Abstract
Deficits in cognitive control are a hallmark characteristic of depression, however less is known about the degree to which they persist beyond symptom remission and might contribute to symptom recurrence in remitted individuals (rMDD). Evidence indicates that stress interferes with cognitive control, highlighting a potential mechanism by which stress precipitates depression relapse. Therefore, this study examined whether stress exposure elicits deficits in error monitoring – a component of cognitive control thought to be particularly implicated in the ability to adaptively respond to negative feedback – in individuals with rMDD. Unmedicated individuals with rMDD (n=30) and healthy controls (n=34) performed an Eriksen Flanker task before and 45 minutes after an acute stressor while 128-channel event-related potentials (ERPs) were recorded. Flanker interference effects and post-error adjustments were examined, and ERP analyses focused on the error-related negativity (ERN) and error positivity (Pe). Standardized low resolution electromagnetic tomography (sLORETA) was used to examine stress-induced changes in current source density. Individuals with rMDD showed blunted cortisol reactivity to the stressor, coupled with heightened self-reported stress reactivity. Although no significant effects of group or stress were observed in scalp-level ERPs, source-level analyses indicated that among the rMDD group only, stress caused a reduction in activation in frontocingulate regions critically implicated in error monitoring. The magnitude of stress-induced decreases in frontocingulate activation correlated with heightened self-reported stress reactivity, and also predicted heightened levels of stress and depression 18 months later in the entire sample. These findings suggest that individuals with rMDD show a stress-induced disruption in frontocingulate function that is linked to heightened stress reactivity, and this disruption prospectively predicts heightened levels of future stress and depressive symptomatology.
Keywords: major depression, stress, remission, ERP, error-related negativity, anterior cingulate
1. Introduction
Major depressive disorder (MDD) is highly recurrent, and although many treatments lead to symptom remission, 30% of individuals experience a recurrence within five years following recovery (Bukh et al., 2016). Mechanisms underlying risk for recurrence in remitted (rMDD) individuals are not fully understood, but may involve changes in cognitive control that persist beyond the resolution of outward signs and symptoms. Furthermore, independent lines of evidence show stress exposure both predicts depression recurrence (Hammen, 2015) and impairs prefrontal function (Arnsten, 2015). Accordingly, stress may trigger recurrence by uncovering or exacerbating cognitive control deficits in rMDD individuals, as cognitive control depends on prefrontal function (Ridderinkhof et al., 2004). However, no study has directly addressed this question.
Cognitive models propose that in MDD, negative affect is prolonged by negative information processing biases and maladaptive responses to negative information, which have been linked to impairments in aspects of cognitive control - notably, performance monitoring (Gotlib and Joormann, 2010). In particular, ruminative thinking and poor regulation of sad mood are associated with difficulty disengaging from negative information and modifying behavior accordingly. For example, on tests of performance monitoring that require rapid, accurate responding (e.g., the Eriksen Flanker task), individuals with MDD perform poorly on trials following a commission error (Holmes and Pizzagalli, 2008; Beard et al., 2015), indicating the presence of an oversensitive error-detection system, or a failure to recruit control systems to adaptively respond to errors.
Adaptive responding to negative information relies on the anterior cingulate cortex (ACC), and abnormalities in ACC function have been well-documented in acute MDD (Pizzagalli, 2011). Consistent with this, individuals with MDD have also been found to have potentiated error-related negativity (ERN) amplitudes following errors (Chiu and Deldin, 2007; Holmes and Pizzagalli, 2008; but see Weinberg et al., 2015). The ERN is a negative-going deflection in the event-related potential (ERP) elicited by commission errors, and peaks at about 80 ms post-error (Falkenstein et al., 2000; Holroyd and Coles, 2002). It is thought to arise from dopamine-modulated ACC activity that signals the mismatch between an error and the representation of the expected correct response (Holroyd and Coles, 2002). This prompts the recruitment of prefrontal systems to adjust behavior. In support of this conceptualization, ERN-related ACC activation predicts subsequent activation of the dorsolateral prefrontal cortex (dlPFC) and adaptive post-error behavioral adjustments in healthy individuals (Kerns et al., 2004)—but this relationship is attenuated in individuals with MDD (Holmes and Pizzagalli, 2008). These findings indicate that in acute MDD, heightened sensitivity to negative information may be linked to dysfunction in frontocingulate regions that are critically involved in error monitoring.
In rMDD, the evidence for deficits in error monitoring, and cognitive control generally, is more mixed. Some studies point to persistently impaired response inhibition (Bora et al., 2013), whereas others have failed to find such deficits (Aker et al., 2016). Similarly, ERN amplitudes have been found to be normal (Schoenberg, 2014), blunted (Alexopoulos et al., 2007), and enhanced (Georgiadi et al., 2011) in rMDD, with some studies revealing abnormal ERN amplitudes in only a subset of rMDD individuals with prior melancholic depression (Weinberg et al., 2016). Clearly, more research is required to determine whether error monitoring deficits persist in rMDD, and are a useful predictor of future depression.
Furthermore, given the wealth of evidence linking stress to prefrontal dysfunction (Arnsten, 2015) and depression onset and maintennce (Hammen, 2015), it would be valuable to know whether depression history increases risk for error monitoring deficits following stress exposure. Although no studies to date have addressed this question, individuals with rMDD may experience greater stress-induced impairments in error monitoring for two reasons: 1) if individuals with rMDD are more sensitive to stress, the increased prefrontal resources required to regulate the stress response may deplete resources available for more cognitive aspects of performance monitoring, and 2) frontocingulate abnormalities that persist beyond remission may reduce the overall capacity for concurrent stress regulation and error monitoring. Consistent with this, theoretical perspectives suggest that effective emotion regulation under stress and effective performance monitoring during cognitive tasks, draw on a set of shared and finite mental resources (with partly overlapping neural underpinings in frontocingulate regions; Ochsner et al., 2009), such that when resources are over-taxed in one domain, impairments arise in the other (Kaplan and Berman, 2010). In support of this theory, effortful suppression of negative emotions resulted in greater interference effects and blunted ERN amplitudes on a non-emotional Stroop task (Inzlicht and Gutsell, 2007). Given that individuals with rMDD may have a stronger stress response and/or a poorer ability to regulate their responses to stress (Bagley et al., 2011), it is possible that they may also experience greater impairments in performance monitoring when faced with an acute stressor, and that the level of impairment may covary with the magnitude of the stress response.
To address these questions, we used high-density ERPs and a distributed source localization to examine the neural correlates of error monitoring in individuals with rMDD and a group of never-depressed healthy controls. Importantly, we implemented an acute stress manipulation to determine whether stress uncovers or exacerbates error monitoring deficits in rMDD. Owing to the literature reviewed above, ERP analyses focused on the ERN as well as the error positivity (Pe). The Pe is a positive-going deflection in the ERP waveform which occurs 150-500 ms following commission errors. In contrast to the ERN, which tracks the automatic processing of errors, the Pe is thought to reflect the subjective evaluation of errors (Falkenstein et al., 2000). By analyzing both the ERN and the Pe, we evaluated more automatic vs. controlled aspects of error processing.
