Abstract
Purpose
The management of endometrial carcinoma no longer amenable to treatment with surgery or radiation has not improved significantly with modern chemotherapy. Alternative therapeutic options are desperately needed.
Experimental Design
We describe 2 heavily pretreated patients with recurrent disease refractory to surgery, radiation and chemotherapy treated with the anti-PD1 immune check-point inhibitor nivolumab.
Results
Patient # 1 harbored an ultra-mutated (Mutation Load/MB = 117.3, total mutations = 4660) tumor driven by mutation in the exonuclease domain of the DNA polymerase ε gene. Patient # 2 harbored a hyper-mutated tumor (Mutation Load/MB = 33.5, total mutations = 1037) due to a germinal MSH6 gene mutation. Both patients demonstrated a remarkable clinical response to the anti-PD1 immune check-point inhibitor nivolumab. Patients’ clinical responses remain unchanged at the time of the writing of this report with no grade 3 or higher side-effects reported to date.
Conclusions
Anti-PD1 inhibitors represent a novel treatment option for recurrent/metastatic ultra/hypermutated human tumors refractory to salvage treatment.
Introduction
Recent next generation sequencing (NGS) studies from the cancer genome atlas (TCGA) network and our group identified a subgroup of ultra- and hyper-mutated endometrial cancer patients harboring large numbers of mutations secondary to DNA polymerase ε gene mutations and/or deficiency in MMR gene functions (1,2). The high mutation numbers and heavy infiltration of tumor-infiltrating lymphocytes (TILs) (3) support the potential high immunogenicity of these tumors commonly arising in the endometrium and gastro-intestinal tract and provided a rationale for testing the activity of immune check-point inhibitors as a novel form of immunotherapy. We describe 2 patients with recurrent ultra- and hyper-mutated endometrial cancers refractory to conventional treatments demonstrating remarkable clinical responses to nivolumab.
Methods
Immunohistochemistry
Briefly, for immunohistochemistry, 4 μm sections were cut from the formalin-fixed paraffin embedded blocks of both cancer patients and stained with the following antibodies according to the manufacturers’ instructions: p53 (clone DO7, 1:6000, DAKO, Carpinteria, CA). CD8 (clone 144B, ready to use, DAKO, Carpinteria, CA) and PD-L1 (clone E1L3N, 1:200 Cell Signaling, Danvers, MA). For antigen retrieval, the sections were pre-treated at low pH for PD-L1 and CD8 and at high pH for p53.
Whole exome sequencing
Briefly, DNA was extracted from FFPE samples using a BiOstic® FFPE Tissue DNA Isolation Kit (MO BIO Laboratories #12250-50) with a modified protocol. Genomic DNA was captured on the NimbleGen 2.1M human exome array and subjected to 74 base paired-end reads on the Illumina HiSeq 2000 instrument as described (2). Sequence reads were mapped to the reference genome (hg19) using the ELAND program. Reads outside the targeted sequences were discarded and statistics on coverage were collected from the remaining reads using in-house Perl scripts as previously described (2).
