Abstract
Introduction
Considering that estradiol (E2) and n-3 polyunsaturated fatty acids (PUFAs) have roles in neurogenesis and in neurotransmission, we examined whether the association of PUFAs with incident depressive symptoms in postmenopausal women is modified by hormone therapy (HT) use or estrogen status.
Methods
Women (N=1616) free of depressive symptoms at baseline (2000-02) in the Multi-Ethnic Study of Atherosclerosis were classified by HT usage and quartiles of dietary eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and the sum EPA+DHA. Women with serum E2 ≤0.073 nmol/L (sample median), were classified low on E2. Poisson regression was used to model incident depressive symptoms at examination 3 (2004-05), defined by the Center for Epidemiological Studies Depression Scale ≥16 or taking an antidepressant, first as a function of HT use and n-3 PUFA quartiles, and second, as a function of low E2 status and n-3 PUFA quartiles.
Results
Among HT non-users, positive, graded relationships (p-trends≤0.003) were found between PUFAs and incident depressive symptoms. Compared to the lowest quartile, the adjusted risk ratios (RRs) for the highest were 2.10, 2.39, and 2.04 for EPA, DHA, and EPA+DHA, respectively. For HT users, no associations were seen. When analyses were run for E2 status, the RRs over quartiles of the PUFAs were positive and graded for low E2 women, but were null for High E2 women.
Conclusions
Higher intakes of DHA and EPA were associated with higher risk of depressive symptoms in nonusers of HT, contrary to hypothesis.
Keywords: estrogen, hormone therapy, menopause, n-3 fatty acids, depressive symptoms, CES-D
1.INTRODUCTION
Eicosapentaenoic acid (EPA) and docosahexanoic acid (DHA) are key n-3 polyunsaturated fatty acids (PUFAs) studied over the past two decades for a relation with depressive disorders or symptoms in a literature that, while quite sizable, has not produced clear and unequivocal findings. The earlier literature supporting a protective association of n-3 PUFAs with depression includes ecologic (Hibbeln, 1998; Hibbeln, 2002) and clinical studies (Peet et al., 1998; Edwards et al., 1998; Maes et al., 1999). Some studies through the 2000s that did not support this association include cross-sectional (Suzuki et al., 2004), prospective (Lucas et al., 2011; Persons et al., 2014), and randomized clinical trials (Marangell et al., 2003; Hakkarainen et al., 2004; Silvers et al., 2005). Although a review and meta-analysis of 31 observational studies concluded that dietary n-3 PUFA intake was associated with lower risk of depression (Grosso et al., 2016), a recent Cochrane review (Appleton et al., 2015) of 26 randomized clinical trials concluded that there was insufficient high quality evidence to determine the effects of n-3 PUFAs as a treatment for major depressive disorder. On the other hand, apart from randomized clinical trials, several cross-sectional (Timonen et al.,2004; Colangelo et al. 2009; Beydoun et al., 2013) and incidence (Sanchez-Villegas et al., 2007; Smith et al., 2014) studies with no fewer than 1300 participants have found inverse associations of n-3 PUFAs or fish, the primary source of n-3 PUFAs, with depressive symptoms or disorders in women but not in men. This gender-specific relation has been attributed to estrogenic effects on n-3 PUFAs (Giltay et al., 2004). Studies indicate that estrogen stimulates and testosterone inhibits the conversion of essential fatty acids into their longer chain metabolites, such as the case with α-linolenic acid conversion into DHA (Decsi and Kennedy, 2011).
Apart from its effects on DHA synthesis, estrogens - namely estradiol - may also have a role in depression in women through its effects on neurotrophic function and the serotonergic system in several brain areas, such as the raphe nucleus, the hippocampus, the amygdala, the anterior cingulate cortex, and the prefrontal cortex (Borrow and Cameron, 2014). Some animal studies have shown that estradiol affects neurogenesis in the dentate gyrus of the hippocampus (Galea et al., 2013) and improves hippocampal synaptic plasticity (Bredemann and McMahon, 2014), processes implicated in the pathophysiology of depression (Christoffel et al., 2011). Given that DHA biosynthesis depends on estrogens (Giltay et al., 2004ab) and considering that estradiol (E2) and n-3 PUFAs have roles in neurogenesis (Crupi et al., 2013) and in neurotransmission, a potential interplay between sex hormones and n-3 PUFAs with respect to depression might be conjectured. The Multi-Ethnic Study of Atherosclerosis (MESA) provides an opportunity to examine whether sex hormones, in particular E2 – endogenous or exogenous – modifies the association of n-3 PUFAs with incident depressive symptoms. We hypothesize that in postmenopausal women, there will be a significant interaction between hormone therapy (HT) use and n-3 PUFA intake such that, in women taking HT, an inverse association between n-3 PUFA intake and incident depressive symptoms will be observed, but in women not taking HT, there will be no association. Additionally, for women with serum E2 levels below the median in the cohort, an inverse association of incident depressive symptoms with n-3 PUFA will be observed and there will be no association for women with E2 above the median.
2. METHODS
2.1 Study population
Initiated in 2000 to investigate the prevalence and progression of subclinical cardiovascular disease, 6,814 non-Hispanic white, African American, Chinese American, and Hispanic men and women without known cardiovascular disease, aged 45-84 years, were recruited from six US communities: Baltimore City and County, MD; Chicago. IL; Forsyth County, NC; New York, NY; Los Angeles County, CA; and St. Paul, MN. Details on the design, recruitment, and cohort examination procedures (Bild et al., 2002) and methods for blood collection (Golden et al., 2007) were published elsewhere. All participants gave informed consent, and the MESA protocol was approved by the Institutional Review Board at each participating site.
2.2 Blood collection and assessment of endogenous sex hormones
Blood specimens from fasting participants were collected in the clinic between 7:30 am and 10:30 am, processed within 30 minutes of phlebotomy, and stored at −70°C using a standardized protocol and shipped to two central laboratories. Using stored blood collected from the baseline MESA exam, serum sex hormone and binding protein concentrations were measured at the University of Massachusetts Medical Center in Worcester, MA. E2 was measured using an ultra-sensitive radioimmunoassay kit from Diagnostic System Laboratories (Webster, TX). Total testosterone (T) and dehydroepiandrosterone (DHEA) were measured directly using RIA kits, and sex hormone binding globulin (SHBG) was measured by chemiluminescent enzyme immunometric assay using Immulite kits obtained from Diagnostic Products Corporation (Los Angeles, CA). Bioavailable T was calculated using total T and SHBG concentrations according to the method of Södergard et al. (1982). Assay variability was monitored by including ~10% blind, quality control samples in each batch. The intra- and inter-assay technical errors were 8.13 and 9.31%, respectively, for total T; 5.22 and 6.39%, respectively, for SHBG; 8.75 and 5.86%, respectively, for E2; and 7.45 and 8.49% for DHEA. Because clinically meaningful cutpoints for sex hormones have not been established, we classified women as having high or low hormone status on a given hormone if her serum hormone level was above/below the median for the cohort. In this cohort the median and standard deviation (SD) for the hormones were: 0.073 (0.171) nmol/L for E2, 0.90 (1.01) nmol/L for total T, 0.21 (0.30 nmol/L for bioavailable T, 10.17 (6.34) nmol/L for DHEA, and 60.10 (55.60) nmol/L for SHBG.
2.3 Menopausal status
Women were classified as postmenopausal if (a) they responded ‘yes’ to the question, ‘Have you gone through menopause (change of life)?’, or (b) had a prior hysterectomy and bilateral oophorectomy. Years of post-menopause were computed as baseline age minus self-reported age at menopause, unless the woman had a hysterectomy and a bilateral oophorectomy, in which case years post-menopause was taken to be years from age at hysterectomy.
2.4 Diet assessment
At the baseline examination a self-administered 120-item food frequency questionnaire (FFQ) assessed the usual dietary intake over the past year. The FFQ was modified from the validated Insulin Resistance Atherosclerosis study in which comparable validity was observed for non-Hispanic white, African American, and Hispanic individuals (Mayer-Davis et al., 1999). The MESA dietary assessment was modified to include foods typically eaten by Chinese groups. The criterion validity of the MESA FFQ was established by quantifying the concordance of the FFQ known relationships between macronutrients and plasma lipid concentrations (Nettleton et al., 2009). De Oliveira Otto et al. (2013) showed in MESA that higher circulating EPA and DHA and higher dietary EPA and DHA were inversely associated with markers of inflammation and with lower cardiovascular disease incidence. A section on vitamins, minerals, and other nutritional supplements – which was not self-administered - was completed at the time of the medication inventory supplement form. Participants were instructed to bring in bottles of any vitamin or other nutritional supplements they took, along with all prescription and over-the-counter medications. Nutrients including n-3 PUFAs were derived from the Minnesota Nutrition Data System NDS software (version 4.02/30; Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN). EPA and DHA were expressed as the percentage of total energy intake.
