Abstract
Associations between temperature and cardiovascular (CVD) mortality have been reported, but the underlying biological mechanisms remain uncertain. We explored the association between apparent temperature and serum biomarkers for CVD. Using linear mixed effects models, we examined the relationships between residence-proximate apparent temperature (same day and 1, 7, and 30 days prior) and several inflammatory, hemostatic, and lipid biomarkers for midlife women from 1999 through 2004. Our study population consisted of 2,306 women with mean age of 51 years (±3 years) enrolled in Study of Women’s Health Across the Nation (SWAN) in Chicago, Illinois; Detroit, Michigan; Los Angeles and Oakland, California; Newark, New Jersey; and Pittsburgh, Pennsylvania. Mean daily apparent temperature was calculated using temperature and relative humidity data provided by the National Climatic Data Center and the US Environmental Protection Agency, while daily data for fine particles, ozone, carbon monoxide, and nitrogen dioxide from the US Environmental Protection Agency Air Quality Data Mart were considered as confounders. All analyses were stratified by warm and cold seasons. More significant (p < 0.10) negative associations were found during the warm season for various lag times, including hs-CRP, fibrinogen, tissue plasminogen activator antigen (tPA-ag), tissue plasminogen activator antigen (PAI-1), high-density lipoprotein (HDL), and total cholesterol. During the cold season, significant negative associations for fibrinogen and HDL, but significant positive associations for tPA-ag, PAI-1, and triglycerides were observed for various lag times. Pollutants did not confound these associations. Apparent temperature was associated with several serum biomarkers of CVD risk in midlife women, shedding light on potential mechanisms.
Introduction
Associations between temperature and morbidity (Turner et al., 2012; Ye et al., 2011) and mortality (Basu, 2009; Basu and Samet, 2002b) have been demonstrated in several studies throughout the world. Consistently, cardiovascular disease (CVD) including acute myocardial infarction (Basu and Ostro, 2008; Bhaskaran et al., 2009; Madrigano et al., 2013; Wichmann et al., 2013), congestive heart failure (Basu and Ostro, 2008; Kolb et al., 2007; Wilker et al., 2012), and ischemic heart disease (Basu and Ostro, 2008; Basu et al., 2012), as well as cerebrovascular outcomes such as stroke (Basu et al., 2012; Miyatake et al., 2011) have been associated with higher ambient temperature. Few studies, however, have been conducted to examine serum markers associated with these CVD outcomes, which may help clarify the mechanisms involved with temperature-induced mortality and morbidity from CVD (Halonen et al., 2011a; Halonen et al., 2011b; Hampel et al., 2010; Hong et al., 2012; Schauble et al., 2012; Schneider et al., 2008; Wilker et al., 2012).
While previous research has investigated the hypothesis that inflammatory, hemostatic and/or lipid markers may be associated with higher ambient temperature, they have yielded mixed results. For example, positive, negative and no associations between temperature and high-sensitivity C-Reactive Protein (hs-CRP) have been reported in several studies, but these inconsistent results may be partially explained by the differences in populations studied and the temperature ranges examined as well as control or lack of control of other confounding factors (Halonen et al., 2010; Hampel et al., 2010; Hong et al., 2012; Schauble et al., 2012; Schneider et al., 2008; Wilker et al., 2012). Furthermore, many studies were conducted among patients with pre-existing diseases or among other vulnerable populations such as the elderly, while fewer were conducted among healthy populations or younger age groups (Benmarhnia et al., 2015). The body’s thermoregulatory capacity and activity patterns may be different between healthy people and people with pre-existing diseases who may spend less time outdoors. Also a large degree of spatial heterogeneity occurs with temperature exposures. Heat impacts may also vary by area due to people’s adaptation to the regional climate, and vectors for diseases are sensitive to different temperature thresholds (Ye et al., 2011). Thus, the effects of temperature on serum biomarkers for CVD are not fully understood.
The Study of Women’s Health Across the Nation (SWAN) is a large multi-racial/ethnic cohort study in the US originally designed to follow middle-aged women approximately annually through menopausal transition. The data provided a unique opportunity to explore the association between temperature and serum biomarkers in a healthy population of women in six geographic locations in the US. We examined the associations between both short- and intermediate-term exposure to temperature, while updating residential addresses annually to capture the relevant exposure over time. The following serum inflammatory/hemostatic biomarkers and lipid blood markers were considered: hs-CRP, plasminogen activator inhibitor-1 (PAI-1), tissue plasminogen activator antigen (tPA-ag), factor VII coagulant activity (Factor VIIc), fibrinogen, total cholesterol, triglycerides, high-density lipoprotein (HDL), low-density lipoprotein (LDL), Lipoprotein A (LPA), and Lipoprotein A-1 (LPA1), respectively.
