Abstract
Due to their ability to kill cancer cells and produce pro-inflammatory cytokines, natural killer (NK) cells have long been of clinical interest for their anti-tumor properties. The recent discovery of NK cell memory demonstrates that NK cell functions, and potentially anti-tumor responses, can be enhanced long-term. Following non-specific activation with the cytokines IL-12, IL-15, and IL-18 or in response to antigens or cytomegalovirus (CMV), human and mouse NK cells exhibit stable, enhanced functional responses with phenotypic and molecular changes. Here we review mechanisms driving differentiation of NK cell memory-like properties, evidence for anti-tumor activity, and the challenges and opportunities in harnessing these for cancer immunotherapy.
NK Cells and Cancer Immunotherapy
Natural killer (NK) cells are innate immune lymphocytes first identified in the 1970's based on their functional ability to kill tumor cells without MHC restriction or prior sensitization [1]. NK cells constitutively express germline-encoded inhibitory receptors that recognize MHC class I molecules and provide inhibitory signals for self. These inhibitory signals are important for tolerance and also shape the responsiveness of NK cells during their development, a process termed education. Additionally, NK cells express activating receptors that recognize stress ligands, pathogen-encoded ligands, and antibodies, and trigger potent effector functions, including killing of tumor cells, largely through perforin- and granzyme- dependent mechanisms [2]. In addition to their cytotoxic abilities, NK cells also rapidly produce cytokines including interferon (IFN) γ and tumor necrosis factor (TNF) α. Conversely, their differentiation, proliferation, and activation are controlled by cytokine signals, including IL-2, -12, -15 and -18, and interactions with accessory cells. While traditionally categorized as innate immune cells, NK cells are now recognized to have features of immunologic memory, including persistent enhanced functionality following activation, and, in some cases, the ability to specifically recognize antigen [3-5].
NK cells have been utilized for the treatment of cancer in patients with varying success, including mismatch of NK inhibitory receptor and MHC ligand interactions in the context of hematopoietic cell transplantation (HCT), NK cell adoptive immunotherapy, and administration of antibodies, cytokines, or drugs aimed at enhancing NK cell function [6-8]. NK cells with memory or memory-like properties are long-lived, have durable enhanced functional activity, and have the potential to be targeted to tumors. Here we review recent advances in NK cell memory, focusing on anti-tumor properties of these cells.
Memory NK Cell Differentiation
NK cells can adapt their behavior based on prior activation, with enhanced functionality after a single activation event [9]. Enhanced NK cell function has been observed in response to antigen-specific stimulation, and antigen-independent cytokine activation. Such NK cells have been referred to as “memory”, “adaptive”, or “memory-like” depending on the context in which NK cells were activated. There are three major differentiation pathways of NK cell memory responses identified to date, including antigen-specific liver NK cell responses, CMV-adapted NK cell memory, and cytokine-induced NK cell memory-like responses [3-5].
Liver-Resident Antigen-Specific NK Memory
The first evidence of specific NK cell responses to antigen came from a study by the Von Andrian laboratory, demonstrating liver NK cell-mediated hapten-specific contact hypersensitivity (CHS) [3]. Liver NK cell memory responses have also been shown in response to several viral antigens and virus-like particles, suggesting the capacity to develop specific responses to a wide variety of antigens [10]. Memory responses are formed in a subset of liver-resident murine NK cells, identified phenotypically as NK1.1+DX5-CD49a+, and express the chemokine receptor CXCR6 and other maturation markers [3, 10-13]. Cytokine signaling, including IL-12, IFN-γ, and IFNαR, is required for liver NK cell-mediated CHS, as evidenced by a failure to generate hapten-specific NK cells in mice lacking IFN-γ or receptors for IL-12 or IFN-α [13]. A recent study demonstrated that liver NK cell-mediated CHS in response to monobenzone also requires activation of tissue-resident macrophages through the NLRP3 inflammasome and IL-18 production [14]. Prior sensitization with monobenzone also enhanced NK cell anti-tumor responses to B16 melanoma tumor cells, a phenomenon thought to be due to monobenzone “haptenizing” melanocyte antigens [14]. Thus, a specific subset of liver-resident NK cells is capable of antigen-specific memory, and is also dependent upon cytokines and accessory cell interactions. The mechanism whereby liver NK cells, which do not somatically re-arrange receptors, are capable of specifically sensing a wide array of antigens remains unknown. Expression of germline-encoded NK receptors is stochastic, and hapten-specific liver-resident NK cells express inhibitory receptors for self, suggesting that these cells are “educated” since recognition of self is required for NK cell tolerance [3, 15]. It is also unknown whether a similar type of memory occurs in humans, although a study in rhesus macaques suggests that liver and splenic NK cells in primates are capable of a similar type of antigen-specific memory [16].
CMV Triggered NK Cell Memory
NK cells are particularly important for the host response against cytomegalovirus (CMV) in mice and humans [17, 18]. Approximately half of murine NK cells in C57BL/6 mice express the germline-encoded activating receptor Ly49H, specific for the murine CMV (MCMV)-encoded ligand m157. Ly49H+ NK cells specifically proliferate and expand following MCMV infection, and the absence of Ly49H on NK cells, or infection with m157-deficient MCMV, renders mice highly susceptible to infection [19-22]. The Lanier laboratory first demonstrated that splenic Ly49H+ NK cells can form long-lasting memory following infection with MCMV, dependent on Ly49H-m157 interactions [5]. MCMV-induced memory NK cells have enhanced recall response to MCMV, and protect neonatal mice from an otherwise lethal infection [5]. Work from the Lanier and Sun laboratories has identified a number of co-stimulatory and molecular requirements for MCMV-induced NK cell memory formation, including the transcription factor Zbtb32, DNAM-1 co-stimulation, pro-inflammatory cytokine signaling, and pro-apoptotic and mitophagy factors [23-28]. Phenotypically, splenic MCMV-induced memory NK cells express Ly49H and high levels of markers associated with maturation and activation including KLRG1 and Ly6C [5] (Key Table, Table I), although there are no specific markers of MCMV memory.
