotin Protein Labeling Kit; Life Technologies Corporation, Carlsbad, CA). In brief, stock antibody solution was diluted to 0.5 mg/mL and desalted using a spin column before labeling. Two hundred microliters of desalted antibody was combined with 20 µL of freshly prepared 1-M NaHCO3 and placed in a reaction tube. DSB-X biotin succinimidyl ester was reconstituted in 40 µL of DMSO, and 2 µL of the DSB-X biotin solution was added to 200 µL of FP receptor polyclonal antibody. The derivatization reaction was carried out at room temperature for 1.5 h with constant stirring. Biotinylated antibody was collected using a spin column containing purification resin and centrifuged 5 min at 1100 × g to remove unbound DSB-X. Labeling of antibody was verified using SDS-PAGE and Western blotting (data not shown).
Biotinylated FP receptor antibody (1 µg) was added to each 35-mm dish and incubated at 37°C in an atmosphere of humidified air and 5% CO2 for 15 min. Medium was then decanted and 2 mL of 1× PBS was added to each dish, gently agitated for approximately 5 s, and decanted. This procedure was repeated 2× to remove unbound antibody. Cells were then incubated with 0.5-nM 605 quantum dots conjugated with streptavidin (Q10101MP; Invitrogen) at room temperature for 2 min. Cells were rinsed 6× using gentle agitation at 20-s interval in 1× PBS to remove unbound quantum dots.
A Zeiss confocal microscope equipped with a high-speed camera (Hamamatsu Photonics, Shizuoka, Japan) was used to record receptor mobility. Quantum dots were excited with UV light and the 605-nm emission was collected using the appropriate filter set. Receptors were observed with a 100× oil objective (N.A. =1.4), and acquisition was set as an image size of 512 × 512 pixels (0.07 µm/pixel × 0.07 µm/ pixel) with 1 × 1 binning. Receptors were recorded at 29 frames/s for a total of 870 frames per video.
Trajectories of individual receptors were generated using Video Spot Tracker v08.01 (University of North Carolina-Chapel Hill, Chapel Hill, NC, USA). Only trajectories with greater than 10 s were used for further analysis. These trajectories were given as X–Y pixel coordinates which were in measurements of 0.07 µm × 0.07 µm. Mean square displacement of trajectories was calculated and plotted as a function of time to determine both microdiffusion and macrodiffusion coefficients according to the equations reported by Daumas et al [29].
Residence time of individual receptors was calculated as previously described by Barisas et al [30]. In brief, a sliding window analysis was used to determine the normalized variance in the position of the receptor within time windows. Windows were then translated alongside of particles time trajectory. The time of the inter-domain jumps was indicated by peaks and collected as n+1. The average diameter of an individual domain was calculated as previously described [29–31]. Domain size was determined by taking the square root of the macrodiffusion coefficient × 4 residence time.
2.2.4. Experiment 3: effects of fish oil and PGF2α on lateral mobility of FP receptors on bovine luteal cells
Mixed luteal cell cultures were prepared from 7 CLs and cultured in 35-mm round bottom glass dishes at 5 × 104 cells/dish. Cells were cultured in control culture medium or medium supplemented with 0.03% (vol/vol) fish oil for 72 h. Cells were then prepared for single particle tracking as described in Experiment 2.
Five luteal cells containing a minimum of 20 labeled FP receptors from each dish were randomly selected for recording. Following the identification of labeled cells, XYZ coordinates were saved to ensure consistent directional positioning along the cell membrane throughout the experiment. Selected cells were recorded without ligand at 0 min to determine initial diffusion coefficients, residence time, and domain size. Cells were then treated with 10-nM PGF2α analog (cloprostenol sodium, Merck Animal Health, Madison, NJ, USA) and recorded at 5, 15, and 30 min after the treatment. Receptors were recorded for 29 s (30 frames/s) for a total of 870 frames per time point and analyzed as described in Experiment 2. All visibly labeled FP receptors were counted at 0 min and 30 min post-PGF2α treatment to estimate percent decrease in receptor number present on the plasma membrane.
2.3. Statistical analysis
Data sets were examined for normality using the Shapiro-Wilk test and transformed accordingly using natural logarithms before statistical analysis. All data are reported as least square means ± standard error of the mean and significance was declared at P < 0.05. In experiment 1, effects of fish oil on fluorescent intensity of lipid microdomains were analyzed using 1-way ANOVA. The statistical model included CL, concentration of fish oil, cell, and residual error. Corpus luteum was used as random variable in the model. The effects of fish oil concentration on fluorescent intensity were further characterized by using linear regression analysis. In experiment 2, influence of fish oil on lateral mobility of FP receptors (microdiffusion and macrodiffusion coefficients), domain sizes, and residence time of receptors were analyzed using 1-way ANOVA. The model included CL, treatment (control, fish oil) cell, receptor, and residual error as sources of variation. Corpus luteum was considered a random variable in the model. In experiment 3, the effects of fish oil and PGF2α on lateral mobility of FP receptors, domain sizes, and residence time were analyzed using 1-way ANOVA. The model included CL, treatment (control, fish oil) cell, receptor, time, and residual error as sources of variation. Corpus luteum was considered as a random variable in the model. The effects of ligand on the disappearance of FP receptor on the plasma membrane 30 min after the treatment in experiment 3 was analyzed using a 1-way ANOVA. The statistical model included CL, treatment (control, 0.03% fish oil, β-MCD), cell, and residual error. Corpus luteum was used as random variable in the model. Calculations were made using the mixed-model procedure or regression procedure of SAS. If main effects or interactions were significant, then means were separated using preplanned t tests and PDIFF option of SAS.
3. Results
3.1. Experiment 1: effects of fish oil on spatial distribution of lipid microdomains on bovine luteal cells
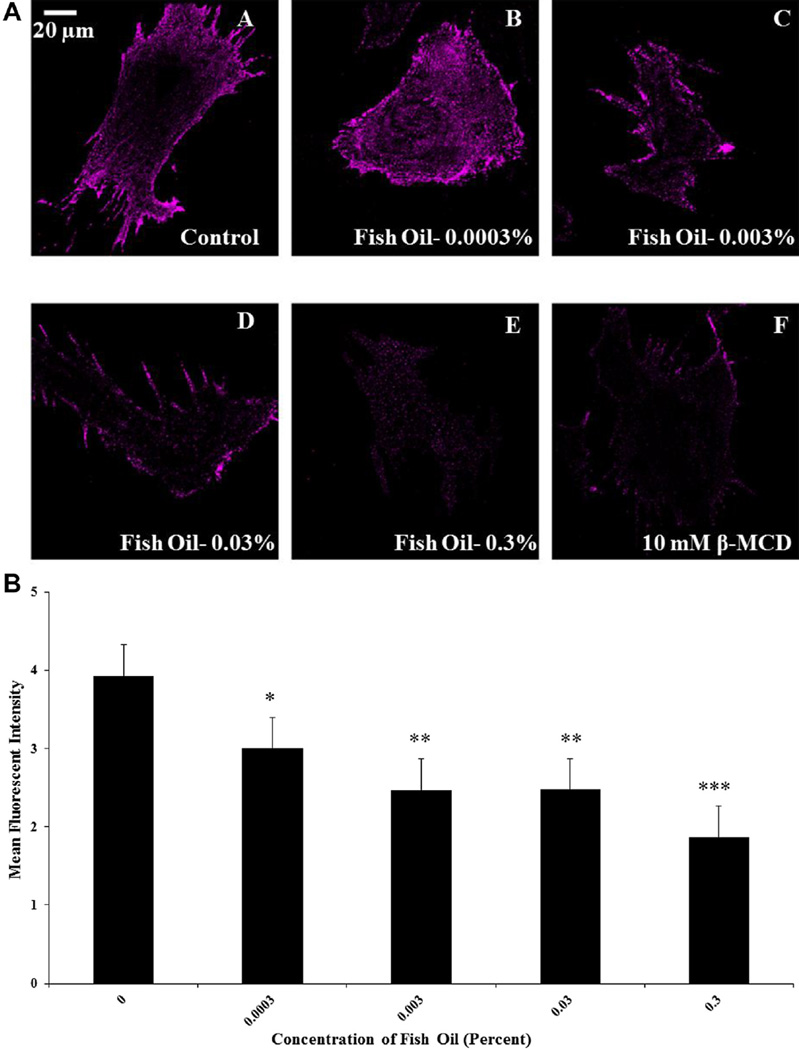
Representative micrographs acquired from control, fish oil, and β-MCD–treated luteal cells are shown in Figure 1A. Lipid microdomains were detected as distinct patches on the plasma membrane of bovine luteal cells. Fish oil and β-MCD treatment had a major impact on structural integrity of these domains. Mean cell fluorescent intensities of microdomains were greater in control cells as compared with cells treated with fish oil or β-MCD (P < 0.05). Fish oil decreased mean cell fluorescent intensity of microdomains in a dose-dependent manner (P < 0.05; Fig. 1B). Increasing fish oil concentrations from 0.0003% to 0.3 % (vol/vol) resulted in a linear disruption of lipid microdomains. Furthermore, removal of membrane cholesterol using β-MCD resulted in further disruption within microdomains, decreasing fluorescent intensity, when compared to control-treated cells (P < 0.05).
