Abstract
The core symptoms of autism spectrum disorder are poorly treated with current medications. Symptoms of autism spectrum disorder are frequently comorbid with a diagnosis of epilepsy and vice versa. Medically-supervised ketogenic diets are remarkably effective nonpharmacological treatments for epilepsy, even in drug-refractory cases. There is accumulating evidence that supports the efficacy of ketogenic diets in treating the core symptoms of autism spectrum disorders in animal models as well as limited reports of benefits in patients. This study tests the behavioral effects of ketogenic diet feeding in the EL mouse, a model with behavioral characteristics of autism spectrum disorder and comorbid epilepsy. Male and female EL mice were fed control diet or one of two ketogenic diet formulas ad libitum starting at 5 weeks of age. Beginning at 8 weeks of age, diet protocols continued and performance of each group on tests of sociability and repetitive behavior was assessed. A ketogenic diet improved behavioral characteristics of autism spectrum disorder in a sex- and test-specific manner; ketogenic diet never worsened relevant behaviors. Ketogenic diet feeding improved multiple measures of sociability and reduced repetitive behavior in female mice, with limited effects in males. Additional experiments in female mice showed that a less strict, more clinically-relevant diet formula was equally effective in improving sociability and reducing repetitive behavior. Taken together these results add to the growing number of studies suggesting that ketogenic and related diets may provide significant relief from the core symptoms of autism spectrum disorder, and suggest that in some cases there may be increased efficacy in females.
Keywords: Autism spectrum disorder, β-Hydroxybutyrate, EL mouse, Glucose, Grooming, Female, Ketogenic diet, Ketosis, Comorbidity, Sex differences, Sociability, Social transmission of food preference
1. Introduction
Autism spectrum disorder (ASD) is frequently comorbid with epilepsy, with 7%–21% of children diagnosed with ASD also having a diagnosis of epilepsy [1–5]. This range is likely due to sampling from different populations, and is likely conservative. Among subgroups of patients, a high seizure frequency or severity correlates with severity of core ASD symptoms (low sociability and communication; high repetitive/stereotyped behavior) and intellectual disability [1,4].
A substantial subset of patients with epilepsy have seizures that are refractory to medications, and this subset is similarly high (up to one-third) of those diagnosed with both ASD and epilepsy [6]; pharmacological attempts to ameliorate the core ASD symptoms of decreased sociability and communication and increased repetitive and stereotyped behaviors have had limited success. Some studies have noted improvements, possibly relating to alleviating the diverse comorbidities found in patients diagnosed with ASD (for instance, see [7]). However there is poor meta-analytical evidence of any drug treatment significantly improving sociability [8]. Serotonin reuptake inhibitors were used to treat repetitive behaviors in the past but are now considered to have little effect [9–11]; atypical antipsychotics might be useful in limiting this symptom [12,13], and the fact that such powerful medications are used highlights the dearth of effective ASD treatments.
A ketogenic diet (KD) is very low in carbohydrate, and high in fat, with a moderate and sufficient level of protein. Multiple versions of this metabolic therapy have been well established as remarkably effective nonpharmacological treatments for epilepsy, even in controlling drug-refractory seizures [14–16] with many decades of clinical use [17,18]. Strikingly, some refractory patients (consistently at least 10%–15% across studies) become seizure-free, and at least 50% achieve over 50% seizure reduction [16,19–22]; a subset of patients can be weaned off the KD and remain seizure-free [23,24]. These outcomes suggest a long-lasting antiepileptogenic effect, possibly through epigenetic mechanisms [25]. The potential of KDs for antiepileptogenesis is supported by diverse animal research [26–32].
Evidence is accumulating for clinically beneficial effect of KDs in ASD in pilot studies [33,34], case studies [35–37], and parental reports [38], as well as in animal models [39–42]. Also, in girls with Rett syndrome (a genetic syndrome in girls which includes seizures and some behavioral features similar to ASD) KDs are clinically helpful with behavior [43–45]. Improved behavior was also seen in animal models of Rett syndrome [46,47]. Reminiscent of the diverse etiology of ASD, there are myriad animal models of ASD, ranging from constitutive or induced, many with known genetic and/or environmental underpinnings.
Previous studies showing beneficial effects of a KD in rodent models of ASD have tested those without a comorbid seizure phenotype: it was important to ascertain if the KD’s effect was on the core behaviors of ASD, or if effects were secondary to reducing the comorbidity of seizures [39]. These studies showed clear improvement in core symptoms, but to date, however, this metabolic therapy has not been tested in a clinically-relevant model of comorbid autism and epilepsy. Furthermore previous studies tested one KD variant - a formula with a >6:1 ratio of fat: (carbohydrate + protein). This formula is typically used in research, but is a much higher ratio than prescribed clinically (typically between 1:1 and 4:1). Additionally, all previous studies were in male animals, and ketogenic diet effects on females are underexplored [46,48].
Here we tested the effects of KDs on behavior and blood chemistry in both sexes of EL mice, an idiopathic model of comorbid epilepsy [49] and ASD [50]. EL mice mimic human autism in that the etiology is unknown and the pathology develops gradually after birth, and complement other mouse ASD models by also developing seizures, a common comorbidity in patients. Previous studies have shown that KD feeding is anticonvulsant and antiepileptic in these mice [27,51]. Therefore, our major goal was to investigate if KD treatment would also ameliorate the core ASD symptoms present in these mice. The effects of two KDs with differing stringency were investigated in several standard assays of ASD-like rodent behavior in EL mice of both sexes.
2. Methods
2.1. Animals
At weaning, EL mice littermates (initial breeders kindly provided by T.N. Seyfried, Boston College, Chestnut Hill, MA) were housed in same-sex cages (3–6/cage). Tests performed here are unaffected by KD feeding in a mouse control strain [39]; here we focused on KD effects and sex differences within the EL strain. At five weeks of age, cages remained on control diet (CD; LabDiet 5001, W.F. Fisher & Son, Somerville, NJ) or were switched to one of two KDs (F3666 or F5140; BioServ, Frenchtown, NJ). Initial weights of all groups were not significantly different (within sexes). All diets were given ad libitum and fresh KD was provided daily. Diet compositions and ketogenic ratios (i.e. the ratio of fat to (protein + carbohydrate) by weight) are listed in Table 1; hereafter, KDs will be referred to by these ratios. Because initial pilot studies indicated that the more stringent 6.6:1 KD improved behavior primarily in females, additional testing of the milder 3.0:1 KD focused on female mice. All testing occurred at 8–9 weeks of age, i.e. between 3 and 4 weeks of dietary treatment. This length of diet treatment was chosen based on our prior observations that rodents are in significant ketosis at these times on these particular KDs and that KDs have significant behavioral and electrophysiological effects at these times including improvements in autistic-type behaviors in the BTBR model [39,52–57]. Spontaneous overt convulsions begin in EL mice of both sexes at ages that depend somewhat on the type and amount of handling, consistent with the expected developmental progression of the phenotype, but typically older than our testing age [58,59]. Behavioral seizures were not an interfering factor in these studies: in viewing behavioral videos, or during handling in behavioral or physiological experiments, we never observed convulsions in our tested animals in any treatment group. However seizures were noted in older adult male breeders -thus confirming the spontaneous seizure phenotype characteristic of the EL strain.
Table 1.