Methods
Study Population
The study design and recruitment of participants for the SWAN cohort has been described in detail previously (Green et al., 2016). Six of the seven clinical SWAN sites participated in this specific study of temperature specifically: Chicago, Illinois; Detroit, Michigan; Los Angeles, California; Newark, New Jersey; Oakland, California; and Pittsburgh, Pennsylvania. This study was based on a longitudinal analysis of temperature and inflammatory, hemostatic, and lipid markers assessed at clinical visits 3 (1999) through 7 (2004). Non-Hispanic white women were examined at each site as well as women selected from the following racial/ethnic groups for specific sites: African-American in Pittsburgh, Detroit, and Chicago; Chinese in Oakland; Japanese in Los Angeles; and Hispanic in New Jersey.
Approximately 450 eligible women at each of the study sites were recruited for the longitudinal cohort study, which conducted annual clinical assessments. hs-CRP values > 10 mg/L were excluded because they may have resulted from severe infection, major trauma, or chronic inflammatory diseases (9.9% of all observations). For other inflammatory, hemostatic, or lipid markers, extreme values (outside the mean + 3 standard deviations) were excluded from the study.
All women provided signed, written informed consent for the SWAN protocols at study recruitment between 1995 and 1997, and the Institutional Review Boards at all six participating sites approved the SWAN protocols for this study of temperature specifically.
Exposure assignment
A residential history was abstracted for each participant throughout the study period. Addresses were geocoded as described in Green et al.(Green et al., 2016)
The main exposure in this study, mean daily apparent temperature, was calculated using temperature and relative humidity data provided by the National Centers for Environnmental Information (NCEI) ((NOAA), 2012) and the US Environmental Protection Agency (EPA). Daily data for fine particles (PM2.5), ozone (O3), carbon monoxide (CO), and nitrogen dioxide (NO2) were abstracted from the US EPA’s Air Quality System Data Mart (US EPA, 2014) to be examined as confounders. Typically, apparent temperature and gaseous pollutants were measured hourly, while PM2.5 was measured every three days but sometimes every six days or daily. Participants with air pollution monitors located within a 20 km radius of each residential address and apparent temperature within 30 km and 12 km for short and intermediate-term exposures, respectively, were included in the study. Short-term apparent temperature exposures consisted of same day (lag0), lag of one day prior (lag1), and cumulative average lag of prior seven days (lag0–6/week), while intermediate-term was defined as a cumulative average of lag 30 days prior (lag0–29/month), inclusive of the blood draw date. ArcGIS v10.0 (ESRI, 2014) was used to assign these exposures. When multiple monitors were available, the monitor that minimized the distance but maximized the number of measurements was selected (Green et al., 2016).
Because PM2.5 began to be routinely monitored in mid-1999 and 2000, our study period began at visit 3, which corresponded to visits starting in 1999. Previous studies examining PM2.5 using the same data found an association with some of these biomarkers, so we wanted to make sure that we adjusted for it in our models (Green et al., 2016; Ostro et al., 2014). The same monitor chosen was used for the 30-day and 7-day metrics to maintain comparability between the exposure periods. However, we allowed single-day lag measurements (0-day and 1-day) to come from different monitors because daily measurements were less available for some exposures. For apparent temperature calculations and gaseous pollutants, if a 30-day period had at least nine days of data, then an average was assigned for the month. For the week-long window, a minimum of three days were required. For PM2.5, if a 30-day period had at least three days of data, then an average was assigned for the month. However, over 75% of the 30-day periods had nine or more measurements. If the participant moved during the year prior to her visit, we took an average of both locations when assigning exposures.
Data Analysis
To study the association between apparent temperature and the serum markers, we used SAS 9.4 Proc Mixed (Inc., 2014) for linear mixed effects regression analyses with first-order autoregressive structure to account for the correlation between repeated measurements for each woman.
We included the serum markers in the regression models as response variables. Study site, race/ethnicity (Caucasian, African-American, Japanese, Chinese and Hispanic) and education (high school or less, some college, or college graduate) were included as time-invariant variables in a base model with the following visit-specific variables: active smoking (yes/no), body mass index (BMI) (continuous) and alcohol consumption in the 24 hours prior to the blood draw (yes/no). In addition, we considered participant’s time-varying age, menopausal status (pre-/early peri-/late peri-/post-/surgically induced/hormone user), and visit number in combination and separately to determine which variable(s) were significant in an effort to adjust for changing trends over time. We stratified all analyses by season of blood draw (warm/cold season). Warm season was defined as May through October for Los Angeles and Oakland and June through September for all non-California sites; cold season was defined as November through April for Los Angeles and Oakland and October through May for all non-California sites. Differences in seasonal definitions by study location were chosen based on local climatology.