Fig. 1.
Effects of fish oil on spatial distribution of lipid microdomains. Panel A shows representative micrographs obtained from (A) control cells, (B) 0.0003% fish oil (vol/vol), (C) 0.003% fish oil (vol/vol), (D) 0.03% fish oil (vol/vol), (E) 0.3% fish oil (vol/vol), and (F) 10-mM beta-methylcyclodextrin (β-MCD). Panel B shows means of the fluorescent intensity for luteal cells obtained from control cells (n = 49), 0.0003% fish oil (vol/vol; n = 48), 0.003% fish oil (vol/vol; n = 48), 0.03% fish oil (vol/vol; n = 49), and 0.3% fish oil (vol/vol; n = 47); control vs fish oil; *P < 0.05; **P < 0.001; ***P < 0.0001.
3.2. Experiment 2: effects of fish oil on lateral mobility of PGF2α receptors on bovine luteal cells
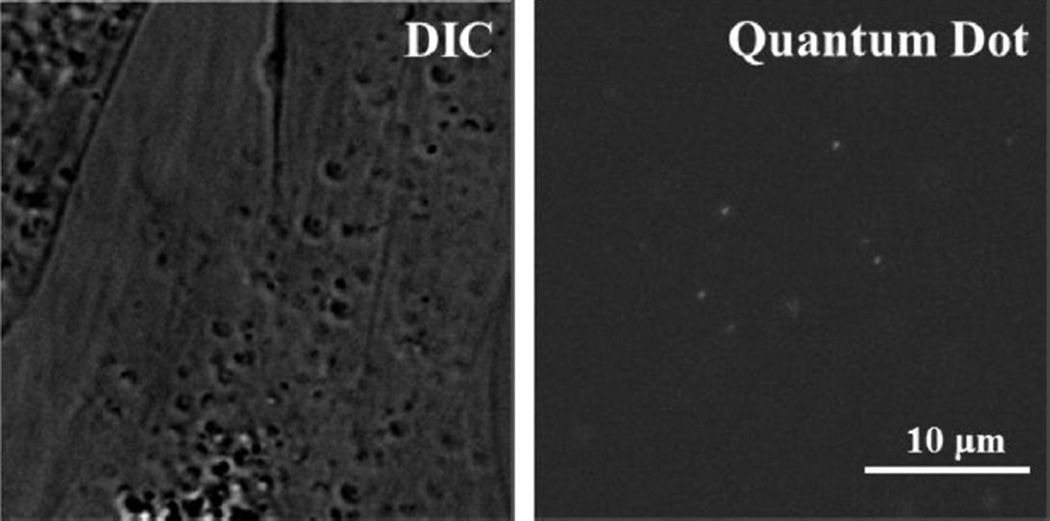
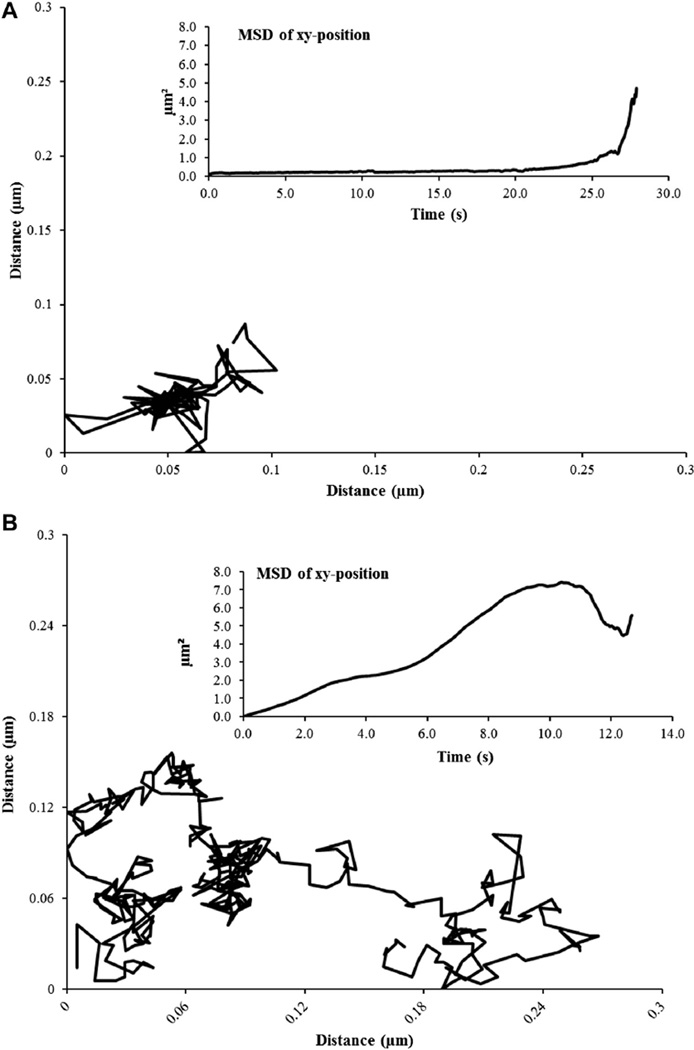
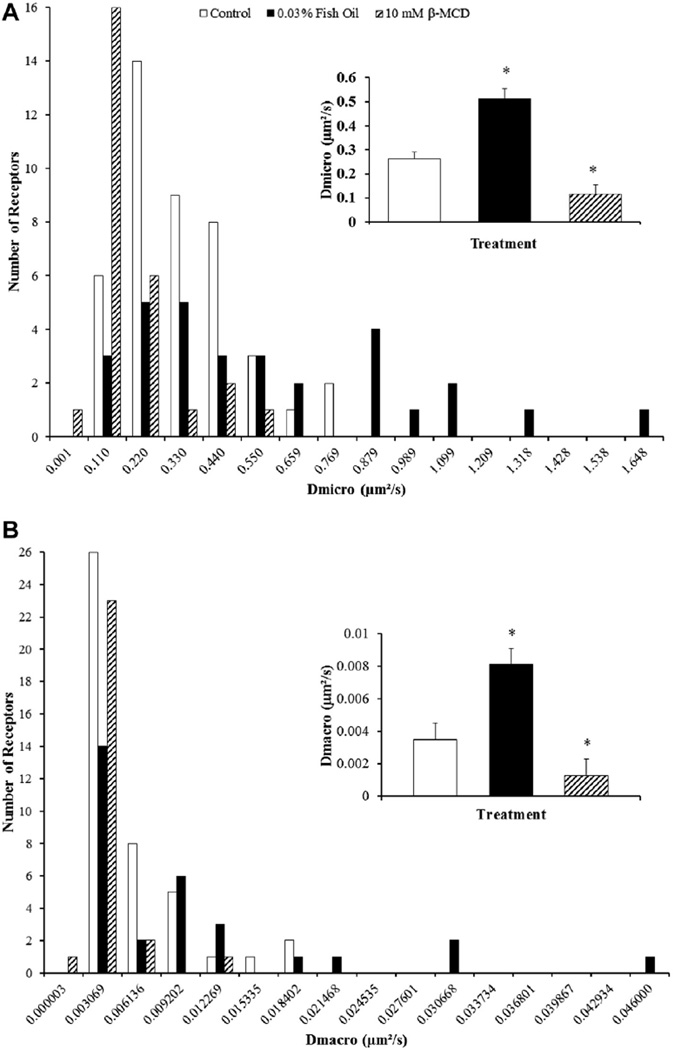
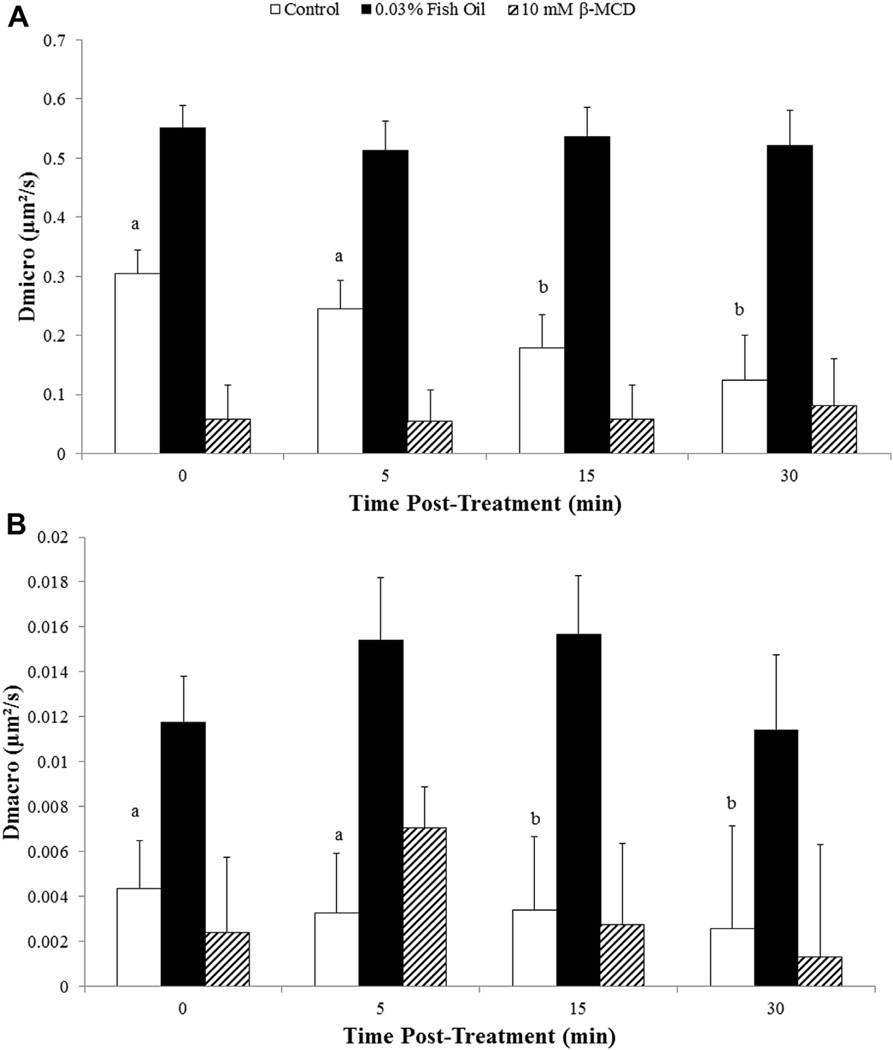
Labeled FP receptors on the plasma membrane of a bovine luteal cell using biotinylated FP receptor antibody and 605-nm streptavidin quantum dots are shown in Figure 2. A representative trajectory from a control and fish oil–treated cell is shown in Figure 3. Fish oil treatment had a significant influence on lateral mobility of FP receptors. Both microdiffusion and macrodiffusion were increased in fish oil–treated cells compared with control cells (P < 0.05). Fish oil treatment increased microdiffusion of the FP receptors on luteal cells by 95% when compared to receptors from control luteal cells (P < 0.05; Fig. 4A). Furthermore, fish oil treatment increased macrodiffusion of FP receptors by 166% when compared to control luteal cells (P < 0.05; Fig. 4B).
Fig. 2.