Results
Patient # 1 is a 57-year old woman with widespread abdominal carcinomatosis secondary to recurrent endometrial cancer. In 2007 she underwent surgical staging for a mixed clear cell and endometrioid (CC/EAC) endometrial cancer, stage IIIA (Figure 1). Post-operatively she received cisplatin/adriamycin/paclitaxel for 7 cycles and vaginal cuff radiation. The patient did well until March 2012, when CT imaging revealed widespread metastatic lesions in the abdominal cavity. She was placed into a clinical trial with cediranib from April to December 2012 and subsequently on Megace from January 2013 to April 2013. Because of disease progression, in June 2013, she underwent an additional laparotomy with tumor debulking, omentectomy and resection and re-anastomosis of the transverse colon. She was thereafter placed on 6 additional cycles of carboplatin/paclitaxel. Disease progression was confirmed in December 2014 by CT guided-biopsies. She was therefore placed on bevacizumab single agent therapy until February 2015 when she was referred to our institution for additional treatment options due to disease progression, proteinuria and hypertension. She was started on dose dense paclitaxel protein-bound particles (100 mg/m2 weekly). A CT scan obtained in September 2015 after 3 cycles of chemotherapy demonstrated interval increase in her retroperitoneal lymphadenopathy measuring up to 2 cm and pelvic and abdominal carcinomatosis with masses up to 8.1 cm in diameter (Figure 2). Next generation sequencing (NGS) testing (Foundation Medicine, (FM) Inc. Cambridge, MA) and Microsatellite instability (MSI) results became available at this time and demonstrated a MSI stable tumor with a POLE (P286R) exonuclease mutation and an ultra-mutated phenotype (i.e., mutations in 87 out of 315 cancer gene tested at FM). Whole exome sequencing performed at the Center for Genome Analysis at Yale University School of Medicine confirmed the tumor to be ultra-mutated (ie, total number of mutations = 4660) and the typical signature of POLE-mutated tumors (ie, high number of C > A transversion) (Figure 3). CD8 T cell infiltration and PD-L1 expression on the pretreatment biopsy were also evaluated. A moderate amount of peri- and intratumoral lymphocytic infiltrate was seen on hematoxylin-eosin (H&E) stain and CD8 immunostain. Approximately 5% of tumor cells demonstrated weak membranous PD-L1 expression by immunohistochemistry, while the peri- and intratumoral lymphocytes were PD-L1 negative (Figure 1). In search of a last attempt to inhibit tumor progression and on the basis of the encouraging report presented at the ASCO 2015 meeting by Le et al., (4) demonstrating a clinical response by anti-PD1 immune check point inhibitors in 2 MSI-H hyper-mutated endometrial cancer patients enrolled in NCT01876511, after extensive counseling and informed consent, the patient was started on nivolumab single agent therapy (3 mg/kg/biweekly) six weeks after her last chemotherapy administration. CA125 tumor levels normalized within 5 weeks of immunotherapy (i.e., baseline 53 U/ML to 8 U/ml). Six weeks after initiation of nivolumab treatment, the patient was clinically improved to the point to resume and maintain all her normal activities. CT imaging obtained 3 months (Figure 2 middle panel) and 5 months thereafter (data not shown) demonstrated a remarkable response (ie, partial response by RECIST v1.1) to the immune checkpoint inhibitor with regression of her large metastatic pelvic and intra-abdominal tumor deposits. A CT scan obtained at 7 months after treatment initiation demonstrated a sustained clinical response with continued regression of her abdominal carcinomatosis (Figure 2 upper right panel) and metastatic tumor deposits located in the pelvis (Figure 2 lower right panel) and retro-peritoneum (data not shown). This patient’s remarkable clinical response remains unchanged at the time of the writing of this report with no side effects reported to date and the patient experiencing good quality of life.
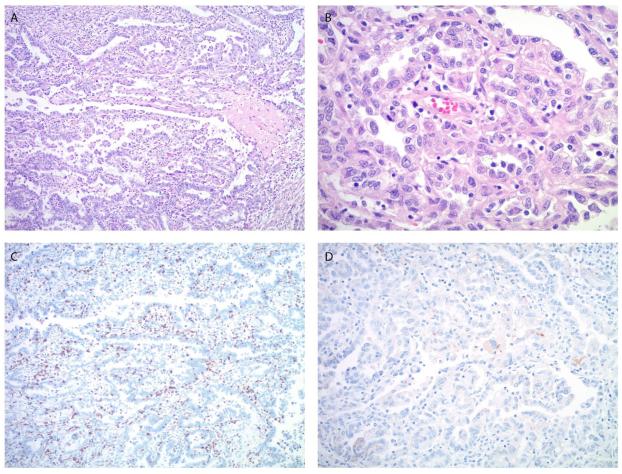
Figure 1.
Microscopic images of the endometrial carcinoma in patient #1. A, B. The tumor shows predominantly a papillary and glandular architecture (A) with high nuclear grade and clear to pale eosinophilic cytoplasm (B), consistent with clear cell carcinoma component. C. Moderate peri- and intratumoral lymphocytic infiltrate is highlighted by CD8 immunostain. D. PD-L1 immunostain shows focal, weak staining predominantly located in the tumor cells. (A. Original magnification 100x, H&E stain, B. Original magnification 400x, H&E stain, C. Original magnification 100x, D. Original magnification 200x).
