Abstract
The capacity for tissues to regenerate often varies during development. Better understanding how developmental context regulates the regenerative capacity remains an important step in our ability to enhance the regenerative capacity of tissues to repair disease or damage. Recent work examining the regeneration of imaginal discs in the fruit fly, Drosophila melanogaster, has begun to identify mechanisms by which developmental progress restricts regeneration, and how Drosophila coordinates regenerative repair with the growth and development of the entire organism. Here we review recent advances in describing the interplay between development and tissue regeneration in Drosophila and identify questions that arise from these recent findings.
Regenerative capacity varies greatly throughout nature. A few animals, such as flatworm planarians, have the ability to regenerate not only individual organs, but entire body axes throughout their life. However, for most animals, regenerative capacity is limited by developmental stage and declines with age. For example, prenatal and one-day-old neonatal mice can regenerate functional cardiac tissue through activation of cardiomyocyte proliferation, but newborn mice lose this capacity by the time they reach seven days of age [1]. The ability to “reactivate” the regenerative capacity once present at earlier stages of development holds promise for the development of new treatments for damaged or diseased tissues.
For this reason, many researchers, working in different experimental systems, have begun to examine the developmental constraints on regenerative capacity. Experiments focused on the regeneration of Drosophila melanogaster imaginal discs are providing unique insights into mechanisms that coordinate regeneration with development.
Regeneration of Drosophila imaginal discs is developmentally constrained
Imaginal discs are larval epithelial tissues that will transform during metamorphosis into most of the visible adult structures (Figure 1). Studies of Drosophila imaginal discs have been crucial to our understanding tissue patterning and growth. In addition, the ability of imaginal discs to regenerate following experimentally-induced damage has long been recognized (reviewed by [2]). Damage to imaginal discs, by either physical injury, X-irradiation, or genetic ablation, produces a regenerative blastema that is characterized by proliferation localized to the site of damage [3,4] and the activation of a complex signaling and transcriptional response. This response includes: 1) Activation of the JNK signaling pathway [5–8] and downstream targets of JNK such as matrix metalloproteinase 1 (MMP1)[9] and the secreted peptide Dilp8 [8,10–12], 2) expression of the Drosophila Wnt1 homologue, wingless (wg) [3,13], 3) increased myc expression [3], 4) activation of the JAK/STAT pathway [12], and 5) Hippo pathway downregulation [4,14]. These coordinated responses in the blastema mediate wound healing, regenerative growth, and cellular respecification (reviewed in [15]).
Figure 1. Drosophila imaginal discs are the larval precursors to adult tissues.
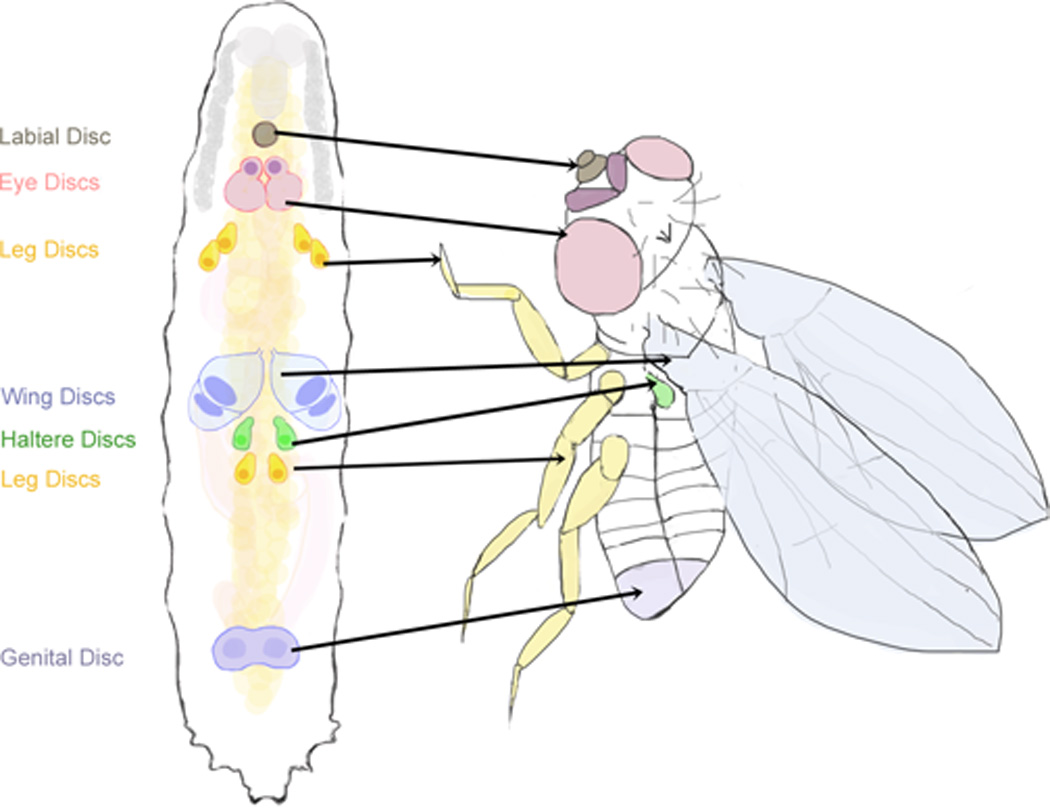
The imaginal discs are epithelial tissues derived from the larval epidermis and are the precursors to most external adult tissues. These include the eye (pink) and antennal (fuschia) discs, the labial disc (brown), the leg discs (yellow), the wing (blue) and haltere (green) discs, and the genital disc (purple).