Verification of prostaglandin F2α receptor labeling on bovine luteal cells. Representative micrograph of bovine luteal cells bound with biotin-conjugated prostaglandin F22α FP antibody and quantum dots.
Fig. 3.
Trajectories of individual prostaglandin F2α receptor on the plasma membrane of control and 0.03% (vol/vol) fish oil–treated bovine luteal cells. Individual trajectories of prostaglandin F2α FP receptor on luteal cell membranes obtained from control (panel A) and 0.03% fish oil (panel B)–treated cells. Inset represents corresponding mean square displacement of each trajectory which was calculated and plotted as a function of time.
Fig. 4.
Effects of fish oil on lateral mobility of prostaglandin F2α receptors on bovine luteal cells. (A) microdiffusion and (B) macrodiffusion coefficient of the prostaglandin F2α (FP) receptor on bovine luteal cells treated with control or 0.03% (vol/vol) fish oil–treated cells. The inset in each panel shows the mean ± standard error of the mean; compared to control *P < 0.05. Control (n = 44 FP receptors), Fish oil 0.03% (vol/vol; n = 29 FP receptors), and 10-mM beta-methylcyclodextrin (β-MCD; n = 26 FP receptors).
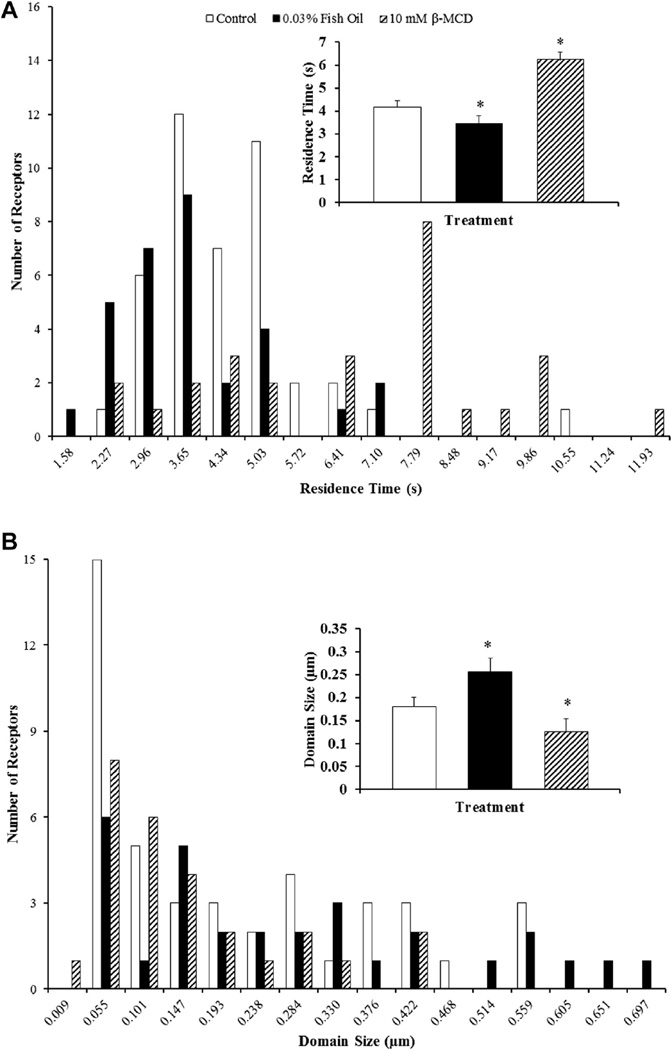
Fish oil treatment also affected domain size and the time a FP receptor resided within a domain, which is referred to as residence time and are shown in Figure 5. The residence time of FP receptors on luteal cells from 0.03% (vol/vol) fish oil treatment was decreased by 25% when compared with receptors on luteal cells from control treatment (P < 0.05; Fig. 5A). Furthermore, fish oil treatment increased the domain size, or corral, of the microdomains by 36% (P < 0.05; Fig. 5B).
