Abstract
Histone acetylation has emerged as a critical factor regulating learning and memory both during and after exposure to stressful stimuli. There are drugs that we now know affect histone acetylation that are already in use in clinical populations. The current study uses these drugs to examine the consequences of acutely increasing or decreasing histone acetylation during exposure to social stress. Using an acute model of social defeat in Syrian hamsters, we systemically and site-specifically administered drugs that alter histone acetylation and measured subsequent behavior and immediate-early gene activity. We found that systemic administration of a histone deacetylase inhibitor enhances social stress-induced behavioral responses in males and females. We also found that systemic administration completely blocks defeat-induced neuronal activation, as measured by Fos-immunoreactivity, in the infralimbic cortex, but not in the amygdala, after a mild social defeat stressor. Lastly, we demonstrated that site-specific administration of histone deacetylase inhibitors in the infralimbic region of the prefrontal cortex, but not in the basolateral amygdala, mimics the systemic effect. Conversely, decreasing acetylation by inhibiting histone acetyltransferases in the infralimbic cortex reduces behavioral responses to defeat. This is the first demonstration that acute pharmacological manipulation of histone acetylation during social defeat alters subsequent behavioral responses in both males and females. These results reveal that even systemic administration of drugs that alter histone acetylation can significantly alter behavioral responses to social stress and highlight the importance of the infralimbic cortex in mediating this effect.
Keywords: Valproic acid, Social defeat, Epigenetics, Medial prefrontal cortex
1. Introduction
DNA transcription is necessary for development and maintenance of experience-dependent, long-term memories that elicit subsequent changes in behavior. The removal or addition of acetyl groups to the histones around which DNA is wrapped by histone deacetylases (HDACs) or histone acetyltransferases (HATs) alters the likelihood of gene transcription. Inhibition of Class I HDACs enhances long-term memory at each stage of memory processing (e.g., acquisition, consolidation, reconsolidation, extinction), while HAT inhibition impairs memory (Kilgore et al., 2010; Reolon et al., 2011). For example, acquisition of conditioned fear is enhanced following the administration of a Class I HDAC inhibitor, as is reconsolidation of that memory (Bredy and Barad, 2008), while inhibition of HATs during fear conditioning blocks acquisition and consolidation of that fear memory (Maddox et al., 2013; Monsey et al., 2015).
HDAC inhibitors, including valproic acid (VPA), are already being used clinically to treat a variety of illnesses such as epilepsy and bipolar disorder, but their effects on learning suggest that they may also be useful in a range of neuropsychiatric illnesses, such as posttraumatic stress disorder (PTSD) or specific phobia, wherein fear learning is potentially aberrant (Bredy and Barad, 2008; Parsons and Ressler, 2013). Further investigation into how these drugs impact long-term behavioral and physiological reactions to stress may lead us to the development of more targeted treatments and interventions, many of which could be immediately available for clinical populations. While the initial data are encouraging, most studies completed to date have used physical stressors (e.g., foot/tail shock) and only a few studies have examined the role of histone acetylation in more ethologically relevant models of stress-induced behavioral change (Covington et al., 2015; Espallergues et al., 2012; Hollis et al., 2011). Social defeat models have strong face and construct validity for human anxiety and depressive behavior (Hollis and Kabbaj, 2014; Huhman, 2006; Toth and Neumann, 2013), but the majority of these models use relatively severe, repeated exposure to social defeat in male mice. While the study of chronic social stress is important, not all social stressors that humans experience are chronic in nature. Acute social stress or trauma can also lead to sudden and discernable changes in behavior, sometimes leading to psychopathology (e.g., PTSD). Furthermore, using an acute model of social stress allows a much more precise determination of when acquisition and consolidation of stress-related learning are occurring, therefore we can test hypotheses about these processes in a way that is not possible in chronic models.
Our laboratory studies acute social defeat stress in Syrian hamsters. Hamsters provide a unique social stress model because both males and females are highly territorial, and these animals do not require complex housing conditions to elicit conspecific aggression or reliable behavioral responses to defeat in the laboratory. Home cage animals of both sexes will readily attack an intruding conspecific. However, after losing one agonistic encounter hamsters abandon all territorial aggression and, instead, become highly submissive and socially avoidant (Huhman, 2006; McCann et al., 2014; McCann and Huhman, 2012). This behavioral change has been termed conditioned defeat and lasts for at least one month in the majority of hamsters (Huhman et al., 2003). The conditioned defeat model is unique among social defeat models for several reasons. First, the agonistic interactions in hamsters are highly ritualized so that they rarely result in physical injury; thus, it is possible to examine the behavioral and physiological effects of social stress in the absence of physical injury or trauma and the concomitant inflammatory response. In addition, striking behavioral and physiological changes, including social avoidance and elevated cortisol, are observed after even a single, relatively mild defeat (Huhman et al., 1991, 2003; McCann and Huhman, 2012). Finally, unlike models using rats or mice, conditioned defeat in hamsters allows examination of defeat-induced behavior in both sexes. Thus, our model of acute social stress provides an excellent opportunity to study the behavioral and physiological responses in both males and females with much more precise temporal specificity compared with chronic defeat models that use only males, that test only extended periods of social stress, or that use species wherein wounding is common during social interactions.
We have made significant progress in delineating the neural circuitry mediating conditioned defeat. It is well established that the amygdala is a crucial site of plasticity necessary for processing and responding to emotional and fearful stimuli (Davis, 1992; Fanselow and Gale, 2003; McGaugh, 2004), and we have demonstrated that the basolateral amygdala (BLA) as well as the medial prefrontal cortex (mPFC) are critical components of the neural circuit mediating conditioned defeat (Jasnow and Huhman, 2001; Jasnow et al., 2005; Markham et al., 2012, 2010). The persistence of the behavioral changes observed after a single social defeat in hamsters suggests that these behavioral changes might be mediated by epigenetic mechanisms. A better understanding of the molecular mechanisms subserving conditioned defeat may lead us to a clearer understanding of how even brief exposure to social stress impacts future social behavior. The purpose of the present study was to test the hypothesis that epigenetic changes within the neural circuit that mediates conditioned defeat contribute to the observed behavioral changes after acute social stress.
2. Material and methods
2.1. Animals
Adult male and female Syrian hamsters (Mesocricetus auratus) were obtained from Charles River Laboratories (Wilmington, MA) or bred in-house from animals obtained from Charles River. Subjects (approximately 12 weeks, 120–130 g) were individually housed in a polycarbonate cage (23 × 43 × 20 cm) and were handled daily for at least one week before any behavioral manipulations began. The colony room was temperature-controlled, and animals were kept on a 14:10 light/dark cycle. All cages contained corncob bedding and cotton nesting material, and food and water were available ad libitum. Same sex resident aggressors (RAs) were used for social defeat training and for social avoidance testing. RAs are larger, individually housed hamsters that readily attack an intruder placed in their home cage. Female subjects were paired with ovariectomized female RAs because aggression in intact females varies over the estrous cycle and aggression in ovariectomized females is more reliable. Behavioral manipulations were done in a dedicated testing suite within the vivarium during the first 3 h of the dark phase of the daily light/dark cycle. All procedures and protocols were approved by the Georgia State University Institutional Animal Care and Use Committee and are in accordance with the standards outlined in the National Institutes of Health Guide for Care and Use of Laboratory Animals.
