Abstract
T cells, as orchestrators of the adaptive immune response, serve important physiological and potentially therapeutic roles, for example in cancer immunotherapy. T cells are readily isolated from patients; however, the yield of antigen-specific T cells is limited, thus making their clinical use challenging. Therefore, the generation of T lymphocytes from hematopoietic stem/progenitor cells (HSPCs) and human pluripotent stem cells (PSCs) in vitro provides an attractive method for large-scale production and genetic manipulation of T cells. In this review, we discuss recent strategies for the generation of T cells from human HSPCs and PSCs in vitro. Continued advancement in the generation of human T cells in vitro will expand their benefits and therapeutic potential in the clinic.
T Cells: Critical Mediators of the Immune Response
T cells are a vital component of the human immune system and carry out numerous functions to protect the individual. Perturbations in T cell function result in reduction or hyper-activation of immunity, culminating in a broad spectrum of diseases. As a result, it is critical to understand T cell development and function, not only to treat disease but also to exploit T cells as therapeutic agents in a variety of applications.
One of the major causes of T cell loss is primary immunodeficiency (PID) [1–3]. PIDs are typically hereditary, often with an early disease onset, and can be treated with an umbilical cord blood (UCB) or bone marrow (BM) transplant for individuals fewer than three years of age [4]. The first hurdle is obtaining human leukocyte antigen (HLA)-matched donor cells and trying to minimize the risk of graft-versus-host disease (GVHD). However, the transplant itself presents a challenge, since the ablation of the recipient’s immune system results in lymphopenia until the transplanted hematopoietic stem/progenitor cells (HSPCs) engraft. The next difficulty lies in generating or expanding sufficient T cell numbers in vivo. T cell repopulation is often delayed by months and it may take years to fully restore normal numbers and functionality, if at all [5, 6].
Besides the many physiological functions of T cells, they also play a critical part in the detection and eradication of cancerous cells [7]. This T cell quality has been utilized in cancer immunotherapy and has shown remarkable promise. For example, tumor-antigen specific T cells, such as tumor infiltrating lymphocytes (TILs), can be isolated, expanded in vitro and re-introduced into the patient as therapeutic agents. Additionally, genetic engineering of T cells allows the generation of T cells bearing custom tumor-specific antigen receptors to improve therapeutic efficiency [8].
Considering the importance of T cells in immunity, it comes as no surprise that many efforts have been made to develop de novo T cells in vitro, including attempts to isolate, expand, and modify patient-derived T cells for therapeutic applications [8] (Figure 1). This review will explore the use of HSPCs, embryonic stem cells (ESCs), and induced pluripotent stem cells (iPSCs) as sources for generating T cells, outline the challenges and advantages of the leading methods in the field for each source, followed by a deeper look into T cell engineering, with a primary focus on cancer immunotherapy.
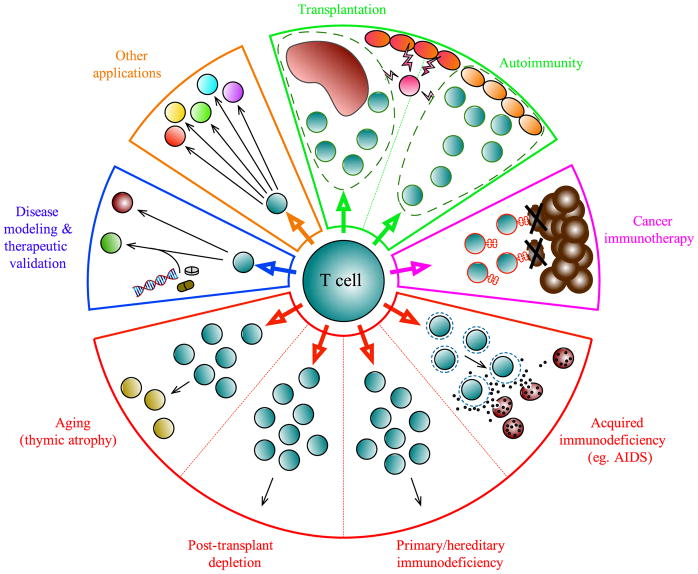
Figure 1. Therapeutic applications for T cell generation and expansion.
T cell-based therapeutics have uses for a broad range of clinical applications. Antigen-specific regulatory T cells (green) could be useful in preventing tissue damage caused by autoimmunity, or following organ transplantation. Tumor-antigen specific effector T cells (pink) isolated from the tumor and surrounding stroma or from peripheral blood can be expanded in vitro to target and eradicate cancer cells. Similarly, T cells can be engineered to express T cell receptor (TCR) or chimeric antigen receptor (CAR) with tumor eradication ability. In lymphopenia (red), when T cells are absent or insufficient to mount an immune response, as in acquired or primary immunodeficiencies (i.e., AIDS or PIDs), after hematopoietic stem cell transplant, or age-related lymphopenia due to thymic involution, T cell therapy can restore immune system function and prevent infections. While the conventional sources of HSPCs for transplant and research rely heavily on bone marrow and cord blood sources, induced pluripotent stem cells (iPSCs) have emerged as a promising source, bypassing issues of HLA-matching. Work pioneered by many groups has helped to develop different approaches for generation, expansion, and/or manipulation of T cells from HSPC sources in vitro in order to gain a deeper understanding of disease mechanisms and validate therapeutic approaches (blue).
Human T Cell Development
Our understanding of T cell development has long relied on the power of the methods and experimental tools available to study human T lymphopoiesis (Box 1). These protocols have traditionally relied on the use of human HSPCs (CD34+CD38−/lo), the hierarchical precursors of T cells, which can be easily isolated (Figure 2).
Box 1. Experimental Systems for Studying Human T Cell Development.
Human fetal thymic organ culture
Human fetal thymuses are isolated from electively terminated human fetuses or thymus tissue that is discarded during pediatric cardiac surgeries. Small thymic fragments are incubated at room temperature to reduce the number of endogenous thymocytes, directly microinjected with progenitor cells to allow for thymic colonization, and incubated on the surface of membranes for study of T cell development.
Hybrid fetal thymic organ culture
Fisher et al. were the first to establish this approach, which involves culture of human progenitors in mouse fetal thymuses. Thymus lobes are micro-dissected from fetal mice at days 14–15 of gestation. The thymic lobes are treated with 2-deoxyguanosine to remove endogenous thymocytes. T cell depleted mouse thymus lobes are reconstituted with human thymocyte progenitors (fetal thymic progenitors, postnatal thymocytes progenitors, fetal liver/UCB progenitors [73–78]) by hanging drop overnight. Lobes are then cultured as fetal thymic organ cultures, and can be manipulated by administration of exogenous cytokines and blocking agents. Flow cytometric analysis of single-cell suspensions permits the analysis of human T cell development.