We hypothesized that stress would impair error monitoring (as indexed by post-error behavioral adjustments, ERN/Pe amplitudes and frontocingulate activation) in individuals with a history of depression, and that the magnitude of impairment would be associated with the magnitude of the stress response (as indexed by self-reported stress reactivity and changes in cortisol). We also hypothesized that the severity of error monitoring impairment following stress would predict increased levels of stress and depressive symptomatology in an 18-month follow-up.
2. Materials and methods
2.1. Participants
Thirty-four healthy control participants and 30 individuals with rMDD were recruited. Inclusion criteria for controls were: no lifetime DSM-IV diagnoses, no first-degree relatives with psychiatric illnesses, a Beck Depression Inventory-II (BDI-II; Beck et al., 1996) score < 13, and no lifetime use of psychotropic medication. Inclusion criteria for rMDD participants were: at least one lifetime major depressive episode (MDE), a BDI-II score < 13 and remission for a minimum of 8 weeks, and no current psychotropic medication (at least 2 weeks for most antidepressants, 6 weeks for venlafaxine). Remission was indexed by a score of 1 on the Depressed Mood and Anhedonia items from the Structured Clinical Interview for DSM-IV (SCID; First et al., 2002) and no SCID score above 2 for the other MDD criteria. Exclusion criteria for all participants were: seizures, loss of consciousness longer than two minutes, or a positive urine drug screen (Amedicheck CLIA-Waived 12-panel cup). All participants provided written informed consent before participating. Additional information on subject eligibility is provided in the Supplement.
Sample characteristics are summarized in Table 1. Groups did not differ in age, gender or education (all ps > 0.05). The rMDD group had significantly higher scores on the BDI-II (although all scores were well below clinical cut-offs) [t(62) = 2.94, p = 0.005] and the Perceived Stress Scale (PSS; Cohen et al., 1983) [t(62) = 3.40, p = 0.001] – a global measure of perceived life stress.
Table 1.
Demographic and clinical characteristics
| Controls (n = 34) | rMDD (n = 30) | test value | p value | |
|---|---|---|---|---|
| Female, N (%) | 23 (67.6) | 21 (70.0) | X2 = 0.04 | .84 |
| Age (yrs.) | 29.8 (10.9) | 31.4 (13.8) | t = −0.50 | .62 |
| Years of education post-high school | 4.6 (2.4) | 4.5 (2.2) | t = 0.27 | .79 |
| BDI-II | 0.8 (1.6) | 2.4 (2.8) | t = −2.94 | .005 |
| PSS | 13.7 (4.7) | 19.0 (7.4) | t = −3.40 | .001 |
| Mean age onset | N/A | 21.2 (12.7) | N/A | |
| Number of prior MDEs | N/A | 2.3 (1.5) | N/A | |
| Past psychotropic medication use, N (%) | N/A | 14 (46.7) | N/A | |
| Time in remission (months) | N/A | 33.4 (26.7) | N/A |
Note: BDI-II (Beck Depression Inventory–II; Beck et al., 1996), PSS (Perceived Stress Scale; Cohen et al., 1983), MDE: Major Depressive Episode
2.2. Procedure
The study involved two sessions. In the first, the SCID was administered by Masters- or PhD-level clinicians, along with self-report measures of depressive symptomatology (the BDI-II). In the second, participants performed an Eriksen Flanker task twice while 128-channel electroencephalography (EEG) data were acquired – once immediately prior to an acute stressor, and then 45 minutes afterwards.
2.3. Eriksen Flanker task
A modified version of the Eriksen Flanker task with an individually titrated response window was used (Eriksen and Eriksen, 1974; Holmes et al., 2010). On each trial, participants viewed four leftward or rightward facing arrows. After 100 ms these arrows were joined by a center arrow (50 ms) pointing in the same direction as the flanking arrows (congruent trials) or in the opposite direction (incongruent trials). Participants had to indicate whether the center arrow pointed to the left or right, and each trial was followed by a fixation cross of 1400 ms. Accuracy and reaction time (RT) were recorded.
2.4. Measures of life stress and residual depression
To assess any residual depressive symptomatology and ongoing stress appraisal, participants were administered the BDI-II again at the EEG session, along with the PSS (Cohen et al., 1983). The PSS is a 14-item measure of the degree to which life situations during the past month are perceived as uncontrollable (e.g., “In the last month, how often have you been upset because of something that happened unexpectedly?”). Responses range from 0 (never) to 4 (very often) and higher scores indicate greater perceived life stress.
2.5. Stress manipulation
Stress was induced using the Maastricht Acute Stress Test (MAST; Smeets et al., 2012). The MAST is a 15-minute acute stress manipulation combining physical pain (a cold pressor), cognitive challenge (a mental arithmetic task), psychosocial evaluative threat (two unempathic evaluators) and uncontrollability (random trial duration), and produces reliable activation of the HPA-axis (Smeets et al., 2012). Additional details about the MAST and Flanker task are presented in the Supplement.
2.6. Measures of stress reactivity
Salivary cortisol samples were collected at five time points (after a 20-minute rest period, immediately after the MAST, 25 minutes post-MAST, 40 minutes post-MAST, 2 hours post-MAST). Salivary cortisol was assayed using a competitive enzyme-linked immunosorbent assay (ELISA). Area under the curve with respect to baseline (AUCi) was calculated and used as an index of the sensitivity of the cortisol response to the MAST (Pruessner et al., 2003).
Subjective ratings of stress were collected at four time points using the State and Trait Anxiety Inventory-I (STAI-I; Spielberger et al., 1983) and Positive and Negative Affect Schedule (PANAS; Watson et al., 1988). Furthermore, mood ratings were measured at five time points using the Visual Analogue Mood Scale (VAMS; Bond and Lader, 1974). The VAMS consisted of three horizontal lines (range: 0 to 100) and were anchored at sad-happy, tense-relaxed, hostile-friendly.
Approximately half-way through data collection, an additional measure of stress appraisals was implemented: the Threat/Challenge Questionnaire (Mendes et al., 2007), which is a self-report measure of the degree to which a person perceives a task (in this instance, the MAST) to be demanding and stressful, and whether they feel they have the resources required to perform well. This measure was administered once immediately after instructions for the MAST were given but before the stress induction had commenced (so that pre-stress ratings could be obtained when participants knew what the stressor would entail but had not been exposed to it yet), and then a second time immediately after the MAST. A demand to resources ratio was computed separately for both time points by adding the scores from demand items and scores from resources items and then dividing the demand total score by the resources total score. Using this metric a score higher than 1 is indicative of a state of threat, whereas a score less than 1 is indicative of a state of challenge. Information about the timing of cortisol and subjective stress measurements is presented in the Supplement.