Fig. 5.
Effects of fish oil on domain size and residence time of prostaglandin F2α receptors on bovine luteal cells. (A) Residence time of prostaglandin F2α (FP) receptors and (B) microdomain diameter on bovine luteal cells treated with control, 0.03% (vol/vol) fish oil, or 10-mM beta-methylcyclodextrin–(β-MCD). The inset in each panel shows the mean ± standard error of the mean; compared to control *P < 0.05. Control (n = 44 FP receptors), fish oil 0.03% (vol/vol; n = 29 FP receptors), and 10-mM β-MCD (n = 26 FP receptors).
Pretreatment of luteal cells with β-MCD to remove cholesterol from the plasma membrane resulted in a 56% reduction in microdiffusion as compared to receptors on luteal cells from control treatment (P < 0.05; Fig. 4A). Furthermore, macrodiffusion was decreased by 66% when compared to control cells (P < 0.05; Fig. 4B). The residence time of the FP receptors on luteal cells pretreated with β-MCD was increased by 53% when compared with receptors on luteal cells from control treatment (P < 0.05; Fig. 5A). Moreover, the depletion of cholesterol by β-MCD decreased the domain size of the microdomains by 35% when compared to control cells (P < 0.05; Fig. 5B).
3.3. Experiment 3: effects of fish oil on lateral mobility of PGF2α receptors on bovine luteal cells in the presence of PGF2α
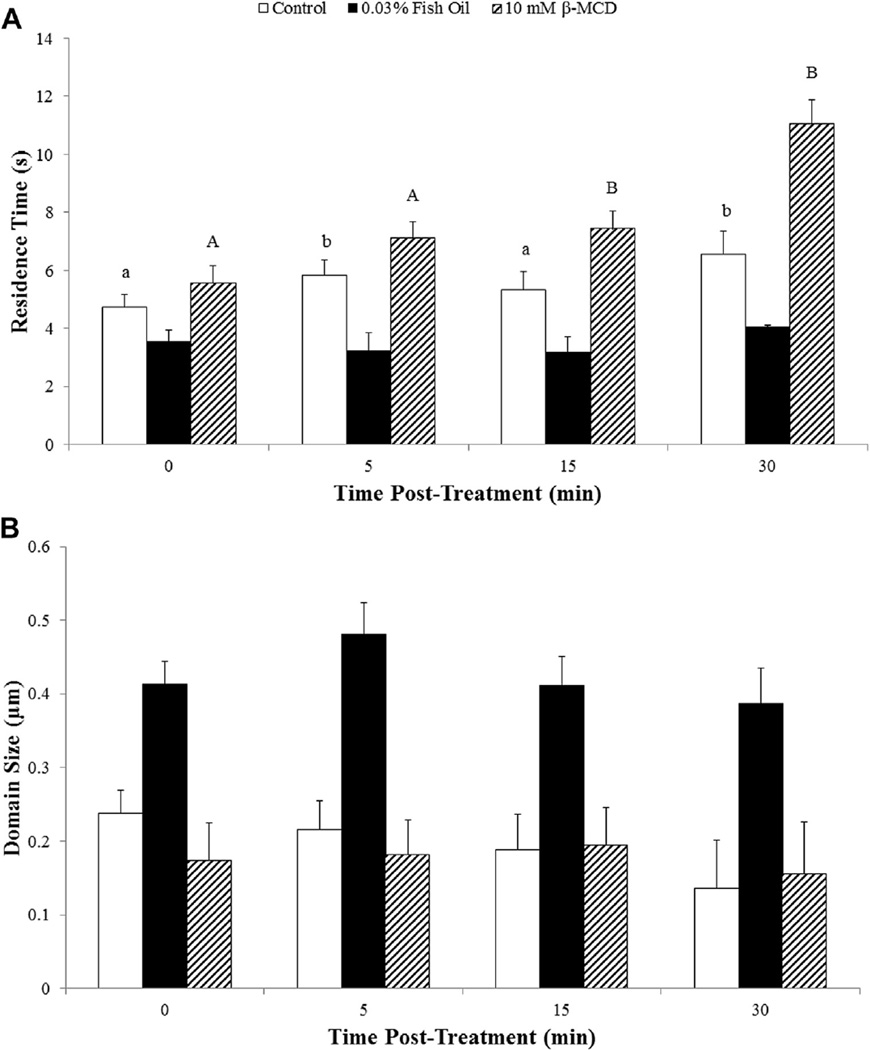
Effects of PGF2α on microdiffusion of FP receptors on bovine luteal cell membranes are shown in Figure 6A. In control luteal cells, there was no difference in microdiffusion at 5 min after addition of ligand (P > 0.10). However, microdiffusion of FP receptors in control luteal cells was reduced at both 15 and 30 min after the addition of ligand when compared prior to the addition of ligand (0 min; P < 0.05). As in experiment 2, microdiffusion of FP receptors was greater for fish oil–treated luteal cells before the addition of ligand as compared with control luteal cells (P < 0.05). Unlike control luteal cells, the addition of PGF2α had no effect on microdiffusion of FP receptors at any of the time points for luteal cells treated with fish oil (P > 0.10). As a result of ligand not affecting mobility of receptors, microdiffusion of FP receptors was greater for fish oil– treated cells at 5, 15, and 30 min after the treatment when compared to control luteal cells (P < 0.05). Depletion of cholesterol from the membrane using β-MCD decreased microdiffusion of FP receptors when compared to control luteal cells as in experiment 2 (P < 0.05). Microdiffusion of FP receptors was unaffected by the addition of PGF2α for luteal cells pretreated with β-MCD (P > 0.10).
Fig. 6.
Effects of fish oil on lateral mobility of prostaglandin F2α receptors in the presence of ligand on bovine luteal cells. (A) Microdiffusion and (B) macrodiffusion coefficient of the prostaglandin F2α (FP) receptors on bovine luteal cells treated with control, 0.03% (vol/vol) fish oil, or 10-mM beta-methylcyclodextrin–(β-MCD) in the presence of 10-nM prostaglandin F2α. Fish oil 0.03% (vol/vol). Ten mM of β-MCD. a, b control cells—means with different letters differ (P < 0.05) when compared to 0 min. Number of receptors analyzed at each time point. Control (n = 80, 52, 31, and 14 FP receptors, respectively), fish oil 0.03% (vol/vol; n = 86, 48, 57, and 32 FP receptors, respectively), and 10-mM β-MCD (n = 35, 26, 22, and 9 FP receptors, respectively).
