Abstract
Dendritic cells (DCs) are central regulators of the adaptive immune response, and as such are necessary for T cell-mediated cancer immunity. In particular, anti-tumoral responses depend upon a specialized subset of conventional DCs that transport tumor antigens to draining lymph nodes and cross-present antigen to activate cytotoxic T lymphocytes. DC maturation is necessary to provide co-stimulatory signals to T cells, but while DC maturation occurs within tumors, it is often insufficient to induce potent immunity, particularly in light of suppressive mechanisms within tumors. Bypassing suppressive pathways or directly activating DCs can unleash a T cell response, and although clinical efficacy has proven elusive, therapeutic targeting of DCs continues to hold translational potential in combinatorial approaches.
Keywords: Dendritic cells, tumor microenvironment, cancer, antigen-presentation, vaccination, immunotherapy
Dendritic Cells in Cancer
The preferential ability of conventional dendritic cells (cDCs) to activate T cells is the foundation of the “cancer-immunity cycle” outlined by Chen and Mellman [1]. Tumor-associated cDCs are thought to endocytose dead neoplastic cells or cellular debris and transport cancer-associated antigens to the draining lymph node where T cell priming and activation can occur. Although multiple other professional antigen-presenting cells exist, including other DC subsets (Box 1), cDCs are particularly adept at initiating a T cell response, directing T cell polarization, and presenting exogenous and endogenous antigen on either major histocompatibility class I (MHC-I) or MHC-II [2].
Box 1. Other DC lineages.
Plasmacytoid DCs
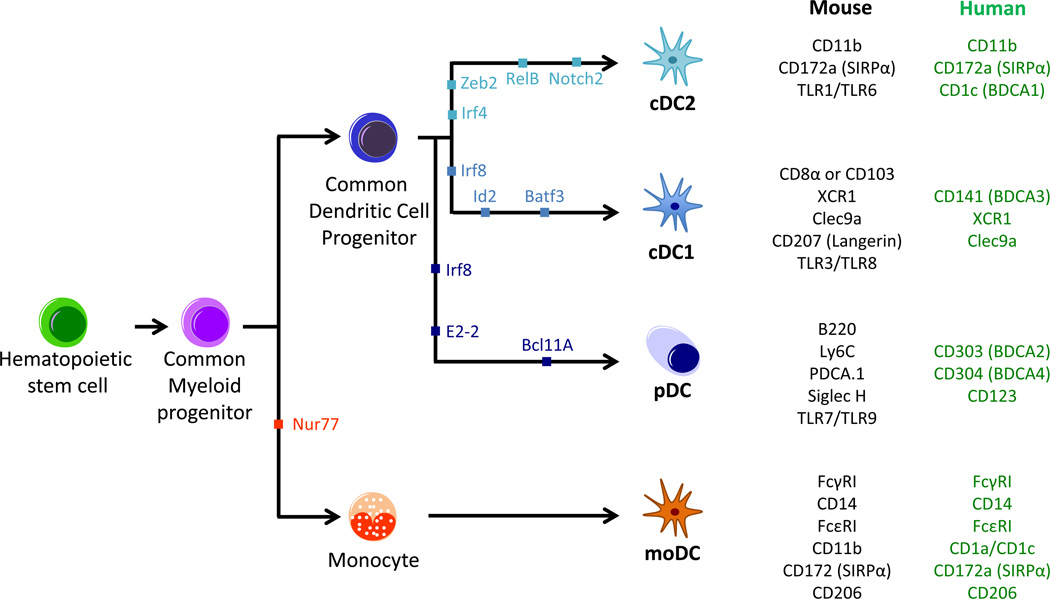
Prominent in the blood and spleen, plasmacytoid DCs (pDCs) are found in small numbers throughout the periphery and are recognized by their expression of B220, Ly6C, and PDCA.1 in mice and CD123, CD303/BDCA2 and CD304/BDCA4 in humans. pDCs selectively express TLR7 and TLR9, and their most important function is thought to be producing significant quantities of type 1 IFN in response to single-stranded viral RNA and DNA [90]. pDCs also have the potential to act as antigen-presenting cells as they express MHC class II and co-stimulatory molecules; however, the ability of pDCs to phagocytose dead cells and present cell-associated antigen is debatable, as is their ability to cross-present exogenous antigen on MHC class I [91]. In tumors, the presence of pDCs correlates with poor prognosis in both breast and ovarian cancers [92, 93], but pDCs can also act as therapeutic targets to elicit IFN-α release and antigen-presentation by cDCs [85, 94].
Monocyte-derived DCs
moDCs differentiate from Ly6C+ or CD14hi monocytes in mice and humans, respectively [95, 96]. moDCs are hard to distinguish as they are phenotypically similar to CD11b+ cDCs or macrophages depending on the markers used for identification, but recent studies have shown that the Fc receptors FcγRI (CD64) and FcεRI can be used successfully [4, 97, 98]. The capacity of moDCs within tissue to transport antigen to the lymph nodes and activate naïve T cells ex vivo represents a fraction of that observable with cDCs [97, 99]. It therefore remains unclear the degree to which moDCs are involved in inducing a de novo T cell response. As recruitment of moDCs is enhanced by infection or TLR ligand injection – conditions that result in the presence of ‘TipDCs’ expressing tumor necrosis factor α (TNF- α) and inducible nitric oxide synthase (iNOS) – one possibility is that they may regulate T cell activity within tumors [100].
cDCs in mice and humans can be further divided into two main lineages distinguished by transcription factor dependency, marker expression, and functionality (Figure 1). cDC1 require the transcription factors IRF8, BATF3, and ID2, preferentially express the chemokine receptor XCR1, and display enhanced ability to cross-present exogenous antigen on MHCI and activate CD8+ T cells. In comparison, cDC2 depend upon IRF4 and ZEB2, preferentially express CD172a, and represent a heterogeneous population that displays enhanced MHCII antigen presentation and preferentially activates CD4+ T cells [2–4].
Figure 1. Dendritic Cell Differentiation.
Dendritic cells (DCs) are part of the hematopoietic cell lineage, originating from hematopoietic stem cells (HSC), which subsequently differentiate into common myeloid progenitors (CMPs). The transcription factor Nur77 drives the differentiation of CMPs through several steps into monocytes, which can further differentiate into monocyte DCs (moDCs) under inflammatory conditions. In the absence of Nur77, CMP will differentiate through multiple stages into a common dendritic cell progenitor (CDP). The conventional type 1 DC (cDC1), conventional type 2 DC (cDC2) and plasmacytoid DC (pDC) subsets arise from the CDP, with the critical transcription factors shown for each lineage. Markers for each DC subset are shown on the right for mouse (black) or human (green). cDC1 in mice can be identified by expression of either CD8 α in the lymphoid organs or CD103 within peripheral tissues.
