Highlights
-
•
Researchers rely on reverse inference to interpret SES-related brain data.
-
•
Neural differences related to SES are posited to be indicative of language delays.
-
•
SES is confounded with cultural language use, bilingualism, and first-language.
-
•
Cognitive tests may under-predict abilities of some children.
-
•
Future neuroimaging studies must examine aspects of children’s language directly.
Keywords: Socioeconomic status (SES), Neuroimaging, Reverse inference, Language, Development, Childhood poverty
Abstract
In the nascent field of the cognitive neuroscience of socioeconomic status (SES), researchers are using neuroimaging to examine how growing up in poverty affects children’s neurocognitive development, particularly their language abilities. In this review we highlight difficulties inherent in the frequent use of reverse inference to interpret SES-related abnormalities in brain regions that support language. While there is growing evidence suggesting that SES moderates children’s developing brain structure and function, no studies to date have elucidated explicitly how these neural findings are related to variations in children’s language abilities, or precisely what it is about SES that underlies or contributes to these differences. This issue is complicated by the fact that SES is confounded with such linguistic factors as cultural language use, first language, and bilingualism. Thus, SES-associated differences in brain regions that support language may not necessarily indicate differences in neurocognitive abilities. In this review we consider the multidimensionality of SES, discuss studies that have found SES-related differences in structure and function in brain regions that support language, and suggest future directions for studies in the area of cognitive neuroscience of SES that are less reliant on reverse inference.
1. Introduction
Recent rapid growth in human neuroimaging is providing the opportunity to examine the relations among socioeconomic status (SES), language development, and brain development, with the ultimate goal of being able to address troubling social inequities more effectively. In this paper we review studies that use neuroimaging to assess the impact of SES on neural features of children’s developing linguistic competence. SES encompasses occupation, income, and education and is typically assessed as either a weighted average of these measures, such as the commonly used Hollingshead Index (HI;Hollingshead, 2011), or one of these measure individually. Family SES has been consistently related to children’s early language environments (Hart and Risley, 1995, Hess and McDevitt, 1984, Hoff, 2003, Rowe, 2008), as well as to their linguistic trajectories and outcomes (Hart and Risley, 1995; Fernald, Marchman, and Weisleder, 2012). Researchers have begun to use methods from cognitive neuroscience to examine the effects of SES on neural structure and function, particularly in the context of language development. These studies in the growing area of the cognitive neuroscience of SES focus specifically on neural aspects of language development, assessing the relation of SES to the structure and function of brain regions that support language comprehension and production. Importantly, however, although many of these studies are methodologically sound, they rely on reverse inference in drawing conclusions about children’s development. Reverse inference refers to the practice within cognitive neuroscience of making inferences about people’s mental states based on the presence of activation within a particular brain region (Poldrack, 2011). The use of reverse inference in this context of the cognitive neuroscience of SES has the unfortunate effect of leading researchers to interpret associations between higher levels of SES and the structure and function of brain regions that support language as prototypic of optimal development, even in the absence of behavioral evidence for such an interpretation.
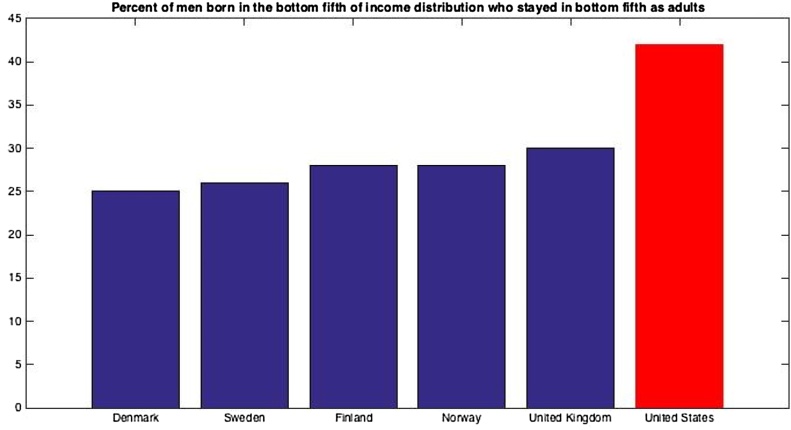
Family SES can be conceptualized as a measure of children’s opportunities. Caregivers who are less educated may not be as equipped to help their children excel in school; those without sufficient money may not be able to purchase extra resources like books and toys; those who work particularly intensive jobs may not have the time or energy to provide the same level of social and emotional support. While education, income, and occupation are interrelated, it is likely that these variables contribute differentially to distinct early childhood experiences (Duncan and Magnuson, 2012). Indeed, there is no clear consensus in the literature about what indices should be used to categorize a family as “lower-SES.” Certainly, there is considerable variability within SES groups, such that children whose families come from similar backgrounds may have exposure to very different sets of opportunities. Still, researchers have consistently found that lower-SES children fare more poorly on a variety of outcome measures. The United States in particular has low rates of relative mobility in comparison with many other developed countries (Fig. 1); thus, children who are born into poverty are more likely to stay in poverty as adults than is the case in many other developed countries (Isaacs et al., 2008). The majority of studies conducted to date examining SES have therefore focused on the United States, although these issues clearly warrant further international research. The dominant view among researchers in the cognitive neuroscience of SES is that these differences in children’s environments affect brain development and, ultimately, cognitive functioning and skills (Johnson et al., 2016). These studies often rely on a model based on animal literature indicating that rodents raised in severe social deprivation ultimately develop fewer and less efficient synaptic connections and abnormal stress reactivity (McLaughlin et al., 2014).
Fig. 1.
Based on data presented by Isaacs et al., 2008.
It is important to recognize, however, that documenting the presence of SES-associated neural effects alone is not sufficient to support this formulation. While lower-SES children may be deprived of certain enriching experiences, they may also have other experiences which are less often considered. For example, researchers have found levels of SES to be related to individual differences not only in such constructs as children’s vocabulary size and reading skill, but also in the ways families use language (such as abiding by culturally-prescribed discourse rules), first language (such as learning another language before English), and bilingualism (Hakimzadeh and Cohn, 2007, Wyatt, 1995). These potential confounds complicate the interpretation of SES-related differences in neural function and structure in regions that support language. While it is difficult to avoid reverse inference altogether in neuroimaging studies (Poldrack, 2011), we believe that a more critical investigation of language is needed to advance the cognitive neuroscience of SES.