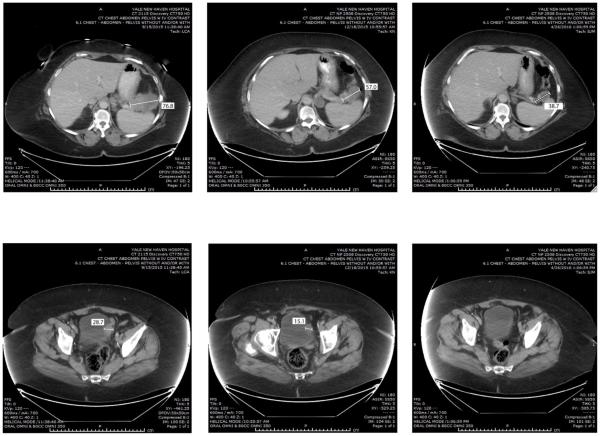
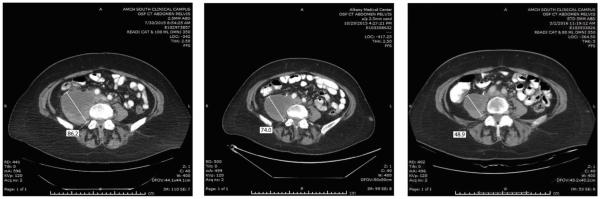
Figure 2.
Representative CAT scans demonstrating activity (ie, partial response) of nivolumab in Patient #1 (ie, POLE-ultra-mutated). Left panels: Pretreatment images with baseline measurements of two representative metastatic tumor deposits [ie, mass abutting the pancreatic tail in the gastro-splenic ligament (Upper Left Panel) and mass involving the bladder dome wall (Lower Left Panel). Middle panels: Regression of the metastatic tumor deposits described above three months after treatment initiation. Right panels: regression (Upper Right) and disappearance (Lower Right) of the metastatic tumor deposits described above seven months after treatment initiation with nivolumab.
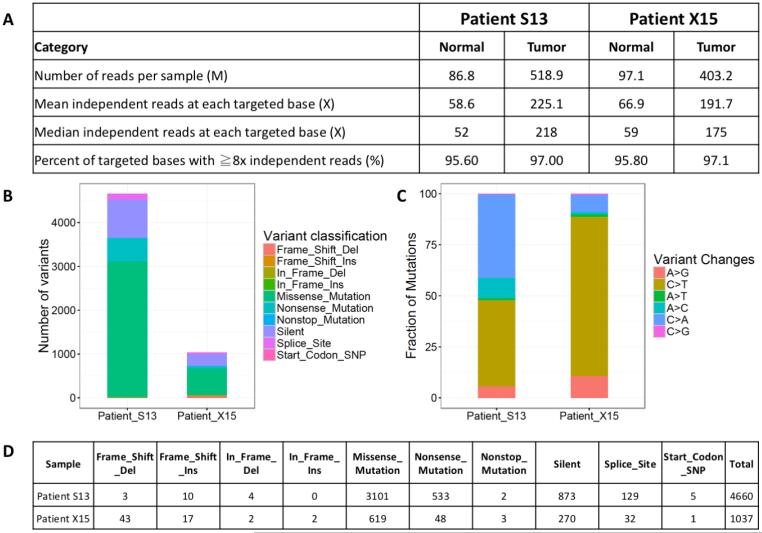
Figure 3.
Whole Exome Sequencing (WES) results of Patient #1 (ie, POLE-ultra-mutated, S13) and Patient # 2 (i.e., MSH6-hyper-mutated, X15). A) Exome sequencing quality statistics for both patients’ tumor and normal DNA. (B) Exonic mutation burden in Patient S13 and X15 with different variant classifications displayed in the barplot. (C) Distribution of six different mutation conversions in Patient S13 and X15. (D) Number and classification of the distinct variant of mutations detected in Patient S13 and X15.