Table 1. Human and Murine NK Cell Memory in Response to CMV and Cytokines.
| Memory stimulus | Human | Mouse |
|---|---|---|
| CMV | ||
| Phenotype Surface | Mature (CD56dimCD57+NKG2A-) NKG2Chigh NKG2C- in NKG2C-deficient individuals |
Mature (KLRG1hiLy6Chi) Ly49H+ |
| Intracellular adaptors | FcεRIγ- subset ↓SYK, EAT-2 |
FcεRIγ- unchanged |
| Differentiation requirements | HCMV-driven, no specific antigen? inflammatory cytokines | MCMV-driven, m157 antigen inflammatory cytokines |
| Epigenetic changes | IFNG, SYK, FCER1G (FcεRIγ), SH2D1B (EAT-2) | ? |
| Function | Antibody-dependent ↑ cytokine production ↓ IFN-γ with IL-12/18 Normal to low degranulation ↑ killing of HLA-E+ tumor targets |
↑ IFN-γ with activating receptors ↓ IFN-γ with IL-12/18 ↑ degranulation |
| In vivo expansion | Expand during HCMV and other viral infections | MCMV, m157-specific expansion |
| In vivo anti-tumor activity | ? | ? |
|
| ||
| Cytokine-induced | ||
| Phenotype | CD25+NKG2A+NKp30+NKp44+ | CD25+ |
| Differentiation requirements | IL-12/18/15, IL-12/18, IL-15/18 anti-CD16 stimulation ↑ formation | IL-12/18 (+/-IL-15)? effect of activating receptors |
| Epigenetic changes | ? | Ifng |
| Function | ↑ IFN-γ with cytokines & tumor targets ↑ cytotoxicity vs. leukemia ↑ Gzmb, perforin |
↑ IFN-γ with cytokines & tumor targets |
| In vivo expansion | Expand with IL-2 | CD4+ T cell-derived IL-2 Macrophage co-factor |
| In vivo anti-tumor activity | + in vivo anti-tumor effects | + in vivo anti-tumor effects |
In humans, a history of infection with human CMV (HCMV) is associated with an increased percentage of peripheral blood NK cells expressing high levels of the activating heterodimeric receptor CD94-NKG2C (referred to as NKG2C) [29, 30]. NKG2C+ NK cells with a mature phenotype (CD56dimCD57+NKG2A-) expand during acute HCMV and re-activation of latent HCMV [18, 31, 32]. Acute infection with other viruses has also been associated with an increased percentage of NKG2C+ NK cells, but only in individuals with a history of HCMV infection [33]. NKG2C+ NK cells have enhanced production of IFNγ in response to activation via the low affinity antibody Fc receptor, FcγRIIIa (CD16) [30]. Due to their association with viral infection and enhanced function, these NK cells have been referred to as “adaptive” or “memory-like”. Epigenetic remodeling of the IFNG locus has been demonstrated in NKG2C+ cells from HCMV positive donors, suggesting a molecular basis for enhanced IFN-γ production [34]. The mechanism of expansion of NKG2C+ NK cells during infection is incompletely understood, but recent studies have suggested that stimulation via FcγRIIIa by antibodies may be responsible for their expansion and persistence. These include HCMV-specific antibodies present in sera from HCMV+ individuals, as well as antibodies specific for other infections, as demonstrated by expansion of this NK cell subset in response to influenza-infected targets in the presence of serum from influenza+ individuals [35, 36].
A subset of human NK cells lacking the signaling adaptor FcεRIγ was also recently identified in CMV+ individuals, and expresses high levels of CD57 and variable, but overall higher expression of NKG2C [36-38]. FcεRIγ- cells have robust antibody-dependent expansion and activation mediated by FcγRIIIa, presumably via utilization of the alternative signaling adapter CD3ζ (Figure 1) [36, 38]. Epigenetic modifications present in FcεRIγ-deficient NK cells were found to alter intracellular signaling proteins and transcription factors, providing a mechanism for altered signaling and function in these HCMV-adapted NK cells [35, 36].
Figure 1. HCMV-Adapted NK Cells Have Enhanced Responses to Engagement of FcγRIIIa.
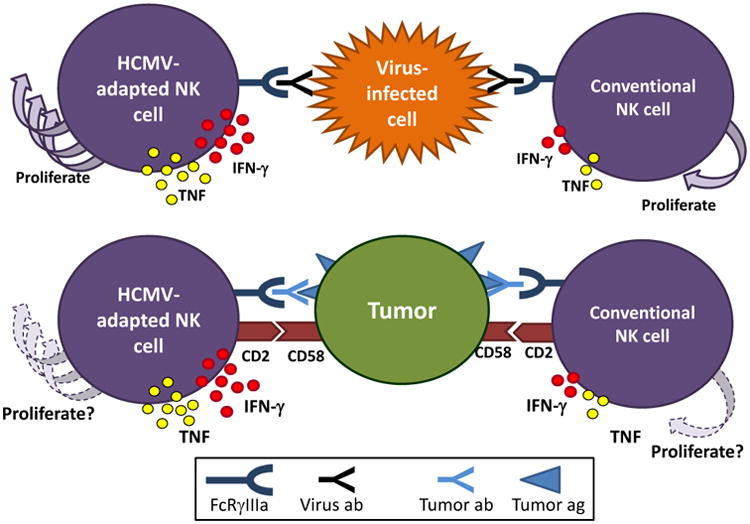
HCMV-adapted NK cells are particularly responsive to stimulation via activating FcγRIIIa (CD16) receptors. (Top) While stimulation of HCMV-adapted NK cells with CMV-infected cells alone leads to minimal cytokine production, the addition of CMV+ serum or viral antibodies leads to significantly enhanced cytokine production by adapted NK cells which also proliferate. These functional responses are superior to those of conventional NK cells. In addition, this response is not HCMV-specific, and was also observed in FcεRIγ-deficient adaptive NK cells cultured with influenza-infected cells in the presence of influenza seropositive autologous serum. (Bottom) CD2 co-stimulation enhances antibody-mediated responses to tumor targets by HCMV-adapted NK cells which produce abundant cytokines, compared to conventional NK cells. The proliferative capacity of NK cells in this setting is not known. Abbreviations used: HCMV: human cytomegalovirus, IFN: interferon, TNF: tumor necrosis factor.