2.2. Social defeat training
For social defeat training, subjects were placed into the home cage of a same-sex RA as described previously (McCann et al., 2014; McCann and Huhman, 2012). Estrous cycles of female subjects were monitored via vaginal swabs for at least two cycles before the experiment, and females were defeated on Diestrus 1 (D1) and tested on Diestrus 2 (D2) because we have previously shown this results in the most pronounced avoidance after social defeat (unpublished observations). A clear cage top was placed on top of the RA's cage to prevent either animal from escaping the cage during a 5 min or 15 min defeat session. The two different training durations were chosen to avoid ceiling and floor effects, respectively, and the choice of which to use was based on a priori hypotheses of the directionality of the expected behavioral effect. Shorter defeat was used when submission and avoidance was expected to increase and longer defeat was used when submission and avoidance was expected to decrease in drug-treated animals as compared with vehicle controls. The holding box used for social avoidance testing, described below, was placed in the RA's cage during training. At the end of the defeat, subjects were returned to their home cages. Animals were monitored during defeat to ensure that no injury occurred to either animal. No-defeat controls were placed in a novel cage with soiled RA bedding and a holding box for the same amount of time as the defeat group and were subsequently returned to their home cage until social avoidance testing. Behavior emitted by RAs and by subjects during defeat training was recorded and scored by trained observers that were blind to experimental condition to ensure that pre-training drug infusions did not alter either the amount of aggression displayed by the RAs toward the subjects or the amount of submission shown by the subjects during defeat training.
2.3. Social avoidance testing
Social avoidance testing was conducted as described previously (McCann et al., 2014; McCann and Huhman, 2012) and was recorded for later analysis. In brief, 24 h after social defeat training, subjects were placed in a clean, novel testing arena (23 × 40 × 20 cm) with an unfamiliar RA placed inside a smaller holding box on one end of the arena. The holding box for the unfamiliar RA was constructed of perforated plastic that allowed the subject to see, hear, and smell the unfamiliar stimulus animal but not to come into direct contact with it. For scoring purposes, the testing arena was divided into eight sections (Fig. 1). Time spent in the far half of the testing arena (operationally defined as avoidance) as well as total number of line crosses (a measure of locomotor behavior) were scored. A line cross was counted when the subject's head and both front paws crossed over a line. Frequencies of specific behaviors (e.g., flees, risk assessments, flank marks), as defined previously (McCann and Huhman, 2012; Song et al., 2014), were also counted.
Fig. 1.
Schematic of testing arena. Dotted lines represent line markers for scoring subjects’ movements during the 5 min testing period. Time spent in the far half of the testing arena (designated by the black dotted line) was operationally defined as avoidance.
2.4. Cannulation and microinjections
For site-specific injections, males were implanted with bilateral cannulae targeting the BLA or with a unilateral cannula targeting the mPFC. Coordinates for guide cannulae used to target the BLA and mPFC were measured from bregma and were as follows for BLA: +0.0AP, ±4.0ML, −3.0DV from dura perpendicular, and for mPFC: +3.0AP, ±1.6ML, −3.2DV from dura at a 20° angle toward the midline to avoid the central sinus. Anesthesia was induced with 5% isoflurane, and animals were maintained at 3–5% isoflurane in a stereotaxic apparatus for the entire surgical procedure. Animals were handled for 1 week after surgery before any experimental manipulations. The compounds and concentrations listed below were injected directly into the site of interest using an infusion pump (Harvard Apparatus) and a Hamilton syringe connected to an injection needle by 50-gauge polyethylene tubing. In order to minimize damage to the area being injected, a shorter guide cannula (26-gauge) was used, and the final depth was reached with a smaller (33-gauge) injection needle that projected from the guide cannula (BLA: 3.3 mm below the guide; mPFC: 1.2 mm below the guide). The injection needle was left in the cannula guide for 1 min post-injection to ensure diffusion of the pharmacological agent from the needle tip. Successful injections were inferred if solution flowed easily from the needle before and after injection and a small air bubble placed between the drug and the saline solution in the tubing moved during microinjection.
2.5. Pharmacological agents
VPA (Sigma-Aldrich, St. Louis, MO) was dissolved in physiological saline. Intraperitoneal (IP; 100 mg/kg, 200 mg/kg, 300 mg/kg) as well as site-specific (100 μg/0.2 μl) injections of VPA were given (Bredy and Barad, 2008; Heinrichs et al., 2013; Kilgore et al., 2010; Kim et al., 2008; Nau and Loscher, 1982). IP injections were administered 2 h before defeat training because it has been clearly established that peak brain histone acetylation occurs 2 h after peripheral administration (Arent et al., 2011; Bredy and Barad, 2008; Bredy et al., 2007; Ploense et al., 2013; Tremolizzo et al., 2002). To test the temporal specificity of peripherally administered VPA in our model, we also completed two control experiments, one in which we administered VPA 1 h before defeat training and another in which we gave VPA 2 h before avoidance testing. These are both time frames in which other pharmacodynamic mechanisms of VPA would be active and are thus good controls to determine if observed effects are likely to be due to changes in histone acetylation versus other actions of VPA. Sodium butyrate (NAB; Alfa Aesar, Ward Hill, MA), another HDAC inhibitor, was used in order to further validate that the observed behavioral effects were indeed the result of HDAC inhibition and not another, more rapid effect of VPA administration such as an enhancement in GABAergic signaling. NAB was given IP (600 mg/kg, 1200 mg/kg in physiological saline) to a small subset of animals, but it induced a temporary and extreme ataxia, so systemic use was discontinued, and it was only tested site-specifically (1.32 μg/0.2 μl) (Blank et al., 2014; Heinrichs et al., 2013; Kilgore et al., 2010; Lattal et al., 2007; Mahan et al., 2012; Simon-O'Brien et al., 2015). Finally, Curcumin (Epigentek, Farmingdale, NY, 1.1 μg/0.2 μl) was dissolved in 55% DMSO. This drug was chosen because it appears to be one of the few, if not only, HAT inhibitors that is currently commercially available that does not have to be dissolved in 100% DMSO. All site-specific injections were given 30 min before social defeat (Simon-O'Brien et al., 2015; Xing et al., 2011) at a total volume of 0.2 μl to limit the spread of the injection.
2.6. Histology
After social avoidance testing, cannulated animals were given an overdose of sodium pentobarbital, and 0.2 μl of ink, to match the volume of drug administration, was injected through the guide cannulae for the purpose of site verification. Brains were sectioned on a cryostat and stained with neutral red for microscopic analysis of cannula placement. Placements more than 300 μm from the target nucleus were used as anatomical, or “miss”, controls to assess site specificity of the drug effects.
2.7. Immunohistochemistry for immediate-early gene c-fos
Animals were given IP injections of either saline or VPA (200 mg/kg) 2 h before a 5 min defeat and were perfused 1 h after the defeat. Postfixed brains were sectioned on a cryostat into cryoprotectant and were stored at −20°C until processing. On Day 1, sections were washed 3 × 5 min with potassium phosphate buffered saline (KPBS) and incubated in 0.3% hydrogen peroxide in KPBS for 30 min. Sections were washed again 3 × 5 min in KPBS and incubated with primary c-fos antibody (rabbit polyclonal IgG, 1:5000, Santa Cruz Biotechnology, Dallas, TX) in KPBS with 1% TritonX-100 and 1% normal goat serum overnight at room temperature. On Day 2, sections were washed 3 × 5 min with KPBS and incubated with 0.4% secondary (biotin-SP-conjugated AffiniPure goat anti-rabbit IgG, Jackson ImmunoResearch, West Grove, PA) in KPBS-T for 90 min at room temperature. Sections were again washed 3 × 5 min in KPBS and then incubated in pre-prepared avidin/biotin blocking solution (Vector Laboratories, Burlingame, CA) at room temperature for 1 h. After incubation, sections were washed 3 × 5 min with KPBS and then incubated in 3,3-diaminobenzidine (Vector Laboratories, Burlingame, CA) for 2–5 min. Sections were rinsed 2 × 5 min in KPBS, mounted using 0.15% gelatin in dH2O and allowed to dry overnight. Sections were then dehydrated for 2 min each in EtOH 50%, 70%, 95%, and 10 min in 100% EtOH, followed by 30 min in Citrosolv and then coverslipped with DPX. For analysis, a template was created for each region of interest and immunoreactive-positive cells within this area were counted using NIH ImageJ software. Bilateral counts from two or three sections per animal were averaged for each brain area.