Patient # 2 is a 60-year old caucasian female with a recurrent/metastatic uterine serous carcinoma (i.e., USC, a highly aggressive variant of Type II endometrial cancer)(Figure 4). Her past surgical history is significant for a robotic-assisted surgical staging procedure performed in September 2011 for stage IIIC2 disease. She received 6 cycles of docetaxel and carboplatin (completed in February 2012), followed by brachytherapy. She was disease free until October 2013 when enlargement of multiple retroperitoneal nodes was noted. She was followed conservatively until November 2014 when she was enrolled in a Phase II Clinical study (MGH study 13-520) comparing two different formulations of doxorubicin. She received two administrations of doxorubicin at 60mg/m2 every 3 weeks. Secondary to grade 4 neutropenia and gastro-intestinal symptoms chemotherapy was discontinued in December 2014. In March 2015, she was referred to our institution for a second opinion and was started on a salvage dose-dense paclitaxel protein-bound particles chemotherapy regimen (80mg/m2/weekly) in April 2015. Unfortunately, a CT scan performed after 3 cycles of therapy demonstrated progression of her carcinomatosis with enlarging retroperitoneal masses up to 7 cm in diameter invading the right psoas muscle (Figure 5 Left panel) and right sided hydroureteronephrosis. Her CA125 level was now elevated at 222 U/ML. NGS testing by FM and MSI results revealed a MSI-High tumor secondary to a MSH6 gene mutation (i.e., mutation = F1088). Thirty-nine additional genes were found mutated out of 315 tested (i.e., hyper-mutated). Whole exome sequencing performed at the Center for Genome Analysis at Yale University School of Medicine confirmed MSH6-related tumor with hyper-mutation (ie, total number of mutations = 1037) (Figure 3). TP53 expression, CD8 T cell infiltration and PD-L1 expression on the pretreatment tumor biopsy were also evaluated. Immunohistochemistry for p53 showed a wild-type staining pattern (weak to moderate staining in approximately 10% of tumor cell nuclei)(Figure 4). The peritumoral lymphocytic infiltrate was moderate, while there were fewer CD8 positive lymphocytes within the tumor cell nests. PD-L1 immunoreactivity was observed in approximately 20% of peri- and intratumoral lymphocytes, and the tumor cells did not show significant PD-L1 expression (Figure 4). The patient’s performance status rapidly deteriorated due to tumor progression and acute development of pulmonary emboli. The patient declined additional chemotherapy after consideration of her options. However, because of early data demonstrating potential clinical activity of pembrolizumab in MSI-H tumors (4) and no good treatment options available for metastatic chemotherapy-resistant endometrial cancer that would preserve quality of life, the patient decided to pursue an off-label trial of nivolumab (intravenous infusions, 3 mg/kg every 2 weeks). She tolerated the nivolumab treatment with no significant adverse effects other than mild fatigue. Her performance status significantly improved after only 3 nivolumab administrations. A CT scan obtained 3 months after treatment initiation revealed a significant decrease in the diameter of the large abdominal metastases (Figure 5 middle panel) with continued improvement at 9 months after treatment initiation (i.e., partial response by RECIST v1.1, Figure 5 right panel). The patient continues to do well at the writing of this report with significant improvement in her performance status and a normalized CA125.
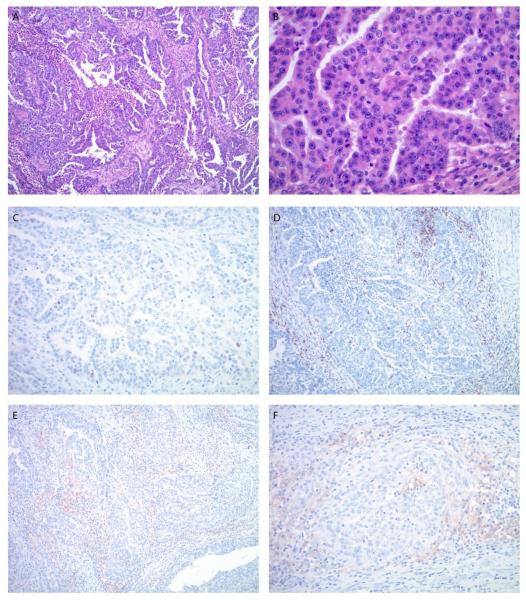
Figure 4.
Microscopic images of the endometrial carcinoma in patient #2. A. The tumor shows a predominant glandular architecture with irregular, slit-like spaces and focal necrosis. The nuclei are large with a high nuclear to cytoplasmic ratio, prominent nucleoli and brisk mitotic activity (B). C. P53 immunostain shows a wild-type staining pattern with weak to moderate nuclear positivity in approximately 10% of tumor cells. D. The lymphocytic infiltrate is predominantly peritumoral; less frequent intratumoral lymphocytes are also highlighted by CD8 immunostain. E, F. PD-L1 immunostain shows diffuse, weak to moderate staining predominantly in peritumoral lymphocytes. (A. Original magnification 100x, H&E stain, B. Original magnification 400x, H&E stain, C. Original magnification 200x, D. Original magnification 100x, E. Original magnification 100x, F. Original magnification 200x).