After embryogenesis, a Drosophila larva hatches and progresses through three larval instars, which are separated by molts (Figure 2a). The larva has the ability to repair imaginal disc damage induced during the first two instars. However, regenerative capacity is lost near the end of the third and final larval instar. Damage to mature imaginal discs (<24 hours before the end of the final larval instar) is incompletely regenerated ([3,13,16], Figure 2a). This loss of regenerative ability is correlated with reduced expression of regenerative signaling pathways in the mature imaginal discs following damage ([3,13], Figure 2b). Interestingly, the activation of JNK appears to be unaffected by developmental progression of the tissue, whereas damage-induced expression of the the JNK-activated genes MMP1 and Dilp8 is reduced in mature discs [13]. Therefore, it is likely that developmental attenuation of regeneration functions downstream of JNK activation in mature discs. To address how the mature discs attenuate the transcriptional responses to damage, Harris et al. examined the regenerative regulation of wg transcription and demonstrated that a defined regulatory element is responsible for the activation of wg expression following damage. They also demonstrate that in mature discs, regenerative activation of wg through this regulatory element is suppressed through Polycomb Group (PcG)-mediated epigenetic silencing [13]. Since PcG regulatory sequences are found in the regulatory regions of other genes whose regenerative induction is attenuated in mature discs, it is possible that this may represent a mechanism for coordinately suppressing the regenerative response to damage. However, the experiments by Harris et al. do not address what determines the timing of PcG silencing in mature discs.
Figure 2. Developmental progression at the end of the larval period limits the regenerative capacity of imaginal discs.
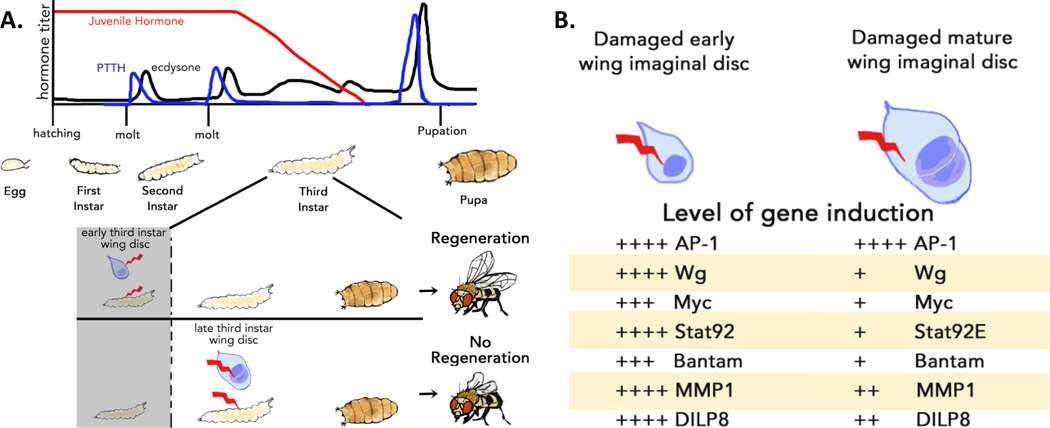
(a) Above: The larval stages of Drosophila development. Drosophila larvae proceed through three larval instars. The transition between each instar is mediated by a larval molt, which is stimulated by pulses of the steroid hormone ecdysone (black) that are stimulated by pulses of PTTH (blue), produced in the brain. Juvenile Hormone (red) is expressed early in larval development to ensure that early ecdysone pulses produce molts instead of an exit from larval development. The larvae demonstrate regenerative ability in their imaginal discs throughout development until late in the third larval instar. Below: An expanded illustration of the third larval instar. Prior to the end of the regeneration competent period of larval development (shaded) damage to imaginal discs can be effectively repaired through regeneration (top). In contrast, damage (bottom) to larvae that have passed the end of the imaginal disc regenerative period (<24 hours before the ecdysone pulse that terminates larval development; unshaded) produces an attenuated regenerative response, resulting in damaged adult tissues. (b) Several pathways that are activated by damage in early imaginal discs are attenuated in mature discs. These include the JNK pathway (AP-1), wingless expression (Wg), myc expression, JAK/STAT pathway activation (Stat92), Hippo downregulation (bantam), and the JNK-pathway targets MMP1 and Dilp8. (Adapted from Harris et al. 2016).