Whether NKG2C directly recognizes an HCMV ligand remains unknown. However, there is evidence that NKG2C is not required for differentiation of HCMV-induced adaptive NK cells, and HCMV-driven adaptive features have been identified in NKG2C-NK cells. Evidence for this comes from patients receiving HCT from donors genetically deficient in NKG2C (∼4% of individuals lack any NKG2C expression), where there was rapid expansion of mature CD56dimNKG2A- NK cells expressing activating and inhibitory killer immunoglobulin-like receptor (KIR) after HCMV infection [39]. A recent study by Liu et al. investigated the molecular and functional characteristics of NKG2C- HCMV-adaptive NK cells and found numerous similarities to NKG2C+ cells, including reduced expression of FcεRIγ and NKG2A, and higher levels of CD57 and self-reactive KIR [40]. NKG2C- adaptive NK cells also had epigenetic changes at the IFNG locus similar to NKG2C+ cells. Both NKG2C+ and NKG2C- adaptive cells from CMV+ donors had decreased responses following cytokine (IL-12+IL-18) or tumor target stimulation, but enhanced FcγRIIIa-dependent antibody mediated IFN-γ and TNF production. CD2 expression was found to be critical for co-stimulation of HCMV-adaptive NK cell antibody-mediated activation (Figure 1) [40]. Since CD2's ligand CD58 is broadly expressed on non-hematopoietic and hematopoietic cells (especially dendritic cells, macrophages), as well as malignant cells, the relevance for a specific anti-tumor NK cell response is currently unclear. However, the potential importance of CD2 and CD58 interaction for NK cell responses to cancer is highlighted by multiple cancers observed to mutate CD58 as an immunoevasion strategy [PMID 27389058].
Together, the identification of enhanced antibody-triggered responses in HCMV-adaptive NK cells (NKG2C+, NKG2C-, and FcεRIγ-) suggest a mechanism whereby anti-HCMV antibodies may promote the differentiation and maintenance of a subset of adaptive NK cells with preferential responsiveness via FcγRIIIa specialized to recognize antibody-opsonized target cells. While these NKG2C+ NK expansions have been observed in HCMV seropositive individuals to date, additional investigation into other scenarios where specific antibodies are chronically elevated, either in health or diseased states, are of interest.
Cytokine-Induced Memory-Like NK Cells
NK cells constitutively express a variety of cytokine receptors, and cytokine signaling is critical for the differentiation, survival, homeostasis, and activation of NK cells. The Yokoyama laboratory first identified that short-term cytokine activation of splenic murine NK cells with high-dose IL-12 and IL-18 gives rise to a population of cells with durable (weeks to months) enhanced responses to re-stimulation, termed cytokine-induced memory-like NK cells [4, 41]. IL-15 was added to culture systems as a survival factor during the differentiation of cells, but was not strictly required for memory-like NK cell differentiation [4]. Mouse cytokine-induced memory-like NK cells have enhanced production of IFN-γ in response to re-stimulation with cytokines, activating receptor ligands, and tumor targets, but do not exhibit enhanced cytotoxicity ex vivo [4, 41]. Memory-like NK cells proliferate and expand following transfer to lymphopenic hosts, and enhanced functionality is passed on to daughter cells, suggesting that heritable changes are involved in the maintenance of memory.
Human NK cells have a similar capacity for cytokine-induced memory following overnight culture with combinations of IL-12/15/18 [42]. NK cells with a history of prior activation had enhanced IFN-γ production in response to re-stimulation with cytokines or a tumor target cell line, 7-21 days after a single activation event [42]. Co-stimulation of NK cells with anti-CD16 (FcγRIIIa) during initial cytokine stimulation led to enhanced NK cell memory-like responses, suggesting that activating receptor engagement can enhance cytokine-induced NK cell memory. Human memory-like NK cells upregulate CD25 (IL-2Rα) expression (resulting in expression of the heterotrimeric high affinity IL-2Rαβγ) and respond with enhanced proliferation, IFN-γ, and cytotoxicity to low-doses of IL-2 [43]. Human memory-like cells were successfully transferred to immunodeficient mice, where enhanced IFN- γ responses persisted when supported by exogenous IL-2 in vivo [43]. It remains to be identified how cytokine-induced memory-like NK cells develop during a physiologic response in vivo, for example, in the context of infection or an antitumor response. Within cancer patients, the inflammatory response that follows chemotherapy, radiotherapy, or HCT represents potential settings of IL-12, IL-15, and IL-18 production, and hence memory-like NK cell induction in vivo. These milieus warrant further investigation as sites of physiologic memory-like NK cell differentiation.
Anti-Tumor Responses of Memory NK Cells
Memory NK cell anti-tumor responses have been best studied in the context of human CMV adaptive NK cells and cytokine-induced memory-like NK cells. Here we will focus on the potential of these types of memory NK cells for cancer immunotherapy, including key differences between mouse and human systems (Table 1).
HCMV Adapted NK Cell Anti-Tumor Responses
Expansion and persistence of NKG2C+CD57+CD56dim adaptive NK cells has been observed in cancer patients with CMV reactivation following HCT [32]. It is currently unclear the extent to which HCMV-adaptive NK cells have enhanced anti-tumor activity in the absence of Fc γ RIIIa based triggering. In two studies, NKG2C+CD57+CD56dim NK cells from healthy HCMV+ donors had modestly increased IFN-γ and TNF production in response to K562 leukemia target cells, but no differences in degranulation, a marker for cytotoxic potential [32, 44]. Other groups found impaired degranulation and reduced cytokine production by NKG2C+ HCMV-adaptive NK cells in response to K562 targets [37, 40]. These differences may potentially be explained by variable expression of the NKG2C ligand HLA-E [45]. Regardless, adaptive NK cells (NKG2C+ or NKG2C-) consistently had enhanced responses to FcγRIIIa cross-linking and antibody-triggered responses to tumor targets (Figure 1) [36, 37, 40].
It has been observed in multiple studies that patients undergoing allogeneic HCT have lower relapse rates when CMV reactivation occurs, although the reasons for this are uncertain [46-48]. Miller and colleagues recently observed increased frequency and numbers of HCMV-adaptive NK cells in patients with HCMV reactivation, with a trend toward lower leukemia early relapse rates [44]. In a separate study, Malmberg and colleagues observed lower leukemia relapse rates in patients who had higher frequencies of “naïve” (NKG2C-CD57-NKG2A+) NK cells following allogeneic HCT when assessed at later time points [49]. These contrasting results highlight the complexity of correlating NK cell phenotype with outcomes in the context of HCT, and may reflect differences in conditioning regimens, stem cell source, KIR-HLA mismatch, and time points chosen for NK cell assessment. Overall, the finding that the HCMV experience status of NK cells may potentially correlate with outcomes following allogeneic HCT suggests that the role of HCMV-induced memory NK cells in anti-tumor responses warrants continued investigation.