Dietary constituents.
| LabDiet 5001 (0.08:1) | Bio-Serv F3666 (3.0:1) | Bio-Serv F5140 (6.6:1) | |
|---|---|---|---|
| Fats | 5.7 | 76.7 | 69.0 |
| Proteins | 23.9 | 8.5 | 18.1 |
| Vitamin mix carbohydrates | NS | 2.04 | 1.95 |
| Mineral mix carbohydrates | NS | 0.41 | 0.77 |
| Other carbohydrates | 48.7 | 0.076 | 2.5 |
| Fuel value | 3.36 kcal/g | 7.24 kcal/g | 6.82 kcal/g |
Values are % by weight of total, except for fuel values. AIN vitamin mix is 97.7% carbohydrate by weight. AIN mineral mixes are 22.1% (F5140) or 11.8% (F3666) carbohydrate by weight. Protein percentages are calculated from casein, which is typically 89% protein, plus methionine. Added values of percentages do not reach 100% as there are unlisted constituents. Ketogenic ratios are given in parentheses. Information obtained from company product data. Abbreviation: NS, not specified.
2.2. Behavioral analysis
Testing occurred as described previously [39]. After 30 min habituation to the testing room mice were tested in the three chamber sociability test [60]. Sociability testing occurred in three 10 min phases each with free movement among the three chambers of a Plexiglas 3-chamber apparatus. After an initial 10 min habituation confined to the center chamber, phase 1 consisted of vacant inverted wire pencil cups (height, 10 cm; width at open end, 10 cm) in each side chamber (a test for side bias). In phase 2, a “stranger” mouse (CD-1, an albino control strain) of the same sex as the test mouse was corralled in the wire cage of one side chamber (a test of sociability). During phase three, the first (now familiar) CD-1 mouse remained in place and a new CD-1 mouse was corralled in the other wire cage (a test for preference for social novelty). Stranger mouse placement was counterbalanced and stranger mice were fed on the respective experimental diet for several days before testing to eliminate a possible confound of diet-related olfactory cues. Time spent in each chamber was quantified for all phases; direct social contact (nose/face/forepaw contact with the cups and/or stranger mice) was quantified only for phases 2 and 3 and only for pencil cups corralling stranger mice.
A standard test of self-grooming, a self-directed repetitive behavior, was performed on a subsequent day: after 30 min habituation to the testing room, mice were placed in a small empty cage (single cage, non-social condition) with no bedding for 20 min; the last 10 min were scored. Grooming was also quantified from video in phases 1 (solitary, non-social situation) and 2 (social situation) in the three-chamber sociability test.
After blood testing of all groups (see below, physiological analysis), passive social communication was assessed by social transmission of a food preference (STFP) as described previously [39]. EL mice were habituated to eating KD or powdered CD, as appropriate, from glass jars (Dyets, Inc., Bethlehem, PA). Two experimental protocols (a 2 day and a 3 day version, described below) were used; only one was administered to a given mouse. Food flavors: For both protocols, flavors were cocoa (2%) and cinnamon (1%) with each being the trained flavor in half the trials, and flavor placement in the cages was counterbalanced. Jars were weighed before and after presentation. 2-day protocol: An isolated demonstrator mouse was fasted for 18 h, and presented with one jar of powdered flavored CD (“trained” flavor) until it had eaten at least 0.5 g. The demonstrator was returned to the home cage for 30 min to interact with cage-mate observer mice (training), which had also been fasted for 18 h. Observer mice then moved to novel cages (one mouse per cage) and were presented with both the “trained” flavor (eaten by the demonstrator) and an “untrained” flavor (novel flavored food) for 2 h. 3-day protocol: An isolated demonstrator mouse was fasted for 18 h, and presented with one jar of powdered flavored food (“trained” flavor) until it had eaten at least 0.5 g. The demonstrator was returned to the home cage for 30 min to interact with cage-mate observer mice (training). Observer mice were fasted for 18 h subsequent to exposure to the demonstrator, and then presented with both the “trained” flavor (eaten by the demonstrator) and an “untrained” flavor (novel flavored food) for 2 h. The 2- and 3-day methods differ in that A) the 3-day method has an additional memory component in that the learning and testing are separated by 18 h, and B) motivation to learn might be lower in the 3-day protocol because observer mice are not in a fasted state during training. In training and testing, all flavored diets were powdered CD. Use of the CD during training and testing is warranted even in KD-fed mice because during training, when associative learning of the food preference occurs (or not), the observer mice have yet to eat any of the powdered CD; it also maintains a consistent diet during the training and the test.
2.3. Physiological analysis
Animals were weighed the day before behavioral testing commenced. Between the small cage grooming test and STFP, mice were lightly anesthetized with isoflurane for tail blood collection; whole blood was analyzed immediately for glucose and β-hydroxybutyrate with Precision Xtra meters (Abbott Laboratories, Bedford, MA). Blood was collected prior to STFP to avoid any confounding metabolic changes due to eating flavored CD in the STFP test phase.
2.4. Data analysis
Social and grooming behavior were scored offline from video by two independent validated scorers, at least one of whom was blind to treatment. Data from each group for each measure were analyzed with Grubb’s test for outliers, which were eliminated from that test; there were no outliers for body weight, blood glucose or blood β-hydroxybutyrate. Data were analyzed using t-tests or ANOVA with Bonferroni post-hoc tests using repeated measures where applicable. Data are reported as mean ± standard error. The significance level was defined as p < 0.05.
3. Results
3.1. Social behavior
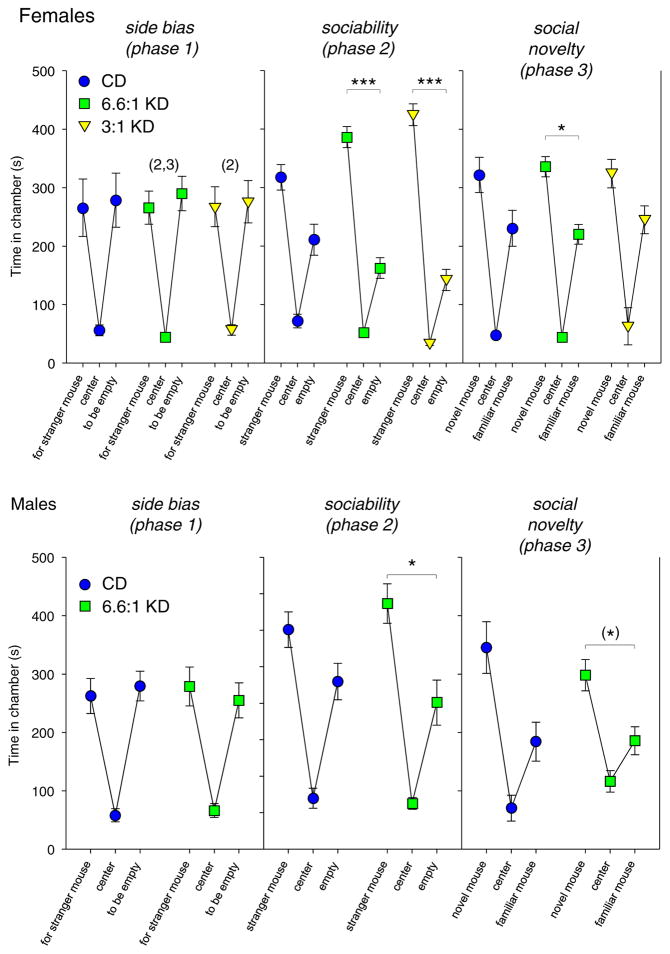
One measure of social behavior in the 3-chamber test is time in the side chambers. EL mice showed no side preference (phase 1) and no social preference: CD-fed EL mice were not significantly social when a mouse was present (phase 2), regardless of sex (Fig. 1; female CD: side F1,34 = 2.6, n.s., phase F2,34 = 3.0, n.s., interaction F2,34 = 0.8 n.s.; male CD: side F1,22 = 3.9, n.s., phase F2,22 = 0.2, n.s., interaction F2,22 = 2.3, n.s.). However, feeding with either KD formula highly increased sociability in females, i.e. increased time spent in the chamber with another mouse (Fig. 1, top; 6.6:1 KD side F1,34 = 13.6, p < 0.01, phase F2,34 = 0.5, n.s., interaction F2,34 = 9.5, p < 0.001; 3:1 KD side F1,32 = 12.2, p < 0.01, phase F2,32 = 7.2, p < 0.01, interaction F2,32 = 9.6, p < 0.001). There was significantly improved sociability in phase 2 with KD feeding in males (Fig. 1, bottom; side F1,24 = 13.8, p < 0.01, phase F2,24 = 8.9, p = 0.001, interaction F2,24 = 0.9, n.s.). In the test of preference for social novelty (introduction of a new mouse, phase 3), only 6.6:1 KD-fed females exhibited significant preference (Fig. 1, top), although the effect size appears small.