The change in regenerative capacity is observed when the larvae are near the end of larval development and the beginning of pupation, a transition coordinated by systemic endocrine signaling through an increase in circulating ecdysteroids. The steroid hormone ecdysone, produced by the prothoracic gland (PG), regulates developmental transitions via its active form 20-hydroxyecdysone (20E). Pulses of ecdysone synthesis during larval development initiate molting and advance the larvae into the pupal phase of development ([17], Figure 2a). The developmental response, whether to molt or pupate, elicited by ecdysone at different stages in larval development is determined by a second hormone, Juvenile hormone (JH). JH is present throughout much of early larval development, and then JH levels decline during the middle of the final instar ([18], Figure 2a). The large pulse of ecdysone that terminates larval development is triggered by prothoracicotropic hormone (PTTH, Figure 2a), a neuropeptide expressed in four neurons in the brain that extend axons out from the brain and contact the PG ([19], Figure 4). In larvae, ecdysone levels can be experimentally manipulated by introducing 20E to the growth medium. Even small amounts of 20E added to the food after damage to imaginal discs substantially limits the regenerative capacity of tissues [16]. Therefore, ecdysone signaling also restricts the regenerative capacity of imaginal discs. To date, there is little experimental evidence to connect ecdysone signaling and changes in PcG-mediated gene silencing, so it remains unclear whether ecdysone acts to limit regeneration in imaginal discs through PcG epigenetic silencing, or whether it limits regenerative capacity through a distinct pathway.
Figure 4. A summary of the molecular pathways that function in regeneration checkpoint timing and growth regulation.
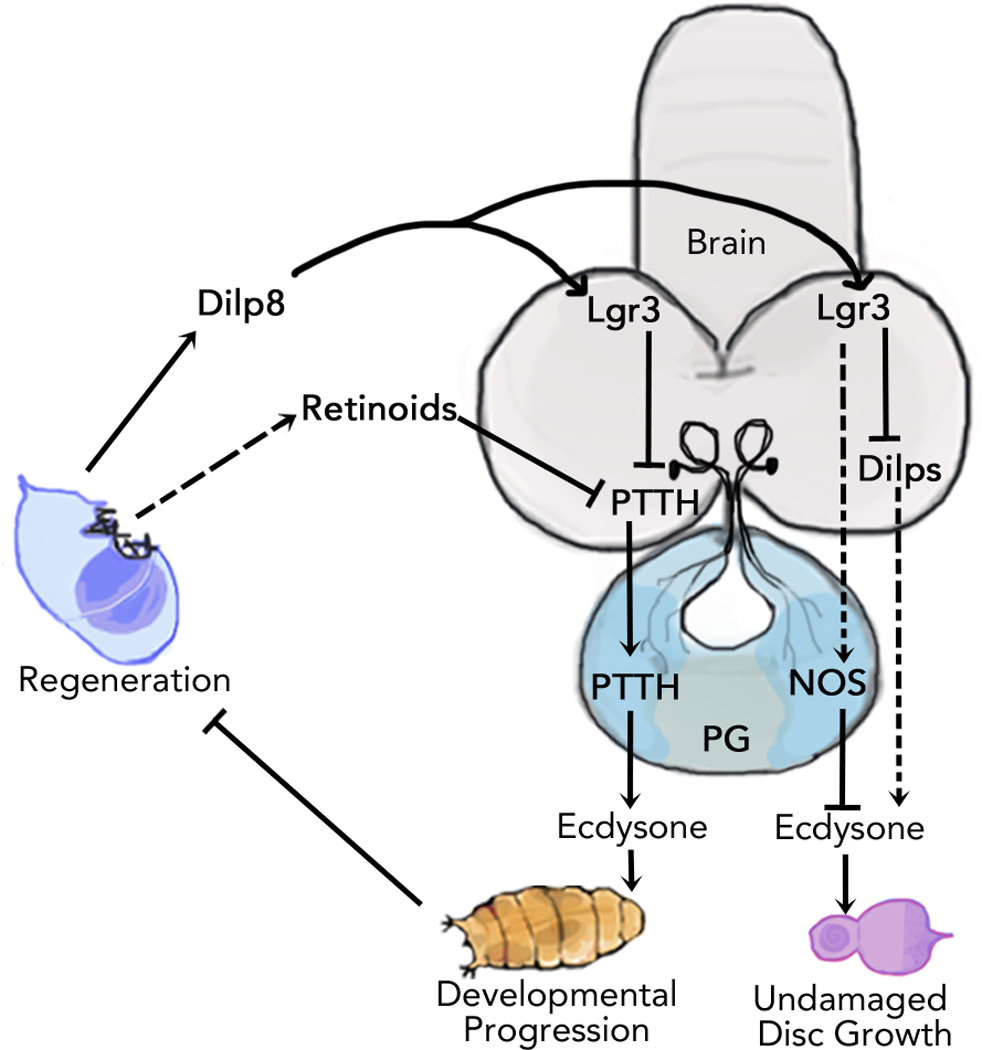
Damage to imaginal discs regulates systemic growth and developmental timing during the regeneration checkpoint. Release of Dilp8 by damaged tissues activates Lgr3+ neurons in the brain that suppress the PTTH-triggered ecdysone pulse, extending the regenerative period. Damaged imaginal discs can also inhibit PTTH through a Dilp8-independent, retinoid-sensitive mechanism. Dilp8 release by damaged tissues also acts to coordinate regenerative growth with developmental growth by reducing ecdysone levels produced by the prothoracic gland (PG) during the larval growth period. The growth coordinating activity of Dilp8 is dependent on the Lgr3+ neurons in the brain and NOS signaling in the prothoracic gland. Dilp8 activation of the Lgr3+ neurons also suppresses transcription of Dilps in the insulin producing cells of the brain which can affect ecdysone expression and growth. Future studies clarifying connections between these mechanisms should help our understanding of how damage signals get integrated to produce complex hormonal signaling outputs.