Although the role of cDC2 in tumor immunity is largely unexplored, the cross-presenting cDC1 population is now established as being necessary for inducing a protective CD8+ T cell response. This has been convincingly demonstrated using Batf3-deficient mice, as these fail to reject highly immunogenic cancer cell lines [5, 6] and do not respond to checkpoint blockade therapy using antibodies against programmed death 1 (PD-1) [7, 8]. Here we will discuss the role of cDCs in delivering antigen to T cells in the lymph node, the stimulatory and suppressive pathways within the tumor microenvironment involved in cDC maturation, and the potential of cDCs as therapeutic targets in cancer immunity.
Antigen Delivery and Presentation
cDCs exist as resident lymphoid tissue cDCs in the spleen and lymph nodes critical for sampling blood and lymph born antigen, respectively, and as nonlymphoid tissue cDCs that can directly transport antigen from the peripheral tissues [2, 3]. The relative importance of lymphoid versus nonlymphoid tissue cDCs in distributing and presenting antigen is highly context specific, depending on both the type of antigen and route of exposure. For example, infection models have been used to demonstrate the necessity of antigen transfer between cDC populations, as well as sequential T cell interactions with different cDC subsets [9].
While lymphoid resident cDC1 (defined by expression of CD8α in mice) were originally thought to be responsible for cross-presenting peripheral antigens, it has become increasingly clear that migratory cDC1 (defined by expression of CD103 in mice) are necessary for transporting cellular antigens from the periphery to the lymph nodes, at least from the skin, lung and intestine in mice [9]. This is true for endogenous antigens and localized infections, as well as the delivery of cellular debris to the lungs via intranasal or intravenous delivery [10, 11]. Only during a bolus subcutaneous injection of dead cells does cellular antigen appear to travel via the lymphatics independently of cDCs [12]. Comparatively less attention has been paid to antigen delivery in tumor models, although naïve T cell activation has long been known to be mediated by DCs within the draining lymph node [13–15]. However, two recent studies have now shown that tumor-associated fluorescent proteins are actively transported by CD103+ cDC1 that migrate from the tumor to the lymph nodes in a CCR7-dependent manner [7, 12]. Importantly this occurs in both implantable and spontaneous tumor models, avoiding experimental artifacts that may occur when injecting large quantities of dead or dying cells.
In addition to delivering tumor antigen, only migratory CD103+ cDC1 displayed a robust ability to activate naïve CD8+ T cells ex vivo following sorting of the various cDC subsets [7, 12]. These findings suggest that CD103+ cDC1 may be the only cDC population required to induce a cytotoxic T cell response against tumors. While this conclusion is consistent with their role in antigen delivery, there are some important caveats to consider. First, ex vivo stimulation would fail to identify a sequential role for cDC subsets. Indeed, using a more stable fluorophore reporter, the Krummel group was able to detect tumor antigen in all of the professional antigen-presenting cells within draining lymph nodes [12], although the relevance of this antigen transfer is unclear. Second, CD8+ T cell effector function is not synonymous with proliferation, and support by CD4+ T cells is required in many tumor models [15–20]. While there appears to be a general defect in the ability of tumor cDCs to present antigen to CD4+ T cells ex vivo [15, 21], it is possible that the ability of cDC2 to activate CD4+ T cells may be involved in regulating multiple aspects of the immune response during tumor development.
It is not completely clear why cDC2 fail to deliver tumor antigen to lymph nodes. In the tumor microenvironment, macrophages, monocytes, and both cDC subsets uptake tumor antigens, with macrophages representing the dominant phagocytic population [7, 21]. Migratory CD11b+ cDC2 (defined by differential expression of CD11c and MHCII in mice) also appear in equivalent numbers to migratory CD103+ cDC1 within tumor draining lymph nodes [12], although it is not evident from the data whether these cells originate from the tumor or other locations. One possibility is an inability of cDC2 to appropriately process cellular antigen. cDC2 express lower levels of endocytic receptors such as CD36 and Clec9a that are involved in recognizing apoptotic cells, and combined with higher levels of lysosomal enzymes and lower phagosomal pH, antigen within cDC2 may simply be degraded during migration. In support of this, delivery of antigen to CD11b+ cDC2 via Ig immune complexes can permit cross-presentation [22, 23]. Reduced antigen presentation may also be a result of cDC2 not receiving the appropriate stimulus within tumors. For example, immune responses induced by anthracyclines or vaccines containing the toll-like receptor 7 (TLR7) agonist R848 provide protection in Batf3-deficient mice [24, 25]. Notably however, prophylactic immunity following vaccination with R848 is evident only when using soluble peptides [24], highlighting the restricted ability of CD11b+ cDCs to cross-present cellular antigens [10, 26].
Activation of Tumor DCs
Based upon the reduced capacity of tumor-associated CD11c+ cells to induce T cell proliferation, DCs within the tumor microenvironment have often been viewed as tolerogenic or immunosuppressive [27]. As discussed however, it is the rare cDC1 subset that is required for CD8+ T cell activation, and it is only recently that this population has been evaluated within tumors (Box 2). Indeed, while the dominant CD11c+ population of macrophages is incapable of activating CD8+ T cells, both tumor resident and migratory CD103+ cDCs display stimulatory capacity ex vivo [7, 12, 21]. This is not to imply that cDCs are operating at peak capacity, especially considering limitations on their infiltration and maturation status [7, 21], as well as suppressive pathways that may block particular functions [28, 29]. However, the success of adoptive cell transfer and immune checkpoint blockade therapies demonstrate that anti-tumor immunity develops in some patients, and therefore – as a prerequisite for the development of these T cell responses – that at least some measure of DC activation is occurring in many tumors.
Box 2. The Problem with CD11c.
CD11c is not a reliable marker of the cDC lineage even under steady state conditions, and this issue is further exaggerated in inflamed tissue. Small populations of macrophages and lymphocytes express CD11c in the secondary lymphoid organs, while migratory cDCs downregulate surface expression. Alveolar macrophages in the lung express high levels of CD11c and macrophages substantially upregulate CD11c following stimulation [3]. Similarly, both populations of cDCs express CD11b, albeit to varying degrees. As such, macrophages cannot be accurately distinguished from cDCs in either human or mouse tumors using only the common markers CD11c, CD11b and MHCII. This has resulted in confusion in the field as properties of macrophages are ascribed to tumor cDCs. IRF8-dependent cDCs can be identified by markers such as XCR1, but depending on the tissue and species being examined, additional macrophage (CD14, CD64, CD115) or cDC markers (CD24, CD26) are necessary to distinguish between macrophages and CD11b+ cDCs [4].