2.5 Outcome variable assessment
Depressive symptoms were measured at the baseline (2000-02) and the third examinations (2004-05) using the 20-item CES-D scale (Radloff, 1977), which has a maximum score of 60. The CES-D, which was self-administered in English, Spanish, Cantonese, and Mandarin, asks participants to indicate how often they experienced each symptom in the past week (“rarely or none of the time (less than 1 day),” “some or a little of the time (1-2 days),” “a moderate amount of the time (3-4 days),” or “most of the time (5-7 days).” Scores for the four possible responses range from 0 to 3 points. Examples of symptoms included in the scale are poor appetite, trouble concentrating, restless sleep, feelings of depressed mood, crying spells, feeling disliked, talking less than usual, and inability to “get going.” A cutoff score of ≥16 is suggested in epidemiologic studies to indicate a high level of depressive symptoms (Radloff, 1977; Radloff and Locke, 1986). We defined depressive symptoms as being present if the CES-D score was ≥16 or if the participant was taking an antidepressant medication. In a secondary analysis, because antidepressants might be prescribed for conditions other than depression, we defined depressive symptoms as present if CES-D ≥16.
2.6 Other participant characteristics
Information on participant demographic and lifestyle characteristics, medical history, and medication use was collected with standardized questionnaires: height and weight were measured and body mass index (BMI) was calculated as weight (kilograms)/height (meters squared). Age, race/ethnicity, years of education, marital status, cigarette smoking history, alcohol intake, medical history and annual income were self-reported. Women were asked to bring in all medications they were taking to each examination, but they were not specifically asked if they were taking a medication for depression. Five categories were used to model smoking status: never smokers, past smokers with fewer than 12.05 pack-years, past smokers with at least 12.05 pack-years, current smokers with fewer than 21.05 pack-years, and current smokers with at least 21.05 pack years. The cutpoints of 12.05 and 21.05 pack years were selected as the medians for past smokers and current smokers, respectively. Physical activity measured by a total intentional exercise variable was categorized into quartiles. The MESA Typical Week Physical Activity Survey is described elsewhere (Bertoni et al., 2009). Income, which was categorized into 13 levels, was treated an ordinal variable.
2.7 Statistical Analysis
Statistical analyses were conducted using SAS for Windows, release 9.4 (SAS Institute Inc., Cary, NC, USA). Descriptive characteristics were tabulated separately for users and non-users of HT across quartiles of EPA + DHA. Multivariable modified Poisson regression (Zou, 2004), implemented through SAS PROC GENMOD with a REPEATED statement to obtain robust error variances, was used to assess the associations of n-3 PUFAs and HT usage/estradiol status with incident depressive symptoms and compute risk ratios (RRs). Tests for interactions between quartiles of n-3 PUFAs and HT status were obtained by specifying the TYPE 3 option in the MODEL statement of PROC GENMOD. The interaction terms were used to obtain RRs over the n-3 PUFA quartiles for HT users and HT non-users. Tests for trend over the quartiles were done by assigning the median n-3 PUFA value in its quartile to all participants in the quartile and modeling it as a continuous variable. We also tested for interactions between quartiles of n-3 PUFAs and E2 status.
We also examined the associations of n-3 PUFA intake with incident depressive symptoms treating the n-3 PUFAs as continuous variables and stratifying by HT use. A generalized additive model (gam), implemented with the “mgcv” package in R.3.1.3 (R foundation for statistical computing), was used to model and illustrate the probability of having depressive symptoms at examination 3 as a function of n-3 PUFAs for HT users and nonusers. This method allows examination for nonlinear associations in the predictor variable on the probability scale (absolute risk). To quantify absolute risk while adjusting for confounders, we used an additive binomial linear model (BLM) available in the R package “blm” (Kovalchik et al. 2013). We computed absolute risk per 1000 women for each n-3 PUFA quartile for nonusers and for users of HT. Statistical models – RR regression models, BLMs and gams – were adjusted for age, race, body mass index, smoking status, intentional exercise, education, income, current drinking status, marital status, diabetes, and energy intake. Exploratory analyses were done assessing multiplicative interactions of n-3 PUFAs with total T, bioavailable T, DHEA, and SHBG.
Subsequent to finding primary results contradictory to our original hypotheses, we conducted additional post hoc exploratory analyses that looked at possible neglected confounders and potential misclassification of menopausal status. We introduced one at a time to the fully adjusted models of tables 2 and 3 variables representing inflammation (via log(C-reactive protein)), cardiovascular disease risk (via Framingham Global CVD Risk Score), reproductive history (via number of live births), and dietary elements associated with depression. The dietary variables consisted of folate, vitamin B12, zinc, vitamin D (Sarris et al., 2016) and selenium (Conner et al., 2015). Because 21 women who self-reported that they were 0 years postmenopause may actually have been perimenopausal by STRAW+10 criteria (Harlow et al., 2012), we conducted the analyses in Tables 2 and 3 again after excluding these women.
Table 2.
Adjusted risk ratios for incident depressive symptoms (CES-D score ≥ 16 or taking antidepressant medication) at examination 3 (2004-2005) according to quartiles of n-3 PUFAs and HT status at baseline (2000-2002).
| Interaction | No Hormone Therapy (n=1080) | Taking Hormone Therapy (n=536) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Quartiles of EPA | p=0.17c | Cases/N (%) | Minimally Adjusted RRa | (95% CI) | Fully Adjusted RRb | (95% CI) | Cases/N (%) | Minimally Adjusted RRa | (95% CI) | Fully Adjusted RRb | (95% CI) |
| 1 | 26/268 (9.7) | 1.00 | 1.00 | 26/136 (19.1) | 1.00 | 1.00 | |||||
| 2 | 30/274 (11.0) | 1.21 | (0.74-2.00) | 1.29 | (0.78-2.13) | 20/130 (15.4) | 0.88 | (0.52-1.49) | 0.96 | (0.57-1.63) | |
| 3 | 28/261 (10.7) | 1.32 | (0.79-2.22) | 1.46 | (0.87-2.44) | 24/143 (16.8) | 0.96 | (0.58-1.58) | 1.10 | (0.67-1.83) | |
| 4 | 37/277 (13.4) | 1.80 | (1.10-2.95) | 2.10 | (1.27-3.48) | 17/127 (13.4) | 0.82 | (0.47-1.44) | 0.93 | (0.53-1.65) | |
| p-trend | 0.02 | 0.003 | 0.49 | 0.89 | |||||||
| Quartiles of DHA | p=0.03c | ||||||||||
| 1 | 25/274 (9.1) | 1.00 | 1.00 | 24/130 (18.5) | 1.00 | 1.00 | |||||
| 2 | 26/260 (10.0) | 1.14 | (0.68-1.92) | 1.23 | (0.73-2.08) | 24/144 (16.7) | 0.92 | (0.55-1.52) | 0.97 | (0.59-1.60) | |
| 3 | 30/277 (10.8) | 1.43 | (0.86-2.38) | 1.55 | (0.93-2.59) | 22/127 (17.3) | 1.01 | (0.60-1.70) | 1.09 | (0.64-1.83) | |
| 4 | 40/269 (14.9) | 2.08 | (1.29-3.38) | 2.39 | (1.45-3.93) | 17/135 (12.6) | 0.77 | (0.43-1.36) | 0.85 | (0.48-1.51) | |
| p-trend | 0.001 | 0.0003 | 0.33 | 0.65 | |||||||
| Quartiles of EPA+DHA | P=0.10c | ||||||||||
| 1 | 27/271 (10.0) | 1.00 | 1.00 | 25/133 (18.8) | 1.00 | 1.00 | |||||
| 2 | 27/265 (10.2) | 1.09 | (0.66-1.81) | 1.17 | (0.71-1.95) | 23/139 (16.6) | 0.91 | (0.55-1.52) | 1.01 | (0.61-1.67) | |
| 3 | 30/274 (11.0) | 1.33 | (0.80-2.20) | 1.46 | (0.88-2.44) | 22/130 (16.9) | 0.97 | (0.58-1.62) | 1.07 | (0.64-1.80) | |
| 4 | 37/270 (13.7) | 1.78 | (1.10-2.90) | 2.04 | (1.24-3.37) | 17/134 (12.7) | 0.76 | (0.43-1.34) | 0.86 | (0.49-1.53) | |
| p-trend | 0.01 | 0.003 | 0.30 | 0.69 | |||||||
RR, risk ratio; HT, hormone therapy; EPA, eicosapentaenoic acid; DHA, docosahexanoic acid; CI, confidence interval.
Minimally adjusted model included variables for age, ethnicity, energy intake, HT status, quartiles of n-3 PUFA, and interaction of HT with n-3 PUFA quartiles.
Fully adjusted model included all variables in the minimally adjusted model plus body mass index, smoking status, quartiles of exercise, education status (3 categories: less than high school, high school or some college, Bachelor's degree or higher), income, current drinker, marital status (4 categories: married, widowed, divorced or separated, and never married or no response), and diabetes.
HT X n-3 PUFA interaction on 3 degrees of freedom from fully adjusted model.
Table 3.