Imaginal disc damage activates a regeneration checkpoint that extends the regenerative period
As early as the 1930s, experiments demonstrated that irradiation of Drosophila larvae extends the period of larval development [20]. Subsequent experiments demonstrated that damage of imaginal discs is necessary and sufficient to produce this developmental delay: 1) If imaginal discs are completely removed, pupation is not inhibited [21–23], but it does prevent developmental delays caused by subsequent irradiation [24]. 2) Transplantation of an ectopic, damaged imaginal disc into an undamaged recipient larva is sufficient to produce delay [25,26]. These experiments suggest that there is some signal produced by damaged imaginal discs that causes developmental delay.
Interestingly, when imaginal discs are damaged early in larval development, the timing of the first two molting transitions are unaffected. Instead, development is extended by increasing the duration of the final larval instar [16,27,28] (Figure 3a). Thus, the timing of imaginal disc damage can be separated from the timing of developmental delay. This observation led us [16] and others [29] to propose that the developmental delay observed in response to imaginal disc damage functions as a developmental or regeneration checkpoint, conceptually similar to a cell-cycle checkpoint. In Drosophila larvae, a checkpoint mechanism monitors the progress of growth and development of the imaginal discs. When damage to the imaginal discs occurs, checkpoint activation will delay a critical developmental transition –the entry into pupal development and metamorphosis – in order to provide essential time for repair of the damaged discs.
Figure 3. A regeneration checkpoint regulates both developmental timing and systemic growth to coordinate regeneration with development.
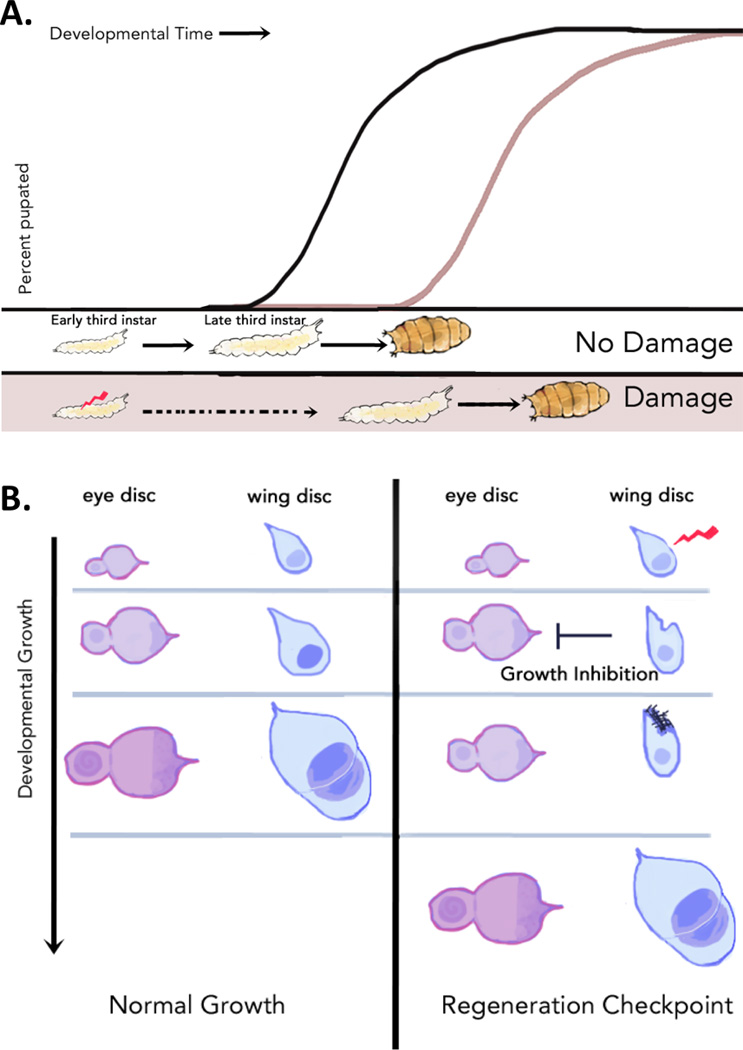
Damage to imaginal discs activates a regenerative checkpoint that (a) extends the regenerative period by producing a developmental delay that extends the first half of the larval third instar, and (b) coordinates regenerative growth with developmental growth by slowing the growth of undamaged imaginal discs.
This checkpoint model predicts that the extended growth period would also extend the period of regenerative competence of imaginal discs. This appears to be the case. Following damage of early third instar imaginal discs, the signaling pathways and localized proliferation that are associated with the regeneration blastema are observed during the extended larval period. This is observed well after the developmental time when new damage to mature discs would normally produce an attenuated regenerative signaling response [3,13]. Therefore, regenerative checkpoint activation extends the period of regenerative competence by inhibiting the pathways that would otherwise lead to PcG and ecdysone-mediated silencing and suppression of regeneration in imaginal discs.