Figure 5.
Representative CAT scans demonstrating activity (ie, partial response) of nivolumab in Patient #2 (ie, MSH6-hyper-mutated). Left panel: Pretreatment image with baseline measurement of a representative metastatic tumor deposit (ie, right common iliac mass invading the psoas muscle and obstructing the right ureter). Middle panel: Regression of the metastatic tumor deposit described above three months after treatment initiation. Right panel: Continue regression (Right Panel) of the metastatic tumor deposit nine months after treatment initiation with nivolumab.
Discussion
To our knowledge, this is the first report demonstrating clinical activity of an immune checkpoint inhibitor in POLE ultra-mutated endometrial cancer patients with recurrent/chemotherapy resistant disease. POLE-ultra-mutated tumors account for 7 to 12 % of all endometrial carcinomas (1, 2, 5, 6) and occur secondary to mutations in the exonuclease domain of POLE, a nuclear DNA polymerase endowed with intrinsic proofreading activity (5). Most of POLE-ultra-mutated cancers (including the POLE tumor of patient #1 described above) are MSI stable and cannot be recognized using clinically-approved IHC and PCR-based MSI assays (1, 5, 6). In contrast, hyper-mutated endometrial tumors may occur secondary to different genetic events such as germline mutations in one of the MMR genes (as observed in patients with Lynch syndrome, including patient # 2 above) or because of epigenetic silencing of the promoters of both alleles of the MLH1 gene (1). Moreover, another group of tumors with MSI phenotype (also referred as “Lynch like” tumors) exist that exhibit loss of expression of 1 or more of the MMR genes secondary to somatic mutations (1, 6). Recent reports analyzing the genetic landscape of endometrial cancers by WES in 232 treatment-naïve endometrial cancer patients (ie, TCGA)(1) and 243 endometrial cancer patients with recurrent disease enrolled in GOG 86P (7) demonstrated that the overall frequency of ultra-mutated and hyper-mutated tumors may range from 35% (ie, 7% POLE, 28% MSI)(1) to 26 % (ie, 2% POLE, 24% MSI)(7), respectively. Taken together, these data suggest that up to 26% of patients developing recurrent endometrial cancer may harbor tumors with either POLE or MMR-gene defects.
While it is currently not understood why patients with ultra-mutated/hyper-mutated phenotype may have improved outcomes when compared to the others endometrial cancer groups (1, 2, 5) it is possible that the large number of somatic mutations present in these tumors, may render these cancers highly immunogenic for the host due to the large number of mutated epitopes. Consistent with this view, POLE mutated and MMR gene defective endometrial cancers are characterized by a high rate of infiltration of T lymphocytes (3) and, in agreement with these results, our research group have recently demonstrated that POLE ultra-mutated endometrial tumors, unlike copy-number low and copy-number high serous-like tumors, may trigger activation of both the T helper arm and the cytotoxic arms of the immune system (8). Anti PD-1 targeting agents may therefore represent a novel therapeutic approach in patients with POLE ultra-mutated MSI stable and MMR gene deficient MSI-H endometrial cancer patients with recurrent disease refractory to standard salvage treatment. Accordingly, a recent report published on the New England Journal of Medicine (4) demonstrated that patients with mismatch repair-deficient cancers including GI malignancies and endometrial cancers had a significantly better clinical response to PD-1 blockade by pembrolizumab than those whose cancers did not have mismatch-repair deficiencies. Importantly, in this report, two patients treated with pembrolizumab harbored MSI high endometrial cancers and both patients experienced a clinical response (ie, a complete and a partial response, respectively) (4). Our current results using nivolumab in POLE ultra-mutated MSI stable and MSH6-hyper-mutated MSI-H endometrial cancer patients are consistent with these data and further support evidence of noteworthy clinical activity of anti-PD1 immune check point inhibitors against ultra/hyper-mutated recurrent/metastatic endometrial cancers resistant to chemotherapy. On the basis of these encouraging results an investigator initiated Phase II study using pembrolizumab in POLE-ultra-mutated and MMR-gene defective hyper-mutated endometrial cancer patients with recurrent disease identified by concurrent assessment of tumor mutation burden (TMB) and microsatellite instability (MSI) status (9) is pending opening at Yale University.