We excluded visits for women who reported having any previous CVD event, such CVD accident, heart attack, stroke, congestive heart failure, percutaneous coronary intervention, or coronary artery bypass graft because these conditions and/or associated medications could potentially affect the serum markers. We also excluded women who did not fast 12 hours before the blood draw at a particular visit (2.8% of the total samples). For fibrinogen and Factor VIIc, which were only measured at visits 3, 5 and 7, medical conditions reported at the measured visit or the visit before were included in the statistical models. Due to the small numbers of New Jersey participants for visits 6 and 7, we censored those visits while keeping women from that site in the model for visits 3, 4, and 5. Since fibrinogen, HDL, LDL, total cholesterol, and LPA1 were normally distributed, results are expressed as unit change in marker per 5.6°C (10°F) increase in apparent temperature. The other serum markers, however, were not normally distributed so were log transformed, and the results were expressed as the percent change per 5.6°C increase using the formula [100% × (exp(β *5.6C) − 1)]. To examine whether apparent temperature was confounded by air pollutants, we considered each pollutant with apparent temperature in separate models. For these models, the same averaging time was used for the pollutant and apparent temperature. The only exception is that the 7-day lag average concentrations of PM2.5 were not available, so the 30-day average PM2.5 concentrations were used instead for the analysis with 7-day average temperature.
Results
Our final study population consisted of 8,940 visits throughout the follow-up years for 2,306 women with sample sizes and population characteristics by site shown in Table 1. Because the distribution of the four metrics of apparent temperature that we examined in this study (lag0, lag 1, lag0–6, and lag0–29) were similar, only lag0 (on the day of the blood draw) apparent temperature was included in Table 1, separately for the cold and warm seasons. Race/ethnicity for study sites are shown as missing for those that were not included in each site by selection of participants. Our study population is well educated, except in Detroit and Newark, where approximately 20% of the women had completed at least a college education. Also, most women were overweight or obese and were not current smokers or consumers of alcohol in the 24 hours prior to the blood draw. Of the pollutants examined, ozone was most strongly correlated with apparent temperature, with Pearson correlation coefficients ranging from 0.44 to 0.72 (p < 0.01) among all sites combined. Moderate or weakly positive correlations between apparent temperature and PM2.5 and NO2, and weak negative correlations with CO concentrations were also found for all sites for the various apparent temperature metrics studied.
Table 1.
Characteristics of the Study Population, SWAN Cohort, 1999–2004
| Detroit, MI n = 436 |
Chicago, IL n = 376 |
Oakland, CA n = 409 |
Los Angeles, CA n = 443 |
Newark, NJ n = 261 |
Pittsburgh, PA N = 381 |
|
|---|---|---|---|---|---|---|
| Mean apparent temp, °C (°F) | ||||||
| Cold season | 5.6 (42) | 3.9 (39) | 5.6 (42) | 5.6 (42) | 13.3 (56) | 10 (50) |
| Warm season | 22.8 (73) | 21.7 (71) | 23.9 (75) | 22.2 (72) | 20 (68) | 15.6 (60) |
| Race/Ethnicity | ||||||
| Black | 61 | 54 | - | - | - | 35 |
| Chinese | - | - | 56 | - | - | - |
| Hispanic | - | - | - | - | 66 | - |
| Japanese | - | - | - | 57 | - | - |
| White | 39 | 46 | 44 | 43 | 34 | 65 |
| Education | ||||||
| ≤High school | 31 | 13 | 19 | 13 | 51 | 23 |
| Some college | 43 | 30 | 24 | 35 | 26 | 36 |
| ≥ College | 24 | 58 | 56 | 52 | 20 | 41 |
| Total number of visits | 1,685 | 1,439 | 1,771 | 2,023 | 535 | 1,556 |
| Body Mass Index | ||||||
| <25, normal | 14 | 20 | 58 | 60 | 22 | 27 |
| 25–30, overweight | 23 | 32 | 23 | 26 | 31 | 32 |
| >30, obese | 60 | 40 | 19 | 13 | 38 | 40 |
| Current smoker | ||||||
| Yes | 23 | 15 | 3 | 9 | 16 | 14 |
| No | 75 | 74 | 96 | 91 | 79 | 85 |
| Alcohol in 24 hours | ||||||
| Yes | 11 | 18 | 15 | 18 | 2 | 13 |
| No | 87 | 81 | 83 | 82 | 93 | 84 |
Note: percentages do not always add up to 100% because of missing data.
The distribution of inflammatory and lipid biomarkers for 2,207 participants across visits 3 to 7 are presented in Table 2. More details on stratification by demographic factors can be found in a previous study (Green et al., 2016). The inclusion of menopausal status offered the best fit to control for time trends, and therefore, was added to the base model with the other variables previously discussed. The association of apparent temperature per every 5.6°C (10°F) increase with changes in the biomarker levels the outcomes of interest varied by season, with more statistically significant negative results observed during the warm season, specifically for tPA-ag (p < 0.01), hs-CRP (p < 0.05) and fibrinogen (p < 0.10) (Table 3). The cold season had significantly positive results for tPA-ag (p < 0.01), and significantly negative results for fibrinogen and PAI-1 (p < 0.10).