Recent work has started to reveal the molecular mechanisms of this regeneration checkpoint. A critical step was the demonstration that Drosophila insulin like peptide 8 (Dilp8), an insulin/relaxin family peptide hormone, is released from damaged imaginal discs and triggers developmental delays [10,11]. JNK activation in the damaged disc is necessary to activate the expression of dilp8 [10]. Dilp8 expression in the regenerating disc also depends on expression of the cytokine unpaired and Jak/STAT pathway activation [12]. Dilp8 is primarily produced within regenerating cells of the blastema [10,12], but can also be observed within some apoptotic cells [10]. Expression of Dilp8 is sufficient to produce a developmental delay similar to that observed during the regeneration checkpoint [10,30], whereas loss of dilp8 function reduces or eliminates developmental delays produced by imaginal disc damage [10,11,31]. The length of the developmental delay correlates with the amount of damage (Halme 2010, Garrelli 2012). Consistent with this, dilp8 transcription levels increase with increasing levels of damage (Garrelli 2012). Interestingly, loss of dilp8 function does not reduce proliferation of the regeneration blastema (Katsuyama 2015), but does produce more regeneration defects (Garrelli 2012). These observations are all consistent with a model in which Dilp8 expression in damaged imaginal discs triggers the regeneration checkpoint, which produces an extension of the regenerative period required for complete regeneration.
Interestingly, developmental delay induced by overexpression of the pro-apoptotic gene reaper in wing imaginal discs is only partially rescued by loss of dilp8 function [11]. However, delays induced by overexpression of the TNFα homologue eiger in wing discs are completely eliminated in dilp8 mutant larvae [31]. This contrast suggests that additional mechanisms may participate in producing developmental delay through Dilp8-independent pathways in reaper-damaged discs. In a genetic screen, mutations that disrupt retinoid metabolism were found to reduce developmental checkpoint delay following damage [16]. However, Dilp8 functions independently of retinoid metabolism [11]. Therefore, Dilp8 and retinoid-dependent pathways may represent distinct mechanisms that produce developmental delay, and possibly reflect differences in the nature of imaginal disc damage and repair.
Dilp8 produced from regenerating imaginal discs is detected in the larval hemolymph suggesting that Dilp8 can function as a long-range signal in larvae [10,11]. Co-culture and in vivo experiments demonstrate that Dilp8 can act as an endocrine signal to limit ecdysone synthesis [10]. Dilp8 does this by delaying the activation of PTTH transcription, which normally triggers the pulse of ecdysone that ends larval development [10,11,16]. Misexpression of PTTH can suppress damage-induced developmental delays suggesting that the expression of PTTH is the rate-limiting step for developmental progression during the regeneration checkpoint [16]. PTTH transcription signals the end of the regeneration checkpoint, but the mechanisms that trigger the progression of development following activation of the regeneration checkpoint remain unclear. Even when damage is persistently induced, or when dilp8 is constitutively expressed, PTTH transcription is eventually induced and larval development progresses to metamorphosis (Halme 2010, Colombani 2012). This suggests that mechanisms other than complete regeneration of imaginal discs or a decrease in Dilp8 production are able to end larval development and initiate metamorphosis upon constitutive activation of the regeneration checkpoint.
Recently, an orphan leucine-rich G-protein coupled receptor, Lgr3, has been identified as a potential receptor of Dilp8 signaling during regeneration. Genetic experiments demonstrate that Lgr3 is necessary for mediating Dilp8-dependent developmental delays [30,32,33], however experiments testing whether Dilp8 directly binds to Lgr3 have produced conflicted results [30,32]. A pair of neurons have been identified in each brain lobe that express Lgr3 (Lgr3+ neurons). These Lgr3+ neurons respond to ectopic Dilp8 by increasing cAMP signaling [30,32], demonstrating that they can be activated by Dilp8. Silencing of the Lgr3+ neurons [30], or inhibition of Lgr3 expression specifically in these neurons [30,32,33], attenuates regeneration checkpoint delay, suggesting that Dilp8 produces delay by signaling through the Lgr3+ neurons. The Lgr3+ neurons make connections with insulin producing cells in the brain [30,32] and the PTTH-expressing neurons [32,33]. Disruption of Lgr3 in the brain leads to misregulation of Dilp3 and Dilp5 as well as JH signaling targets [32]. Many factors, including Dilps, provide competence cues for the PG, determining when and how much ecdysone is produced (reviewed [34]). Therefore, it remains unclear whether the Lgr3+neurons directly regulate ecdysone production through PTTH neuron activity, or indirectly through Dilps, JH, or other targets.
The regeneration checkpoint coordinates growth between regenerating and undamaged tissues
The growth of each imaginal disc is thought to be primarily regulated by the disc’s own autonomous program [35]. The activation of a regenerative program in an individual tissue, which extends the imaginal disc growth period, poses a challenge for the organism: how to coordinate the additional growth required for regeneration with the growth of undamaged tissues. Several groups have observed that Drosophila larvae have a mechanism to address this challenge: damage to the wing imaginal discs inhibits growth of distal, undamaged imaginal discs resulting in adults that retain appropriate proportional tissue size [10,28,31] (Figure 3b). This growth coordination is also observed between developmental compartments (the anterior and posterior compartments) within the same imaginal disc [36]. Thus, activation of the regenerative checkpoint also acts to coordinate growth between regenerating and undamaged tissues.