Cytokine-Induced Memory-Like NK Cell Anti-Tumor Responses
The Cerwenka laboratory first demonstrated that mouse cytokine pre-activated NK cells exhibit antitumor activity, using an in vivo model of established tumors in irradiated mice [50]. Adoptive transfer of IL-12/15/18-activated NK cells led to proliferation of memory-like NK cells, delayed tumor growth, and enhanced survival of hosts. NK cells in this model expressed high levels of CD25, and proliferation was dependent on CD4+ T cell-derived IL-2 [50]. Tumor control in this model was dependent on NK cell IFN-γ and perforin. A recent study from this same group showed that IL-12/15/18 pre-activated murine NK cells have partial demethylation of the CNS1 region controlling the IFN-γ gene 11 days following adoptive transfer, suggesting epigenetic changes during memory-like differentiation [51]. In addition, CD4+ T cell help of NK cell anti-tumor effects depended on the presence of macrophages in lymphopenic hosts [51]. The complete complement of receptor and/or cytokine-based interactions between NK cells, T cells, and macrophages, and whether T cell dependent help is antigen specific, remains to be identified.
We previously demonstrated that IL-12/15/18-induced human memory-like NK cells produced increased IFN-γ in response to K562 myeloid leukemia targets [42]. Recently, we performed a pre-clinical investigation of cytokine-induced memory-like NK cell responses to acute myeloid leukemia (AML) [52]. Cytokine-induced memory-like human cells exhibited enhanced IFN-γ and TNF production in response to re-stimulation with primary AML blasts in vitro. In addition, memory-like NK cells had increased granzyme B and perforin expression, and were more effective at killing K562 leukemia targets in vitro. Using mass cytometry, we found that memory-like NK cells demonstrated increased expression of the inhibitory receptor NKG2A, but also enhanced expression of the activating receptors NKG2D, NKp44, NKp30, NKp46 and TRAIL. These changes combined with other lineage and maturation markers (e.g. CD27, CD62L) suggest that multidimensional analyses may be able to distinguish memory-like from naïve/conventional NK cell populations.
We also investigated whether classic NK cell KIR-based interactions and licensing altered memory-like NK cell responses to leukemia targets. Unexpectedly, inhibitory KIR-matched memory-like NK cells (cells expressing an inhibitory KIR recognizing a ligand present on primary AML blasts) still exhibited increased IFN-γ responses compared to control cells (Figure 2) [52]. The concept of NK cell licensing (or education) predicts that only NK cells expressing inhibitory receptors for self will acquire functional competence, as a mechanism to ensure self-tolerance [15]. In a separate study, the ‘licensing status’ of cytokine-induced memory-like NK cells was examined, and revealed that while licensed memory-like NK cells had the greatest functionality, unlicensed memory-like NK cells responded to leukemia targets at a similar level as licensed control cells [53]. Together, these studies demonstrate that cytokine-induced memory-like NK cells have an enhanced capacity to recognize and respond to cancer targets previously invisible to those individuals' conventional, naïve NK cells.
Figure 2. Cytokine-Induced Memory-Like NK Cells Respond to Primary AML Cells Expressing Inhibitory MHC Class I Ligands.
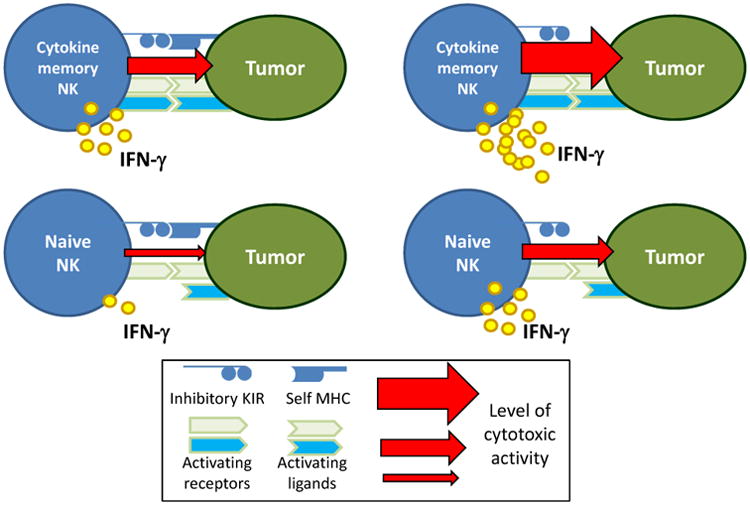
(Left) Engagement of inhibitory killer-cell immunoglobulin-like receptors (KIR) expressed by conventional, naïve NK cells normally limits their activation. However, cytokine-induced memory-like NK cells expressing inhibitory KIR recognizing MHC class I expressed by primary AML blasts exhibited enhanced IFN-γ production, compared to control or naïve NK cells. The precise activating signal in this context is currently unknown, although cytokine-induced memory-like NK cells have increased expression of a number of activating receptors. In addition to IFN-γ production, human memory-like NK cell exhibited enhanced cytotoxicity against leukemia targets, compared to control NK cells from the same donors. (Right) In the setting of KIR to KIR-ligand mismatch, memory-like NK cells also have superior responses to conventional or naïve control cells.
In vivo human memory-like NK cell responses were also demonstrated in a murine xenograft model [43, 52]. A single injection of human memory-like NK cells into NSG mice xenografted with K562 leukemia provided enhanced tumor control and survival in vivo when compared to control NK cells [52]. In a similar human xenograft model, the Cerwenka group demonstrated protection against a human melanoma cell line in vivo [51]. NK cells were supported by exogenous IL-2 in both models. Thus, pre-clinical human xenograft models support the enhanced activity of IL-12/15/18 pre-activated NK cells against both hematologic and solid malignancies.
Based on the original pre-clinical studies, we initiated a phase I first-in-human clinical trial (NCT#01898793) of adoptive immunotherapy with cytokine-induced memory-like NK cells in patients with relapsed/refractory acute myeloid leukemia (AML) in 2014 (Figure 3). Patients with active AML that failed prior therapy received immunosuppressive chemotherapy prior to transfer of related-donor MHC-haploidentical NK cells that were pre-activated with IL-12/15/18. Low dose IL-2 was provided to patients for 2 weeks to support the memory-like NK cells. Donor NK cells proliferated and expanded in recipients, peaking in number in the blood and bone marrow 7-14 days post transfer, comprising >90% of the NK cell population within the patients. Functional analysis revealed responses to leukemia targets ex vivo, with the percentage and magnitude of IFN-γ+ donor NK cells significantly exceeding recipient NK cells. Together, these results indicate that human IL-12/15/18 pre-activated NK cells transferred into cancer patients are able to proliferate, differentiate, and exhibit enhanced anti-cancer responsiveness.