DC maturation is marked by the movement of MHC/peptide complexes to the cell membrane, upregulation of the costimulatory molecules CD80/CD86, and expression of cytokines that drive T cell proliferation and differentiation (e.g. IL-12). These molecules are referred to as signal 1, 2, and 3, respectively, and are required for proper T cell activation [30]. DC activation is normally considered to result from detection of pathogen-associated molecular patterns (PAMPs) by pattern recognition receptors (PRRs) such as the TLRs. Within tumors many of these same receptors recognize endogenous, constitutively expressed damage-associated molecular patterns (DAMPs) that are released or expressed on the surface of dead/dying cells [31]. In contrast to apoptotic cells, dead ‘immunogenic’ cells induce expression of MHCII, CD40, CD80, and CD86 on DCs, along with the release of the inflammatory cytokines IL-1β, IL-6, IL-12 and TNF-α.
Immunogenic cell death is best described in cancer cells treated with anthracycline chemotherapies and involves: 1) translocation of intracellular calreticulin and other endoplasmic reticulum proteins to the cell surface; 2) secretion of ATP during the blebbing phase of apoptosis; and 3) release of the chromatin-binding protein high-mobility group box 1 (HMGB1) [32]. ATP binding to P2RX7 appears important for inducing myeloid cell recruitment and activation [25, 33–35], as well as the release of active IL-1β, while recognition of calreticulin by CD91 is necessary for engulfment of cellular antigens [36, 37]. The mechanism by which HMGB1-TLR4 promotes immunity is less clear, but could involve recruitment, enhanced antigen processing, and/or direct DC activation [38–40]. Despite this knowledge gap, HMGB1 expression is the most distinguishing characteristic of immunogenic cell death, as ATP release occurs under many conditions of cellular stress [34, 41]. Interestingly, CD11c+ myeloid cell activation within tumors may be limited by binding of HMGB1 to T-cell immunoglobulin and mucin-domain containing (TIM)-3, although this receptor impacts tumor growth independently of a T cell response and therefore the role of this pathway in regulating cDC activity is unclear [42].
In contrast to their critical role in mediating immunological responses to anthracycline chemotherapies, mice deficient in TLR or IL-1 receptor signaling display no defect in spontaneous or radiation-induced T cell responses against tumors [43, 44]. P2RX7 was similarly dispensable for spontaneous T cell priming [43]. Instead, anti-tumor immune responses were found to be highly dependent upon expression of the type I interferon receptor (IFNAR1) [45]. Type I IFNs (IFN-α, IFN-β) promote DC activation, migration and cross-presentation, and in an elegant series of experiments IFNAR1 expression by Batf3-dependent cDC1 was found to be specifically required [6, 46]. Although expression of Ifna genes was not examined, Ifnb expression in these models localized to CD11c+ cells within tumors and draining lymph nodes, hinting at the migration of activated cDCs [43]. In addition, Batf3-deficiency did not alter Ifnb expression levels [6], suggesting that CD11b+ cDC2 might be an important source of IFN-β, at least within the lymph nodes. It remains to be determined whether type I IFN expression by macrophages, plasmacytoid DCs (pDCs), or even autocrine production by cDC1 might also be important. Finally, it is unclear whether IFN-β is functionally necessary within tumors or draining lymph nodes, and correspondingly whether IFNAR1-expression by migratory or non-migratory cDC1 cells is required.
Surprisingly, incubating bone marrow-derived DCs with apoptotic or necrotic tumor cells fails to induce Ifnb expression. Instead, the entry of DNA into the cytoplasm is required for recognition by cyclic-GMP-AMP synthase (cGAS), followed by activation of presence of stimulator of interferon genes complex (STING) and phosphorylation of the transcription factor IRF3 [43, 44, 47]. Although this is not observed in vitro, tumor DNA and phosphorylated IRF3 are readily found within CD11c+ leukocytes within tumors, and mice deficient in either STING or IRF3 fail to develop immunity. This could suggest that specific receptors not expressed by bone marrow-derived cells are required to allow DNA into the cytoplasm. For example, Clec9a binding to exposed F-actin filaments regulates intracellular trafficking and promotes cross-presentation by cDC1 during viral infection [31]. Alternatively, cDCs may require the appropriate stimulatory signals, as demonstrated for TLR7 stimulation of CD11b+ cDC2 [24]. The source of this potential activating signal is unclear, as TLR signaling in the host is dispensable for spontaneous or radiation-induced immunity. One possibility is cell death via necroptosis, a programed form of necrosis linked to inflammation [48]. In addition to release of DAMPs, necroptosis has recently been shown to induce inflammatory cytokine gene expression within dying cells and to drive cross-priming of CD8+ T cells in vivo [49], a process that can be utilized for prophylactic vaccination against tumors [50]. The discrepancies in the role of TLRs and IFN genes between spontaneous and chemotherapy-induced T cell responses remain to be reconciled.
Suppression of Tumor DCs
Several features distinguish immunogenic from non-immunogenic tumors. The frequency of neoantigens appears to be a major determinant, based upon the importance of immunoediting as well as the relationship between mutational burden and response to immune checkpoint blockade [51–54]. As discussed above, a second factor may be the degree of DC maturation that results from the type and extent of cell death within tumors. A third factor is likely the level of local and systemic immune suppression caused by the tumor. Direct suppression of effector T cells is well characterized, but the switch from immunogenic to immunosuppressive that occurs during tumor progression also correlates with a phenotypic change in DCs [55]. In particular the balance between stimulatory and suppressive signals within the tumor microenvironment is probably critical in dictating the ability of cDCs to induce and maintain a T cell response, and understanding this relationship will be important in the development of therapies designed to augment T cell immunity.
A number of molecules found in the tumor microenvironment inhibit DC activation in vitro. This includes vascular endothelial growth factor (VEGF), prostaglandin E2 (PGE2), and IL-10. Additionally, VEGF, IL-6, IL-10, and colony-stimulating factor 1 (CSF-1) have been shown to inhibit maturation of bone marrow progenitors or monocytes into DCs, instead driving monocytes toward a suppressive phenotype [27]. The relevance of these suppressive pathways in vivo is not well established, especially as monocytes are not a source of cDCs within tumors. However, increased numbers of CD11c+CD83+ DCs are found in murine tumors following blockade of VEGF receptor 2 [56], and we have reported a higher percentage of CD103+ cDC1 and CD11b+ cDC2 in tumors following CSF-1 neutralization or blockade of the IL-10 receptor during paclitaxel chemotherapy [28]. IL-10 production by tumor macrophages also suppresses expression of IL-12 by tumor CD103+ cDC1 [28], which may be sensitized to respond via TLR2-mediated upregulation of the IL-10 receptor [57].