To date, studies of the cognitive neuroscience of SES have been important in demonstrating that there are significant differences between children from low- and high-SES families in neural structure and function. In addition to examining potential language differences, these researchers have also examined executive function and emotional processing. Many of these studies, however, have not explicitly linked the neural differences they documented to environmental or cognitive variables. Instead, they have used children’s SES as a proxy for these constructs, making an assumption that children from low-SES backgrounds grow up in sub-optimal environments and have reduced cognitive skills. In this context, therefore, higher-SES children’s brains are viewed as optimal, leading to interpretations of SES-related group differences in neural function and structure as reflecting a deficit in lower-SES children.
In this review we focus on language development as an exemplar of this phenomenon within the field of the cognitive neuroscience of SES. Advances in technology now allow researchers interested in language to assess more systematically how children’s language environments affect their developing linguistic skills. Investigators working in the cognitive neuroscience of SES can profitably utilize these advancements to gain a more comprehensive understanding of the relations among SES, language outcomes, and the developing brain. Indeed, we argue that in order to move forward effectively in the cognitive neuroscience of SES, researchers should focus more explicitly on elucidating the associations between SES-related neural findings and environmental and cognitive variables. Specifically, investigators must examine how variations in children’s environments are related to their neural trajectories and linguistic outcomes.
2. Unpacking SES: a multidimensional variable
Researchers in psychology and cognitive neuroscience differ considerably in their operationalization of SES (Braveman et al., 2005). Whereas some focus on parental education and occupation, others focus on income, and still others use a composite score that encompasses multiple variables. For the purposes of this review, in discussing particular studies we will use the term SES as each investigator has operationalized it (see Table 1). Several researchers have provided more comprehensive reviews of this variability and its significance within the cognitive neuroscience of SES (D'Angiulli et al., 2012, Hackman and Farah, 2009, Johnson et al., 2016, Ursache and Noble, 2015). It is clear that there is a need to consider the varying contributions of income, education, and occupation (Duncan and Magnuson, 2012), and to examine the concept of causality in the cognitive neuroscience of SES (Lipina, 2016). In addition, it is important to note that although in many cases researchers have attempted to recruit socioeconomically diverse samples, the families included in these studies nevertheless tend to be skewed toward the higher end of SES.
Table 1.
Studies included in the present review.
| References | Measure of SES |
|---|---|
| Noble et al. (2006) | Composite of parental education, occupation, income-to-needs ratio |
| Raizada et al. (2008) | Hollingshead four-factor index (weighted average of parental education and occupation) |
| Noble et al. (2012) | Income-to-needs ratio; parental education |
| Jednoróg et al. (2012) | Hollingshead two-factor index of social position (weighted average of maternal education and occupation) |
| Hanson et al. (2013) | Percentage relative to federal poverty level (FPL) |
| Noble et al. (2015) | Parental education; family income |
| Hair et al. (2015) | Percentage relative to federal poverty level (FPL) |
| Brito et al. (2016) | Income-to-needs ratio; maternal education; family income |
Investigators have consistently documented associations between SES and school performance. Not only are children from lower-SES homes less likely to graduate from college, but those who do graduate are less likely to pursue graduate education or to obtain lucrative employment (Walpole, 2003). In fact, there is evidence that SES-related language difficulties are observable within the first two years of life (Fernald et al., 2012); perhaps not surprisingly, lower-SES children already seem to lag behind their peers in reading readiness by the time they enter school (Coley, 2002). Investigators who have tried to understand this phenomenon have noted large, and likely inter-related, differences between children from high- and low-SES families in their social and physical environments. Compared to the neighborhoods of higher-SES children, lower-SES children tend to grow up in neighborhoods with more concentrated poverty (Desmond, 2016, Sharkey, 2013) that are less safe, and that have fewer resources (Leventhal and Brooks-Gunn, 2000). Their homes are more likely to be crowded and chaotic (Evans et al., 2005), and family members are less likely to have the monetary resources, education, time, or energy to navigate complex systems like healthcare and education (Roscigno and Ainsworth-Darnell, 1999).
It is also important to note that in the United States, where the majority of studies of the cognitive neuroscience of SES have been conducted, SES is indelibly tied to race and ethnicity. Between 2007 and 2011, 29% of Blacks and 23% of Hispanics were living below the national poverty line, compared to only 10% of Non-Hispanic Whites (Macartney et al., 2013). Children from racial and ethnic minorities are subjected to harsher disciplinary treatment in school (Okonofua et al., 2016) and have lower academic performance, possibly due in part to stereotype threat (Steele, 1997). In fact, race predicts academic outcomes above and beyond the effects of SES (Sirin, 2005). Importantly, minority families often have different cultural norms, which tend to be particularly pronounced among those whose parents are least integrated into mainstream “white” America (Trueba, 1988). It is crucial, therefore, that researchers be cognizant of the complex interaction of SES with race and ethnicity and how this interaction may contribute to the effects of SES obtained in the literature.
Social psychologists have long recognized that the circumstances under which we are raised affect how we behave in, and think about, the world. For example, researchers have found that lower-SES children rely more on environmental explanations for events and outcomes, whereas higher-SES children tend to emphasize individual agency (Kraus et al., 2012). Some developmental psychologists suggest that deemphasizing individual agency in the context of school puts students at risk for poorer academic performance (Chapman and Skinner, 1989). There is also evidence, however, that relying on environmental attributions can foster social intelligence. For example, lower-SES adults exhibit greater empathic accuracy than do their higher-SES peers, which is mediated by their tendency to emphasize the role of the environment over that of the individual (Kraus et al., 2010). Similarly, experimental manipulations show that both adults and children assigned to a lower social status condition engage in more prosocial behaviors (Guinote et al., 2015). Overall, social psychological studies of status converge to suggest that lower-SES individuals rely on a framework rooted in contextualism, a belief that the environment plays an important role in shaping cognition and behavior, while higher-SES people rely on a framework of solipsism, which is more focused on the individual (Kraus et al., 2012). Importantly, these frameworks constitute underlying SES-based psychological differences that do not support inferences about the superiority of the different frameworks adopted by lower- or higher-SES individuals. The recognition of psychological differences due to the environment, which are not inherently positive or negative, has been termed cultural relativism (Herskovits, 1948).