OP9-DL System
Stem cells are seeded onto monolayers of Delta-like 1 or 4-expressing OP9 cells (OP9-DL1/OP9-DL4, respectively). Cells are induced to differentiate in the presence of exogenous interleukin-7 (IL-7), Fms-related tyrosine kinase 3-ligand (FLT3-L), and stem cell factor (SCF), which support early lymphopoiesis. The length of the culture period depends on the desired stage of T cell differentiation that is to be studied. This system is compatible with several types and sources of stem cells and progenitors, including UCB and BM HSPCs, as well as PSCs [17, 21, 38–43].
Humanized Mice
The study of development and function of the human immune system in vivo is largely restricted to humanized mice, which are immunodeficient mice engrafted with human hematopoietic cells or tissues, or transgenic mice expressing human genes. Several humanized mouse strains and engraftment protocols exist (reviewed in [79, 80]).
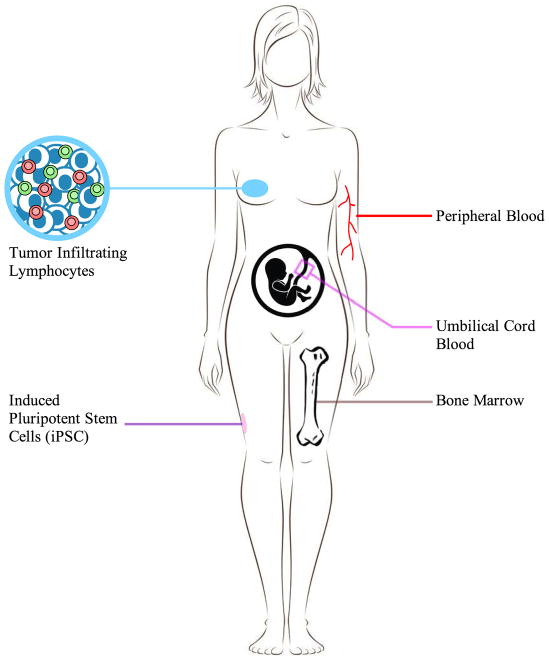
Figure 2. Sources for HSPC or T cell isolation.
HSPCs and T cells can be derived from various sites within the human body including peripheral blood, umbilical cord blood and bone marrow. Tumor-infiltrating lymphocytes can be isolated from cancerous tissues and be expanded in vitro. Induced pluripotent stem cells (iPSCs) are reprogrammed from isolated somatic cells, such as skin fibroblast or T cells, then differentiated into hematopoietic cells, and subsequently T cells in vitro.
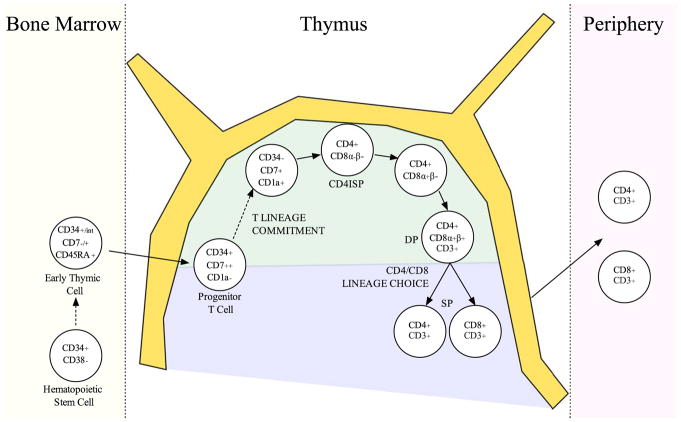
Human hematopoietic stem cells can be found within the lineage-marker negative (Lin−) CD45+CD34+CD38− subset of the BM. The human thymus starts to become colonized by blood-borne BM-progenitors by weeks 8–9 of gestation [9]. Although, the exact nature of the BM-derived thymus-seeding cell is unclear, candidate populations with T cell potential have been described: CD34+CD45RA+CD7+CD1a− [10], Lin−CD34+CD7− [11], and more recently, CD45+CD7+CD34int [9]. In response to intrathymic Notch signals, CD7 is up-regulated [10–12], and the subsequent acquisition of CD1a is strongly associated with T lineage commitment, with a concomitant diminished capacity to generate non-T lineage cells [12, 13]. Downstream of the CD34+CD7+CD1a+ stage, cells express CD4, but not yet CD8, and are termed CD4 intermediate single positive (ISP) cells. Following this stage, cells undergo proliferation and reach the CD4+CD8+ double positive (DP) stage. After positive and negative selection events, there is emigration of mature CD4+CD8− and CD4−CD8+ single positive (SP) T cells to the periphery (Figure 3).
Figure 3. Human T cell development in the thymus.
CD34+/int multipotent blood-borne progenitors reconstitute the thymus and pass through distinct stages that are distinguished based on cell surface marker expression and state of TCR rearrangements [9–13]. The earliest intrathymic cells are double negative for CD4 and CD8 expression. Upon induction of intrathymic Notch signals, human thymocytes up-regulate CD7 followed by CD5. The subsequent acquisition of CD1a marks commitment to the T lineage, and thymocytes lose their capacity to generate non-T lineage cells [12, 13]. Expression of CD4, but not CD8, on the cell surface occurs next, marking CD4 immature single positive (CD4ISP) cells. The CD4ISP then develops into a CD4+CD8+ double positive (DP) cell, with CD4+CD8α+β− early DPs preceding CD4+CD8α+β+ late DPs. Following expression of a successful TCRαβ heterodimer, thymocytes undergo positive and negative selection events in the cortex (green) and medulla (blue), respectively. Surviving mature CD4+CD8− and CD4−CD8+ single positive (SP) T cells migrate to the periphery.
Approaches for Generating T Cells
The mere fact that T cells require their own specialized organ for development, the thymus, serves to highlight the importance of niche in cellular specification. As a result, there have been many experimental attempts to mimic the thymic niche in order to generate T cells in vitro (reviewed in [14, 15]). This has resulted in several different protocols that provide a 2D and 3D structural environment to support T cell differentiation (Box 1). However, although the 2D culture approach has shown some promising results, as experimental model systems, feeder-free differentiation systems remain of special interest considering they are capable of producing T cells that can be used in a clinical setting.
OP9-DL Co-culture System
The OP9-DL co-culture method is a widely used and powerful tool for producing T cells in vitro (Box 1). In this system, HSPCs are co-cultured with OP9 cells ectopically expressing Dll1 (DL1) or Dll4 (DL4) to induce T cell development [16, 17]. A considerable advantage of this system is the ease in generating a large number of T cell progenitors for adoptive transfer, an insurmountable limitation of previous protocols. OP9-DL1 and OP9-DL4 cells co-cultured with UCB-HSPC yield similar results in terms of T cell differentiation outcomes [18]. In the OP9-DL system, UCB-HSPCs undergo T lineage differentiation, giving rise to CD34+CD7+ progenitor T (proT)-cells followed by continued differentiation into mature SP cells that are capable of responding to CD3/CD28 T cell receptor (TCR) stimulation [19]. Furthermore, the cells derived from this system resemble ex vivo-derived mature SPs by proliferation and pro-inflammatory effector cytokine production. Despite the robust generation of T cells, the OP9-DL co-culture system has some limitations. Given the lack of major histocompatibility antigen class II or CD1d expression by OP9 cells, there is no support for the selection of CD4+ T cells and NKT cells. Nevertheless, since functionally mature T cells including CD4+ and CD8+ were obtained from HSPCs by OP9-DL co-culture, there appears to be some level of positive selection that is induced by interaction between T precursor cells [20].
