Abstract
Tuberculosis is caused by Mycobacterium tuberculosis and provided original proof that an infectious agent can cause human disease. However, key steps in tuberculosis pathogenesis remain poorly understood. We propose that autoimmunity is a critical and overlooked process driving pathology in tuberculosis, and present clinical and experimental observations supporting this hypothesis.
Keywords: tuberculosis, host immune response, autoimmunity, pathology, CD1
Tuberculosis (TB) is the leading cause of death by an infectious disease. Robert Koch identified the causative organism, Mycobacterium tuberculosis (Mtb), providing “Koch’s postulates” to prove that a disease is caused by an infectious agent. Furthermore, many lines of evidence in humans and mice demonstrate that the immune system is essential in protecting the host against Mtb. Therefore, it seems counter-intuitive to suggest that TB has an autoimmune component. However, diverse evidence suggests that autoimmunity is a critical process exacerbating pathology in TB, leading to cavitation and transmission.
The Host Immune Response Is Essential to TB Transmission
Mtb is transmitted by patients with pulmonary TB. The host immune response is central to containing Mtb, as patients with HIV-induced immunocompromise have a greatly increased TB risk, but conversely also drives the underlying lung pathology. Patients with advanced HIV infection rarely develop cavitation, whereas during immune system reconstitution tissue damage often occurs. Pulmonary disease most frequently occurs in young adults with the strongest immunological response [1]. Similarly, in rabbit models, cavitation is accelerated by pre-sensitisation with purified protein derivative (PPD) to drive a strong delayed-type hypersensitivity reaction, but inhibited by immunosuppression. Immunity is thought to be directed against Mtb antigens, with progressive accumulation of total mycobacterial antigenic load over time precipitating inflammation and lung tissue destruction [2]. However, a striking feature of TB granulomas is the paucity of mycobacteria, while extensive pathology develops. It remains unclear how so few mycobacteria drive such a florid inflammatory immune response.
Diverse Autoimmune Phenomena Occur in Human TB
Physicians treating TB patients observe numerous clinical events associated with autoimmune diseases that are unexplained by current disease paradigms. For example, autoantibodies associated with autoimmune diseases such as Wegener’s granulomatosis and systemic lupus erythematosus are detected in 40% of TB patients [3]. Poncet’s disease is an inflammatory polyarthritis occurring in TB patients in the absence of detectable mycobacteria in the joint spaces. In the eye, TB can cause uveitis without mycobacteria, which resolves with anti-mycobacterial treatment. Uveitis is typically associated with autoimmune diseases such as inflammatory bowel disease, Behcet’s disease and ankylosing spondylitis. Similarly, erythema nodosum, an inflammatory cutaneous disorder affecting the shins, occurs in TB and autoimmune diseases such as Crohn’s disease, ulcerative colitis and sarcoidosis.
Genomic analyses show single nucleotide polymorphisms that associate with TB severity also associate with autoimmune disease, and CTLA-4 autoimmunity associated genotype contributes to TB severity [4]. Additionally, autoreactive T cells are reported to be increased in TB patients. Systemic autoimmunity can be trigged by intra-vesical administration of Mycobacterium bovis BCG for bladder cancer. When considered together, these phenomena suggest that mycobacteria induce inappropriate host responses to self-antigens, causing autoimmune inflammation.
Furthermore, experimental studies link Mtb with development of autoimmunity. For example, Mtb antigens are used in Freund’s adjuvant in animal models of autoimmune diseases [5], suggesting that these antigens seem to overcome tolerance to host antigens when co-administered. In experimental models, immunisation to Mtb can drive autoimmune arthritis by causing cross-reactivity with proteoglycan in cartilage [6]. Similarly, an acetone-precipitable fraction of Mtb can specifically drive release of the autoantigen proliferating cell nuclear antigen (PCNA) from human cells [7]. Whilst these attributes of Mtb inducing autoimmune phenomena have been extensively used experimentally for modelling of autoimmune diseases, the concept that the same process is occurring in TB patients is not considered.
