Abstract
The goals of this study were to use multiple informative markers to define and characterize the neurochemically distinct compartments of the pigeon basal ganglia, especially striatum and accumbens. To this end, we used antibodies against 12 different neuropeptides, calcium-binding proteins or neurotransmitter-related enzymes that are enriched in the basal ganglia. Our results clarify boundaries between previously described basal ganglia subdivisions in birds, and reveal considerable novel heterogeneity within these previously described subdivisions. Sixteen regions were identified that each displayed a unique neurochemical organization. Four compartments were identified within the dorsal striatal region. The neurochemical characteristics support previous comparisons to part of the central extended amygdala, somatomotor striatum, and associational striatum of mammals, respectively. The medialmost part of the medial striatum, however, has several unique features, including prominent pallidal-like woolly fibers and thus may be a region unique to birds. Four neurochemically distinct regions were identified within the pigeon ventral striatum: the accumbens, paratubercular striatum, ventrocaudal striatum, and the ventral area of the lateral part of the medial striatum that is located adjacent to these regions. The pigeon accumbens is neurochemically similar to the mammalian rostral accumbens. The pigeon paratubercular and ventrocaudal striatal regions are similar to the mammalian accumbens shell. The ventral portions of the medial and lateral parts of the medial striatum, which are located adjacent to accumbens shell-like areas, have neurochemical characteristics as well as previously reported limbic connections that are comparable to the accumbens core. Comparisons to neurochemically identified compartments in reptiles, mammals, and amphibians indicate that, although most of the basic compartments of the basal ganglia were highly conserved during tetrapod evolution, uniquely avian compartments may exist as well.
Keywords: striatum, accumbens, globus pallidus, ventral pallidum, bed nucleus of stria terminalis, avian
1. Introduction
The basal ganglia play a critical role in modulating motor functions. Similar types of neurons and fibers have been identified in the basal ganglia of birds, reptiles, amphibians and mammals, which co-express similar neuropeptides and appear to have similar functions (Anderson and Reiner, 1990a; Reiner and Anderson, 1990). Homologues of the main components of the basal ganglia have been identified in mammals, birds, and reptiles, including striatum, nucleus accumbens, bed nucleus of stria terminalis, globus pallidus (or dorsal pallidum), and ventral pallidum (Medina and Reiner, 1997; Smeets et al., 2000; Roberts et al., 2002; Reiner et al., 2004b; Balint and Csillag, 2007; Kuenzel et al., 2011). The boundaries of these major subdivisions have, however, not necessarily been clearly defined in all cases.
The main components of the avian striatum are the medial striatum, lateral striatum, and accumbens, each of which has a distinct neurochemical expression pattern (Reiner et al., 1994, 2004b). The medial striatum (previously named the lobus parolfactorius) has not been associated with a specific homologous field with the mammalian striatum, although it has some markers in common with the nucleus accumbens, and others in common with striatum proper (Reiner et al., 2004b). Similarly, the lateral striatum (previously the paleostriatum augmentatum) in birds is clearly striatal in nature but has not been definitively related to any one specific part of mammalian striatum. Neurochemical and connectional studies have suggested that the avian nucleus accumbens consists of regions corresponding to the mammalian accumbens core, shell, and rostrum (Balint and Csillag, 2007), but the boundaries of these regions have been elusive. Thus, in spite of numerous studies on avian basal ganglia, boundaries between and within its major subdivisions remain poorly documented, particularly in the ventral striatum, in part because only a limited number of neurochemical markers have been used for identifying different compartments. Moreover, prior studies show regional neurochemical heterogeneity throughout avian basal ganglia that needs to be better defined for a clearer understanding of avian basal ganglia organization and to facilitate its comparison to the basal ganglia in other vertebrate groups.
The goals of this study were to use multiple informative markers to define and characterize the neurochemically distinct compartments of the pigeon basal ganglia, especially those of the striatum and accumbens. To this end, we used antibodies against 10 different neuropeptides, calcium-binding proteins or neurotransmitter-related enzymes known to be enriched in the basal ganglia: (1) calbindin (CALB), (2) cholecystokinin (CCK), (3) choline acetyl transferase (ChAT), (4) glutamic acid decarboxylase (GAD), (5) leucine-enkephalin-(ENK), (6) neuropeptide Y (NPY), (7) parvalbumin (PARV), (8) substance P (SP), (9) tyrosine hydroxylase (TH), and (10) vasoactive intestinal polypeptide (VIP). In addition, antibodies against calretinin (CR) and the neuropeptide cocaine- and amphetamine-regulated transcript (CART) were used to discriminate striatal accumbens territories. Our results clarify boundaries between previously described basal ganglia subdivisions in birds, and reveal considerable novel heterogeneity within these previously described subdivisions. Comparisons to neurochemically identified compartments in reptiles, mammals, and amphibians indicate that although most of the basic compartments of the basal ganglia were highly conserved during tetrapod evolution, as previously noted, uniquely avian compartments may exist as well.
2. Materials and methods
The brains of sixteen adult homing pigeons (Columba livia), which were immunostained for CCK, ChAT, CR, GAD, ENK, NPY, TH, SP, VIP, CALB or PARV, were used in these studies. Sections from one of these were stained sequentially with the first 8 antibodies and provide the images in figs. 2–8. All procedures employed in this study were approved by the Animal Care Committees at State University of New York at Stony Brook and the University of Tennessee. The pigeons were deeply anesthetized with sodium pentobarbital (70 mg/kg) and perfused through the left ventricle with in 0.1M phosphate buffer (PB; pH 7.4) with 0.75% sodium chloride followed by 4% paraformaldehyde in PB. Brains were removed from the skulls, placed in fixative at 4°C for 3–4 hrs, and then transferred to a cryoprotective solution of 30% sucrose and 0.1% sodium azide in PB for 3–4 days. Brains were sectioned at 30–40 μm on a sliding, freezing microtome in the transverse plane used in the Karten and Hodos (1967) brain atlas. For each antibody, sections were washed in PB, immersed in 0.3% H2O2 in PB for 10 min, and rinsed again in PB prior to antibody incubation.
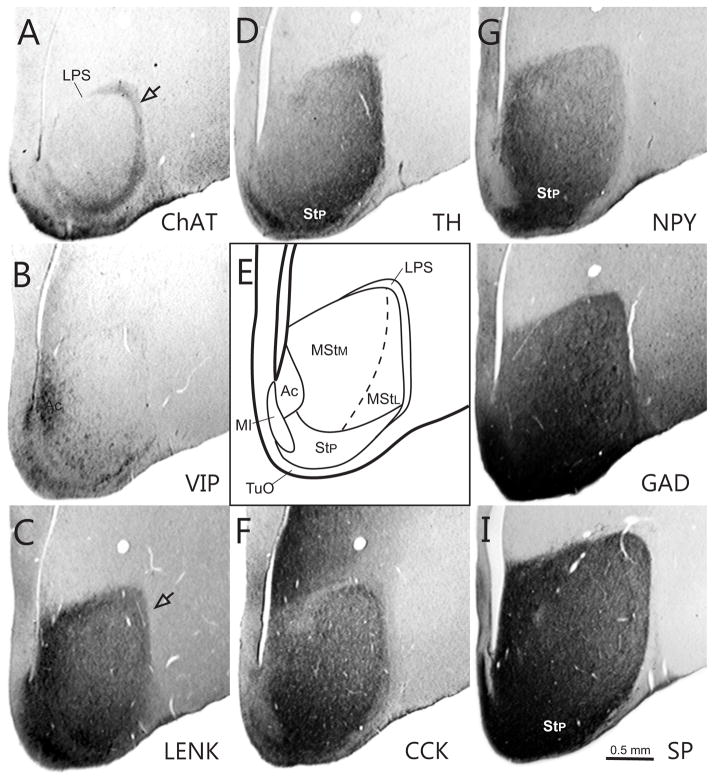
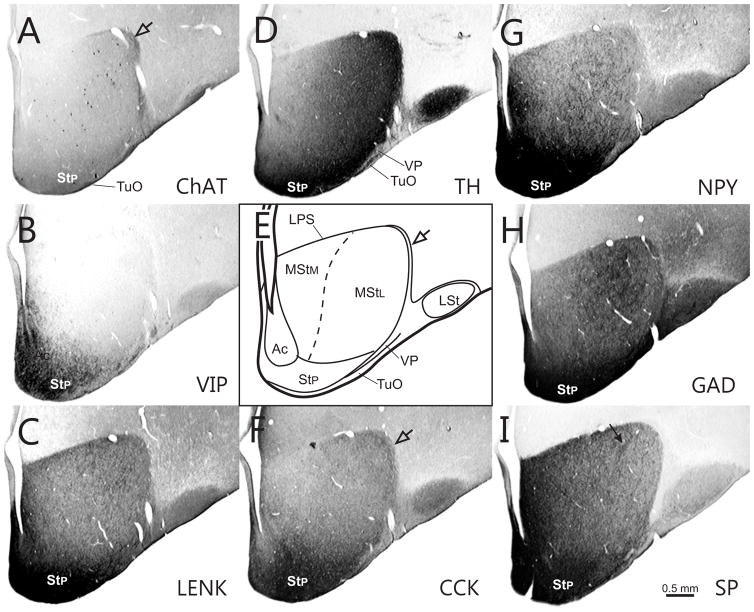
Fig. 2.
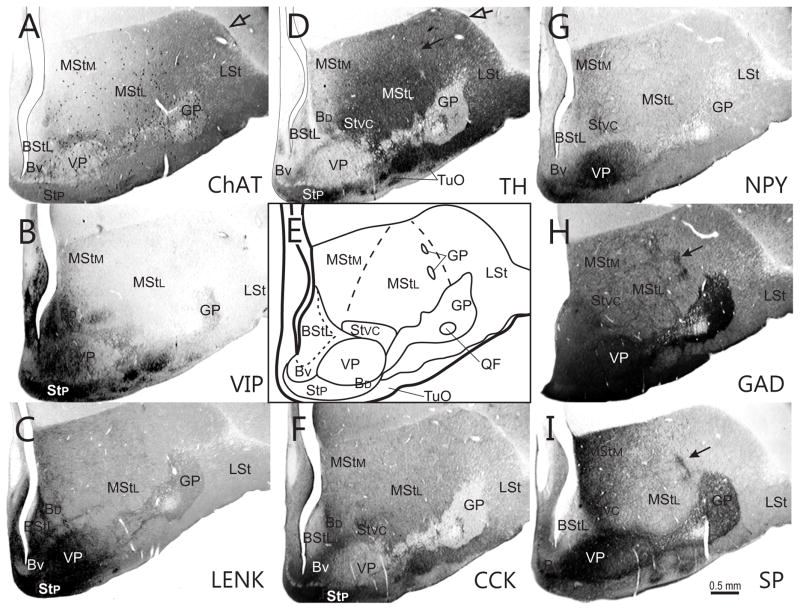
Series of transverse sections through the rostral basal ganglia comparing immunostaining patterns of ChAT (A), VIP (B), ENK (C), TH(D), CCK (F), NPY G), GAD (H), and SP (I) in different compartments of the striatum and ventral pallidum. These are illustrated in a schematic diagram (E). The series is arranged from rostral (A) to caudal (I). Note that two patches of neurons, the MI (open arrowhead) and islands of cells within the LPS (open arrows) express different neurochemical patterns than the adjacent striatal matrix. Rostrocaudal level is approximately A13.50.
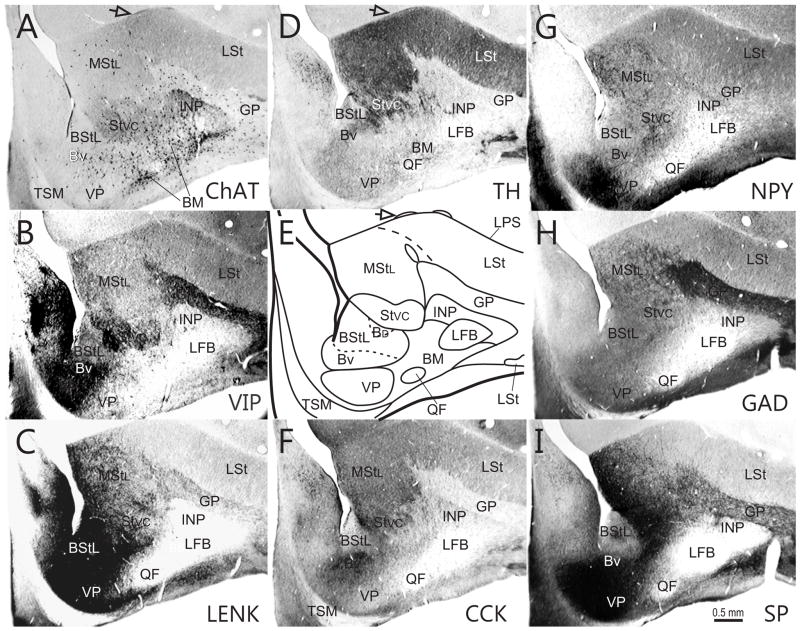
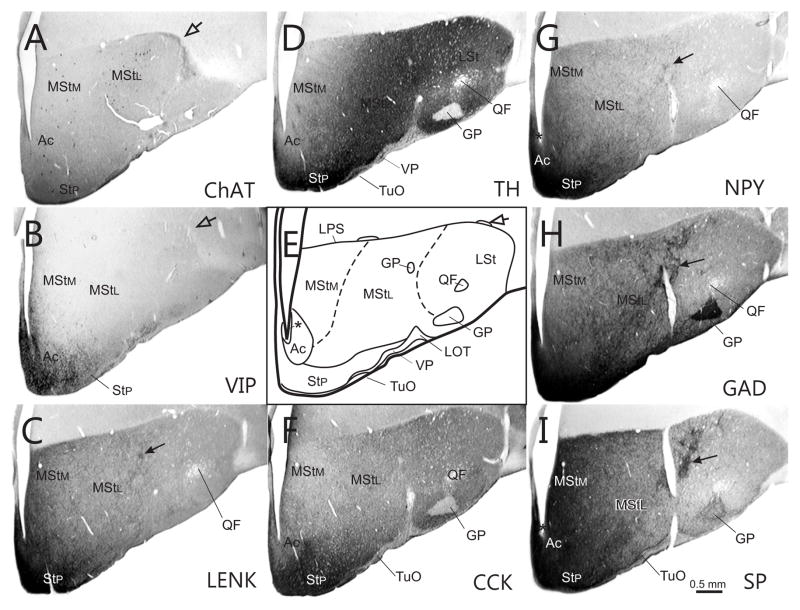
Fig. 8.
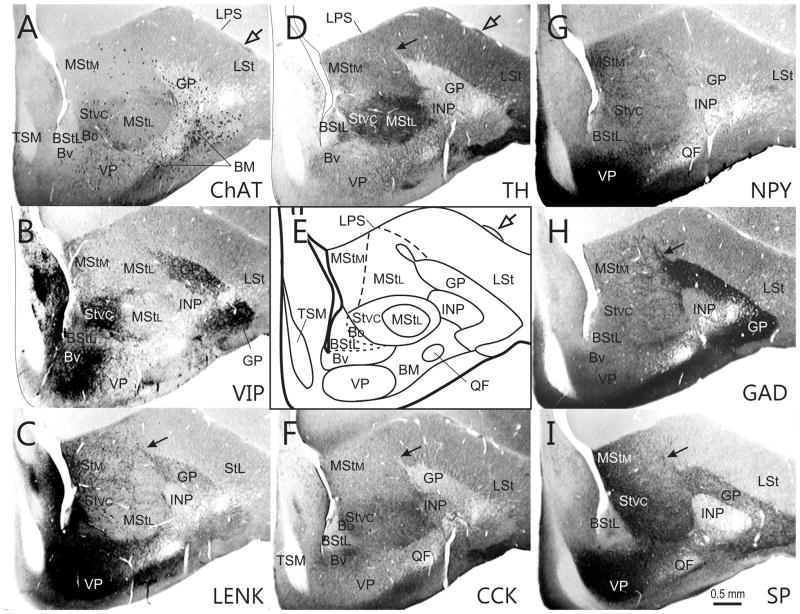
Series of transverse sections through the rostral basal ganglia at approximately A9.00, arranged as explained in Fig. 2. Open arrows - islands of cells in the LPS.
Some of the tissue used in this study was used for analyses of other brain systems in earlier reports (Erichsen et al., 1991; Krebs et al., 1991; Riters et al. 1999), and the immunohistochemical procedures we employed are described there in greater detail. For the immunohistochemical staining employed to generate the tissue illustrated in figures 2–8, the following standard procedure was used (see Table 1 for optimal primary antibody concentrations and antibody sources). All sections were incubated in a 1.5% solution of normal serum in 0.1 M PB with 0.3% Triton X-100 (Sigma Chemical, St. Louis, MO) (PBX) appropriate for the animal source of the primary antibody. Following a brief wash in PB as noted above, each series of sections was then incubated in a primary antibody diluted in PBX containing 1.5% normal serum for 65–89 hours at 4°C. Several washes preceded a 1 hour incubation in biotinylated IgG (Vector Labs, Burlingame, CA) (diluted 1:200 in PBX) directed against the species originating each of the primary antibodies used in the study. After another wash in PB, the sections were preincubated in ABC solution (1:50 dilution; Vector Labs) for one hour before being placed in a solution of DAB with H2O2 for an additional 15 min. Sections were then washed, mounted onto slides, osmicated and coverslipped for subsequent examination. For immunolabeling with CALB and PARV, the method described in Laverghetta et al. (2006) was used. For SP, ENK and VIP, colchicine-treated material that had been prepared for use in prior studies was also available (Anderson and Reiner, 1990a, b, 1991).
Table 1.