Figure 3. Schema of the First-In-Human Phase 1 Study of Adoptive Immunotherapy with Allogeneic Memory-Like NK Cells.
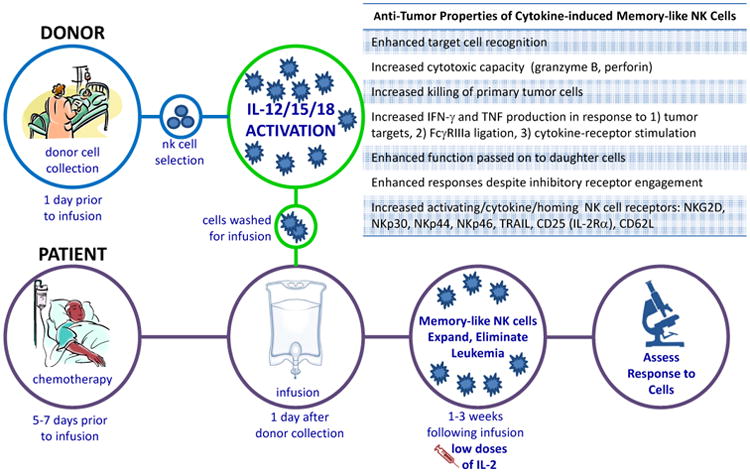
Patients with relapsed or refractory acute myeloid leukemia (AML) are treated with chemotherapy to provide immunosuppression that allows the engraftment of allogeneic donor NK cells. Related donors undergo leukapheresis to collect peripheral blood mononuclear cells, and NK cells are purified by CD3 negative and CD56 positive selection (CliniMACs device that results in >90% CD56+CD3- NK cells). These purified NK cells are then pre-activated with IL-12, IL-15, and IL-18 for 12-16 hours in a GMP setting. Cells are extensively washed to remove cytokines and infused into the cancer patient. Memory-like NK cell differentiate and expand, peaking 1-2 weeks post infusion, supported by low dose IL-2 therapy. Clinical responses are assessed at day 14 and 30 post-infusion using international working group criteria. The anti-tumor properties of cytokine-induced memory-like NK cells are summarized in the box inset.
Of the nine patients evaluable thus far, five (56%) patients had clinical responses with four (44%) having complete remissions, and none experienced dose limiting toxicity or graft versus host disease [52]. These preliminary results compare favorably to other published data using highly purified NK cells without IL-12/15/18 pre-activation, which resulted in 1 complete remission within 15 patients (7%) with active AML treated across two published studies [54, 55]. Thus, this first-in-human study of human NK cell memory-like cells indicates promising confirmation of their biology in vivo in cancer patients, and provides key initial evidence of their anti-leukemia effectiveness in humans.
Opportunities to Enhance Memory-Like and Adaptive NK Cell Responses to Cancer
Improving Tumor Cell Recognition and Triggering of Memory NK Cells
Multiple studies have demonstrated that HCMV epigenetically programs adaptive NK cell for enhanced FcγRIIIa-triggered cytokine responses [36, 38, 40]. Similarly, cytokine-induced memory-like NK cells exhibit enhanced responsiveness when triggered via FcγRIIIa [53], and have evidence of epigenetic alterations in the Ifng gene in the mouse system [51], identifying a potential common activating receptor strategy to direct human memory NK cells against cancer. Therapeutic monoclonal antibodies specific for a tumor restricted antigen provide a low affinity protein link between FcγRIIIa and a tumor target. More recent advances have optimized therapeutic mAbs for augmented antibody dependent cellular cytotoxicity (ADCC) and cytokine release (ADCR). Newer approaches include bi-specific and tri-specific proteins that bind FcγRIIIa on NK cells, and direct cells to a tumor target(s) or include a cytokine such as IL-15 [56-59]. Combining such agents with adaptive or cytokine-induced memory-like NK cell therapy may capitalize on the unique biology of these cells, and pre-clinical testing will be important to define the optimal NK cell memory type to combine with FcγRIIIa-triggering agents.
The activating receptors expressed or utilized by memory NK cell populations also differ from conventional NK cells, and may directly impact their ability to respond to tumor targets. Cytokine-induced human memory-like NK cells have increased expression of a number of activating receptors, including NKp30, NKG2D, NKp44, NKp46, and TRAIL [52]. In contrast, HCMV-adaptive NK cells exhibited pronounced NKG2C expression, but reduced NKp46 and NKp44 expression, with more modest reductions in FcγRIIIa expression levels (in spite of higher FcγRIIIa-induced responses), and intact expression of NKG2D and DNAM-1 [37]. Thus, distinct types of memory NK cells may be more effectively triggered by tumors expressing ligands recognized by NK cell activating receptors. In addition, costimulatory receptors may be required for optimal adaptive NK cell responses. Recent work has shown that HCMV-adaptive NK cells require CD2 co-activation for optimal functional responses [40], thus expression of its ligand CD58 may be important.
The recent success of chimeric antigen receptor (CAR) T cells in blood cancers also suggests that genetic modification of NK cells with an optimized triggering receptor may be feasible. Proof of principle studies have shown generation of CAR NK cells is possible [60, 61], but reports using primary human NK cells are limited. Exploration of CAR modification of memory-like and adaptive NK cells is warranted, and should potentially be designed for optimal responses based on the biology of memory-like or adaptive NK cells.
Memory NK Cell Checkpoints and The Tumor Microenvironment
Inhibitory receptors constitutively expressed on NK cells have been studied for decades, and clinical mAbs that block inhibitory KIR and NKG2A are under clinical development [7]. While not yet comprehensively reported, emerging evidence suggests that adaptive NK cells may have alterations of ‘induced’ inhibitory checkpoints that facilitate responses within the tumor microenvironment [7]. For example, the Miller laboratory recently identified that HCMV-adaptive NK cells express lower amounts of the inhibitory receptor TIGIT than conventional NK cells, thereby mitigating their inhibition by myeloid derived suppressor cells [62]. Cytokine-induced memory-like NK cells also have altered expression of inhibitory receptors, for example, globally increased inhibitory CD94-NKG2A, suggesting that combinations with anti-NKG2A mAbs may optimize their functional responses against HLA-E+ targets. Moreover, despite expression of KIR and NKG2A, cytokine-induced memory-like NK cells respond to MHC class I+ tumor targets [52, 53], and it remains to be determined what mechanisms are responsible for such effective responses despite inhibitory receptor signaling.