Dilp8 is both necessary and sufficient for inhibiting growth of undamaged imaginal discs during the regenerative checkpoint [10,31], and the Lgr3+ neurons in the brain mediate Dilp8 coordination of imaginal disc growth [33]. The mechanism by which Lgr3 activation in the brain controls imaginal disc growth has not been directly demonstrated. However, Dilp8 released from damaged discs also activates nitric oxide synthase (NOS) in the PG, and NOS is necessary and sufficient for growth coordination during the regeneration checkpoint [31]. During larval development, NOS activation in the PG reduces the basal levels of ecdysone biosynthesis, systemically reducing ecdysone signaling. Increasing ecdysone levels through hormone feeding during the regeneration checkpoint, or following misexpression of Dilp8, rescues the growth of undamaged imaginal discs, demonstrating that reduced ecdysone signaling is an essential element of growth coordination.
In addition to regulating developmental timing, ecdysone also regulates developmental growth. Ecdysone acts as an antagonist of insulin signaling in tissues such as the prothoracic gland and fatbody, inhibiting growth across the whole larvae [37–39]. However, ecdysone signaling also promotes the growth of imaginal discs (for a recent illustration, see [40]). Imaginal disc clones expressing a dominant-negative form of the ecdysone receptor grow more slowly [39] demonstrating that ecdysone signaling can directly promote growth in imaginal discs.
During the regeneration checkpoint, Dilp8 released from damaged tissues regulates both: 1) Growth – through reduction in the basal levels of ecdysone during the larval growth period, and 2) Timing – by delaying the PTTH-triggered pulse of ecdysone synthesis that ends the larval period of development (Figure 4). While Dilp8 produces both of these checkpoint responses by regulating ecdysone signaling, the regulation of developmental growth and developmental timing are genetically separable: loss of NOS function rescues damage-induced distal growth inhibition, but does not prevent developmental delay [31]. Therefore, there are at least two distinct mechanisms by which regeneration and Dilp8 regulate hormone production and signaling.
What’s next?
While our understanding of the hormonal regulation of regeneration is advancing, the findings described in this review raise questions that could direct future studies. For instance, what is the role of endocrine signaling in the local regulation of regenerative growth? In contrast to the experiments we describe in this review that suggest that ecdysone signaling may limit regeneration, experiments in other insect and crustacean models [41,42] suggest that regenerative growth is locally dependent on ecdysone signaling. However, regenerative growth in Drosophila imaginal discs is able to proceed despite systemic reductions in ecdysone signaling. Is the systemic reduction of ecdysone necessary for regeneration, or do regenerating tissues act to accommodate reduced ecdysone signaling by alternate pathways to promote growth in the blastema? What does the role of ecdysone in the progressive differentiation of the imaginal discs play in this process? Is there an “optimal” amount of ecdysone signaling for regeneration that permits regenerative growth but prevents the developmental progression of tissues into a non-regenerative stage? Experiments designed to address these questions should not only improve our understanding of how regeneration and endocrine signaling are orchestrated in the context of a developing organism, but could also reveal novel pathways that could be manipulated to promote regenerative capacity of diseased or damaged tissues.
Acknowledgments
The authors are grateful to many colleagues for thoughts on the manuscript including Dina Halme for editing. We would especially like to thank Cristina D’Ancona for her beautiful artwork for all of the figures in this review. This work is funded by a grants from the National Institutes of Heath to AH (RO1 GM099803) and JSJ (T32 GM008136) and in part by a March of Dimes Basil O’Connor Starter Scholar award to AH (#5-FY12-60).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
* special interest;
** outstanding interest
- 1.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Worley MI, Setiawan L, Hariharan IK. Regeneration and transdetermination in Drosophila imaginal discs. Annu. Rev. Genet. 2012;46:289–310. doi: 10.1146/annurev-genet-110711-155637. [DOI] [PubMed] [Google Scholar]
- 3. Smith-Bolton RK, Worley MI, Kanda H, Hariharan IK. Regenerative growth in Drosophila imaginal discs is regulated by Wingless and Myc. Dev. Cell. 2009;16:797–809. doi: 10.1016/j.devcel.2009.04.015. This report describes the development of an important new experimental model for producing damage and assessing regeneration in wing imaginal discs, and demonstrates that Wingless and Myc activity are necessary for effective disc regeneration.