Table 2.
Biomarker levels for all SWAN participants, 1999–2004.
| Variable | Number of samples | Mean ± standard deviation |
|---|---|---|
| hs-CRP (mg/L) | 7,955 | 2.4 ± 2.4 |
| tPA-ag (ng/ml) | 8,463 | 8.4 ± 12.5 |
| PAI-1 (ng/ml) | 8,431 | 25 ± 40 |
| Factor VIIc (%) | 4,046 | 125 ± 40 |
| Fibrinogen (mg/dl) | 4,100 | 276 ± 59 |
| HDL (mg/dl) | 8,909 | 59 ± 16 |
| LDL (mg/dl) | 8,551 | 118 ± 33 |
| Triglycerides (mg/dl) | 8,668 | 130 ± 111 |
| Total Cholesterol (mg/dl) | 8,916 | 202 ± 37 |
| Lipoprotein(a) (%) | 8,545 | 32 ± 39 |
Table 3.
Association of apparent temperature per 5.6°C (10°F) with inflammatory/hemostatic markers for SWAN cohort, 1999–2004.
| hs-CRP (%) | Fibrinogen (%) | Factor VII (%) | tPA (%) | PAI-1 (%) | |
|---|---|---|---|---|---|
| Warm Season | |||||
| Day 0 | −3.7 (−6.7, −0.6)** | −0.5 (−1.4, 0.5) | −0.9 (−2.0, 0.2) | −2.2 (−3.7, −0.6)*** | −2.1 (−5.0, 1.0) |
| Day 1 | −2 (−5.1, 1.2) | −0.4 (−1.3, 0.6) | −1.1 (−2.2, 0)* | −1.9 (−3.4, −0.3)** | −1.4 (−4.4, 1.7) |
| 7-day | −1.5 (−5.3, 2.5) | −0.6 (−1.7, 0.6) | −0.8 (−2.1, 0.6) | −2.8 (−4.7, −0.9)*** | −2.6 (−6.3, 1.3) |
| 30-day | −1 (−5.4, 3.6) | −1.2 (−2.4, 0.1)* | −0.3 (−1.8, 1.2) | −3.1 (−5.2, −0.8)*** | −4.2 (−8.3, 0.1)* |
| Cold Season | |||||
| Day 0 | −0.5 (−2.5, 1.5) | −0.6 (−1.2, 0)* | 0.4 (−0.3, 1.1) | 1.2 (0.2, 2.2)*** | 1.7 (−0.1, 3.6)* |
| Day 1 | −0.8 (−2.7, 1.2) | −0.6 (−1.2, 0.1)* | 0.03 (−0.71, 0.77) | 1.4 (0.5, 2.4)*** | 1.1 (−0.7, 2.9) |
| 7-day | −0.5 (−2.8, 1.9) | −0.1 (−0.9, 0.7) | 0.3 (−0.6, 1.2) | 1.9 (0.8, 3.1)*** | 1.3 (−0.8, 3.5) |
| 30-day | 1.9 (−0.8, 4.7) | −0.03 (−0. 9, 0.8) | 0.3 (−0.7, 1.4) | 1.3 (0, 2.6)* | 0.9 (−1.5, 3.4) |
Adjusted for study site, visit number, race/ethnicity, education, menopausal status, BMI, active smoking status, alcohol consumption in the past 24 hours before blood draw, hormone use since last visit.
p≤0.01
0.01<≤0.05
0.05<p≤0.10
As shown in Table 4, significantly negative associations were consistently found for HDL (p < 0.01) during either the warm or cold seasons for all exposure metrics. Triglycerides and total cholesterol also had significantly positive and negative associations, respectively (p < 0.10).
Table 4.
Association of apparent temperature per 5.6°C (10°F) with lipid markers for SWAN cohort, 1999–2004.