2.8. Statistical analysis
Statistics for group comparisons were completed using SPSS for Windows (PASW Statistics 22.0). Student's t-tests or ANOVA with LSD post-hoc analysis were used for all analyses. All significant results reported here had a p-value of less than 0.05. Following statistical analysis, all avoidance data were graphed as percent of control for each experiment because baseline avoidance among the experiments was somewhat variable. This variability among experiments is to be expected, particularly given that some experiments involved a 5 min and others a 15 min defeat stressor.
3. Results
3.1. Systemic administration of an HDAC inhibitor before social stress enhances conditioned defeat learning in males
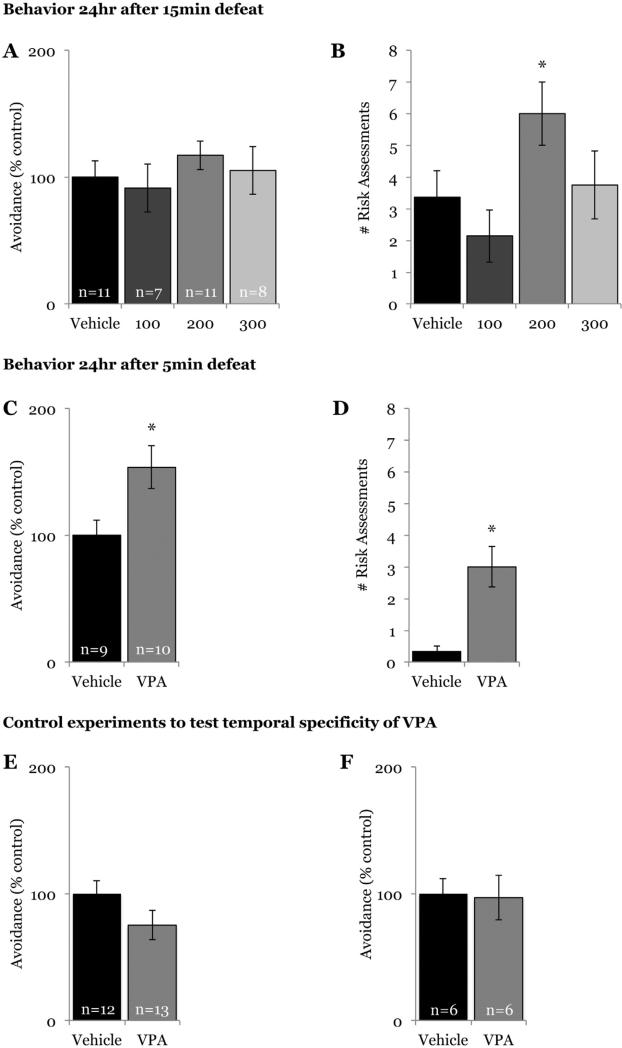
VPA or saline was administered IP 2 h before defeat training, and 24 h later we measured social avoidance and submission in response to a caged stimulus animal. Following a 15 min defeat, there was no difference in social avoidance during testing among animals given VPA (regardless of dose) and those given saline (F(3,33) = 0.527, p = 0.667; Fig. 2a); however, animals receiving 200 mg/kg of VPA displayed a significant increase in the number of risk assessments (F(3,33) = 2.883, p = 0.05; Fig. 2b). VPA did not alter seconds of avoidance (vehicle: 72.67 ± 7.71, 100 mg/kg: 75.63 ± 11.06, 200 mg/kg: 60 ± 5.06, 300 mg/kg: 95.29 ± 19.08; p = 0.517) or number of risk assessments (vehicle: 0 ± 0, 100 mg/kg: 0.5 ± 0.38, 200 mg/kg: 0 ± 0, 300 mg/kg: 0.14 ± 0.14; p = 0.264) in no-defeat controls, suggesting that the increase in risk assessments observed in defeated animals given VPA was not a non-specific effect of the drug on agonistic or anxiety-like behavior.
Fig. 2.
Systemic administration of VPA enhances conditioned defeat learning in males. Systemic VPA did not increase (A) social avoidance when given before a 15 min defeat regardless of drug dose (0 mg/kg, 100 mg/kg, 200 mg/kg, 300 mg/kg); however, animals given 200 mg/kg VPA exhibited an increase during testing in the number of (B) risk assessments (p = 0.041 compared with saline). When given 2 h before a 5 min defeat training session, systemic VPA increased both (C) social avoidance and (D) number of risk assessments observed during testing 24 h later. VPA (200 mg/kg) did not alter social avoidance when given either (E) 1 h before defeat or when given (F) 2 h before testing. *p < 0.05 compared with vehicle.
In the first experiment, all defeated animals, regardless of group, exhibited social avoidance when compared with no-defeat controls, suggesting a potential ceiling effect on avoidance following a 15 min defeat. To test this possibility, animals were given 200 mg/kg VPA (the dosage shown to increase risk assessment in the first experiment) or saline IP 2 h before a 5 min defeat. Animals given VPA before this shorter defeat experience exhibited both increased social avoidance (t(17) = −2.569, p = 0.02; Fig. 2c) and increased risk assessments (t(17) = −3.882, p = 0.001; Fig. 2d) during testing compared with animals given saline. Again, there was no effect of VPA on behavior of no-defeat controls during testing (seconds of avoidance, vehicle: 71.4 ± 8.68, VPA: 78.2 ± 1.95; p = 0.482; no risk assessments observed).
To test the temporal specificity of VPA, animals were given VPA 1 h before social defeat training. Animals given drug did not differ in social avoidance (t(23) = 1.593, p = 0.125; Fig. 2e) or risk assessment during testing compared with animals given saline. To further determine if VPA-enhanced conditioned defeat was specific to the acquisition of the memory of defeat, we tested defeat-induced social avoidance in animals given VPA 2 h before social avoidance testing to examine whether increased histone acetylation in the brain also had an effect on the expression of conditioned defeat. There was no difference in avoidance displayed by animals given VPA or saline (t(10) = 0.15, p = 0.883; Fig. 2f).