- 4.Sun G, Irvine KD. Regulation of Hippo signaling by Jun kinase signaling during compensatory cell proliferation and regeneration, and in neoplastic tumors. Dev. Biol. 2011;350:139–151. doi: 10.1016/j.ydbio.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bosch M, Serras F, Martín-Blanco E, Baguñà J. JNK signaling pathway required for wound healing in regenerating Drosophila wing imaginal discs. Dev. Biol. 2005;280:73–86. doi: 10.1016/j.ydbio.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Mattila J, Omelyanchuk L, Kyttälä S, Turunen H, Nokkala S. Role of Jun N-terminal Kinase (JNK) signaling in the wound healing and regeneration of a Drosophila melanogaster wing imaginal disc. Int. J. Dev. Biol. 2005;49:391–399. doi: 10.1387/ijdb.052006jm. [DOI] [PubMed] [Google Scholar]
- 7.Bergantiños C, Corominas M, Serras F. Cell death-induced regeneration in wing imaginal discs requires JNK signalling. Development. 2010;137:1169–1179. doi: 10.1242/dev.045559. [DOI] [PubMed] [Google Scholar]
- 8.Skinner a, Khan SJ, Smith-Bolton RK. Trithorax regulates systemic signaling during Drosophila imaginal disc regeneration. Development. 2015;142:3500–3511. doi: 10.1242/dev.122564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McClure KD, Sustar A, Schubiger G. Three genes control the timing, the site and the size of blastema formation in Drosophila. Dev. Biol. 2008;319:68–77. doi: 10.1016/j.ydbio.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Colombani J, Andersen DS, Leopold P. Secreted Peptide Dilp8 Coordinates Drosophila Tissue Growth with Developmental Timing. Science. 2012;336:582–585. doi: 10.1126/science.1216689. This report, together with ref. 11, identifies Dilp8 as the signal which activates the developmental delay during the regeneration checkpoint. This report also demonstrates that Dilp8 is the diffusable signal that regulates neuroendocrine activity and the regeneration checkpoint.
- 11. Garelli A, Gontijo AM, Miguela V, Caparros E, Dominguez M. Imaginal Discs Secrete Insulin-Like Peptide 8 to Mediate Plasticity of Growth and Maturation. Science. 2012;336:579–582. doi: 10.1126/science.1216735. This report, together with ref. 10, identifies Dilp8 as the signal which activates the developmental delay during the regeneration checkpoint. Addtionally, this work demonstrates that Dilp8 is required for adult wing symmetry in the absence of damage.
- 12.Katsuyama T, Comoglio F, Seimiya M, Cabuy E, Paro R. During Drosophila disc regeneration, JAK/STAT coordinates cell proliferation with Dilp8-mediated developmental delay. Proc. Natl. Acad. Sci. U.S.A. 2015;112:E2327–E2336. doi: 10.1073/pnas.1423074112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harris RE, Setiawan L, Saul J, Hariharan IK. Localized epigenetic silencing of a damage-activated WNT enhancer limits regeneration in mature Drosophila imaginal discs. Elife. 2016;5:1–28. doi: 10.7554/eLife.11588. This study identifies a damage-responsive enhancer near the Wnt genes that is epigenetically suppressed by Polycomb silencing at the end of the regenerative period. This finding connects larval age and regenerative capacity with localized epigenetic silencing of regeneration-activated genes.
- 14.Grusche FA, Degoutin JL, Richardson HE, Harvey KF. The Salvador/Warts/Hippo pathway controls regenerative tissue growth in Drosophila melanogaster. Dev. Biol. 2011;350:255–266. doi: 10.1016/j.ydbio.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 15.Sun G, Irvine KD. Control of Growth During Regeneration. Curr. Top. Dev. Biol. 2014;108:95–120. doi: 10.1016/B978-0-12-391498-9.00003-6. [DOI] [PubMed] [Google Scholar]
- 16.Halme A, Cheng M, Hariharan IK. Retinoids Regulate a Developmental Checkpoint for Tissue Regeneration in Drosophila. Curr. Biol. 2010;20:458–463. doi: 10.1016/j.cub.2010.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rewitz KF, Yamanaka N, O’Connor MB. Developmental checkpoints and feedback circuits time insect maturation. Curr. Top. Dev. Biol. 2013;103:1–33. doi: 10.1016/B978-0-12-385979-2.00001-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou X, Riddiford LM. Broad specifies pupal development and mediates the “status quo” action of juvenile hormone on the pupal-adult transformation in Drosophila and Manduca. Development. 2002;129:2259–2269. doi: 10.1242/dev.129.9.2259. [DOI] [PubMed] [Google Scholar]
- 19.McBrayer Z, Ono H, Shimell M, Parvy J-P, Beckstead RB, Warren JT, Thummel CS, Dauphin-Villemant C, Gilbert LI, O’Connor MB. Prothoracicotropic hormone regulates developmental timing and body size in Drosophila. Dev. Cell. 2007;13:857–871. doi: 10.1016/j.devcel.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tennant R. A maximum point in and effect of prolonged X-ray irradiation upon Drosophila larvae. Science. 1931;73:567–568. doi: 10.1126/science.73.1899.567. [DOI] [PubMed] [Google Scholar]
- 21.Shearn a, Rice T, Garen a, Gehring W. Imaginal disc abnormalities in lethal mutants of Drosophila. Proc. Natl. Acad. Sci. U.S.A. 1971;68:2594–2598. doi: 10.1073/pnas.68.10.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simpson P, Berreur P, Berreur-Bonnenfant J. The initiation of pupariation in Drosophila: dependence on growth of the imaginal discs. J. Embryol. Exp. Morphol. 1980;57:155–165. [PubMed] [Google Scholar]