BM-derived HSPCs, and mobilized peripheral blood HSPCs reach similar developmental milestones on OP9-DL cells, although requiring longer timeframes and generating fewer CD1a+ T lineage committed progenitors [21]. Interestingly, using an alternative approach, HSPCs derived from the fetal thymus or liver arrest at the DP stage of development in the presence of Notch ligands in vitro [22]. It is likely that these differences are cell-intrinsic, highlighting the differential ability of various HSPC sources to generate T lineage cells on different inducing feeder cell systems (Table 1). Understanding the differences between various sources of HSPCs will provide insights into their clinical potential. Currently, many approaches are being established to overcome the need for rigorous selection processes in vitro towards the implementation of a clinically applicable system.
Table 1.
Generation of T Cells from Human Cell Sources
| Starting cell type | In vitro differentiation methods | Approx. fold expansion | Duration (weeks) | CD8+ T cells? | References |
|---|---|---|---|---|---|
| Cord blood HSC | OP9-DL co-culture | 10,000 | 7 | Yes | [17] |
| OP9-DL co-culture | 100,000 | 6 | Yes | [21] | |
| LSC-mDL1 co-culture | 50,000 | 10 | Yes | [22] | |
| Fibroblast-DL co-culture | 50,000,000 | 2 | No | [86] | |
| DL-coated ICC (feeder-free) | ~1.5 | 2 | Yes | [87] | |
| S17-DL co-culture | N.D. | 6 | Yes | [88] | |
| Conditioned medium and immobilized DL | N.D. | 3.5 | Yes | [27] | |
|
| |||||
| Bone marrow HSC | OP9-DL co-culture | 10,000 | 6 | Yes | [21] |
| LSC-mDL1 co-culture | 500 | 8 | Yes | [22] | |
| DL-coated microbeads | ~2 | 1 | N.D. | [26] | |
|
| |||||
| ESC | OP9-DL | N.D. | 4 | Yes | [38] |
|
| |||||
| iPSC | OP9-DL | ~25–150 | 4 | No | [38,43] |
Feeder Cell Free Systems
In order for these T cells to have a clinical application, there is a need for Notch-based feeder-cell-free systems capable of supporting T precursor generation as alternatives to xenogeneic OP9-DL cells. Varnum-Finney et al. demonstrated that immobilized Delta-like 1 (DL1)-Fc chimeric molecules, allowed for robust generation of T cell progenitors with in vivo thymus repopulating ability [23]. In another study, the immobilization of Delta-like-4 (DL4), another Notch ligand, supported enhanced in vitro generation of T cell progenitors from mouse HSPCs given moderate IL-7, while increased IL-7 favored self-renewal [24]. Importantly, similar approaches have been used to generate human T lineage cells with immobilized DL4-Fc chimeric molecules [25]. While this work begins to provide a basis for rapid translation of culture systems into clinical procedures for T progenitor production, other groups continue to explore alternative protocols. To this end, Taqvi et al enabled the generation of T cells from BM progenitors using DL4 immobilized on magnetic polystyrene beads [26] and others have focused on the generation of antigen-specific T cells to extend the potential for clinical applications further [27]. Fernandez et al utilized surface-immobilized DL1 and stromal cell conditioned medium to induce the development of CD1a+CD7+ committed T cell precursors, as well as CD4+CD8+ double positive cells from UCB-derived HSPCs. Further culture with cytomegalovirus or Influenza-A viral epitopes resulted in the generation of a polyclonal population of antigen-specific CD8+ T cells with cytolytic functionality. Although promising, the overall conclusions from these findings appear to be overreaching, and there remains a need to validate the ability to generate antigen-specific T cells using this approach.
In Vivo T Cell Reconstitution: In Vitro Generated Progenitor T Cells
Generation of mature T cells in vitro is limited, and their transplant into immunodeficient patients often leads to GVHD [28]. However, HSPC-derived T cell precursors, proT cells, undergo positive and negative selection on the recipient’s thymus, bypassing the need for selection in vitro, and overcoming the clinical challenge associated with GVHD. Furthermore, a key advantage of the use of proT cells for immunotherapy is that this strategy does not require strict histocompatibility between donors and recipients. In vitro-derived proT cells from the OP9-DL system engraft the thymus of humanized mice (Box 1) and develop into host-tolerant CD4+ and CD8+ mature polyclonal T lymphocytes [29]. In an allogeneic mouse transplant, adoptively transferred T cell precursors, transferred together with bone marrow, could give rise to improved T cell dependent immunity against infection, robust graft versus tumor activity, with an absolute lack of GVHD [30]. The use of proT cells during periods of immunodeficiency following HSPC transplantation is also well established [25, 31]. Use of immobilized DL4 was also used to differentiate human CD34+ HSPCs to proT cells, and these progenitors possessed the phenotypic and molecular signatures of immature thymic precursors [25]. Furthermore, upon xenotransplantation, they were capable of thymic settling and differentiation into mature polyclonal T cells, which provides encouraging prospects for future clinical applications.
Generating T Cells from Various Cell Sources
Hematopoietic Stem/Progenitor Cell Expansion Strategies
As highlighted during the first section of this review, HSPCs are a powerful source of T cell production. However, one of the challenges of using HSPCs as a T cell source is their rarity and limited cell recovery from a single donor [32–34]. As a result, groups have taken advantage of small molecules, including SR1 and UM171 [35, 36], which in combination with cytokine cocktails can expand HSPCs to a clinically relevant threshold in vitro. Culture of UCB-derived CD34+ cells in the presence of SR1 resulted in a 669-fold and 17,100-fold expansion in short-term and long-term cultures, respectively [35]. Pre-clinical engraftment of SR1-expanded UCB-HSPCs into immunodeficient mice demonstrated comparable reconstitution of peripheral T cells between mice transplanted with SR1-treated versus un-manipulated HSPCs [35], but the kinetics of T cell recovery have not been reported. Although the exact mechanism of action for SR1 remains elusive, SR1 (HSC835)-expanded UCB-HSPCs are being evaluated in a clinical trial sponsored by Novartis [37]. The T cell potential of SR1-derived progenitors also warrants further investigation.