3.2. Systemic administration of VPA also enhances conditioned defeat learning in females
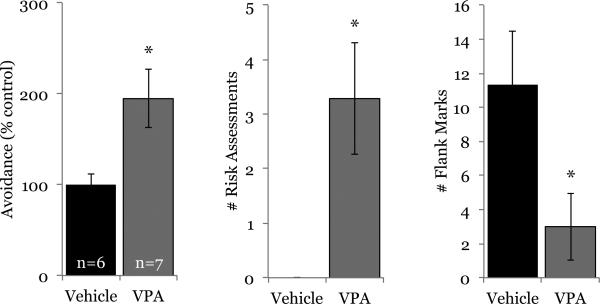
Subjects in the above experiments were males, and the purpose of the next experiment was to test if systemic VPA administration also enhances the acquisition of conditioned defeat in females. Like males, females given VPA (200 mg/kg) 2 h before a 5 min defeat displayed increased social avoidance (t(11) = −2.609, p = 0.02) and risk assessments (t(11) = −2.972, p = 0.01) compared with females given saline (Fig. 3). VPA also significantly decreased flank marking exhibited by defeated females (t(11) = 2.328, p = 0.04). One animal receiving vehicle was removed from analysis because its avoidance score during testing was an outlier (z-score = 2.24). Again, there was no effect on behavior of no-defeat controls during testing (seconds of avoidance, vehicle: 88.75 ± 24.04, VPA: 93.25 ± 16.73; p = 0.883; no risk assessments observed), indicating that the behavioral effects of systemic HDAC inhibition were specific to defeated females.
Fig. 3.
Systemic administration of VPA also enhances acquisition of conditioned defeat in females. VPA (200 mg/kg) increased defeat-induced social avoidance and risk assessments and decreased flank marking in females compared with their controls given saline. *p < 0.05.
3.3. Systemic administration of VPA decreases defeat-induced immediate-early gene activation in the mPFC
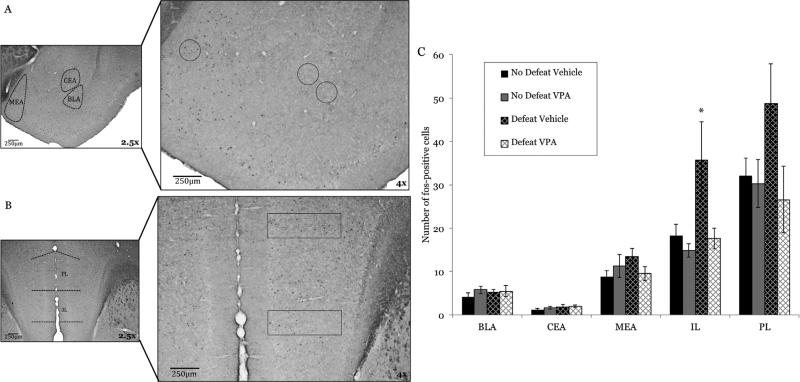
We next used immunohistochemistry for c-fos to localize where systemically administered VPA might be acting within the neural circuit mediating conditioned defeat to enhance behavioral responses to a 5 min defeat. Fos-immunoreactive cells were counted in male hamsters in several nuclei that are crucial to conditioned defeat learning, including regions of the amygdala (basolateral, central, medial) (Fig. 4a) and mPFC (prelimbic, infralimbic) (Fig. 4b). Surprisingly, no differences from control were observed in the number of fos-positive cells in amygdala following defeat with or without HDAC inhibition (BLA: F(1,20) = 0.946, p = 0.342; central amygdala: F(1,20) = 0.556, p = 0.465; medial amygdala (F(1,20) = 0.154, p = 0.669; Fig. 4c). VPA blocked defeat-induced neuronal activation, however, in the mPFC of defeated animals that received systemic VPA (Fig. 4c), as evidenced by a decrease in the number of Fos-positive cells. There was a main effect of HDAC inhibition in the infralimbic cortex (IL) (F(1,20) = 4.897, p = 0.039) and a trend for a 5 min defeat, alone, to increase Fos activation (F(1,20) = 4.27, p = 0.052). While trending in the same direction as the IL, no main effects were observed in the prelimbic cortex (PL) (HDAC inhibition: F(1,20) = 3.075, p = 0.095; defeat: F(1,20) = 0.882, p = 0.359).
Fig. 4.
Systemic HDAC inhibition modulates neural activity in the infralimbic cortex. Animals (n = 6 per group) were given VPA (200 mg/kg) or saline 2hr before a 5 min defeat and were sacrificed 1 h after defeat. Photomicrographs illustrate the sub-regions wherein Fos-positive cells were quantified: (A) amygdala (BLA: basolateral, CEA: central, MEA: medial) and (B) mPFC (PL: prelimbic, IL: infralimbic). (C) No significant differences were found in the amygdala (BLA, p = 0.342; CEA, p = 0.465.; MEA, p = 0.699) or PL (p = 0.095), but animals given vehicle before defeat had significantly higher fos-immunoreactivity in the IL than did all other groups. Animals given VPA exhibited fos-immunoreactivity that was comparable to no-defeat controls. *p < 0.05.
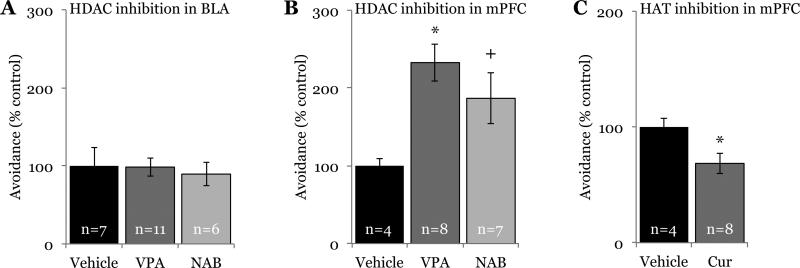
3.4. Site-specific HDAC inhibition in the mPFC, but not in the BLA, alters behavioral responses to social defeat
To test if HDAC inhibition in specific regions of the conditioned defeat circuit enhances conditioned defeat learning, we next administered an HDAC inhibitor (either VPA or NAB) directly into specific nuclei. Not surprisingly given our lack of cellular activation in the amygdala after systemic HDAC inhibition, animals given drug in the BLA before defeat exhibited the same amount of avoidance (F(2,21) = 0.095; p = 0.91; Fig. 5a) as did animals given saline, further suggesting the role of the BLA in the acquisition of conditioned defeat may be independent of HDAC activity. Conversely, the activation changes observed in mPFC after systemic administration of VPA were paralleled by a behavioral effect of the drug in the mPFC. There was a main effect of HDAC inhibition in the mPFC on seconds of social avoidance exhibited during testing (F(2,16) = 4.897, p = 0.022; Fig. 5b). Animals given VPA displayed significantly more avoidance than did animals given saline (p = 0.006). Animals given NAB exhibited a strong trend towards increased avoidance over those given saline (p = 0.063) and did not differ from those given VPA (p = 0.218).
Fig. 5.
HDAC and HAT inhibition in the mPFC, but not the BLA, modulate behavioral responses to social defeat. HDAC inhibition (valproic acid (VPA) or sodium butyrate (NAB)) in the (A) BLA before social defeat training did not alter social avoidance during testing 24 h later. HDAC inhibition in the (B) mPFC during social defeat training significantly increased social avoidance during testing, while (C) HAT inhibition (curcumin (CUR)) specifically in the IL decreased social avoidance. *p < 0.05, +p = 0.06 compared with vehicle.
There was no effect of central HDAC inhibition on seconds of avoidance exhibited by no-defeat controls (BLA, vehicle: 114.25 ± 20.14, VPA: 97.33 ± 9.71, NAB: 115.67 ± 9.82; p = 0.341; mPFC, vehicle: 85.8 ± 11.5, VPA: 92.75 ± 3.57, NAB: 95.75 ± 11.48; p = 0.768). Furthermore, HDAC inhibition in the anatomical (“miss”) controls (n = 3) for mPFC, which were located in the cingulate cortex more than 300 μm from the target nucleus, did not cause significant increases in social avoidance compared with controls (t(5) = −0.810, p = 0.455), supporting anatomical specificity of the drug effect. VPA given in the IL appeared to have a more robust effect on social avoidance (220.2s ± 22.28s, n = 5) than did VPA given in the PL (159s ± 30.57s, n = 3), but because this was not statistically significant (p = 0.151) these groups were collapsed for analysis.