Fig. 1.
Sociability assessed by side or center chamber time in the 3-chamber test. Top: In females, there was no baseline side preference (left). In the sociability test, CD-fed females were not significantly social (p = 0.11), but those fed either KD were highly social (middle). In the test for preference for social novelty, only those females fed the stricter KD significantly preferred social novelty (right). *p < 0.05, ***p < 0.001, side-to-side comparisons; (2) - side times different from phase 2, p < 0.001, (3) - side times different from phase 3, p < 0.05); n = 18 all groups. Center chamber times are shown but were not used for analysis. Bottom: In males, there was no baseline side preference (left). In the sociability test, CD-fed males were not social, but KD-fed males were (middle). There was no significant preference for social novelty regardless of diet, although there was a p = 0.053 trend in KD fed males (right); n = 11–13.
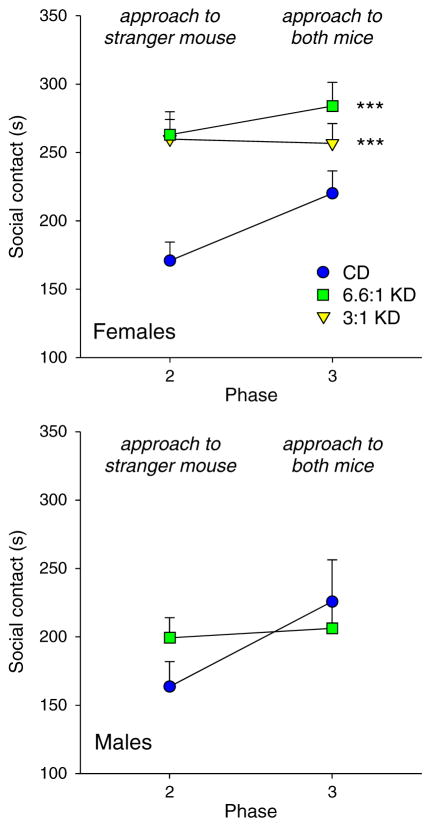
Another measure of social behavior in the 3-chamber test is social contact. Treatment with either KD significantly increased social contact in females, regardless of whether there was one mouse or two (phases 2 and 3, respectively; Phase F1,50 = 3.2, p = 0.08; Diet F2,50 = 13.4, p < 0.001; Interaction F2,50 = 1.5, n.s.) There was no significant effect of KD feeding on social contact in males (Fig. 2; Phase F1,22 = 4.8, p = 0.04; Diet F1,22 = 0.2, n.s.; Interaction F1,22 = 2.9, n.s.), which was generally low and in the same range as the CD-fed females. This pattern indicates that KD-fed males were preferring to spend time in the chamber with the stranger mouse but were not highly investigative of or interacting directly with the mouse - thus indicating only a limited boost to sociability by KD feeding.
Fig. 2.
Sociability assessed with social contact time in the 3-chamber test. For phase three, social contact with both inhabited corrals was combined. Top: In females, feeding with either KD increased social contact in both phases 2 and 3. Post-hoc tests were performed for the Diet factor. ***p < 0.001, comparison to CD group; n = 17–18. Bottom: In males, KD treatment did not change social contact in either phase; n = 11–13.
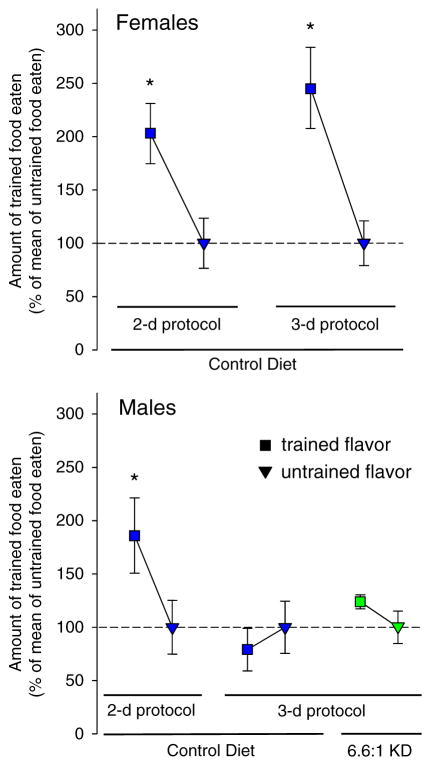
STFP test results were sex- and protocol-dependent. CD-fed female EL mice preferred the trained flavor and thus performed STFP successfully in protocols with or without a long-term memory component (3 day vs. 2 day protocol, respectively; Fig. 3), and this result was maintained and not changed significantly when female mice were fed KD in either protocol (data not shown). Male EL mice fed CD successfully performed STFP in the 2-day protocol but not the 3-day protocol, suggesting a failure either to learn the flavor or to remember the food preference after an 18 h delay. Similar to the lack of a KD influencing STFP in females, successful performance in the 2-day protocol was not altered and the performance deficit in the 3-day protocol was not alleviated by KD feeding in males (Fig. 3).
Fig. 3.
Diet effects in the social transmission of food preference test. Amount of trained flavor eaten is expressed as percent of untrained food eaten, which was normalized at 100%. Top: Females fed the control diet successfully performed the task in 2-day and 3-day protocols. *p < 0.05, n = 13–14. Bottom: Males fed the control diet preferred the trained flavor in the 2 day protocol, but showed no preference in the 3-day protocol which includes an additional memory demand; this outcome was not changed by feeding with a KD. *p < 0.05, n = 10–14.