Effects of PGF2α on macrodiffusion of FP receptors on bovine luteal cell membranes are shown in Figure 6B. In control luteal cells, there was no difference in macrodiffusion at 5 min after the addition of ligand (P > 0.10). However, macrodiffusion of FP receptors was reduced at both 15 and 30 min after the addition of ligand when compared prior to the addition of ligand (0 min; P < 0.05). Macrodiffusion of FP receptors on fish oil–treated luteal cells prior to the addition of ligand was increased by 198% as compared with receptors on luteal cells from control treatment (P < 0.05). Macro diffusion was not affected by the addition of PGF2α in luteal cells treated with fish oil or β-MCD (P > 0.10).
Effects of PGF2α on residence time of FP receptors in a domain are shown in Figure 7A. The addition of PGF2α increased residence time of FP receptors in a domain for control luteal cells within 5 min after the addition of ligand (P < 0.05). Residence time of FP receptors in a domain was 20% less for luteal cells treated with fish oil treatment when compared with control luteal cells before addition of PGF2α (P < 0.05). Unlike control luteal cells, PGF2α treatment had no effect on residence time of FP receptors at 5, 15, or 30 min after the treatment (P > 0.10). The residence time for FP receptors was greater for luteal cells treated with β-MCD before PGF2α treatment when compared with control luteal cells (P < 0.05). The addition of PGF2α further increased residence of FP receptors for luteal cells pretreated with β-MCD (P < 0.05).
Fig. 7.
Effects of fish oil on domain size and residence time of prostaglandin F2α receptors in the presence of ligand on bovine luteal cells. (A) Residence time of prostaglandin F2α (FP) receptors and (B) microdomain diameter on bovine luteal control, 0.03% (vol/vol) fish oil, or 10-mM beta-methylcyclodextrin–(β-MCD) in the presence of 10-nM prostaglandin F2α. Fish oil 0.03% (vol/vol). Ten mM of β-MCD. a, b control cells—means with different letters differ (P < 0.05) when compared to 0 min. A,B β-MCD cells—means with different letters differ (P < 0.05) when compared to 0 min. Number of receptors analyzed at each time point. Control (n = 80, 52, 31, and 14 FP receptors, respectively), fish oil 0.03% (vol/vol; n = 86, 48, 57, and 32 FP receptors, respectively), and 10-mM β-MCD (n = 35, 26, 22, and 9 FP receptors, respectively).
Effects of PGF2α on domain size are shown in Figure 7B. Size of domain was influenced by fish oil treatment. Domains were larger when compared to control luteal cells (P < 0.05). Prostaglandin F2α treatment did not affect domain size for control, fish oil–treated, or pretreated β-MCD luteal cells (P > 0.10).
The decreased number of FP receptors visible on the plasma membrane for control, β-MCD, and 0.03% fish oil– treated cells at 30 min after the treatment were 81 ± 6, 91 ± 6, and 37 ± 6%, respectively. This decrease in the percentage of visible receptors did not differ between control and β-MCD–treated cells (P > 0.10). However, when compared to fish oil–treated cells, there was a decrease in the percentage of visible receptors for both control and β-MCD (P < 0.05).
4. Discussion
Biological membranes including the plasma membrane of cells are made of a bilayer of lipids with associated integral and peripheral proteins [32]. The major lipids of membranes include glycerophospholipids, sterols, and sphingolipids [12–14]. Once postulated to be homogeneous, the plasma membrane is asymmetric in regard to lipid composition and is very dynamic [33]. Early studies in the 1970s using artificial membranes showed that the lipids of the bilayer segregate into lipid-ordered states, now referred to as lipid microdomains [34]. Lipid microdomains were detected as distinct patches on the plasma membrane of control luteal cells using cholera toxin binding to monosialotetrahexosylganglioside, a known marker of lipid microdomains, and antibody crosslinking. The present study shows that culturing bovine luteal cells in the presence of fish oil had a dramatic effect on lipid microdomain structure. Fish oil resulted in an increase in the spatial distribution of these domains in a dose-dependent manner. The results from this study are in agreement with previous observations that supplementation with fish oil, either in vitro or in vivo, affects the order of lipid microdomains on the plasma membrane [35–38].
The mechanism by which fish oil affects lipid microdomain structure is still largely unknown. It is postulated that long-chain polyunsaturated fatty acids, specifically the omega-3 fatty acids eicosapentaenoate and docosahexaenoate, may play a key role in the disruption of the structural integrity of lipid microdomains. Generally, long-chain fatty acids associated with microdomains are saturated, allowing for tight packing, thereby increasing lipid order [39]. The long-chain omega-3 polyunsaturated fatty acids prevalent in fish oil can incorporate into the lipid microdomains [25,35], which may increase the fluidity of this ordered region of the plasma membrane. Alteration of membrane fluidity or microdomain structure with fish oil may affect mobility of integral and peripheral proteins associated with the plasma membrane.
To our knowledge, this is the first study examining lateral mobility of FP receptors on the plasma membrane of bovine luteal cells using single particle tracking methodology. Receptors were successfully labeled and tracked, and resulting trajectories from individual receptors allowed for the estimation of diffusion coefficients, domain sizes, and residence times. Microdiffusion and macrodiffusion coefficients, domain size, and residence time of FP receptors obtained from control luteal cells were slightly greater than values reported in the literature for membrane-bound receptors. Most studies reported in the literature with lower diffusion coefficients for membrane-bound receptors were conducted using transfected cells [29,40,41]. The introduction of membrane-bound receptors to the plasma membrane using transfection may lead to overexpression of receptors causing an increase in protein crowding. Increased protein crowding has been reported to decrease mobility of membrane proteins in computer-simulated membrane models [42,43] and artificial membranes [44] which may account for differences in mobility characteristics in the present study and other studies using transfected cell lines. Moreover, the steroidogenic nature and potential-increased cholesterol content within the plasma membrane of bovine luteal cells [45] could play a role in the increased diffusion coefficients observed in this study as well. However, more importantly, luteal cells cultured in the presence of fish oil had increased lateral mobility of the FP receptor when compared with control luteal cells. Furthermore, the average time a receptor resided in a domain was decreased compared with receptors from controls. Likewise, increased domain sizes were observed with fish oil–treated cells, confirming spatial distribution results, indicating increased structural disruption of lipid microdomains. Taken together, the disruption of spatial distribution of lipid microdomains and increased lateral mobility of the FP receptors indicate that fish oil supplementation could potentially influence sensitivity of FP receptors to PGF2α.
Effects of ligand on the lateral mobility of the FP receptor in bovine luteal cells were also examined in the present study. Lateral mobility of FP receptors (both microdiffusion and macrodiffusion) in control luteal cells decreased following the addition of ligand. Furthermore, the number of visible receptors on the membrane decreased following the addition of ligand. As previously discussed, lipid microdomains may play a role in colocalization of membrane-bound receptors with its associated intracellular signaling proteins. In addition to cell signaling, these domains may play a role in endocytosis of ligand-bound receptors, leading to desensitization to additional ligand [46]. It is hypothesized that binding of PGF2α to its receptor resulted in receptor docking within the lipid microdomains between 5 and 15 min after the treatment, allowing for downstream signaling. Within 30 min after the treatment, there was clearly a decrease in number of receptors visible on the membrane, indicating possible endocytosis of ligand-bound FP receptors. Endocytosis of the FP receptor may be occurring through a negative feedback mechanism, which includes the phosphorylation of receptors by protein kinases [47] and binding of βarrestin, following the addition of ligand [48]. However, unlike in control luteal cells, the lateral mobility of the FP receptor in fish oil–treated cells remained unchanged during the 30 min of stimulation. Likewise, the number of visibly labeled receptors on the cell membrane 30 min after the treatment was higher than control luteal cells, possibly indicating a potential disruption in downstream cell signaling and (or) endocytosis of ligand-bound receptors.