Antibodies Used in Immunohistochemistry1
| Antibody | Immunogen | Optimal [1°] | Source |
|---|---|---|---|
| Mouse anti-CALB | Bovine kidney calbindin-D-28K | 1:500 | C9848; Sigma-Aldrich, St. Louis, MO |
| Mouse anti-CART | Rat CART (54–102) conjugated to ovalbumin | 1:2000 | Dr. L. Thim, Novo Nordisk A/S, Bagsvaerd, Denmark |
| Rabbit anti-CCK | Sulfated CCK-8 (26–33) | 1:1000 | 20078; Incstar, Stillwater, MN |
| Rabbit anti-CR | recombinant human calretinin | 1:500 | 7699/3; Swant (Bellinzona, Switzerland); |
| Mouse anti-Leu-ENK | Leu5-enkephalin conjugated to bovine serum albumin | 1:1000 | MAS083; Sera-Lab; Crawley Down, UK; Cuello et al., 1984 |
| Sheep anti-GAD | Partially purified rat brain synaptosomes | 1:3000–4000 | 1440-4; National Institutes of Health; Oertel et al., 1981 |
| Rabbit anti-NPY | 36 amino acid sequence | 1:1,500 | RAS7172N; Peninsula Labs |
| Mouse anti-PARV | Purified frog muscle parvalbumin | 1:500–1:1000 | P3088; Sigma-Aldrich, St. Louis, MO; Heizmann et al., 1987 |
| Rat anti-SP | Carboxylic terminal fragment of human SP | 1:1000 | MAS035b; Sera-Lab, Crawley Down, Sussex, UK |
| Rabbit anti-TH | TH purified from bovine adrenal medulla | 1:1000–2000 | TE101; Eugene Tech, Allendale, NJ; Armstrong et al., 1981 |
| Rabbit anti-VIP | Carboxyl terminal 18–28 region of VIP | 1:1000 | 7916; Dr. J.H. Walsh, Univ. California Los Angeles |
For abbreviations, see text.
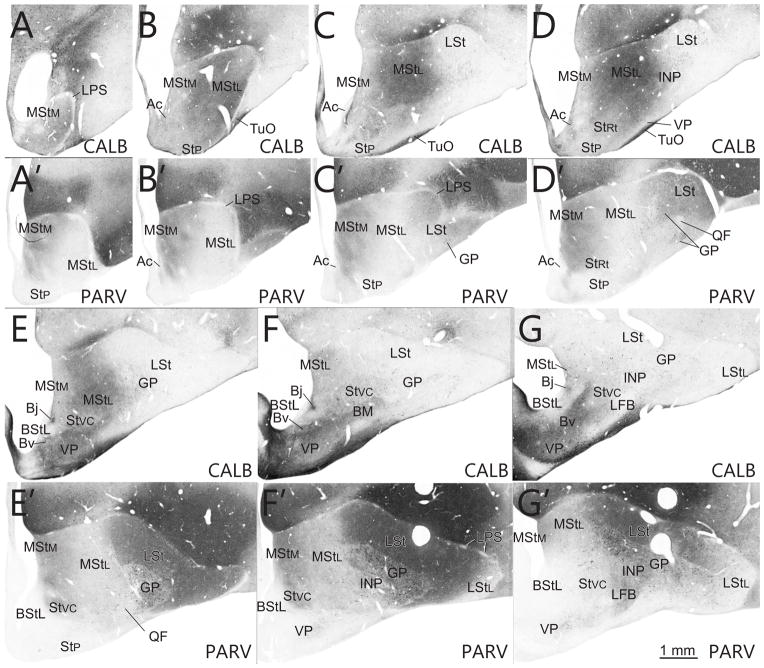
The distribution patterns of ChAT, VIP, ENK, TH, CCK, NPY, GAD, SP, CALB, PARV, CR, and CART within the lateral wall of the pigeon subpallium were analyzed in a parallel series of transverse sections through the forebrain (Figs. 2–10). Sections were imaged using a Nikon USB 5 megapixel CCD camera attached to a Nikon Optiphot microscope and connected to a High-End Desktop M55 computer. The gray scale and image contrast were adjusted, and figures labeled and formatted, using CorelDraw and Corel PhotoPaint 12. Six transverse planes through the striatum rostral to the anterior commissure were selected to illustrate the expression patterns within each neurochemical compartment using 8 stains (Fig. 1). The regional intensity of immunolabeling was densitometrically measured to quantitatively distinguish compartments (Table 2). The types of perikarya, and the dendrites and terminals that contained the markers were qualitatively analyzed to define the limits of the known subregions within the striatal and pallidal part of the subpallium, as well as to identify novel subdivisions. Immunolabeling of neuropil intensity was quantitatively measured in images prior to any contrast adjustments by recording intensity levels at three points within each compartment and at each level using the Corel Photo-Paint eyedropper tool (Table 2). Measurements were ranked at 4 levels: very intense or abundant immunolabeling, intense or abundant immunolabeling, moderately abundant or intense immunolabeling, and background or near background immunolabeling). Abundance of labeled perikarya was qualitatively rated separately (Table 3). The revised avian nomenclature of Reiner et al. (2004b) is used, but expanded upon where labeling patterns suggest there are previously unrecognized compartments.
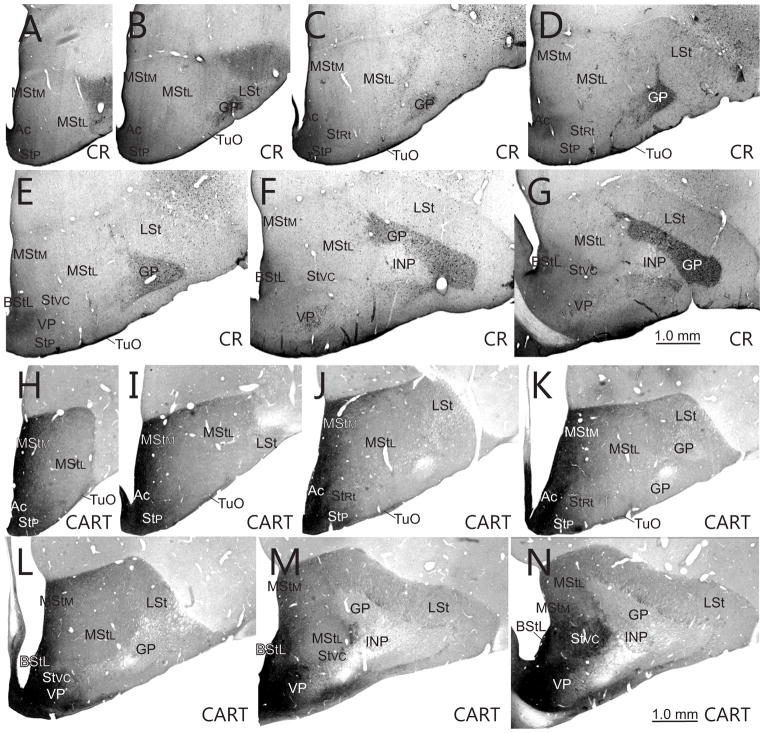
Fig. 10.
Transverse sections through the rostral striatum immunostaining patterns of CR (A–G) and CART (H–N). Note that the neuropil of nucleus accumbens stains with high levels of CR and CART, whereas the neuropil of the medial part of the paratubercular striatum stains with high levels of CART+ but low levels of CR.
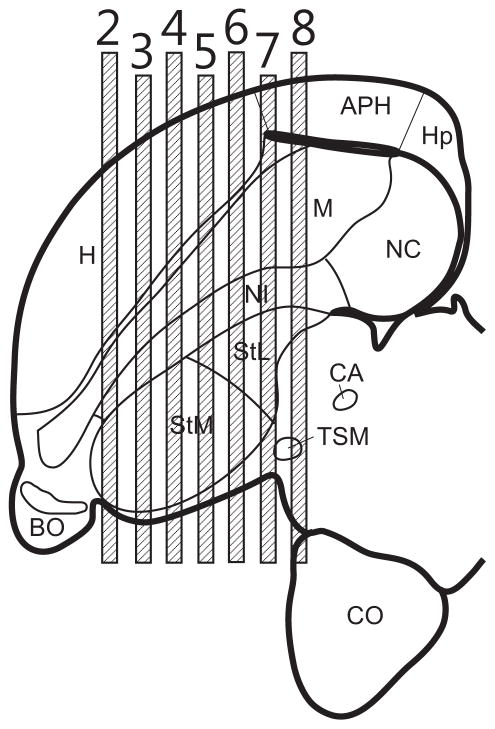
Fig. 1.
Lateral view of the forebrain of Columbia livia. Numbers on top refer to the figure number that illustrates transverse sections from that level.
Table 2.
Relative densities of neuropil immunostaining for markers examined in subregions of the subpallium
| Dorsal Subpallium | Ventral Subpallium | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MStM | MStL | LStM | LStL | LPS | GP | INP | Ac | StP | StR | StVC | VP | TuO | BSTL | BV | BD | |
| CALB | 2 | 2 | 1 | 1 | 2 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 |
| CART | 4 | 3 | 3 | 3 | 3 | 2 | 2 | 4 | 4-3 | 3 | 4 | 4 | 4 | 4 | 4 | 4 |
| CCK | 2 | 2 | 2 | 3 | 1 | 1 | 1 | 3 | 4 | 3 | 4 | 2 | 1 | 2 | 4 | 2 |
| ChAT | 1 | 2 | 2 | 2 | 3 | 1 | 2 | 2 | 3 | 3 | 3 | 2 | 2 | 2 | 1 | 1 |
| CR | 3 | 2 | 2 | 3 | 2 | 4 | 2 | 4 | 4 | 3 | 3 | 3 | 4 | 4 | 4 | 3 |
| GAD | 3 | 3 | 3 | 3 | 2 | 4 | 2 | 3 | 4 | 3 | 3 | 4 | 1 | 3 | 3 | 2 |
| LENK | 3 | 2 | 2 | 2 | 2 | 2 | 1 | 4 | 4 | 3 | 3 | 4 | 1 | 4 | 4 | 1 |
| NPY | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 4 | 3 | 3 | 3 | 4 | 1 | 2 | 3 | 1 |
| PARV | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| SP | 4 | 2 | 2 | 2 | 1 | 3 | 1 | 4 | 4 | 4 | 4 | 4 | 1 | 2 | 3 | 1 |
| TH | 2 | 3 | 3 | 4 | 1 | 1 | 2 | 2 | 4 | 3 | 4 | 3 | 1 | 2 | 1 | 1 |
| VIP | 1 | 1 | 2 | 3 | 1 | 3 | 1 | 4 | 4 | 4 | 3 | 3 | 3 | 3 | 4 | 2 |
Intensity of antibody expression: 1 – low; 2 – moderate; 3 – high; 4 – very high
Table 3.
Relative abundance of immunostained neurons for markers examined in subregions of the subpallium
| Dorsal Subpallium | Ventral Subpallium | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MStM | MStL | LStM | LStL | LPS | GP | INP | Ac | StP | StR | StVC | VP | TuO | BSTL | BV | BD | |
| CALB | 2 | 4 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 3 | 3 | 3 | 3 |
| CART | 3 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 2 | 1 | 1 | 0 | 2 | 1 | 0 |
| CCK | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ChAT | 1 | 2 | 1 | 1 | 1 | 3 | 4 | 2 | 2 | 2 | 3 | 4 | 3 | 3 | 3 | 3 |
| CR | 3 | 2 | 2 | 3 | 2 | 4 | 2 | 4 | 4 | 3 | 3 | 3 | 4 | 4 | 4 | 3 |
| GAD | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| LENK | 4 | 4 | 4 | 4 | 4 | 2 | 3 | 4 | 4 | 4 | 4 | 1 | 3 | 4 | 4 | 4 |
| NPY | 4 | 3 | 1 | 1 | 1 | 1 | 1 | 4 | 3 | 3 | 2 | 1 | 3 | 3 | 2 | 3 |
| PARV | 1 | 2 | 2 | 1 | 2 | 4 | 1 | 1 | 1 | 1 | 1 | 3 | 1 | 1 | 1 | 1 |
| SP | 4 | 4 | 4 | 4 | 4 | 2 | 4 | 4 | 4 | 4 | 4 | 1 | 3 | 1 | 2 | 2 |
| TH | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| VIP | 2 | 1 | 1 | 1 | 1 | 0 | 1 | 2 | 2 | 2 | 1 | 0 | 1 | 1 | 1 | 1 |
Abundance of labeled cells: 0 – none; 1 – negligible; 2 – few; 3 – some; 4 – many
2.1 Antibody characterization and specificity
A list of all antibodies used in the present study is shown in Table 1, including the immunogens and dilutions used and their sources. Each antibody produced characteristic patterns of immunostaining that were expected based on previous reports in mammals or birds.
Western blot studies of the monoclonal anti-calbindin-D-28K by the manufacturer showed a single band at 28 kD, and also showed that it does not react with other members of the EF-hand family. It recognizes calbindin, but not PARV or CR (Conde et al., 1994). Our data are consistent with a study that described the distribution pattern of CALB in the bed nucleus of stria terminalis and accumbens in the pigeon using another CALB antibody (Husband and Shimizu, 2012). In mammals calbindin immunostaining demarcates rostral associative and paralimbic subdivisions of the striatum (Morel, 2002), which it also does in the present study.
The polyclonal anti-calretinin antibody recognizes a 29 kDa protein in amphibians, lizards, and chicks (Hack et al., 2000; Morona and González, 2008; Yan et al., 2010) and has been widely used in studies of diverse vertebrate species. Within the rodent basal ganglia this antibody is a marker for terminals in the olfactory tubercle, medial parts of nucleus accumbens, as well as numerous small interneurons throughout the basal ganglia (Bubser et al., 2000). The present study focused on the pattern of CR terminal labeling in the basal ganglia, which we found is consistent with results in rodents.
The immunogen for the polyclonal anti-cholecystokinin octapeptide antibody is sulfated CCK-8 (26–33) coupled to bovine thyroglobulin (BTg) with glutaraldehyde. Immunostaining with this antibody is abolished by preadsorption with CCK-8. The antibody has been used studies of numerous vertebrate and invertebrate species. Although glutaraldehyde was used to produce the immunogen, this antibody works very well with paraformaldehyde-fixed tissues, as do other similarly produced antibodies against immunogens (e.g., Veenman and Reiner, 1996). This antibody is a marker for terminals in the ventral accumbens and the olfactory tubercle of rodents (Zaborszky et al., 1985; Zahm and Heimer, 1988), which is consistent with the labeling pattern seen in pigeons in the present study.
The monoclonal cocaine- and amphetamine-regulated transcript peptide antibody was generated against a rat CART (54–102) fragment (Thim et al., 1998). The specificity of this antibody has been demonstrated by omission of primary antibodies and by immunoblot analysis showed a single precipitin band that migrates at about 14 kD (Subhedar et al., 2011; Singru et al., 2007). Within the basal ganglia, CART is a marker for neurons in the medial part of the accumbens shell in diverse vertebrate species, (Smith et al., 1999; Lázár et al., 2004; Barsagade et al., 2011; Subhedar et al., 2011), which is consistent with the labeling pattern observed in the present study.
The anti-glutamic acid decarboxylase polyclonal antibody preferentially recognizes GAD65 and also binds to GAD67 (Oertel et al., 1981; Kaufman et al., 1991). It has been characterized by Western blot analyses using zebra finch cerebellum and forebrain, revealing bands at 61 and 59kD (Spiro et al., 1995). The rodent and pigeon basal ganglia contain numerous GAD-expressing neurons throughout, and fibers and terminals in pallidal areas stain particularly heavily with anti-GAD (Veenman et al., 1995; Sun et al. 2005), which is consistent with the labeling pattern in the present study.
The mouse anti-leu-enkephalin monoclonal antibody to leu-enkephalin has been previously characterized for specificity by immunodot-blotting and by specific adsorption (Cuello et al., 1984; Milner et al., 1989), and has been used extensively in a variety of vertebrate species. In the rodent and pigeon basal ganglia anti-leu-encephalin antibodies have been used as a marker of neurons located throughout the striatal part of the basal ganglia, as well as for terminals in pallidal territories, including woolly fibers in the external segment of globus pallidus and in the ventral pallidum (Reiner et al., 1984a; Anderson and Reiner, 1990a). The present study is confirms these labeling patterns. The pattern of neuronal labeling is also consistent with prior studies using in situ hybridization histochemistry (Molnar et al., 1994).
The rabbit polyclonal anti-neuropeptide Y antiserum was raised against a 36 amino acid sequence that cross reacts with human, rat, and porcine NPY, but not other closely related peptides (Kienzler et al., 2009). Within the mammalian basal ganglia, NPY is a marker for identifying the accumbens shell and ventral pallidum (Bálint and Csillag, 2007; Brauer et al., 2000), which we found useful in the present study.
The mouse parvalbumin monoclonal antibody reacts with parvalbumin (12 kDa) from a variety of mammals as well as frog and fish but does not react with closely related peptides of the EF-hand family such as calmodulin and intestinal calcium-binding protein. The specificity has been examined by the manufacturer and researchers (Heizmann and Celio, 1987; Sigma-Aldrich). It has been used previously in pigeons to describe the distribution of PARV neurons in the basal ganglia (Reiner and Anderson, 1993; Lavergetta et al., 2006), and the present results are consistent with their findings. Our data are qualitatively similar to the staining patterns shown in a previous study in in the pigeon using another PARV antibody (Husband and Shimizu, 2012). In mammals, parvalbumin neuropil immunostainings demarcates a caudolateral somatomotor subdivision of the striatum (Morel, 2002), which it also does in the present study.
lmmunohistochemical localization of substance P utilized a monoclonal substance P antibody (supplier: Sera-Lab, Crawley, England) raised in tissue culture from rat spleen hybridoma. The details of the production of this antibody and the specificity have been described elsewhere (Cuello et al., 1979). The specificity of the immunoreactivity for SP was assessed by the use of a blocked control in which the primary antibody was preabsorbed with synthetic SP (Reiner et al., 1983). In prior studies in mammals and birds, the striatum was found to contain numerous SP+ neurons and varying intensities of neuropil stain depending on location, whereas the neuropil of the globus pallidus and ventral pallidum are characterized by heavy SP staining but few SP+ neurons, consistent with the present results (Cuello et al., 1979; Reiner et al., 1983).