3.5. HAT inhibition in the mPFC blocks the acquisition of conditioned defeat
To test whether histone acetylation in the mPFC is necessary for behavioral responses to social defeat, we administered the HAT inhibitor Curcumin (Balasubramanyam et al., 2004; Kang et al., 2005) to determine if this treatment would decrease the acquisition of conditioned defeat (i.e., have the opposite effect of HDAC inhibition). Curcumin administration resulted in decreased avoidance when compared with vehicle (t(10) = 2.328, p = 0.042; Fig. 5c). For this experiment, we localized injections to the IL because HDAC inhibition had a more robust effect in this region, both systemically and site-specifically. Injections in the PL or the cingulate cortex (“miss” controls, n = 6) did not cause a significant decrease in avoidance when compared with animals receiving vehicle (t(8) = 1.795, p = 0.11), demonstrating anatomical specificity of the drug effect.
3.6. Nonspecific behavioral effects of HDAC and HAT inhibition
To test if drug administration before social defeat altered the initial defeat experience and thereby caused the subsequent behavioral changes, we scored the behavior of both the aggressor and the subject during defeat training. Pharmacological manipulation of histone acetylation did not affect the amount of aggression shown by RAs during training nor the amount of submission shown by the subjects in any of the experiments. Furthermore, with the exception of animals given the highest dose of VPA in Experiment 1, drug manipulations did not affect locomotor activity during testing, as measured by number of line crosses (see Fig. 1). These data are shown in Table 1.
Table 1.
Nonspecific behavioral effects of HDAC and HAT inhibition.
| Aggression by RA (s) | Submission by Subject (s) | # Line Crosses | ||
|---|---|---|---|---|
|
Experiment 1: Systemic VPA Aggression: p=0.172 Submission: p=0.446 Line crosses: p=0.004 |
Vehicle | 304.45 ± 53.75 | 513.73 ± 58.41 | 88.55 ± 6.28 |
| 100mg/kg | 155.86 ± 26.43 | 448.57 ± 60.78 | 96.86 ± 7.95 | |
| 200mg/kg | 190.64 ± 43.1 | 381.36 ± 56.27 | 87.45 ± 5.04 | |
| 300mg/kg | 259.13 ± 61.58 | 421.25 ± 77.57 | 63.25 ± 2.05* | |
|
Experiment 2: Systemic VPA (5min defeat) Aggression: p=0.641 Submission: p=0.873 Line crosses: p=0.332 |
Vehicle | 72.78 ± 15.02 | 120.33 ± 26.64 | 86.89 ± 3.9 |
| VPA | 64.80 ± 7.75 | 125.40 ± 17.27 | 94.2 ± 5.98 | |
|
Experiment 3: Systemic VPA in females Aggression: p=0.612 Submission: p=0.394 Line crosses: p=0.12 |
Vehicle | 85 ± 20.54 | 87 ± 24.92 | 79 ± 6 |
| VPA | 70.86 ± 17.91 | 122 ± 29.57 | 67.57 ± 3.62 | |
|
Experiment 4: Control systemic VPA Aggression: p=0.194 Submission: p=0.753 Line crosses (1hr): p=0.082 Line crosses (expression): p=0.459 |
Vehicle | 173 ± 33.66 | 323.08 ± 57.36 | 91.92 ± 4.98 81.83 ± 9.05 |
| VPA | 241.46 ± 37.45 | 354.31 ± 77.76 | 79.69 ± 4.55 71.83 ± 9.31 |
|
|
Experiment 5: Intra-BLA HDAC inhibition Aggression: p=0.369 Submission: p=0.899 Line crosses: p=0.092 |
Vehicle | 104 ± 22.57 | 161 ± 23.03 | 63.71 ± 5.22 |
| VPA | 136.36 ± 22.31 | 152.27 ± 18.93 | 76.73 ± 4.78 | |
| NAB | 93 ± 19.69 | 144.33 ± 29.96 | 80.67 ± 4.52 | |
|
Experiment 6: Intra-PFC HDAC inhibition Aggression: p=0.317 Submission: p=0.113 Line crosses: p=0.694 |
Vehicle | 289.75 ± 101.50 | 519.25 ± 83.53 | 65.25 ± 14.03 |
| VPA | 224.71 ± 35.31 | 561.71 ± 105.4 | 79.43 ± 13.01 | |
| NAB | 165.67 ± 33.16 | 318.17 ± 21.44 | 67.71 ± 10.3 | |
|
Experiment 7: Intra-PFC HAT inhibition Aggression: p=0.108 Submission: p=0.403 Line crosses: p=0.475 |
Vehicle | 253.25 ± 27.2 | 386.25 ± 56.73 | 93.25 ± 7.35 |
| Curcumin | 153 ± 38.9 | 302 ± 64.18 | 80.5 ± 11.35 |
No differences in seconds of aggression produced by the RA or seconds of submission exhibited by the subject were observed between groups in any experiment. Animals exhibited no difference in locomotor activity, as measured by the number of line crosses, during social avoidance testing with the exception of animals given the highest dose of VPA in Experiment 1. While there were no obvious signs of ataxia, animals given 300 mg/kg VPA exhibited significantly fewer line crosses than all other groups in that experiment (*p ± 0.05). All data are shown as mean ± standard error of the mean.
4. Discussion
In summary, the data presented here suggest that manipulation of histone acetylation, even via systemically administered drugs, may offer a novel and effective way to alter behavioral responses to acute social stress in both males and females. The data further suggest that these treatments act, at least in part, via their action in the IL and emphasize the importance of prefrontal epigenetic regulation in mediating behavioral changes observed after exposure to acute social stress.
Systemic administration of VPA before a single social defeat experience intensified subsequent behavioral responses to defeat. Our customary defeat procedure uses a 15 min, inescapable defeat. This is a relatively mild social stressor, but it is sufficient to lead to robust and quantifiable behavioral changes observed during subsequent testing (Gray et al., 2015; Jasnow and Huhman, 2001; McCann and Huhman, 2012). In our original experiment, we did not observe a change in social avoidance in animals given VPA, but this could be due to a ceiling effect. We did, however, observe a significant increase in risk assessment, which is a defensive/submissive behavior in which subjects cautiously stretch forward to investigate a potential threat. This increase in risk assessments suggests that there indeed was an increase in submission after systemic HDAC inhibition that was not captured by measuring seconds of avoidance. To test if systemic HDAC inhibition was in fact enhancing conditioned defeat learning, we next tested subjects using a shorter 5 min defeat. A 5 min defeat is not usually sufficient to elicit pronounced long-term behavioral changes; therefore, we reasoned that this defeat might provide a better starting point with which to discern possible enhancement. Using a 5 min defeat, we demonstrated that hamsters given systemic VPA did exhibit significant increases in social avoidance and risk assessment. Overall, these data demonstrate that a systemically administered HDAC inhibitor can enhance behavioral responses to acute social stress.