Adjusted risk ratios for incident depressive symptoms (CES-D score ≥ 16 or taking antidepressant medication) at examination 3 (2004-2005) according to quartiles of n-3 PUFAs and E2 status at baseline (2000-2002).
| Interaction | E2 ≤ median (n=824) | E2 > median (n=792) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Quartiles of EPA | p=0.48c | Cases/N (%) | Minimally Adjusted RRa | (95% CI) | Fully Adjusted RRb | (95% CI) | Cases/N (%) | Minimally Adjusted RRa | (95% CI) | Fully Adjusted RRb | (95% CI) |
| 1 | 19/199 (9.6) | 1.00 | 1.00 | 33/205 (16.1) | 1.00 | 1.00 | |||||
| 2 | 24/211 (11.4) | 1.29 | (0.73-2.28) | 1.36 | (0.77-2.41) | 26/193 (13.5) | 0.90 | (0.56-1.44) | 0.98 | (0.61-1.58) | |
| 3 | 26/199 (13.1) | 1.63 | (0.93-2.86) | 1.70 | (0.97-2.98) | 26/205 (12.7) | 0.88 | (0.55-1.41) | 1.03 | (0.63-1.67) | |
| 4 | 27/215 (12.6) | 1.70 | (0.96-3.02) | 2.00 | (1.12-3.60) | 27/189 (14.3) | 1.08 | (0.67-1.72) | 1.22 | (0.75-1.98) | |
| p-trend | 0.08 | 0.04 | 0.66 | 0.30 | |||||||
| Quartiles of DHA | p=0.16c | ||||||||||
| 1 | 19/209 (9.1) | 1.00 | 1.00 | 30/195 (15.4) | 1.00 | 1.00 | |||||
| 2 | 19/194 (9.8) | 1.14 | (0.62-2.09) | 1.24 | (0.68-2.28) | 31/210 (14.8) | 0.97 | (0.61-1.53) | 1.01 | (0.64-1.60) | |
| 3 | 28/213 (13.2) | 1.74 | (1.00-3.04) | 1.86 | (1.06-3.26) | 24/191 (12.6) | 0.90 | (0.55-1.48) | 0.98 | (0.60-1.60) | |
| 4 | 30/208 (14.4) | 1.99 | (1.14-3.47) | 2.32 | (1.31-4.09) | 27/196 (13.8) | 1.05 | (0.65-1.70) | 1.15 | (0.70-1.87) | |
| p-trend | 0.008 | 0.004 | 0.81 | 0.47 | |||||||
| Quartiles of EPA+DHA | P=0.32c | ||||||||||
| 1 | 21/209 (10.1) | 1.00 | 1.00 | 31/195 (15.9) | 1.00 | 1.00 | |||||
| 2 | 20/196 (10.2) | 1.09 | (0.61-1.95) | 1.18 | (0.66-2.11) | 30/208 (14.4) | 0.94 | (0.59-1.48) | 1.03 | (0.65-1.63) | |
| 3 | 28/211 (13.3) | 1.60 | (0.93-2.76) | 1.72 | (0.99-2.98) | 24/193 (12.4) | 0.86 | (0.52-1.40) | 0.97 | (0.59-1.59) | |
| 4 | 27/208 (13.0) | 1.64 | (0.94-2.85) | 1.90 | (1.08-3.36) | 27/196 (13.8) | 1.02 | (0.64-1.65) | 1.15 | (0.70-1.87) | |
| p-trend | 0.06 | 0.03 | 0.86 | 0.48 | |||||||
RR, risk ratio; HT, hormone therapy; EPA, eicosapentaenoic acid; DHA, docosahexanoic acid; CI, confidence interval.
Minimally adjusted model included variables for age, ethnicity, energy intake, E2 status, quartiles of n-3 PUFA, and interaction of E2 status with n-3 PUFA quartiles.
Fully adjusted model included all variables in the minimally adjusted model plus body mass index, smoking status, quartiles of exercise, education status (3 categories: less than high school, high school or some college, Bachelor's degree or higher), income, current drinker, marital status (4 categories: married, widowed, divorced or separated, and never married or no response), diabetes, and HT.
E2 X n-3 PUFA interaction on 3 degrees of freedom from fully adjusted model.
From the 3,601 women in the MESA cohort, we excluded 592 without sex hormone levels, 3 missing menopause status, 47 who were not menopausal, 62 missing age at menopause, 15 missing CES-D score at baseline, 403 who did not return to the third examination or who were missing CES-D score at examination 3, 31 who were missing information on HT, 115 who were missing covariate data, and 522 who had clinically significant depression at baseline, defined as CES-D score of 16 or higher and/or taking antidepressant medication at baseline, leaving 1811 women in the analytic cohort. From the 1811, an additional 195 were excluded for having extremely high (>6000 kcal/d) or low energy (<600 kcal/d) intake, leaving 1616 in the final analytic cohort.
We compared the baseline characteristics of the 1616 women included in the analytic cohort to the 1126 who were excluded and not depressed. Compared to those excluded, those included were on average older (mean 64.5 vs 59.4 years, p<0.001), had lower BMI (mean 28.3 vs 29.0 kg/m2, p=0.004), higher median income ($35K-$39.9K vs $30K-$34.9K, p=0.09), were more likely to have at least a bachelor's degree (32.5% vs 28.6%, p=0.04) and consume alcohol (51.4% vs 45.9%, p=0.005), and were less likely to have diabetes (10.8% vs 13.4%, p=0.03) or to smoke (9.0% vs 13.4%, p<0.001).
3. RESULTS
Mean age (± standard deviation) in the cohort of 1616 women was 64.5 (±8.8) years at baseline and 536 (33%) women were HT users. Mean follow-up (± standard deviation) was 3.2 (±0.3) years. Overall n-3 PUFA intakes were low. Mean ± SD intakes of EPA and DHA were 0.04 (0.04) gms and 0.08 (0.06) gms, respectively. The maximum EPA+DHA was 1.06 gms. Among 1080 HT nonusers, there were 121 (11%) incident cases of high depressive symptoms at examination 3 and among 536 HT users, there were 87 (16%) cases. Table 1 shows characteristics of the HT nonusers and users by quartile of EPA+DHA. HT users were on average fewer years postmenopause than nonusers and both HT users and nonusers were, on average, overweight. Whites were more often represented among current users. HT users were more likely to be married and less likely to be widowed. MET-minutes/week of intentional exercise also tended to be greater and family income and level of education was higher among HT users. For both HT nonusers and users, the highest quartile of EPA+DHA had lowest mean energy intake.
Table 1.
Baseline characteristics of participants by hormone therapy status and quartiles of EPA+DHA(% of energy).