The specificity of the immunostaining produced with the rabbit tyrosine hydroxylase polyclonal antiserum is well established (Pickel et al., 1975; Hervonen et al., 1980; Armstrong et al., 1981). Tyrosine hydroxylase was partially purified from bovine adrenal medulla by precipitation with ammonium sulfate and column chromatography. The antibody specificity was based on immunoelectrophoresis of the antibody run against either partially purified tyrosine hydroxylase from bovine or rat adrenal medulla which yielded a single precipitin band. No precipitin bands formed when the antibody was run against other catecholamine-synthesizing enzymes, including dopa-decarboxylase, dopamine beta-hydroxylase, or phenylethanolamine N-methyltransferase (Pickel et al., 1975). TH is a marker for dopaminergic and adrenergic neurons and fibers. For example, TH immunostaining detects dopaminergic neurons of the substantia nigra pars compacta and their terminals in the striatum. In the present study, TH immunostaining of dopaminergic terminals was used to delineate pigeon striatal subterritories.
The rabbit polyclonal antiserum to vasoactive intestinal polypeptide was generously provided by Dr. J. Walsh (UCLA) and has been widely used in numerous species. The VIP antiserum is specific for the carboxyl terminal 18–28 region of VIP. Tests for specificity showed negligible reactivity with glucagon, secretin, and gastric inhibitory polypeptide (Furness et al., 1981). VIP is widespread in the brain, and we used it as a marker to delineate striatal and pallidal sub-territories.
3. Results
The avian subpallium is traditionally divided into dorsal and ventral subdivisions, each of which contains striatal and pallidal territories (Reiner et al., 1994). The dorsal and ventral subpallia are associated with somatic and limbic functions of the basal ganglia, respectively. The divide between dorsal and ventral subpallial regions can be established most notably by the rich dopamine beta-hydroxylase (DBH) neuropil in the ventral subpallial striatal areas, whereas the dorsal subpallium is DBH poor (Reiner et al., 1994). In addition, we have found that a subset of regions within the DBH-poor dorsal subpallium is rich in parvalbumin, whereas the DBH-rich ventral subpallial regions, including the bed nucleus of the stria terminalis, are the opposite. This rationale for the dorsal-ventral somatic/limbic distinction in birds does not appear to apply for distinguishing mammalian dorsal and ventral subpallial regions. The differential expression patterns of Islet1, cPax6, cLmo4, and cLmo3 are consistent with this dorsal-ventral somatic/limbic distinction in birds (Abellán and Medina, 2009), and will be considered further in the Discussion. Note that we focus on subpallial territories of the lateral telencephalic wall anterior to the anterior commissure, and do not include amygdalar or septal subpallial territories.
3.1. Dorsal Subpallium
Based on their neurochemistry, the medial and lateral striatum are each further divided into medial and lateral zones. The nucleus intrapeduncularis is included here as a striatal part of the dorsal subpallium, although this assignment is not unambiguous. Finally, we also recognize distinct striatal cellular islets in the boundary territory between pallium and subpallium. Only one large pallidal territory is recognized in the dorsal subpallium, namely the globus pallidus.
3.1.1. Medial part of the medial striatum
Neurons
As is true of dorsal striatum in general, the medial MSt contains medium spiny neurons expressing ENK, SP, and low levels of GAD immunolabeling (Fig. 11B). The sparse GAD perikaryal immunolabeling is, however, deceptive, because in situ hybridization for GAD65 and immunolabeling for GABA shows this region to be rich in GAD+/GABA+ perikarya (Veenman et al., 1995; Sun et al. 2005). Scattered ChAT+ neurons are present, although less frequent than in the more lateral parts of MSt. NPY+ and CART+ neurons are sparsely scattered throughout the medial MSt (Fig. 11A). Some VIP+ perikarya are observed in colchicine-treated material. This area has fewer CALB+ neurons than the adjacent lateral part of MSt. PARV+ neurons are also sparse in the medial MSt.
Fig. 11.
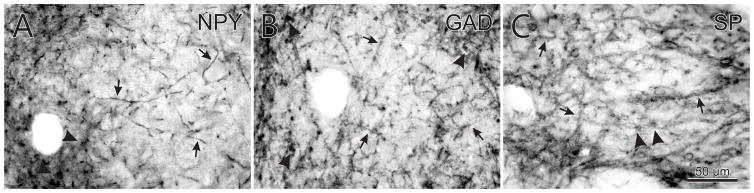
Images of woolly fibers stained with NPY (A), GAD (B), and SP (C) in the medial MSt (A, B) and GP (C). Note that NPY+ and GAD+ fibers (arrows), terminals, and cells (arrowheads) are evident in the medial MST (A, B) and SP+ fibers (arrows) and cells (arrowheads) are evident in the globus pallidus (C).
Neuropil
The neuropil of the medialmost part of the MSt expresses very high levels of SP, high levels of GAD and ENK, moderate levels of TH, CCK, NPY, CALB and PARV, and low levels of ChAT and VIP (Figs. 2–8). The medialmost MSt contains subtle heterogeneously distributed areas of slightly lighter and darker PARV expression, particularly near the transition with the lateralmost MSt (Fig. 9A′–D′). The low TH and CALB and higher PARV expression distinguishes the medialmost MSt, and show that it extends caudally to the level at which the tractus septopallio-mesencephalicus (TSM) reaches the basal telencephalon. Within the neuropil of medialmost MSt are “woolly fibers”, which are tight rows of terminals presumed to be from the medium spiny neurons in the striatum that are rich in SP, ENK, NPY and GAD, and that synapse along unlabeled dendrites (Fig. 11A, B). Based on prior findings for the globus pallidus (Reiner and Caraway, 1987), these woolly fiber terminals are likely to end on long, smooth dendrites of neurons that lightly express LANT6 and/or PARV. Whether these striatal neurons are interneurons or projection neurons is unknown, although PARV+/LANT6+ expressing pallidal projection neurons, but not interneurons, receive woolly fiber inputs (Reiner et al., 2004a, b).
Fig. 9.
Series of transverse sections through the rostral basal ganglia comparing immunostaining patterns of CALB (A–G) and PARV (A′–G′). Images from the 7 levels shown in Fig. 1 are arranged from rostral (A, A′) to caudal (G, G′).
3.1.2. Lateral part of the medial striatum
Neurons
Neurons containing ChAT are more plentiful in the lateral MSt than in more medial or lateral striatal compartments. The lateral MSt, like the medial MSt, is rich in medium-sized spiny projection neurons containing either SP or ENK, as evident in colchicine-treated material. Scattered NPY+ neurons were also present throughout lateral MSt. Neurons immunolabeled for GAD are scarce in the lateral MSt, although GABA immunolabeling and GAD65 in situ hybridization studies have shown that GAD-synthesizing perikarya are in fact abundant throughout both medial and lateral MSt (Veenman and Reiner, 1994; Sun et al., 2005). A few neurons immunolabeled for VIP are observed in colchicine-treated cases. Numerous PARV+ and CALB+ neurons are also present, in contrast with the medial MSt.
Neuropil
The striatal neuropil contains high levels of TH and GAD, moderate levels of ChAT, ENK, CCK, NPY, SP, and CALB, and low levels of VIP and PARV. The neuropil of the lateralmost MSt is distinguished from the more medial MSt by its higher expression of TH, ChAT and CALB and lower expression of PARV. The lateralmost MSt extends medially to the ventricle at its more caudal levels, that is from the level at which the TSM reaches the basal telencephalon to the anterior commissure (Figs. 8, 9F, G, G′). The lateralmost medial striatum contains some woolly fibers, which are strongly labeled for SP, NPY, and ENK, and which emanate as finger-like extensions from globus pallidus into the lateral MSt. Woolly fibers are, nonetheless, less abundant in the lateral than medial MSt. VIP and CCK are sparse in the neuropil of both the medial and lateral MSt.
3.1.3. Medial part of lateral striatum
Neurons
Like other striatal territories, the medial LSt is rich in medium-sized spiny projection neurons that contain either SP or ENK. The medial LSt is very poor in both ChAT and NPY neurons, in contrast to the MSt. VIP+ neurons were rarely observed, even in colchicine-treated tissue. The medial LSt contains numerous PARV+ neurons but few CALB+ neurons. Large GAD+ neurons resembling the PARV+ neurons in size and frequency are present, consistent with previous studies showing that GAD and PARV are co-expressed in the same neurons of LSt (Reiner and Anderson, 1993).
Neuropil
The medial part of the LSt contains high levels of TH, GAD, and PARV, moderate levels of ChAT, VIP, ENK, CCK, NPY, and SP, and low levels of CALB and (Figs. 2–8; Table 2). The medial part of the LSt extends medially to the ventricle at levels near the anterior commissure. Woolly fibers are rare, except along the border with the globus pallidus (GP), where they extend from the GP into the striatum. Except when present in woolly fibers, NPY, GAD, CCK, and VIP are expressed fairly evenly throughout the LSt neuropil. TH is present in a graded pattern that is lowest in the medial most MSt, higher in the lateralmost MSt, slightly lower in the medialmost LSt, and highest in the lateralmost LSt (Figs. 2–8). CALB is found in higher levels in the lateral part of the medial striatum than in adjacent striatal territories, whereas PARV and CART are less abundant in the lateral part of the medial striatum than in adjacent striatal territories (Figs. 9, 10).
3.1.4. Lateral part of lateral striatum
Neurons
The lateral LSt is very similar to the medial LSt. It is rich in SP+ and ENK+ medium-sized spiny projection neurons, but is very poor in ChAT+, NPY+, VIP+, and CALB+ neurons. There are few CALB+ neurons. Numerous large PARV+ and GAD+ neurons likely represent the same neuronal population (Reiner and Anderson, 1993).
Neuropil
The neuropil in the lateralmost part of the LSt contains very high levels of TH, high levels of VIP, CCK, and GAD, moderate levels of ChAT, ENK, NPY, and SP, and low levels of CALB and PARV. The lateralmost part of the LSt along the lateral margins of the globus pallidus is a neurochemically unique area, distinguished by its much higher expressions of VIP, TH, and CCK in its neuropil (Figs. 6–7), suggesting that this area may have a specialized function and thus differ from the remainder of lateral LSt. Woolly fibers are rare, except along the border with the globus pallidus (GP), where they extend from the GP into the striatum. Except when present in woolly fibers, NPY, GAD, CCK, and VIP are expressed fairly evenly throughout the lateral LSt neuropil. The lateral LSt can be readily distinguished from the medial LSt by the paucity of PARV in its neuropil. Of the markers examined in this study, the localization of TH and the calcium binding proteins provides the best means to distinguish the different striatal areas (Figs. 2–8). In the case of the lateral striatum, CALB is found in higher levels in the neuropil of the lateral striatum than in the medial striatum, whereas PARV is less abundant in the neuropil of the lateral part of the lateral striatum than in the medial parts of the lateral striatum (Fig. 9).
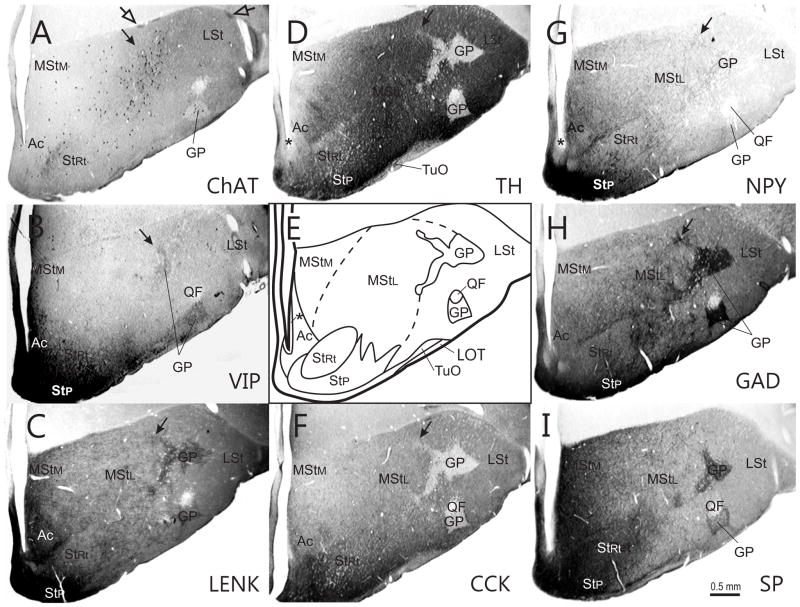
Fig. 6.
Series of transverse sections through the rostral basal ganglia at approximately A10.00, arranged as explained in Fig. 2. Closed arrows - finger-like extensions of the GP into the medial striatum; open arrows - islands of cells in the LPS.
Fig. 7.
Series of transverse sections through the rostral basal ganglia at approximately A9.50, arranged as explained in Fig. 2. Closed arrows - finger-like extensions of the GP into the medial striatum; open arrows - islands of cells in the LPS.
3.1.4. Intrapeduncular Nucleus (INP)
Neurons
The INP is located along the ventromedial border of globus pallidus and lateral to the MSt (Figs. 7–8). The neuronal profile of INP is largely striatal. For example, many medium-sized SP+ and ENK+ neurons are present in colchicine-treated material in INP, resembling those seen in MSt and LSt. Prior in situ hybridization studies confirm that the INP contains many SP+ neurons (Abellán and Medina, 2009) and scattered ENK+ neurons (Molnar et al., 1994), as well as medium-sized GAD+ neurons (Sun et al., 2005). NPY+ and CALB+ neurons are sparse in the INP, as they are in most other striatal areas. On the other hand, ChAT+ neurons are much more plentiful in the INP than in the medial or lateral striatum, whereas PARV+ neurons are scarcer.
Neuropil
The neuropil of INP contains moderate levels of ChAT, TH, GAD, and PARV, and low levels of VIP, ENK, CCK, NPY, SP, and CALB. The neuropil of the INP can be distinguished from that of the globus pallidus by the absence of woolly fibers that contain GAD, ENK and/or SP, and a more intensely PARV+ neuropil, whereas the GP neuropil is rich in woolly fibers that contain GAD, ENK and/or SP. The neuropil of INP, in general, resembles that of the striatum. The INP contains many ChAT+ fibers, likely reflecting its enrichment with cholinergic neurons.
3.1.5. Cell islands within the lamina pallio-subpallialis
Neurons
The dorsal and lateral edge of the striatum is formed by a band of fibers, the lamina pallio-subpallialis. Tightly clustered neurons form compact islands within it, which are particularly large and prominent rostrally (Figs. 2–3), but smaller more caudally (Figs. 4–8). Abellán and Medina have identified these islands in embryonic chick and termed them the striatal capsule. Neurons within these islands appear to contain medium-sized striatal projection neuron markers, notably SP+ and ENK+. According to Abellán and Medina (2009), these cells also express the striatal gene cLmo4 and GAD67 (their fig. 13D–F). These neurons are enriched in DARPP32+ as well (Reiner et al., 1998b).
Fig. 3.
Series of transverse sections through the rostral basal ganglia at approximately A12.50, arranged as explained in Fig. 2. Open arrows - islands of cells in the lamina pallio-subpallialis.
Fig. 4.
Series of transverse sections through the rostral basal ganglia at approximately A11.75, arranged as explained in Fig. 2. Asterisk - thin rostral extension of the BStL; closed arrows - finger-like extensions of the GP into the medial striatum; open arrows - islands of cells within the LPS.
Neuropil
The neuropil of the islands of the striatal capsule contain high levels of ChAT, moderate levels of ENK, TH, CALB and PARV, and low levels of VIP, GAD, CCK, NPY, and SP. The islands are most easily identified by their ChAT immunostaining because the adjacent striatum stains less intensely, whereas immunostaining for other markers is either absent or similar to that in the adjacent striatum.
3.1.6 Globus Pallidus
Neurons
The globus pallidus appears rostrally at approximately A11.75 as small finger-like extensions into rostral striatum (Figs. 4–5) arising from the main body of the more caudal pallidal zone (Figs. 6–8). The large GABAergic pallidal neurons are enriched in PARV, and so PARV+ neurons are plentiful in globus pallidus (Laverghetta et al., 2006). ChAT-immunolabeled neurons are relatively plentiful within the globus pallidus as well, although less numerous than in the INP. Scattered spiny SP+ and ENK+ neurons are also seen in globus pallidus (Fig. 11C), as noted previously (Reiner et al., 1983; Molnar et al., 1994), but NPY+, CCK+, and CALB+ neurons are largely absent.
Fig. 5.
Series of transverse sections through the rostral basal ganglia at approximately A10.75, arranged as explained in Fig. 2. Asterisk - thin rostral extension of the BStL; closed arrows - finger-like extensions of the GP into the medial striatum; open arrows - islands of cells within the LPS.
Neuropil
Woolly fibers dominate the neuropil of the globus pallidus and contain very high levels of GAD, high levels of ENK and SP, and moderate levels of VIP (Fig. 11C). The woolly fibers arise from medium spiny neurons in the LSt, which are likewise rich in GAD, SP, and ENK, and the terminals of LSt neurons form rows of synapses along the sides of the dendrites of globus pallidus neurons. Since the globus pallidus also contains some VIP+ woolly fibers, it seems likely that some LSt medium spiny neurons do synthesize VIP, although VIP+ neurons were not detected in it by immunolabeling. The globus pallidus contains few fibers labeled for ChAT, TH, CCK, NPY, CALB or PARV, with the paucity of TH typical of a pallidal zone.