Peripheral VPA crosses the blood brain barrier quickly, with peak concentrations of the drug found in the brain 15 min after administration, dropping to non-detectable levels at 8 h post-administration (Nau and Loscher, 1982). VPA is known to be a potent and reliable HDAC inhibitor (Gottlicher et al., 2001; Phiel et al., 2001) with peak acetylation occurring in brain 2 h after systemic administration (Tremolizzo et al., 2002). This timeframe has been established as a reliable timeframe for observing behavioral effects of brain HDAC inhibition as well (Bredy and Barad, 2008) and coincides with our main behavioral effect. VPA did not affect behavior when given 1 h before defeat, a time when the drug has already crossed the blood brain barrier but before peak brain acetylation occurs, nor when given before avoidance testing, supporting the role of histone acetylation in mediating the observed behavioral changes. There was also no effect of the drugs on no-defeat controls or on the behavior observed during defeat training when the drug is known to be in the brain and pharmacologically active. Together, these findings indicate that systemic VPA specifically enhances the acquisition of the memory of a mild social defeat stressor and that this effect coincides with the time of known peak brain acetylation.
Our site-specific microinjections offer further support for a role of histone acetylation in the behavioral changes observed in response to acute social defeat. It has previously been demonstrated that ventricular and intra-mPFC administration of VPA or NAB decreases HDAC activity in the mPFC (Arent et al., 2011). Here, we demonstrate that infusion of HDAC inhibitors in the mPFC enhances conditioned defeat learning while administration of a HAT inhibitor impairs it. The opposing behavioral effects of HDAC and HAT inhibition in the mPFC strongly support a role of histone acetylation in this nucleus in mediating the behavioral responses to acute social stress. Recent evidence has demonstrated that chronic administration of an HDAC inhibitor into the mPFC after repeated defeat also decreases social avoidance (Covington et al., 2015). Together with our current data, these findings highlight an important role for epigenetic regulation in the mPFC, both during and after defeat, in modifying behavioral responses to social stress.
We have previously demonstrated that the BLA is critical for acquisition and expression of conditioned defeat (Jasnow and Huhman, 2001; Markham et al., 2010). Temporary inactivation of this nucleus with a GABA-A receptor agonist blocks the acquisition and expression of defeat-induced behavioral changes (Jasnow and Huhman, 2001; Markham et al., 2010) as does an NMDA glutamate receptor antagonist (Jasnow et al., 2004), and de novo protein synthesis in this nucleus is necessary for the behavioral changes characterizing conditioned defeat (Markham and Huhman, 2008). We were thus surprised to find that acute HDAC inhibition within the BLA did not affect the acquisition of conditioned defeat. There is evidence, however, that ventricular administration of VPA or NAB and intra-amygdalar administration of VPA does not decrease HDAC activity in the BLA (Arent et al., 2011). Thus, it is entirely possible that our treatment with these two known HDAC inhibitors did not, in fact, alter histone acetylation in the BLA.
Another prominent use for VPA is as an anticonvulsant or a mood stabilizer because of the drug's pharmacodynamic effect of increasing GABAergic neurotransmission (Nau and Loscher, 1982; Tunnicliff, 1999). While it might seem possible that some of the observed behavioral effects in this study result from an increase in GABA signaling, it is important to note that the enhanced avoidance and submission observed after acute systemic HDAC inhibition is specific to the time of peak brain histone acetylation. Histone acetylation (specifically at H3) reaches a peak 2 h after systemic administration, corresponding with our main behavioral effect, whereas increased GABA signaling in the brain is observed within 15 min after systemic VPA and remains elevated for up to 8 h (Nau and Loscher, 1982). We demonstrated that there was no effect of VPA on behavior when the drug was given 1 h before social defeat, a time when GABA signaling in the brain is enhanced, nor when it was given before avoidance testing, a time when GABAergic receptor agonists potently inhibit the expression of conditioned defeat (Jasnow and Huhman, 2001). Similarly, if VPA were acting primarily via a GABAergic mechanism, then we would certainly expect to see a decrease in the acquisition of conditioned defeat when VPA was given in the BLA as is seen when a GABA-A agonist is administered there (Jasnow and Huhman, 2001). Finally, NAB administration, which does not directly affect GABA signaling, caused a similar enhancement of defeat-induced behavior to VPA, while HAT inhibition in the IL, which reduces histone acetylation, reduced the acquisition of conditioned defeat. The primary shared mechanism of action for these drugs is their direct effect on the enzymes that regulate histone acetylation. Together, these data strongly dispute the argument that the observed behavioral changes resulted from an effect of VPA on GABAergic signaling and, instead strongly support the hypothesis that histone acetylation is an epigenetic mechanism contributing to the observed behavioral changes after exposure to acute social stress.
Furthermore, we observed less defeat-induced cellular activation, as measured by Fos-immunoreactivity, in the IL after systemic VPA administration compared with saline. No other brain region analyzed exhibited differential Fos-immunoreactivity after HDAC inhibition. Interestingly, the activation patterns in the PL were similar to that observed in the IL, although they did not reach statistical significance. This is perhaps surprising given the literature indicating that these two regions of the mPFC have opposing roles in the expression of conditioned fear (Sierra-Mercado et al., 2011). Our results, using drugs that target histone acetylation, however, suggest that these nuclei may have similar roles in mediating the acquisition of behavioral responses to acute social stress. We have shown previously that Fos-immunoreactivity increases in the BLA after a 15 min social defeat (Markham et al., 2010); here, we show that a 5 min defeat is not sufficient to increase immediate-early gene activation in the amygdala. It is notable that there was a trend for defeat to increase Fos activation in the IL, suggesting that the IL is sensitive even to an extremely mild, 5 min social defeat stressor.
We were also at first surprised to find that systemic administration of an HDAC inhibitor decreased fos-immunoreactivity in the IL. HDAC inhibition is associated with an increase in acetylation and thus an increase in gene transcription. It is important to note, however, that fos-immunoreactivity is not necessarily indicative of activation of excitatory neurons. The IL has strong inhibitory connections to the BLA and, although we did not see a corresponding increase in Fos-immunoreactivity in the BLA, it is possible that the decrease in defeat-induced neural activity in IL following HDAC inhibition leads to disinhibition of a subset of BLA neurons and that this is the mechanism by which the acquisition of conditioned defeat is enhanced after systemic or intra-mPFC HDAC inhibition.
Finally, one of the unique benefits of using hamsters to model social stress is that females also display agonistic behavior to same-sex conspecifics. Females are often overlooked in other translational models of social stress because of the difficulty in eliciting spontaneous female aggression in rats and mice. Female hamsters typically exhibit more aggression during agonistic encounters than do males, and their expression of conditioned defeat after losing a fight may be less marked than that observed in males when tested with a non-aggressive opponent (Huhman et al., 2003). Defeated females, however, respond to a caged opponent with similar social avoidance to that observed in males. Here, we demonstrate that systemic VPA also has the same social stress-promoting effect in females as it does in males. Interestingly, VPA also reduced the number of flank marks in defeated females. Flank marking is a mode of social communication in which a hamster rubs its flank glands along the wall of the cage. This behavior is produced more often by dominant animals and is thought to communicate information about social status (Albers and Prishkolnik, 1992; Ferris et al., 1987). There are also significant sex differences in flank marking, with females flank marking more often than do males. In the current study, males exhibited few, if any, flank marks (mean of less than 1 flank mark per animal during a 5 min test), while most females marked during testing. The decrease in flank marking observed in the defeated females given VPA is an additional measure of submission or loss of territoriality. Together, these data are the first to show that systemic HDAC inhibition in both males and females enhances the acquisition of stress-induced behavioral changes following acute social defeat.