Harnessing the Potential of Pluripotent Stem Cells for T Cell Generation
While HSPCs have long been used as the conventional source for T cell generation, several groups have shown that PSCs can indeed generate T cells in vitro [38–43]. However, the acquisition of this fate from PSCs remains inefficient in comparison to UCB or BM progenitors [44], resulting in low T cell yields in vitro. Additionally, PSC-derived CD34+ progenitors and their downstream outcomes differ from their UCB-derived counterparts, by a reduced proliferative and/or self-renewal capacity. This is thought to be, in part, a result of the activation of pathways in PSC-derived progenitors that are inhibitory to T lineage induction [45].
In order to make T cells from PSCs in vitro, it is necessary to replicate the many sequential milestones that PSCs must reach before gaining T cell potential. Namely, the PSC must first be induced to the mesoderm fate, then instructed to produce CD34+ hemogenic endothelial cells that will round up in morphology and gain the hematopoietic marker CD45 in a process known as endothelial-to-hematopoietic transition [46]. However, making a CD45+ hematopoietic cell in vitro from PSCs does not guarantee T cell potential. There are two waves of hematopoiesis that occur in the mammalian embryo – the primitive and definitive, which amongst many other differing characteristics also contain differing lineage potential. Definitive hematopoiesis follows the primitive wave and is the only hematopoietic program that contains T cell potential [38]. So adding to the complexity of reproducing the many stages of hematopoietic development in vitro, in order to create progenitors with T cell potential, the definitive hematopoietic program must be specified throughout differentiation.
Kennedy et al. used PSCs to generate embryoid bodies (EB) that were directed towards CD34+ HSPCs, which were then isolated and co-cultured to generate T-lineage cells using the OP9-DL system [38]. The EB method provides a defined, serum-free differentiation system to produce T cells [38, 39]. Within this system, PSCs are aggregated into 10–15 cell spheric clusters. During differentiation, growth factors are added to skew the developmental outcome of the PSC to a T cell fate. Although many of the xenogenic concerns present in serum- or cell-based differentiation systems are circumvented using this method, it too poses its own sets of challenges. The highly precise nature of the work makes it very complex to use and amongst other variables, the success of the differentiation is highly dependent upon the physical robustness and size of the EB spheres, which are formed manually and are therefore susceptible to variability.
Although these techniques have been in use for a number of years now, the T cell yield remains low mostly due to difficulties in generating a bona fide HSPC in vitro. It has been shown that inhibition of Activin/Nodal or Wnt signaling during mesoderm specification favours the induction of the definitive hematopoietic program, thus creating a hematopoietic progenitor that is enriched in T cell potential [38, 39]. However, large-scale production of T cells from PSCs will be dependent on a better understanding of the signals needed to specify definitive self-renewing HSPCs with T cell potential in vitro.
Induced Pluripotent Stem Cells as a T Cell Source: Challenges and Potential
As the pivotal discovery of iPSCs was made [47], the opportunity opened up for generating a boundless supply of T cells from patient-derived iPSCs. iPSCs provide a limitless source of pluripotent stem cells that are free of ethical concerns due to their non-embryonic origin. Additionally, the use of iPSCs bypasses the issue of HLA-matching as they are patient-derived, and also allows for the generation of T cells from patients who are completely T cell deficient, since any somatic cell can be corrected and reprogrammed, although it should be noted that T cell generation from non-T cell sourced iPSCs is far less efficient than from T cell-derived iPSCs. Still, this enables the study of T cells in their normal and aberrant forms, elucidating novel disease mechanisms [43] and validating gene-correction therapies in JAK3- and IL-2Rγ-deficient iPSCs by gene-specific correction using CRISPR or TALEN-mediated approaches [41, 42]. In another study involving IL-2R-γ deficiency, CD34+ HSPCs of patients, for which there was no suitable HLA identical donor, received γ-retroviral treatment [48]. While the long-term effects have not yet been established, this report suggests superior T cell development stemming from these cells when compared to patients that received a haploidentical human stem cell transplantation, bringing to light that gene-correction of patient-derived cells as a preferential practice in the future.
However, the efficiency with which fibroblast-derived iPSCs convert to the T cell fate, even when compared to ESCs, has been low. Kim et al. showed that mouse iPSCs derived from non-hematopoietic somatic cells did not adopt the hematopoietic lineage as robustly as iPSCs of hematopoietic origin [49]. Additionally, the loci of many hematopoietic genes were methylated and therefore silenced in neural or fibroblast derived-iPSCs, with methylation patterns even distinguishing the myeloid or lymphoid origin of the iPSC. When similar comparisons were made with human iPSCs of UCB or of neonatal keratinocyte origin, again, residual epigenetic memory affecting differentiation potential and outcome was detected [50].
Considering the importance of iPSC generation, strangely, not much is known about the processes that govern the reprogramming of a somatic cell. To address this lapse in literature, Project Grandiose culminated in back-to-back papers describing the existence of a new murine pluripotent stem cell – the fuzzy iPSC – that was markedly different from traditional flat, compact iPSCs or ESCs [51–55]. These F-class iPSCs, normally discarded, show a propensity to respond well to large-scale production due to increased proliferation rates and low adhesiveness. However, F-class iPSCs are highly susceptible to differentiation and require sustained reprogramming factor expression to maintain their pluripotency. A similar cell type displaying signs of an alternative pluripotent state was isolated from human ESCs and called region-selective PSC [56]. Region-selective PSCs showed greater cloning efficiency, allowing for ease of genetic manipulation, and tolerance of large-scale cell production. Although much remains to be discovered about these cells, including their T cell potential, they remain an interesting future avenue of research.
Tumor Infiltrating Lymphocytes and Beyond
Considering the difficulty in generating T cells de novo from PSC sources, attempts have been made to isolate, manipulate and utilize mature T cells from patients. TILs can be found in several solid tumors, including in gastrointestinal [57] and pancreatic cancers [58] and their presence correlates with a more positive clinical outcome. Therefore, the methodology used for the isolation, ex vivo expansion and reintroduction of TILs to the patient provides a compelling option for cancer treatment.
However, not all antigen-specific T cells need to be TILs. Carluccio et al. isolated and expanded tumor-specific T cells from peripheral blood [59]. While isolation of these T cells provides a method to expand these cells in vitro for adoptive immunotherapy, iPSCs provide a source for expansion of antigen-specific T cells (reviewed in [28, 60]). To this end, Saito et al. have reprogrammed TILs isolated from melanoma to generate iPSCs [61].
Although applications of custom antigen-specific TCRs have primarily moved towards tools for cancer immunotherapeutics, their potential uses appear, as the TCR repertoire itself, seemingly boundless. Efforts by van Lent et al demonstrate that human antigen-specific TCRs can be retrovirally introduced into CD34+ CD1a− HSPCs and yield mature, functional T cells with specificity against melanoma, viral, and minor histocompatability antigens [62]. In a study stimulating human PBMCs with anti-4-1BB and viral peptide, virus-specific T cells were obtained [63]. Introduction of adenovirus specific TCR into α/β and γ/δ T cells was proposed as a treatment against infection post allogeneic HSCT [64]. These, and other studies, extend the range of applications for custom-tailored antigen-specific T cells well beyond their use as cancer immunotherapeutics, and allows for merging with newly emerging technologies.