The mechanism(s) by which fish oil affects lateral mobility of membrane-bound FP receptors on bovine luteal cells is unknown. The increased mobility most likely is due to greater membrane fluidity in both lipid microdomains and bulk lipid allowing receptors to diffuse at a greater rate. The increased domain size in response to fish oil may be due to the incorporation of omega-3 polyunsaturated fatty acids. The triglycerides in fish oil must be hydrolyzed to release the omega-3 polyunsaturated fatty acids prior to the incorporation of free eicosapentaenoate and docosahexaenoate into the plasma membrane of the luteal cells. It is, however, possible that the triglycerides are hydrolyzed catalytically by the lipases present in the fetal bovine serum present in the culture media. In addition, it has been reported that intact triglycerides can diffuse through plasma membranes; thus the triglycerides may be hydrolyzed by luteal lipases [49]. Experiments are ongoing in our laboratory investigating the influence of individual omega-3 polyunsaturated fatty acids on lipid microdomain structure and lateral mobility of FP receptors on bovine luteal cells.
In this study, β-MCD was used as a disruptor of lipid microdomains. Cholesterol content within the plasma membrane of bovine luteal cells also affected lipid microdomain structure, mobility of FP receptors, and residence time within domains which differed greatly from fish oil–treated cells. These data are in agreement wherein cholesterol is critical for microdomain integrity [50]. Removal of cholesterol from these domains can allow for the rearrangement of lipids [51], membrane proteins [52], or the cytoskeleton [53]. This reorganization of the plasma membrane may lead to larger or disordered lipid microdomains. Further investigation of interaction of cholesterol with the cytoskeleton and lipids in microdomains is warranted in bovine luteal cells. Removal of cholesterol by β-MCD reduced lateral mobility of FP receptors and increased residence time. These data are in agreement with previous studies where acute depletion of cholesterol reduces mobility of membrane proteins and increases confinement [53,54].
The role of cholesterol in regulating lipid microdomain structure and mobility of lipids and proteins within the plasma membrane still needs defining. The hydrocarbon rings of cholesterol interact with the acyl chains of long-chain fatty acids esterified to glycerophospholipids that regulate plasma membrane fluidity. The concentration of β-MCD used in this study removes approximately 80% of the cholesterol [55] which would allow for packing of acyl chains reducing fluidity of the membrane and most likely lateral mobility of the FP receptor. Acute depletion of cholesterol in the present study also increased residence time of FP receptors which has been shown in previous studies in which acute or chronic depletion of cholesterol increased confinement of membrane-bound proteins [53,54]. Depletion of membrane cholesterol results in reorganization of the cytoskeleton and phosphatidylinositol which may lead to increased confinement of membrane-bound receptors. A similar mechanism may be occurring with the FP receptor in cholesterol-depleted luteal cells and warrants further investigation.
Inclusion of omega-3 fatty acids from fish by-products in the diet of breeding cows may prevent the embryonic loss during early gestation. It has been estimated that 20% to 30% of potentially viable embryos die within the first 30 d of pregnancy [56]. Inadequate control of PGF2α secretion by reproductive tissues may contribute to this embryonic loss. The conceptus must effectively regulate uterine PGF2α secretion during early pregnancy allowing for continuous secretion of progesterone by the CL and establishment of pregnancy. The trophoblastic cells of the conceptus secrete interferon-τ which inhibits uterine PGF2α. Timing of interferon-τ secretion and (or) its concentration is critical for maternal recognition of pregnancy. Mann and Lamming [57] reported that a delay in postovulatory progesterone secretion can lead to a poorly developed conceptus that produced nondetectable levels of interferon-τ which may lead to failure in pregnancy.
A single luteolyic pulse of PGF2α from the uterus may set in motion the mechanisms that cause regression of the CL and loss of the pregnancy for a poorly developed conceptus. Reducing the sensitivity of the CL to PGF2α may increase the window of opportunity for a conceptus to appropriately inhibit uterine PGF2α secretion. Prostaglandin F2α binds to a G-protein–coupled receptor, activating an intracellular signaling cascade that leads to inhibition of progesterone synthesis and induction of apoptosis. The interaction of ligand-bound receptors with its associated G-proteins is unclear, but may involve lipid microdomains. This study shows that fish oil influences plasma membrane ultra-structure and FP receptor dynamics in the absence and presence of ligand in bovine luteal cells. Therefore, inclusion of fish oil or other fish by-products such as fish meal in the diet of dairy and beef cows may be a novel approach to alter sensitivity of the CL to PGF2α, delaying regression of the gland, and thus allowing for an extended window for the establishment of pregnancy.
5. Conclusion
The data presented here provide strong evidence that fish oil supplementation leads to disruption in lipid microdomains. This disruption resulted in increased spatial distribution of lipid microdomains and lateral mobility of the FP receptor. Furthermore, addition of PGF2α restricted lateral mobility of FP receptors and increased residence time of receptors in domains in control cells, but had no effect on fish oil–treated cells. This study showed that fish oil causes an increase in lateral mobility in the presence of ligand, indicating that fish oil supplementation could potentially influence sensitivity of FP receptors to PGF2α.
Acknowledgments
The authors thank Kenneth Cochran and Chad Wangeline at the University of Northern Colorado Instrumentation and Fabrication Services for their assistance with microscopy. Supported by Agriculture and Food Research Initiative competitive grant 2013-6715-20966 from the U.S. Department of Agriculture to P. D. B. and National Institutes of Health, award number CA175937 to B. G. B.