5. Conclusions
The current study focused on the effect of acute HDAC or HAT inhibition during the experience of a mild social stressor. Social stress is particularly relevant in that it is argued to be the most common stressor experienced by humans (Bjorkqvist, 2001), and perceptions of social defeat are strongly associated with depression, anxiety disorders, social withdrawal, and submissiveness (Agid et al., 2000; Heim and Nemeroff, 2001; Nemeroff, 1998). Understanding the role that histone acetylation plays in the acquisition of socially relevant fear memories could be an important step in elucidating the molecular mechanisms underlying stress-related neuropsychiatric diseases including mood and anxiety disorders and in potentially developing better treatments to alter maladaptive behavioral responses to stressful events. Increased focus on interventions involving HAT inhibition may hold particular translational relevance because this treatment reduced behavioral responses to acute social stress. Furthermore, while systemic administration lacks anatomical resolution, it has valuable translational implications for the potential usefulness of the drugs for clinical populations, particularly when there are drugs that are already FDA-approved. The data presented here demonstrate for the first time that altering the enzymes that regulate histone acetylation, even systemically, can alter behavioral responses to acute social stress in both males and females.
Acknowledgements
The authors would like to acknowledge AD Guzman Bambaren, BM Thompson, KA Partrick, and TM Kahl for their assistance with this project.
Role of funding source
Research reported here was supported by the National Institute of Mental Health of the National Institutes of Health under Award Number R01MH062044 awarded to KLH and a Brains and Behavior Fellowship, a Honeycutt Fellowship, and a Dissertation Grant from Georgia State University awarded to KEM. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or Georgia State University.
Footnotes
Contributors
KEM and KLH designed all experiments; KEM conducted all experiments; AMR, GMFJ, AN, and DCC contributed to design and implementation of individual experiments; KEM and KLH prepared the manuscript; all authors edited and approved the manuscript.
Conflicts of interest
None
References
- Agid O, Kohn Y, Lerer B. Environmental stress and psychiatric illness. Biomed. Pharmacother. 2000;54:135–141. doi: 10.1016/S0753-3322(00)89046-0. [DOI] [PubMed] [Google Scholar]
- Albers HE, Prishkolnik J. Sex differences in odor-stimulated flank marking in the golden hamster (Mesocricetus auratus). Horm. Behav. 1992;26:229–239. doi: 10.1016/0018-506x(92)90044-v. [DOI] [PubMed] [Google Scholar]
- Arent CO, Valvassori SS, Fries GR, Stertz L, Ferreira CL, Lopes-Borges J, Mariot E, Varela RB, Ornell F, Kapczinski F, Andersen ML, Quevedo J. Neuroanatomical profile of antimaniac effects of histone deacetylases inhibitors. Mol. Neurobiol. 2011;43:207–214. doi: 10.1007/s12035-011-8178-0. [DOI] [PubMed] [Google Scholar]
- Balasubramanyam K, Varier RA, Altaf M, Swaminathan V, Siddappa NB, Ranga U, Kundu TK. Curcumin, a novel p300/CREB-binding protein-specific inhibitor of acetyltransferase, represses the acetylation of histone/nonhistone proteins and histone acetyltransferase-dependent chromatin transcription. J. Biol. Chem. 2004;279:51163–51171. doi: 10.1074/jbc.M409024200. [DOI] [PubMed] [Google Scholar]
- Bjorkqvist K. Social defeat as a stressor in humans. Physiol. Behav. 2001;73:435–442. doi: 10.1016/s0031-9384(01)00490-5. [DOI] [PubMed] [Google Scholar]
- Blank M, Dornelles AS, Werenicz A, Velho LA, Pinto DF, Fedi AC, Schroder N, Roesler R. Basolateral amygdala activity is required for enhancement of memory consolidation produced by histone deacetylase inhibition in the hippocampus. Neurobiol. Learn. Mem. 2014;111:1–8. doi: 10.1016/j.nlm.2014.02.009. [DOI] [PubMed] [Google Scholar]
- Bredy TW, Barad M. The histone deacetylase inhibitor valproic acid enhances acquisition, extinction, and reconsolidation of conditioned fear. Learn. Memory. 2008;15:39–45. doi: 10.1101/lm.801108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredy TW, Wu H, Crego C, Zellhoefer J, Sun YE, Barad M. Histone modifications around individual BDNF gene promoters in prefrontal cortex are associated with extinction of conditioned fear. Learn. Memory. 2007;14:268–276. doi: 10.1101/lm.500907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington HE, 3rd, Maze I, Vialou V, Nestler EJ. Antidepressant action of HDAC inhibition in the prefrontal cortex. Neuroscience. 2015;298:329–335. doi: 10.1016/j.neuroscience.2015.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annu. Rev. Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Espallergues J, Teegarden SL, Veerakumar A, Boulden J, Challis C, Jochems J, Chan M, Petersen T, Deneris E, Matthias P, Hahn CG, Lucki I, Beck SG, Berton O. HDAC6 regulates glucocorticoid receptor signaling in serotonin pathways with critical impact on stress resilience. J. Neurosci. 2012;32:4400–4416. doi: 10.1523/JNEUROSCI.5634-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Gale GD. The amygdala, fear, and memory. Ann. N. Y. Acad. Sci. 2003;985:125–134. doi: 10.1111/j.1749-6632.2003.tb07077.x. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Axelson JF, Shinto LH, Albers HE. Scent marking and the maintenance of dominant/subordinate status in male golden hamsters. Physiol. Behav. 1987;40:661–664. doi: 10.1016/0031-9384(87)90114-4. [DOI] [PubMed] [Google Scholar]
- Gottlicher M, Minucci S, Zhu P, Kramer OH, Schimpf A, Giavara S, Sleeman JP, Lo Coco F, Nervi C, Pelicci PG, Heinzel T. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J. 2001;20:6969–6978. doi: 10.1093/emboj/20.24.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray CL, Norvelle A, Larkin T, Huhman KL. Dopamine in the nucleus accumbens modulates the memory of social defeat in Syrian hamsters (Mesocricetus auratus). Behav. Brain Res. 2015;286:22–28. doi: 10.1016/j.bbr.2015.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol. Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Leite-Morris KA, Rasmusson AM, Kaplan GB. Repeated valproate treatment facilitates fear extinction under specific stimulus conditions. Neurosci. Lett. 2013;552:108–113. doi: 10.1016/j.neulet.2013.07.035. [DOI] [PubMed] [Google Scholar]
- Hollis F, Kabbaj M. ILAR J. Vol. 55. Institute of Laboratory Animal Resources; 2014. Social defeat as an animal model for depression. pp. 221–232. [DOI] [PubMed] [Google Scholar]
- Hollis F, Duclot F, Gunjan A, Kabbaj M. Individual differences in the effect of social defeat on anhedonia and histone acetylation in the rat hippocampus. Horm. Behav. 2011;59:331–337. doi: 10.1016/j.yhbeh.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhman KL, Hebert MA, Meyerhoff JL, Bunnell BN. Plasma cyclic AMP increases in hamsters following exposure to a graded footshock stressor. Psychoneuroendocrinology. 1991;16:559–563. doi: 10.1016/0306-4530(91)90039-v. [DOI] [PubMed] [Google Scholar]
- Huhman KL, Solomon MB, Janicki M, Harmon AC, Lin SM, Israel JE, Jasnow AM. Conditioned defeat in male and female Syrian hamsters. Horm. Behav. 2003;44:293–299. doi: 10.1016/j.yhbeh.2003.05.001. [DOI] [PubMed] [Google Scholar]