Cognitive psychologists have generally not integrated the concept of cultural relativism into their research. While a small number of cognitive psychologists have argued that lower-SES children may reach the same level of skill as their higher-SES peers through alternate developmental pathways, which would explain differences in SES at the same level of measured cognition at various ages (D'Angiulli et al., 2012), most investigators in this field seem to posit that impaired general cognitive functioning underlies the poorer academic performance of lower-SES children. Certainly, it is possible that lower-SES children do, in fact, have poorer cognitive skills than do their higher-SES peers. Alternatively, however, it is also possible that differences in test performance and educational attainment arise in part from other factors, including stereotype threat (Steele, 1997), academic expectations and resources (Tenenbaum and Ruck, 2007), acute distraction due to environmental stressors (Sharkey, 2010), and discomfort in formal academic settings (Walton and Cohen, 2007). In this case, as D'Angiulli et al. (2012) point out, links between cognitive tests and academic achievement may be nothing more than confirmation of a form of bias such that both of these measures systematically advantage one group over another (Suzuki and Aronson, 2005). Importantly, however, the position within cognitive psychology that lower-SES children have poorer cognitive abilities than do their higher-SES peers has influenced the developing field of the cognitive neuroscience of SES.
Cognitive neuroscience offers exciting opportunities to examine how different environments that have been associated with levels of SES affect cognitive development at the level of the brain. Studies in this field can reduce our dependence on behavioral measures of cognition, which, in addition to their general insensitivity (Raizada, 2010, Raizada et al., 2008), may be influenced by confounds such as stereotype threat (Walton and Spencer, 2009) and outcome bias (D'Angiulli et al., 2012, van de Vijver and Poortinga, 1997). At this point in its development, however, the field of the cognitive neuroscience of SES is also characterized by a distinct set of difficulties. Because the brain is highly plastic and functional specialization is largely dependent on early experiences, complex behaviors can be achieved through non-normative pathways with no discernable reduction in skill (Fox et al., 2010). Given the differing environments of lower- and higher-SES children, it is not surprising that there are SES-related differences in regional brain structure and patterns of functional activation. In this context, however, it is important to recognize that the environmental differences that these neural effects might reflect − more than simply deprivation of experience in lower-SES children − represent a complex amalgamation of varying social and cultural practices. Thus, we should be wary of interpreting nonspecific neural effects that are associated with lower levels of SES as necessarily reflecting SES-related differences in cognitive development.
3. SES, language, and brain structure
The brain undergoes significant changes over the course of development (Johnson et al., 2016). Synaptic density increases rapidly in the first few years of life, during which time neurons form connections and become organized into specialized functional regions. Synapses undergo an extended period of adjustment and pruning, and axons become increasingly myelinated through early adulthood. Importantly, this process has been shown to be experience-dependent (Fox et al., 2010). Although structural MRI is generally insensitive to specific neuronal processes like axonal pruning and myelination, it is nevertheless useful as a method to quantify large-scale morphometric changes in vivo, including the measurement of such variables as regional volume, cortical thickness, and surface area. Each of these measures change nonlinearly over the course of development. Indeed, measures of brain structure have been found to be correlated with differences in cognition and are posited to be sensitive to experience-dependent neural plasticity (Galván, 2010, Johnson et al., 2016, Noble et al., 2015).
Two primary models based on the animal literature have been used to understand socioeconomic differences within the cognitive neuroscience of SES. The first suggests that being deprived of meaningful stimulation causes early proliferation and pruning, resulting in global inefficiencies. The second suggests that living in particularly stressful conditions blunts autonomic reactivity and alters neural systems involved in emotional processing, thus inhibiting fear learning. In this context, theorists have posited that lower-SES children have been raised in environments in which they have been deprived of stimulation and subject to chronic stressors, both of which may negatively affect their development (McLaughlin et al., 2014).
A number of researchers in the field of the cognitive neuroscience of SES have now studied brain structure in probing cognitive differences between lower- and higher-SES children. For example, in attempting to elucidate the effects of SES on disparities in neurocognitive development, Noble et al. (2012) examined regional volumes in a sample of 5- to 17-year-olds from lower- and higher-SES backgrounds. Previous work demonstrated that higher-SES children outperformed their economically disadvantaged peers on neurocognitive tasks thought to assess the functioning of the brain’s language system (Noble et al., 2007). Thus, Noble and colleagues attempted to examine more directly the neural basis of these SES-associated differences. They examined five neural regions of interest (ROIs) on the basis of their presumed role in the development of language and reading skills: the left superior temporal gyrus (LSTG), left middle temporal gyrus, left inferior temporal gyrus (LITG), left inferior frontal gyrus (LIFG), and left fusiform gyrus. Noble et al. found that, after controlling for age and whole-brain volume, only LITG volume showed a trend-level association with SES. They did, however, find a significant interaction of SES and age in both the LIFG and LSTG. Whereas in the lower-SES children volumes of both ROIs were negatively related to age, this relation was reversed in the higher-SES children, who showed a positive relation between ROI volumes and age.
In interpreting this finding, Noble et al. (2012) drew on preexisting research regarding the impact of SES on development, relying on evidence that the early language environment is poorer in impoverished homes. They suggested that language deprivation is compounded throughout children’s lives, citing Hart and Risley (1995), who measured children’s language input only until they were three years of age. Next, Noble et al. turned to a separate body of research suggesting that lower-SES children perform more poorly on neurocognitive assessments of language than do their higher-SES peers (Noble et al., 2007). Integrating these findings, Noble et al. reasoned that because higher-SES children are posited to have more advanced language skills, presumably due to compounding differences in the home language environment, and because the higher-SES children in their sample had increasing LIFG and LSTG volume with greater age, the late increase of volume in these ROIs (compared to a decrease among the lower-SES children) must be indicative of superior language development in the higher-SES children. Noble et al. then suggested that this difference arises due to an extended period of neurodevelopment, specifically that pruning occurs over a longer timeframe, which gives these children a prolonged period of plasticity that aids in learning (Noble et al., 2012). It is important to note that Noble et al. do not present any evidence concerning the language experience and home environment of the children in their study; there are no direct correlations presented among their neuroimaging findings, children’s language experience, and language performance.