2.7. Measures of stress and depression at follow-up
At 12 and 18 months after their initial assessment, participants were invited to take part in a follow-up assessment to assess for emergence of any Axis-I psychopathology during the follow-up period, along with levels of depressive symptomatology and life stress. Participants who consented to take part were first screened over the phone for Axis-I symptomatology, and those who reported evidence of some symptomatology since their initial assessment were invited into the lab for a Longitudinal Interval Follow-up Evaluation (LIFE; Keller et al., 1987), which was performed by a Masters- or PhD-level clinical interviewer. Participants also completed the BDI-II and PSS at this session. Those who did not have a LIFE interview conducted were sent a link to complete the BDI-II and PSS online.
2.8. Behavioral and physiological data reduction
2.8.1. Behavioral data
For the Flanker task, only trials in which participants made a response were considered. Data were subjected to a pre-defined quality control check (see Supplement). The primary variables of interest were interference effects, characterized as poorer accuracy and longer RTs on incongruent compared to congruent trials, and post-error behavioral adjustment, characterized by longer RT and increased accuracy on trials following incorrect compared to correct trials.
2.8.2. ERPs
ERPs were recorded using a 128-channel Hydrocel Geodesic Sensor Net system (Electrical Geodesics, Inc., Eugene, Oregon) and sampled at 250 Hz (bandwidth, 0.1-100 Hz; impedances < 100 kΩ) referenced to the vertex. Data processing occurred offline using Brain Vision Analyzer 2.0 (Brain Products GmbH, Gilching, Germany). Artifacts were removed using independent components analysis and corrupted channels were interpolated using a spline interpolation. Data were then re-referenced to the average reference and filtered (0.1-30 Hz). Epochs were extracted from −200 ms to 750 ms around markers locked to incorrect responses on incongruent trials (there were too few segments to examine epochs locked to incorrect responses on congruent trials). Epochs were visually inspected and remaining artifacts removed, baseline-corrected (−200 ms to −100 ms) and averaged. Based on the grand average waveforms we defined the ERN as the most negative point for each participant occurring −20 ms to 150 ms after incorrect responses, at midline electrodes Fz, FCz, Cz and Pz. The Pe was identified as the most positive peak occurring 100-250 ms following incorrect responses. For both components, the mean amplitude (±40 ms around the peak) was extracted. To test the specificity of putative findings, the mean amplitude of the correct-related negativity (CRN) and correct positivity (Pc) was also extracted from epochs time-locked to correct responses on incongruent trials. Mean CRN and Pc amplitude was extracted ±40 ms around the time point during which the ERN (for CRN) and Pe (for Pc) peaks occurred for that subject.
2.8.3. sLORETA
We performed a current density analysis of the scalp-recorded ERPs using standardized low resolution electromagnetic tomography (sLORETA). This method provides a 3D, linear, minimum norm solution to the inverse problem (Pascual-Marqui, 2002). An earlier version of this method, LORETA, has received considerable validation from studies combining LORETA with structural and functional MRI and PET (Pizzagalli, 2007). The newer version, sLORETA, has been found to have improved localization properties over LORETA (Sekihara et al., 2005). [For cross-modal validation of the sLORETA algorithm and its limitation, see Supplement.]
sLORETA calculates the standardized current density at 6239 voxels in the grey matter and hippocampus of the MNI-reference brain (MNI-Average-305-T1; voxel size: 5 mm3). sLORETA analyses were conducted using activity extracted from 28-128 ms (for ERN) and 128-228 ms (for Pe) post-response (areas defined based on the range of individual ERN and Pe peak latencies).
2.9. Statistical analyses
2.9.1. Behavioral data
The impact of stress on interference effects was analyzed using separate mixed Group (controls, rMDD) × Stress (pre-stress, post-stress) × Trial type (congruent, incongruent) analyses of variance (ANOVAs). Stress effects on post-error behavioral accuracy and RT were analyzed using separate Group × Stress × Response (post-correct, post-error) ANOVAs.
2.9.2. ERP data
The ERN was maximal at Fz and FCz; accordingly, the effects of stress on ERN amplitude were examined using a Group (control, rMDD) × Stress (pre-stress, post-stress) × Response (post-correct, post-incorrect) × Site (Fz, FCz) ANOVA. The same analyses were conducted for the Pe, using electrodes FCz and Cz, where the Pe was maximal.
2.9.3. sLORETA
To identify regions showing group differences in stress-induced changes in error-related activity, we performed voxelwise unpaired t-tests comparing groups in their pre-minus post-stress difference scores (a significant t-test effect using difference scores is equivalent to a significant Group × Stress interaction). Clusters were defined as areas showing a significant group difference in stress-related changes in current density at p < 0.005 (uncorrected) with a minimum size of 5 contiguous voxels.
2.9.4. Predictors of symptoms at follow-up
Step-wise multiple regression was used to determine the degree to which stress-induced changes in error monitoring could predict the severity of perceived life stress and depressive symptomatology at the 12- and 18-month follow-up evaluations, after controlling for baseline symptom severity. For these analyses, a difference score was computed by subtracting post-stress metrics error monitoring from pre- stress values, and then entering this difference score, along with the baseline symptom score into the model as predictors, and the follow-up score as the dependent variable. Separate regression models were used for predicting life stress (PSS scores) and depressive symptomatology (BDI-II scores). Regression analyses were only conducted on those measures of error monitoring for which significant Group × Stress interactions emerged.
3. Results
3.1. Group differences in stress reactivity
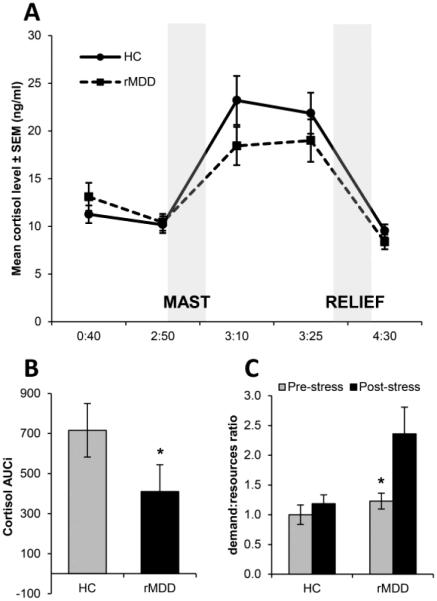
Fig. 1 shows mean raw cortisol levels across five time points in each group, along with cortisol AUCi A Group × Time ANOVA revealed a main effect of Time, F(4,248) = 49.96, p < 0.001, ηp2 = 0.45. Across groups, cortisol at time 3 and 4 was higher than all other time points (all ps < 0.001), indicating the MAST elicited robust and sustained activation of the HPA-axis across both groups. The rMDD group had a significantly lower AUCi index than controls, t(62) = 2.01, p = 0.049, Cohen’s d = 0.51, indicative of a more blunted HPA-axis reactivity.