3.2. Repetitive behavior
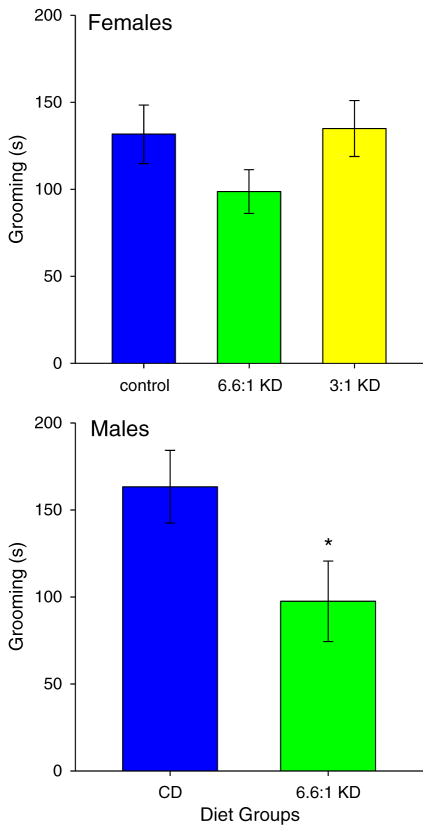
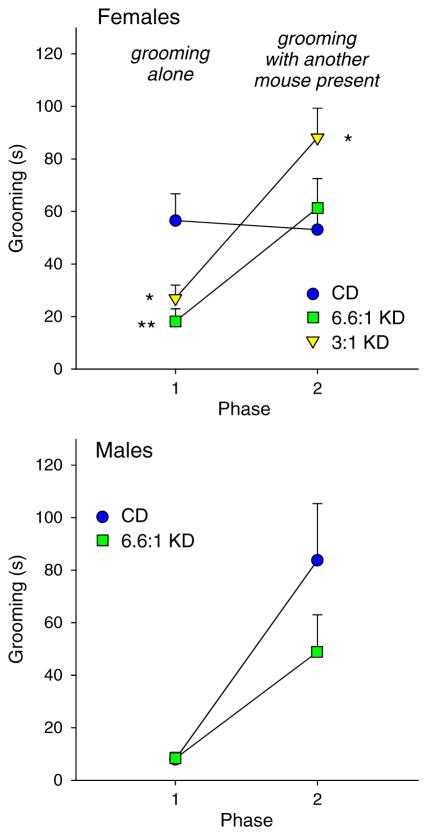
Effects of KDs on repetitive behavior (self-grooming) were mixed based on sex and environment. In the small cage (solitary, non-social) test, grooming was decreased significantly in males (t = 2.1, *p < 0.05), but was not changed by KD feeding in females (Fig. 4; F2,51 = 1.5, n.s.). In the 3-chamber test, grooming was high in phase 1 (non-social) in CD-fed females, and was lowered significantly by either KD; self-grooming in phase 2 (social) was not affected by the strict KD but was significantly elevated by the moderate KD (Fig. 5 top; Phase F1,49 = 38.9, p < 0.001; Diet F2,49 = 1.7, n.s.; Interaction F2,49 = 13.0, p < 0.001). For males in the 3-chamber test, grooming was low in phase 1 and was not affected by KD feeding; phase 2 self-grooming was also not affected (Fig. 5 bottom; Phase F1,23 = 20.8, p < 0.001; Diet F1,23 = 1.9, n.s.; Interaction F1,23 = 1.9, n.s.).
Fig. 4.
Grooming in the small cage (solitary, non-social) test. There were no significant diet effects in females (top, n = 18 all groups), but grooming was decreased by the 6.6:1 KD in males (bottom, n = 12–14).
Fig. 5.
Grooming in the 3-chamber test. Grooming was assessed in phase 1 (non-social, test mouse alone) and 2 (social, stranger mouse present with test mouse). Top: In females, both KDs reduced grooming in phase 1, but not in phase 2. The moderate KD increased grooming in phase 2. Post hoc tests were restricted to within-phase comparisons. *p < 0.05, **p < 0.01, comparison to CD group; n = 17–18. Bottom: In males, KD feeding did not affect grooming in either phase: grooming increased regardless of diet in phase 2; n = 11–14.
3.3. Blood chemistry and body mass
Consistent with previous findings, feeding on either KD raised levels of circulating ketones in all groups, but with some sex-specific effects: in females the stronger 6.6:1 KD caused stronger ketosis than the milder KD (Table 2). Blood glucose results were more variable based on diet and sex: blood glucose was mildly but significantly reduced in females by the 6.6:1 KD but not by the 3:1 KD; curiously, the stricter KD had no significant effect on glucose in males (Table 2). We replicated this unexpected latter result in a separate group of males (n = 5, data not shown). In addition, another group of males was left on the KD for a 6 wk. extended period of time: again, blood glucose was not significantly reduced (n = 6, data not shown). The 6.6:1 KD did not significantly alter weight in either sex, but females on the 3:1 KD had body weights elevated compared to females fed on either the CD or 6.6:1 KD (Table 2). All diets were fed ad libitum, and these findings indicate that animals were not self-restricting and that caloric restriction was not necessary for the beneficial behavioral effects observed here.
Table 2.
Physiological parameters.
| Sex and diet | Female CD n = 18 |
Female 6.6:1 KD n = 18 |
Female 3:1 KD n = 18 |
Male CD n = 12 |
Male 6.6:1 KD n = 14 |
|---|---|---|---|---|---|
| Body weight (g) | 22.4 ± 0.4 | 20.7 ± 0.4 | 28.2 ± 0.7***,§§§ | 25.6 ± 0.4 | 25.4 ± 0.8 |
| Blood glucose (mg/dL) | 154 ± 4 | 135 ± 6* | 145 ± 4 | 147 ± 8 | 153 ± 5 |
| Blood β-HB (mM) | 0.30 ± 0.02 | 1.62 ± 0.13*** | 0.91 ± 0.08***,§§§ | 0.28 ± 0.03 | 1.04 ± 0.09*** |
Females: weight ANOVA F2,51 = 54.8, p < 0.001; glucose ANOVA F2,51 = 4.0, p < 0.05; β-hydroxybutyrate ANOVA F2,51 = 60.3, p < 0.001.
Bonferroni post hocs -
p < 0.05,
p < 0.001 compared to CD;
p < 0.001 compared to 6:6.1 KD. Males: weight t-test t = 0.2, n.s.; glucose t = −0.6, n.s.; β-hydroxybutyrate t = −7.6,
p < 0.001.
4. Discussion
Effects of two ketogenic diet formulas on core behavioral symptoms of ASD were assessed in both sexes of EL mice, a model of idiopathic comorbid epilepsy and autism. The EL mouse behavioral phenotype develops over time, consistent with many clinical cases of autism. In addition, the ketogenic diet formulas applied here included a ratio of fat to carbohydrate and protein within the range of that administered routinely to children. Overall effects of two KD formulas were positive or neutral on behavioral characteristics associated with ASD.
Consistent with core behavioral symptoms of ASD EL mice of both sexes fed a CD were asocial in the 3-chamber test of sociability. These findings are consistent with previous work characterizing EL mice as having low or abnormal sociability in various other paradigms [50,61–63]. Notably, after KD feeding, females became highly social in the 3-chamber test; there was moderately increased sociability in males. However, a sex-specific deficit in males in a socially-cued learning and memory test (3-day STFP protocol) was not improved by a KD. Beyond improved sociability, the relative amount of time spent on repetitive self-directed behavior (grooming) in non-social situations was reduced by ketogenic diet feeding in a test- and sex-specific manner and behavioral improvements were very similar with 6.6:1 and 3:1 KDs. Phase 1 grooming in the CD-fed females was higher than in any other ASD model that we have studied ([39]; unpublished data), and was strikingly reduced by KD feeding. In general, these data are in accord with prior studies finding beneficial effects of KD feeding in other rodent models of ASD (BTBR mouse, Engrailed 2 knockout mouse; prenatal valproic acid treatment in rats) [39–42] and with limited clinical reports with similar diets in ASD patients [33–38].
STFP results were sex-dependent as well. Female EL mice fed the control diet performed the STFP test successfully in either a 2-day or 3-day protocol; results in males were mixed: control diet-fed male EL mice were apparently sufficiently social during training in the 2-day protocol to learn the association of the demonstrator mouse and the trained flavor and thus prefer the trained flavor in the immediate food preference test (also showing that EL mice do not have impaired olfaction). Males did not show this preference in the 3 day protocol which incurs an additional memory component. Therefore, the poor performance of male EL mice in the 3-day protocol is not due to low sociability during training, suggesting a more specific memory deficit, and perhaps a social memory deficit. However the 2- and 3-day protocols differ in an important respect besides the delay between training and testing: in the 2-day, but not the 3-day, protocol, test mice have been fasted prior to (and may be more motivated during) training. To address this confound, we performed a modified 3-day protocol with fasting before training and test mice also showed no preference for the trained flavor. Therefore, differences in motivation during learning appear not to be involved, and we conclude that the poor performance of male EL mice in the 3-day protocol is due to a memory deficit. A much shorter delay might have been suffficient to impair memory, as EL mice have a social recognition memory deficit over 30 min [62]. Nevertheless, KD feeding did not improve male EL mouse memory in STFP. Similarly, KD feeding did not improve EL mouse acquisition of an associative procedural task in the radial water maze [27].