For investigators to be able to make claims about language, they must examine the language experiences of the children in their studies instead of relying on reverse inference (Poldrack, 2011). Many of the children in Noble et al.’s (2012) sample were Hispanic/Latino (42%) or African American (13%). Due largely to differences in parental education − which is how Noble et al. operationalized SES − Hispanic-American children from lower SES backgrounds are significantly more likely than are their higher-SES counterparts to grow up speaking Spanish in the home (Hakimzadeh and Cohn, 2007). Similarly, lower-SES Black-American children are significantly more likely to grow up hearing a variation of Standard American English (SAE) often referred to as African American Vernacular English (AAVE) (Smitherman, 2003, Wyatt, 1995). Bilingualism has shown to be related to differences in patterns of neural activation (Buchweitz and Prat, 2013). Thus, children’s language, and in particular bilingualism, may be confounded with SES in a way that the researchers did not control.
Given this confound, it is worth considering the following alternative explanation for Noble et al.’s (2012) finding of an interaction of age and SES in the LIFG. Schools in the United States, among other more formal settings, demand the use of SAE (Smitherman, 2003, Valdes-Fallis, 1978), but most lower-SES Hispanic and Black children continue to use Spanish and AAVE, respectively, when interacting with friends and family (Rickford and Rickford, 2002, Valdes-Fallis, 1978). This is particularly true when these individuals express strong emotion (Harris, 2004). This switching between language variants, termed code-switching, has received considerable attention in the study of bilingual children as a possible mechanism through which bilinguals may develop superior executive function capacities (Bialystok, 1999, Carlson and Meltzoff, 2008, Emmorey et al., 2008), although we should note that this is still an area in which there is considerable debate (Duñabeitia and Carreiras, 2015). Typically developing children recruit bilateral IFG for executive function tasks, particularly those that require switching between tasks (Aron et al., 2003). In fact, one study showed that bilinguals are more likely than monolinguals to differentially recruit the LIFG, and this increased recruitment of LIFG during switching tasks mediated their superior performance on the task itself (Garbin et al., 2010). Thus, because the lower-SES children in Noble et al.’s study may have had superior task-switching abilities relative to their peers, the decreasing volume of LIFG with age may be evidence of an advanced trajectory of executive function development, such that synaptic pruning begins at earlier ages, allowing them to reach adult-level proficiency more rapidly (Shaw et al., 2006).
While this alternative explanation is speculative, it highlights the difficulty of using reverse inference to interpret neural differences, particularly over development. One can interpret these differences as reflecting either a deficiency or an advantage simply by drawing on different literatures. Indeed, in the absence of longitudinal data, structural MRI measures during childhood and adolescence may be particularly subject to different interpretations (Mills and Tamnes, 2014). In this example, it is unclear whether decreased cortical volume represents underdevelopment of synaptic connections or more advanced pruning.
Noble et al. (2012) were meticulous in implementing controls for such confounding factors as gender and age, and were appropriately cautious in offering their interpretations of their data. Nevertheless, it is important to recognize that the reification inherent in cross-group studies of SES makes the isolation of any particular variable of interest, and hence the interpretability of any findings, particularly difficult. At this point, we believe that there is simply not sufficient evidence to support linking structural brain differences to underlying language deficits in lower-SES children.
Other investigators have also interpreted structural differences between lower- and higher-SES children, in the absence of data concerning the participants’ language functioning, as evidence of a neural basis for language delays in the former group. For example, Jednoróg et al. (2012) studied a sample of 8- to 10-year-old children and found a positive correlation between SES and gray matter volume of bilateral middle temporal gyri, left fusiform gyrus, and right inferior occipito-temporal region. Because these regions have been found in previous studies to be associated with the processing of written language, Jednoróg et al. (2012) suggest that their obtained volumetric differences are indicative of poorer reading and writing skills in lower-SES children. They found no significant correlation, however, between SES and tests of phonological skill or non-verbal IQ, nor did they include any tests of reading and writing skills in the children whom they studied. Therefore, it is difficult to evaluate the validity of their interpretations of their study.
Two recent studies have made significant headway in investigating the interactions among SES, brain structure, and language development. In a groundbreaking study, Noble et al. (2015) analyzed data from the brains of over 1000 children from a number of sites across the United States. These investigators found that after controlling for genetic ancestry, there was a logarithmic association between SES and cortical surface area, such that children whose families earned the lowest income had significantly reduced cortical surface area, particularly in temporal regions that have been posited to support language. Although this measure of cortical surface area partially mediated the relation between SES and a test of executive function, it was not associated with any measures of language. In a separate study, Hair et al. (2015) assessed a large sample of socioeconomically diverse children. They found that between ages 4–22 years, children below the poverty line were below developmental norms in regional gray matter volume, particularly in the temporal lobe. As expected, these structural differences partially mediated the link between SES and standardized tests thought to measure intelligence and academic achievement (Hair et al., 2015). Hair et al. (2015) did not, however, include any direct measures of language comprehension or production. Moreover, the developmental norms were modeled from their sample, which was predominantly higher-SES; thus, the trajectories may be more representative of the trajectories of higher-SES children.
These two studies are groundbreaking within the cognitive neuroscience of SES in both their sample size and scope. Both are also limited, however, in furthering our understanding of hypothesized language deficits associated with SES: neither study elucidates specifically what it is about poverty that drives these neurodevelopmental differences. These two studies also rely on standardized tests, which, due to the associations among SES, race, ethnicity, and native language, as we noted above, may produce artificially low scores in lower-SES children (Walton and Spencer, 2009).