Other factors likely to suppress DC function relate to the metabolic dysfunction within tumors. For example, hypoxia and lactic acid regulate macrophage function within tumors [58, 59] and suppress DC activation in vitro [60, 61]. Metabolic reprograming within DCs themselves is also important during TLR-mediated activation and could mediate dysfunction within tumors [62], as was recently demonstrated for activation of the ER stress response factor XBP1 and the ensuing accumulation of intracellular lipids [63]. Presumably migration of cDCs to the lymph node will also be impacted by the tumor microenvironment, but this has yet to be described. Finally, CD103+ and BDCA3+ cDC1 represent the least prevalent myeloid population in mouse and human tumors, respectively [7, 21, 28]. The functional relevance of these cells may therefore be restricted due to a lack of cell numbers. This has been demonstrated in melanoma, with either systemic expansion or intratumoral injection of cDCs able to augment response to checkpoint blockade [7, 29]. With the ability to clearly differentiate tumor cDCs from macrophages, it will be important to begin to validate some of the suspected suppressive pathways in vivo, as well as to examine their potential to regulate anti-tumor immunity in a therapeutic context.
Non-Migratory Tumor DCs
The primary function of cDCs in cancer immunity is to sequentially acquire tumor antigen, migrate to the lymph node, and activate a de novo T cell response (Figure 2). However, only a small fraction of tumor cDCs will end up migrating to the lymph nodes, possibly related to controlled expression of CCR7 [12]. This raises the possibility that the remaining cells may be involved in regulating the local immune microenvironment. It has already been described in the skin that clustering of effector/memory T cells with macrophages and cDCs is important for an immune response following re-exposure to a hapten [64]. These clusters are also involved in the maintenance of anti-viral memory CD4+ T cells in mucosa [65]. Although these situations are not synonymous with the continuous presence of antigen or the immunosuppressive environment within tumors, it is possible that non-migratory intratumoral cDCs may have a similar role in maintaining cytotoxic T cell activity, either through direct antigen presentation or through establishment of a favorable cytokine milieu [28]. In support of this, removing tumor-draining lymph nodes does not impact the immune response during anthracycline chemotherapy [25], and intratumoral CD103+ cDC1 are important for tumor regression after adoptive T cell transfer, irrespective of any activity in the lymph node [21]. While it is technically challenging to differentiate between a local and systemic role for cDCs, this distinction may have important implications when optimizing dosing strategy for combination immunotherapies.
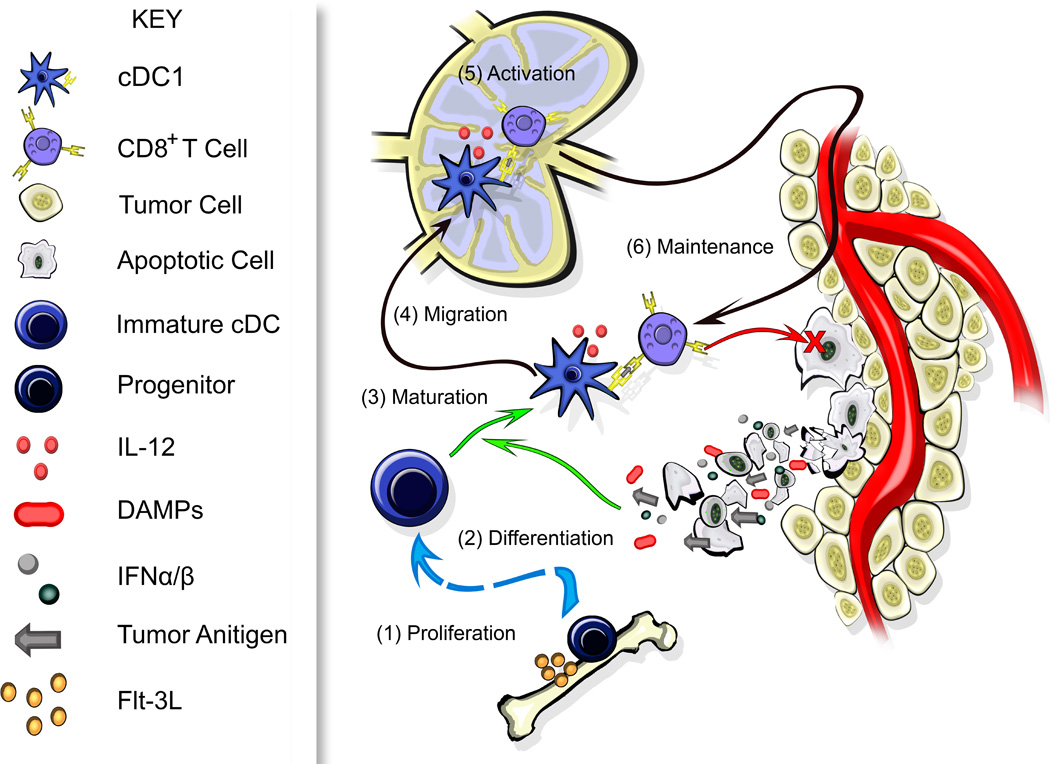
Figure 2. DCs in Anti-Tumor Immunity.
Conventional type 1 dendritic cells (cDC1) are necessary for the generation of a de novo T cell response against tumors. Expansion of cDC progenitors occurs in the bone marrow driven by FMS-related tyrosine kinase 3 ligand (Flt-3L) (1). These cells differentiate into immature cDC1 within tissues, including tumors as depicted here. There, they can acquire antigens but are unable to stimulate T cells (2). DC activation/maturation is driven by damage-associated molecular patterns (DAMPs) released from necrotic cells and/or type I interferons (IFNs) released by cells within the tumor microenvironment (3). A subset of mature cDC1 expressing CCR7 migrate to the lymph node (4) where they prime a CD8+ T cell response (5). Activated T cells may then migrate to the tumor site – depending on the condition of the vasculature and stroma – where they can mediate their cytotoxic effector function (red X). Non-migratory cDC1 that remain in the tumor may interact with CD8+ T cells to regulate the anti-tumor response, with cDC1 secreting interleukin-12 (IL-12) or other cytokines to promote T cell effector function (6). Under ideal conditions this may release additional tumor antigens and DAMPs, and increase expression of inflammatory cytokines, further augmenting an immune response.