Sustaining NK Cell Memory in vivo
Classical studies of mouse NK cells identified a half-life at the population level of approximately 7-17 days [63, 64]. Studies of MCMV memory and cytokine-induced memory-like NK cells in mice suggest that these cells or their progeny with similar biological responsiveness persist following adoptive transfer into syngeneic hosts for months, identifying these cells as ‘long lived’ [4, 5, 41]. The persistence of cytokine-induced memory-like functional responses after transfer into a lymphopenic host decreases after ∼1 month, likely due to extensive homeostatic proliferation of both control and memory-like cells [41]. However, IL-2 supports memory-like NK cell responses in syngeneic, lymphopenic conditions [43, 50, 51]. Our current clinical approach utilizes low-dose IL-2 to support memory-like NK cells in patients, with minimal side effects. IL-15, which also signals through the shared IL-2/15βγcomplex is an obvious alternative, and has not been implicated in Treg stimulation. Clinical development of IL-15, and IL-15:IL-15Rα complexes have shown promise in augmenting conventional NK cell numbers and anti-tumor responses [65, 66], and may result in an improved expansion of memory-like NK cells in vivo.
While less data is available to define the longevity of human cytokine-induced memory-like NK cells, parallels between mouse and human biology (Table I), and persistence of human cells for 6 weeks in vitro [42], suggest a similar biology. To date, human memory-like NK cells have only been adoptively transferred into MHC-incompatible recipients [52], making determination of their half-life impossible, as they are ultimately rejected by the patient's T cells. Future studies exploring transfer into MHC- and immune-compatible environments, for example in the HCT setting, will be informative.
Following HCMV infection or after other viral infections in HCMV+ individuals, HCMV-adaptive NK2GC+ NK cells expand and may remain elevated for months or even years in humans [29, 33, 67, 68]. It remains unclear if these cells possess an intrinsically prolonged half-life, or if they are sustained by persistent stimulation, possibly through virus-specific mAbs as has been shown in vitro [35, 36]. However, this aspect of their biology may potentially be harnessed for cancer immunotherapy, for example via provision of anti-tumor therapeutic mAbs that chronically stimulate these highly FcγRIIIa-dependent NK cells [36-38]. Similarly, if antibodies to HCMV are critical, the use of CMV vaccines that induced HCMV specific mAbs could result the support and/or expansion of adaptive NK cell populations.
Concluding Remarks
The emergence of the concept of long-lasting NK cell memory responses has led to investigation of their anti-tumor properties. There are many remaining questions regarding the biology of NK cell memory and applicability to the treatment of cancer (see Outstanding Questions). Studies of mouse and human cytokine-driven memory-like NK cells suggest that they have enhanced anti-tumor responses, and led to the first clinical trial administering these cells to patients with AML. Human CMV adaptive NK cells have been studied in the context of HCT, and recent data supports enhanced antibody-dependent responses, suggesting translational approaches to enhance their efficacy in vivo. In addition, while we have focused here on CMV and cytokine-driven cancer immunotherapy, there is recent evidence that for hapten-driven NK cell memory anti-tumor response is based on a shared antigen specificity. Additional investigation of the anti-tumor properties of hapten-induced memory cells, and continued investigation of the immunotherapy potential of CMV and cytokine-driven NK cell memory in humans will be important as we continue to develop novel NK-based immunotherapy strategies.
Trends Box
Over the past decade it has been recognized that human and murine NK cells have properties of immunologic memory, with long-lasting enhanced functionality following stimulation with antigens, cytomegalovirus, or cytokines.
NK cell immunotherapy strategies for the treatment of cancer are currently under clinical investigation, and there is now evidence that some types of memory NK cells have enhanced anti-tumor properties.
A better understanding of the mechanisms of NK cell memory formation and functional properties of different types of memory cells is essential for harnessing the anti-tumor properties of these lymphocytes for the treatment of human disease.
Outstanding Questions Box
What forms of NK cell memory are most relevant for health and disease in humans? Do cytokine-induced memory-like NK cells arise in humans under physiologic conditions and how can they be identified? What is the physiologic importance of HCMV-induced adaptive NK cells?
Are HCMV-adaptive NK cells beneficial in the setting of cancer and hematopoietic cell transplantation? Will antibody-mediated targeting of these cells to cancer targets enhance their anti-tumor properties in vivo? Are HCMV-induced adaptive NK cell long-lived or continually formed in vivo?
What are the molecular pathways responsible for differentiation of cytokine-induced memory-like NK cells? Can cytokine-induced NK cell memory be generated in situ in patients by administration of cytokines or other treatments? How long do cytokine induced memory-like NK cells persist in patients and how can survival be enhanced?
What is the mechanism by which liver resident NK cell memory specifically recognize antigen? Can this be utilized for cancer immunotherapy?
What strategies can be used to target memory NK cells to tumor cells and what is the physiological in vivo distribution of these cells?