Clearly, it is challenging to understand the significance of SES-related structural differences in the brain. At best, large-scale measures of structure using MRI are proxies for more precise neural mechanisms. Moreover, further complicating the interpretability of morphometric SES-associated differences, the confounds associated with SES may systematically affect behavioral measures of cognition even in the absence of real cognitive differences. Studies with longitudinal data sets, large samples, and varied cognitive assessments advance the field. That said, however, given the concerns described above, no single study has yet presented compelling evidence that structural brain differences underlie language deficits in lower-SES children.
4. SES, language, and brain function
Using task-based functional MRI (fMRI), investigators have assessed whether brain function underlies SES-related differences in language skill. FMRI permits the examination of brain activity during language tasks, which allows researchers to probe which regions of the brain are specifically recruited for particular components of language processing. In an early study, Noble et al. (2006) attempted to elucidate how brain activation differs by level of SES by controlling for children’s language skill. They recruited children who had been identified in New York Public Schools as delayed readers. Children from both high- and low-SES families in this sample tested similarly on a task meant to measure phonological awareness, a skill crucial for reading proficiency. During the task, the lower-SES children activated the left fusiform region, a region that adults and normally-reading children generally recruit for such a task. As would be expected given these past findings, left fusiform activation was related to these children’s performance on the task. The higher-SES children, however, showed a different pattern of activation: at the same levels of phonological awareness, they were less likely than were lower-SES children to activate the left fusiform region. Furthermore, the extent to which they recruited this region was not related to their phonological awareness. Thus, there was a stronger brain-behavior relation in the lower- than in the higher-SES children (Noble et al., 2006).
This early study in the field of the cognitive neuroscience of SES has important implications. First, it suggests that lower-SES children with poor reading skills − compared to performance-matched higher-SES children − are not underperforming because of underlying neurological impairment. Rather, their brains are activating as we would hypothesize they should be. Therefore, their underperformance must be explained by other factors. Second, given the use of SES as a proxy for the environment, Noble et al.’s (2006) findings suggest that the environments in which children grow up can moderate their patterns of neural activation in ways that do not necessarily present behaviorally. As a related point, differential patterns of activation can produce the same level of phonological skill. Finally, these findings indicate that even with evidence of true neurological dysfunction, children may learn to compensate by recruiting other regions of the brain. Noble and colleagues suggest that the higher-SES children have been exposed to more books in their homes, allowing them to develop such compensatory strategies. Because they did not directly measure book-reading in the home, however, it is not clear whether this − or a completely different factor that differentiates higher- from lower-SES children − underlies such a finding. For example, the potential impact of different amounts of school resources (Carter and Welner, 2013), different levels of comfort in educational settings (Walton and Cohen, 2007), and different attitudes toward the importance of tests (Steele, 1997), all might contribute to these findings. Future studies should examine more explicitly and systematically what specific aspects of SES may be driving such a result.
Regardless of potential environmental mediation, Noble et al. (2006) demonstrated that SES is related to differences in neural recruitment in the absence of language differences. Critically, they were able to do this by studying children at the lowest end of language proficiency − those with severe reading delays. This sampling, however, limits the extent to which their findings can be used to understand the neurological basis of observed SES-based language deficits in children within the normal range of abilities. Raizada et al. (2008) attempted to address this question by examining patterns of neural activation in 5-year-old children as they completed a rhyming task in the MRI scanner. In addition to collecting information about family SES, they asked children to complete a number of behavioral tests that measure intelligence and language skill. Next, they examined correlations among measures of language and cognition, task performance, functional activation, and SES. The only significant correlation was that between SES and left-minus-right recruitment of the IFG during the task. Whereas higher-SES children recruited the LIFG more than they did the RIFG, lower-SES children showed less hemispheric specialization. This difference in specialization, however, was not related either to any behavioral measures of language or to the children’s performance on the rhyming task.
To examine whether differential neural activation underlies language differences, Raizada et al. (2008) tested the potential mediation of language skill on the association between SES and functional activation. Yet when behavioral measures of language were controlled − together or individually − the relation remained statistically significant. Raizada and colleagues suggest that their finding points to early SES-related neural substrates of language delay that behavioral measures cannot yet identify. In fact, there is evidence that hemispheric specialization in adulthood is related to language outcomes (Josse and Tzourio-Mazoyer, 2004). Raizada et al. (2008), however, assessed children who were 5 years old, an age when hemispheric specialization is just beginning to emerge (Amunts et al., 2003). There is little evidence to suggest that early development of hemispheric specialization − as opposed to protracted development which, as Noble et al. (2012) and others have suggested, might be beneficial − leads to, or predicts the development of, superior language abilities. Similarly, relations between SES and any behavioral measures of language in Raizada et al.’s (2008) sample did not reach significance. Thus, even functional findings that are unrelated to any measures other than SES are difficult to interpret. This difficulty is compounded by the fact that scholars too often interpret any SES-related differences in neural function as indicative of an underlying linguistic delay, even when there is no evidence in their study to support such an assumption.
Taken together, studies of both neuroanatomy and neural functional activation yield SES-related differences in brain regions that support language; these findings, however, have not reliably been linked to differences in language functioning. Importantly, a recent study has shown no measurable differences in electrophysiological brain activity between lower- and higher-SES newborn babies, although EEG power at birth was associated with infants’ language and memory outcomes 15 months later (Brito et al., 2016), Similarly, another study showed no early differences in temporal gray matter, but different frontal and parietal growth trajectories over the first months of life (Hanson et al., 2013). These findings provide preliminary support for the formulation that, over early sensitive periods, children’s environments systematically influence functional brain development. Yet the majority of studies in the area of the cognitive neuroscience of SES rely on SES as a crude proxy for both children’s environment and their language skill. SES-related brain differences are presumed to be indicative of language deficits in lower-SES children, regardless of whether a relation between SES and language skill was confirmed in a given study. Taken together, evidence for brain-based differences in language skill between higher- and lower-SES children remains negligible.