3.2. Ventral Subpallium
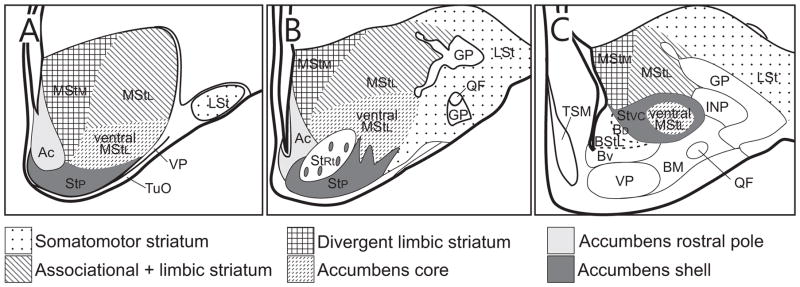
The ventral subpallium is divided into striatal, pallidal, and mixed populations as determined by their neurochemical patterns (Abellán and Medina, 2009). Based on our findings and prior studies, we identify the following four striatal regions within the ventral subpallium: (1) nucleus accumbens, defined by Reiner et al. (2004b) as a ventral part of rostral striatum, nearest the ventricle; (2) an area lateral to nucleus accumbens that we here refer to as reticular ventral striatum; (3) a region deep to the olfactory tubercle but external to the reticular ventral striatum, which we term the paratubercular striatum; (4) a region that extends dorsal and caudal to the reticular striatum and ventral pallidum that we call the ventrocaudal striatum (Stvc). Our paratubercular striatum and Stvc corresponds to the “accumbens shell” of chicken embryos identified by Abellán and Medina (2009). A single pallidal territory of the ventral subpallium, the ventral pallidum, is considered pallidal based on the presence of woolly fibers, namely closely spaced parallel rows of synaptic terminals that immunostain strongly for DARPP32, SP, ENK, CALB or neurotensin (Reiner and Carraway, 1987; Reiner et al., 1998b). Finally, several subpallial regions have a mixture of striatal and pallidal characteristics: (1) the lateral bed nucleus of stria terminals (BStL), which is adjacent to the tip of the lateral ventricle and extends as a thin sheet rostrally along the ventricle at the medial edge of nucleus accumbens; (2) the juxtacapsular (Bjx) subdivision, which forms the lateral border of the BStL (N.B. earlier avian studies often called the BStL and Bjx the ‘nucleus accumbens’) (Reiner et al., 2004b); and (3) the olfactory tubercle, which occupies the superficial surface of the lateral subpallium and receives olfactory input (Abellán and Medina, 2009).
3.2.1. Nucleus accumbens
Neurons
Nucleus accumbens, as defined in Reiner et al. (2004b) is rich in SP+ and ENK+ neurons, as well as GABAergic neurons (Veenman and Reiner, 1994; Sun et al., 2005), as typical of striatal domains. ChAT+, VIP+, NPY+, CALB+, and CART+ neurons also are scattered throughout.
Neuropil
The neuropil of nucleus accumbens is heterogeneous, and largely characterized by very high levels of VIP, ENK, NPY, and SP, high levels of CCK and GAD, moderate levels of ChAT, TH, and CALB, and low levels of PARV (Figs. 2–5). The neurochemical characteristics of its neuropil are particularly useful for defining nucleus accumbens. For example, the rich levels of SP immunoreactivity distinguish it from adjacent dorsal striatal regions, except the medialmost MSt, which also has high SP neuropil levels. In contrast, TH is less abundant in the accumbens compared to the dorsal striatal regions, except for the medialmost MSt. The accumbens neuropil is richer in VIP, ENK, CCK, and NPY than other parts of the striatum. The VIP fibers, in particular, differentially delineate accumbens from striatal territory above and lateral to it. The NPY and ENK fibers tend to be distributed in patches of high and low abundance, not seen with other markers (Figs. 5 C, G). Caudally, the accumbens is contiguous with the rostral BStL (Fig. 5), but can be easily distinguished by the much higher levels of SP in accumbens compared to the BStL (Figs. 2–5). In addition, nucleus accumbens is one of the few striatal areas with high levels of CR and CART in the neuropil (Fig. 10).
3.2.2. Paratubercular striatum
Overview
The paratubercular striatum (Stp) is located superficial (i.e. ventral or external) to the accumbens and reticular striatum, but deep (i.e. ventral or internal) to the thin, rostral extension of the ventral pallidum and to the olfactory tubercle, which itself is the thin olfactory-recipient zone along the ventral surface of the subpallium. Its neuropil exhibits a unique pattern because it immunostains intensely for most markers in contrast to other striatal regions, as described below, which is the major reason for recognizing it as a distinct territory (Figs. 2–6). However, it is difficult to distinguish it from the olfactory tubercle, as both have similar staining characteristics and some neurons of Stp may receive olfactory bulb input, like the olfactory tubercle, via dendrites that extend into the olfactory terminal zone of olfactory tubercle. Nonetheless, olfactory terminals do not end in the Stp (Reiner and Karten, 1985; Atoji and Wild, 2014), and for this reason, we do not include it as part of the olfactory tubercle. The Stp expands dorsolaterally towards the MSt at the level of the reticular striatum (see below) just anterior to the rostral pole of the ventral pallidum (Fig. 5). Around the lateral aspect of VP, it blends with the Stvc (Fig. 6).
Neurons
The Stp contains SP+ and ENK+ neurons, but only scattered ChAT+, NPY+, and PARV+ neurons. VIP+ neurons were rarely seen. CART neurons are also present within the Stp (Fig. 10), which differentiates the Stp from most other striatal areas.
Neuropil
The Stp neuropil contains very high levels of VIP, ENK, TH, CCK, GAD, and SP, high levels of ChAT and NPY, moderate levels of CALB, and low levels of PARV (Figs. 2–9). Woolly fibers rich in VIP, ENK, GAD, and SP, and non-woolly fibers rich in TH and CCK are more abundant rostromedially than caudolaterally. The medial Stp neuropil also displays high levels of CART (Fig. 10H–I).
3.2.3. Reticular ventral striatum
Overview
The reticular part of the ventral striatum (StR) lies lateral to nucleus accumbens of Reiner et al. (2004b) and anterior to the rostral pole of the ventral pallidum (Fig. 5). It is better distinguished by its neuropil traits than by its constituent neuronal populations, as further detailed below. In particular, it is characterized by and named for the reticulated appearance imparted by the numerous fiber bundles that traverse this region, as most evident in the TH and CCK immunolabeled tissue.
Neurons
As a striatal region, it contains neurons rich in ENK or SP, which probably co-contain GABA (Reiner and Anderson, 1993). ChAT+ neurons are scattered throughout, in a somewhat greater abundance than in nucleus accumbens. Scattered NPY+ and CALB+ neurons are also present.
Neuropil
The reticular part of the ventral striatum contains very high levels of VIP and SP, high levels of ChAT, ENK, TH, CCK, NPY and GAD, but moderate levels of CALB and low levels of PARV. The high SP and VIP combined with low GAD and NPY distributions especially define this region. It exhibits a unique combination of features that distinguish it from the accumbens and the overlying MSt. First, compared to the adjacent accumbens the StR neuropil is richer in ChAT and TH, but poorer in ENK and NPY. Second, compared to the medialmost MSt, the neuropil is much richer in VIP, TH, CCK, and GAD, but poorer in PARV. Third, it is poor in ENK and NPY woolly fibers, and the more numerous ENK and NPY woolly fibers of the MSt form a clear border with the StR. Finally, the abundance of neuropil zones with lower levels of ENK, TH, CCK, NPY, GAD, and PARV, interspersed with zones displaying higher levels, distinguish it from the olfactory tubercle and paratubercular striatum below (Figs. 2–6). The pattern of TH and CCK immunolabeled fibers also characterizes this reticular region, with small zones of richly labeled neuropil interdigitating with small, poorly labeled zones, in contrast to the uniform neuropil labeling for TH and CCK throughout the dorsal striatum.
3.2.4. Ventrocaudal part of the striatum
Overview
The ventrocaudal part of the striatum (Stvc) appears rostrally just above the reticular striatum and extends caudally above the ventral pallidum and medial to the globus pallidus, forming a cup-shaped zone at the caudoventral pole of the lateral MSt (Figs. 7–8). It is usually included in the lateral part of the MSt because of its location just below it. Abellán and Medina (2009), however, considered this region as the caudal continuation of the paratubercular striatum, and the similar immunohistochemical labeling patterns we observed are consistent with their grouping. Although the ventrocaudal region and the MSt have some neuronal and neuropil characteristics in common, many other features readily distinguish the two regions, suggesting it is distinct from the MSt.
Neurons
The ventrocaudal part of MSt, like the other parts of MSt, is rich in medium-sized spiny projection neurons containing either SP or ENK, as evident in colchicine-treated material. Scattered NPY+ neurons are present, but there are few PARV+ and CALB+ neurons, similar to the medial MSt but in contrast to the lateral MSt. Neurons immunolabeled for GAD are scarce, but GABA immunolabeling and GAD65 in situ hybridization studies have shown that GAD-synthesizing perikarya are abundant in this area (Veenman and Reiner, 1994; Sun et al., 2005). Rare neurons immunolabeled for VIP are observed in colchicine-treated cases. The Stvc has scattered ChAT+ neurons, which are more abundant than in the adjacent lateral part of MSt, but far less abundant than in the adjacent globus pallidus or INP.
Neuropil
The neuropil of the ventrocaudal part of the striatum contains very high levels of TH and CCK, high levels of ChAT, VIP, ENK, NPY and GAD, moderate levels of CALB, and low levels of PARV. It is highly enriched in SP and ChAT. A number of these features distinguish the ventrocaudal striatum from the MSt. Notably, woolly fibers enriched with SP, ENK and/or VIP are more numerous in the Stvc. GAD woolly fibers, however, are less intensely labeled in the Stvc than in the MSt. The non-woolly part of the neuropil contains more ChAT, VIP, TH, and NPY compared to the MSt. Unlike the medial part of the MSt, but like the lateral part of the MSt, the neuropil of the ventrocaudal striatal region is rich in TH but poor in PARV. At its more rostral levels (Figs. 6, 7), it has especially high levels of TH+ and CCK+ fibers and terminals. The enrichment in CALB is similar to the lateral MSt. The similar distributions of TH and CCK in terminals in the paratubercular, reticulated, and ventrocaudal striatal regions are consistent with the possibility of their co-localization in the terminals of dopaminergic midbrain neurons in these regions. CCK has been colocalized to dopaminergic terminals in mammals (Seroogy et al., 1988).
3.2.5. Ventral Pallidum
Neurons
The ventral pallidum (VP) appears rostrally as a very thin band between the striatum and olfactory tubercle, and is best distinguished by its very low TH expression compared to these adjacent regions (Figs. 5D–7D). At approximately A10.75 in the Karten and Hodos atlas (1967), the VP expands dorsally, and occupies an oval zone ventrolateral to the bed nucleus of the stria terminalis (Fig. 6). It is rich in large aspiny GABAergic and PARV+ neurons, which are likely to be co-labeled projection neurons. Very few neurons with medium spiny projection neuron markers such as SP, ENK, and CALB are present, although ChAT+ neurons are scattered throughout the VP.
Neuropil
The neuropil of the VP contains very high levels of ENK, NPY, GAD, and SP, high levels of VIP, moderate levels of ChAT, CCK, and CALB, and low levels of PARV. The SP, ENK, GAD and NPY immunolabeling take the form of woolly fibers, which occur throughout the ventral pallidum, whereas VIP+ woolly fibers are only seen in the medial VP. ChAT, TH, and CCK immunolabeling are conspicuously poorer in VP than in adjacent regions, and thereby serve to delineate it. The complementarity between the few TH+ or CCK+ fibers, and the many SP+, ENK+, and NPY+ woolly fibers is especially striking.
3.2.6. Olfactory Tubercle
Overview
The olfactory tubercle is defined here as the thin subpallial region at the base of the telencephalon that receives olfactory bulb input (Figs. 2–6).
Neurons
The rostral and ventral olfactory tubercle contain neurons that express striatal markers, including ENK and SP. GAD+ neurons are also present in the rostral and ventromedial olfactory tubercle as expected for a striatal region. ChAT+, NPY+, VIP+, CALB+ and PARV+ neurons are scarce in this territory.
Neuropil
Like other striatal regions, the neuropil of the rostral ventral olfactory tubercle is rich in ENK, TH, CCK, NPY, GAD, SP, and CALB, moderate in VIP, and poor in PARV. More caudally and laterally, the olfactory tubercle has some pallidal characteristics, since it is poor in TH.
3.2.7. Lateral part of the Bed Nucleus of Stria Terminalis
Overview
The lateral bed nucleus of the stria terminalis (BStL) is largest at levels caudal to the nucleus accumbens (Figs. 6–8). It extends rostrally as a thin sheet along the ventricular edge of the accumbens, and can be distinguished from the accumbens by its neurochemical traits (Figs. 4–5, asterisk), as described below.
Neurons
The BStL is characterized by the presence of many ENK+ neurons, which are also known to be GABAergic, whereas neurons containing ChAT, NPY, SP, CALB, and PARV are scarce. By contrast, both SP+ and ENK+ neurons are abundant in nucleus accumbens.
Neuropil
Most of the BStL neuropil is rich in ENK but poor by comparison in VIP, although it contains smaller patches that are poor in ENK and rich in VIP. Other markers are distributed more homogeneously, and the BSTL is characterized by moderate levels of GAD and NPY, and only very low levels of ChAT, TH, CCK, and SP. The neurochemistry of the neuropil of BStL is distinctive, since it is conspicuously poor in SP, ChAT, CCK, and TH compared to the surrounding striatum, although a TH+ neuropil zone is present at caudal levels (Figs. 7D, 8D). The neuropil of the BStL and striatum express similar levels of GAD and NPY. Our results are consistent with a previous suggestion (Abellán and Medina, 2009) that the BStL has both pallidal and striatal traits, since we observed the pallidal traits of low TH and CCK, and the striatal trait of many ENK+ neurons. The BStL pallidal traits stem from its development from the pallidal sector of the lateral ventricle where it is located, and striatal neurons such as those containing ENK migrate into the pallidal territory (Abellán and Medina, 2009). The BStL expresses very low levels of CALB and PARV, in agreement with the description of Husband and Shimizu (2011).
3.2.8. External parts of the Lateral Bed Nucleus of Stria Terminalis
Overview
The BStL is bordered at its dorsal and ventral edges by an external zone, with the external zone border being located ventrally by the VP and dorsally by the MSt. We identify this region because it differs from the BSTL itself in its neurochemistry, but also appears distinct from the adjacent MSt and VP. Husband and Shimizu (2011) subdivided this external region into dorsal and ventral parts.
Neurons
The external region of the BStL is richer than the BStL in ChAT+ neurons, but poorer in ENK+ neurons. Neurons containing NPY and PARV are also scarce, although CALB+ neurons are scattered within it, as previously noted by Husband and Shimizu (2011).
Neuropil
The neuropil of the dorsal part of the external region is rich in ENK and VIP, moderate in ChAT, TH, CCK, and SP, but is poor in NPY and PARV and contains patches of CALB. The enrichment of the neuropil of the ventral part of the external region in CCK and VIP readily distinguishes it from the lighter immunostaining for these in the adjacent BSTL, ventral pallidum, and septum. ChAT, TH, GAD, ENK, and SP do not differentiate these two BStL regions. However, CALB expression is distributed more evenly in the ventral than in the dorsal part.
4. Discussion
Previous studies of the neurochemistry of the avian basal ganglia identified the fundamental regions that are similar to the mammalian striatum, accumbens, globus pallidus, and ventral pallidum (Reiner et al., 1998; Mezey and Csillag, 2002; Reiner et al., 2004b; Balint and Csillag, 2007). The present neurochemical analysis, together with previous neurochemical and connectional studies of the basal ganglia, confirms these fundamental observations, and furthermore identifies several additional compartments and their boundaries. These data lead us to consider several possible previously unrecognized homologies between the avian and mammalian basal ganglia, as well as note that some may be unique to birds or, more broadly, to birds and reptiles.
4.1. Medial and Lateral Striatum
The dorsal striatum of birds comprises the dorsalmost part of most of the lateral subpallium, and is composed of the medial and lateral striatum, formerly known as the lobus parolfactorius and paleostriatum augmentatum, respectively (Karten and Hodos, 1967; Reiner et al., 2002, 2004b). Our results illustrate striking differences in immunolabeling intensities between the medial and lateral striatum, which confirms and extends the differences noted by many other investigators (Reiner et al., 1983; Wynne and Gunturkun, 1995; Gallatioto et al., 1998; Ballint and Csillag, 2007). The histochemical differences may reflect functional differences, as for example reflected in connectional differences between the medial and lateral striatum. The avian medial striatum is connected with areas often characterized as limbic (Yamamoto and Reiner, 2005). For example, it receives its principal inputs from the prehippocampal area, olfactory cortex, caudolateral nidopallium, and posterior amygdala, and projects to the dopaminergic substantial nigra (Karten and Dubbeldam, 1973; Brauth et al., 1978; Reiner et al., 1983; Bottjer et al., 1989; Veenman et al., 1995). The lateral striatum receives inputs predominantly related to sensory and motor processing, including the hyperpallium (visual and somatosensory cortical-like areas), and lateral nidopallium (Veenman et al., 1995). The lateral striatum projects primarily to the globus pallidus, whereas the medial striatum projects primarily to the substantia nigra pars compacta and ventral tegmental area (Karten and Dubbeldam, 1973; Brauth et al., 1978; Lewis et al., 1981; Reiner et al., 1983, 1998a; Bottjer et al., 1989; Bottjer, 1993; Castro and Ball, 1994; Grisham and Arnold, 1994; Reiner et al., 1994; Medina and Reiner, 1995; Soha et al., 1996; Luo and Perkel, 1999; Sun and Reiner, 2000). As detailed below, however, our results support division of medial striatum into neurochemically distinct medial and lateral zones, as suggested by Abellán and Medina (2009), and that the lateral striatum of birds is also divided into neurochemically distinct medial and lateral zones.
Most striatal regions are enriched with dopaminergic terminals, identifiable by intense granular TH immunolabeling, and a high density of medium-sized GABAergic neurons with spiny dendrites containing SP, ENK, GAD and dopamine-regulated neuronal phosphoprotein (DARPP32) (Reiner et al., 1983; Anderson and Reiner 1990a, b; Reiner et al., 1994; Veenman and Reiner, 1994). Medium-sized spiny projection neuronal perikarya containing either SP or ENK are evident in colchicine-treated material (Anderson and Reiner, 1990a, b). Although all medium-sized spiny neurons are thought to be GABAergic, their perikarya do not immunolabel well for either GAD or GABA (Veenman et al., 1994), but can be detected by in situ hybridization for GAD65 (Sun et al., 2005). Various striatal interneuron types are also characteristic of striatum, including large neurons containing ChAT, large GABAergic neurons containing parvalbumin, and medium-sized interneurons co-containing somatostatin, NPY and NADPHd (nicotinamide adenine dinucleotide phosphate-diaphorase) (Reiner et al., 1998a). Note that somatostatin, NPY and NADPHd are largely found in the same striatal interneurons in both mammals and birds, but in birds, somatostatin and NPY also occur in many medium spiny projection neurons (Anderson and Reiner, 1990b). Thus, NADPHd (which is known to represent the enzymatic activity of nitric oxide synthase) selectively identifies the interneuron cells in pigeons, while somatostatin and NPY immunolabeling do not. Other studies show that NADPHd+ neurons are present in both medial and lateral striatum in birds (Brüning, 1993; Brüning et al., 1994; Atoji et al., 2001). The lateral striatum with its paucity of cholinergic interneurons differs from medial striatum due to its relative abundance in cholinergic interneurons, as noted here and previously (Medina and Reiner, 1994), whereas both striatal sectors contain NADPHd+ interneurons and PARV+ interneurons. The predominant outputs of the medial and lateral striatum also differ, as the medial striatum largely projects to the midbrain nigral region, whereas the lateral striatum projects mainly to globus pallidus (Karten and Dubbeldam, 1973; Reiner et al., 2004b). Further details about medial and lateral striatum are considered in the next section in which the avian dorsal striatum is compared to that in mammals.