In addition to behavioral problems, maturing EL mice develop epilepsy that can be treated and prevented by KD feeding [27]. This improvement is thought to depend on reduced blood glucose: decreased blood glucose correlated with decreased seizures [31,64]. Indeed, caloric restriction alone has seizure-reducing effects in EL mice [64,65]. In parallel, evidence is building that limiting glucose is anticonvulsant through an adenosine-based mechanism [54,56,66] and we hypothesized that ASD core symptoms could be alleviated by this same mechanism [67]. Here, for ASD-like symptoms, there appears to be a dissociation among caloric restriction, KD, and blood glucose. First, behavioral improvement occurred in females with the 6.6:1 KD without significant weight loss and, strikingly, also with the 3:1 KD which incurred significant weight gain. Secondly, although blood glucose was significantly lowered in females fed the 6.6:1 KD, this drop did not occur in those fed the 3:1 KD yet the behavioral effects of both KDs were similar. Also, the mild behavioral improvements in males occurred without changes in weight or blood glucose. We conclude that a caloric restriction component to KDs [68] is not necessary for behavioral benefits in this model and appears not to be necessary in at least some of the clinical ASD reports [33,34,37]. Glucose is not likely to be irrelevant, however: blood levels of glucose are notably stable on minutes-to-hours timescales during KD feeding [69,70], and recently a low glycemic index diet was shown to improve ASD-like behaviors in the BTBR mouse model [71]. In this latter paper, however, dietary treatment was started in the mothers of the test mice before conception, and so beneficial effects could be developmental rather than therapeutic.
In addition to reduced glucose, the eponymous blood chemistry hallmark of KDs is ketosis. Male EL mice fed the 6.6:1 KD had elevated β-hydroxybutyrate (similar to females fed the milder 3:1 KD) and showed improved sociability but not strong and significant improvements like females. Thus ketosis is not proven to be sufficient for improved sociability, though it may be necessary. A high fat content is likely also not sufficient: a high-fat non-ketotic diet worsens behavior and cognition in the BTBR ASD model [72]. However, a recent case report described a striking improvement in ASD behaviors (as well as seizures) with a diet rich in medium-chain triglycerides [37]. This type of fat is rapidly absorbed and metabolized to ketone bodies in the absence of dietary carbohydrate restriction (although downstream effects of this fat are difficult to disentangle from glucose metabolism [66,73]). Also, concerning seizures, evidence is mounting for medium-chain fatty acid actions apart from ketone body elevation [74,75]. More studies of medium-chain triglycerides and fatty acids and ketone body-augmenting treatments such as ketone esters are warranted in ASD patients and models.
Of additional clinical importance, KDs do not worsen ASD-associated behaviors and there is evidence they may restore homoeostasis [25]. Previously we and others published the lack of a KD effect in control mouse and rat strains on behavior and electrophysiology in a variety of paradigms. In normally social control strains, we found KD feeding produced no significant change in sociability or in STFP; in control strains with normal grooming levels, KD similarly produced no change [39]. We also found a lack of effect of KD feeding on working memory in control mouse strains [48] and a lack of effect on baseline hippocampal electrophysiology in vitro in control strains (but an impact on chemically-induced epileptiform activity; [56]). We found normal in vivo baseline hippocampal field potential activity (but a lessening of induced seizures or tetanus-induced potentiation; [53,54,57]. Work from other laboratories is largely in agreement (for instance [76,77]). Overall, KDs and ketosis have negligible effects in animals that are normal and in systems that are unperturbed.
Given the lack of effective drug treatments against poor sociability and communication, the limited effectiveness of drug treatments against stereotypical behaviors, and the undesirable side effects of these drugs [78], KDs and similar treatments are particularly attractive for ASD as non-pharmacological approaches that aid with these core symptoms. A metabolic approach is well established and represents a mechanistically new type of treatment for ASD. In epilepsy, KDs are as effectively anticonvulsant as the commonly-used anticonvulsant drugs [16,79–82]; KDs may prove to surpass medications in effectiveness against ASD core symptoms. Based on the present and prior research [27,51], KD feeding treats both seizures and ASD-like symptoms in the EL mouse model of comorbid epilepsy/ASD - therefore, KDs may merit special consideration as a treatment for autistic patients with this same comorbidity. In epilepsy, chronic KD treatment can produce seizure control that extends well beyond cessation of the diet (e.g. [83–85]) suggesting a persistent, possibly epigenetic, effect [32] – it remains to be seen if KD amelioration of ASD symptoms can persist in a similar manner. Finally, although ASD is more common in males, our positive results in females may provide additional hope for the 4 in 1000 females (650,000 individuals in the USA) who have ASD [86].
HIGHLIGHTS.
Drug treatments are poorly effective against core symptoms of autism.
Ketogenic diets were tested in EL mice, a model of comorbid autism and epilepsy.
Sociability was improved and repetitive behaviors were reduced in female mice.
In males behavioral improvements were more limited.
Metabolic therapy may be especially beneficial in comorbid autism and epilepsy.
Acknowledgments
Supported by NIH grants NS066392, AT008742, and NS065957. We thank Michelle I. Murphy, Lisa Saa, Sierra L. Slade, and Sarah R. Nunes for technical assistance.
Contributor Information
David N. Ruskin, Email: david.ruskin@trincoll.edu.
Jessica A. Fortin, Email: jessica.fortin@umassmed.edu.
Subrina N. Bisnauth, Email: sbisnauth112@gmail.com.
Susan A. Masino, Email: susan.masino@trincoll.edu.