Figure 1.
Figure shows (A) the mean cortisol across the five sampling points (shown on the x-axis as the hours and minutes from session start; control group n = 34, rMDD group n = 30), (B) cortisol AUCi, in the control and rMDD groups, and (C) changes in the demand to resources ratio on the Threat/Challenge Questionnaire (control group n = 13, rMDD group n = 13). * p < 0.05.
There was a main effect of Time for all self-report measures of stress reactivity (all ps < 0.05), indicating the MAST was also effective at inducing a subjective state of stress. Post-hoc tests showed stress increased scores on PANAS negative affect, STAI, all VAMS scales, and decreased scores on PANAS positive affect (all ps < 0.01). There was also a main effect of Group for PANAS negative affect (p = 0.02) and the STAI (p = 0.01), due to overall (averaged across time) higher levels of negative affect and state anxiety in the rMDD relative to control group. Full descriptive statistics are provided in Supplementary Table 1.
There was a main effect of Group, which was qualified by a Group × Stress interaction for the demand:resources ratio derived from the Threat/Challenge Questionnaire, F(1,24) = 4.84, p = 0.04, ηp2 = 0.17. Post-hoc tests on the interaction revealed no stress-induced changes in the demand:resources ratio for controls (p = 0.55) but a stress-induced increase for the rMDD group (p = 0.001). Furthermore, although there were no group differences at pre-stress (p = 0.31), at post-stress the demand:resources ratio was higher for the rMDD relative to the control group (p = 0.02).
3.2. Behavioral analyses
After excluding participants who did not pass the quality criteria (see Supplement), there were 33 controls and 30 rMDD participants for analysis of Flanker interference effects, and 25 controls and 21 rMDD participants for analysis of post-error adjustments.
For interference effects, the Group (controls, rMDD) × Stress (pre-stress, post-stress) × Trial type (congruent, incongruent) ANOVA on RT revealed main effects of Stress, F(1,61) = 29.96, p < 0.001, ηp2 = 0.33, and Trial type, F(1,61) = 438.73, p < 0.001, ηp2 = 0.88, where RTs were faster following stress (mean ± SD: pre-stress, 390 ms ± 51 ms; post-stress, 377 ms ± 48 ms) and on congruent (360 ms ± 39 ms) vs. incongruent (407 ms ± 49 ms) trials. For accuracy, a main effect of Trial type emerged, F(1,61) = 204.90, p < 0.001, ηp2 = 0.77, due to higher accuracy on congruent (98.8% ± 2.0) vs. incongruent (73.5% ± 15.7) trials. These findings indicate the task elicited the intended effects, but there were no effects of Group.
For post-error adjustments, there was a main effect of Stress for RT, F(1,44) = 40.48, p < 0.001, ηp2 = 0.48 and a main effect of Response, F(1,44) = 14.39, p < 0.001, ηp2 = 0.25, with faster RTs following stress (pre-stress, 384 ms ± 37 ms; post-stress, 367 ms ± 33 ms) and on trials following correct (371 ms ± 33 ms) vs. erroneous (380 ms ± 38 ms) responses. No effects emerged for post-error accuracy and no effects involved Group.
3.3. ERP analyses
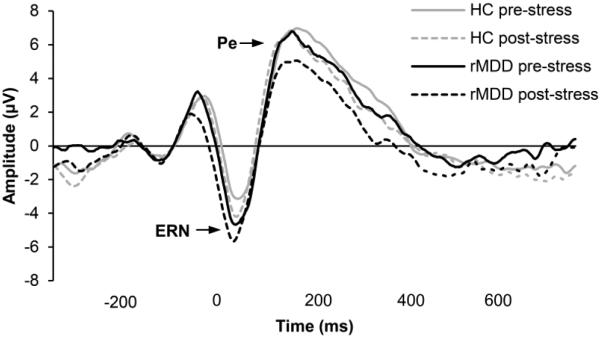
Twenty-nine controls and 24 rMDD participants had enough artifact-free ERP segments to be included in ERP analyses. Grand average ERP waveforms for the groups elicited by incorrect responses are shown in Fig. 2. A comparison of ERP waveforms following correct vs. incorrect responses across both stress conditions is shown in Supplemental Fig.1.
Figure 2.
Average ERP waveform time-locked to errors on incongruent trials in the rMDD and controls groups, pre- and post-stress, at electrode FCz.
3.3.1. ERN/CRN
The only significant effect to emerge from the Group × Stress × Response × Site (Fz, FCz) ANOVA was a main effect of Response, F(1,51) = 23.13, p < 0.001, ηp2 = 0.31, due to larger (i.e., more negative) ERN amplitude following incorrect compared to correct responses. Contrary to our hypotheses, there were no main effects or interactions involving Group (or Stress).
3.3.2. Pe/Pc
An analogous Group × Stress × Response × Site (FCz, Cz) ANOVA revealed a significant Stress × Response interaction, F(1,51) = 5.53, p = 0.03, ηp2 = 0.10, due to the fact that stress increased Pc amplitude (p = 0.04) and decreased Pe amplitude (p = 0.09). Additional effects involving Site (but not Stress or Group) are outlined in the Supplement.
3.4. sLORETA analyses
3.4.1. Stress-induced changes in CSD at the time of the ERN
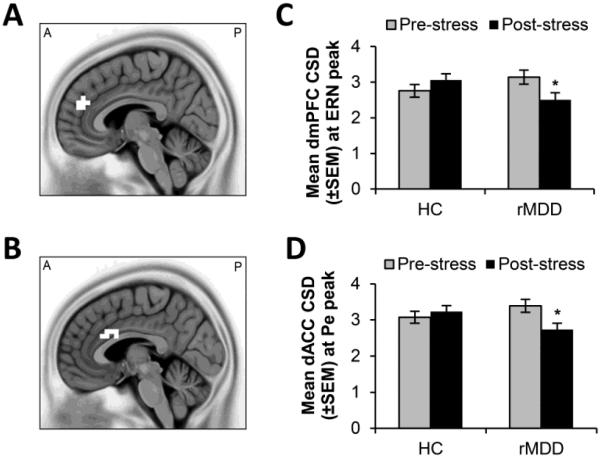
A cluster of nine voxels in the dorsomedial prefrontal cortex (dmPFC; BA9; peak voxel x = 5, y = 45, z = 25) emerged as showing greater stress-related changes in the rMDD group relative to controls at the time of the ERN (p < 0.005, Fig. 3A). To interpret this finding, mean current source density (CSD) in this region was extracted and post-hoc tests of simple effects conducted maintaining the initial threshold of p < 0.005. Stress reduced activation in the rMDD group (p = 0.002) but not in controls (p = 0.10). Furthermore, no differences emerged between the groups at pre-stress (p = 0.16, Cohen’s d = 0.40), however activation in the rMDD group was lower than the control group at post-stress but this did not survive the more conservative p < 0.005 threshold (p = 0.04, Cohen’s d = 0.58).