| HDL (mg/dl) | LDL (mg/dl) | Triglycerides (%) | Total cholesterol (mg/dl) | Lipoprotein(a) (%) | Lipoprotein A1 (mg/dl) | |
|---|---|---|---|---|---|---|
| Warm Season | ||||||
| Day 0 | −0.8 (−1.4, − 0.2)*** | −0.3 (−1.1, 0.6) | −0.9 (−2.3, 0.6) | −0.5 (−1.1, 0)* | 1 (−1.1, 3.1) | −0.2 (−1.4, 1.1) |
| Day 1 | −0.7 (−1.3, − 0.1)** | 0.04 (−0.8, 0. 9) | 0.02 (−1.4, 1.5) | −0.2 (−0.8, 0.3) | 1.5 (−0.7, 3.7) | −0.4 (−1.6, 0.9) |
| 7-day average | −0.3 (−1.0, 0.4) | 0.3 (−0.8, 1.3) | −0.3 (−2.1, 1.5) | −0.1 (−0.8, 0.6) | 1.4 (−1.3, 4.1) | −0.3 (−1.8, 1.1) |
| 30-day average | −0.2 (−1.0, 0.7) | 0.6 (−0.6, 1.8) | 1.5 (−0.6, 3.6) | 0.3 (−0.5, 1.1) | 2 (−1.1, 5.1) | −0.5 (−2.2, 1.2) |
| Cold Season | ||||||
| Day 0 | −0.4 (−0.8, 0)** | 0.05 (−0.5, 0.6) | 0.5 (−0.4, 1.4) | −0.04 (−0.4, 0.3) | 0.05 (−1.4, 1.5) | 0.3 (−0.4, 1.1) |
| Day 1 | −0.6 (−1, − 0.3)*** | −0.2 (−0.7, 0.4) | 0.7 (−0.2, 1.5) | −0.2 (−0.5, 0.2) | 0.03 (−1.4, 1.5) | 0.1 (−0.7, 0.9) |
| 7-day average | −0.8 (−1.2, − 0.3)*** | 0.05 (−0.6, 0.7) | 0.6 (−0.4, 1.7) | −0.1 (−0.5, 0.3) | 0.5 (−1.2, 2.3) | 0.2 (−0.7, 1.2) |
| 30-day average | −0.5 (−1.0, 0)** | −0.03 (−0.7, 0.7) | 1.1 (−0.1, 2.3)* | −0.01 (−0.5, 0.4) | −0.02 (−2.0, 2.0) | 0.5 (−0.5, 1.6) |
Adjusted for study site, visit number, race/ethnicity, education, menopausal status, BMI, active smoking status, alcohol consumption in the past 24 hours before blood draw, hormone use since last visit.
p≤0.01
0.01<≤0.05
0.05<≤0.10
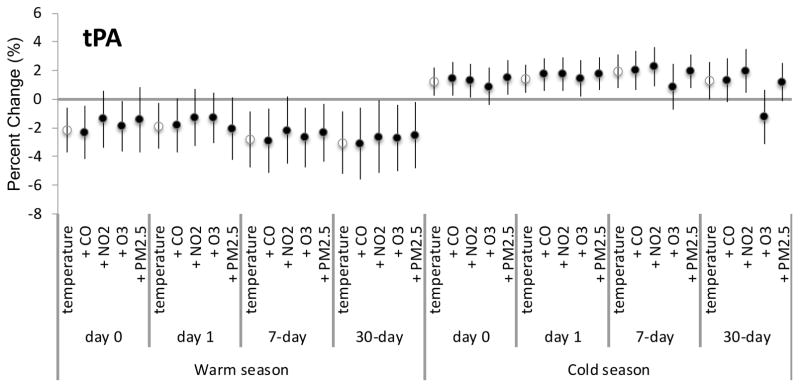
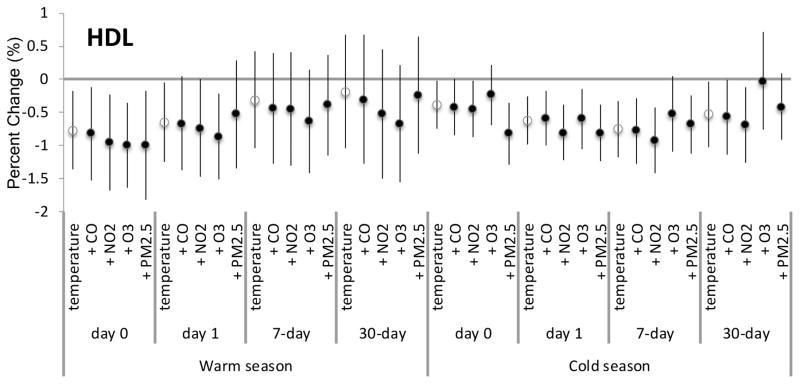
None of the pollutants examined showed evidence for confounding for any exposure metrics during the warm or cold seasons. Since tPA-ag and HDL had the most consistently significant associations for the main analyses, we showed the confounding results for the air pollutants for these biomarkers only in Figures 1 and 2, respectively.
Figure 1.
Percent change for tPA per every 5.56°C (10°F) increase in apparent temperature for various lag times adjusted for each specified air pollutant.
Figure 2.
Percent change for HDL per every 5.56°C (10°F) increase in apparent temperature for various lag times adjusted for each specified air pollutant.
Discussion
In this multi-site follow-up study of midlife women in the US, we found some hemostatic and lipid markers to be associated with both short- and intermediate-term average apparent temperature exposures. The most notable findings were for tPA, a protein that is involved in blood clot resorption and quantified by measuring tPA-ag in this study, for both the warm and cold seasons. Hemostatic markers responsible for coagulation, such as fibrinogen and Factor VIIc, were also associated with various metrics of apparent temperature during the warm season, while fibrinogen was also associated with short-term apparent temperature. Fibrinogen is a soluble plasma glycoprotein that is converted to fibrin to promote clot formation, and Factor VIIc contributes to a high thrombotic tendency. We also found some evidence for negative assoications for hs-CRP, which is another inflammatory marker.