Clinical Potential and Application of T Cells
Induced Pluripotent Stem Cell-Derived T Cell Immunotherapy
Using mature CD8+ single positive T cells as the source of iPSCs, several groups [65, 66] elegantly demonstrated the therapeutic potential of iPSC-derived antigen-specific T cells. Mature CD8+ T cells specific for the melanoma-associated antigen MART-1 were converted into iPSCs. These CD8+ MART-1 reactive iPSCs were then differentiated on OP9 and OP9-DL1 cell co-cultures to the DP stage and stimulated to expand the iPSC-derived CD8+CD3+ MART-1 reactive T cells. Furthermore, these CD8+CD3+MART-1+ cells were tested for maturity and functionality by assessing interferon-γ production after stimulation. Crucial to the concept of antigen-specific immunotherapeutics, these newly regenerated T cells showed antigen-specific activation in vitro. These published findings demonstrate that T cells generated from T cell-derived iPSCs retain their original TCR, and that by reverting the cells to an iPSC state, the potential for large-scale production of T cells bearing a TCR of interest is gained. However, all functional assays were restricted to an in vitro setting and did not yet show any clinical correlates or whether the killing capacity of these MART-1-specific T cells would be sustained in vivo, particularly within the immunosuppressive tumor microenvironment that is known to promote T cell dysfunction.
Chimeric Antigen Receptor Modified T Cells
The use of TIL therapy and of antigen-specific T cells as sources for iPSCs is predicated on the successful detection, isolation, cloning and expansion of patient-derived T cells expressing a TCR of interest. The challenges faced in the isolation and handling of these rare populations expressing valuable TCRs were circumvented with the advent of chimeric antigen receptors (CARs) and their ability to manipulate both patient-derived and de novo generated T cells [67] (Box 2).
Box 2. Genetic Engineering of T cells for Therapeutic Use.
Chimeric antigen receptors (CARs)
CARs are single polypeptide chains composed of an extracellular immunoglobulin-derived single-chain variable fragment that activates the T cell via the intracellular CD3ζ signaling chain [67]. This allows for T cell activation in an HLA-independent fashion, where the antigen is directly recognized by the immunoglobulin-derived portion but exerts downstream T cell functions.
Generations of CAR technology
There have been many modifications of the CAR technology, with “second-generation” CARs containing a co-stimulatory domain such as CD28, CD134 or CD137 to the signaling part in order to promote T cell activation and survival [81, 82], and “third-generation” CARs with two co-stimulatory domains flanking CD3ζ domain thus maximizing T cell function in the absence of any co-stimulation from the antigen-presenting cells [83].
It was the second-generation CARs, containing the CD137 co-stimulatory domain that were used in the first report of successful leukemia treatment using patient-isolated T cells engineered to express CD19-specific CARs, a marker abundantly expressed in B-cell leukemia [68].
TRUCK T cells: adaptive T cell immunotherapy rallies the innate system
The “fourth-generation” CARs, named TRUCKs, attempts to address previous concerns about the applicability of this therapy in solid tumours, which often display a high level of heterogeneity, or even in leukemic malignancies where expression of the targeted antigen, such as CD19, may be down-regulated in certain cells. In this scenario, the specificity of this therapy, often cited as a benefit, can also narrow the scope of treatment and render the patient susceptible to either relapse from non-antigen expressing cancer cells or may not achieve complete remission due to the cellular heterogeneity of the tumour.
In response to this setback, CAR T cells are engineered to carry an IL-12 payload, and upon CAR T cell activation, in addition to lysing their targets they also release IL-12, attracting neighbouring innate cells and thus mounting a secondary immune response to the tumour while simultaneously invigorating their own activation [84, 85]. The strength of this technology lies in harnessing the targeting specificity of the CAR by directing the CAR T cells to the site of interest, and then once in the tumour, delivering signals needed to recruit immune cells that will attack cancerous cells in a non-antigen specific manner. However, the increased might of these cells can be a double-edged sword, as the more powerful CAR T cells become, so does the toxicity of any “on-target, off-tumour” effects, where the targeted antigen is expressed on non-cancerous cells.
Patient-isolated CAR-modified T cells were used in the first report of successful leukemia treatment in humans [68] demonstrating the expansion and persistence of CAR-modified T cells in vivo. Furthermore, clinical trials of anti-CD19 CAR-modified T cells showed complete remission in patients with acute or chronic lymphoblastic leukemia [68–71].
Chimeric Antigen Receptors Meet Induced Pluripotent Stem Cell Technology
Considering the success of CARs, Themeli et al. considered whether the power of this approach could be augmented by coupling it with iPSC technology [72]. Whereas most CAR therapeutics rely on patient-derived peripheral T cells to be manipulated into CAR-expressing T cells, this report utilized iPSC technology to provide a limitless supply of anti-CD19 CAR-modified iPSC-derived T cells for patient infusions. Peripheral blood T cells were converted to iPSCs and then these T cell-derived iPSCs were modified to express CD19-specific CARs and re-differentiated to the T cell fate. Anti-CD19 CAR iPSC-derived T cells transplanted into humanized immunodeficient mice with CD19+ lymphoma cells showed significant anti-tumor activity and increased survival when compared to the untreated mice. Additionally, mice that received anti-CD19 CAR iPSC-derived T cells showed similar results to those receiving anti-CD19 CAR αβ or γδ T cells. These data show that CAR-modified iPSC-derived T cells possess the same functionality and anti-tumor activity as CAR-modified peripheral blood-isolated T cells, giving promise for future combined iPSC and CAR treatments.
Concluding Remarks
The field of T cell development has rapidly progressed and expanded to incorporate new sources and applications thanks to the refinement of T cell generation strategies. To date, the most reliable source of T cell generation in vitro still remains the HSPC. However, one of the most significant limitations of HSPC-based T cell production is the small number of HSPCs that can be harvested from individuals. Major strides have been made in the field to address this problem by focusing on the expansion of HSPCs through the use of small molecules like SR1/UM171.
After HSPCs, one of the most promising sources of T cell generation is the PSC. The potential of these cells for clinical as well as academic study of T cell development is impressive. However, one of the largest obstacles in using PSCs as robust sources of T lymphopoiesis remains our lack of understanding of how to truly recapitulate hematopoietic development in vitro. In order to be able to generate T cells from PSC-derived precursors, one must first understand how to generate a physiologically relevant and stable HSPC in vitro.
iPSC-derived T lymphopoiesis faces similar impediments as ESC-derived T lymphopoiesis, however, this difficulty is compounded by the preservation of the epigenetic memory of these cells. De-methylating agents are currently being explored as potential solutions to this problem. However, it is important to note that T cell-derived iPSCs produce abundant T cell progeny with full functionality. In fact, iPSC-derived T cells, combined with other gene editing technologies, have already shown anti-tumor immunogenicity and may become a powerful immunotherapeutic tool in the clinic.