5. Toward a nuanced understanding: conclusions and future directions
In this review we highlighted several concerns within the field of the cognitive neuroscience of SES. Early findings have relied on reverse inference, using group-related neural differences to guide discussions about participants’ cognitive abilities and early environments. This is especially complicated in the case of children, whose brains undergo varied and nonlinear changes over the course of development. Thus, making inferences about developmental trajectories on the basis of group differences in neural structure or task-related activation is difficult in the absence of longitudinal data. As a related point, drawing strong conclusions from comparisons of two groups that differ markedly in one or more respects (e.g., SES, race, ethnicity, first language, bilingualism) can be problematic. Moreover, standardized cognitive tests may under-predict the abilities of certain groups of children, which further complicates the interpretability of group-related differences in cognitive functioning. Certainly, these issues are not specific to studies of SES, language, and the brain. Developmental neuroscience more broadly would benefit from careful consideration of these concerns. Nevertheless, because the cognitive neuroscience of SES is at a nascent stage of development and is rapidly gaining momentum, we have the opportunity to improve our methods at this early stage of development.
Recent studies designed to examine how SES-related differences in environments might influence children’s neural trajectories represent a promising avenue for understanding the effects of income inequality. Indeed, there is strong evidence that SES moderates children’s brain development. What these developmental differences mean for children’s language skills, however, or why they emerge, has yet to be clearly tested. Future studies should move toward a more careful evaluation of proposed mechanisms through which SES might act on the developing brain to influence language outcomes. Crucial to this approach is removing or controlling for confounds that are linked with SES, such as culture or first language, that may be influencing neural development in ways that have not yet been examined. Moreover, the cognitive neuroscience of SES should, whenever possible, move away from reverse inference and toward more systematic testing of variables of interest.
New technology is allowing researchers to examine more systematically variations in the home language environment that are hypothesized to be related to language outcomes. For example, the LENA digital language processor (DLP) is a digital recorder worn in children’s front pockets that can unobtrusively sample children’s home language environments by recording proximal speech for up to 16 h (Xu et al., 2008). Using LENA analysis software to measure child-directed speech (CDS) may reduce bias introduced in researcher-prescribed settings and allow for a more accurate estimate of the speech children hear regularly. In fact, measures of naturally-occurring CDS derived using LENA have been shown to be related to children’s early trajectories of language development (Weisleder and Fernald, 2013). Importantly, these investigators recruited children who came from similar neighborhoods, shared a common ethnicity, were exposed to the same first language in the home, and had minimal exposure to any second language. The cognitive neuroscience of SES would benefit from recruiting children in a similar manner. Investigators should capitalize on these advancements within the field of language development to examine whether specific elements of CDS, including measures of both language quantity and quality, are related to brain structure and function, and test whether these neural differences mediate the association between CDS and language skill.
Although reverse inference is difficult to avoid − and indeed, has been useful − in studies of the brain (Poldrack, 2011), it is important to be cautious in interpreting results of studies concerning SES. Not only is SES a broad and poorly defined variable (Braveman et al., 2005), but it is linked to many other factors that may confound the interpretability of potential results. Moreover, SES is highly politicized; the popular press picks up on studies of SES and the brain with alarming alacrity and distortion. Already, the New Yorker has published an article declaring that poverty results in a “weakened” brain due to the deprivation of the environment, stating, in fact, that “the scientific consensus has become clear” (Ostrander, 2015, p. 3). This is the case even when research findings are discussed with appropriate caution, and highlights the need for researchers to seek training in interacting effectively with the press.
Of course, the scientific evidence to date is far from clear. In the absence of a demonstrated link between brain structure and the language environment or language skill, observed SES-related differences in brain regions that support language are difficult to interpret. While this state of affairs means that the cognitive neuroscience of SES has the exciting prospect of developing a better understanding these relations, it is important that researchers in this field are circumspect about their recruitment of participants, their definitions of constructs and tools for measurement, and their interpretation of results.
Funding
Preparation of this article was facilitated by NIMH grant R01-MH101495 to IHG.
References
- Amunts K., Schleicher A., Ditterich A., Zilles K. Broca’s region: cytoarchitectonic asymmetry and developmental changes. J. Comp. Neurol. 2003;465(1):72–89. doi: 10.1002/cne.10829. [DOI] [PubMed] [Google Scholar]
- Aron A.R., Fletcher P.C., Bullmore E.T., Sahakian B.J., Robbins T.W. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat. Neurosci. 2003;6(2):115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Bialystok E. Cognitive complexity and attentional control in the bilingual mind. Child Dev. 1999;70(3):636–644. [Google Scholar]
- Braveman P.A., Cubbin C., Egerter S., Chideya S., Marchi K.S., Metzler M.R., Posner S. Socioeconomic status in health research. Jama. 2005;294(22):2879–2888. doi: 10.1001/jama.294.22.2879. [DOI] [PubMed] [Google Scholar]
- Brito N.H., Fifer W.P., Myers M.M., Elliott A.J., Noble K.G. Associations among family socioeconomic status, EEG power at birth, and cognitive skills during infancy. Dev. Cognit. Neurosci. 2016;19:144–151. doi: 10.1016/j.dcn.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchweitz A., Prat C. The bilingual brain: flexibility and control in the human cortex. Phys. Life Rev. 2013;10(4):428–443. doi: 10.1016/j.plrev.2013.07.020. [DOI] [PubMed] [Google Scholar]
- Carlson S.M., Meltzoff A.N. Bilingual experience and executive functioning in young children. Dev. Sci. 2008;11(2):282–298. doi: 10.1111/j.1467-7687.2008.00675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter P.L., Welner K.G. University Press; Oxford: 2013. Closing the Opportunity Gap. [Google Scholar]
- Chapman M., Skinner E.A. Children’s agency beliefs, cognitive performance, and conceptions of effort and ability: individual and developmental differences. Child Dev. 1989;60(5):1229–1238. [PubMed] [Google Scholar]
- Coley, R. J., 2002. An Uneven Start. pp. 1–77.
- D'Angiulli A., Lipina S.J., Olesinska A. Explicit and implicit issues in the developmental cognitive neuroscience of social inequality. Front. Hum. Neurosci. 2012;6:1–17. doi: 10.3389/fnhum.2012.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond, M., 2016. Evicted. Crown.