| No Hormone Therapy | On Hormone Therapy | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1: 0.0 – 0.03288 | Q2: 0.03290-0.05648 | Q3: 0.05649-0.09485 | Q4: 0.09493-0.56529 | Q1: 0.0 – 0.03288 | Q2: 0.03290-0.05648 | Q3: 0.05649-0.09485 | Q4: 0.09493-0.56529 | |||||||||
| N | 271 | 265 | 274 | 270 | 133 | 139 | 130 | 134 | ||||||||
| Age, years | 66.1 | 9.4 | 65.2 | 8.8 | 65.3 | 8.9 | 65.7 | 8.7 | 63.5 | 8.8 | 62.4 | 7.7 | 62.1 | 8.8 | 61.2 | 7.3 |
| Years postmenopause | 18.4 | 11.4 | 16.9 | 10.6 | 17.5 | 10.7 | 16.9 | 10.3 | 17.1 | 10.5 | 14.4 | 9.0 | 14.5 | 9.7 | 12.9 | 9.5 |
| E2 > median (0.073 nmol/L) (%) | 74 | 27.3 | 83 | 31.3 | 80 | 29.2 | 79 | 29.3 | 121 | 91.0 | 125 | 89.9 | 113 | 86.9 | 117 | 87.3 |
| BMI | 28.8 | 5.4 | 28.8 | 6.1 | 28.8 | 6.1 | 28.3 | 5.9 | 28.2 | 5.9 | 27.6 | 5.2 | 27.4 | 5.5 | 26.5 | 5.2 |
| Race (%) | ||||||||||||||||
| White | 103 | 38.0 | 93 | 35.1 | 85 | 31.0 | 71 | 26.3 | 71 | 53.4 | 90 | 64.8 | 76 | 58.5 | 72 | 53.7 |
| Chinese | 21 | 7.8 | 23 | 8.7 | 38 | 13.9 | 72 | 26.7 | 5 | 3.7 | 6 | 4.3 | 12 | 9.2 | 25 | 18.7 |
| Black | 41 | 15.1 | 76 | 28.7 | 113 | 41.2 | 101 | 37.4 | 25 | 18.8 | 26 | 18.7 | 29 | 22.3 | 29 | 21.6 |
| Hispanic | 106 | 39.1 | 73 | 27.6 | 38 | 13.9 | 26 | 9.6 | 32 | 24.1 | 17 | 12.2 | 13 | 10.0 | 8 | 6.0 |
| Marital status (%) | ||||||||||||||||
| Married | 123 | 45.4 | 141 | 53.2 | 129 | 47.1 | 140 | 51.9 | 79 | 59.4 | 95 | 68.4 | 70 | 53.9 | 89 | 66.4 |
| Widowed | 69 | 25.5 | 63 | 23.8 | 78 | 28.5 | 60 | 22.2 | 21 | 15.8 | 13 | 9.4 | 20 | 15.4 | 9 | 6.7 |
| Divorced or separated | 53 | 19.6 | 39 | 14.7 | 41 | 15.0 | 42 | 15.6 | 25 | 18.8 | 25 | 18.0 | 25 | 19.2 | 26 | 19.4 |
| Never married or no answer | 26 | 9.6 | 22 | 8.3 | 26 | 9.5 | 28 | 10.4 | 8 | 6.0 | 6 | 4.3 | 15 | 11.5 | 10 | 7.5 |
| Current smoker (%) | 21 | 7.8 | 27 | 10.2 | 27 | 9.9 | 19 | 7.0 | 16 | 12.0 | 10 | 7.2 | 13 | 10.0 | 13 | 9.7 |
| Current drinker (%) | 102 | 37.6 | 128 | 48.3 | 141 | 51.5 | 130 | 48.2 | 68 | 51.1 | 88 | 63.3 | 86 | 66.2 | 88 | 65.7 |
| Education Level (%) | ||||||||||||||||
| Less than HS | 85 | 31.4 | 61 | 23.0 | 38 | 13.9 | 46 | 17.0 | 16 | 12.0 | 14 | 10.1 | 8 | 6.2 | 10 | 7.5 |
| HS or some college | 143 | 52.8 | 135 | 50.9 | 148 | 54.0 | 122 | 45.2 | 84 | 63.2 | 68 | 48.9 | 58 | 44.6 | 55 | 41.0 |
| BS or higher degree | 43 | 15.9 | 69 | 26.0 | 88 | 32.1 | 102 | 37.8 | 33 | 24.8 | 57 | 41.0 | 64 | 49.2 | 69 | 51.5 |
| Total Intentional exercise, MET-minutes/week | 1048 | 1618 | 1136 | 1655 | 1356 | 1871 | 1489 | 1891 | 1196 | 1892 | 1633 | 1960 | 1409 | 1641 | 1685 | 1989 |
| Total family income. median | $25,000-$29,999 | $30,000-$34,999 | $35,000-$39,999 | $30,000-$34,999 | $35,000-$39,999 | $50,000-$74,999 | $50,000-$74,999 | $50,000-$74,999 | ||||||||
| Diabetes (%) | 27 | 10.0 | 38 | 14.3 | 32 | 11.7 | 30 | 11.1 | 17 | 12.8 | 8 | 5.8 | 12 | 9.2 | 11 | 8.2 |
| Energy, kcal | 1545 | 728 | 1555 | 715 | 1446 | 621 | 1338 | 615 | 1557 | 677 | 1519 | 593 | 1451 | 587 | 1351 | 624 |
Data are mean (SD) or n (%) unless otherwise noted
Table 2 shows the minimally adjusted and fully adjusted RRs for incident depressive symptoms over the quartiles of n-3 PUFAs by HT status. For HT nonusers, the minimally adjusted RRs reveal significant positive and graded associations of each n-3 PUFA with incident depressive symptoms. After full adjustment, the RRs increased in magnitude with DHA maintaining the greatest magnitude in the highest quartile, RR=2.4. For HT users, there were no associations for any n-3 PUFA in either minimally or fully adjusted models.
Figure 1 shows the fully adjusted associations of EPA+DHA as a continuous variable with probability of having depressive symptoms for HT nonusers on the left and HT users on the right. Using the gam model, the association appears positive and linear for the HT nonusers whereas the association is absent for the HT users. Using the fully adjusted BLM, we computed the fitted absolute risks and standard errors (SE) of depressive symptoms per 1000 women for the 4 quartiles of EPA+DHA by HT user/nonuser status. For HT nonusers, in order of quartiles, the fitted risks (SE) were: 93.4 (12.2), 96.0 (11.6), 111.5 (13.1), and 159.0 (18.4). For HT users the fitted risks (SE) were: 155.1 (28.6), 167.0 (29.4), 171.4 (29.9), and 133.8 (24.0).
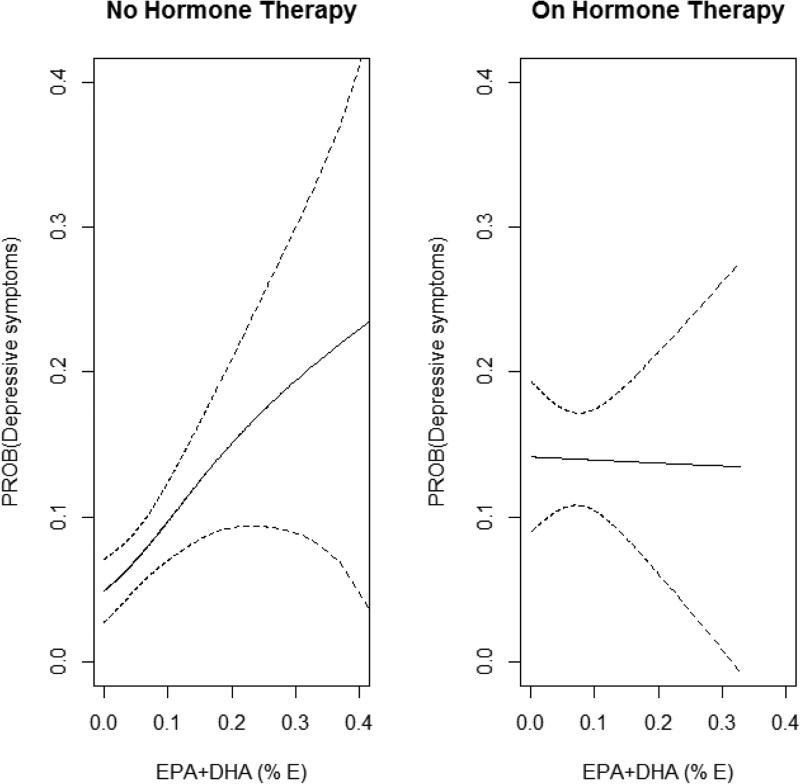
Figure.
Left panel: Adjusted relationship by spline regression between probability of having incident depressive symptoms at examination 3 and EPA+DHA as a percent of energy intake for HT nonusers (n=1087). Spline (with 95% pointwise confidence band) is adjusted for age, ethnicity, energy intake, body mass index, smoking status, quartiles of exercise, education status, income, current drinker, marital status, and diabetes.
Right panel: Adjusted relationship by spline regression between probability of having incident depressive symptoms at examination 3 and EPA+DHA as a percent of energy intake for HT users (n=538). Spline (with 95% pointwise confidence band) is adjusted for age, ethnicity, energy intake, body mass index, smoking status, quartiles of exercise, education status, income, current drinker, marital status, and diabetes.
When analyses for the RR regression models were rerun substituting high E2 status (i.e., above median E2) for HT status (Table 3), for women at or below the median E2 level, the RRs over the quartiles of each n-3 PUFA had similar patterns to those seen in Table 2. For women above the median E2 level, there were no associations.
In secondary analyses defining depressive symptoms by CES-D ≥16 without consideration of antidepressant use, although the RRs for incident depressive symptoms were all attenuated for HT nonusers (Table 4), they remained positive and graded in the fully adjusted models. The trends for DHA remained significant, but for EPA and for EPA+DHA, the trends were marginally significant. There were still no significant associations for the HT users (Table 4). In the secondary analyses by E2 status (Table 5), the RRs for women with E2 ≤ median E2 level for all PUFAs were again attenuated and only the trend for DHA was marginally significant (p=0.05) in the fully adjusted model. For women with E2 above the median, the RRs over the PUFA quartiles showed positive trends departing from the null, but they did not reach significance.
Table 4.