TB and Sarcoidosis Are Virtually Indistinguishable
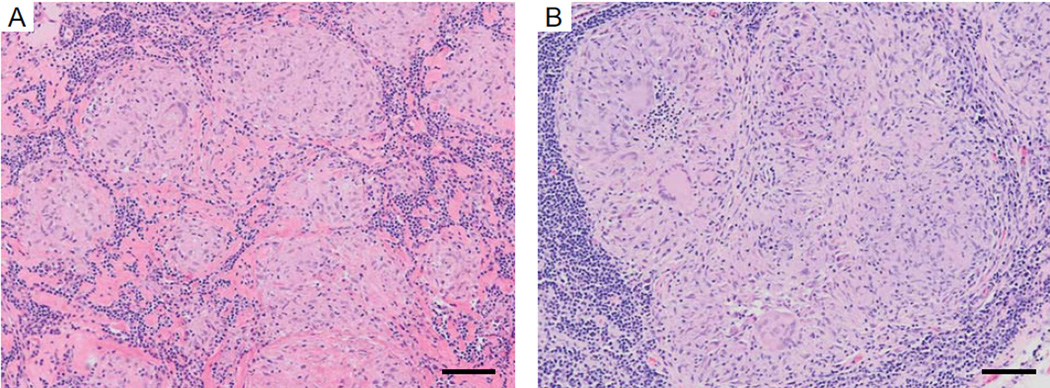
Further evidence for autoimmunity TB comes from sarcoidosis, a human disease even considered part of the same disease spectrum as TB by some. Sarcoidosis is an autoimmune disease with no proof of an infectious aetiology, despite extensive investigation for mycobacteria within lesions, that resolves with systemic corticosteroid treatment. Sarcoidosis has several similarities with TB, suggesting a common fundamental process. Firstly, histological analysis shows well-organised granulomas formed from activated macrophages, multinucleate giant cells and peripheral T cells, and lesions are often histologically indistinguishable from TB granulomas (Figure 1). Secondly, both TB and sarcoidosis typically affect the lung upper lobes and the mediastinal lymph nodes. Thirdly, sarcoidosis and TB can affect other organs, including the central nervous system, and neurosarcoidosis and TB meningitis can be clinically indistinguishable. Finally, analysis of peripheral blood gene signatures show highly similar expression patterns [8]. Therefore, similarities between the autoimmune disease sarcoidosis and the infectious disease TB suggest common antigens drive the two diseases. Since sarcoidosis is an autoimmune disease that resolves with corticosteroids, the implication is that similar autoantigens contribute to pathology in TB. Adjunctive corticosteroids are routinely used in treatment of pericardial and meningeal TB and intriguingly, corticosteroids accelerate radiographic resolution in pulmonary TB and potentially reduce TB mortality.
Figure 1. The Histological Appearances of Sarcoidosis and Tuberculosis Are Often Indistinguishable.
A) Lymph node biopsy of sarcoidosis; the patient improved with systemic immunosuppression with corticosteroids. B) Lymph node biopsy of tuberculosis; Mycobacterium tuberculosis culture was positive and the patient recovered with antibiotic therapy. Scale bar 100µm.
Investigating an Autoimmune Process of Unknown Aetiology
The clinical phenomena presented suggest that the interaction of host with mycobacterial antigens elicits the subsequent development of an additional autoimmune inflammatory process, exacerbating pathology in TB. However, the host antigens involved are unknown, presenting a significant challenge. Nevertheless, the clinical and experimental associations are so strong that ignoring this possibility risks neglecting a central process in TB pathogenesis.
A bioinformatic approach may identify pathways up-regulated in granulomas, and indicate the antigen presenting molecules and T cell receptors most likely involved. One approach could be RNA-Seq analysis of TB granulomas, sarcoid granulomas and suitable control tissues. Analysis TB and sarcoid tissue, combined with protein interaction networks, may indicate pathways induced to inform ex vivo analyses. Alternatively, a hypothesis-driven approach may identify antigens. Mtb causes extensive extracellular matrix destruction and cleavage of collagen and elastin releases novel epitopes that may be autoreactive, which have been identified in other destructive pulmonary pathologies [9]. Therefore, investigating T cell responses to lung structural fibrils may be fruitful. Alternatively, bioinformatic approaches suggest cross-reactive T cell epitopes between the Mtb and human proteomes, leading these authors to suggest that autoimmunity contributes to pathology [10]. Additionally, direct evidence of cross-reactivity of antibodies to mycobacterial 65-kDa heat shock protein and human lactoferrin has been demonstrated.
Finally, and perhaps most likely, key antigens may be shared between Mtb and humans. For example, specific lipid antigens are common to both Mtb and the host, such as glycerophospholipids. These antigens include phosphatidylglycerol and phosphatidylinositol, which are presented by CD1d to NKT cells. Phosphatidylglycerol is also presented by CD1b molecules to CD1b self-reactive T cells. CD1-restricted presentation may explain why the HLA associations in TB, although described, are weak, and why mice do not develop typical human pathology, since they lack Group I CD1 molecules. These host lipids may include stress antigens such as cholesterol esters or squalene, presented by CD1c and CD1a respectively, to self-reactive T cells. We hypothesise that host lipids presented by Group I CD1 are the common antigens for TB and sarcoidosis.
Concluding Remarks
Diverse evidence from both clinical observation and animal studies suggests that development of autoimmunity is a fundamental process in human TB. This concept has wide implications and the evidence is sufficiently strong to justify further investigation. Experimental data rarely challenge established dogma; instead, concepts must evolve and then experiments investigate these hypotheses [11]. Apoptosis of infected cells has recently been shown to induce self-reactive T cells to promote auto-inflammation in other infections [12], and extensive macrophage apoptosis occurs in TB, providing a potential central mechanism. Whilst it may seem implausible that Mtb induces autoimmunity to transmit, such ingenious immunological deception may explain why pathological processes in TB remain so enigmatic.