4.1.1. Comparisons of the avian striatum to striatum in mammals
The avian striatum, like the mammalian striatum, develops from a Dlx1/2-rich and Nkx2.1-poor neuroepithelium (Fernandez et al., 1998; Puelles et al., 2000). The mammalian striatum contains neurochemically and hodologically distinct striosomes (or patches) dispersed within the striatal matrix, but such a segregation of patches within the striatal matrix is not obvious in birds (Karten and Dubbeldam, 1973; Brauth et al., 1978; Reiner et al., 1983, 1994, 1998a; Bottjer et al., 1989; Bottjer, 1993; Castro and Ball, 1994; Grisham and Arnold, 1994; Medina and Reiner, 1995; Soha et al., 1996; Durstewitz et al., 1999; Luo and Perkel, 1999). The matrix compartment of mammalian striatum is further divided into functional territories (termed T1–T3 by Morel et al., 2002) that are distinguished by their neuropil content of different markers, in particular CALB and PARV (Morel et al., 2002, François et al., 1994; Holt et al., 1997; Joel and Winer, 1997; Prensa et al, 2003; Riedel et al., 2002). The accumbens core comprises the T4 region of Morel et al., (2002) and is discussed with the ventral striatum below.
The matrix of the caudal and lateral putamen (T1 region of Morel et al., 2002) of mammals is characterized by low levels of CALB and very high levels of PARV. It receives its dominant input from somatosensory and motor cortical areas, and for this reason is sometimes called somatomotor striatum (Morel et al., 2002; Künzle, 1975; Parent and Hazrati, 1995; Prensa 1999; Riedel et al., 2002). This compartment in mammals resembles the medial part of the lateral striatum in pigeons, which likewise contains low CALB and high PARV levels (present data). The medial part of the LSt receives a prominent input from the rostral Wulst of the hyperpallium (a motor cortical area) and projects to globus pallidus, which together form the motor output circuit of the avian basal ganglia (Veenman et al., 1995; Medina et al., 1997; Wild and Williams, 2000; Shimizu et al., 1995). Additionally, the medial LSt and mammalian somatomotor striatum have similar developmental origins (Abellán and Medina, 2009). Although the medial LSt of pigeons is certainly at least analogous to the somatomotor striatum of mammals, the immunohistochemical, connectional, and developmental similarities between it and the mammalian somatomotor striatum suggest that they are most likely homologous rather than independently derived similarities.
The matrix of the rostral dorsal caudoputamen (T2 of Morel et al., 2002) is characterized by high levels of CALB and very little PARV; it receives its main inputs from associative cortical territories such as the visual, sensory-motor, cingulate, and entorhinal cortices as well as the basolateral amygdala, and projects to the globus pallidus (Mesulam, 1985; McGeorge and Faull, 1989; Parent and Hazrati, 1995; Haber and McFarland, 1999; Morel et al., 2002; Prensa et al., 2003; Riedel et al., 2002; Künzle, 2005). The matrix of the rostral ventral caudoputamen (T3 region of Morel et al., 2002) is also characterized by high levels of CALB and very little PARV, similar to the T2 region in mammals. In addition, the main inputs to T3 arise from paralimbic cortical territories such as prefrontal, insular, perirhinal and entorhinal cortices, and amygdala (Mesulam, 1985; Haber and McFarland, 1999; Morel et al., 2002; Prensa et al., 2003; Riedel et al., 2002; Künzle, 2005). Our results show that immunochemistry of the lateral part of the medial striatum of birds resembles both the rostral dorsal and rostral ventral matrix (T2 and T3) of the caudoputamen, as it, also, has high CALB/low PARV. The connections further support these comparisons with associative-like inputs arising predominantly from the caudal Wulst of the hyperpallium, a largely visual cortical area, from the caudolateral nidopallium, a limbic associative-like pallial area and limbic-like projections from periolfactory and paralimbic pallial areas and amygdalar areas (Veenman et al., 1995; Kröner and Güntürkün, 1999), and output projections to the globus pallidus (Farries et al., 2005; Abellán and Medina, 2009; Kuenzel et al., 2011). In addition to these hodological and immunohistochemical similarities, developmental parallels also support the view that the lateral part of MSt is homologous to the mammalian associative caudoputamen (Abellán and Medina, 2009; Kuenzel et al., 2011). Thus, the immunohistochemical, connectional and developmental characteristics of the lateral part of the MSt resemble both the associational and limbic striatal areas of mammals. Further work is needed to determine if separate limbic and association striatal areas can be differentiated within the lateral MSt, and to what degree the lateral MSt is homologous or independently derived from the ancestral amniote striatal organization.
The projections to the medialmost part of the MSt resemble those to the mammalian T3 matrix, as medialmost MSt receives its input from such limbic-associated regions as the prehippocampal area, the pyriform cortex, prepyriform cortex, and nucleus taeniae (olfactory amygdala area), the core of the arcopallium, and the caudolateral nidopallium (Veenman et al., 1995; Atoji and Wild 2014). Moreover, medialmost MSt projects to dopamine neurons in the ventral tegmental area, as does the mammalian limbic striatum (Mezey and Csillag, 2002). The medialmost MSt of pigeons, however, shows less CALB and more PARV than the mammalian T3, and also shows fewer TH+ fibers and more SP+ woolly fibers than any of the mammalian striatal matrix areas (Voorn et al., 1989; Morel et al., 2002; Holt et al., 1997). Thus, it is uncertain if the two regions are homologous but with divergent neurochemistry, or if they are merely similar in connectivity but evolutionarily separately derived (i.e., analogous). The possibility has also been raised that medialmost MSt might correspond to a mammalian striosome-like compartment that has not been interwoven into a matrix compartment (Bálint and Csillag, 2007; Kuenzel et al., 2011). The low CALB and PARV, moderate TH, and heavy SP levels of medial MSt are consistent with this possibility, as is its limbic-associated input. The rostroventral striosomes near the ventricle appear late in neurogenesis in mammals, in agreement with the late neurogenesis of the medial striatum in birds (Tsai et al., 1981a, b; Song & Harlan, 1994). However, the medialmost MSt in birds is unique in its enrichment of woolly fibers, which are absent in the mammalian striatal striosomes and matrix. Additionally, high mu opiate receptor levels are a characteristic of striosomes, and the medial striatum possesses low levels, although slightly higher than in more lateral striatal areas (Reiner et al., 1989; Wang et al., 2007), which led Reiner et al. (1989) to suggest that neurons homologous to striosomes may be homogeneously distributed in the striatum of birds. Thus, a clear mammalian homologue of the medial MSt is uncertain and it appears to be a divergent region.
Finally, the caudal and lateralmost part of the lateral striatum of pigeons expresses low levels of both PARV and CALB, and does not readily compare to any part of the mammalian striatum. Several prior studies have suggested that at least part of the caudalmost part of LSt may correspond to the central extended amygdala of mammals (Abellán and Medina, 2009; Bruce, 2012).
4.1.2. Comparisons of the avian striatum to striatum in reptiles and amphibians
The reptilian striatum has a medial–lateral differentiation similar to most species that have been studied. In Caiman, the subpallial region termed the ventrolateral area is comparable to the striatum and contains heterogeneous distributions of markers that define at least three regions comparable to those identified here in pigeons (Brauth, 1984; Brauth et al., 1985; Brauth, 1988; Brauth et al., 1988). Those regions are: (1) a rostromedial region that is CALB poor but contains high levels of ENK+, SP+ and ChAT+ in neuronal perikarya and neuropil, and is thus comparable to the medial MSt of pigeons; (2) a dorsolateral region that has a CALB+, ENK+, TH+, and serotonin+ neuropil, and is thus comparable to pigeon lateral medial striatum; and (3) a ventrolateral region with a TH+ neuropil, comparable to pigeon lateral striatum. It is uncertain whether the lateral striatum of Caiman can be further divided into sectors, as we have here for pigeons. The striatum (paleostriatum augmentatum) of the turtle Pseudemys contains a medial region with higher levels of ChAT+, ENK+, NPY+, SP+, and somatostatin+ cells and neuropil and thus is comparable to avian medial striatum, and a lateral region with a similar neuronal profile but higher levels of dopaminergic terminals in the neuropil and thus is comparable to avian lateral striatum (Reiner et al., 1984; Reiner, 1987; Reiner and Oliver, 1987; Smeets et al., 1987; Powers and Reiner, 1993). In lizards, the neuropil of the medial striatum contains higher levels of ENK and somatostatin than the lateral striatum (Russchen et al., 1987; Pérez-Clausell and Fredens, 1988), but other markers (dopamine and acetylcholinesterase) did not reveal additional compartments (Smeets et al, 1986a, b). Studies of CALB and PARV localization are needed to determine if the striatum of turtles and lizards possesses two medial striatum and two lateral striatum sectors, as in pigeons.
The amphibian striatum can also be divided into neurochemical compartments. In contrast to pigeons, the medial striatal region in amphibians contains higher levels of TH and ChAT and lower levels of SP and ENK compared to the lateral striatal region, although occasional patches that express high levels of ENK are present near the ventricle (Marin et al., 1997, 1998; Mühlenbrock-Lenter et al., 2005). The neuropil at the border of medial and lateral regions contains higher levels of CALB (Morona and González, 2008). Together, these data suggest that there may be three neurochemical compartments in amphibian striatum. In amphibians, however, the features of the medial and lateral striatal compartments do not correspond to those of the similarly located compartments in birds. Moreover, striatal neurons tend to be concentrated along the ventricle in amphibians, and few are located more laterally. By contrast, in birds they are uniformly distributed in striatum. Thus, the compartments in amphibians may be independently derived and unrelated to those in birds.
4.2. Ventral Striatum
The avian ventral striatum consists of the nucleus accumbens, the paratubercular striatum, the reticular striatum, Stvc, and the olfactory tubercle. In addition, the lateral StM extends ventrally so that it is adjacent to ventral striatal territories, and its ventralmost part will be discussed as a possible ventral striatal territory. Each compartment is distinguished by unique combinations of neurochemical characteristics, as noted above. The olfactory tubercle is clearly similar to its mammalian namesake, but homologs of the other compartments are less straightforward. In mammals, the accumbens core and accumbens shell regions are often subdivided into medial and lateral regions based on their neurochemical properties.
4.2.1. Nucleus Accumbens: Core-like region
The human and marmoset nucleus accumbens can be divided into medial and lateral compartments based on their CR+, CALB- and PARV-neuropil content (medial and lateral T4 region of Morel et al., 2002). The medial T4 region corresponds with the rodent rostral accumbens (Meredith et al., 1996) and will be considered with the shell-like regions below. The characteristics of the lateral subdivision (lateral T4 region of Morel et al., 2002) of the human and rodent accumbens core closely resemble those seen in the ventralmost lateral MSt of pigeons. The primate accumbens core contains a lateral compartment (lateral T4) that is neurochemically similar to the adjacent (T2 and T3) striatal matrix, with high levels of CALB and very low levels of PARV (Meredith et al., 1996; Morel et al., 2002). This region also contains high levels of TH+ fibers, moderate levels of ENK and SP, and low levels of NPY (Zaborsky et al., 1985; Voorn et al., 1989; Zahm and Brog, 1992; Heimer et al., 1997; Brauer et al., 2000; Riedel et al., 2002, Prensa et al., 2003). Thus, the similar characteristics and location of the ventralmost lateral MSt suggest it may be homologous to the lateral subdivision of the accumbens core compartment of humans and rodents.
4.2.2. Nucleus accumbens: Shell-like regions
The accumbens shell region of rodents is distinguished from the core region by its low levels of CALB (Voorn et al., 1989; Zahm and Brog, 1992; Jongen-Rêlo et al., 1994). By this definition, the main shell region of rodents is often subdivided into medial and lateral divisions, but also includes most of the rostral pole of accumbens. The rodent rostral pole is comparable to the human medial striatal subdivision (medial T4 region of Morel et al., 2002), a medial core region of humans, and is dealt with separately below.
The main part of the mammalian accumbens shell (medial and lateral divisions) contains higher levels of TH+, SP+, VIP+, and CCK+ fibers than the accumbens core (Zaborsky et al., 1985; Voorn et al., 1989; Zahm and Brog, 1992; Heimer et al., 1997; Brauer et al., 2000; Riedel et al., 2002, Prensa et al., 2003). CART+ perikarya and a CR+ neuropil are located predominantly in the medial accumbens shell (Bubser et al., 2000; Fagergren and Hurd 2007). In birds, the homologue of the mammalian accumbens shell has been identified relatively recently (Roberts et al., 2002; Reiner et al., 2004b; Ballint and Csillag, 2007; Abellán and Medina, 2009), although its boundaries remained unclear. Based on similar neurochemical similarities, both the paratubercular and Stvc regions resemble the mammalian shell of the accumbens, although the Stvc immunostains slightly less for VIP, ENK, and GAD than the paratubercular region. The Lmo4 gene has been identified as a marker for the accumbens shell in chicks and mammals (Abellán and Medina, 2009), and this Lmo4+ region in chicks roughly coincides with the paratubercular striatum plus Stvc of pigeons, consistent with a homology with the mammalian medial and lateral shell regions. In pigeons, striatal CART+ cells are primarily located in the medial part of the medial part of the paratubercular region, suggesting it may be comparable to the mammalian medial accumbens shell. The location of the mammalian medial and lateral accumbens shell also resembles the paratubercular and Stvc of pigeons, being sandwiched between the accumbens core and the ventral pallidum. Interestingly, in pigeons, the Stvc forms a cup around the caudal pole of the ventral part of the lateral MSt, much like the accumbens shell that surrounds the caudal accumbens core in mammals (Zahm and Brog, 1992), giving this homology a further topological similarity.
4.2.3 Nucleus accumbens: Rostromedial-like regions
In humans, the neuropil of the medial subdivision of the accumbens core (medial T4 region of Morel et al., 2002) immunostains at very high levels for CR, moderate levels of CALB, and low levels of PARV (Morel, 2002). In rodents, this compartment appears to correspond to a low CALB region in the rostral accumbens and the striosomal patches that extend from it into the accumbens core, which label with very high levels of SP, low CALB and PARV (Voorn et al., 1989; Zahm and Brog, 1992; Zahm and Heimer, 1993; Meredith et al., 1996; Riedel et al., 2002). In rodents this region is often considered a shell-like region because of its low CALB+ neuropil and its projections to the ventral pallidum, hypothalamus, and ventral tegmental area (Zahm and Heimer, 1993). The region identified as the avian nucleus accumbens in Reiner et al. (2004b) is similarly characterized by a neuropil with very high levels of SP and CR, moderate levels of CALB, and low levels of PARV, compared to the overlying dorsal striatum. Thus, the similar combination of neurochemical staining and the rostral medial location suggests that the avian accumbens of Reiner et al. (2004b) may be comparable to the medial accumbens core of humans and to the rostral accumbens clusters and striosome-like extensions of rodents.
4.2.4. Nucleus accumbens: Connectional and functional correlations
Connectional similarities further support these shell and core comparisons between mammals and pigeons. For example, in mammals, the ventral pallidum receives projections from the medial and lateral shell and core, and in pigeons, it receives projections from the paratubercular region, the Stvc, and from the ventral part of the lateral MSt (Groenewegen et al., 1999; Medina and Reiner, 1997; Bálint et al., 2011). Both the avian and mammalian accumbens shell and core exhibit largely similar efferent projections, including their major projections to the ventral pallidum, basal nucleus of Meynert, nucleus of the diagonal band, lateral hypothalamus, lateral preoptic area, subthalamic nucleus, substantia nigra and parabrachial region, although a projection from neurotensin-labeled neurons in the accumbens (likely corresponding to our Stvc) and BSTL project to the parabrachial nucleus appears to be unique to birds (Bálint et al., 2011, 2016). The accumbens core and shell of both birds and mammals are distinguished by some of their afferent projections. The hippocampal subicular and CA1 regions project predominantly to the rostral and medial parts of the shell (Groenewegen et al., 1999; Groenewegen et al., 1987; Brog et al., 1993). Similarly, the pigeon hippocampal formation projects to the accumbens and paratubercular regions (Atoji and Wild, 2004). Furthermore, the avian Stvc, ventral part of the lateral MSt (proposed accumbens shell and core), and BSTL receive projections from the dorsal arcopallium, which may be comparable in part to the mammalian projection from the pallial amygdala (Hanics et al., 2016).
The subdivisions of the mammalian accumbens shell and core have distinct connections and distinct roles in processing appetitive behaviors, (e.g., Kelley, 1999; Zahm, 1999; Heimer et al., 1997). The mammalian nucleus accumbens plays a critical role in learning and regulating reward-associated motivational responses, with the shell and core regions contributing distinct functions (Mogenson et al., 1980; Swerdlow and Koob, 1987; LeMoal and Simon 1991; Heimer et al., 1991; Zahm and Brog, 1992; Kalivas et al., 1999; Haber and McFarland, 1999). The shell receives motivationally relevant information from areas such as the ventral tegmental area, the medial part of the ventral pallidum, and the prefrontal cortex. This information is integrated to determine the intensity of the response and reaches the accumbens core. The accumbens core then regulates the initiation of the motor response through projections to motor regions such as the substantia nigra, subthalamic nucleus, and pedunculopontine nuclei. Comparable studies in birds are important for resolving this possible homology. Further investigations are needed to determine if the potential accumbens shell and core subterritories that we have identified histochemically have functional interactions similar to those in mammals.