Figure 3.
Regions showing significant differences in stress-related changes in current density between the control and rMDD groups at the time of the ERN (A&C) and Pe (B&D). * p < 0.005, uncorrected.
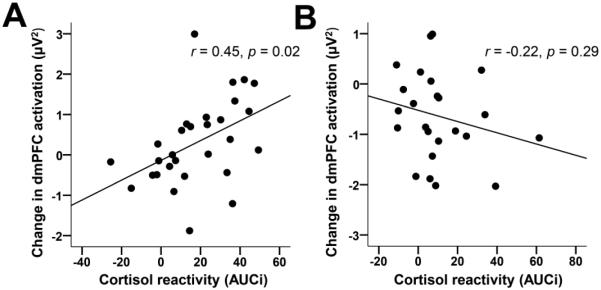
Among the control group, stress-induced changes in dmPFC activation correlated with cortisol reactivity. Specifically, smaller cortisol AUCi was associated with greater reductions in dmPFC activation from pre- to post-stress (control group: r = 0.45, p = 0.02; rMDD group r = −0.22, p = 0.29, Fig. 4A&B). Fisher’s test for independent correlations revealed that these correlations were significantly different from one another, Z = 2.41, p < 0.05. Furthermore, within the rMDD group, a greater number of prior MDEs was associated with lower dmPFC activation at pre-stress (r = −0.42, p = 0.046) and at trend-level post-stress (r = −0.39, p = 0.06).
Figure 4.
Significant correlations between cortisol reactivity and changes in dmPFC current source density from pre- to post-stress at the time of the ERN in the (A) control group (n = 29) and (B) rMDD group (n = 24).
3.4.2. Stress-induced changes in CSD at the time of the Pe
At the time of the Pe, a cluster of 13 voxels in the dorsal ACC (dACC; BAs 24, 32, 33, peak voxel x = −5, y = 10, z = 25) showed greater stress-induced changes in CSD in the rMDD group relative to controls (p < 0.005, Fig. 3B). Specifically, stress reduced dACC activation in the rMDD group (p = 0.003) but not in controls (p = 0.41). There were no group differences pre-stress (p = 0.20, Cohen’s d = 0.36), and although dACC activation in the rMDD group was lower than in the control group at post-stress, this post-hoc test did not survive the more stringent threshold of p < 0.005 (p = 0.04, Cohen’s d = 0.57).
Stress-induced decreases in dACC activation were associated with self-reported stress reactivity across the entire sample. Specifically, stress-induced decreases in dACC activation were associated with increases in PANAS negative affect (r = −0.41, p = 0.002) and state anxiety (r = −0.32, p = 0.02), and decreases in PANAS positive affect (r = 0.30, p = 0.03) in response to the MAST. Furthermore, greater stress-induced decreases in dACC activation were associated with a greater increase in the demand:resources ratio score on the Threat/Challenge Questionnaire (r = −0.54, p = 0.01). Scatter plots revealed that the correlations involving the PANAS subscales were driven largely by 1-2 individuals and therefore were not considered to be robust (Supplemental Fig. 2).
Although greater activation in both of these regions was associated with greater post-error slowing (dmPFC: r = 0.28, p = 0.046; dACC: r = 0.30, p = 0.03) and increased post-error accuracy (dmPFC: r = 0.31, p = 0.03, dACC: r = 0.43, p = 0.001) at pre-stress, activation in these regions was not associated with ERN or Pe amplitudes (all ps > 0.05). This indicates that although these regions were clearly involved in post-error behavioral adjustments, they might be distinct from the neural generators of the ERN and Pe. Subsequent analyses evaluated this hypothesis (see Supplement) and confirmed that the neural generators of the ERN and Pe were located in the supplementary motor area (SMA) and more caudal part of the ACC, respectively, and did not overlap with the dmPFC and dACC regions.
3.5. Predictors of stress and depressive symptomatology at follow-up
Thirty-eight and 28 participants completed the 12- and 18-month follow-up evaluations, respectively. Over the 18-month follow-up period, four individuals experienced a major depressive episode (two from the healthy control group and two from the rMDD group). We confirmed that all initial findings held when these two healthy controls were excluded from analysis. Given the low rate of recurrence it was not possible to identify predictors of new depressive episode onset; accordingly, analyses focused on determining whether cortisol AUCi as well as stress-induced changes in dmPFC and dACC activity (i.e., the variables showing group differences at the baseline assessment) predicted symptom severity at follow-up across the groups. After controlling for baseline symptomatology, greater stress-induced decreases in dACC activation at the time of the Pe predicted elevated levels of stress at both 12 (β = −0.36, p = 0.03) and 18 months (β = −0.42, p = 0.02), and predicted increased levels of depressive symptomatology on the BDI-II at 18 months (β = −0.42, p = 0.03). These effects remained significant even when cortisol AUCi and stress-induced changes in dmPFC activation at the time of the ERN were entered into the model (all ps < 0.05), indicating that error-related decreases in dACC activation following stress may be a unique predictor of heightened stress and depressive symptoms over 18 months. Neither cortisol AUCi nor stress-induced changes in dmPFC activity were significant predictors of follow-up symptoms when entered into the model alone (all ps > 0.05).
4. Discussion
This study examined whether error monitoring deficits observed in acute MDD are evident in rMDD individuals, and if exposure to acute stress exacerbates or precipitates such deficits. Results showed that both prior to and following stress, the rMDD and control groups did not differ on behavioral (post-error adjustments) or ERP (ERN and Pe amplitude) indices of error monitoring. However, stress reduced error-related activation within regions critical for performance monitoring – the dmPFC and dACC – specifically for those with rMDD. Consistent with the absence of scalp-level ERP effects, these regions were spatially distinct from the neural generators of the ERN and Pe, which were localized in the SMA and more caudal portions of the ACC, respectively.