Adjusted risk ratios for incident depressive symptoms (CES-D score ≥ 16) at examination 3 (2004-2005) according to quartiles of n-3 PUFAs and HT status at baseline (2000-2002).
| Interaction | No Hormone Therapy (n=1080) | Taking Hormone Therapy (n=536) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Quartiles of EPA | p=0.85c | Cases/N (%) | Minimally Adjusted RRa | (95% CI) | Fully Adjusted RRb | (95% CI) | Cases/N (%) | Minimally Adjusted RRa | (95% CI) | Fully Adjusted RRb | (95% CI) |
| 1 | 26/268 (9.7) | 1.00 | 1.00 | 12/136 (8.8) | 1.00 | 1.00 | |||||
| 2 | 25/274 (9.1) | 1.03 | (0.61-1.74) | 1.10 | (0.64-1.88) | 9/130 (6.9) | 0.92 | (0.41-2.07) | 1.04 | (0.46-2.37) | |
| 3 | 22/261 (8.4) | 1.08 | (0.62-1.90) | 1.23 | (0.70-2.15) | 12/143 (8.4) | 1.12 | (0.53-2.36) | 1.31 | (0.60-2.82) | |
| 4 | 28/277 (10.1) | 1.38 | (0.80-2.36) | 1.67 | (0.95-2.94) | 9/127 (7.1) | 1.00 | (0.44-2.24) | 1.18 | (0.52-2.70) | |
| p-trend | 0.21 | 0.05 | 0.92 | 0.57 | |||||||
| Quartiles of DHA | p=0.73c | ||||||||||
| 1 | 24/274 (8.8) | 1.00 | 1.00 | 9/130 (6.9) | 1.00 | 1.00 | |||||
| 2 | 22/260 (8.5) | 1.00 | (0.58-1.72) | 1.09 | (0.63-1.90) | 12/144 (8.3) | 1.24 | (0.55-2.81) | 1.31 | (0.58-2.97) | |
| 3 | 25/277 (9.0) | 1.28 | (0.74-2.20) | 1.40 | (0.81-2.41) | 11/127 (8.7) | 1.39 | (0.60-3.22) | 1.50 | (0.64-3.52) | |
| 4 | 30/269 (11.2) | 1.67 | (0.98-2.84) | 1.99 | (1.14-3.46) | 10/135 (7.4) | 1.24 | (0.53-2.92) | 1.44 | (0.60-3.44) | |
| p-trend | 0.04 | 0.009 | 0.65 | 0.25 | |||||||
| Quartiles of EPA+DHA | P=0.88 c | ||||||||||
| 1 | 26/271 (9.6) | 1.00 | 1.00 | 10/133 (7.5) | 1.00 | 1.00 | |||||
| 2 | 24/265 (9.1) | 1.02 | (0.60-1.73) | 1.10 | (0.65-1.87) | 11/139 (7.9) | 1.16 | (0.52-2.58) | 1.28 | (0.57-2.85) | |
| 3 | 23/274 (8.4) | 1.09 | (0.63-1.90) | 1.24 | (0.71-2.17) | 11/130 (8.5) | 1.27 | (0.57-2.85) | 1.41 | (0.62-3.22) | |
| 4 | 28/270 (10.4) | 1.42 | (0.83-2.43) | 1.68 | (0.96-2.96) | 10/134 (7.5) | 1.17 | (0.51-2.68) | 1.38 | (0.59-3.20) | |
| p-trend | 0.18 | 0.05 | 0.74 | 0.30 | |||||||
RR, risk ratio; HT, hormone therapy; EPA, eicosapentaenoic acid; DHA, docosahexanoic acid; CI, confidence interval.
Minimally adjusted model included variables for age, ethnicity, energy intake, HT status, quartiles of n-3 PUFA, and interaction of HT with n-3 PUFA quartiles.
Fully adjusted model included all variables in the minimally adjusted model plus body mass index, smoking status, quartiles of exercise, education status (3 categories: less than high school, high school or some college, Bachelor's degree or higher), income, current drinker, marital status (4 categories: married, widowed, divorced or separated, and never married or no response), and diabetes.
HT X n-3 PUFA interaction on 3 degrees of freedom from fully adjusted model.
Table 5.
Adjusted risk ratios for incident depressive symptoms (CES-D score ≥ 16) at examination 3 (2004-2005) according to quartiles of n-3 PUFAs and E2 status at baseline (2000-2002).
| Interaction | E2 ≤ median (n=824) | E2 > median (n=792) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Quartiles of EPA | p=0.87 c | Cases/N (%) | Minimally Adjusted RRa | (95% CI) | Fully Adjusted RRb | (95% CI) | Cases/N (%) | Minimally Adjusted RRa | (95% CI) | Fully Adjusted RRb | (95% CI) |
| 1 | 19/199 (9.6) | 1.00 | 1.00 | 19/205 (9.3) | 1.00 | 1.00 | |||||
| 2 | 20/211 (9.5) | 1.13 | (0.62-2.06) | 1.18 | (0.65-2.15) | 14/193 (7.3) | 0.86 | (0.45-1.65) | 0.97 | (0.50-1.90) | |
| 3 | 20/199 (10.1) | 1.30 | (0.71-2.40) | 1.43 | (0.78-2.62) | 14/205 (6.8) | 0.89 | (0.46-1.71) | 1.06 | (0.54-2.09) | |
| 4 | 19/215 (8.8) | 1.24 | (0.66-2.35) | 1.49 | (0.77-2.88) | 18/189 (9.5) | 1.28 | (0.70-2.35) | 1.53 | (0.81-2.88) | |
| p-trend | 0.57 | 0.27 | 0.27 | 0.10 | |||||||
| Quartiles of DHA | p=0.99 c | ||||||||||
| 1 | 18/209 (8.6) | 1.00 | 1.00 | 15/195 (7.7) | 1.00 | 1.00 | |||||
| 2 | 17/194 (8.8) | 1.07 | (0.57-2.02) | 1.19 | (0.63-2.25) | 17/210 (8.1) | 1.06 | (0.55-2.04) | 1.13 | (0.59-2.16) | |
| 3 | 21/213 (9.9) | 1.41 | (0.77-2.59) | 1.52 | (0.83-2.79) | 15/191 (7.9) | 1.18 | (0.60-2.34) | 1.31 | (0.65-2.63) | |
| 4 | 22/208 (10.6) | 1.60 | (0.86-2.96) | 1.93 | (1.03-3.63) | 18/196 (9.2) | 1.45 | (0.76-2.78) | 1.68 | (0.86-3.27) | |
| p-trend | 0.13 | 0.05 | 0.17 | 0.06 | |||||||
| Quartiles of EPA+DHA | P=0.89 c | ||||||||||
| 1 | 21/209 (10.1) | 1.00 | 1.00 | 15/195 (7.7) | 1.00 | 1.00 | |||||
| 2 | 17/196 (8.7) | 0.96 | (0.52-1.77) | 1.02 | (0.55-1.88) | 18/208 (8.7) | 1.20 | (0.63-2.28) | 1.32 | (0.70-2.52) | |
| 3 | 20/211 (9.5) | 1.19 | (0.65-2.15) | 1.32 | (0.72-2.42) | 14/193 (7.3) | 1.09 | (0.55-2.17) | 1.24 | (0.61-2.50) | |
| 4 | 20/208 (9.6) | 1.25 | (0.68-2.31) | 1.50 | (0.80-2.83) | 18/196 (9.2) | 1.46 | (0.77-2.79) | 1.70 | (0.87-3.31) | |
| p-trend | 0.47 | 0.21 | 0.22 | 0.09 | |||||||
RR, risk ratio; HT, hormone therapy; EPA, eicosapentaenoic acid; DHA, docosahexanoic acid; CI, confidence interval.
Minimally adjusted model included variables for age, ethnicity, energy intake, E2 status, quartiles of n-3 PUFA, and interaction of E2 status with n-3 PUFA quartiles.
Fully adjusted model included all variables in the minimally adjusted model plus body mass index, smoking status, quartiles of exercise, education status (3 categories: less than high school, high school or some college, Bachelor's degree or higher), income, current drinker, marital status (4 categories: married, widowed, divorced or separated, and never married or no response), diabetes, and HT.
E2 X n-3 PUFA interaction on 3 degrees of freedom from fully adjusted model.
In prespecified exploratory analyses we assessed whether there were any interactions of EPA+DHA with total T, bioavailable T, DHEA, or SHBG. Interactions were tested using quartiles of EPA+DHA with each log-transformed hormone and with square-root transformed EPA+DHA with the log-transformed hormones. A significant interaction was apparent only for SHBG (p=0.04 for square root EPA+DHA × logSHBG). For participants with SHBG at or below the median level, the RRs (95% CI) for quartiles 1 through 4 of EPA+DHA were: 1, 1.23 (0.68, 2.124), 1.87 (1.06, 3.29), and 1.89 (1.04, 3.44), respectively. For participants with SHBG above the median level, all RRs over the quartiles were nonsignificant and close to unity.
In the post hoc exploratory analyses, only the inclusion of dietary selenium to the fully adjusted models affected the RRs for the n-3 PUFA quartiles. Whereas the fully adjusted RR (95% CI) for the highest quartile of DHA for non-HT users was 2.39 (1.45-3.93) in the original analysis, after including selenium it became 1.91 (1.09-3.33).