References
- 1.Cuccaro ML, Tuchman RF, Hamilton KL, Wright HH, Abramson RK, Haines JL, et al. Exploring the relationship between autism spectrum disorder and epilepsy using latent class cluster analysis. J Autism Dev Disord. 2012;42:1630–1641. doi: 10.1007/s10803-011-1402-y. [DOI] [PubMed] [Google Scholar]
- 2.Kantzer AK, Fernell E, Gillberg C, Miniscalco C. Autism in community pre-schoolers: developmental profiles. Res Dev Disabil. 2013;34:2900–2908. doi: 10.1016/j.ridd.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 3.Carlsson LH, Norrelgen F, Kjellmer L, Westerlund J, Gillberg C, Fernell E. Coexisting disorders and problems in preschool children with autism spectrum disorders. TheScientificWorldJOURNAL. 2013;2013:213979. doi: 10.1155/2013/213979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amiet C, Gourfinkel-An I, Laurent C, Bodeau N, Génin B, Leguern E, et al. Does epilepsy in multiplex autism pedigrees define a different subgroup in terms of clinical characteristics and genetic risk? Mol Autism. 2013;4:47. doi: 10.1186/2040-2392-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doshi-Velez F, Ge Y, Kohane I. Comorbidity clusters in autism spectrum disorders: an electronic health record time-series analysis. Pediatrics. 2014;133:e54–e63. doi: 10.1542/peds.2013-0819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sansa G, Carlson C, Doyle W, Weiner HL, Bluvstein J, Barr W, et al. Medically refractory epilepsy in autism. Epilepsia. 2011;52:1071–1075. doi: 10.1111/j.1528-1167.2011.03069.x. [DOI] [PubMed] [Google Scholar]
- 7.Pearson DA, Santos CW, Aman MG, Arnold LE, Casat CD, Mansour R, et al. Effects of extended release methylphenidate treatment on ratings of attention-deficit/hyperactivity disorder (ADHD) and associated behavior in children with autism spectrum disorders and ADHD symptoms. J Child Adolesc Psychopharmacol. 2013;23:337–351. doi: 10.1089/cap.2012.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farmer C, Thurm A, Grant P. Pharmacotherapy for the core symptoms in autistic disorder: current status of the research. Drugs. 2013;73:303–314. doi: 10.1007/s40265-013-0021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doyle CA, McDougle CJ. Pharmacologic treatments for the behavioral symptoms associated with autism spectrum disorders across the lifespan. Dialogues Clin Neurosci. 2012;14:263–279. doi: 10.31887/DCNS.2012.14.3/cdoyle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams K, Brignell A, Randall M, Silove N, Hazell P. Selective serotonin reuptake inhibitors (SSRIs) for autism spectrum disorders (ASD) Cochrane Database Syst Rev. 2013;8:CD004677. doi: 10.1002/14651858.CD004677.pub3. [DOI] [PubMed] [Google Scholar]
- 11.Baribeau DA, Anagnostou E. An update on medication management of behavioral disorders in autism. Curr Psychiatry Rep. 2014;16:437. doi: 10.1007/s11920-014-0437-0. [DOI] [PubMed] [Google Scholar]
- 12.Ching H, Pringsheim T. Aripiprazole for autism spectrum disorders (ASD) Cochrane Database Syst Rev. 2012;5:CD009043. doi: 10.1002/14651858.CD009043.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Dove D, Warren Z, McPheeters ML, Taylor JL, Sathe NA, Veenstra-VanderWeele J. Medications for adolescents and young adults with autism spectrum disorders: a systematic review. Pediatrics. 2012;130:717–726. doi: 10.1542/peds.2012-0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kinsman SL, Vining EP, Quaskey SA, Mellits D, Freeman JM. Efficacy of the ketogenic diet for intractable seizure disorders: review of 58 cases. Epilepsia. 1992;33:1132–1136. doi: 10.1111/j.1528-1157.1992.tb01770.x. [DOI] [PubMed] [Google Scholar]
- 15.Kankirawatana P, Jirapinyo P, Kankirawatana S, Wongarn R, Thamanasiri N. Ketogenic diet: an alternative treatment for refractory epilepsy in children. J Med Assoc Thail. 2001;84:1027–1032. [PubMed] [Google Scholar]
- 16.Hallböök T, Sjolander A, Amark P, Miranda M, Bjurulf B, Dahlin M. Effectiveness of the ketogenic diet used to treat resistant childhood epilepsy in Scandinavia. Eur J Paediatr Neurol. 2015;19:29–36. doi: 10.1016/j.ejpn.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Wilder RM. High fat diets in epilepsy. Mayo Clin Bull. 1921;2:308. [Google Scholar]
- 18.Mike EM. Practical guide and dietary management of children with seizures using the ketogenic diet. Am J Clin Nutr. 1965;17:399–409. doi: 10.1093/ajcn/17.6.399. [DOI] [PubMed] [Google Scholar]
- 19.Hassan AM, Keene DL, Whiting SE, Jacob PJ, Champagne JR, Humphreys P. Ketogenic diet in the treatment of refractory epilepsy in childhood. Pediatr Neurol. 1999;21:548–552. doi: 10.1016/s0887-8994(99)00045-4. [DOI] [PubMed] [Google Scholar]
- 20.Kang HC, Lee YM, Kim HD, Lee JS, Slama A. Safe and effective use of the ketogenic diet in children with epilepsy and mitochondrial respiratory chain complex defects. Epilepsia. 2007;48:82–88. doi: 10.1111/j.1528-1167.2006.00906.x. [DOI] [PubMed] [Google Scholar]
- 21.Neal EG, Chaffe H, Schwartz RH, Lawson MS, Edwards N, Fitzsimmons G, et al. A randomized trial of classical and medium-chain triglyceride ketogenic diets in the treatment of childhood epilepsy. Epilepsia. 2009;50:1109–1117. doi: 10.1111/j.1528-1167.2008.01870.x. [DOI] [PubMed] [Google Scholar]
- 22.Suo C, Liao J, Lu X, Fang K, Hu Y, Chen L, et al. Efficacy and safety of the ketogenic diet in Chinese children. Seizure. 2013;22:174–178. doi: 10.1016/j.seizure.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 23.Martinez CC, Pyzik PL, Kossoff EH. Discontinuing the ketogenic diet in seizure-free children: recurrence and risk factors. Epilepsia. 2007;48:187–190. doi: 10.1111/j.1528-1167.2006.00911.x. [DOI] [PubMed] [Google Scholar]
- 24.Patel A, Pyzik PL, Turner Z, Rubenstein JE, Kossoff EH. Long-term outcomes of children treated with the ketogenic diet in the past. Epilepsia. 2010;51:1277–1282. doi: 10.1111/j.1528-1167.2009.02488.x. [DOI] [PubMed] [Google Scholar]
- 25.Boison D, Sandau U, Ruskin DN, Kawamura M, Jr, Masino SA. Homeostatic control of brain function - new approaches to understand epileptogenesis. Front Cell Neurosci. 2013;7:109. doi: 10.3389/fncel.2013.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muller-Schwarze AB, Tandon P, Liu Z, Lang Y, Holmes GL, Stafstrom CE. Ketogenic diet reduces spontaneous seizures and mossy fiber sprouting in the kainic acid model. Neuroreport. 1999;10:1517–1522. doi: 10.1097/00001756-199905140-00023. [DOI] [PubMed] [Google Scholar]
- 27.Todorova MT, Tandon P, Madore RA, Stafstrom CE, Seyfried TN. The ketogenic diet inhibits epileptogenesis in EL mice: a genetic model for idiopathic epilepsy. Epilepsia. 2000;41:933–940. doi: 10.1111/j.1528-1157.2000.tb00275.x. [DOI] [PubMed] [Google Scholar]
- 28.Su SW, Sogawa MRCY, Silveira DC, Holmes GL, Stafstrom CE. Timing of ketogenic diet initiation in an experimental epilepsy model. Dev Brain Res. 2000;125:131–138. doi: 10.1016/s0165-3806(00)00130-9. [DOI] [PubMed] [Google Scholar]
- 29.Hu X-L, Cheng X, Fei J, Xiong Z-Q. Neuron-restrictive silencer factor is not required for the antiepileptic effect of the ketogenic diet. Epilepsia. 2011;52:1609–1616. doi: 10.1111/j.1528-1167.2011.03171.x. [DOI] [PubMed] [Google Scholar]