It is also probable that tumor-associated cDCs are involved in priming T cell responses within tumors when found in ectopic or tertiary lymphoid structures (TLS). These clusters of T cells, B cells, and cDCs occur during chronic inflammation and are capable of inducing and supporting a T cell response during infection [66]. Although they may not be involved in the initial detection of the tumor, TLS could play an important role in the response to neoantigens that develop during later stages of neoplastic progression. Critically, when present in tumors, TLS are associated with a positive prognosis across a range of malignancies [67]. TLS have proven difficult to evaluate experimentally as they do not normally develop in murine models. However, expression of ovalbumin by tumor cells is sufficient to drive TLS formation and T cell activation even in mice lacking lymph nodes [68, 69]. TLS can also be generated by intratumoral injection of DCs expressing CCL21, overexpression of tumor necrosis factor superfamily member 14 (TNFSF14), or injection of lymphotoxin α, and in all cases this is sufficient to drive an anti-tumor T cell response [70–72].
DCs in Cancer Therapy
Vaccines
Therapeutic vaccination for cancer continues to be an active area of research and clinical investigation. However, as this has been expertly reviewed elsewhere [73], we will attempt only to highlight some key concepts here. All vaccines depend upon the ability of DCs to act as antigen-presenting cells to T cells and can be classified by their approach into: 1) nontargeted; 2) in vivo targeted; or 3) ex vivo loaded [73]. One of the first cancer vaccines to significantly advance in the clinic was GVAX, which involves irradiated tumor cells modified to express GM-CSF, thereby recruiting and maturing DCs at the site of vaccination to promote antigen uptake and delivery. Building off of this concept has included the addition of stimulating agents such as cyclic dinucleotides to activate STING [74], or alternative delivery vehicles for GM-CSF such as oncolytic viruses [75].
In the more traditional and widely tested approach, recombinant peptides/proteins are mixed with adjuvants containing various formulations of TLR agonists. These can be injected directly or fused with antibodies that target DCs in vivo, including DEC205, Clec9a, or DC-SIGN. While the in vivo targeting approach has not shown much clinical advancement, multiple trials with peptide vaccines are ongoing [73]. Efficacy has been observed in humans using allogeneic monocyte-derived DC (moDCs) loaded with peptides ex vivo, an approach first approved with Sipuleucel-T for metastatic castration-resistant prostate cancer [76]. In this case, peripheral blood mononuclear cells are isolated and pulsed with a fusion protein made up of GM-CSF and human prostatic acid phosphatase [77]. GM-CSF induced moDCs can also be matured ex vivo with CD40 ligand, IFN-γ, and/or TLR agonists [78, 79]. However, as moDCs display a limited capacity to cross-present antigen or migrate to the lymph nodes, it is unclear the degree to which these cells are acting as antigen-presenting cells or delivery vehicles for antigen, with several studies demonstrating that endogenous DCs are actually required for T cell priming [80–82].
In general these single-agent cancer vaccines are well tolerated and produce a systemic immune reaction against the tumor antigen, but have failed to demonstrate substantial efficacy in late stage clinical studies. One possible explanation for this failure is a suppressive tumor microenvironment that can limit T cell infiltration and effector function. The current testing of vaccines in combination with antagonist antibodies against programmed death-1 should address whether this pathway has been a significant impediment. Another possibility is that the target antigens have not been optimized, given that they have been mostly limited to overexpressed or aberrantly expressed antigens that are not tumor-specific. The next generation use of vaccines containing patient-specific neoantigens will help to determine whether this has been a critical barrier to efficacy [79]. Alternatively, perhaps the lack of observed efficacy has simply been due to insufficient or inappropriate immune stimulation. In support of this, a DC vaccine formulated with tetanus toxoid has been reported to show efficacy in glioblastoma in both mice and patients [83], and an attenuated strain of Listeria expressing mesothelin increased overall survival in pancreatic cancer patients when used to boost a GVAX priming injection [84]. Approaches that trigger IFN-α release by pDCs may be an alternative way to exploit anti-pathogen defenses while avoiding these complex vaccine formulations [85].
In vivo expansion/activation
Many of the same stimulatory pathways used in vaccine development could also prove useful for enhancing endogenous DC activity within tumors. This approach has the potential advantage of targeting a broader spectrum of antigens, allowing neoantigen targeting without patient-specific vaccine development, and minimizing the complexities associated with live cell approaches. Intratumoral injection of TLR (CpG or Poly[I:C]) or STING agonists has already been shown to suppress tumor growth in mice by enhancing a CD8+ T cell-response [7, 86–88]. As well as the expected increase in cDC maturation, these agonists may promote lymph node migration and could theoretically increase antigen delivery. However, as with vaccination, monotherapy with these agonists is unlikely to show substantial clinical efficacy. Instead, targeting multiple pathways to abrogate immune suppression while augmenting cDC activation may be required. One of the first examples of this involved treating tumor-bearing animals with CpG and an anti-IL-10 receptor antibody [89], with potentially comparable results obtained when using cytotoxic therapy as a surrogate immune agonist [28]. In addition, systemic injection of FMS-related tyrosine kinase 3 ligand (Flt-3L) induces a 4-fold expansion of CD103+ cDCs in B16 melanomas, overcoming limited tumor infiltration and delaying tumor growth equivalent to treatment with Poly(I:C) [7]. Importantly, combining Flt-3L with Poly(I:C) shows excellent tumor control that is further augmented by immune stimulation or checkpoint blockade [7, 8]. Identifying additional pathways that can be manipulated to increase cDC infiltration, activation, or effector function should prove useful for enhancing the efficacy of any treatment modalities that are dependent upon an anti-tumor immune response.
Concluding Remarks and Future Directions
As inducers of a T cell response, DCs are the foundation of oncoimmunology, and it will likely become clear that they are critical for responses to cytotoxic and targeted agents as the immunological components of these treatments are uncovered. Although DC activation has been thoroughly examined in the context of infection, our understanding of the signals driving activation under sterile conditions remains incomplete, as does our understanding of the immunosuppressive pathways within tumors that prevent immune recognition (see Outstanding Questions). This knowledge gap has inhibited the translational potential of DC-targeted therapies, but is beginning to be addressed with significant discoveries regarding the importance of the cDC1 subset in delivering antigen to the lymph nodes and inducing the anti-tumor T cell response. One of the major shifts in focus resulting from the success of checkpoint blockade is the realization that combination immunotherapy will be required for the majority of patients to experience durable complete responses. Molecules that drive the antigen presenting and stimulatory capacity of DCs should prove to be important ‘adjuvants’ to enhance the efficiency of these therapies in the case of poorly immunogenic tumors.
Outstanding Questions.
What role (if any) do monocytic DCs play in priming T cells? Do they regulate immune responses within tumors?
What is the role of CD11b+ cDCs in anti-tumor immunity? Are they responsible for activating CD4+ T cells?
Are intratumoral cDCs necessary for sustaining a T cell response?
What is the relative importance of soluble versus cell-associated tumor antigens?
What factors are critical for DC activation within tumors? Does this vary with the type of therapeutic intervention? What suppressive pathways block DC activation?
How important are tertiary lymphoid structures in anti-tumor immunity? Do they regulate response to therapy?