4. DISCUSSION
To the best of our knowledge this is the first cohort study to examine whether estrogen status of postmenopausal women modifies the association of n-3 PUFA intake with incident depressive symptoms. Recently, Jin et al. (2016) reported cross-sectional inverse correlations of erythrocyte levels of n-3 PUFAs with Beck Depression Inventory scores only in postmenopausal women using HT, and no associations in the women not using HT, supporting an interaction between n-3 PUFA and estrogen with depressive symptoms. While the findings of Jin et al. are consistent with the hypotheses we advanced at the outset of our study, they are in stark contrast to our findings, as our findings are in contrast to our hypotheses. Although we did find a qualitative interaction of HT with n-3 PUFA intake on incident depressive symptoms, the nature and directions of the n-3 PUFA – depression associations were contrary to our hypothesis in the HT users and the HT nonusers: the association was null in HT users and positive in the nonusers. Moreover, the magnitude of the associations in nonusers in our study was non trivial with RRs ≥ 2 in the highest quartile of n-3 PUFA. This raises the possibility of either a spurious association due to selection bias or uncontrolled confounding – and these circumstances are potential in either study. In the cross-sectional study by Jin et al., details on the process of selecting the 214 participants were not provided. While the potential confounders adjusted for in the present study were numerous and included demographics, SES, and lifestyle factors, an omitted confounder or residual confounding cannot be excluded. Our post hoc exploratory analyses only revealed dietary selenium with the potential to attenuate the RRs for the quartiles of n-3 PUFAs. However, it is important to note that selenium is not typically associated with n-3 PUFAs (Park et al. 2011) and the preferred method of estimating selenium levels is from a biochemical marker such as with neutron activation analysis of toenails (He, 2011). Using an FFQ to estimate selenium exposure is problematic due to selenium varying widely within individual foods, resulting in inaccurate food composition tables for selenium (Willett, p 175, 1998). Therefore, the RRs resulting with selenium from FFQ in the statistical models should be interpreted with caution.
While this still does not address the issue of an unmeasured confounder, we note that if there is an unmeasured confounder U, then an extension of the “Cornfield conditions” (Ding and VanderWeele, 2016) implies we should consider scenarios where the n-3 PUFA – U relative risk would have to be at least as large as the observed n-3 PUFA- depression relative risk (which is ~2 for the highest n-3 PUFA quartile) and the U – depression relative risk would also have to be at least as large as the observed n-3 PUFA – depression relative risk (Ding and VanderWeele, 2016).
It is also possible that the association is real, but an incomplete state of knowledge permits only speculation about the existence of an underlying mechanism that could produce a positive relation of n-3 PUFAs with incident depressive symptoms in postmenopausal women who have a low E2 status. Estrogen has a role in the biosynthesis of DHA (Kitson et al., 2010), as indicated by the works of Giltay and colleagues (2004a, 2004b). Postmenopausal women taking estrogen replacement have increased circulating DHA compared to postmenopausal women not using hormone therapy (Giltay et al. 2004a) and in ovariectomized female-to-male transsexuals the effect of receiving testosterone was to decrease the plasma cholesteryl ester DHA whereas in male-to-female transexuals the effect of receiving estradiol was to increase DHA in plasma cholesteryl ester (Giltay et al., 2004b). It has been suggested estrogen might increase the concentration or activity of DHA synthesis enzymes (Kitson et al., 2010). These enzymes include the delta-6 and delta-5 desaturases, elongases, and peroxisomal β-oxidation enzymes (Kitson et al., 2010). The pathways of enzymatic conversion of essential fatty-acids to longer-chain metabolites have been detailed elsewhere (Kitson et al, 2010; Decsi and Kennedy, 2011).
There are limited studies that consider an interplay of ovarian hormones with DHA composition in the brain, and these were in rat models (Allessandri et al., 2011; Fabelo et al., 2011; McNamara et al., 2009). One such study was initiated subsequent to finding that DHA was significantly reduced in the postmortem prefrontal cortex of female, but not male, patients with major depression (McNamara et al., 2007). McNamara et al. (2009) evaluated erythrocyte and brain DHA composition in intact male and female rats as well as in oophorectomized rats with or without cyclic estradiol treatment. Oophorectomy resulted with decreased DHA in the hippocampus, but not in the prefrontal cortex or midbrain and estradiol treatment did not prevent the decrease of hippocampal DHA, supporting the conclusion that regional brain DHA is in part regulated by ovarian hormones. Other studies (Allesandri et al., 2011; Fabelo et al., 2011) also support an effect of estradiol regulating brain DHA in the female rat.
There is a separate literature examining estradiol's effects on hippocampal neurogenesis in the female rodent (Barha and Galea, 2010; Galea et al., 2013; Pawluski et al, 2009), although it does not examine these effects on regulation of brain DHA. The neurogenesis hypothesis of depression was first introduced around 1999 since then its viability has grown in acceptance (Eisch and Petrik, 2012). Hippocampal neurogenesis is altered throughout the adult female lifespan and is influenced by fluctuations in endogenous and exogenous estrogens (Barha and Galea, 2010). Experiments have shown that age and reproductive experience (Barha and Galea, 2010), and in particular multiparity and nulliparity (Barha et al., 2015) also have a role in estrogen's ability to influence neurogenesis. Whether these findings in female rodents have implications for female humans is to be determined.
While experiments in animal models reveal a complex relation of estradiol to neurogenesis -- and it is not known what action estradiol might have on neurogenesis in humans -- it has been observed in human experiments that the responses of depression to estradiol may differ between perimenopausal and postmenopausal women, with perimenopausal depression tending to be improved by HT and menopausal depression being less responsive (Toffol et al., 2015). While the efficacy of estradiol treatment for postmenopausal depression is questionable, two randomized experiments in non-depressed postmenopausal women revealed untoward effects with estradiol administration (Newhouse et al,. 2008) and with estradiol withdrawal (Schmidt et al, 2015). In an experiment that subjected postmenopausal women free of current or past Axis I psychiatric disorders to a series of challenge studies intended to examine differences in sensitivity to acute monoamine depletion and psychosocial stress, contrary to the hypothesized expectation that E2 administration would blunt negative mood effects induced by the combination of monoamine depletion and stress test, the investigators observed in the women randomized to E2 a “markedly exaggerated negative emotional response” (Newhouse et al., 2008). In another experiment that studied the effect of estradiol withdrawal in asymptomatic postmenopausal women (Schmidt et al., 2015), those with a history of perimenopausal depression experienced a recurrence of depressive symptoms upon E2 withdrawal, whereas those with no history of perimenopausal depression did not experience an occurrence of depressive symptoms. Importantly, in both studies no women were currently taking antidepressants, so the observed responses could not be attributed to an antidepressant. These studies suggest that estradiol administered alone does not have simple, linear actions on behavior in female humans in any reproductive stage.
The findings of the present study indicate a statistical qualitative interaction of high estradiol status or HT status with dietary intake of n-3 PUFAs in postmenopausal women. If these findings can be replicated in other cohort studies there may be implications for randomized clinical trials investigating efficacy of n-3 PUFAs for depressive disorders. A recent Cochrane Review (Appleton et al., 2015) found insufficient evidence to determine the effects of n-3 PUFAs as a treatment. It may be that randomized clinical trials need to stratify on menopausal status and within menopausal status, estrogen status to identify the best target population.
Other large cohort studies of older women that do not support an inverse association of n-3 PUFAs with incident clinical depression or depressive symptoms include the Nurses’ Health Study (Lucas et al., 2011), which included over 54,000 US women aged 50-77 years, and the Women's Health Initiative Memory Study (Persons et al., 2014), which included 7086 postmenopausal women aged 63-81 years. In the Nurses’ Health Study, although an inverse association of α-linolenic acid with clinical depression was found, there was no association for n-3 PUFA. On the other hand, in the Women's Health Initiative Memory Study, a positive association of dietary DHA+EPA with baseline depressive symptoms was found, though no association was seen at follow-up. Both of these studies have ample numbers of HT users and nonusers and an analysis conditioning on HT status might be informative.
Our study has some limitations. Diet was evaluated at a single time point, as were the serum hormones. Reliability and validity of FFQ are not high and may result in misclassification. The STRAW criteria for staging reproductive aging (Harlow et al, 2012), introduced in 2001 and updated in 2011, currently classifies a woman as perimenopausal if she is within 1 year of her final menstrual period. It is possible some women who self-identified as menopausal in 2000-02 with 0 years postmenopause were actually perimenopausal by STRAW+10 criteria. We did not have specific information on type of HT, that is, whether estrogen alone or estrogen in combination with progesterone was used. Other research indicates that a progestogenic component may have effects on mood (Toffol et al., 2015). There were temporal changes in HT use during the study, with only 2.5% of the women in the HT nonuser group using HT at exam 3, but with 66.6% of women in the HT user group curtailing the use of HT by the third exam. However, within the HT user group, additional analysis revealed the null association of n-3 PUFA with depressive symptoms was still present for both HT consistent users and the HT curtailers. Also, we could only assess depressive symptoms as determined by the CES-D score rather than by a clinical structured interview for depressive disorders.
In addition, although this study had CES-D measures at baseline and follow-up so that it was possible to examine incident depressive symptoms, history of depressive symptoms prior to baseline was not available. Thus, the “incident” depressive symptoms do not necessarily represent first onset.