References
- 1.Poyser NL. The control of prostaglandin production by the endometrium in relation to luteolysis and menstruation. Prostaglandins Leukot Essent Fatty Acids. 1995;53:147–195. doi: 10.1016/0952-3278(95)90115-9. [DOI] [PubMed] [Google Scholar]
- 2.Okuda K, Miyamoto Y, Skarzynski DJ. Regulation of endometrial prostaglandin F(2alpha) synthesis during luteolysis and early pregnancy in cattle. Domest Anim Endocrinol. 2002;23:255–264. doi: 10.1016/s0739-7240(02)00161-3. [DOI] [PubMed] [Google Scholar]
- 3.McCracken JA, Carlson JC, Glew ME, Goding JR, Baird DT, Gréen K, Samuelsson B. Prostaglandin F 2 identified as a luteolytic hormone in sheep. Nat New Biol. 1972;238:129–134. doi: 10.1038/newbio238129a0. [DOI] [PubMed] [Google Scholar]
- 4.McCracken JA, Einer-Jensen N, Fried J. Prostaglandin F2 alpha and its 13-dehydro analogs: comparative luteolytic effects in vivo. Adv Exp Med Biol. 1979;112:577–601. doi: 10.1007/978-1-4684-3474-3_65. [DOI] [PubMed] [Google Scholar]
- 5.Kindahl H, Edquist LE, Bane A, Granström E. Blood levels of progesterone and 15-keto-13,14-dihydro-prostaglandin F2alpha during the normal oestrous cycle and early pregnancy in heifers. Acta Endocrinol (Copenh) 1976;82:134–149. doi: 10.1530/acta.0.0820134. [DOI] [PubMed] [Google Scholar]
- 6.Kindahl H, Granström E, Edqvist LE, Eneroth P. Prostaglandin levels in peripheral plasma during the reproductive cycle. Adv Prostaglandin Thromboxane Res. 1976;2:667–671. [PubMed] [Google Scholar]
- 7.Kindahl H, Edqvist LE, Granström E, Bane A. The release of prostaglandin F2alpha as reflected by 15-keto-13,14-dihydroprostaglandin F2alpha in the peripheral circulation during normal luteolysis in heifers. Prostaglandins. 1976;11:871–878. doi: 10.1016/0090-6980(76)90194-5. [DOI] [PubMed] [Google Scholar]
- 8.Sakamoto K, Ezashi T, Miwa K, Okuda-Ashitaka E, Houtani T, Sugimoto T, Ito S, Hayaishi O. Molecular cloning and expression of a cDNA of the bovine prostaglandin F2 alpha receptor. J Biol Chem. 1994;269:3881–3886. [PubMed] [Google Scholar]
- 9.Sharif NA, Xu SX, Williams GW, Crider JY, Griffin BW, Davis TL. Pharmacology of [3H]prostaglandin E1/[3H]prostaglandin E2 and [3H]prostaglandin F2alpha binding to EP3 and FP prostaglandin receptor binding sites in bovine corpus luteum: characterization and correlation with functional data. J Pharmacol Exp Ther. 1998;286:1094–1102. [PubMed] [Google Scholar]
- 10.Mamluk R, Chen D, Greber Y, Davis JS, Meidan R. Characterization of messenger ribonucleic acid expression for prostaglandin F2 alpha and luteinizing hormone receptors in various bovine luteal cell types. Biol Reprod. 1998;58:849–856. doi: 10.1095/biolreprod58.3.849. [DOI] [PubMed] [Google Scholar]
- 11.Davis JS, Weakland LL, Weiland DA, Farese RV, West LA. Prostaglandin F2 alpha stimulates phosphatidylinositol 4,5-bisphosphate hydrolysis and mobilizes intracellular Ca2+ in bovine luteal cells. Proc Natl Acad Sci U S A. 1987;84:3728–3732. doi: 10.1073/pnas.84.11.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang TY, Silvius JR. Sphingolipid partitioning into ordered domains in cholesterol-free and cholesterol-containing lipid bilayers. Biophys J. 2003;84:367–378. doi: 10.1016/S0006-3495(03)74857-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramstedt B, Slotte JP. Interaction of cholesterol with sphingomyelins and acyl-chain-matched phosphatidylcholines: a comparative study of the effect of the chain length. Biophys J. 1999;76:908–915. doi: 10.1016/S0006-3495(99)77254-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown DA, London E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J Biol Chem. 2000;275:17221–17224. doi: 10.1074/jbc.R000005200. [DOI] [PubMed] [Google Scholar]
- 16.Schroeder R, London E, Brown D. Interactions between saturated acyl chains confer detergent resistance on lipids and glycosylphosphatidylinositol (GPI)-anchored proteins: GPI-anchored proteins in liposomes and cells show similar behavior. Proc Natl Acad Sci U S A. 1994;91:12130–12134. doi: 10.1073/pnas.91.25.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pike LJ. The challenge of lipid rafts. J Lipid Res. 2009;50(Suppl):S323–S328. doi: 10.1194/jlr.R800040-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pike LJ. Rafts defined: a report on the Keystone Symposium on Lipid Rafts and Cell Function. J Lipid Res. 2006;47:1597–1598. doi: 10.1194/jlr.E600002-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.Oh P, Schnitzer JE. Segregation of heterotrimeric G proteins in cell surface microdomains. G(q) binds caveolin to concentrate in caveolae, whereas G(i) and G(s) target lipid rafts by default. Mol Biol Cell. 2001;12:685–698. doi: 10.1091/mbc.12.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chini B, Parenti M. G-protein coupled receptors in lipid rafts and caveolae: how, when and why do they go there? J Mol Endocrinol. 2004;32:325–338. doi: 10.1677/jme.0.0320325. [DOI] [PubMed] [Google Scholar]
- 21.Kitagawa S, Endo J, Kametani F. Effects of long-chain cis-unsaturated fatty acids and their alcohol analogs on aggregation of bovine platelets and their relation with membrane fluidity change. Biochim Biophys Acta. 1985;818:391–397. doi: 10.1016/0005-2736(85)90014-8. [DOI] [PubMed] [Google Scholar]
- 22.Zeron Y, Sklan D, Arav A. Effect of polyunsaturated fatty acid supplementation on biophysical parameters and chilling sensitivity of ewe oocytes. Mol Reprod Dev. 2002;61:271–278. doi: 10.1002/mrd.1156. [DOI] [PubMed] [Google Scholar]
- 23.Tappia PS, Ladha S, Clark DC, Grimble RF. The influence of membrane fluidity, TNF receptor binding, cAMP production and GTPase activity on macrophage cytokine production in rats fed a variety of fat diets. Mol Cell Biochem. 1997;166:135–143. doi: 10.1023/a:1006875010120. [DOI] [PubMed] [Google Scholar]
- 24.Lund EK, Harvey LJ, Ladha S, Clark DC, Johnson IT. Effects of dietary fish oil supplementation on the phospholipid composition and fluidity of cell membranes from human volunteers. Ann Nutr Metab. 1999;43:290–300. doi: 10.1159/000012797. [DOI] [PubMed] [Google Scholar]
- 25.Rockett BD, Teague H, Harris M, Melton M, Williams J, Wassall SR, Shaikh SR. Fish oil increases raft size and membrane order of B cells accompanied by differential effects on function. J Lipid Res. 2012;53:674–685. doi: 10.1194/jlr.M021782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan YY, Ly LH, Barhoumi R, McMurray DN, Chapkin RS. Dietary docosahexaenoic acid suppresses T cell protein kinase C theta lipid raft recruitment and IL-2 production. J Immunol. 2004;173:6151–6160. doi: 10.4049/jimmunol.173.10.6151. [DOI] [PubMed] [Google Scholar]
- 27.Miyamoto A, Schams D. Oxytocin stimulates progesterone release from microdialyzed bovine corpus luteum in vitro. Biol Reprod. 1991;44:1163–1170. doi: 10.1095/biolreprod44.6.1163. [DOI] [PubMed] [Google Scholar]
- 28.Mattos R, Guzeloglu A, Badinga L, Staples CR, Thatcher WW. Polyunsaturated fatty acids and bovine interferon-tau modify phorbol ester-induced secretion of prostaglandin F2 alpha and expression of prostaglandin endoperoxide synthase-2 and phospholipase-A2 in bovine endometrial cells. Biol Reprod. 2003;69:780–787. doi: 10.1095/biolreprod.102.015057. [DOI] [PubMed] [Google Scholar]