Importantly, stress-induced decreases in dmPFC and dACC activation correlated with distinct components of the stress response. Relative to controls, the rMDD group showed a blunted cortisol response coupled with a heightened subjective stress response. Notably, a greater blunting of cortisol responses was associated with a larger stress-induced decrease in dmPFC activation at the time of automatic error detection (ERN peak) in healthy controls, whereas greater subjective stress-reactivity (stress-related increases in state anxiety and perceived ratio of task demands versus coping resources) was associated with a larger stress-induced decrease in dACC activation at the time of more elaborated error evaluation (Pe peak) across the entire sample. Furthermore, results showed that across the entire sample, greater stress-induced dACC deactivation predicted more severe stress and depressive symptomatology 18 months later. These findings of abnormal stress responding and greater stress-induced frontocingulate impairment in rMDD, along with the observed association between frontocingulate impairment and indices of stress reactivity and future depressive symptoms, provide some support for our initial hypothesis that disruptions in frontocingulate function during error monitoring in rMDD may be linked to abnormal patterns of stress reactivity, and may be a potential mechanism by which stress makes depression-prone individuals vulnerable to relapse.
The unmasking of frontocingulate dysfunction in rMDD following stress links separate lines of evidence indicating the presence of performance monitoring deficits and increased stress sensitivity in depression-prone individuals, and evidence of the detrimental effects of acute stress on prefrontal function. Specifically, prior research using Eriksen Flanker and Stroop paradigms in acute MDD have shown evidence of poorer post-error adjustments (Beard et al., 2015), abnormal ERN (Chiu and Deldin, 2007) and Pe amplitudes (Schrijvers et al., 2008; Olvet et al., 2010), as well as disrupted frontocingulate activation (Holmes and Pizzagalli, 2008), and neuroimaging studies have consistently observed depression-related deficits in regions of the dlPFC, dmPFC and ACC (Fitzgerald et al., 2008) that may contribute to these impairments. These regions are known to be highly sensitive to stress-related excesses in catecholamine and glucocorticoid levels (Arnsten, 2009), and this may explain the exacerbation of prefrontal dysfunction in stress-sensitive populations, such as those with rMDD. Furthermore, stress-related impairments in the recruitment of control systems during processing of negative information may result in rMDD individuals having a poorer ability to regulate responses to negative feedback, making them vulnerable to subsequent depressive episodes when faced with stress. However, given that stress-induced frontocingulate impairment remained a unique predictor of symptom severity at follow-up even after controlling for endocrine and subjective stress reactivity, this suggests that it is not the magnitude of the stress response per se that confers risk for future depressive symptoms, but rather, the degree to which stress impairs frontocingulate function.
Our source localization findings align well with a wealth of prior research identifying neural generators of the ERN in the SMA and the Pe in more ventral regions of the ACC (Holroyd et al., 1998; Mathalon et al., 2002). However, the lack of group differences in scalp-recorded ERN amplitude adds to the growing picture of a complex relationship between depression vulnerability and ERN magnitude. Specifically, whereas some studies have demonstrated an increased ERN amplitude in MDD (Chiu and Deldin, 2007; Holmes and Pizzagalli, 2008), others have found ERN reduction in severely depressed samples (Schrijvers et al., 2008; Olvet et al., 2010), suggesting that the relationship between ERN amplitude and depression severity may be non-linear (Olvet et al., 2010). An alternative explanation is that differences arise due to diagnostic heterogeneity, as blunted ERN amplitudes have been linked with psychomotor retardation (Schrijvers et al., 2008), and the melancholic depression subtype (Weinberg et al., 2016). The lack of group differences in scalp-level ERN and Pe amplitudes must be interpreted with caution however, as our sample of subjects with sufficient artifact-free ERP data at both pre- and post-stress, was smaller than prior studies.
We also found that the rMDD group demonstrated a blunted cortisol response to stress. Although this may seem at odds with findings of consistently enhanced self-reported stress reactivity in depression, there is considerable variability in the literature regarding the precise direction of abnormality in cortisol responding that accompanies heightened subjective stress reactivity. Research using acute stressors similar to the MAST have observed blunted cortisol reactivity in rMDD individuals (Morris et al., 2014), particularly in females (Bagley et al., 2011). In contrast, findings of enhanced cortisol reactivity in rMDD appear to be more consistent for studies using minor stressors. Elevated cortisol reactivity in rMDD was observed in response to aversive images (Admon et al., 2015) and enhanced cortisol reactivity to a low- but not a high-stress version of the Trier Social Stress Test predicted increased depression at follow-up (Morris et al., 2012). One explanation for the blunted cortisol response coupled with heightened subjective stress reactivity in those with rMDD is that in individuals who are especially sensitive to the effects of stress, repeated hypersecretion of cortisol could cause desensitization of the HPA axis, eventually resulting in reduced adrenocortical responsiveness (Heim et al., 2000). This interpretation remains speculative in regard to our sample however, as we cannot confirm that our rMDD participants had been exposed to repeated life stress.
Some limitations must be considered when interpreting the current findings. In particular, future studies using a neutral stress condition are needed to rule out the possibility that our effects are not simply due to practice or fatigue. Additionally, although we observed no group differences in ERN amplitude, recent research using the Flanker task found that only rMDD individuals with prior melancholic depression, showed blunted ERN amplitudes (Weinberg et al., 2016). Unfortunately the SCID-IV assessment only captures MDE specifiers for current depressive episodes, so we were unable to classify our rMDD sample into melancholic versus non-melancholic subtypes to address this question. Given the strong links between stress and anhedonia (Pizzagalli, 2014; a core feature of melancholic depression), future research should evaluate whether the effects of stress on error monitoring are greater in individuals with prior melancholic depression. Moreover, although we observed interactive effects of stress and depression history on the ratio of perceived demands to resources on the Threat/Challenge Questionnaire, this measure was introduced after the study had started and was thus available only for a subset of participants, so these findings should be treated as preliminary. Finally, we used an uncorrected threshold for our sLORETA analyses (p < 0.005 with a minimum cluster extent), and therefore these results await replication in a larger sample. Given the low spatial resolution of sLORETA, these findings also need to be confirmed using functional imaging techniques that have higher spatial resolution (e.g., fMRI).
The current findings also stimulate several areas for future research. Specifically, it would be valuable to know whether interventions that enhance prefrontal functioning are able to improve the recruitment of frontocingulate regions under stress. For example, cognitive behavioral therapy has been shown to modulate frontocingulate functioning (Goldapple et al., 2004), and the novel antidepressant agent, vortioxetine, has been found to improve several aspects of executive functioning in depressed individuals (McIntyre et al., 2014). It is possible that reductions in depression resulting from these interventions may be associated with fewer deficits in performance monitoring following stress. Additionally, although we suggest that frontocingulate deficits under stress are sequelae of prior depressive episodes, an alternative explanation is that the propensity for frontocingulate dysfunction under stress may be a predisposing factor that precedes depression onset. Studies examining frontocingulate function prior to the onset of MDD are limited, however, the evidence runs counter to this interpretation. For example, a prospective longitudinal study found that cognitive control during early and mid-adolescence (as measured by performance on a Stroop task), was not predictive of later MDD onset (Vijayakumar et al., 2016). Furthermore, research has failed to find evidence of cognitive control deficits in high-risk offspring of depressed mothers (Klimes-Dougan et al., 2006), except in cases where the offspring also has current MDD (Micco et al., 2009). However, a critical caveat is that none of these studies evaluated whether the emergence of cognitive control deficits under stress predicted future depression onset. Therefore, future longitudinal studies that incorporate stress manipulations are needed to evaluate this possibility.