4.2.5. Striatal reticular area
The striatal reticular area is distinguished by small interdigitating zones that stain lightly vs. darkly with ENK, TH, CCK, NPY, GAD, and PARV. The darker staining zones appear to represent the neuropil of the paratubercular region as it passes over the rostral pole of the ventral pallidum to fuse with the Stvc, which is located dorsal to the enlarged ventral pallidum. The interdigitating lighter staining zones may represent the rostral pole of the ventral pallidum, or an axonal tract that passes along the rostral pole of the ventral pallidum, or both. Because we could not distinguish between these possibilities, we recognize this as a distinct region that requires further investigation.
4.2.6. Comparisons of ventral striatum compartments to reptiles and amphibians
A mammalian-like accumbens region associated with the rostral striatum has been identified in both reptiles and amphibians. In frogs it is characterized by very high levels of TH, SP, and ENK (Marin et al., 1998), and thus appears to be comparable to the shell-like regions of pigeons, StP and Stvc, and to the mammalian accumbens shell. In the lizard Psamodromus, two accumbens regions have been identified (Guirado et al., 1999). The rostromedial accumbens expresses high levels of TH, SP, GABA, whereas the caudolateral accumbens expresses levels more similar to the adjacent striatum, suggesting a possible shell and core division, respectively, similar to those in birds and mammals.
4.3. Olfactory tubercle
The similarities between the olfactory tubercle of birds, reptiles, and mammals are striking. In birds, reptiles, and mammals, it receives a projection from, and projects to, the olfactory bulb (Martinez Garcia et al., 1991; Lohman and Smeets, 1993; Lanuza and Halpern, 1998; Atoji and Wild, 2014). Embryologically, it is formed predominantly by pallidal derivatives medially and striatal derivatives laterally (including the islands of Calleja), based on gene expression in birds and mammals (Abellán and Medina, 2009). Most neurochemical markers are richly abundant in the neuropil (ENK, TH, CCK, NPY, GAD and SP) and in cells (ENK, SP, and GAD). This region has been identified in multiple studies, and its similarity to the like-named mammalian region has been noted (Striedter et al., 1998; Puelles et al., 2000; Roberts et al., 2002; García-López et al., 2008; Reiner et al., 2004b; Abellán and Medina 2009).
4.3. Globus Pallidus and Ventral Pallidum
The avian pallidum, like that of mammals, includes the globus (dorsal) pallidus and ventral pallidum, which are distinguished from striatal areas by an abundance of woolly fibers that at least contain substance P or enkephalin, and low levels of dopaminergic and cholinergic fibers and acetylcholinesterase (Young et al, 1984; Heimer et al., 1987; Medina and Reiner, 1995; Heimer et al., 1999), which are largely pallidal characteristics. Our data confirm these earlier findings. Furthermore, the pallidal nature of these groups is also indicated by their origin from comparable Nkx2.1-expressing regions during development, the medial ganglionic eminence in mammals or its equivalent in birds, as well as their expression of chicken Lhx6 and chicken Lhx7/8 (Puelles et al., 2000; Abellán and Medina, 2009). Nonetheless, in reptiles and birds the globus pallidus is not divided into internal and external pallidal segments as in mammals, since SP+ and ENK+ woolly fibers have overlapping distributions in birds, unlike in mammals, in which they are segregated to the internal and external pallidal segments, respectively. Of interest, our data indicate the existence of a prominent population of VIP+ striatal neurons that project to GP, given its enrichment in VIP+ woolly fibers. This population was not seen with colchicine treatment and will require sensitive in situ hybridization to detect. Similarly, the presence of VIP+ and NPY+ woolly fibers in VP indicates the existence of VIP+ and NPY+ striatal projection neurons projecting to VP. The present and prior studies indicate these are likely to reside in MSt and nucleus accumbens (Anderson et al., 1990b). The boundaries of the ventral pallidum have occasionally been misidentified in previous studies in birds, often locating the ventral pallidum more rostral or medial than its true location as defined here by woolly fiber localization, or mislabeling it as the accumbens shell (e.g., Kuenzel et al., 2011). Recognizing the full extent of the VP boundary is a challenge even with histochemical markers such as VIP, CCK and NPY, because they do not occupy the entire the ventral pallidum. In this regard, SP, ENK, and GAD provide useful positive markers for identifying VP, as they heavily stain the entire nucleus. TH and CCK define the VP and GP by their absence.
4.4. Intrapeduncular nucleus
The intrapeduncular nucleus was once considered a pallidal region because of its location between the globus pallidus and the ventral pallidum (Karten and Dubbeldam, 1973; Medina and Reiner, 1994), and suggested to be comparable to globus pallidus internus of mammals. Indeed, it contains relatively few ChAT+ and TH+ terminals in its neuropil, giving it this pallidal characteristic. However, the intrapeduncular nucleus is devoid of SP, ENK, and VIP, and GAD woolly fibers, which is the most defining feature of the globus pallidus and ventral pallidum (Reiner et al., 2004b). Moreover, it contains neurons expressing striatal markers Lmo4, SP, and Cdh8, but has few cells expressing pallidal markers Nkx2.1, Lhx6, or Lhx7/8 (Abellán and Medina, 2009, Vicario et al., 2014). These findings suggest that INP is a dorsal striatal structure that contains a small number of cells originating from pallidal areas during development. A clear mammalian homologue is uncertain, and further studies are needed to determine if this cell group represents a part of the central extended amygdalar complex, part of the striatum, or part of the pallidum. As no clear homologue is evident in reptiles, INP may be uniquely evolved in avians. Along these lines, it is important to note that INP is highly enriched in ChAT+ neurons, as observed here and reported previously by Medina and Reiner (1994). It may be that, in the absence of intrinsic cholinergic neurons, these are the source of the cholinergic terminals in lateral striatum, and enable a role of the type of muscarinic mechanisms in motor plasticity as seen in mammals (Pisani et al., 2007).
4.5. Bed nucleus of stria terminalis: Lateral and external parts
Although the location of the BSTL varied in earlier studies in pigeons, it is recognized and clearly differentiated from the striatal and accumbens regions by its low levels of TH, ChAT, NPY, SP, DARPP-32, parvalbumin and calbindin and its relative richness in ENK and calretinin (Reiner et al., 1998b; Durstewitz et al., 1999; Reiner et al., 2004b; Balint and Csillag, 2007; Husband and Shimizu, 2011; this study). Our data confirm the low levels of ChAT and SP and show that the BSTL can also be identified by lower expression levels of VIP, TH and NPY, relative to adjacent areas. The expression of VIP, ENK and CCK is more complex, with regions of higher and lower intensity within the BSTL. Our BSTL appears to correspond to what was termed the dorsomedial part of the BSTL (BSTLdm) in embryonic chicks, which contains lower levels of cLmo4 and cSP, relative to adjacent areas (Abellán and Medina, 2009).
The external part of the BSTL forms the dorsal and ventral margins of the BSTL, respectively. The dorsal part of this border region displays higher levels of ChAT (or AChE), CCK, NPY, TH and VIP than the BSTL (present study). The ventral part displays higher levels of TH, CCK, and NPY, but lower levels of ChAT (or AChE). The ventral external BSTL corresponds to an area that expresses higher levels of calbindin, and the dorsal BSTL corresponds to a heterogeneous area with clusters of parvalbumin, calretinin, and calbindin neuropil, called the ventral and dorsal regions of nucleus accumbens by Husband and Shimizu (2011).
Based on these characteristics, the pigeon dorsal external BSTL is comparable to the dorsal capsular and dorsal central parts of the rodent BSTL (Alheid et al., 1995). The pigeon ventral external BSTL is comparable to rodent juxtacapsular and ventral BSTL groups (Riedel et al., 2002; Alheid et al., 1995). The pigeon dorsal external BSTL appears to correspond to the dorsolateral BSTL of embryonic chicks, in which cells express cLmo4, cPax6, cCdh8. The ventral external BSTL corresponds to the ventral BSTL of embryonic chicks, with cells that express cLmo4 (Abellán and Medina, 2009).
4.5.1. Bed nucleus of stria terminalis: Comparison to reptiles and amphibians
A homologue of the mammalian BSTL, a rostral part of the central extended amygdala, has been recognized in amphibians, but not yet in reptiles, although labeling patterns suggest it is present (Smeets et al., 1986a, b; Reiner et al., 1984b, 1987; Russchen et al., 1987; Marin et al., 1998). In frogs, the bed nucleus of the stria terminalis expresses low levels of TH and high levels of ENK, as in birds and mammals.
4.6. Summary
The analysis of a combination of markers in the pigeon basal ganglia provides a powerful tool for identification of discrete, neurochemically distinct compartments and for comparisons to compartments in other species. This study provides a detailed description of the distribution of some of the major neurochemical markers of the basal ganglia. By combining multiple markers known to discriminate different regions of the mammalian basal ganglia and comparing their distribution over the full rostrocaudal extent of the subpallium, the present study has yielded a more comprehensive view of the heterogeneous organization of the basal ganglia in birds and a better resolution of the boundaries of the various groups. The combination of neuronal markers used here distinguished sixteen compartments in the pigeon basal ganglia. Four compartments were identified in the dorsal striatum, including a medial compartment that appears to be unique to birds based in part on its high content of woolly fibers, an associational striatal compartment, a lateral somatomotor striatal compartment, and a lateralmost compartment that is part of the central extended amygdala. Regions within the ventral striatum were identified that appear to be comparable to the rostral pole, shell, and core of the mammalian accumbens (Fig. 12). The distinct compartments align in many cases with particular sets of projections reported in various mammalian species. Comparative analyses of our results demonstrate that most basal ganglia compartments are highly conserved among tetrapods. However, there are also divergent areas that may have evolved independently in birds, notably the medialmost MSt and the INP.
Fig. 12.
Schematic drawings summarizing the neurochemical subdivisions observed in this study, and proposed striatal and accumbens relationships. A, B, and C correspond to rostrocaudal levels A12.50, A10.75, and A9.50, respectively.
The compartments of the basal ganglia may have arisen from distinct genetic domains that are recognized differently by ingrowing axons. It will be important to determine how the neurochemical compartments relate to particular sets of projections, and to identify the genetic mechanisms that control their origins and development.
Highlights.
Sixteen distinct compartments were identified in the pigeon basal ganglia using multiple neurochemical markers.
The striatum contains neurochemical regions comparable to the mammalian somatomotor and associational striatum.
A neurochemically distinct area located in the medialmost striatum of pigeons appears to be unique to birds.
The ventral striatum contains neurochemical regions similar to the mammalian accumbens core, shell, and rostral areas.
Most of the main compartments of the basal ganglia were highly conserved during tetrapod evolution, yet unique avian compartments representing diversification have also evolved.
Acknowledgments
Supported by NS-19620, NS-28721, and NS-57722 The Methodist Hospitals Endowed Professorship in Neuroscience (AR).). We are grateful for the technical assistance of Gary Henderson, Sherry Cuthbertson, Ellen Karle, and Marion Joni.
Abbreviations
- Ac
accumbens
- BM
nucleus basalis magnocellularis
- BSTL
bed nucleus of stria terminalis, lateral part
- BJ
juxtacapsular part of BSTL
- Bv
ventral part of the BSTL
- GP
globus pallidus
- INP
intrapeduncular nucleus
- LFB
lateral forebrain bundle
- LOT
lateral olfactory tractl
- LPS
lamina pallio-subpallialis
- LSt
lateral striatum
- MSt
medial striatum
- MStM
medial part of the medial striatum
- LStM
lateral part of the medial striatum
- StVC
ventral striatum, ventrocaudal part
- StP
ventral striatum, paratubercular region
- QF
quintofrontal tract
- TSM
tractus septopallio-mesencephalicus
- TuO
tuberculum olfactorium
- VP
ventral pallidum
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6.0 References
- 1.Abellán A, Medina L. Subdivisions and derivatives of the chicken subpallium based on expression of LIM and other regulatory genes and markers of neuron subpopulations during development. J Comp Neurol. 2009;515:465–501. doi: 10.1002/cne.22083. [DOI] [PubMed] [Google Scholar]
- 2.Alheid GF, De Olmos JS, Beltramino CA. Amygdala and extended amygdala. In: Paxinos G, editor. The Rat Nervous System. Academic Press; San Diego: 1995. pp. 495–578. [Google Scholar]
- 3.Anderson KD, Reiner A. Extensive co-occurrence of substance P and dynorphin in striatal projection neurons: an evolutionarily conserved feature of basal ganglia organization. J Comp Neurol. 1990a;295:339–369. doi: 10.1002/cne.902950302. [DOI] [PubMed] [Google Scholar]
- 4.Anderson KD, Reiner A. Distribution and relative abundance of neurons in the pigeon forebrain containing somatostatin, neuropeptide Y, or both. J Comp Neurol. 1990b;299:261–282. doi: 10.1002/cne.902990302. [DOI] [PubMed] [Google Scholar]
- 5.Anderson KD, Reiner A. Striatonigral projection neurons: a retrograde labeling study of the percentages that contain substance P or enkephalin in pigeons. J Comp Neurol. 1991;303:658–673. doi: 10.1002/cne.903030410. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong DM, Pickel VM, Joh TH, Reis DJ, Miller RJ. Immunocytochemical localization of catecholamine synthesizing enzymes and neuropeptides in area postrema and medial nucleus tractus solitarius of rat brain. J Comp Neurol. 1981;196:505–517. doi: 10.1002/cne.901960312. [DOI] [PubMed] [Google Scholar]
- 7.Atoji Y, Yamamoto Y, Suzuki Y. Distribution of NADPH diaphorase-containing neurons in the pigeon central nervous system. J Chem Neuroanat. 2001;21:1–22. doi: 10.1016/s0891-0618(00)00103-4. [DOI] [PubMed] [Google Scholar]
- 8.Atoji Y, Wild JM. Fiber connections of the hippocampal formation and septum and subdivisions of the hippocampal formation in the pigeon as revealed by tract tracing and kainic acid lesions. J Comp Neurol. 2004;475:426–61. doi: 10.1002/cne.20186. [DOI] [PubMed] [Google Scholar]
- 9.Atoji Y, Wild JM. Efferent and afferent connections of the olfactory bulb and prepiriform cortex in the pigeon (Columba livia) J Comp Neurol. 2014;522:1728–1752. doi: 10.1002/cne.23504. [DOI] [PubMed] [Google Scholar]
- 10.Bálint E, Csillag A. Nucleus accumbens subregions: hodological and immunohistochemical study in the domestic chick (Gallus domesticus) Cell Tissue Res. 2007;327:221–230. doi: 10.1007/s00441-006-0295-0. [DOI] [PubMed] [Google Scholar]
- 11.Bálint E, Balázsa T, Zachar G, Mezey S, Csillag A. Neurotensin: revealing a novel neuromodulator circuit in the nucleus accumbens-parabrachial nucleus projection of the domestic chick. Brain Struct Funct. 2016;221:605–616. doi: 10.1007/s00429-014-0928-0. [DOI] [PubMed] [Google Scholar]
- 12.Bálint E, Mezey S, Csillag A. Efferent connections of nucleus accumbens subdivisions of the domestic chicken (Gallus domesticus): an anterograde pathway tracing study. J Comp Neurol. 2011;519:2922–2953. doi: 10.1002/cne.22672. [DOI] [PubMed] [Google Scholar]
- 13.Barsagade VG, Mazumdar M, Singru PS, Thim L, Clausen JT, Subhedar N. Reproductive phase-related variations in cocaine- and amphetamine-regulated transcript (CART) in the olfactory system, forebrain, and pituitary of the female catfish, Clarias batrachus (Linn) J Comp Neurol. 2010;518:2503–2524. doi: 10.1002/cne.22349. [DOI] [PubMed] [Google Scholar]
- 14.Bottjer SW. The distribution of tyrosine hydroxylase immunoreactivity in the brains of male and female zebra finches. J, Neurobiol. 1993;24:51–69. doi: 10.1002/neu.480240105. [DOI] [PubMed] [Google Scholar]