Acknowledgments
Work in the Fehniger laboratory is supported by R01 AI102924, Leukemia SPORE P50 CA171963, the Siteman Cancer Center P30 CA91842, Gabrielle's Angel Foundation for Cancer Research, and the Leukemia and Lymphoma Society. Work in the Cooper laboratory is supported by the Rheumatology Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yokoyama WM. In: Natural Killer Cells, in Fundamental Immunology. Paul W, editor. Lippincott, Williams &Wilkins; Philadelphia: 2013. pp. 395–430. [Google Scholar]
- 2.Vivier E, et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Leary JG, et al. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7:507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- 4.Cooper MA, et al. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci U S A. 2009;106:1915–1919. doi: 10.1073/pnas.0813192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun JC, et al. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knorr DA, et al. Clinical utility of natural killer cells in cancer therapy and transplantation. Semin Immunol. 2014;26:161–172. doi: 10.1016/j.smim.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berrien-Elliott MM, et al. Improving natural killer cell cancer immunotherapy. Curr Opin Organ Transplant. 2015;20:671–680. doi: 10.1097/MOT.0000000000000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruggeri L, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 9.Cooper MA, Yokoyama WM. Memory-like responses of natural killer cells. Immunol Rev. 2010;235:297–305. doi: 10.1111/j.0105-2896.2010.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paust S, et al. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat Immunol. 2010;11:1127–1135. doi: 10.1038/ni.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng H, et al. Liver-resident NK cells confer adaptive immunity in skin-contact inflammation. J Clin Invest. 2013;123:1444–1456. doi: 10.1172/JCI66381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paust S, et al. Adaptive immune responses mediated by natural killer cells. Immunol Rev. 2010;235:286–296. doi: 10.1111/j.0105-2896.2010.00906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Majewska-Szczepanik M, et al. Natural killer cell-mediated contact sensitivity develops rapidly and depends on interferon-alpha, interferon-gamma and interleukin-12. Immunology. 2013;140:98–110. doi: 10.1111/imm.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van den Boorn JG, et al. Inflammasome-Dependent Induction of Adaptive NK Cell Memory. Immunity. 2016;44:1406–1421. doi: 10.1016/j.immuni.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 15.Elliott JM, Yokoyama WM. Unifying concepts of MHC-dependent natural killer cell education. Trends Immunol. 2011;32:364–372. doi: 10.1016/j.it.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reeves RK, et al. Antigen-specific NK cell memory in rhesus macaques. Nat Immunol. 2015;16:927–932. doi: 10.1038/ni.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biron CA, et al. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med. 1989;320:1731–1735. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- 18.Kuijpers TW, et al. Human NK cells can control CMV infection in the absence of T cells. Blood. 2008;112:914–915. doi: 10.1182/blood-2008-05-157354. [DOI] [PubMed] [Google Scholar]
- 19.Dokun AO, et al. Specific and nonspecific NK cell activation during virus infection. Nat Immunol. 2001;2:951–956. doi: 10.1038/ni714. [DOI] [PubMed] [Google Scholar]
- 20.Smith HR, et al. Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc Natl Acad Sci U S A. 2002;99:8826–8831. doi: 10.1073/pnas.092258599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arase H, et al. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science. 2002;296:1323–1326. doi: 10.1126/science.1070884. [DOI] [PubMed] [Google Scholar]
- 22.Cheng TP, et al. Ly49h is necessary for genetic resistance to murine cytomegalovirus. Immunogenetics. 2008;60:565–573. doi: 10.1007/s00251-008-0313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun JC, et al. Proinflammatory cytokine signaling required for the generation of natural killer cell memory. J Exp Med. 2012;209:947–954. doi: 10.1084/jem.20111760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nabekura T, et al. Costimulatory molecule DNAM-1 is essential for optimal differentiation of memory natural killer cells during mouse cytomegalovirus infection. Immunity. 2014;40:225–234. doi: 10.1016/j.immuni.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Sullivan TE, et al. Natural Killer Cell Memory. Immunity. 2015;43:634–645. doi: 10.1016/j.immuni.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beaulieu AM, et al. The transcription factor Zbtb32 controls the proliferative burst of virus-specific natural killer cells responding to infection. Nat Immunol. 2014;15:546–553. doi: 10.1038/ni.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Sullivan TE, et al. BNIP3- and BNIP3L-Mediated Mitophagy Promotes the Generation of Natural Killer Cell Memory. Immunity. 2015;43:331–342. doi: 10.1016/j.immuni.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Min-Oo G, et al. Proapoptotic Bim regulates antigen-specific NK cell contraction and the generation of the memory NK cell pool after cytomegalovirus infection. J Exp Med. 2014;211:1289–1296. doi: 10.1084/jem.20132459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guma M, et al. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood. 2004;104:3664–3671. doi: 10.1182/blood-2004-05-2058. [DOI] [PubMed] [Google Scholar]
- 30.Rolle A, Brodin P. Immune Adaptation to Environmental Influence: The Case of NK Cells and HCMV. Trends Immunol. 2016;37:233–243. doi: 10.1016/j.it.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 31.Lopez-Verges S, et al. Expansion of a unique CD57(+)NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc Natl Acad Sci U S A. 2011;108:14725–14732. doi: 10.1073/pnas.1110900108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foley B, et al. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood. 2012;119:2665–2674. doi: 10.1182/blood-2011-10-386995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malmberg KJ, et al. Spotlight on NKG2C and the human NK-cell response to CMV infection. Eur J Immunol. 2012;42:3141–3145. doi: 10.1002/eji.201243050. [DOI] [PubMed] [Google Scholar]
- 34.Luetke-Eversloh M, et al. Human cytomegalovirus drives epigenetic imprinting of the IFNG locus in NKG2Chi natural killer cells. PLoS Pathog. 2014;10:e1004441. doi: 10.1371/journal.ppat.1004441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schlums H, et al. Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity. 2015;42:443–456. doi: 10.1016/j.immuni.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee J, et al. Epigenetic modification and antibody-dependent expansion of memory-like NK cells in human cytomegalovirus-infected individuals. Immunity. 2015;42:431–442. doi: 10.1016/j.immuni.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hwang I, et al. Identification of human NK cells that are deficient for signaling adaptor FcRgamma and specialized for antibody-dependent immune functions. Int Immunol. 2012;24:793–802. doi: 10.1093/intimm/dxs080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang T, et al. Cutting edge: antibody-dependent memory-like NK cells distinguished by FcRgamma deficiency. J Immunol. 2013;190:1402–1406. doi: 10.4049/jimmunol.1203034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Della Chiesa M, et al. Human cytomegalovirus infection promotes rapid maturation of NK cells expressing activating killer Ig-like receptor in patients transplanted with NKG2C-/- umbilical cord blood. J Immunol. 2014;192:1471–1479. doi: 10.4049/jimmunol.1302053. [DOI] [PubMed] [Google Scholar]