Previous studies have not considered these markers of inflammation with temperature, apart from hs-CRP. In Boston (Wilker et al., 2012), each 5°C increase in 4-day moving averages of apparent temperature was found to be associated with a 21.6% increase in hs-CRP. Schauble et al. (2012) reported that for every 5°C decrease in the 5-day average of temperature, there was an 8.4% decrease in hs-CRP. These prior results were both observed among patients with pre-existing disease or genetic susceptibility whereas our study had mostly healthy women. Furthermore, previous studies focused primarily on cold stress and CVD markers rather than on associations with heat exposure (Halonen et al., 2011b; Hampel et al., 2010; Hong et al., 2012; Schauble et al., 2012; Schneider et al., 2008).
Prior research on lipid biomarker associations with temperature is scarce. In a previous cohort study of men living in Boston, investigators reported that a 5°C increase in mean ambient temperature was negatively associated with HDL but positively associated with LDL (1.76% decrease and 1.87% increase, respectively; 2-day lag) (Halonen et al., 2011a). We also found consistently negative associations between HDL and increasing temperature during both the warm and cold seasons, but less consistent results with LDL. HDL serves as a marker of “good cholesterol,” which has been shown to be protective for CVD outcomes, so negative associations indicate potential harm. Other findings from our study were less conclusive with respect to lipid markers, but we did find significantly negative assocations for total cholesterol and triglycerides during the warm and cold seasons, respectively. LPA was also found to be associated with risk of coronary heart disease and stroke (Emerging Risk Factors Collaboration, 2009). However, no prior study has examined whether LPA in human body is influenced by heat or cold stress. We found that increased apparent temperature, particularly during the warm season, was associated with decreased LPA, which is a marker for lower CVD risk. These lipid biomarkers provide some insight into the reasons for greater CVD risk during periods of higher ambient heat exposure.
In a previous study using the same data, one-year exposure to PM2.5 was associated with hs-CRP, tPA-ag, and PAI-1, and one-year O3 was associated with Factor VIIc (Green et al., 2016). Previous literature on particulate matter (PM) and Factor VIIc has largely concentrated on particles less than 10 microns in diameter. A couple of these studies have shown an inverse relationship between PM and Factor VIIc specifically (Pekkanen et al., 2000; Ruckerl et al., 2006). Although PM2.5 did not confound the associations observed in our study, gaseous pollutants slightly reduced some of the estimates for biomarkers such as Factor VIIc and fibrinogen, although the associations still remained. We did not consider exposures longer than 30-day averages in our study because our exposure of interest was apparent temperature, and we were interested in mostly short-term exposures within one week. Fewer studies have been conducted on apparent temperature and these particular biomarkers than on air pollution.
Physiologic efforts to thermoregulate efficiently seem to be at the root of many of the CVD associations triggered by apparent temperature exposure. Inflammation may lead to stress on the heart with one of the primary responses, vasodilation, to reduce blood pressure during heat exposure (Charles L. Schauf, 1993), while the opposite may occur during cold weather (Halonen et al., 2011b). Increasing the volume of blood flow to extremities and away from vital organs in an effort to cool down may also increase heart rate, thereby leading to overstressing the heart. Increased blood viscosity, or changes in coagulation may lead to increased risk of thrombosis (Leon and Helwig, 2010). Despite cellular uptake of cholesterol as a protective response to heat exposure, elevated cholesterol levels have also been reported particularly during hot weather (Halonen et al., 2011a).
We acknowledge several limitations that complicate interpreting our study results. Because of the site-specific race/ethnicity selection criteria used to recruit study participants to SWAN, we were unable to consider effect modification by race/ethnicity. Also because of participant selection criteria, the study population may not be representative of the general US population, although it was community-based likely reflecting a healthy population, and not clinic-based which likely reduced the bias of including a sick sample, but the results need to be interpreted with that caveat in mind. While we had residential history, including changes of address over time for each woman, we did not have occupational addresses. A previous study, however, has observed that Californians spend, on average, 62% of their time at home indoors (Jenkins et al., 1992). This percentage is greater in other parts of the US where weather restrictions do not allow people to spend as much time outdoors throughout the year. A national survey revealed that on average, the general US population spends 75% (equivalent to 1,078 minutes/day) of their time at home (US EPA, 2011). However, we did not have time-activity diaries to verify whether this was true for our SWAN participants, or data on other factors, such as housing characteristics and air contioning use, that may influence the degree to which outdoor measurements reflect indoor exposures. However, ambient temperature, which served as a proxy for heat exposure in our study, has been shown to be a good marker of skin temperature in an exposure assessment study of elderly inviduals (Basu and Samet, 2002a). We did not consider extreme temperatures in this study, since we were interested in overall apparent temperature exposure during the warm and cold seasons. Previous studies have shown that associations fo heat and cold waves may differ by exposure and duration (Barnett et al., 2012), and by heat wave definition (Kent et al., 2014).