STATEMENT OF TRANSLATIONAL RELEVANCE.
Endometrial cancer patients have extremely limited therapeutic options when the disease becomes resistant to platinum and taxane chemotherapy. Recent next generation sequencing (NGS) studies in recurrent endometrial cancer patients demonstrated that about 25% of the patients with recurrent endometrial cancer harbor an ultra- and hyper-mutated endometrial tumor harboring large numbers of somatic mutations attributable to mutations in DNA polymerase epsilon and/or DNA mismatch repair genes. Our report represents the first demonstration of the clinical activity of an immune checkpoint inhibitor in POLE ultra-mutated endometrial cancer patients with recurrent/chemotherapy resistant disease. These results suggest that anti PD-1 may prove efficacious in other patients with POLE ultra-mutated MSI stable and MMR gene deficient, MSI-H endometrial cancer patients with recurrent disease refractory to standard salvage treatment.
Acknowledgments
Funding Informations:
This work was supported in part by grants from NIH U01 CA176067-01A1, the Deborah Bunn Alley Foundation, the Tina Brozman Foundation, the Discovery to Cure Foundation and the Guido Berlucchi Foundation to Alessandro D. Santin. This investigation was also supported by NIH Research Grant CA-16359 from NCI. Richard Lifton is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Conflicts of interest: None
References
- 1.Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H, et al. Integrated genomic characterization of endometrial carcinoma. The Cancer Genome Atlas Research Network. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao S, Choi M, Overton JD, Bellone S, Roque DM, Cocco E, et al. Landscape of somatic single-nucleotide and copy-number mutations in uterine serous carcinoma. Proceedings of the National Academy of Sciences of the USA. 2013;110:2916–21. doi: 10.1073/pnas.1222577110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howitt BE, Shukla SA, Sholl LM, Ritterhouse SA, Watkins JC, Rodig S, et al. Association of Polymerase e–Mutated and Microsatellite-Instable Endometrial Cancers with Neoantigen Load, Number of Tumor-Infiltrating Lymphocytes, and Expression of PD-1 and PD-L1. JAMA Oncol. 2015;1:1319–23. doi: 10.1001/jamaoncol.2015.2151. [DOI] [PubMed] [Google Scholar]
- 4.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372(26):2509–20. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rayner E, van Gool IC, Palles C, Kearsey SE, Bosse T, Tomlinson I, et al. A panoply of errors: polymerase proofreading domain mutations in cancer. Nature Reviews Cancer. 2016;16:71–81. doi: 10.1038/nrc.2015.12. [DOI] [PubMed] [Google Scholar]
- 6.Konstantinopoulos PA, Matulonis UA. POLE Mutations as an Alternative Pathway for Microsatellite Instability in Endometrial Cancer: Implications for Lynch Syndrome Testing. Cancer. 2015;121:331–334. doi: 10.1002/cncr.29057. [DOI] [PubMed] [Google Scholar]
- 7.Aghajanian C, Filiaci VL, Dizon DS, Carlson J, Powell MA, Alvarez-Secorde A, et al. A randomized phase II study of paclitaxel/carboplatin/bevacizumab, paclitaxel/carboplatin/temsirolimus and ixabepilone/carboplatin/bevacizumab as initial therapy for measurable stage III or IVA, stage IVB or recurrent endometrial cancer, GOG-86P. J Clin Oncol. 2015;33 suppl; abstr 5500. [Google Scholar]
- 8.Bellone S, Centritto F, Black J, Schwab C, English D, Cocco E, et al. Polymerase ε (POLE) ultra-mutated tumors induce robust tumor-specific CD4+ T cell responses in endometrial cancer patients. Gynecol Oncol. 2015;138:11–7. doi: 10.1016/j.ygyno.2015.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santin AD, Moore KN, Gunderson C, Gowen K, Fabrizio D, Frampton GM, et al. Immunotherapy (IO) versus targeted therapy triage in endometrial adenocarcinoma (EA) by concurrent assessment of tumor mutation burden (TMB), microsatellite instability (MSI) status, and targetable genomic alterations (GA) J Clin Oncol. 2016;34 suppl; abstr 5591. [Google Scholar]