Outstanding Questions.
Will proT cell injection prove to be clinically relevant for improving the success of HSPC engraftment?
Cell-free in vitro systems would represent an important tool for the clinical production of T cells. What are the niche requirements that need to be fulfilled to efficiently support T lineage differentiation?
SR1 allows the mobilization of a clinically relevant number of HPSCs. What is the mechanism behind SR1-mediated HSPC expansion? Is the T lineage capacity of these cells comparable to non-manipulated HSPCs?
How can PSC (ESC and iPSC) differentiation be improved to allow for T cell yields and outcomes comparable to HSPCs (CB or BM CD34+ progenitors)?
Can PSCs be manipulated in vitro to generate bona fide CD34+ HSPCs capable of engraftment?
Will iPSC technology prove to be safe and effective for therapeutic applications, generally and in the specific case of T cell production?
Trends.
The generation of T cells in vitro is an attractive tool to produce therapeutic agents in cancer immunotherapy and immunodeficiencies.
Better in vitro 3D, 2D, and particularly feeder-free systems could provide clinically relevant methods to generate and expand T cells for therapeutic applications.
Injection of T cell progenitors may potentially enhance the production of new donor-derived T cells following hematopoietic stem cell transplant (HSCT).
Genetic engineering of patient-derived or induced pluripotent stem cells (iPSC)-derived T cells with chimeric antigen receptors specific for tumor antigens has shown remarkable clinical promise in cancer immunotherapy.
Autologous cell types, such as iPSCs, may overcome histocompatibility issues, although their potential to generate T cells, is currently limited.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Russell SM, et al. Mutation of Jak3 in a patient with SCID: essential role of Jak3 in lymphoid development. Science. 1995;270:797–800. doi: 10.1126/science.270.5237.797. [DOI] [PubMed] [Google Scholar]
- 2.Noguchi M, et al. Interleukin-2 receptor gamma chain mutation results in X-linked severe combined immunodeficiency in humans. Cell. 1993;73:147–157. doi: 10.1016/0092-8674(93)90167-o. [DOI] [PubMed] [Google Scholar]
- 3.Macchi P, et al. Mutations of Jak-3 gene in patients with autosomal severe combined immune deficiency (SCID) Nature. 1995;377:65–68. doi: 10.1038/377065a0. [DOI] [PubMed] [Google Scholar]
- 4.Notarangelo LD. Primary immunodeficiencies. The Journal of allergy and clinical immunology. 2010;125:S182–194. doi: 10.1016/j.jaci.2009.07.053. [DOI] [PubMed] [Google Scholar]
- 5.Storek J, et al. Infectious morbidity in long-term survivors of allogeneic marrow transplantation is associated with low CD4 T cell counts. American journal of hematology. 1997;54:131–138. doi: 10.1002/(sici)1096-8652(199702)54:2<131::aid-ajh6>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 6.Storek J, et al. Reconstitution of the immune system after hematopoietic stem cell transplantation in humans. Seminars in immunopathology. 2008;30:425–437. doi: 10.1007/s00281-008-0132-5. [DOI] [PubMed] [Google Scholar]
- 7.Vesely MD, Schreiber RD. Cancer immunoediting: antigens, mechanisms, and implications to cancer immunotherapy. Annals of the New York Academy of Sciences. 2013;1284:1–5. doi: 10.1111/nyas.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morgan RA, et al. Recognition of glioma stem cells by genetically modified T cells targeting EGFRvIII and development of adoptive cell therapy for glioma. Human gene therapy. 2012;23:1043–1053. doi: 10.1089/hum.2012.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farley AM, et al. Dynamics of thymus organogenesis and colonization in early human development. Development. 2013;140:2015–2026. doi: 10.1242/dev.087320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haddad R, et al. Dynamics of thymus-colonizing cells during human development. Immunity. 2006;24:217–230. doi: 10.1016/j.immuni.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Hao QL, et al. Human intrathymic lineage commitment is marked by differential CD7 expression: identification of CD7− lympho-myeloid thymic progenitors. Blood. 2008;111:1318–1326. doi: 10.1182/blood-2007-08-106294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spits H. Development of alphabeta T cells in the human thymus. Nature reviews. Immunology. 2002;2:760–772. doi: 10.1038/nri913. [DOI] [PubMed] [Google Scholar]
- 13.Taghon T, et al. Notch signaling during human T cell development. Current topics in microbiology and immunology. 2012;360:75–97. doi: 10.1007/82_2012_230. [DOI] [PubMed] [Google Scholar]
- 14.Dellatore SM, et al. Mimicking stem cell niches to increase stem cell expansion. Current opinion in biotechnology. 2008;19:534–540. doi: 10.1016/j.copbio.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roh KH, Roy K. Engineering approaches for regeneration of T lymphopoiesis. Biomaterials research. 2016;20:20. doi: 10.1186/s40824-016-0067-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmitt TM, Zuniga-Pflucker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17:749–756. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- 17.La Motte-Mohs RN, et al. Induction of T-cell development from human cord blood hematopoietic stem cells by Delta-like 1 in vitro. Blood. 2005;105:1431–1439. doi: 10.1182/blood-2004-04-1293. [DOI] [PubMed] [Google Scholar]
- 18.Besseyrias V, et al. Hierarchy of Notch-Delta interactions promoting T cell lineage commitment and maturation. The Journal of experimental medicine. 2007;204:331–343. doi: 10.1084/jem.20061442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Awong G, et al. Human CD8 T cells generated in vitro from hematopoietic stem cells are functionally mature. BMC immunology. 2011;12:22. doi: 10.1186/1471-2172-12-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Coppernolle S, et al. Functionally mature CD4 and CD8 TCRalphabeta cells are generated in OP9-DL1 cultures from human CD34+ hematopoietic cells. Journal of immunology. 2009;183:4859–4870. doi: 10.4049/jimmunol.0900714. [DOI] [PubMed] [Google Scholar]
- 21.De Smedt M, et al. T-lymphoid differentiation potential measured in vitro is higher in CD34+CD38−/lo hematopoietic stem cells from umbilical cord blood than from bone marrow and is an intrinsic property of the cells. Haematologica. 2011;96:646–654. doi: 10.3324/haematol.2010.036343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel E, et al. Diverse T-cell differentiation potentials of human fetal thymus, fetal liver, cord blood and adult bone marrow CD34 cells on lentiviral Delta-like-1-modified mouse stromal cells. Immunology. 2009;128:e497–505. doi: 10.1111/j.1365-2567.2008.03013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varnum-Finney B, et al. Combined effects of Notch signaling and cytokines induce a multiple log increase in precursors with lymphoid and myeloid reconstituting ability. Blood. 2003;101:1784–1789. doi: 10.1182/blood-2002-06-1862. [DOI] [PubMed] [Google Scholar]
- 24.Ikawa T, et al. An essential developmental checkpoint for production of the T cell lineage. Science. 2010;329:93–96. doi: 10.1126/science.1188995. [DOI] [PubMed] [Google Scholar]