Conclusions
This is the first cohort study of which we are aware to consider the associations between both short- and intermediate-term apparent temperature exposure and these specific serum biomarkers for CVD at multiple sites thoughout the US. Because we could track changes of addresses annually during blood draws, we were able to adjust exposure as needed for each woman over time rather than assuming that exposure at baseline was the correct exposure for the entire study period. By examining these serum biomarkers, we were able to shed further light to the mechanisms involved in apparent temperature contributing to the risk of CVD.
Highlights.
Significant negative associations found during the warm season for various lag times.
During the cold season, significant negative associations for fibrinogen and HDL.
Positive associations for tPA, PAI-1, and triglyceride observed during cold season.
Criteria air pollutants did not confound the associations observed.
Results shed light on mechanisms between temperature and cardiovascular risk.
Acknowledgments
The opinions expressed in this paper are solely those of the authors and do not represent the policy or position of the State of California or the California Environmental Protection Agency. The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
Clinical Centers: University of Michigan, Ann Arbor – Siobán Harlow, PI 2011 – present, MaryFran Sowers, PI 1994-2011; Massachusetts General Hospital, Boston, MA – Joel Finkelstein, PI 1999 – present; Robert Neer, PI 1994 – 1999; Rush University, Rush University Medical Center, Chicago, IL – Howard Kravitz, PI 2009 – present; Lynda Powell, PI 1994 – 2009; University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles – Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011 – present, Rachel Wildman, PI 2010 – 2011; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994 – 2004; and the University of Pittsburgh, Pittsburgh, PA – Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD – Winifred Rossi 2012 - present; Sherry Sherman 1994 – 2012; Marcia Ory 1994 – 2001; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Maria Mori Brooks, PI 2012 - present; Kim Sutton-Tyrrell, PI 2001 – 2012; New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001.
Steering Committee: Susan Johnson, Current Chair, Chris Gallagher, Former Chair
We thank the study staff at each site and all the women who participated in SWAN. Conflict of interest: None
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arc GIS Version 10.0. Environmental Systems Research Institute; Redlands, CA: 2014. [Google Scholar]
- Barnett AG, et al. Cold and heat waves in the United States. Environ Res. 2012;112:218–24. doi: 10.1016/j.envres.2011.12.010. [DOI] [PubMed] [Google Scholar]
- Basu R. High ambient temperature and mortality: a review of epidemiologic studies from 2001 to 2008. Environ Health. 2009:8. doi: 10.1186/1476-069X-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu R, Ostro BD. A multicounty analysis identifying the populations vulnerable to mortality associated with high ambient temperature in California. Am J Epidemiol. 2008;168:632–7. doi: 10.1093/aje/kwn170. [DOI] [PubMed] [Google Scholar]
- Basu R, et al. The effect of high ambient temperature on emergency room visits. Epidemiology. 2012;23:813–20. doi: 10.1097/EDE.0b013e31826b7f97. [DOI] [PubMed] [Google Scholar]
- Basu R, Samet JM. An exposure assessment study of ambient heat exposure in an elderly population in Baltimore, Maryland. Environ Health Perspect. 2002a;110:1219–24. doi: 10.1289/ehp.021101219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu R, Samet JM. Relation between elevated ambient temperature and mortality: a review of the epidemiologic evidence. Epidemiol Rev. 2002b;24:190–202. doi: 10.1093/epirev/mxf007. [DOI] [PubMed] [Google Scholar]
- Benmarhnia T, et al. Review Article: Vulnerability to Heat-related Mortality: A Systematic Review, Meta-analysis, and Meta-regression Analysis. Epidemiology. 2015;26:781–93. doi: 10.1097/EDE.0000000000000375. [DOI] [PubMed] [Google Scholar]
- Bhaskaran K, et al. Effects of ambient temperature on the incidence of myocardial infarction. Heart. 2009;95:1760–9. doi: 10.1136/hrt.2009.175000. [DOI] [PubMed] [Google Scholar]
- Schauf Charles L, Stacie Moffett DM. Human Physiology: Foundations and Frontiers. Oxford: Wm. C. Brown Publishers; 1993. [Google Scholar]