- 15.Bottjer SW, Halsema KA, Brown SA, Miesner EA. Axonal connections of a forebrain nucleus involved with vocal learning in zebra finches. J Comp Neurol. 1989;279:312–326. doi: 10.1002/cne.902790211. [DOI] [PubMed] [Google Scholar]
- 16.Brauer K, Häusser M, Härtig W, Arendt T. The core-shell dichotomy of nucleus accumbens in the rhesus monkey as revealed by double-immunofluorescence and morphology of cholinergic interneurons. Brain Res. 2000;858:151–162. doi: 10.1016/s0006-8993(00)01938-7. [DOI] [PubMed] [Google Scholar]
- 17.Brauth SE. Enkephalin-like immunoreactivity within the telencephalon of the reptile Caiman crocodilus. Neuroscience. 1984;11:345–358. doi: 10.1016/0306-4522(84)90028-9. [DOI] [PubMed] [Google Scholar]
- 18.Brauth SE. The organization and projections of the paleostriatal complex of Caiman crocodilus. In: Schwerdtfeger WK, Smeets WJAJ, editors. The Forebrain of Reptiles. Int. Symp. Recent Advances in Understanding the Structure and Function of the Forebrain in Reptiles. Karger (Basel); Frankfurt: 1988. pp. 60–76. [Google Scholar]
- 19.Brauth SE, Ferguson JL, Kitt CA. Prosencephalic pathways related to the paleostriatum of the pigeon (Columba livia) Brain Res. 1978;147:205–221. doi: 10.1016/0006-8993(78)90836-3. [DOI] [PubMed] [Google Scholar]
- 20.Brauth SE, Kitt CA, Gerfen CR. Calcium binding protein in the basal ganglia system of a non-mammalian vertebrate: an immunohistochemical study in the reptile Caiman crocodilus. Brain Res. 1988;452:367–372. doi: 10.1016/0006-8993(88)90041-8. [DOI] [PubMed] [Google Scholar]
- 21.Brauth SE, Kitt CA, Price DL, Wainer BH. Cholinergic neurons in the telencephalon of the reptile Caiman crocodilus. Neurosci Lett. 1985;58:235–240. doi: 10.1016/0304-3940(85)90170-3. [DOI] [PubMed] [Google Scholar]
- 22.Brog JS, Salyapongse A, Deutch AY, Zahm DS. The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol. 1993;338:255–278. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- 23.Bruce LL. Evolution of the amygdala. In: Yilmazer-Hanke D, editor. Insights into the Amygdala. Nova Science; New York: 2012. pp. 1–24. [Google Scholar]
- 24.Brüning G. Localization of NADPH-diaphorase in the brain of the chicken. J Comp Neurol. 1993;334:192–208. doi: 10.1002/cne.903340204. [DOI] [PubMed] [Google Scholar]
- 25.Brüning G, Wiese S, Mayer B. Nitric oxide synthase in the brain of the turtle Pseudemys scripta elegans. J Comp Neurol. 1994;348:183–206. doi: 10.1002/cne.903480203. [DOI] [PubMed] [Google Scholar]
- 26.Bubser M, Scruggs JL, Young CD, Deutch AY. The distribution and origin of the calretinin-containing innervation of the nucleus accumbens of the rat. Eur J Neurosci. 2000;12:1591–1598. doi: 10.1046/j.1460-9568.2000.00052.x. [DOI] [PubMed] [Google Scholar]
- 27.Castro JM, Ball GF. Characterization and localization of D1 dopamine receptors in the sexually dimorphic vocal control nucleus, area X, and the basal ganglia of European starlings. J, Neurobiol. 1994;25:767–780. doi: 10.1002/neu.480250703. [DOI] [PubMed] [Google Scholar]
- 28.Condé F, Lund JS, Jacobowitz DM, Baimbridge KG, Lewis DA. Local circuit neurons immunoreactive for calretinin, calbindin D-28k or parvalbumin in monkey prefrontal cortex: distribution and morphology. J Comp Neurol. 1994;341:95–116. doi: 10.1002/cne.903410109. [DOI] [PubMed] [Google Scholar]
- 29.Cuello AC, Galfré G, Milstein C. Detection of substance P in the central nervous system by a monoclonal antibody. Proc Natl Acad Sci USA. 1979;76:3532–3536. doi: 10.1073/pnas.76.7.3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cuello AC, Milstein C, Coutre R, Wright B, Priestley JV, Jarvis J. Characterization and immunocytochemical application of monoclonal antibodies against enkephalins. J Histochem Cytochem. 1984:32947–957. doi: 10.1177/32.9.6086744. [DOI] [PubMed] [Google Scholar]
- 31.Durstewitz D, Kroner S, Gunturkun O. The dopaminergic innervation of the avian telencephalon. Prog Neurobiol. 1999;59:161–195. doi: 10.1016/s0301-0082(98)00100-2. [DOI] [PubMed] [Google Scholar]
- 32.Erichsen JT, Bingman VP, Krebs JR. The distribution of neuropeptides in the dorsomedial telencephalon of the pigeon (Columba livia): a basis for regional subdivisions. J Comp Neurol. 1991;314:478–492. doi: 10.1002/cne.903140306. [DOI] [PubMed] [Google Scholar]
- 33.Fagergren P, Hurd Y. Human cocaine- and amphetamine-regulated transcript (CART) mRNA is highly expressed in limbic- and sensory-related brain regions. J Comp Neurol. 2000;425:583–598. doi: 10.1002/1096-9861(20001002)425:4<583::aid-cne8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 34.Farries MA, Meitzen J, Perkel DJ. Electrophysiological properties of neurons in the basal ganglia of the domestic chick: conservation and divergence in the evolution of the avian basal ganglia. J Neurophysiol. 2005;94:454–467. doi: 10.1152/jn.00539.2004. [DOI] [PubMed] [Google Scholar]
- 35.Fernandez AS, Pieau C, Repérant J, Boncinelli E, Wassef M. Expression of the Emx-1 and Dlx-1 homeobox genes define three molecularly distinct domains in the telencephalon of mouse, chick, turtle and frog embryos: implications for the evolution of telencephalic subdivisions in amniotes. Development. 1998;125:2099–2111. doi: 10.1242/dev.125.11.2099. [DOI] [PubMed] [Google Scholar]
- 36.François C, Yelnik J, Percheron G, Tande D. Calbindin D-28k as a marker for the associative cortical territory of the striatum in macaque. Brain Res. 1994;633:331–336. doi: 10.1016/0006-8993(94)91557-1. [DOI] [PubMed] [Google Scholar]
- 37.Furness JB, Costa M, Walsh JH. Evidence for and significance of the projection of VIP neurons from the myenteric plexus to the taenia coli in the guinea pig. Gastroenterology. 1981;80:1557–1561. [PubMed] [Google Scholar]
- 38.Galatioto S, Abbate F, Laura R, Naccari F, Germanà G. Morphological and immunohistochemical considerations on the basal ganglia in pigeon (Columba livia) Anat Histol Embryol. 1998;27:173–178. doi: 10.1111/j.1439-0264.1998.tb00176.x. [DOI] [PubMed] [Google Scholar]
- 39.García-López M, Abellán A, Legaz I, Rubenstein JL, Puelles L, Medina L. Histogenetic compartments of the mouse centromedial and extended amygdala based on gene expression patterns during development. J Comp Neurol. 2008;506:46–74. doi: 10.1002/cne.21524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grisham W, Arnold AP. Distribution of GABA-like immunoreactivity in the song system of the zebra finch. Brain Res. 1994;651:115–122. doi: 10.1016/0006-8993(94)90686-6. [DOI] [PubMed] [Google Scholar]
- 41.Groenewegen HJ, Wright CI, Beijer AV, Voorn P. Convergence and segregation of ventral striatal inputs and outputs. Ann N Y Acad Sci. 1999;877:49–63. doi: 10.1111/j.1749-6632.1999.tb09260.x. [DOI] [PubMed] [Google Scholar]
- 42.Groenewegen HJ, Vermeulen-Van der Zee E, te Kortschot A, Witter MP. Organization of the projections from the subiculum to the ventral striatum in the rat. A study using anterograde transport of Phaseolus vulgaris leucoagglutinin. Neuroscience. 1987;23:103–120. doi: 10.1016/0306-4522(87)90275-2. [DOI] [PubMed] [Google Scholar]
- 43.Guirado S, Dávila JC, Real MA, Medina L. Nucleus accumbens in the lizard Psammodromus algirus: Chemoarchitecture and cortical afferent connections. J Comp Neurol. 1999;405:15–31. doi: 10.1002/(sici)1096-9861(19990301)405:1<15::aid-cne2>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 44.Haber SN, McFarland NR. The concept of the ventral striatum in nonhuman primates. Ann NY Acad Sci. 1999;877:33–48. doi: 10.1111/j.1749-6632.1999.tb09259.x. [DOI] [PubMed] [Google Scholar]
- 45.Hack NJ, Wride MC, Charters KM, Kater SB, Parks TN. Developmental changes in the subcellular localization of calretinin. J Neurosci. 2000;20:RC67. doi: 10.1523/JNEUROSCI.20-07-j0001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hanics J, Teleki G, Alpár A, Székely AD, Csillag A. Multiple amygdaloid divisions of arcopallium send convergent projections to the nucleus accumbens and neighboring subpallial amygdala regions in the domestic chicken: a selective pathway tracing and reconstruction study. Brain Struct Funct. 2016 Apr 6; doi: 10.1007/s00429-016-1219-8. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heimer L, Zahm DS, Churchill L, Kalivas PW, Wohltmann C. Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience. 1991;41:89–125. doi: 10.1016/0306-4522(91)90202-y. [DOI] [PubMed] [Google Scholar]
- 48.Heimer L, Alheid GF, de Olmos JS, Groenewegen HJ, Haber SN, Harlan RE, Zahm DS. The accumbens: beyond the core-shell dichotomy. J Neuropsychiatry Clin Neurosci. 1997;9:354–381. doi: 10.1176/jnp.9.3.354. [DOI] [PubMed] [Google Scholar]
- 49.Heimer L, de Olmos JS, Alheid GF, Pearson J, Sakamoto N, Shinoda K, Marsteiner J, Switzer RC., III . The human basal forebrain. Part II. In: Bloom FE, Bjorklund A, Hokfelt T, editors. Handbook of Chemical Neuroanatomy. Vol 15. The Primate Nervous System, Part 3. Elsevier; Amsterdam: 1999. pp. 57–226. [Google Scholar]
- 50.Hervonen A, Pickel VM, Joh TH, Reis DJ, Linnoila I, Kanerva L, Miller RJ. Immunocytochemical demonstration of the catecholamine-synthesizing enzymes and neuropeptides in the catecholamine-storing cells of human fetal sympathetic nervous system. Adv Biochem Psychopharmacol. 1980;25:373–378. [PubMed] [Google Scholar]
- 51.Holt DJ, Graybiel AM, Saper CB. Neurochemical architecture of the human striatum. J Comp Neurol. 1997;384:1–25. doi: 10.1002/(sici)1096-9861(19970721)384:1<1::aid-cne1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 52.Husband SA, Shimizu T. Calcium-binding protein distributions and fiber connections of the nucleus accumbens in the pigeon (Columba livia) J Comp Neurol. 2011;519:1371–1394. doi: 10.1002/cne.22575. [DOI] [PubMed] [Google Scholar]
- 53.Joel D, Weiner I. The organization of the basal ganglia–thalamocortical circuits: open interconnected rather than closed segregated. Neuroscience. 1997;63:363–379. doi: 10.1016/0306-4522(94)90536-3. [DOI] [PubMed] [Google Scholar]
- 54.Jongen-Rêlo AL, Voorn P, Groenewegen HJ. Immunohistochemical characterization of the shell and core territories of the nucleus accumbens in the rat. Eur J Neurosci. 1994;6:1255–1264. doi: 10.1111/j.1460-9568.1994.tb00315.x. [DOI] [PubMed] [Google Scholar]
- 55.Kalivas PW, Churchill L, Romanides A. Involvement of the pallidal-thalamocortical circuit in adaptive behavior. Ann N Y Acad Sci. 1999;877:64–70. doi: 10.1111/j.1749-6632.1999.tb09261.x. [DOI] [PubMed] [Google Scholar]
- 56.Karten HJ, Dubbeldam JL. The organization and projections of the paleostriatal complex in the pigeon (Columba livia) J Comp Neurol. 1973;148:61–89. doi: 10.1002/cne.901480105. [DOI] [PubMed] [Google Scholar]
- 57.Karten HJ, Hodos W. Columba livia. The Johns Hopkins University Press; Baltimore: 1967. A Stereotaxic Atlas of the Brain of the Pigeon. [Google Scholar]
- 58.Kaufman DL, Houser CR, Tobin A. Two forms of the gamma-aminobutyric acid synthetic enzyme glutamate decarboxylase have distinct intraneuronal distributions and cofactor interactions. J Neurochem. 1991;56:720–723. doi: 10.1111/j.1471-4159.1991.tb08211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kelley AE. Functional specificity of ventral striatal compartments in appetitive behaviors. Ann NY Acad Sci. 1999;877:71–90. doi: 10.1111/j.1749-6632.1999.tb09262.x. [DOI] [PubMed] [Google Scholar]
- 60.Krebs JR, Erichsen JT, Bingman VP. The distribution of neurotransmitters and neurotransmitter-related enzymes in the dorsomedial telencephalon of the pigeon (Columba livia) J Comp Neurol. 1991;314:467–477. doi: 10.1002/cne.903140305. [DOI] [PubMed] [Google Scholar]
- 61.Kröner S, Güntürkün O. Afferent and efferent connections of the caudolateral neostriatum in the pigeon (Columba livia): a retro- and anterograde pathway tracing study. J Comp Neurol. 1999;407:228–260. doi: 10.1002/(sici)1096-9861(19990503)407:2<228::aid-cne6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 62.Kuenzel SJ, Medina L, Csillag A, Perkel DJ, Reiner A. The avian subpallium: new insights into structural and functional subdivisions occupying the lateral subpallial wall and their embryological origins. Brain Res. 2011;1424:67–101. doi: 10.1016/j.brainres.2011.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Künzle H. Bilateral projections from precentral motor cortex to the putamen and other parts of the basal ganglia. An autoradiographic study in Macaca fascicularis. Brain Res. 1975;88:195–209. doi: 10.1016/0006-8993(75)90384-4. [DOI] [PubMed] [Google Scholar]
- 64.Künzle H. The striatum in the hedgehog tenrec: Histochemical organization and cortical afferents. Brain Res. 2005;1034:90–113. doi: 10.1016/j.brainres.2004.11.047. [DOI] [PubMed] [Google Scholar]
- 65.Lanuza E, Halpern M. Efferents and centrifugal afferents of the main and accessory olfactory bulbs in the snake Thamnophis sirtalis. Brain Behav Evol. 1998;51:1–22. doi: 10.1159/000006525. [DOI] [PubMed] [Google Scholar]
- 66.Laverghetta AV, Toledo CA, Veenman CL, Yamamoto K, Wang H, Reiner A. Cellular localization of AMPA type glutamate receptor subunits in the basal ganglia of pigeons (Columba livia) Brain Behav Evol. 2006;67:10–38. doi: 10.1159/000088856. [DOI] [PubMed] [Google Scholar]
- 67.Lázár G, Calle M, Roubos EW, Kozicz T. Immunohistochemical localization of cocaine- and amphetamine-regulated transcript peptide in the central nervous system of the frog Rana esculenta. J Comp Neurol. 2004;477:324–339. doi: 10.1002/cne.20264. [DOI] [PubMed] [Google Scholar]
- 68.Le Moal M, Simon H. Mesocorticolimbic dopaminergic network: functional and regulatory roles. Physiol Rev. 1991;71:155–234. doi: 10.1152/physrev.1991.71.1.155. [DOI] [PubMed] [Google Scholar]
- 69.Lewis JW, Ryan SM, Arnold AP, Butcher LL. Evidence for a catecholaminergic projection to area X in the zebra finch. J Comp Neurol. 1981;196:347–354. doi: 10.1002/cne.901960212. [DOI] [PubMed] [Google Scholar]
- 70.Lohman AH, Smeets WJ. Overview of the main and accessory olfactory bulb projections in reptiles. Brain Behav Evol. 1993;41:147–55. doi: 10.1159/000113832. [DOI] [PubMed] [Google Scholar]
- 71.Luo M, Perkel DJ. Long-range GABAergic projection in a circuit essential for vocal learning. J Comp Neurol. 1999;403:68–84. [PubMed] [Google Scholar]
- 72.Marin O, Smeets WJ, Gonzalez A. Distribution of choline acetyltransferase immunoreactivity in the brain of anuran (Rana perezi, Xenopus laevis) and urodele (Pleurodeles waltl) amphibians. J Comp Neurol. 1997;382:499–534. doi: 10.1002/(sici)1096-9861(19970616)382:4<499::aid-cne6>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 73.Marin O, Smeets WJ, Gonzalez A. Basal ganglia organization in amphibians: chemoarchitecture. J Comp Neurol. 1998;392:285–312. [PubMed] [Google Scholar]
- 74.Martinez-Garcia F, Olucha FE, Teruel V, Lorente MJ, Schwerdtfeger WK. Afferent and efferent connections of the olfactory bulbs in the lizard Podarcis hispanica. J Comp Neurol. 1991;305:337–347. doi: 10.1002/cne.903050214. [DOI] [PubMed] [Google Scholar]
- 75.McGeorge AJ, Faull RL. The organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience. 1989;29:503–537. doi: 10.1016/0306-4522(89)90128-0. [DOI] [PubMed] [Google Scholar]
- 76.Medina L, Reiner A. Distribution of choline acetyltransferase immunoreactivity in the pigeon brain. J Comp Neurol. 1994;342:497–537. doi: 10.1002/cne.903420403. [DOI] [PubMed] [Google Scholar]
- 77.Medina L, Reiner A. Neurotransmitter organization and connectivity of the basal ganglia in vertebrates: implications for the evolution of basal ganglia. Brain Behav Evol. 1995;46:235–258. doi: 10.1159/000113277. [DOI] [PubMed] [Google Scholar]
- 78.Medina L, Reiner A. The efferent projections of the dorsal and ventral pallidal parts of the pigeon basal ganglia, studied with biotinylated dextran amine. Neuroscience. 1997;81:773–802. doi: 10.1016/s0306-4522(97)00204-2. [DOI] [PubMed] [Google Scholar]
- 79.Meredith GE, Pattiselanno A, Groenewegen HJ, Haber SN. Shell and core in monkey and human nucleus accumbens identified with antibodies to calbindin- D28k. J Comp Neurol. 1996;365:628–639. doi: 10.1002/(SICI)1096-9861(19960219)365:4<628::AID-CNE9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 80.Mesulam MM. Patterns in behavioral neuroanatomy: Association areas, the limbic system, and hemispheric specialization. In: Plum F, Baringer JR, Gilman S, editors. Principles of Behavioral Neurology. Davis; Philadelphia: 1985. pp. 1–192. [Google Scholar]
- 81.Mezey SE, Csillag A. Selective striatal connections of midbrain dopaminergic nuclei in the chick (Gallus domesticus) Cell Tissue Res. 2002;308:35–46. doi: 10.1007/s00441-002-0514-2. [DOI] [PubMed] [Google Scholar]