- 23.Szabad J, Bryant PJ. The mode of action of “discless” mutations in Drosophila melanogaster. Dev. Biol. 1982;93:240–256. doi: 10.1016/0012-1606(82)90256-1. [DOI] [PubMed] [Google Scholar]
- 24.Poodry CA, Woods DF. Control of the developmental timer for Drosophila pupariation. Roux’s Arch. Dev. Biol. 1990;199:219–227. doi: 10.1007/BF01682081. [DOI] [PubMed] [Google Scholar]
- 25.Rahn P. Untersuchungen zur Entwicklung von Ganz und Teilimplantaten der Flugelimaginalscheibe von Ephestia kuhniella Z. Wilhelm Roux. Arch. Entwickl. Mech. Org. 1972;170:48–82. doi: 10.1007/BF00575521. [DOI] [PubMed] [Google Scholar]
- 26.Dewes E. Entwicklungsleistungen Implantierter Ganzer und Halbierter Männlicher Genitalimaginalscheiben von Ephestia kuehniella Z. und Entwicklungsdauer der Wirtstiere. Wilhelm Roux Arch Entwickl Mech Org. 1975;178:167–183. doi: 10.1007/BF00848395. [DOI] [PubMed] [Google Scholar]
- 27.Hackney JF, Zolali-Meybodi O, Cherbas P. Tissue Damage Disrupts Developmental Progression and Ecdysteroid Biosynthesis in Drosophila. PLoS One. 2012;7 doi: 10.1371/journal.pone.0049105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parker NF, Shingleton AW. The coordination of growth among Drosophila organs in response to localized growth-perturbation. Dev. Biol. 2011;357:318–325. doi: 10.1016/j.ydbio.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Cherbas L. EcR isoforms in Drosophila: testing tissue-specific requirements by targeted blockade and rescue. Development. 2003;130:271–284. doi: 10.1242/dev.00205. [DOI] [PubMed] [Google Scholar]
- 30.Garelli A, Heredia F, Casimiro AP, Macedo A, Nunes C, Koyama T, Gontijo AM. Dilp8 requires the neuronal relaxin receptor Lgr3 to couple growth to developmental timing. Nat. Commun. 2015;6:1–14. doi: 10.1038/ncomms9732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaszczak JS, Wolpe JB, Dao AQ, Halme A. Nitric oxide synthase regulates growth coordination during Drosophila melanogaster imaginal disc regeneration. Genetics. 2015;200:1219–1228. doi: 10.1534/genetics.115.178053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vallejo DM, Juarez-Carreño S, Bolivar J, Morante J, Dominguez M. A brain circuit that synchronizes growth and maturation revealed through Dilp8 binding to Lgr3. Science. 2015;350 doi: 10.1126/science.aac6767. This report, along with refs. 30 and 33, identify Lgr3 to be the receptor for Dilp8, formally demonstrating the biochemical interaction between Dilp8 and Lgr3.
- 33.Colombani J, Andersen DS, Boulan L, Boone E, Romero N, Virolle V, Texada M, Léopold P. Drosophila Lgr3 Couples Organ Growth with Maturation and Ensures Developmental Stability. Curr. Biol. 2015;25:2723–2729. doi: 10.1016/j.cub.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 34.Yamanaka N, Rewitz KF, O’Connor MB. Ecdysone Control of Developmental Transitions: Lessons from Drosophila Research. Annu. Rev. Entomol. 2013;58:497–516. doi: 10.1146/annurev-ento-120811-153608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hariharan IK. Organ Size Control Lessons from Drosophila. Dev. Cell. 2015;34:255–265. doi: 10.1016/j.devcel.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Repiso A, Bergantiños C, Serras F. Cell fate respecification and cell division orientation drive intercalary regeneration in Drosophila wing discs. Development. 2013;140:3541–3551. doi: 10.1242/dev.095760. [DOI] [PubMed] [Google Scholar]
- 37.Colombani J, Bianchini L, Layalle S, Pondeville E, Dauphin-Villemant C, Antoniewski C, Carré C, Noselli S, Léopold P. Antagonistic actions of ecdysone and insulins determine final size in Drosophila. Science. 2005;310:667–670. doi: 10.1126/science.1119432. [DOI] [PubMed] [Google Scholar]
- 38.Mirth C, Truman JW, Riddiford LM. The role of the prothoracic gland in determining critical weight for metamorphosis in Drosophila melanogaster. Curr. Biol. 2005;15:1796–1807. doi: 10.1016/j.cub.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 39.Delanoue R, Slaidina M, Léopold P. The steroid hormone ecdysone controls systemic growth by repressing dMyc function in drosophila fat cells. Dev. Cell. 2010;18:1012–1021. doi: 10.1016/j.devcel.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 40.Herboso L, Oliveira MM, Talamillo A, Pérez C, González M, Martín D, Sutherland JD, Shingleton AW, Mirth CK, Barrio R. Ecdysone promotes growth of imaginal discs through the regulation of Thor inD. melanogaster. Sci. Rep. 2015;5:12383. doi: 10.1038/srep12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Das S, Durica DS. Ecdysteroid receptor signaling disruption obstructs blastemal cell proliferation during limb regeneration in the fiddler crab, Uca pugilator. Mol. Cell. Endocrinol. 2013;365:249–259. doi: 10.1016/j.mce.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 42.Madhavan K, Schneiderman H. Hormonal control of imaginal disc regeneration in Galleria mellonella (Lipidoptera) Biol. Bull. 1969;137:321–331. [Google Scholar]