- 25.Reimann C, et al. Human T-lymphoid progenitors generated in a feeder-cell-free Delta-like-4 culture system promote T-cell reconstitution in NOD/SCID/gammac(−/−) mice. Stem cells. 2012;30:1771–1780. doi: 10.1002/stem.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taqvi S, et al. Biomaterial-based notch signaling for the differentiation of hematopoietic stem cells into T cells. Journal of biomedical materials research. Part A. 2006;79:689–697. doi: 10.1002/jbm.a.30916. [DOI] [PubMed] [Google Scholar]
- 27.Fernandez I, et al. Generation of functional, antigen-specific CD8+ human T cells from cord blood stem cells using exogenous Notch and tetramer-TCR signaling. Stem cells. 2014;32:93–104. doi: 10.1002/stem.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Themeli M, et al. New cell sources for T cell engineering and adoptive immunotherapy. Cell stem cell. 2015;16:357–366. doi: 10.1016/j.stem.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Awong G, et al. Characterization in vitro and engraftment potential in vivo of human progenitor T cells generated from hematopoietic stem cells. Blood. 2009;114:972–982. doi: 10.1182/blood-2008-10-187013. [DOI] [PubMed] [Google Scholar]
- 30.Zakrzewski JL, et al. Adoptive transfer of T-cell precursors enhances T-cell reconstitution after allogeneic hematopoietic stem cell transplantation. Nature medicine. 2006;12:1039–1047. doi: 10.1038/nm1463. [DOI] [PubMed] [Google Scholar]
- 31.Awong G, et al. Human proT-cells generated in vitro facilitate hematopoietic stem cell-derived T-lymphopoiesis in vivo and restore thymic architecture. Blood. 2013;122:4210–4219. doi: 10.1182/blood-2012-12-472803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bari S, et al. Expansion and homing of umbilical cord blood hematopoietic stem and progenitor cells for clinical transplantation. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2015;21:1008–1019. doi: 10.1016/j.bbmt.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 33.Lund TC, et al. Advances in umbilical cord blood manipulation-from niche to bedside. Nature reviews. Clinical oncology. 2015;12:163–174. doi: 10.1038/nrclinonc.2014.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Broxmeyer HE. Enhancing the efficacy of engraftment of cord blood for hematopoietic cell transplantation. Transfusion and apheresis science: official journal of the World Apheresis Association: official journal of the European Society for Haemapheresis. 2016;54:364–372. doi: 10.1016/j.transci.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boitano AE, et al. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science. 2010;329:1345–1348. doi: 10.1126/science.1191536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fares I, et al. Cord blood expansion. Pyrimidoindole derivatives are agonists of human hematopoietic stem cell self-renewal. Science. 2014;345:1509–1512. doi: 10.1126/science.1256337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wagner JE, Jr, et al. Phase I/II Trial of StemRegenin-1 Expanded Umbilical Cord Blood Hematopoietic Stem Cells Supports Testing as a Stand-Alone Graft. Cell stem cell. 2016;18:144–155. doi: 10.1016/j.stem.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kennedy M, et al. T lymphocyte potential marks the emergence of definitive hematopoietic progenitors in human pluripotent stem cell differentiation cultures. Cell reports. 2012;2:1722–1735. doi: 10.1016/j.celrep.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 39.Sturgeon CM, et al. Wnt signaling controls the specification of definitive and primitive hematopoiesis from human pluripotent stem cells. Nature biotechnology. 2014;32:554–561. doi: 10.1038/nbt.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang CW, et al. Broad T-cell receptor repertoire in T-lymphocytes derived from human induced pluripotent stem cells. PloS one. 2014;9:e97335. doi: 10.1371/journal.pone.0097335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang CW, et al. Modeling Human Severe Combined Immunodeficiency and Correction by CRISPR/Cas9-Enhanced Gene Targeting. Cell reports. 2015;12:1668–1677. doi: 10.1016/j.celrep.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 42.Menon T, et al. Lymphoid regeneration from gene-corrected SCID-X1 subject-derived iPSCs. Cell stem cell. 2015;16:367–372. doi: 10.1016/j.stem.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brauer PM, et al. Modeling altered T-cell development with human induced pluripotent stem cells from patients with RAG1 mutations and distinct immunological phenotypes. Blood. 2016 doi: 10.1182/blood-2015-10-676304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fleming HE, Scadden DT. Embryonic stem cells make human T cells. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:12213–12214. doi: 10.1073/pnas.0605344103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martin CH, et al. Differences in lymphocyte developmental potential between human embryonic stem cell and umbilical cord blood-derived hematopoietic progenitor cells. Blood. 2008;112:2730–2737. doi: 10.1182/blood-2008-01-133801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ditadi A, Sturgeon CM. Directed differentiation of definitive hemogenic endothelium and hematopoietic progenitors from human pluripotent stem cells. Methods. 2016;101:65–72. doi: 10.1016/j.ymeth.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 47.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 48.Touzot F, et al. CD45RA depletion in HLA-mismatched allogeneic hematopoietic stem cell transplantation for primary combined immunodeficiency: A preliminary study. The Journal of allergy and clinical immunology. 2015;135:1303–1309. e1301–1303. doi: 10.1016/j.jaci.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 49.Kim K, et al. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467:285–290. doi: 10.1038/nature09342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim K, et al. Donor cell type can influence the epigenome and differentiation potential of human induced pluripotent stem cells. Nature biotechnology. 2011;29:1117–1119. doi: 10.1038/nbt.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tonge PD, et al. Divergent reprogramming routes lead to alternative stem-cell states. Nature. 2014;516:192–197. doi: 10.1038/nature14047. [DOI] [PubMed] [Google Scholar]
- 52.Hussein SM, et al. Genome-wide characterization of the routes to pluripotency. Nature. 2014;516:198–206. doi: 10.1038/nature14046. [DOI] [PubMed] [Google Scholar]
- 53.Lee DS, et al. An epigenomic roadmap to induced pluripotency reveals DNA methylation as a reprogramming modulator. Nature communications. 2014;5:5619. doi: 10.1038/ncomms6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clancy JL, et al. Small RNA changes en route to distinct cellular states of induced pluripotency. Nature communications. 2014;5:5522. doi: 10.1038/ncomms6522. [DOI] [PubMed] [Google Scholar]
- 55.Benevento M, et al. Proteome adaptation in cell reprogramming proceeds via distinct transcriptional networks. Nature communications. 2014;5:5613. doi: 10.1038/ncomms6613. [DOI] [PubMed] [Google Scholar]