5. Conclusion
To conclude, the findings of the current study indicate that individuals with rMDD display normative error monitoring under stress-free conditions. However, following exposure to acute uncontrollable stress, aberrant activity within key performance monitoring systems emerges during discrete stages of error processing, and this disruption is associated with abnormalities stress responding. Critically, across the entire sample, these abnormalities predicted greater levels of depressive and stress-related symptomatology 18 months later. These findings add to the growing body of evidence pointing to the critical role of stress in depression recurrence, but also highlight a novel role for stress-induced frontocingulate dysfunction in the emergence of future depressive symptomatology.
Supplementary Material
Highlights.
Stress is a trigger of depression relapse and impairs performance monitoring.
In rMDD, stress impaired frontocingulate activation in an error monitoring task.
Greater frontocingulate impairment predicted increased depression at 18 months.
Results highlight a mechanism by which stress may trigger future depression.
Acknowledgements
The authors wish to acknowledge Eva Woodward and Nancy Hall-Brooks for their contribution to diagnostic screening of participants in the current study.
Role of Funding Source: This research was supported by National Institute of Mental Health grants R01 MH068376 and R37 MH068376 awarded to Dr. Pizzagalli and The William Rosenberg Family Foundation (Carol Silverstein and Jill Gotlieb). Dr. Whitton was partially supported by Fellowships from the American Australian Association; the Brain & Behavior Foundation (grant 24168); and the National Health and Medical Research Council of Australia (grant APP1110773). No funding sources played a role in the study design, collection, analyses and interpretation of the data, in the writing of the report or the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest: Over the past three years, Dr. Pizzagalli has received honoraria/consulting fees from Akili Interactive Labs, BlackThorn Therapeutics, Otsuka America Pharmaceutical and Pfizer for activities unrelated to this project. All other authors declare no conflicts of interest.
Contributors. DAP designed the study, PK, AVV, and AH performed data collection, DJC performed the stress manipulations with AEW, AEW processed electrophysiological data (with MLI), analyzed the data, and wrote the first draft of the manuscript with input from DAP. DGD assisted with interpretation of behavioral findings, and provided input on subsequent drafts of the manuscript, along with DAP. All authors have approved the final manuscript.
Ethical standards: The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Supplemental information = Additional information about: inclusion/exclusion criteria, Eriksen Flanker Task parameters and quality control criteria, the Maastricht Acute Stress Test, ERP peak detection, ERN and Pe source localization, and supplementary analyses of behavioral and stress-reactivity data.
References
- Admon R, Holsen LM, Aizley H, Remington A, Whitfield-Gabrieli S, Goldstein JM, Pizzagalli DA. Striatal hypersensitivity during stress in remitted individuals with recurrent depression. Biol. Psychiatry. 2015;78:67–76. doi: 10.1016/j.biopsych.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aker M, Bø R, Harmer C, Stiles TC, Landrø NI. Inhibition and response to error in remitted major depression. Psychiatry Res. 2016;235:116–122. doi: 10.1016/j.psychres.2015.11.038. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Murphy CF, Gunning-Dixon FM, Kalayam B, Katz R, Kanellopoulos D, Etwaroo GR, Klimstra S, Foxe JJ. Event-related potentials in an emotional go/no-go task and remission of geriatric depression. Neuroreport. 2007;18:217–221. doi: 10.1097/WNR.0b013e328013ceda. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat. Rev. Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF. Stress weakens prefrontal networks: molecular insults to higher cognition. Nat. Neurosci. 2015;18:1376–1385. doi: 10.1038/nn.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagley SL, Weaver TL, Buchanan TW. Sex differences in physiological and affective responses to stress in remitted depression. Physiol. Behav. 2011;104:180–186. doi: 10.1016/j.physbeh.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Beard C, Donahue R, Dillon D, Van't Veer A, Webber C, Lee J, Barrick E, Hsu K, Foti D, Carroll F. Abnormal error processing in depressive states: a translational examination in humans and rats. Transl. Psychiatry. 2015;5:e564. doi: 10.1038/tp.2015.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck depression inventory-II. Psychological Corporation; San Antonio, TX: 1996. p. b9. [Google Scholar]
- Bond A, Lader M. The use of analogue scales in rating subjective feelings. Brit. J. Med. Psychol. 1974;47:211–218. [Google Scholar]
- Bora E, Harrison B, Yücel M, Pantelis C. Cognitive impairment in euthymic major depressive disorder: a meta-analysis. Psychol. Med. 2013;43:2017–2026. doi: 10.1017/S0033291712002085. [DOI] [PubMed] [Google Scholar]
- Bukh J, Andersen P, Kessing L. Rates and predictors of remission, recurrence and conversion to bipolar disorder after the first lifetime episode of depression–a prospective 5-year follow-up study. Psychol. Med. 2016;46:1–11. doi: 10.1017/S0033291715002676. [DOI] [PubMed] [Google Scholar]
- Chiu PH, Deldin PJ. Neural evidence for enhanced error detection in major depressive disorder. Am. J. Psychiatry. 2007;164:608–616. doi: 10.1176/ajp.2007.164.4.608. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J. Health Soc. Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept. Psychophys. 1974;16:143–149. [Google Scholar]
- Falkenstein M, Hoormann J, Christ S, Hohnsbein J. ERP components on reaction errors and their functional significance: a tutorial. Biol. Psychol. 2000;51:87–107. doi: 10.1016/s0301-0511(99)00031-9. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ. A meta-analytic study of changes in brain activation in depression. Hum. Brain Mapp. 2008;29:683–695. doi: 10.1002/hbm.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiadi E, Liotti M, Nixon NL, Liddle PF. Electrophysiological evidence for abnormal error monitoring in recurrent major depressive disorder. Psychophysiology. 2011;48:1192–1202. doi: 10.1111/j.1469-8986.2011.01198.x. [DOI] [PubMed] [Google Scholar]
- Goldapple K, Segal Z, Garson C, Lau M, Bieling P, Kennedy S, Mayberg H. Modulation of cortical-limbic pathways in major depression: treatment-specific effects of cognitive behavior therapy. Arch. Gen. Psychiatry. 2004;61:34–41. doi: 10.1001/archpsyc.61.1.34. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J. Cognition and depression: current status and future directions. Ann. Rev. Clin. Psychol. 2010;6:285. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen CL. Stress and depression: old questions, new approaches. Curr. Opin. Psychol. 2015;4:80–85. [Google Scholar]
- Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Holmes AJ, Bogdan R, Pizzagalli DA. Serotonin transporter genotype and action monitoring dysfunction: a possible substrate underlying increased vulnerability to depression. Neuropsychopharmacology. 2010;35:1186–1197. doi: 10.1038/npp.2009.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes AJ, Pizzagalli DA. Spatiotemporal dynamics of error processing dysfunctions in major depressive disorder. Arch. Gen. Psychiatry. 2008;65:179–188. doi: 10.1001/archgenpsychiatry.2007.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB, Coles MG. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol. Rev. 2002;109:679. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Dien J, Coles MG. Error-related scalp potentials elicited by hand and foot movements: evidence for an output-independent error-processing system in humans. Neurosci. Lett. 1998;242:65–68. doi: 10.1016/s0304-3940(98)00035-4. [DOI] [PubMed] [Google Scholar]
- Inzlicht M, Gutsell JN. Running on empty: neural signals for self-control failure. Psychol. Sci. 2007;18:933–937. doi: 10.1111/j.1467-9280.2007.02004.x. [DOI] [PubMed] [Google Scholar]
- Kaplan S, Berman MG. Directed attention as a common resource for executive functioning and self-regulation. Perspect. Psychol. Sci. 2010;5:43–57. doi: 10.1177/1745691609356784. [DOI] [PubMed] [Google Scholar]
- Keller MB, Lavori PW, Friedman B, Nielsen E, Endicott J, McDonald-Scott P, Andreasen NC. The Longitudinal Interval Follow-up Evaluation: a comprehensive method for assessing outcome in prospective longitudinal studies. Arch. Gen. Psychiatry. 1987;44:540–548. doi: 10.1001/archpsyc.1987.01800180050009. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Klimes-Dougan B, Ronsaville D, Wiggs EA, Martinez PE. Neuropsychological functioning in adolescent children of mothers with a history of bipolar or major depressive disorders. Biol. Psychiatry. 2006;60:957–965. doi: 10.1016/j.biopsych.2006.03.031. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Fedor M, Faustman WO, Gray M, Askari N, Ford JM. Response-monitoring dysfunction in schizophrenia: an event-related brain potential study. J. Abnorm. Psychol. 2002;111:22–41. [PubMed] [Google Scholar]
- McIntyre RS, Lophaven S, Olsen CK. A randomized, double-blind, placebo-controlled study of vortioxetine on cognitive function in depressed adults. Int. J. Neuropsychopharmacol. 2014;17:1557–1567. doi: 10.1017/S1461145714000546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes WB, Gray HM, Mendoza-Denton R, Major B, Epel ES. Why egalitarianism might be good for your health: physiological thriving during stressful intergroup encounters. Psychol. Sci. 2007;18:991–998. doi: 10.1111/j.1467-9280.2007.02014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micco JA, Henin A, Biederman J, Rosenbaum JF, Petty C, Rindlaub LA, Murphy M, Hirshfeld-Becker DR. Executive functioning in offspring at risk for depression and anxiety. Depress. Anxiety. 2009;26:780–790. doi: 10.1002/da.20573. [DOI] [PubMed] [Google Scholar]
- Morris MC, Rao U, Garber J. Cortisol responses to psychosocial stress predict depression trajectories: social-evaluative threat and prior depressive episodes as moderators. J. Affect. Disord. 2012;143:223–230. doi: 10.1016/j.jad.2012.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MC, Rao U, Wang L, Garber J. Cortisol reactivity to experimentally manipulated psychosocial stress in young adults at varied risk for depression. Depress. Anxiety. 2014;31:44–52. doi: 10.1002/da.22125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Hughes B, Robertson ER, Cooper JC, Gabrieli JD. Neural systems supporting the control of affective and cognitive conflicts. J. Cognitive Neurosci. 2009;21:1841–1854. doi: 10.1162/jocn.2009.21129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvet DM, Klein DN, Hajcak G. Depression symptom severity and error-related brain activity. Psychiatry Res. 2010;179:30–37. doi: 10.1016/j.psychres.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui RD. Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods Find. Exp. Clin. Pharmacol. 2002;24D:5–12. [PubMed] [Google Scholar]
- Pizzagalli DA. Electroencephalography and high-density electrophysiological source localization. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of Psychophysiology. 3rd Cambridge University Press; Cambridge, UK: 2007. pp. 56–84. [Google Scholar]
- Pizzagalli DA. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2011;36:183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA. Depression, stress, and anhedonia: toward a synthesis and integrated model. Annu. Rev. Clin. Psychol. 2014;10:393. doi: 10.1146/annurev-clinpsy-050212-185606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Schoenberg PL. The error processing system in major depressive disorder: Cortical phenotypal marker hypothesis. Biol. Psychol. 2014;99:100–114. doi: 10.1016/j.biopsycho.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Schrijvers D, de Bruijn ERA, Maas Y, De Grave C, Sabbe BGC, Hulstijn W. Action monitoring in major depressive disorder with psychomotor retardation. Cortex. 2008;44:569–579. doi: 10.1016/j.cortex.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Sekihara K, Sahani M, Nagarajan SS. Localization bias and spatial resolution of adaptive and non-adaptive spatial filters for MEG source reconstruction. Neuroimage. 2005;25:1056–1067. doi: 10.1016/j.neuroimage.2004.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeets T, Cornelisse S, Quaedflieg CW, Meyer T, Jelicic M, Merckelbach H. Introducing the Maastricht Acute Stress Test (MAST): A quick and non-invasive approach to elicit robust autonomic and glucocorticoid stress responses. Psychoneuroendocrinology. 2012;37:1998–2008. doi: 10.1016/j.psyneuen.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Spielberger C, Gorsuch R, Lushene R, Vagg P, Jacobs G. Manual for the State-Trait Anxiety Inventory. CA Consulting Psychologists Press; Palo Alto: 1983. [Google Scholar]
- Vijayakumar N, Whittle S, Yücel M, Byrne ML, Schwartz O, Simmons JG, Allen NB. Impaired maturation of cognitive control in adolescents who develop major depressive disorder. J. Clin. Child. Adolesc. Psychol. 2016;45:31–43. doi: 10.1080/15374416.2014.987381. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark L, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J. Pers. Soc. Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Kotov R, Proudfit GH. Neural indicators of error processing in generalized anxiety disorder, obsessive-compulsive disorder, and major depressive disorder. J. Abnorm. Psychol. 2015;124:172. doi: 10.1037/abn0000019. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Liu H, Shankman SA. Blunted neural response to errors as a trait marker of melancholic depression. Biol. Psychol. 2016;113:100–107. doi: 10.1016/j.biopsycho.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.