- Huhman KL. Social conflict models: can they inform us about human psychopathology? Horm. Behav. 2006;50:640–646. doi: 10.1016/j.yhbeh.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Huhman KL. Activation of GABA(A) receptors in the amygdala blocks the acquisition and expression of conditioned defeat in Syrian hamsters. Brain Res. 2001;920:142–150. doi: 10.1016/s0006-8993(01)03054-2. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Cooper MA, Huhman KL. N-methyl-d-aspartate receptors in the amygdala are necessary for the acquisition and expression of conditioned defeat. Neuroscience. 2004;123:625–634. doi: 10.1016/j.neuroscience.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Shi C, Israel JE, Davis M, Huhman KL. Memory of social defeat is facilitated by cAMP response element-binding protein overexpression in the amygdala. Behav. Neurosci. 2005;119:1125–1130. doi: 10.1037/0735-7044.119.4.1125. [DOI] [PubMed] [Google Scholar]
- Kang J, Chen J, Shi Y, Jia J, Zhang Y. Curcumin-induced histone hypoacetylation: the role of reactive oxygen species. Biochem. Pharmacol. 2005;69:1205–1213. doi: 10.1016/j.bcp.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Kilgore M, Miller CA, Fass DM, Hennig KM, Haggarty SJ, Sweatt JD, Rumbaugh G. Inhibitors of class 1 histone deacetylases reverse contextual memory deficits in a mouse model of Alzheimer's disease. Neuropsychopharmacology. 2010;35:870–880. doi: 10.1038/npp.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WY, Kim S, Kim JH. Chronic microinjection of valproic acid into the nucleus accumbens attenuates amphetamine-induced locomotor activity. Neurosci. Lett. 2008;432:54–57. doi: 10.1016/j.neulet.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Lattal KM, Barrett RM, Wood MA. Systemic or intrahippocampal delivery of histone deacetylase inhibitors facilitates fear extinction. Behav. Neurosci. 2007;121:1125–1131. doi: 10.1037/0735-7044.121.5.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox SA, Watts CS, Doyere V, Schafe GE. A naturally-occurring histone acetyltransferase inhibitor derived from Garcinia indica impairs newly acquired and reactivated fear memories. PLoS One. 2013;8:e54463. doi: 10.1371/journal.pone.0054463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahan AL, Mou L, Shah N, Hu JH, Worley PF, Ressler KJ. Epigenetic modulation of Homer1a transcription regulation in amygdala and hippocampus with pavlovian fear conditioning. J. Neurosci. 2012;32:4651–4659. doi: 10.1523/JNEUROSCI.3308-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham CM, Huhman KL. Is the medial amygdala part of the neural circuit modulating conditioned defeat in Syrian hamsters? Learn. Memory. 2008;15:6–12. doi: 10.1101/lm.768208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham CM, Taylor SL, Huhman KL. Role of amygdala and hippocampus in the neural circuit subserving conditioned defeat in Syrian hamsters. Learn. Memory. 2010;17:109–116. doi: 10.1101/lm.1633710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham CM, Luckett CA, Huhman KL. The medial prefrontal cortex is both necessary and sufficient for the acquisition of conditioned defeat. Neuropharmacology. 2012;62:933–939. doi: 10.1016/j.neuropharm.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann KE, Huhman KL. The effect of escapable versus inescapable social defeat on conditioned defeat and social recognition in Syrian hamsters. Physiol. Behav. 2012;105:493–497. doi: 10.1016/j.physbeh.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann KE, Bicknese CN, Norvelle A, Huhman KL. Effects of inescapable versus escapable social stress in Syrian hamsters: the importance of stressor duration versus escapability. Physiol. Behav. 2014;129:25–29. doi: 10.1016/j.physbeh.2014.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu. Rev. Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- Monsey MS, Gerhard DM, Boyle LM, Briones MA, Seligsohn M, Schafe GE. A diet enriched with curcumin impairs newly acquired and reactivated fear memories. Neuropsychopharmac. 2015;40:1278–1288. doi: 10.1038/npp.2014.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nau H, Loscher W. Valproic acid: brain and plasma levels of the drug and its metabolites, anticonvulsant effects and gamma-aminobutyric acid (GABA) metabolism in the mouse. J. Pharmacol. Exp. Ther. 1982;220:654–659. [PubMed] [Google Scholar]
- Nemeroff CB. The neurobiology of depression. Sci. Am. 1998;278:42–49. doi: 10.1038/scientificamerican0698-42. [DOI] [PubMed] [Google Scholar]
- Parsons RG, Ressler KJ. Implications of memory modulation for post-traumatic stress and fear disorders. Nat. Neurosci. 2013;16:146–153. doi: 10.1038/nn.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phiel CJ, Zhang F, Huang EY, Guenther MG, Lazar MA, Klein PS. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J. Biol. Chem. 2001;276:36734–36741. doi: 10.1074/jbc.M101287200. [DOI] [PubMed] [Google Scholar]
- Ploense KL, Kerstetter KA, Wade MA, Woodward NC, Maliniak D, Reyes M, Uchizono RS, Bredy TW, Kippin TE. Exposure to histone deacetylase inhibitors during Pavlovian conditioning enhances subsequent cue-induced reinstatement of operant behavior. Behav. Pharmacol. 2013;24:164–171. doi: 10.1097/FBP.0b013e32836104ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reolon GK, Maurmann N, Werenicz A, Garcia VA, Schroder N, Wood MA, Roesler R. Posttraining systemic administration of the histone deacetylase inhibitor sodium butyrate ameliorates aging-related memory decline in rats. Behav. Brain Res. 2011;221:329–332. doi: 10.1016/j.bbr.2011.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon-O'Brien E, Alaux-Cantin S, Warnault V, Buttolo R, Naassila M, Vilpoux C. The histone deacetylase inhibitor sodium butyrate decreases excessive ethanol intake in dependent animals. Addict. Biol. 2015;20:676–689. doi: 10.1111/adb.12161. [DOI] [PubMed] [Google Scholar]
- Song Z, McCann KE, McNeill J.K. t., Larkin TE, 2nd, Huhman KL, Albers HE. Oxytocin induces social communication by activating arginine-vasopressin V1a receptors and not oxytocin receptors. Psychoneuroendocrinology. 2014;50:14–19. doi: 10.1016/j.psyneuen.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth I, Neumann ID. Animal models of social avoidance and social fear. Cell Tissue Res. 2013;354:107–118. doi: 10.1007/s00441-013-1636-4. [DOI] [PubMed] [Google Scholar]
- Tremolizzo L, Carboni G, Ruzicka WB, Mitchell CP, Sugaya I, Tueting P, Sharma R, Grayson DR, Costa E, Guidotti A. An epigenetic mouse model for molecular and behavioral neuropathologies related to schizophrenia vulnerability. Proc. Natl. Acad. Sci. U. S. A. 2002;99:17095–17100. doi: 10.1073/pnas.262658999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunnicliff G. Actions of sodium valproate on the central nervous system. J. Physiol. Pharmacol. 1999;50:347–365. [PubMed] [Google Scholar]
- Xing B, Zhao Y, Zhang H, Dang Y, Chen T, Huang J, Luo Q. Microinjection of valproic acid into the ventrolateral orbital cortex exerts an antidepressant-like effect in the rat forced swim test. Brain Res. Bull. 2011;85:153–157. doi: 10.1016/j.brainresbull.2011.03.007. [DOI] [PubMed] [Google Scholar]