- 30.Jiang Y, Yang Y, Wang S, Ding Y, Guo Y, Zhang M-M, et al. Ketogenic diet protects against epileptogenesis as well as neuronal loss in amygdaloid-kindling seizures. Neurosci Lett. 2012;508:22–26. doi: 10.1016/j.neulet.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Mantis JG, Meidenbauer JJ, Zimick NC, Centeno NA, Seyfried TN. Glucose reduces the anticonvulsant effects of the ketogenic diet in EL mice. Epilepsy Res. 2014;108:1137–1144. doi: 10.1016/j.eplepsyres.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 32.Lusardi TA, Akula KK, Coffman SQ, Ruskin DN, Masino SA, Boison D. Ketogenic diet prevents epileptogenesis and disease progression in adult mice and rats. Neuropharmacology. 2015;99:500–509. doi: 10.1016/j.neuropharm.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Evangeliou A, Vlachonikolis I, Mihailidou H, Spilioti M, Skarpalezou A, Makaronas N, et al. Application of a ketogenic diet in children with autistic behavior: pilot study. J Child Neurol. 2003;18:113–118. doi: 10.1177/08830738030180020501. [DOI] [PubMed] [Google Scholar]
- 34.Spilioti M, Evangeliou AE, Tramma D, Theodoridou Z, Metaxas S, Michailidi E, et al. Evidence for treatable inborn errors of metabolism in a cohort of 187 Greek patients with autism spectrum disorder (ASD) Front Hum Neurosci. 2013;7:858. doi: 10.3389/fnhum.2013.00858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arvio M, Kuisma L, Pöntinen M. The modified Atkins diet brought back the joy of life to a developmentally severely disabled youth. Duodecim. 2010;126:557–560. [PubMed] [Google Scholar]
- 36.Masino SA, Svedova J, Kawamura M, Jr, DiMario FD, Jr, et al. Adenosine and autism - recent research and a new perspective. In: Eapen V, editor. Autism - A Neurodevelopmental Journey From Genes to Behaviour. InTech; Rijeka, Croatia: 2011. pp. 103–122. [Google Scholar]
- 37.Herbert MR, Buckley JA. Autism and dietary therapy: case report and review of the literature. J Child Neurol. 2013;28:975–982. doi: 10.1177/0883073813488668. [DOI] [PubMed] [Google Scholar]
- 38.Frye RE, Sreenivasula S, Adams JB. Traditional and non-traditional treatments for autism spectrum disorder with seizures: an on-line survey. BMC Pediatr. 2011;11:37. doi: 10.1186/1471-2431-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruskin DN, Svedova J, Cote JL, Sandau U, Rho JM, Kawamura M, et al. Ketogenic diet improves core symptoms of autism in BTBR mice. PLoS One. 2013;8:e65021. doi: 10.1371/journal.pone.0065021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahn Y, Narous M, Tobias R, Rho JM, Mychasiuk R. The ketogenic diet modifies social and metabolic alterations identified in the prenatal valproic acid model of autism spectrum disorder. Dev Neurosci. 2014;36:371–380. doi: 10.1159/000362645. [DOI] [PubMed] [Google Scholar]
- 41.Verpeut JL, DiCicco-Bloom E, Bello NT. Ketogenic diet exposure during the juvenile period increases social behaviors and forebrain neural activation in adult Engrailed 2 null mice. Physiol Behav. 2016;161:90–98. doi: 10.1016/j.physbeh.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 42.Castro K, Baronio D, Perry IS, Riesgo RdS, Gottfried C. The effect of ketogenic diet in an animal model of autism induced by prenatal exposure to valproic acid. Nutr Neurosci. 2016 doi: 10.1080/1028415X.2015.1133029. (in press) [DOI] [PubMed] [Google Scholar]
- 43.Haas RH, Rice MA, Tauner DA, Merritt TA. Therapeutic effects of a ketogenic diet in Rett syndrome. Am J Med Genet Suppl. 1986;1:225–246. doi: 10.1002/ajmg.1320250525. [DOI] [PubMed] [Google Scholar]
- 44.Bujas-Petković Z, Matijasić R, Divcić B. Rett’s syndrome: differential diagnosis in relation to autism with case report. Lijeć Vjesn. 1989;111:458–460. [PubMed] [Google Scholar]
- 45.Liebhaber GM, Riemann E, Baumeister FA. Ketogenic diet in Rett syndrome. J Child Neurol. 2003;18:74–75. doi: 10.1177/08830738030180011801. [DOI] [PubMed] [Google Scholar]
- 46.Mantis JG, Fritz CL, Marsh J, Heinrichs SC, Seyfried TN. Improvement in motor and exploratory behavior in Rett syndrome mice with restricted ketogenic and standard diets. Epilepsy Behav. 2009;15:133–141. doi: 10.1016/j.yebeh.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 47.Park MJ, Aja S, Li Q, Degano AL, Penati J, Zhuo J, et al. Anaplerotic triheptanoin diet enhances mitochondrial substrate use to remodel the metabolome and improve lifespan, motor function, and sociability in MeCP2-null mice. PLoS One. 2014;9:e109527. doi: 10.1371/journal.pone.0109527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ruskin DN, Ross JL, Kawamura M, Jr, Ruiz TL, Geiger JD, et al. A ketogenic diet delays weight loss and does not impair working memory or motor function in the R6/2 1J mouse model of Huntington’s disease. Physiol Behav. 2011;103:501–507. doi: 10.1016/j.physbeh.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murashima YL, Tada H, Kasamo K, Morooka K, Suzuki J. Antiepileptic effects of allopurinol involved in hippocampal specific SOD (superoxide dismutase) induction in the mutant EL mouse. Jpn J Psychiatry Neurol. 1993;47:374–377. doi: 10.1111/j.1440-1819.1993.tb02111.x. [DOI] [PubMed] [Google Scholar]
- 50.Meidenbauer JJ, Mantis JG, Seyfried TN. The EL mouse: a natural model of autism and epilepsy. Epilepsia. 2011;52:347–357. doi: 10.1111/j.1528-1167.2010.02898.x. [DOI] [PubMed] [Google Scholar]
- 51.Mantis JG, Centeno NA, Todorova MT, McGowan R, Seyfried TN. Management of multifactorial idiopathic epilepsy in EL mice with caloric restriction and the ketogenic diet: role of glucose and ketone bodies. Nutr Metab. 2004;1:11. doi: 10.1186/1743-7075-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruskin DN, Kawamura M, Jr, Masino SA. Reduced pain and inflammation in juvenile and adult rats fed a ketogenic diet. PLoS One. 2009;4:e8349. doi: 10.1371/journal.pone.0008349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koranda JL, Ruskin DN, Masino SA, Blaise JH. A ketogenic diet reduces long-term potentiation in the dentate gyrus of freely-behaving rats. J Neurophysiol. 2011;106:662–666. doi: 10.1152/jn.00001.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Masino SA, Li T, Theofilas P, Sandau U, Ruskin DN, Fredholm BB, et al. A ketogenic diet suppresses seizures in mice through adenosine A1 receptors. J Clin Invest. 2011;121:2679–2683. doi: 10.1172/JCI57813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruskin DN, Suter TACS, Ross JL, Masino SA. Ketogenic diets and thermal pain: dissociation of hypoalgesia, elevated ketones, and lowered glucose in rats. J Pain. 2013;14:467–474. doi: 10.1016/j.jpain.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kawamura M, Jr, Ruskin DN, Geiger JD, Boison D, Masino SA. Ketogenic diet sensitizes glucose control of hippocampal excitability. J Lipid Res. 2014;55:2254–2260. doi: 10.1194/jlr.M046755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Masino SA, Koranda JL, Ruskin DN, Blaise JH. Effects of a ketogenic diet on hippocampal plasticity in freely-moving juvenile rats. Physiol Rep. 2015;3:e12411. doi: 10.14814/phy2.12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Todorova MT, Dangler CA, Drage MG, Sheppard BJ, Fox JG, Seyfried TN. Sexual dysfunction and sudden death in epileptic male EL mice: inheritance and prevention with the ketogenic diet. Epilepsia. 2003;44:25–31. doi: 10.1046/j.1528-1157.2003.11402.x. [DOI] [PubMed] [Google Scholar]