- 82.Milner TA, Pickel VM, Reis DJ. Ultrastructural basis for interactions between central opioids and catecholamines. I Rostral ventrolateral medulla. J Neurosci. 1989;9:2114–2130. doi: 10.1523/JNEUROSCI.09-06-02114.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- 84.Molnar M, Casini G, Davis BM, Bagnoli P, Brecha NC. Distribution of preproenkephalin mRNA in the chicken and pigeon telencephalon. J Comp Neurol. 1994;348:419–432. doi: 10.1002/cne.903480308. [DOI] [PubMed] [Google Scholar]
- 85.Morel A, Loup F, Magnin M, Jeanmonod D. Neurochemical organization of the human basal ganglia: Anatomofunctional territories defined by the distributions of calcium-binding proteins and SMI-32. J Comp Neurol. 2002;443:86–103. doi: 10.1002/cne.10096. [DOI] [PubMed] [Google Scholar]
- 86.Morona R, González A. Calbindin-D28k and calretinin expression in the forebrain of anuran and urodele amphibians: further support for newly identified subdivisions. J Comp Neurol. 2008;511:187–220. doi: 10.1002/cne.21832. [DOI] [PubMed] [Google Scholar]
- 87.Mühlenbrock-Lenter S, Endepols H, Roth G, Walkowiak W. Immunohistological characterization of striatal and amygdalar structures in the telencephalon of the fire-bellied toad Bombina orientalis. Neuroscience. 2005;134:705–719. doi: 10.1016/j.neuroscience.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 88.Oertel WH, Schmechel DE, Tappaz ML, Kopin IJ. Production of a specific antiserum to rat brain glutamic acid decarboxylase by injection of an antigen-antibody complex. Neuroscience. 1981;61:2689–2700. doi: 10.1016/0306-4522(81)90113-5. [DOI] [PubMed] [Google Scholar]
- 89.Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I The cortico-basal ganglia-thalamo-cortical loop. Brain Res Rev. 1995;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- 90.Pérez-Clausell J, Fredens K. Chemoarchitectonics in the telencephalon of the lizard Podarcis hispanica. In: Schwerdtfeger WK, Smeets WJAJ, editors. The Forebrain of Reptiles Int Symp Recent Advances in Understanding the Structure and Function of the Forebrain in Reptiles. Karger (Basel); Frankfurt: 1988. pp. 85–96. [Google Scholar]
- 91.Pickel VM, Joh TH, Reis DJ. Ultrastructural localization of tyrosine hydroxylase in noradrenergic neurons of brain. Proc Natl Acad Sci USA. 1975;72:659–663. doi: 10.1073/pnas.72.2.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pisani A, Bernardi G, Ding J, Surmeier DJ. Re-emergence of striatal cholinergic interneurons in movement disorders. Trends Neurosci. 2007;30:545–553. doi: 10.1016/j.tins.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 93.Powers AS, Reiner A. The distribution of cholinergic neurons in the central nervous system of turtles. Brain, Behav Evol. 1993;41:326–345. doi: 10.1159/000113853. [DOI] [PubMed] [Google Scholar]
- 94.Prensa L, Giménez-Amaya JM, Parent A. Chemical heterogeneity of the striosomal compartment in the human striatum. J Comp Neurol. 1999;413:603–618. [PubMed] [Google Scholar]
- 95.Prensa L, Richard S, Parent A. Chemical anatomy of the human ventral striatum and adjacent basal forebrain structures. J Comp Neurol. 2003;460:345–367. doi: 10.1002/cne.10627. [DOI] [PubMed] [Google Scholar]
- 96.Puelles L, Kuwana E, Puelles E, Bulfone A, Shimamura K, Keleher J, Smiga S, Rubenstein JL. Pallial and subpallial derivatives in the embryonic chick and mouse telencephalon, traced by the expression of the genes Dlx-2, Emx-1, Nkx-2.1, Pax-6, and Tbr-1. J Comp Neurol. 2000;424:409–438. doi: 10.1002/1096-9861(20000828)424:3<409::aid-cne3>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 97.Reiner A. The distribution of proenkephalin-derived peptides in the central nervous system of turtles. J Comp Neurol. 1987;259:65–91. doi: 10.1002/cne.902590106. [DOI] [PubMed] [Google Scholar]
- 98.Reiner A, Anderson KD. The patterns of neurotransmitter and neuropeptide co-occurrence among striatal projection neurons: conclusions based on recent findings. Brain Res Brain Res Rev. 1990;15:251–265. doi: 10.1016/0165-0173(90)90003-7. [DOI] [PubMed] [Google Scholar]
- 99.Reiner A, Anderson KD. Co-occurrence of gamma-aminobutyric acid, parvalbumin and the neurotensin-related neuropeptide LANT6 in pallidal, nigral and striatal neurons in pigeons and monkeys. Brain Res. 1993;624:317–325. doi: 10.1016/0006-8993(93)90096-6. [DOI] [PubMed] [Google Scholar]
- 100.Reiner A, Brauth SE, Kitt CA, Quirion R. Distribution of mu, delta, and kappa opiate receptor types in the forebrain and midbrain of pigeons. J Comp Neurol. 1989;280:359–382. doi: 10.1002/cne.902800304. [DOI] [PubMed] [Google Scholar]
- 101.Reiner A, Carraway RE. Immunohistochemical and biochemical studies on Lys8-Asn9-neurotensin8-13 (LANT6)- related peptides in the basal ganglia of pigeons, turtles, and hamsters. J Comp Neurol. 1987;257:453–476. doi: 10.1002/cne.902570312. [DOI] [PubMed] [Google Scholar]
- 102.Reiner A, Davis BM, Brecha NC, Karten HJ. The distribution of enkephalinlike immunoreactivity in the telencephalon of the adult and developing domestic chicken. J Comp Neurol. 1984a;228:245–262. doi: 10.1002/cne.902280210. [DOI] [PubMed] [Google Scholar]
- 103.Reiner A, Karle EJ, Anderson KD, Medina L. Catecholaminergic perikarya and fibers in the avian nervous system. In: Smeets WJAJ, Reiner A, editors. Phylogeny and Development of Catecholamine Systems in the CNS of Vertebrates. Cambridge Univ; Cambridge: 1994. pp. 135–181. [Google Scholar]
- 104.Reiner A, Karten HJ. Comparison of olfactory bulb projections in pigeons and turtles. Brain Behav Evol. 1985;27:11–27. doi: 10.1159/000118717. [DOI] [PubMed] [Google Scholar]
- 105.Reiner A, Karten HJ, Solina AR. Substance P: localization within paleostriatal-tegmental pathways in the pigeon. Neuroscience. 1983;9:61–85. doi: 10.1016/0306-4522(83)90047-7. [DOI] [PubMed] [Google Scholar]
- 106.Reiner A, Krause JE, Keyser KT, Eldred WD, McKelvy JF. The distribution of substance P in turtle nervous system: a radioimmunoassay and immunohistochemical study. J Comp Neurol. 1984b;226:50–75. doi: 10.1002/cne.902260105. [DOI] [PubMed] [Google Scholar]
- 107.Reiner A, Laverghetta AV, Meade CA, Cuthbertson SL, Bottjer SW. An immunohistochemical and pathway tracing study on the striatopallidal organization of area X in the male zebra finch. J Comp Neurol. 2004a;469:239–261. doi: 10.1002/cne.11012. [DOI] [PubMed] [Google Scholar]
- 108.Reiner A, Medina L, Veenman CL. Structural and functional evolution of the basal ganglia in vertebrates. Brain Res Rev. 1998a;28:235–285. doi: 10.1016/s0165-0173(98)00016-2. [DOI] [PubMed] [Google Scholar]
- 109.Reiner A, Oliver JR. Somatostatin and neuropeptide Y are almost exclusively found in the same neurons in the telencephalon of turtles. Brain Res. 1987;426:149–156. doi: 10.1016/0006-8993(87)90434-3. [DOI] [PubMed] [Google Scholar]
- 110.Reiner A, Perera M, Paullus R, Medina L. Immunohistochemical localization of DARPP32 in striatal projection neurons and striatal interneurons in pigeons. J Chem Neuroanat. 1998b;16:17–33. doi: 10.1016/s0891-0618(98)00056-8. [DOI] [PubMed] [Google Scholar]
- 111.Reiner A, Perkel DJ, Bruce LL, Butler AB, Csillag A, Kuenzel W, Medina L, Paxinos G, Shimizu T, Striedter G, Wild M, Ball GF, Durand S, Gunturkun O, Lee DW, Mello CV, Powers A, White SA, Hough G, Kubikova L, Smulders TV, Wada K, Dugas-Ford J, Husband S, Yamamoto K, Yu J, Siang C, Jarvis ED Avian Brain Nomenclature Forum. Revised nomenclature for avian telencephalon and some related brainstem nuclei. J Comp Neurol. 2004b;473:377–414. doi: 10.1002/cne.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Riedel A, Härtig W, Seeger G, Gärtner U, Brauer K, Arendt T. Principles of rat subcortical forebrain organization: a study using histological techniques and multiple fluorescence labeling. J Chem Neuroanat. 2002;23:75–104. doi: 10.1016/s0891-0618(01)00142-9. [DOI] [PubMed] [Google Scholar]
- 113.Riters LV, Erichsen JT, Krebs JR, Bingman VP. Neurochemical evidence for at least two regional subdivisions within the homing pigeon (Columba livia) caudolateral neostriatum. J Comp Neurol. 1999;412:469–487. doi: 10.1002/(sici)1096-9861(19990927)412:3<469::aid-cne7>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 114.Roberts TF, Hall WS, Brauth SE. Organization of the avian basal forebrain: chemical anatomy in the parrot (Melopsittacus undulatus) J Comp Neurol. 2002;454:383–408. doi: 10.1002/cne.10456. [DOI] [PubMed] [Google Scholar]
- 115.Russchen FT, Smeets WJ, Hoogland PV. Histochemical identification of pallidal and striatal structures in the lizard Gekko gecko: evidence for compartmentalization. J Comp Neurol. 1987;256:329–341. doi: 10.1002/cne.902560303. [DOI] [PubMed] [Google Scholar]
- 116.Seroogy K, Tsuruo Y, Hökfelt T, Walsh J, Fahrenkrug J, Emson PC, Goldstein M. Further analysis of presence of peptides in dopamine neurons. Cholecystokinin, peptide histidine-isoleucine/vasoactive intestinal polypeptide and substance P in rat supramammillary region and mesencephalon. Exp Brain Res. 1988;72:523–534. doi: 10.1007/BF00250598. [DOI] [PubMed] [Google Scholar]
- 117.Shimizu T, Cox K, Karten HJ. Intratelencephalic projections of the visual wulst in pigeons (Columba livia) J Comp Neurol. 1995;359:551–572. doi: 10.1002/cne.903590404. [DOI] [PubMed] [Google Scholar]
- 118.Singru PS, Mazumdar M, Sakharkar AM, Lechan RM, Thim L, Clausen TJ, Subhedar NK. Immunohistochemical localization of cocaine- and amphetamine-regulated transcript peptide in the brain of the catfish, Clarias batrachus (Linn. ) J Comp Neurol. 2007;502:215–235. doi: 10.1002/cne.21295. [DOI] [PubMed] [Google Scholar]
- 119.Smeets WJ, Hoogland PV, Lohman AH. A forebrain atlas of the lizard Gekko gecko. J Comp Neurol. 1986a;254:1–19. doi: 10.1002/cne.902540102. [DOI] [PubMed] [Google Scholar]
- 120.Smeets WJ, Hoogland PV, Voorn P. The distribution of dopamine immunoreactivity in the forebrain and midbrain of the lizard Gekko gecko: an immunohistochemical study with antibodies against dopamine. J Comp Neurol. 1986b;253:46–60. doi: 10.1002/cne.902530105. [DOI] [PubMed] [Google Scholar]
- 121.Smeets WJ, Jonker AJ, Hoogland PV. Distribution of dopamine in the forebrain and midbrain of the red-eared turtle, Pseudemys scripta elegans, reinvestigated using antibodies against dopamine. Brain, Behav Evol. 1987;30:121–142. doi: 10.1159/000118642. [DOI] [PubMed] [Google Scholar]
- 122.Smeets WJ, Marin O, Gonzalez A. Evolution of the basal ganglia: new perspectives through a comparative approach. J Anat. 2000;196(Pt 4):501–517. doi: 10.1046/j.1469-7580.2000.19640501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Smith Y, Kieval J, Couceyro PR, Kuhar MJ. CART peptide-immunoreactive neurones in the nucleus accumbens in monkeys: ultrastructural analysis, colocalization studies, and synaptic interactions with dopaminergic afferents. J Comp Neurol. 1999;407:491–511. doi: 10.1002/(sici)1096-9861(19990517)407:4<491::aid-cne3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 124.Soha J, Shimizu T, Doupe AJ. Development of the catecholaminergic innervation of the song system of the male zebra finch. J Neurobiol. 1996;29:473–489. doi: 10.1002/(SICI)1097-4695(199604)29:4<473::AID-NEU5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 125.Spiro JE, Dalva MB, Mooney R. Long-range inhibition within the zebra finch song nucleus RA can coordinate the firing of multiple projection neurons. J Neurophysiol. 1999;81:3007–3020. doi: 10.1152/jn.1999.81.6.3007. [DOI] [PubMed] [Google Scholar]
- 126.Striedter GF, Marchant TA, Beydler S. The “neostriatum” develops as part of the lateral pallium in birds. J Neurosci. 1998;18:5839–5849. doi: 10.1523/JNEUROSCI.18-15-05839.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Subhedar N, Barsagade VG, Singru PS, Thim L, Clausen JT. Cocaine- and amphetamine-regulated transcript peptide (CART) in the telencephalon of the catfish, Clarias gariepinus: Distribution and response to Fasting, 2-Deoxy-D-glucose, glucose, insulin, and leptin Treatments. J Comp Neurol. 2011;519:1281–1300. doi: 10.1002/cne.22569. [DOI] [PubMed] [Google Scholar]
- 128.Sun Z, Reiner A. Localization of dopamine D1A and D1B receptor mRNAs in the forebrain and midbrain of the domestic chick. J Chem Neuroanat. 2000;19:211–224. doi: 10.1016/s0891-0618(00)00069-7. [DOI] [PubMed] [Google Scholar]
- 129.Sun Z, Wang HB, Laverghetta A, Yamamoto K, Reiner A. The distribution and cellular localization of glutamic acid decarboxylase-65 (GAD65) mRNA in the forebrain and midbrain of domestic chick. J Chem Neuroanat. 2005;29:265–281. doi: 10.1016/j.jchemneu.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 130.Swerdlow NR, Koob GF. Dopamine, schizophrenia, mania, and depression: Toward a unified hypothesis of cortico-striatopallido-thalamic function. Behav Brain Sci. 1987;10:197–245. [Google Scholar]
- 131.Thim L, Nielsen PF, Judge ME, Andersen AS, Diers I, Egel-Mitani M, Hastrup S. Purification and characterization of a new hypothalamic satiety peptide, cocaine and amphetamine regulated transcript (CART), produced in yeast. FEBS Lett. 1998;428:263–268. doi: 10.1016/s0014-5793(98)00543-2. [DOI] [PubMed] [Google Scholar]
- 132.Veenman CL, Albin RL, Richfield EK, Reiner A. Distributions of GABAA, GABAB, and benzodiazepine receptors in the forebrain and midbrain of pigeons. J Comp Neurol. 1994;344:161–189. doi: 10.1002/cne.903440202. [DOI] [PubMed] [Google Scholar]
- 133.Veenman CL, Reiner A. The distribution of GABA-containing perikarya, fibers, and terminals in the forebrain and midbrain of pigeons, with particular reference to the basal ganglia and its projection targets. J Comp Neurol. 1994;339:209–250. doi: 10.1002/cne.903390205. [DOI] [PubMed] [Google Scholar]
- 134.Veenman CL, Wild JM, Reiner A. Organization of the avian “corticostriatal” projection system: a retrograde and anterograde pathway tracing study in pigeons. J Comp Neurol. 1995;354:87–126. doi: 10.1002/cne.903540108. [DOI] [PubMed] [Google Scholar]
- 135.Vicario A, Abellán A, Desfilis E, Medina L. Genetic identification of the central nucleus and other components of the central extended amygdala in chicken during development. Front Neuroanat. 2014;8:90. doi: 10.3389/fnana.2014.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Voorn P, Gerfen CR, Groenewegen HJ. Compartmental organization of the ventral striatum of the rat: immunohistochemical distribution of enkephalin, substance P, dopamine, and calcium-binding protein. J Comp Neurol. 1989;289:189–201. doi: 10.1002/cne.902890202. [DOI] [PubMed] [Google Scholar]
- 137.Wild JM, Williams MN. Rostral wulst in passerine birds. I Origin, course, and terminations of an avian pyramidal tract. J Comp Neurol. 2000;416:429–450. [PubMed] [Google Scholar]
- 138.Wynne B, Güntürkün O. Dopaminergic innervation of the telencephalon of the pigeon (Columba livia): a study with antibodies against tyrosine hydroxylase and dopamine. J Comp Neurol. 1995;357:446–464. doi: 10.1002/cne.903570309. [DOI] [PubMed] [Google Scholar]
- 139.Yamamoto K, Reiner A. Distribution of the limbic system-associated membrane protein (LAMP) in pigeon forebrain and midbrain. J Comp Neurol. 2005;486:221–242. doi: 10.1002/cne.20562. [DOI] [PubMed] [Google Scholar]
- 140.Yan K, Tank YZ, Carr CE. Calcium-binding protein immunoreactivity characterizes the auditory system of Gekko gecko. J Comp Neurol. 2010;518:3409–3426. doi: 10.1002/cne.22428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Young WS, 3rd, Alheid GF, Heimer L. The ventral pallidal projection to the mediodorsal thalamus: a study with fluorescent retrograde tracers and immunohistofluorescence. J Neurosci. 1984;4:1626–1638. doi: 10.1523/JNEUROSCI.04-06-01626.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zaborszky L, Alheid GF, Beinfeld MC, Eiden LE, Heimer L, Palkovits M. Cholecystokinin innervation of the ventral striatum: a morphological and radioimmunological study. Neuroscience. 1985;14:427–453. doi: 10.1016/0306-4522(85)90302-1. [DOI] [PubMed] [Google Scholar]
- 143.Zahm DS. Functional-anatomical implications of the nucleus accumbens core and shell subterritories. Ann NY Acad Sci. 1999;877:113–128. doi: 10.1111/j.1749-6632.1999.tb09264.x. [DOI] [PubMed] [Google Scholar]
- 144.Zahm DS, Brog JS. On the significance of subterritories in the “accumbens” part of the rat ventral striatum. Neuroscience. 1992;50:751–767. doi: 10.1016/0306-4522(92)90202-d. [DOI] [PubMed] [Google Scholar]
- 145.Zahm DS, Heimer L. Specificity in the efferent projections of the nucleus accumbens in the rat: comparison of the rostral pole projection patterns with those of the core and shell. J Comp Neurol. 1993;327:220–232. doi: 10.1002/cne.903270205. [DOI] [PubMed] [Google Scholar]