What is the best way to target DCs therapeutically? How can DC-based therapies be combined with other immunotherapies?
Trends Box.
cDC1 are necessary for inducing anti-tumor T cell responses. This appears to trace to the ability of migratory cDC1 to deliver tumor antigen and cross-present to CD8+ T cells.
Spontaneous anti-tumor immunity is dependent upon activation of cDCs by type I IFN. Expression of type I IFNs is induced in myeloid cells via recognition of cytosolic DNA and activation of the STING pathway.
Intratumoral cDC1 are capable of restimulating CD8+ T cells and may be important within tumors for antigen-presentation and/or cytokine expression.
The next generation of vaccines consisting of patient-specific neoantigens or attenuated pathogens may demonstrate single agent efficacy or find utility in combination with checkpoint blockade.
Acknowledgments
This work was supported NCI/NIH grant R00CA185325-02 and Moffitt Cancer Center’s Shula Breast Cancer Award to B.R.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Mildner A, Jung S. Development and function of dendritic cell subsets. Immunity. 2014;40(5):642–656. doi: 10.1016/j.immuni.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 3.Merad M, et al. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31:563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guilliams M, et al. Unsupervised High-Dimensional Analysis Aligns Dendritic Cells across Tissues and Species. Immunity. 2016 doi: 10.1016/j.immuni.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hildner KE, B T, Purtha WE, Diamond M, Matsushita H, Kohyama M, Calderon B, Schraml BU, Unanue ER, Diamond MS, Schreiber RD, Murphy TL, Murphy KM. Batf3 Deficiency Reveals a Critical Role for CD8a+ Dendritic Cells in Cytotoxic T Cell Immunity. Science. 2008;322(5904):1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuertes MB, et al. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J Exp Med. 2011;208(10):2005–2016. doi: 10.1084/jem.20101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salmon H, et al. Expansion and Activation of CD103(+) Dendritic Cell Progenitors at the Tumor Site Enhances Tumor Responses to Therapeutic PD-L1 and BRAF Inhibition. Immunity. 2016;44(4):924–938. doi: 10.1016/j.immuni.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanchez-Paulete AR, et al. Cancer Immunotherapy with Immunomodulatory Anti-CD137 and Anti-PD-1 Monoclonal Antibodies Requires BATF3-Dependent Dendritic Cells. Cancer Discov. 2015 doi: 10.1158/2159-8290.CD-15-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Segura E, Amigorena S. Cross-Presentation in Mouse and Human Dendritic Cells. Adv Immunol. 2015;127:1–31. doi: 10.1016/bs.ai.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Desch AN, et al. CD103+ pulmonary dendritic cells preferentially acquire and present apoptotic cell-associated antigen. J Exp Med. 2011;208(9):1789–1797. doi: 10.1084/jem.20110538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Headley MB, et al. Visualization of immediate immune responses to pioneer metastatic cells in the lung. Nature. 2016 doi: 10.1038/nature16985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts EW, et al. Critical Role for CD103(+)/CD141(+) Dendritic Cells Bearing CCR7 for Tumor Antigen Trafficking and Priming of T Cell Immunity in Melanoma. Cancer Cell. 2016;30(2):324–336. doi: 10.1016/j.ccell.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engelhardt JJ, et al. Marginating dendritic cells of the tumor microenvironment cross-present tumor antigens and stably engage tumor-specific T cells. Cancer Cell. 2012;21(3):402–417. doi: 10.1016/j.ccr.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson MJ, et al. Tolerization of tumor-specific T cells despite efficient initial priming in a primary murine model of prostate cancer. J Immunol. 2007;178(3):1268–1276. doi: 10.4049/jimmunol.178.3.1268. [DOI] [PubMed] [Google Scholar]
- 15.Gerner MY, Casey KA, Mescher MF. Defective MHC class II presentation by dendritic cells limits CD4 T cell help for antitumor CD8 T cell responses. J Immunol. 2008;181(1):155–164. doi: 10.4049/jimmunol.181.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu Z, et al. CD4+ T Cell Help Selectively Enhances High-Avidity Tumor Antigen-Specific CD8+ T Cells. J Immunol. 2015;195(7):3482–3489. doi: 10.4049/jimmunol.1401571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bos R, Sherman LA. CD4+ T-cell help in the tumor milieu is required for recruitment and cytolytic function of CD8+ T lymphocytes. Cancer Res. 2010;70(21):8368–8377. doi: 10.1158/0008-5472.CAN-10-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schietinger A, et al. Bystander killing of cancer requires the cooperation of CD4(+) and CD8(+) T cells during the effector phase. J Exp Med. 2010;207(11):2469–2477. doi: 10.1084/jem.20092450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomita Y, et al. Identification of promiscuous KIF20A long peptides bearing both CD4+ and CD8+ T-cell epitopes: KIF20A-specific CD4+ T-cell immunity in patients with malignant tumor. Clin Cancer Res. 2013;19(16):4508–4520. doi: 10.1158/1078-0432.CCR-13-0197. [DOI] [PubMed] [Google Scholar]
- 20.Marzo AL, et al. Tumor-specific CD4+ T cells have a major "post-licensing" role in CTL mediated anti-tumor immunity. J Immunol. 2000;165(11):6047–6055. doi: 10.4049/jimmunol.165.11.6047. [DOI] [PubMed] [Google Scholar]
- 21.Broz ML, et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell. 2014;26(5):638–652. doi: 10.1016/j.ccell.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.den Haan JM, Bevan MJ. Constitutive versus activation-dependent cross-presentation of immune complexes by CD8(+) and CD8(−) dendritic cells in vivo. J Exp Med. 2002;196(6):817–827. doi: 10.1084/jem.20020295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Platzer B, et al. IgE/FcεRI-Mediated Antigen Cross-Presentation by Dendritic Cells Enhances Anti-Tumor Immune Responses. Cell reports. 2015;10(9):1487–1495. doi: 10.1016/j.celrep.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desch AN, et al. Dendritic cell subsets require cis-activation for cytotoxic CD8 T-cell induction. Nat Commun. 2014;5:4674. doi: 10.1038/ncomms5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma Y, et al. Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity. 2013;38(4):729–741. doi: 10.1016/j.immuni.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Jakubzick C, et al. Optimization of methods to study pulmonary dendritic cell migration reveals distinct capacities of DC subsets to acquire soluble versus particulate antigen. J Immunol Methods. 2008;337(2):121–131. doi: 10.1016/j.jim.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zong J, et al. Tumor-derived factors modulating dendritic cell function. Cancer Immunol Immunother. 2016;65(7):821–833. doi: 10.1007/s00262-016-1820-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruffell B, et al. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell. 2014;26(5):623–637. doi: 10.1016/j.ccell.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature. 2015 doi: 10.1038/nature14404. [DOI] [PubMed] [Google Scholar]