- 59.Leussis MP, Heinrichs SC. Routine tail suspension husbandry facilitates onset of seizure susceptibility in EL mice. Epilepsia. 2006 doi: 10.1111/j.1528-1167.2006.00525.x. [DOI] [PubMed] [Google Scholar]
- 60.Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, et al. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- 61.Bond TL, Drage M, Heinrichs SC. Seizure-prone EL mice exhibit deficits in pup nursing and retrieval assessed using a novel method of maternal behavior phenotyping. Epilepsy Behav. 2003;4:57–64. doi: 10.1016/s1525-5050(02)00645-5. [DOI] [PubMed] [Google Scholar]
- 62.Lim CE, Turner LH, Heinrichs SC. Short-term social recognition memory deficit and atypical social and physiological stressor reactivity in seizure-susceptible EL mice. Seizure. 2007;16:59–68. doi: 10.1016/j.seizure.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 63.Turner LH, Lim CE, Heinrichs SC. Antisocial and seizure susceptibility phenotypes in an animal model of epilepsy are normalized by impairment of brain corticotropin-releasing factor. Epilepsy Behav. 2007;10:8–15. doi: 10.1016/j.yebeh.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 64.Greene AE, Todorova MT, McGowan R, Seyfried TN. Caloric restriction inhibits seizure susceptibility in epileptic EL mice by reducing blood glucose. Epilepsia. 2001;42:1371–1378. doi: 10.1046/j.1528-1157.2001.17601.x. [DOI] [PubMed] [Google Scholar]
- 65.Meidenbauer JJ, Roberts MF. Reduced glucose utilization underlies seizure protection with dietary therapy in epileptic EL mice. Epilepsy Behav. 2014;39:48–54. doi: 10.1016/j.yebeh.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Socała K, Nieoczym D, Pieróg M, WlaŸ P. Role of the adenosine system and glucose restriction in the acute anticonvulsant effect of caprylic acid in the 6 Hz psychomotor seizure test in mice. Prog Neuro-Psychopharmacol Biol Psychiatry. 2015;57C:44–51. doi: 10.1016/j.pnpbp.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 67.Masino SA, Kawamura M, Jr, Plotkin LM, Svedova J, DiMario FJ, et al. The relationship between the neuromodulator adenosine and behavioral symptoms of autism. Neurosci Lett. 2011;500:1–5. doi: 10.1016/j.neulet.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Napoli E, Dueñas N, Giulivi C. Potential therapeutic use of the ketogenic diet in autism spectrum disorders. Front Pediatr. 2014;2:69. doi: 10.3389/fped.2014.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Noakes M, Foster PR, Keogh JB, James AP, Mamo JC, Clifton PM. Comparison of isocaloric very low carbohydrate/high saturated fat and high carbohydrate/low saturated fat diets on body composition and cardiovascular risk. Nutr Metab. 2006;3:7. doi: 10.1186/1743-7075-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nuttall FQ, Almokayyad RM, Gannon MC. Comparison of a carbohydrate-free diet vs. fasting on plasma glucose, insulin and glucagon in type 2 diabetes. Metabolism. 2015;64:253–262. doi: 10.1016/j.metabol.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 71.Currais A, Farrokhi C, Dargusch R, Goujon-Svrzic M, Maher P. Dietary glycemic index modulates the behavioral and biochemical abnormalities associated with autism spectrum disorder. Mol Psychiatry. 2016;21:426–436. doi: 10.1038/mp.2015.64. [DOI] [PubMed] [Google Scholar]
- 72.Zilkha N, Kuperman Y, Kimchi T. High-fat diet exacerbates cognitive rigidity and social deficiency in the BTBR mouse model of autism. Neuroscience. 2016 doi: 10.1016/j.neuroscience.2016.01.070. http://dx.doi.org/10.1016/j.neuroscience.2016.01.070. [DOI] [PubMed]
- 73.McDonald TS, Tan KN, Hodson MP, Borges K. Alterations of hippocampal glucose metabolism by even versus uneven medium chain triglycerides. J Cereb Blood Flow Metab. 2014;34:153–160. doi: 10.1038/jcbfm.2013.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maciejak P, Szyndler J, Turzyńska D, Sobolewska A, Kołosowska K, Krząścik P, et al. Is the interaction between fatty acids and tryptophan responsible for the efficacy of a ketogenic diet in epilepsy? The new hypotheses of action. Neuroscience. 2016;313:130–148. doi: 10.1016/j.neuroscience.2015.11.029. [DOI] [PubMed] [Google Scholar]
- 75.Chang P, Augustin K, Boddum K, Williams S, Sun M, Terschak JA, et al. Seizure control by decanoic acid through direct AMPA receptor inhibition. Brain. 2016;139:431–443. doi: 10.1093/brain/awv325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stafstrom CE, Wang C, Jensen FE. Electrophysiological observations in hippocampal slices from rats treated with the ketogenic diet. Dev Neurosci. 1999;21:393–399. doi: 10.1159/000017389. [DOI] [PubMed] [Google Scholar]
- 77.Thio LL, Wong M, Yamada KA. Ketone bodies do not directly alter excitatory or inhibitory hippocampal synaptic transmission. Neurology. 2000;54:325–331. doi: 10.1212/wnl.54.2.325. [DOI] [PubMed] [Google Scholar]
- 78.Masino SA, Fortin JA, Murphy MI, Saa L, Ruskin DN. Autism spectrum disorder and homeostasis. In: Boison D, Masino SA, editors. Homeostatic Control of Brain Function. Oxford University Press; New York: 2015. pp. 586–609. [Google Scholar]
- 79.Freeman JM, Vining EP, Pillas DJ, Pyzik PL, Casey JC, Kelly LM. The efficacy of the ketogenic diet-1998: a prospective evaluation of intervention in 150 children. Pediatrics. 1998;102:1358–1363. doi: 10.1542/peds.102.6.1358. [DOI] [PubMed] [Google Scholar]
- 80.Hemingway C, Freeman JM, Pillas DJ, Pyzik PL. The ketogenic diet: a 3- to 6-year follow-up of 150 children enrolled prospectively. Pediatrics. 2001;108:898–905. doi: 10.1542/peds.108.4.898. [DOI] [PubMed] [Google Scholar]
- 81.Neal EG, Chaffe H, Schwartz RH, Lawson MS, Edwards N, Fitzsimmons G, et al. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurol. 2008;7:500–506. doi: 10.1016/S1474-4422(08)70092-9. [DOI] [PubMed] [Google Scholar]
- 82.Dressler A, Trimmel-Schwahofer P, Reithofer E, Mühlebner A, Gröppel G, Reiter-Fink E, et al. Efficacy and tolerability of the ketogenic diet in Dravet syndrome - comparison with various standard antiepileptic drug regimen. Epilepsy Res. 2015;109:81–89. doi: 10.1016/j.eplepsyres.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 83.Kossoff EH, Rho JM. Ketogenic diets: evidence from short- and long-term efficacy. Neurotherapeutics. 2009;6:406–414. doi: 10.1016/j.nurt.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Caraballo R, Vaccarezza M, Cersósimo R, Rios V, Soraru A, Arroyo H, et al. Long-term follow-up of the ketogenic diet for refractory epilepsy: multicenter Argentinean experience in 216 pediatric patients. Seizure. 2011;20:640–645. doi: 10.1016/j.seizure.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 85.Taub KS, Kessler SK, Bergqvist AGC. Risk of seizure recurrence after achieving initial seizure freedom on the ketogenic diet. Epilepsia. 2014;55:579–583. doi: 10.1111/epi.12583. [DOI] [PubMed] [Google Scholar]
- 86.Centers for Disease Control and Prevention. Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2010. Morb Mortal Wkly Rep. 2014;63(SS02):1–21. [PubMed] [Google Scholar]