- Duñabeitia J.A., Carreiras M. The bilingual advantage: acta est fabula? Cortex. 2015;73:371–372. doi: 10.1016/j.cortex.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Duncan G.J., Magnuson K. Socioeconomic status and cognitive functioning: moving from correlation to causation. Wiley Interdiscip. Rev. Cognit. Sci. 2012;3:377–386. doi: 10.1002/wcs.1176. [DOI] [PubMed] [Google Scholar]
- Emmorey K., Luk G., Bialystok E. The source of enhanced cognitive control in bilinguals. Psychol. Sci. 2008;19(12):1201–1206. doi: 10.1111/j.1467-9280.2008.02224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans G.W., Gonnella C., Marcynyszyn L.A., Gentile L., Salpekar N. The role of chaos in poverty and children’s socioemotional adjustment. Psychol. Sci. 2005;16(7):560–565. doi: 10.1111/j.0956-7976.2005.01575.x. [DOI] [PubMed] [Google Scholar]
- Fernald A., Marchman V.A., Weisleder A. SES differences in language processing skill and vocabulary are evident at 18 months. Dev. Sci. 2012;16(2):234–248. doi: 10.1111/desc.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox S.E., Levitt P., Nelson C.A. How the timing and quality of early experiences influence the development of brain architecture. Child Dev. 2010;81(1):28–40. doi: 10.1111/j.1467-8624.2009.01380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galván A. Neural plasticity of development and learning. Hum. Brain Mapp. 2010;31(6):879–890. doi: 10.1002/hbm.21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbin G., Sanjuan A., Forn C., Bustamante J.C., Rodriguez-Pujadas A., Belloch V. Bridging language and attention: brain basis of the impact of bilingualism on cognitive control. Neuroimage. 2010;53(4):1272–1278. doi: 10.1016/j.neuroimage.2010.05.078. [DOI] [PubMed] [Google Scholar]
- Guinote A., Cotzia I., Sandhu S., Siwa P. Social status modulates prosocial behavior and egalitarianism in preschool children and adults. Proc. Natl. Acad. Sci. 2015;112(3):731–736. doi: 10.1073/pnas.1414550112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman D.A., Farah M.J. Socioeconomic status and the developing brain. Trends Cogn. Sci. 2009;13(2):65–73. doi: 10.1016/j.tics.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hair N.L., Hanson J.L., Wolfe B.L., Pollak S.D. Association of child poverty, brain development, and academic achievement. JAMA Pediatr. 2015;169(9):822. doi: 10.1001/jamapediatrics.2015.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakimzadeh S., Cohn D. Pew Hispanic Center; 2007. English Usage Among Hispanics in the United States; pp. 1–27. [Google Scholar]
- Hanson J.L., Hair N., Shen D.G., Shi F., Gilmore J.H., Wolfe B.L., Pollak S.D. Family poverty affects the rate of human infant brain growth. PLoS One. 2013;8(12):e80954–e80959. doi: 10.1371/journal.pone.0080954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris C.L. Bilingual speakers in the lab: psychophysiological measures of emotional reactivity. J. Multiling. Multicult. Dev. 2004;25(2–3):223–247. [Google Scholar]
- Hart B., Risley T.R. Paul H Brookes Publishing Company; 1995. Meaningful Differences in the Everyday Experience of Young American Children. [Google Scholar]
- Herskovits M.J. A.A. Knopf; New York: 1948. Man and His Works. [Google Scholar]
- Hess R.D., McDevitt T.M. Some cognitive consequences of maternal intervention techniques: a longitudinal study. Child Dev. 1984;55(6):2017. [Google Scholar]
- Hoff E. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2003. Language Development in Childhood. [Google Scholar]
- Hollingshead A.A. Four-factor index of social status. Yale J. Sociol. 2011;8:21–51. [Google Scholar]
- Isaacs J.B., Sawhill I.V., Haskins R. Brookings Institution; 2008. Getting Ahead or Losing Ground: Economic Mobility in America. [Google Scholar]
- Jednoróg K., Altarelli I., Monzalvo K., Fluss J., Dubois J., Billard C. The influence of socioeconomic status on children’s brain structure. PLoS One. 2012;7(8):e42486–e42489. doi: 10.1371/journal.pone.0042486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S.B., Riis J.L., Noble K.G. State of the art review: poverty and the developing brain. Pediatrics. 2016;137(4):e20153075. doi: 10.1542/peds.2015-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josse G., Tzourio-Mazoyer N. Hemispheric specialization for language. Brain Res. Rev. 2004;44(1):1–12. doi: 10.1016/j.brainresrev.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Kraus M.W., Cote S., Keltner D. Social class, contextualism, and empathic accuracy. Psychol. Sci. 2010;21(11):1716–1723. doi: 10.1177/0956797610387613. [DOI] [PubMed] [Google Scholar]
- Kraus M.W., Piff P.K., Mendoza-Denton R., Rheinschmidt M.L., Keltner D.1. Social class, solipsism, and contextualism: how the rich are different from the poor. Psychol. Rev. 2012;119(3):546–572. doi: 10.1037/a0028756. [DOI] [PubMed] [Google Scholar]
- Leventhal T., Brooks-Gunn J. The neighborhoods they live in: the effects of neighborhood residence on child and adolescent outcomes. Psychol. Bull. 2000;126(2):309–337. doi: 10.1037/0033-2909.126.2.309. [DOI] [PubMed] [Google Scholar]
- Lipina S.J. Critical considerations about the use of poverty measures in the study of cognitive development. Int. J. Psychol. 2016 doi: 10.1002/ijop.12282. [DOI] [PubMed] [Google Scholar]
- Macartney S., Bishaw A., Fontenot K. U.S. Department of Commerce; 2013. Poverty Rates for Selected Detailed Race and Hispanic Groups by State and Place: 2007–2011; pp. 1–20. [Google Scholar]