- Emerging Risk Factors Collaboration et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302:412–23. doi: 10.1001/jama.2009.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R, et al. Long- and Short-term Exposure to Air Pollution and Inflammatory/Hemostatic Markers in Midlife Women. Epidemiology. 2016;27:211–220. doi: 10.1097/EDE.0000000000000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halonen JI, et al. Associations between outdoor temperature and markers of inflammation: a cohort study. Environ Health. 2010;9:42. doi: 10.1186/1476-069X-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halonen JI, et al. Outdoor temperature is associated with serum HDL and LDL. Environ Res. 2011a;111:281–7. doi: 10.1016/j.envres.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halonen JI, et al. Relationship between outdoor temperature and blood pressure. Occup Environ Med. 2011b;68:296–301. doi: 10.1136/oem.2010.056507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampel R, et al. Air temperature and inflammatory and coagulation responses in men with coronary or pulmonary disease during the winter season. Occup Environ Med. 2010;67:408–16. doi: 10.1136/oem.2009.048660. [DOI] [PubMed] [Google Scholar]
- Hong YC, et al. Association of cold ambient temperature and cardiovascular markers. Sci Total Environ. 2012;435–436:74–9. doi: 10.1016/j.scitotenv.2012.02.070. [DOI] [PubMed] [Google Scholar]
- SAS Statistical Software Inc. Cary; North Carolina: 2014. [Google Scholar]
- Jenkins PL, et al. Activity patterns of Californians: use of and proximity to indoor pollutant sources. Atmos Environ. 1992;26A:2141–2148. [Google Scholar]
- Kent ST, et al. Heat Waves and Health Outcomes in Alabama (USA): The Importance of Heat Wave Definition. Environ Health Perspect. 2014;122:151–8. doi: 10.1289/ehp.1307262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb S, et al. The short-term influence of weather on daily mortality in congestive heart failure. Arch Environ Occup Health. 2007;62:169–76. doi: 10.3200/AEOH.62.4.169-176. [DOI] [PubMed] [Google Scholar]
- Leon LR, Helwig BG. Heat stroke: role of the systemic inflammatory response. Journal of Applied Physiology. 2010;109:1980–8. doi: 10.1152/japplphysiol.00301.2010. [DOI] [PubMed] [Google Scholar]
- Madrigano J, et al. Temperature, myocardial infarction, and mortality: effect modification by individual- and area-level characteristics. Epidemiology. 2013;24:439–46. doi: 10.1097/EDE.0b013e3182878397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyatake N, et al. The relation between ambulance transports stratified by heat stroke and air temperature in all 47 prefectures of Japan in August, 2009: ecological study. Environ Health Prev Med. 2011 doi: 10.1007/s12199-011-0221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Oceanic and Atmospheric Administration (NOAA). National Centers for Environmental Information (NCEI) 2012 [Google Scholar]
- Ostro B, et al. Chronic PM2.5 exposure and inflammation: determining sensitive subgroups in mid-life women. Environ Res. 2014;132:168–75. doi: 10.1016/j.envres.2014.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekkanen J, et al. Daily concentrations of air pollution and plasma fibrinogen in London. Occup Environ Med. 2000;57:818–22. doi: 10.1136/oem.57.12.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruckerl R, et al. Air pollution and markers of inflammation and coagulation in patients with coronary heart disease. Am J Respir Crit Care Med. 2006;173:432–41. doi: 10.1164/rccm.200507-1123OC. [DOI] [PubMed] [Google Scholar]
- Schauble CL, et al. Short-term effects of air temperature on blood markers of coagulation and inflammation in potentially susceptible individuals. Occup Environ Med. 2012;69:670–8. doi: 10.1136/oemed-2011-100469. [DOI] [PubMed] [Google Scholar]
- Schneider A, et al. Air temperature and inflammatory responses in myocardial infarction survivors. Epidemiology. 2008;19:391–400. doi: 10.1097/EDE.0b013e31816a4325. [DOI] [PubMed] [Google Scholar]
- Turner LR, et al. Ambient Temperature and Cardiorespiratory Morbidity: A Systematic Review and Meta-analysis. Epidemiology. 2012;23:594–606. doi: 10.1097/EDE.0b013e3182572795. [DOI] [PubMed] [Google Scholar]
- U. S. E. P.A. Exposure Factors Handbook 2011 Edition (Final) U.S. Environmental Protection Agency; Washington, DC: 2011. [Google Scholar]
- U. S. E. P.A. Air Quality System Data Mart. U.S. Environmental Protection Agency; Washingon, D.C: 2014. [Google Scholar]
- Wichmann J, et al. Association between ambient temperature and acute myocardial infarction hospitalisations in Gothenburg, Sweden: 1985–2010. PLoS One. 2013;8:e62059. doi: 10.1371/journal.pone.0062059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilker EH, et al. Ambient temperature and biomarkers of heart failure: a repeated measures analysis. Environ Health Perspect. 2012;120:1083–7. doi: 10.1289/ehp.1104380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, et al. Ambient Temperature and Morbidity: A Review of Epidemiological Evidence. Environ Health Perspect. 2011 doi: 10.1289/ehp.1003198. [DOI] [PMC free article] [PubMed] [Google Scholar]