Supplementary Material
Text box. Directions for Future Research.
What is the mechanism whereby Mycobacterium tuberculosis induces immune cells to respond to host antigens?
Do Mtb adjuvants prime a response to bystander host antigens, and if so, what are these host antigens?
Alternatively, are there antigens that are shared between host and pathogen?
In addition to auto-immunity related phenomena, does immune dysregulation similar to human auto-inflammatory conditions play a role?
- What are the optimal experimental approaches and model systems to investigate this phenomenon?
-
◦Unbiased genomic and proteomic approaches
-
◦Hypothesis driven approaches, such as lung structural fibrils or CD1-presented lipids
-
◦Autoantibody profiling as a surrogate marker for T cell immunity
-
◦Human clinical investigation versus tractable model systems
-
◦
What novel experimental tools are required, such as for characterisation of CD1-restricted T cell responses in the peripheral circulation and in lung granulomas?
If mycobacterial infection drives autoimmunity as an evolutionary strategy, what are the implications for novel vaccination approaches?
Is autoimmune inflammation also the principal mechanism driving the TB-Immune Reconstitution Inflammatory Syndrome that occurs during HIV treatment?
Will inhibiting autoimmune inflammation, whilst concurrently preserving anti-mycobacterial effector immune responses, reduce mortality of patients with advanced TB?
Acknowledgments
We thank Dr Sanjay Jogai for providing the histological images. Samples used in this study were sourced from the Southampton Research Biorepository, University Hospital Southampton NHS Foundation Trust and University of Southampton. PE is supported by the Antimicrobial Resistance Cross Council Initiative funded by the Biotechnology and Biological Sciences Research Council and the Medical Research Council MR/N006631/1, and the US National Institute for Health R33AI102239. MT is supported by a Clinical Lectureship provided by the UK National Institute for Health Research and funding from the UK Technology Strategy Board / Innovate UK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interest.
References
- 1.Comstock GW, et al. The prognosis of a positive tuberculin reaction in childhood and adolescence. Am J Epidemiol. 1974;99(2):131–138. doi: 10.1093/oxfordjournals.aje.a121593. [DOI] [PubMed] [Google Scholar]
- 2.Hunter RL. Tuberculosis as a three-act play: A new paradigm for the pathogenesis of pulmonary tuberculosis. Tuberculosis (Edinb) 2016;97:8–17. doi: 10.1016/j.tube.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kakumanu P, et al. Patients with pulmonary tuberculosis are frequently positive for anti-cyclic citrullinated peptide antibodies, but their sera also react with unmodified arginine-containing peptide. Arthritis Rheum. 2008;58(6):1576–1581. doi: 10.1002/art.23514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pagan AJ, Ramakrishnan L. Immunity and Immunopathology in the Tuberculous Granuloma. Cold Spring Harb Perspect Med. 2015;5(9) doi: 10.1101/cshperspect.a018499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Billiau A, Matthys P. Modes of action of Freund's adjuvants in experimental models of autoimmune diseases. J Leukoc Biol. 2001;70(6):849–860. [PubMed] [Google Scholar]
- 6.van Eden W, et al. Arthritis induced by a T-lymphocyte clone that responds to Mycobacterium tuberculosis and to cartilage proteoglycans. Proc Natl Acad Sci U S A. 1985;82(15):5117–5120. doi: 10.1073/pnas.82.15.5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haftel HM, et al. Induction of the autoantigen proliferating cell nuclear antigen in T lymphocytes by a mycobacterial antigen. J Clin Invest. 1994;94(4):1365–1372. doi: 10.1172/JCI117471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maertzdorf J, et al. Common patterns and disease-related signatures in tuberculosis and sarcoidosis. Proc Natl Acad Sci U S A. 2012;109(20):7853–7858. doi: 10.1073/pnas.1121072109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee SH, et al. Antielastin autoimmunity in tobacco smoking-induced emphysema. Nat Med. 2007;13(5):567–569. doi: 10.1038/nm1583. [DOI] [PubMed] [Google Scholar]
- 10.Chodisetti SB, et al. Potential T cell epitopes of Mycobacterium tuberculosis that can instigate molecular mimicry against host: implications in autoimmune pathogenesis. BMC Immunol. 2012;13:13. doi: 10.1186/1471-2172-13-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Behr MA, Waters WR. Is tuberculosis a lymphatic disease with a pulmonary portal? Lancet Infect Dis. 2014;14(3):250–255. doi: 10.1016/S1473-3099(13)70253-6. [DOI] [PubMed] [Google Scholar]
- 12.Campisi L, et al. Apoptosis in response to microbial infection induces autoreactive TH17 cells. Nat Immunol. 2016;17(9):1084–1092. doi: 10.1038/ni.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.