- 56.Wu J, et al. An alternative pluripotent state confers interspecies chimaeric competency. Nature. 2015;521:316–321. doi: 10.1038/nature14413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tran E, et al. Immunogenicity of somatic mutations in human gastrointestinal cancers. Science. 2015;350:1387–1390. doi: 10.1126/science.aad1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meng Q, et al. Expansion of Tumor-reactive T Cells From Patients With Pancreatic Cancer. Journal of immunotherapy. 2016;39:81–89. doi: 10.1097/CJI.0000000000000111. [DOI] [PubMed] [Google Scholar]
- 59.Carluccio S, et al. Generation of tumor-specific cytotoxic T-lymphocytes from the peripheral blood of colorectal cancer patients for adoptive T-cell transfer. Journal of cellular physiology. 2015;230:1457–1465. doi: 10.1002/jcp.24886. [DOI] [PubMed] [Google Scholar]
- 60.Rami F, et al. Induced Pluripotent Stem Cell as a New Source for Cancer Immunotherapy. Genetics research international. 2016;2016:3451807. doi: 10.1155/2016/3451807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saito H, et al. Reprogramming of Melanoma Tumor-Infiltrating Lymphocytes to Induced Pluripotent Stem Cells. Stem cells international. 2016;2016:8394960. doi: 10.1155/2016/8394960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Lent AU, et al. Functional human antigen-specific T cells produced in vitro using retroviral T cell receptor transfer into hematopoietic progenitors. Journal of immunology. 2007;179:4959–4968. doi: 10.4049/jimmunol.179.8.4959. [DOI] [PubMed] [Google Scholar]
- 63.Imahashi N, et al. Simple and efficient generation of virus-specific T cells for adoptive therapy using anti-4-1BB antibody. Journal of immunotherapy. 2015;38:62–70. doi: 10.1097/CJI.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 64.Dorrie J, et al. Human adenovirus-specific gamma/delta and CD8+ T cells generated by T-cell receptor transfection to treat adenovirus infection after allogeneic stem cell transplantation. PloS one. 2014;9:e109944. doi: 10.1371/journal.pone.0109944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vizcardo R, et al. Regeneration of human tumor antigen-specific T cells from iPSCs derived from mature CD8(+) T cells. Cell stem cell. 2013;12:31–36. doi: 10.1016/j.stem.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 66.Nishimura T, et al. Generation of rejuvenated antigen-specific T cells by reprogramming to pluripotency and redifferentiation. Cell stem cell. 2013;12:114–126. doi: 10.1016/j.stem.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 67.Gross G, et al. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:10024–10028. doi: 10.1073/pnas.86.24.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kalos M, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Science translational medicine. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brentjens RJ, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–4828. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kochenderfer JN, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–2720. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brentjens RJ, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Science translational medicine. 2013;5:177ra138. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Themeli M, et al. Generation of tumor-targeted human T lymphocytes from induced pluripotent stem cells for cancer therapy. Nature biotechnology. 2013;31:928–933. doi: 10.1038/nbt.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fisher AG, et al. Human thymocyte development in mouse organ cultures. International immunology. 1990;2:571–578. doi: 10.1093/intimm/2.6.571. [DOI] [PubMed] [Google Scholar]
- 74.Merkenschlager M, Fisher AG. CD45 isoform switching precedes the activation-driven death of human thymocytes by apoptosis. International immunology. 1991;3:1–7. doi: 10.1093/intimm/3.1.1. [DOI] [PubMed] [Google Scholar]
- 75.Plum J, et al. In vitro intrathymic differentiation kinetics of human fetal liver CD34+CD38− progenitors reveals a phenotypically defined dendritic/T-NK precursor split. Journal of immunology. 1999;162:60–68. [PubMed] [Google Scholar]
- 76.Yeoman H, et al. Human bone marrow and umbilical cord blood cells generate CD4+ and CD8+ single-positive T cells in murine fetal thymus organ culture. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:10778–10782. doi: 10.1073/pnas.90.22.10778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Robin C, et al. Identification of human T-lymphoid progenitor cells in CD34+ CD38low and CD34+ CD38+ subsets of human cord blood and bone marrow cells using NOD-SCID fetal thymus organ cultures. British journal of haematology. 1999;104:809–819. doi: 10.1046/j.1365-2141.1999.01266.x. [DOI] [PubMed] [Google Scholar]
- 78.Robin C, et al. Identification of lymphomyeloid primitive progenitor cells in fresh human cord blood and in the marrow of nonobese diabetic-severe combined immunodeficient (NOD-SCID) mice transplanted with human CD34(+) cord blood cells. The Journal of experimental medicine. 1999;189:1601–1610. doi: 10.1084/jem.189.10.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brehm MA, et al. Humanized mouse models to study human diseases. Current opinion in endocrinology, diabetes, and obesity. 2010;17:120–125. doi: 10.1097/MED.0b013e328337282f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ito R, et al. Current advances in humanized mouse models. Cellular & molecular immunology. 2012;9:208–214. doi: 10.1038/cmi.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bridgeman JS, et al. Building better chimeric antigen receptors for adoptive T cell therapy. Current gene therapy. 2010;10:77–90. doi: 10.2174/156652310791111001. [DOI] [PubMed] [Google Scholar]
- 82.Hoyos V, et al. Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia. 2010;24:1160–1170. doi: 10.1038/leu.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hombach AA, Abken H. Costimulation by chimeric antigen receptors revisited the T cell antitumor response benefits from combined CD28-OX40 signalling. International journal of cancer. 2011;129:2935–2944. doi: 10.1002/ijc.25960. [DOI] [PubMed] [Google Scholar]
- 84.Chmielewski M, et al. IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively Muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer research. 2011;71:5697–5706. doi: 10.1158/0008-5472.CAN-11-0103. [DOI] [PubMed] [Google Scholar]
- 85.Zhang L, et al. Improving adoptive T cell therapy by targeting and controlling IL-12 expression to the tumor environment. Molecular therapy: the journal of the American Society of Gene Therapy. 2011;19:751–759. doi: 10.1038/mt.2010.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mohtashami M, et al. Induction of T-cell development by Delta-like 4-expressing fibroblasts. International immunology. 2013;25:601–611. doi: 10.1093/intimm/dxt027. [DOI] [PubMed] [Google Scholar]
- 87.Poznansky MC, et al. Efficient generation of human T cells from a tissue-engineered thymic organoid. Nature biotechnology. 2000;18:729–734. doi: 10.1038/77288. [DOI] [PubMed] [Google Scholar]
- 88.Jaleco AC, et al. Differential effects of Notch ligands Delta-1 and Jagged-1 in human lymphoid differentiation. The Journal of experimental medicine. 2001;194:991–1002. doi: 10.1084/jem.194.7.991. [DOI] [PMC free article] [PubMed] [Google Scholar]