- McLaughlin K.A., Sheridan M.A., Lambert H.K. Childhood adversity and neural development: deprivation and threat as distinct dimensions of early experience. Neurosci. Biobehav. Rev. 2014;47:578–591. doi: 10.1016/j.neubiorev.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills K.L., Tamnes C.K. Methods and considerations for longitudinal structural brain imaging analysis across development. Dev. Cognit. Neurosci. 2014;9:172–190. doi: 10.1016/j.dcn.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble K.G., Wolmetz M.E., Ochs L.G., Farah M.J., McCandliss B.D. Brain–behavior relationships in reading acquisition are modulated by socioeconomic factors. Dev. Sci. 2006;9(6):642–654. doi: 10.1111/j.1467-7687.2006.00542.x. [DOI] [PubMed] [Google Scholar]
- Noble K.G., McCandliss B.D., Farah M.J. Socioeconomic gradients predict individual differences in neurocognitive abilities. Dev. Sci. 2007;10(4):464–480. doi: 10.1111/j.1467-7687.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- Noble K.G., Houston S.M., Kan E., Sowell E.R. Neural correlates of socioeconomic status in the developing human brain. Dev. Sci. 2012;15(4):516–527. doi: 10.1111/j.1467-7687.2012.01147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble K.G., Houston S.M., Brito N.H., Bartsch H., Kan E., Kuperman J.M. Family income, parental education and brain structure in children and adolescents. Nat. Neurosci. 2015;18(5):773–778. doi: 10.1038/nn.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okonofua J.A., Walton G.M., Eberhardt J.L. A vicious cycle: a social-psychological account of extreme racial disparities in school discipline. Perspect. Psychol. Sci. 2016;11(3):381–398. doi: 10.1177/1745691616635592. [DOI] [PubMed] [Google Scholar]
- Ostrander M. The New Yorker; 2015. What Poverty Does to the Young Brain. June. [Google Scholar]
- Poldrack R.A. Inferring mental states from neuroimaging data: from reverse inference to large-Scale decoding. Neuron. 2011;72(5):692–697. doi: 10.1016/j.neuron.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raizada R.D.S., Richards T.L., Meltzoff A., Kuhl P.K. Socioeconomic status predicts hemispheric specialisation of the left inferior frontal gyrus in young children. Neuroimage. 2008;40(3):1392–1401. doi: 10.1016/j.neuroimage.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raizada R. Effects of socioeconomic status on brain development, and how cognitive neuroscience may contribute to leveling the playing field. Front. Hum. Neurosci. 2010:1–11. doi: 10.3389/neuro.09.003.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickford J.R., Rickford R.J. John Wiley & Sons; 2002. Spoken Soul. [Google Scholar]
- Roscigno V.J., Ainsworth-Darnell J.W. Race, cultural capital, and educational resources: persistent inequalities and achievement returns. Sociol. Educ. 1999;72(3):158–178. [Google Scholar]
- Rowe M.L. Child-directed speech: relation to socioeconomic status, knowledge of child development and child vocabulary skill. J. Child Lang. 2008;35(01):185–205. doi: 10.1017/s0305000907008343. [DOI] [PubMed] [Google Scholar]
- Sharkey P. The acute effect of local homicides on children’s cognitive performance. PNAS. 2010;107(26):11733–11738. doi: 10.1073/pnas.1000690107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey P. University of Chicago Press; 2013. Stuck in Place. [Google Scholar]
- Shaw P., Greenstein D., Lerch J., Clasen L., Lenroot R., Gogtay N. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440(7084):676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Sirin S.R. Socioeconomic status and academic achievement: a meta-analytic review of research. Rev. Educ. Res. 2005;75(3):417–453. [Google Scholar]
- Smitherman G. Routledge; 2003. Talkin That Talk. [Google Scholar]
- Steele C.M. A threat in the air. Am. Psychol. 1997;52(6):613–629. doi: 10.1037//0003-066x.52.6.613. [DOI] [PubMed] [Google Scholar]
- Suzuki L., Aronson J. The cultural malleability of intelligence and its impact on the racial/ethnic hierarchy. Psychol. Public Policy Law. 2005;11(2):320–327. [Google Scholar]
- Tenenbaum H.R., Ruck M.D. Are teachers’ expectations different for racial minority than for European American students? A meta-analysis. J. Educ. Psychol. 2007;99(2):253–273. [Google Scholar]
- Trueba H.T. Culturally based explanations of minority students’ academic achievement. Anthropol. Educ. Q. 1988;19(3):270–287. [Google Scholar]
- Ursache A., Noble K.G. Neurocognitive development in socioeconomic context: multiple mechanisms and implications for measuring socioeconomic status. Psychophysiology. 2015;53(1):71–82. doi: 10.1111/psyp.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes-Fallis G. Code switching and the classroom teacher. Lang. Educ. Theory Pract. 1978;4:1–31. [Google Scholar]
- Walpole M. Socioeconomic status and college: how SES affects college experiences and outcomes. Rev. Higher Educ. 2003;27(1):45–73. [Google Scholar]
- Walton G.M., Cohen G.L. A question of belonging: race, social fit, and achievement. J. Pers. Soc. Psychol. 2007;92(1):82–96. doi: 10.1037/0022-3514.92.1.82. [DOI] [PubMed] [Google Scholar]
- Walton G.M., Spencer S.J. Latent ability. Psychol. Sci. 2009;20(9):1132–1139. doi: 10.1111/j.1467-9280.2009.02417.x. [DOI] [PubMed] [Google Scholar]
- Weisleder A., Fernald A. Talking to children matters: early language experience strengthens processing and builds vocabulary. Psychol. Sci. 2013;24(11):2143–2152. doi: 10.1177/0956797613488145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt T.A. Language development in African American English child speech. Ling. Educ. 1995;7:7–22. [Google Scholar]
- Xu D., Yapanel U., Gray S., Gilkerson J., Richards J., Hansen J. ISCA Archive; 2008. Signal Processing for Young Child Speech Language Development; pp. 1–6. [Google Scholar]
- van de Vijver F.J.R., Poortinga Y.H. Towards an integrated analysis of bias in cross-Cultural assessment. Eur. J. Psychol. Assess. 1997;13(1):29–37. [Google Scholar]