In summary, this study supports an interaction of estrogen status with dietary n-3 PUFAs in postmenopausal women. Confirmation of this interaction in other cohorts is essential as uncontrolled confounding or selection bias cannot be excluded. While this study adjusted for many potential confounders, it did not control for lifetime endogenous estrogen exposure (Georgakis et al 2016). Also, assessing for selection bias can be a nontrivial undertaking, as evidenced by the Harvard Study of Moods and Cycles (Harlow et al., 2013) – a study that invoked the use of directed acyclic graphs in their evaluation for bias. However, given these cautions, the findings in this study may have implications for the design of future randomized clinical trials using PUFAs for prevention of depression. Ideally, a randomized clinical trial conducted in postmenopausal women with a 2 × 2 factorial design testing the effects of HT and n-3 PUFA for depression could be done in the future to avoid biases that may have occurred in the current study.
Highlights.
We studied the interaction of n-3 fatty acids with hormone therapy (HT) for incident depressive symptoms postmenopause
Graded, positive associations were found for n-3 fatty acids and depressive symptoms in HT non-users
There were no associations in postmenopausal HT users
The findings suggest menopause and HT status should be considered in the design of studies examining n-3 fatty acids for depressive disorders
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Role of the funding source
This work was supported by R01 HL074406, R01 HL074338 and contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants UL1-TR-000040 and UL1-TR-001079 from NCRR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions
LAC designed the analysis, conducted the statistical analysis, and wrote the manuscript. PO, SMG, and KL were responsible for acquisition of the data and obtained the original funding. All authors contributed to interpretation of the data, critical review and revision for important intellectual content, and approved the final manuscript for publication.
Conflict of interest
All authors have no financial disclosures and no conflicts of interests to report.
REFERENCES
- Alessandri J-M, Extier A, Al-Gubory KH, Langelier B, Baudry C, LePoupon C, Lavialle M, Guesnet P. Ovariectomy and 17β-estradiol alter transcription of lipid metabolism genes and proportions of neo-formed n3 and n-6 long-chain polyunsaturated fatty acids differently in brain and liver. J Nutr Biochem. 2011;22:820–827. doi: 10.1016/j.jnutbio.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Appleton KM, Sallis HM, Perry R, Ness AR, Churchill R. Omega-3 fatty acids for depression in adults. Cochrane Database Syst Rev. 2015;11:CD004692. doi: 10.1002/14651858.CD004692.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barha CK, Galea LAM. Influence of different estrogens on neuroplasticity and cognition in the hippocampus. Biochim Biophys Acta. 2010;1800:1056–1067. doi: 10.1016/j.bbagen.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Barha CK, Lieblich SE, Chow C, Galea LAM. Multiparity-induced enhancement of hippocampal neurogenesis and spatial memory depends on ovarian hormone status in middle age. Neurobiol Aging. 2015;36:2391–2405. doi: 10.1016/j.neurobiolaging.2015.04.007. [DOI] [PubMed] [Google Scholar]
- Bertoni AG, Whitt-Glover MC, Chung H, Le KY, Barr RG, Mahesh M, Jenny NS, Burke GL, Jacobs DR. The association between physical activity and subclinical atherosclerosis. The Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2009;169:444–454. doi: 10.1093/aje/kwn350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beydoun MA, Kuczmarski MTF, Beydoun HA, Hibbeln JR, Evans MK, Zonderman AB. ω-3 fatty acid intakes are inversely related to elevated depressive symptoms among United States women. J Nutr. 2013;143:1743–1752. doi: 10.3945/jn.113.179119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Rouz AV, Folsom AR, Greenland P, Jacobs DR, Jr, Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic Study of Atherosclerosis: Objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- Borrow A, Cameron NM. Estrogenic mediation of serotonergic and neurotrophic systems: implications for female mood disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2014;54:13–25. doi: 10.1016/j.pnpbp.2014.05.009. [DOI] [PubMed] [Google Scholar]
- Bredemann TM, McMahon LL. 17β estradiol increases resilience and improves hippocampal synaptic function in helpless ovariectomized rats. Psychoneuroendocrinology. 2014;42:77–88. doi: 10.1016/j.psyneuen.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffel DJ, Golden SA, Russo SJ. Structural and synaptic plasticity in stress-related disorders. Rev Neurosci. 2011;22:535–549. doi: 10.1515/RNS.2011.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colangelo LA, He K, Whooley MA, Daviglus ML, Liu K. Higher dietary intake of long-chain omega-3 polyunsaturated fatty acids is inversely associated with depressive symptoms in women. Nutrition. 2009;25:1011–1019. doi: 10.1016/j.nut.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner TS, Richardson AC, Miller JC. Optimal serum selenium concentrations are associated with lower depressive symptoms and negative mood among young adults. J Nutr. 2015;145:59–65. doi: 10.3945/jn.114.198010. [DOI] [PubMed] [Google Scholar]
- Crupi R, Marino A, Cuzzocrea S. n-3 fatty acids: role in neurogenesis and neuroplasticity. Curr Med Chem. 2013;20:2953–2963. doi: 10.2174/09298673113209990140. [DOI] [PubMed] [Google Scholar]
- Decsi T, Kennedy K. Sex-specific differences in essential fatty acid metabolism. Am J Clin Nutr. 2011;94(suppl):1914S–1919S. doi: 10.3945/ajcn.110.000893. [DOI] [PubMed] [Google Scholar]
- De Oliveira Otto MC, Wu JHY, Baylin A, Vaidya D, Ricj SS, Tsai MY, Jacobs Jr DR, Mozaffarian D. Circulating and dietary omega-3 and omega-6 polyunsaturated fatty acids and incidence of CVD in the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2013;2:e000506. doi: 10.1161/JAHA.113.000506. doi: 10.1161/JAHA.113.000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding P, VanderWeele TJ. Sensitivity analysis without assumptions. Epidemiology. 2016;27:368–377. doi: 10.1097/EDE.0000000000000457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards R, Peet M, Shay J, Horrobin D. Omega-3 polyunsaturated fatty acid levels in the diet and in red blood cell membranes of depressed patients. J Affect Disord. 1998;48:149–155. doi: 10.1016/s0165-0327(97)00166-3. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Petrik D. Depression and hippocampal neurogenesis: a road to remission? Science. 2012;338(6103):72–75. doi: 10.1126/science.1222941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabelo N, Martin V, Gonzalez C, Alonso A, Diaz M. Effects of oestradiol on brain lipid class and fatty acid composition: comparison between pregnant and ovariectomised oestradiol-treated rats. J Neuroendocrinol. 2011;24:292–309. doi: 10.1111/j.1365-2826.2011.02242.x. [DOI] [PubMed] [Google Scholar]
- Galea LAM, Wainwright SR, Roes MM, Duarte-Guteman P, Chow C, Hamson DK. Sex, hormones and neurogenesis in the hippocampus: hormonal modulation of neurogenesis and potential functional implications. J Neuroendocrinol. 2013;25:1039–1061. doi: 10.1111/jne.12070. [DOI] [PubMed] [Google Scholar]
- Georgakis MK, Thomopoulos TP, Diamantaras A-A, Kalogirou EI, Skalkidou A, Daskalopoulou SS, Petridou ET. Association of age at menopause and duration of reproductive period with depression after menopause. A systematic review and meta-analysis. JAMA Psychiatry. 2016;73:139–149. doi: 10.1001/jamapsychiatry.2015.2653. [DOI] [PubMed] [Google Scholar]
- Giltay EJ, Duschek EJJ, Katan MB, Zock PL, Neele SJ, Netelenbos JC. Raloxifene and hormone replacement therapy increase arachidonic acid and docosahexaenoic acid levels in postmenopausal women. J Endocrinol. 2004;182:399–408. doi: 10.1677/joe.0.1820399. [DOI] [PubMed] [Google Scholar]
- Giltay EJ, Gooren LJG, Toorians AWFT, Katan MB, Zock PL. Docosahexaenoic acid concentrations are higher in women than in men because of estrogenic effects. Am J Clin Nutr. 2004;80:1167–1174. doi: 10.1093/ajcn/80.5.1167. [DOI] [PubMed] [Google Scholar]
- Golden SH, Dobs AS, Vaidya D, Szklo M, Gapstur S, Kopp P, Liu K, Ouyang P. Endogenous sex hormones and glucose tolerance status in postmenopausal women. J Clin Endocrinol Metab. 2007;92:1289–1295. doi: 10.1210/jc.2006-1895. [DOI] [PubMed] [Google Scholar]
- Grosso G, Micek A, Marventano S, Castellano S, Mistretta A, Pajak A, Galvano F. Dietary n-3 PUFA, fish consumption and depression: a systematic review and meta-analysis of observational studies. Journal of Affective Disorders. 2016 doi: 10.1016/j.jad.2016.08.011. http://dx.doi.org/10.1016/j.jad.2016.08.011. [DOI] [PubMed]