- 30.Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106(3):255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 31.Zelenay S, Reis e Sousa C. Adaptive immunity after cell death. Trends Immunol. 2013;34(7):329–335. doi: 10.1016/j.it.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 32.Kroemer G, et al. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 33.Müller T, et al. The purinergic receptor P2Y2 receptor mediates chemotaxis of dendritic cells and eosinophils in allergic lung inflammation. Allergy. 2010;65(12):1545–1553. doi: 10.1111/j.1398-9995.2010.02426.x. [DOI] [PubMed] [Google Scholar]
- 34.Ghiringhelli F, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15(10):1170–1178. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 35.Idzko M, et al. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat Med. 2007;13(8):913–919. doi: 10.1038/nm1617. [DOI] [PubMed] [Google Scholar]
- 36.Obeid M, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13(1):54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 37.Obeid M, et al. Calreticulin exposure is required for the immunogenicity of gamma-irradiation and UVC light-induced apoptosis. Cell Death Differ. 2007;14(10):1848–1850. doi: 10.1038/sj.cdd.4402201. [DOI] [PubMed] [Google Scholar]
- 38.Apetoh L, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13(9):1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 39.Messmer D, et al. High mobility group box protein 1: an endogenous signal for dendritic cell maturation and Th1 polarization. The Journal of Immunology. 2004;173(1):307–313. doi: 10.4049/jimmunol.173.1.307. [DOI] [PubMed] [Google Scholar]
- 40.Schiraldi M, et al. HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J Exp Med. 2012;209(3):551–563. doi: 10.1084/jem.20111739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pfirschke C, et al. Immunogenic Chemotherapy Sensitizes Tumors to Checkpoint Blockade Therapy. Immunity. 2016;44(2):343–354. doi: 10.1016/j.immuni.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chiba S, et al. Tumor-infiltrating DCs suppress nucleic acid-mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1. Nat Immunol. 2012;13(9):832–842. doi: 10.1038/ni.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woo SR, et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity. 2014;41(5):830–842. doi: 10.1016/j.immuni.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deng L, et al. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity. 2014;41(5):843–852. doi: 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fuertes MB, et al. Type I interferon response and innate immune sensing of cancer. Trends Immunol. 2013;34(2):67–73. doi: 10.1016/j.it.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diamond MS, et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med. 2011;208(10):1989–2003. doi: 10.1084/jem.20101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Corrales L, et al. The host STING pathway at the interface of cancer and immunity. J Clin Invest. 2016;126(7):2404–2411. doi: 10.1172/JCI86892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pasparakis M, Vandenabeele P. Necroptosis and its role in inflammation. Nature. 2015;517(7534):311–320. doi: 10.1038/nature14191. [DOI] [PubMed] [Google Scholar]
- 49.Yatim N, et al. RIPK1 and NF-kappaB signaling in dying cells determines cross-priming of CD8(+) T cells. Science. 2015;350(6258):328–334. doi: 10.1126/science.aad0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aaes TL, et al. Vaccination with Necroptotic Cancer Cells Induces Efficient Anti-tumor Immunity. Cell Rep. 2016;15(2):274–287. doi: 10.1016/j.celrep.2016.03.037. [DOI] [PubMed] [Google Scholar]
- 51.Rizvi NA, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Allen EM, et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 2015;350(6257):207–211. doi: 10.1126/science.aad0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Le DT, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372(26):2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DuPage M, et al. Expression of tumour-specific antigens underlies cancer immunoediting. Nature. 2012;482(7385):405–409. doi: 10.1038/nature10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scarlett UK, et al. Ovarian cancer progression is controlled by phenotypic changes in dendritic cells. J Exp Med. 2012;209(3):495–506. doi: 10.1084/jem.20111413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roland CL, et al. Cytokine levels correlate with immune cell infiltration after anti-VEGF therapy in preclinical mouse models of breast cancer. PloS one. 2009;4(11):e7669. doi: 10.1371/journal.pone.0007669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tang M, et al. Toll-like Receptor 2 Activation Promotes Tumor Dendritic Cell Dysfunction by Regulating IL-6 and IL-10 Receptor Signaling. Cell Rep. 2015;13(12):2851–2864. doi: 10.1016/j.celrep.2015.11.053. [DOI] [PubMed] [Google Scholar]
- 58.Doedens AL, et al. Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression. Cancer Res. 2010;70(19):7465–7475. doi: 10.1158/0008-5472.CAN-10-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Colegio OR, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513(7519):559–563. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liang D, et al. A2B adenosine receptor activation switches differentiation of bone marrow cells to a CD11c+ Gr − 1+ dendritic cell subset that promotes the Th17 response. Immunity, inflammation and disease. 2015;3(4):360–373. doi: 10.1002/iid3.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gottfried E, et al. Tumor-derived lactic acid modulates dendritic cell activation and antigen expression. Blood. 2006;107(5):2013–2021. doi: 10.1182/blood-2005-05-1795. [DOI] [PubMed] [Google Scholar]
- 62.O'Neill LA. Immunometabolism governs dendritic cell and macrophage function. J Exp Med. 2016;213(1):15–23. doi: 10.1084/jem.20151570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cubillos-Ruiz JR, et al. ER Stress Sensor XBP1 Controls Anti-tumor Immunity by Disrupting Dendritic Cell Homeostasis. Cell. 2015;161(7):1527–1538. doi: 10.1016/j.cell.2015.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Natsuaki Y, et al. Perivascular leukocyte clusters are essential for efficient activation of effector T cells in the skin. Nat Immunol. 2014;15(11):1064–1069. doi: 10.1038/ni.2992. [DOI] [PubMed] [Google Scholar]
- 65.Iijima N. T cell memory. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science. 2014;346(6205):93–98. doi: 10.1126/science.1257530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pitzalis C, et al. Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat Rev Immunol. 2014;14(7):447–462. doi: 10.1038/nri3700. [DOI] [PubMed] [Google Scholar]
- 67.Dieu-Nosjean MC, et al. Tertiary lymphoid structures, drivers of the antitumor responses in human cancers. Immunol Rev. 2016;271(1):260–275. doi: 10.1111/imr.12405. [DOI] [PubMed] [Google Scholar]