- 29.Daumas F, Destainville N, Millot C, Lopez A, Dean D, Salomé L. Confined diffusion without fences of a g-protein-coupled receptor as revealed by single particle tracking. Biophys J. 2003;84:356–366. doi: 10.1016/S0006-3495(03)74856-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barisas BG, Smith SM, Liu J, Song J, Hagen GM, Pecht I, Roess DA. Compartmentalization of the Type I Fcε receptor and MAFA on mast cell membranes. Biophys Chem. 2007;126:209–217. doi: 10.1016/j.bpc.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 31.Murase K, Fujiwara T, Umemura Y, Suzuki K, Iino R, Yamashita H, Saito M, Murakoshi H, Ritchie K, Kusumi A. Ultrafine membrane compartments for molecular diffusion as revealed by single molecule techniques. Biophys J. 2004;86:4075–4093. doi: 10.1529/biophysj.103.035717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singer SJ, Nicolson GL. The fluid mosaic model of the structure of cell membranes. Science. 1972;175:720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- 33.Bretscher MS. Asymmetrical lipid bilayer structure for biological membranes. Nat New Biol. 1972;236:11–12. doi: 10.1038/newbio236011a0. [DOI] [PubMed] [Google Scholar]
- 34.Phillips MC, Ladbrooke BD, Chapman D. Molecular interactions in mixed lecithin systems. Biochim Biophys Acta. 1970;196:35–44. doi: 10.1016/0005-2736(70)90163-x. [DOI] [PubMed] [Google Scholar]
- 35.Fan YY, McMurray DN, Ly LH, Chapkin RS. Dietary (n-3) polyunsaturated fatty acids remodel mouse T-cell lipid rafts. J Nutr. 2003;133:1913–1920. doi: 10.1093/jn/133.6.1913. [DOI] [PubMed] [Google Scholar]
- 36.Corsetto PA, Cremona A, Montorfano G, Jovenitti IE, Orsini F, Arosio P, Rizzo AM. Chemical-physical changes in cell membrane microdomains of breast cancer cells after omega-3 PUFA incorporation. Cell Biochem Biophys. 2012;64:45–59. doi: 10.1007/s12013-012-9365-y. [DOI] [PubMed] [Google Scholar]
- 37.Kim W, Fan YY, Barhoumi R, Smith R, McMurray DN, Chapkin RS. n-3 polyunsaturated fatty acids suppress the localization and activation of signaling proteins at the immunological synapse in murine CD4+ T cells by affecting lipid raft formation. J Immunol. 2008;181:6236–6243. doi: 10.4049/jimmunol.181.9.6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma DW, Seo J, Davidson LA, Callaway ES, Fan YY, Lupton JR, Chapkin RS. n-3 PUFA alter caveolae lipid composition and resident protein localization in mouse colon. FASEB J. 2004;18:1040–1042. doi: 10.1096/fj.03-1430fje. [DOI] [PubMed] [Google Scholar]
- 39.Lazzarini A, Macchiarulo A, Floridi A, Coletti A, Cataldi S, Codini M, Lazzarini R, Bartoccini E, Cascianelli G, Ambesi-Impiombato FS, Beccari T, Curcio F, et al. Very-long-chain fatty acid sphingomyelin in nuclear lipid microdomains of hepatocytes and hepatoma cells: can the exchange from C24:0 to C16:0 affect signal proteins and vitamin D receptor? Mol Biol Cell. 2015;26:2418–2425. doi: 10.1091/mbc.E15-04-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calebiro D, Rieken F, Wagner J, Sungkaworn T, Zabel U, Borzi A, Cocucci E, Zürn A, Lohse MJ. Single-molecule analysis of fluorescently labeled G-protein-coupled receptors reveals complexes with distinct dynamics and organization. Proc Natl Acad Sci U S A. 2013;110:743–748. doi: 10.1073/pnas.1205798110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suzuki K, Ritchie K, Kajikawa E, Fujiwara T, Kusumi A. Rapid hop diffusion of a G-protein-coupled receptor in the plasma membrane as revealed by single-molecule techniques. Biophys J. 2005;88:3659–3680. doi: 10.1529/biophysj.104.048538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goose JE, Sansom MS. Reduced lateral mobility of lipids and proteins in crowded membranes. PLoS Comput Biol. 2013;9:e1003033. doi: 10.1371/journal.pcbi.1003033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jin S, Haggie PM, Verkman AS. Single-particle tracking of membrane protein diffusion in a potential: simulation, detection, and application to confined diffusion of CFTR Cl- channels. Biophys J. 2007;93:1079–1088. doi: 10.1529/biophysj.106.102244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frick M, Schmidt K, Nichols BJ. Modulation of lateral diffusion in the plasma membrane by protein density. Curr Biol. 2007;17:462–467. doi: 10.1016/j.cub.2007.01.069. [DOI] [PubMed] [Google Scholar]
- 45.Carlson JC, Buhr MM, Wentworth R, Hansel W. Evidence of membrane changes during regression in the bovine corpus luteum. Endocrinology. 1982;110:1472–1476. doi: 10.1210/endo-110-5-1472. [DOI] [PubMed] [Google Scholar]
- 46.Freedman NJ, Lefkowitz RJ. Desensitization of G protein-coupled receptors. Recent Prog Horm Res. 1996;51:319–351. [discussion: 352–3] [PubMed] [Google Scholar]
- 47.Hausdorff WP, Bouvier M, O’Dowd BF, Irons GP, Caron MG, Lefkowitz RJ. Phosphorylation sites on two domains of the beta 2-adrenergic receptor are involved in distinct pathways of receptor desensitization. J Biol Chem. 1989;264:12657–12665. [PubMed] [Google Scholar]
- 48.Benovic JL, Pike LJ, Cerione RA, Staniszewski C, Yoshimasa T, Codina J, Caron MG, Lefkowitz RJ. Phosphorylation of the mammalian beta-adrenergic receptor by cyclic AMP-dependent protein kinase. Regulation of the rate of receptor phosphorylation and dephosphorylation by agonist occupancy and effects on coupling of the receptor to the stimulatory guanine nucleotide regulatory protein. J Biol Chem. 1985;260:7094–7101. [PubMed] [Google Scholar]
- 49.Bailey JM, Howard BV, Tillman SF. Lipid metabolism in cultured cells. XI. Utilization of serum triglycerides. J Biol Chem. 1973;248:1240–1247. [PubMed] [Google Scholar]
- 50.Kabouridis PS, Janzen J, Magee AL, Ley SC. Cholesterol depletion disrupts lipid rafts and modulates the activity of multiple signaling pathways in T lymphocytes. Eur J Immunol. 2000;30:954–963. doi: 10.1002/1521-4141(200003)30:3<954::AID-IMMU954>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 51.McMullen TPW, Lewis RNAH, McElhaney RN. Cholesterol–phospholipid interactions, the liquid-ordered phase and lipid rafts in model and biological membranes. Curr Opin Colloid Interf Sci. 2004;8:459–468. [Google Scholar]
- 52.Ilangumaran S, Hoessli DC. Effects of cholesterol depletion by cyclodextrin on the sphingolipid microdomains of the plasma membrane. Biochem J. 1998;335(Pt 2):433–440. doi: 10.1042/bj3350433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kwik J, Boyle S, Fooksman D, Margolis L, Sheetz MP, Edidin M. Membrane cholesterol, lateral mobility, and the phosphatidylinositol 4,5-bisphosphate-dependent organization of cell actin. Proc Natl Acad Sci U S A. 2003;100:13964–13969. doi: 10.1073/pnas.2336102100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brejchová J, Sýkora J, Ostašov P, Merta L, Roubalová L, Janáček J, Hof M, Svoboda P. TRH-receptor mobility and function in intact and cholesterol-depleted plasma membrane of HEK293 cells stably expressing TRH-R-eGFP. Biochim Biophys Acta. 2015;1848:781–796. doi: 10.1016/j.bbamem.2014.11.029. [DOI] [PubMed] [Google Scholar]
- 55.Kilsdonk EP, Yancey PG, Stoudt GW, Bangerter FW, Johnson WJ, Phillips MC, Rothblat GH. Cellular cholesterol efflux mediated by cyclodextrins. J Biol Chem. 1995;270:17250–17256. doi: 10.1074/jbc.270.29.17250. [DOI] [PubMed] [Google Scholar]
- 56.Diskin MG, Morris DG. Embryonic and early foetal losses in cattle and other ruminants. Reprod Domest Anim. 2008;43(Suppl 2):260–267. doi: 10.1111/j.1439-0531.2008.01171.x. [DOI] [PubMed] [Google Scholar]
- 57.Mann GE, Lamming GE. Relationship between maternal endocrine environment, early embryo development and inhibition of the luteolytic mechanism in cows. Reproduction. 2001;121:175–180. doi: 10.1530/rep.0.1210175. [DOI] [PubMed] [Google Scholar]