- Hakkarainen R, Partonen T, Haukka J, Virtamo J, Albanes D, Lönnqvist J. Is low dietary intake of omega-3 fatty acids associated with depression? Am J Psychiatry. 2004;161:567–569. doi: 10.1176/appi.ajp.161.3.567. [DOI] [PubMed] [Google Scholar]
- Harlow BL, MacLehose RF, Smolenski DJ, Soares CN, Otto MW, Joffe H, Cohen LS. Disparate rates of new-onset depression during the menopausal transition in 2 community-based populations: real, or really wrong? Am J Epidemiol. 2013;177:1148–1156. doi: 10.1093/aje/kws365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, Sherman S, Sluss PM, de Villiers TJ, STRAW 10 Collaborative Group Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. Menopause. 2012;19:387–395. doi: 10.1097/gme.0b013e31824d8f40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He K. Trace elements in nails as biomarkers in clinical research. Eur J Clin Invest. 2011;41:98–102. doi: 10.1111/j.1365-2362.2010.02373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbeln JR. Fish consumption and major depression (letter). Lancet. 1998;351:1213. doi: 10.1016/S0140-6736(05)79168-6. [DOI] [PubMed] [Google Scholar]
- Hibbeln JR. Seafood consumption, the DHA content of mothers’ milk and prevalence rates of postpartum depression: a cross-national, ecological analysis. J Affect Disord. 2002;69:15–29. doi: 10.1016/s0165-0327(01)00374-3. [DOI] [PubMed] [Google Scholar]
- Jin Y, Kim T-H, Park Y. Association between erythrocyte levels of n-3 polyunsaturated fatty acids and depression in postmenopausal women using or not using hormone therapy. Menopause. 2016 doi: 10.1097/GME.0000000000000667. doi:10.1097/GME.0000000000000667. [DOI] [PubMed] [Google Scholar]
- Kitson AP, Stroud CK, Start KD. Elevated production of docosahexaenoic acid in females: potential molecular mechanisms. Lipids. 2010;45:209–224. doi: 10.1007/s11745-010-3391-6. [DOI] [PubMed] [Google Scholar]
- Kovalchik SA, Varadhan R, Fetterman B, Poitras NE, Wacholder S, Katki HA. A general binomial regression model to estimate standardized risk differences from binomial response data. Stat Med. 2013;32:808–821. doi: 10.1002/sim.5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas M, Mirzaei F, O'Reilly EJ, Pan A, Willett WC, Kawachi I, Koenen K, Ascherio A. Dietary intake of n-3 and n-6 fatty acids and the risk of clinical depression in women: a 10-y prospective follow-up study. Am J Clin Nutr. 2011;93:1337–1343. doi: 10.3945/ajcn.111.011817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, Christophe A, Delanghe J, Altamura C, Neels H, Melzer HY. Lowered ω3 polyunsaturated fatty acids in serum phospholipids and cholesteryl esters of depressed patients. Psychiatry Res. 1999;85:275–291. doi: 10.1016/s0165-1781(99)00014-1. [DOI] [PubMed] [Google Scholar]
- Marangell LB, Martinez JM, Zboyan HA, Kertz B, Kim HFS, Puryear LJ. A double-blind, placebo-controlled study of the omega-3 fatty acid docosahexaenoic acid in the treatment of major depression. Am J Psychiatry. 2003;160:996–998. doi: 10.1176/appi.ajp.160.5.996. [DOI] [PubMed] [Google Scholar]
- Mayer-Davis EJ, Vitolins MZ, Carmichael SL, Hemphill S, Tsaroucha G, Rushing J, Levin S. Validity and reproducibility of a food frequency interview in a multi-cultural epidemiologic study. Ann Epidemiol. 1999;9:314–324. doi: 10.1016/s1047-2797(98)00070-2. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Able J, Jandacek R, Rider T, Tso P. Gender differences in rat erythrocyte and brain docosahexaenoic acid composition: role of ovarian hormones and dietary omega-3 fatty acid composition. Psychoneuroendocrinology. 2009;34:532–539. doi: 10.1016/j.psyneuen.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Hahn C-G, Jandacek R, Rider T, Tso P, Stanford KE, Richtand NM. Selective deficits in the omega-3 fatty acid docosahexaenoic acid in the postmortem orbitofrontal cortex of patients with major depressive disorder. Biol Psychiatry. 2007;62:17–24. doi: 10.1016/j.biopsych.2006.08.026. [DOI] [PubMed] [Google Scholar]
- Nettleton JA, Rock CL, Wang Y, Jenny NS, Jacobs DR., Jr. Associations between dietary macronutrient intake and plasma lipids demonstrate criterion performance of the Multi-Ethnic Study of Atherosclerosis (MESA) food-frequency questionnaire. Br J Nutr. 2009;102:1220–1227. doi: 10.1017/S0007114509382161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhouse PA, Dumas J, Hancur-Bucci C, Naylor M, Sites CK, Benkelfat C, Young SN. Estrogen administration negatively alters mood following monoaminergic depletion and psychosocial stress in postmenopausal women. Neuropsychopharmacology. 2008;33:1514–1527. doi: 10.1038/sj.npp.1301530. [DOI] [PubMed] [Google Scholar]
- Park K, Rimm E, Siscovick D, Spiegelman D, Morris JS, Mozaffarian D. Demographic and lifestyle factors and selenium levels in men and women in the US. Nutr Res Pract. 2011;5:357–364. doi: 10.4162/nrp.2011.5.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawluski JL, Brummelte S, Barha CK, Crozier TM, Galea LAM. Effects of steroid hormones on neurogenesis in the hippocampus of the adult female rodent during the estrous cycle, pregnancy, lactation and aging. Front Neuroendocrinol. 2009;30:343–357. doi: 10.1016/j.yfrne.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Peet M, Murphy B, Shay J, Horrobin D. Depletion of omega-3 fatty acid levels in red blood cell membranes of depressive patients. Biol Psychiatry. 1998;43:315–319. doi: 10.1016/s0006-3223(97)00206-0. [DOI] [PubMed] [Google Scholar]
- Persons JE, Robinson JG, Ammann EM, Coryell WH, Espeland MA, Harris WS, Manson JE, Fiedorowicz JG. Omega-3 fatty acid biomarkers and subsequent depressive symptoms. Int J Geriatr Psychiatry. 2014;29:747–757. doi: 10.1002/gps.4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- Radloff LS, Locke BZ. The Community Mental Health Assessment Survey and the CES-D Scale. In: Weissman MM, Myers JK, Ross CE, editors. Community Surveys of Psychiatric Disorders. Vol. 4. Rutgers University Press; New Brunswick, NJ: 1986. pp. 177–187. [Google Scholar]
- Sanchez-Villegas A, Henriquez P, Figueiras A, Ortuno F, Lahortiga F, Martinez-Gonzalez MA. Long chain omega-3 fatty acids intake, fish consumption and mental disorders in the SUN cohort study. Eur J Nutr. 2007;46:337–346. doi: 10.1007/s00394-007-0671-x. [DOI] [PubMed] [Google Scholar]
- Sarris J, Murphy J, Mischoulon D, Papakostas GI, Fava M, Berk M, Ng CH. Adjunctive nutraceuticals for depression: a systematic review and meta-analyses. Am J Psychiatry. 2016;173:575–587. doi: 10.1176/appi.ajp.2016.15091228. [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Ben Dor R, Martinez PE, Guerrieri GM, Harsh VL, Thompson K, Koziol DE, Nieman LK, Rubinow DR. Effects of estradiol withdrawal on mood in women with past perimenopausal depression. A randomized clinical trial. JAMA Psychiatry. 2015;72:714–726. doi: 10.1001/jamapsychiatry.2015.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers KM, Woolley CC, Hamilton FC, Watts PM, Watson RA. Randomised double-blind placebo-controlled trial of fish oil in the treatment of depression. Prostaglandins Leukot Essent Fatty Acids. 2005;72:211–218. doi: 10.1016/j.plefa.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Smith KJ, Sanderson K, McNaughton SA, Gall SL, Dwyer T, Venn AJ. Longitudinal associations between fish consumption and depression in young adults. Am J Epidemiol. 2014;179:1228–1235. doi: 10.1093/aje/kwu050. [DOI] [PubMed] [Google Scholar]
- Södergard R, Backstrom T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;16:801–810. doi: 10.1016/0022-4731(82)90038-3. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Akechi T, Kobayashi M, Taniguchi K, Goto K, Sasaki S, Tsugane S, Nishiwaki Y, Miyaoka H, Uchitomi Y. Daily omega-3 fatty acid intake and depression in Japanese patients with newly diagnosed lung cancer. Br J Cancer. 2004;90:787–793. doi: 10.1038/sj.bjc.6601621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timonen M, Horrobin D, Jokelainen J, Laitinen J, Herva A, Räsänen P. Fish consumption and depression: the Northern Finland 1966 birth cohort study. J Affect Disord. 2004;82:447–452. doi: 10.1016/j.jad.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Toffol E, Heikinheimo O, Partonen T. Hormone therapy and mood in perimenopausal and postmenopausal women: a narrative review. Menopause. 2015;22:564–578. doi: 10.1097/GME.0000000000000323. [DOI] [PubMed] [Google Scholar]
- Willett WC. Nutritional Epidemiology. 2nd edition. Oxford University Press; New York, NY: 1998. [Google Scholar]
- Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]