- 68.Thompson ED, et al. Tumor masses support naive T cell infiltration, activation, and differentiation into effectors. The Journal of experimental medicine. 2010;207(8):1791–1804. doi: 10.1084/jem.20092454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Joshi NS, et al. Regulatory T Cells in Tumor-Associated Tertiary Lymphoid Structures Suppress Anti-tumor T Cell Responses. Immunity. 2015;43(3):579–590. doi: 10.1016/j.immuni.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schrama D, et al. Immunological tumor destruction in a murine melanoma model by targeted LTα independent of secondary lymphoid tissue. Cancer Immunology, Immunotherapy. 2008;57(1):85–95. doi: 10.1007/s00262-007-0352-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yu P, et al. Priming of naive T cells inside tumors leads to eradication of established tumors. Nature immunology. 2004;5(2):141–149. doi: 10.1038/ni1029. [DOI] [PubMed] [Google Scholar]
- 72.Kirk CJ, Hartigan-O'Connor D, Mule JJ. The dynamics of the T-cell antitumor response: chemokine-secreting dendritic cells can prime tumor-reactive T cells extranodally. Cancer Res. 2001;61(24):8794–8802. [PubMed] [Google Scholar]
- 73.Palucka K, Banchereau J. Dendritic-cell-based therapeutic cancer vaccines. Immunity. 2013;39(1):38–48. doi: 10.1016/j.immuni.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fu J, et al. STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Science Translational Medicine. 2015;7(283) doi: 10.1126/scitranslmed.aaa4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Russell SJ, Peng KW, Bell JC. Oncolytic virotherapy. Nat Biotechnol. 2012;30(7):658–670. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kantoff PW, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. New England Journal of Medicine. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 77.Burch PA, et al. Priming tissue-specific cellular immunity in a phase I trial of autologous dendritic cells for prostate cancer. Clinical Cancer Research. 2000;6(6):2175–2182. [PubMed] [Google Scholar]
- 78.Carreno BM, et al. IL-12p70–producing patient DC vaccine elicits Tc1-polarized immunity. The Journal of clinical investigation. 2013;123(8):3383–3394. doi: 10.1172/JCI68395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Carreno BM, et al. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science. 2015;348(6236):803–808. doi: 10.1126/science.aaa3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kleindienst P, Brocker T. Endogenous dendritic cells are required for amplification of T cell responses induced by dendritic cell vaccines in vivo. J Immunol. 2003;170(6):2817–2823. doi: 10.4049/jimmunol.170.6.2817. [DOI] [PubMed] [Google Scholar]
- 81.Yewdall AW, et al. CD8+ T cell priming by dendritic cell vaccines requires antigen transfer to endogenous antigen presenting cells. PLoS One. 2010;5(6):e11144. doi: 10.1371/journal.pone.0011144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Petersen TR, et al. Exploiting the role of endogenous lymphoid-resident dendritic cells in the priming of NKT cells and CD8+ T cells to dendritic cell-based vaccines. PLoS One. 2011;6(3):e17657. doi: 10.1371/journal.pone.0017657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mitchell DA, et al. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature. 2015;519(7543):366–369. doi: 10.1038/nature14320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Le DT, et al. Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J Clin Oncol. 2015;33(12):1325–1333. doi: 10.1200/JCO.2014.57.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kranz LM, et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534(7607):396–401. doi: 10.1038/nature18300. [DOI] [PubMed] [Google Scholar]
- 86.Kawarada Y, et al. NK- and CD8(+) T cell-mediated eradication of established tumors by peritumoral injection of CpG-containing oligodeoxynucleotides. J Immunol. 2001;167(9):5247–5253. doi: 10.4049/jimmunol.167.9.5247. [DOI] [PubMed] [Google Scholar]
- 87.Heckelsmiller K, et al. Peritumoral CpG DNA elicits a coordinated response of CD8 T cells and innate effectors to cure established tumors in a murine colon carcinoma model. J Immunol. 2002;169(7):3892–3899. doi: 10.4049/jimmunol.169.7.3892. [DOI] [PubMed] [Google Scholar]
- 88.Ohkuri T, et al. STING contributes to antiglioma immunity via triggering type I IFN signals in the tumor microenvironment. Cancer Immunol Res. 2014;2(12):1199–1208. doi: 10.1158/2326-6066.CIR-14-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vicari AP, et al. Reversal of Tumor-induced Dendritic Cell Paralysis by CpG Immunostimulatory Oligonucleotide and Anti–Interleukin 10 Receptor Antibody. The Journal of Experimental Medicine. 2002;196(4):541–549. doi: 10.1084/jem.20020732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Swiecki M, Colonna M. The multifaceted biology of plasmacytoid dendritic cells. Nat Rev Immunol. 2015;15(8):471–485. doi: 10.1038/nri3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Villadangos JA, Young L. Antigen-presentation properties of plasmacytoid dendritic cells. Immunity. 2008;29(3):352–361. doi: 10.1016/j.immuni.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 92.Conrad C, et al. Plasmacytoid dendritic cells promote immunosuppression in ovarian cancer via ICOS costimulation of Foxp3+ T-regulatory cells. Cancer research. 2012;72(20):5240–5249. doi: 10.1158/0008-5472.CAN-12-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Treilleux I, et al. Dendritic cell infiltration and prognosis of early stage breast cancer. Clinical Cancer Research. 2004;10(22):7466–7474. doi: 10.1158/1078-0432.CCR-04-0684. [DOI] [PubMed] [Google Scholar]
- 94.Le Mercier I, et al. Tumor promotion by intratumoral plasmacytoid dendritic cells is reversed by TLR7 ligand treatment. Cancer Res. 2013;73(15):4629–4640. doi: 10.1158/0008-5472.CAN-12-3058. [DOI] [PubMed] [Google Scholar]
- 95.Leon B, Ardavin C. Monocyte-derived dendritic cells in innate and adaptive immunity. Immunol Cell Biol. 2008;86(4):320–324. doi: 10.1038/icb.2008.14. [DOI] [PubMed] [Google Scholar]
- 96.Dominguez PM, Ardavin C. Differentiation and function of mouse monocyte-derived dendritic cells in steady state and inflammation. Immunol Rev. 2010;234(1):90–104. doi: 10.1111/j.0105-2896.2009.00876.x. [DOI] [PubMed] [Google Scholar]
- 97.Tamoutounour S, et al. Origins and functional specialization of macrophages and of conventional and monocyte-derived dendritic cells in mouse skin. Immunity. 2013;39(5):925–938. doi: 10.1016/j.immuni.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 98.Plantinga M, et al. Conventional and monocyte-derived CD11b+ dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity. 2013;38(2):322–335. doi: 10.1016/j.immuni.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 99.Jakubzick C, et al. Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity. 2013;39(3):599–610. doi: 10.1016/j.immuni.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Marigo I, et al. T Cell Cancer Therapy Requires CD40-CD40L Activation of Tumor Necrosis Factor and Inducible Nitric-Oxide-Synthase-Producing Dendritic Cells. Cancer Cell. 2016;30(3):377–390. doi: 10.1016/j.ccell.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]