- 40.Liu LL, et al. Critical Role of CD2 Co-stimulation in Adaptive Natural Killer Cell Responses Revealed in NKG2C-Deficient Humans. Cell Rep. 2016;15:1088–1099. doi: 10.1016/j.celrep.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keppel MP, et al. Murine NK cell intrinsic cytokine-induced memory-like responses are maintained following homeostatic proliferation. J Immunol. 2013;190:4754–4762. doi: 10.4049/jimmunol.1201742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Romee R, et al. Cytokine activation induces human memory-like NK cells. Blood. 2012;120:4751–4760. doi: 10.1182/blood-2012-04-419283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leong JW, et al. Preactivation with IL-12, IL-15, and IL-18 induces CD25 and a functional high-affinity IL-2 receptor on human cytokine-induced memory-like natural killer cells. Biol Blood Marrow Transplant. 2014;20:463–473. doi: 10.1016/j.bbmt.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cichocki F, et al. CD56dimCD57+NKG2C+ NK cell expansion is associated with reduced leukemia relapse after reduced intensity HCT. Leukemia. 2016;30:456–463. doi: 10.1038/leu.2015.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bigley AB, et al. Latent cytomegalovirus infection enhances anti-tumour cytotoxicity through accumulation of NKG2C+ NK cells in healthy humans. Clin Exp Immunol. 2016;185:239–251. doi: 10.1111/cei.12785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elmaagacli AH, et al. Early human cytomegalovirus replication after transplantation is associated with a decreased relapse risk: evidence for a putative virus-versus-leukemia effect in acute myeloid leukemia patients. Blood. 2011;118:1402–1412. doi: 10.1182/blood-2010-08-304121. [DOI] [PubMed] [Google Scholar]
- 47.Ito S, et al. CMV reactivation is associated with a lower incidence of relapse after allo-SCT for CML. Bone Marrow Transplant. 2013;48:1313–1316. doi: 10.1038/bmt.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Green ML, et al. CMV reactivation after allogeneic HCT and relapse risk: evidence for early protection in acute myeloid leukemia. Blood. 2013;122:1316–1324. doi: 10.1182/blood-2013-02-487074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bjorklund AT, et al. Naive Donor NK Cell Repertoires Associated with Less Leukemia Relapse after Allogeneic Hematopoietic Stem Cell Transplantation. J Immunol. 2016;196:1400–1411. doi: 10.4049/jimmunol.1501434. [DOI] [PubMed] [Google Scholar]
- 50.Ni J, et al. Sustained effector function of IL-12/15/18-preactivated NK cells against established tumors. J Exp Med. 2012;209:2351–2365. doi: 10.1084/jem.20120944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ni J, et al. Adoptively transferred natural killer cells maintain long-term anti-tumor activity by epigenetic imprinting and CD4+ T cell help. Oncoimmunology. 2016 doi: 10.1080/2162402X.2016.1219009. Published online 05 Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Romee R, et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci Transl Med. 2016 doi: 10.1126/scitranslmed.aaf2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wagner JA, et al. Mechanisms governing human memory-like natural killer cell function. AAI 2016 Immunology Meeting, May 13-17 2016 [Google Scholar]
- 54.Bachanova V, et al. Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein. Blood. 2014;123:3855–3863. doi: 10.1182/blood-2013-10-532531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Curti A, et al. Successful transfer of alloreactive haploidentical KIR ligand-mismatched natural killer cells after infusion in elderly high risk acute myeloid leukemia patients. Blood. 2011;118:3273–3279. doi: 10.1182/blood-2011-01-329508. [DOI] [PubMed] [Google Scholar]
- 56.Reusch U, et al. A novel tetravalent bispecific TandAb (CD30/CD16A) efficiently recruits NK cells for the lysis of CD30+ tumor cells. MAbs. 2014;6:728–739. doi: 10.4161/mabs.28591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gleason MK, et al. CD16xCD33 bispecific killer cell engager (BiKE) activates NK cells against primary MDS and MDSC CD33+ targets. Blood. 2014;123:3016–3026. doi: 10.1182/blood-2013-10-533398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rothe A, et al. A phase 1 study of the bispecific anti-CD30/CD16A antibody construct AFM13 in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2015;125:4024–4031. doi: 10.1182/blood-2014-12-614636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schmohl JU, et al. Enhanced ADCC and NK Cell Activation of an Anticarcinoma Bispecific Antibody by Genetic Insertion of a Modified IL-15 Cross-linker. Mol Ther. 2016;24:1312–1322. doi: 10.1038/mt.2016.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chu J, et al. CS1-specific chimeric antigen receptor (CAR)-engineered natural killer cells enhance in vitro and in vivo antitumor activity against human multiple myeloma. Leukemia. 2014;28:917–927. doi: 10.1038/leu.2013.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hermanson DL, Kaufman DS. Utilizing chimeric antigen receptors to direct natural killer cell activity. Front Immunol. 2015;6:195. doi: 10.3389/fimmu.2015.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sarhan D, et al. Adaptive NK cells with low TIGIT expression are inherently resistant to myeloid-derived suppressor cells. Cancer Res. 2016 doi: 10.1158/0008-5472.CAN-16-0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koka R, et al. Interleukin (IL)-15R[alpha]-deficient natural killer cells survive in normal but not IL-15R[alpha]-deficient mice. J Exp Med. 2003;197:977–984. doi: 10.1084/jem.20021836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jamieson AM, et al. Turnover and proliferation of NK cells in steady state and lymphopenic conditions. J Immunol. 2004;172:864–870. doi: 10.4049/jimmunol.172.2.864. [DOI] [PubMed] [Google Scholar]
- 65.Waldmann TA. The shared and contrasting roles of IL2 and IL15 in the life and death of normal and neoplastic lymphocytes: implications for cancer therapy. Cancer Immunol Res. 2015;3:219–227. doi: 10.1158/2326-6066.CIR-15-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Romee R, et al. Utilizing cytokines to function-enable human NK cells for the immunotherapy of cancer. Scientifica (Cairo) 2014;2014:205796. doi: 10.1155/2014/205796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bjorkstrom NK, et al. Rapid expansion and long-term persistence of elevated NK cell numbers in humans infected with hantavirus. J Exp Med. 2011;208:13–21. doi: 10.1084/jem.20100762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Petitdemange C, et al. Unconventional repertoire profile is imprinted during acute chikungunya infection for natural killer cells polarization toward cytotoxicity. PLoS Pathog. 2011;7:e1002268. doi: 10.1371/journal.ppat.1002268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Min-Oo G, Lanier LL. Cytomegalovirus generates long-lived antigen-specific NK cells with diminished bystander activation to heterologous infection. J Exp Med. 2014;211:2669–2680. doi: 10.1084/jem.20141172. [DOI] [PMC free article] [PubMed] [Google Scholar]


