Abstract
Prolonged exposure of CD8+T cells to their cognate antigen can result in exhaustion of effector functions enabling the persistence of infected or transformed cells. Recent advances in strategies to rejuvenate host effector function using Immune Checkpoint Blockade have resulted in tremendous success towards the treatment of several cancers. However, it is unclear if T-cell rejuvenation results in long-lived antitumor functions. Emerging evidence suggests that T-cell exhaustion may also represent a significant impediment in sustaining long-lived antitumor activity by chimeric antigen receptor T-cells. Here, we discuss current findings regarding transcriptional regulation during T-cell exhaustion and address the hypothesis that epigenetics may be a potential barrier to achieving the maximum benefit of T-cell based immunotherapies.
Keywords: Immune checkpoint blockade, chimeric antigen receptor T cells, T cell exhaustion, epigenetics, histone modification, DNA methylation
T-Cell Exhaustion
Recent insights into T-cell differentiation have resulted in considerable advances in our ability to exploit the host immune response to treat chronic infections and cancer. Many of these new developments have been borne out of studies aimed at gaining a deeper understanding of the mechanisms involved in a T cell’s ability to acquire stable functional properties. Upon antigen exposure, naive antigen-specific CD8+ T cells undergo a differentiation program that promotes their clonal expansion and development into highly functional cytotoxic effector T cells that can traffic to, and kill pathogen-infected or tumor cells. Once the antigen is cleared, the majority of effector T cells die with only a subset of surviving cells differentiating into long-lived memory cells that can confer long-term protection against pathogen rechallenge [1]. However, if the source of antigen is not cleared, the combination of prolonged T-cell receptor (TCR) stimulation and exposure to inflammatory signals results in progressive repression of effector function, termed T-cell exhaustion (see Glossary). Originally discovered using the mouse model of chronic lymphocytic choriomeningitis virus (LCMV) infection [2–4], the concept of T-cell exhaustion has now emerged as a general mechanism for restricting T-cell effector functions during infection with chronic viruses and the development of several human cancers [5–9]. Initial investigations into the mechanisms involved in repression of the effector response focused on changes in gene regulation among functional memory and exhausted T cells. Genome-wide gene expression profiles of effector and functional memory cells in comparison with exhausted CD8+ T cells revealed that CD8+ T cells acquire a distinct transcriptional program during the development of exhaustion. Specifically, it was reported that functionally exhausted CD8+ T cells maintained an altered metabolism, a defect in proliferation, and a high level of expression of multiple inhibitory receptors (IRs), including programmed cell death protein 1 (PD-1), cytotoxic T lymphocyte antigen 4 (CTLA-4), and T cell immunoglobulin mucin receptor 3 (Tim-3) [10–13]. Because exhausted T cells sustain expression of IRs, several investigators sought to determine their role in suppressing T-cell function during the development of exhaustion and to develop therapeutic strategies limiting IR signaling. It is now believed that T-cell exhaustion can be at least partially overcome with the administration of monoclonal antibodies (mAbs) against surface IRs, supporting the idea that effector function can be restored by blocking sustained inhibitory signals [11, 14–16]. Harnessing the therapeutic potential of host CD8+ T cells to treat various diseases has also been extended through genetic engineering of human T cells, either by cloning of endogenous TCR receptors or, by the introduction of a chimeric antigen receptor (CAR) that recognizes antigen presenting cells. Indeed, recent evidence suggests that the specificity of a T cell can be redirected to recognize and eliminate tumors, with dramatic clinical responses seen in patients with metastatic melanoma [17] and B-cell malignancies [18, 19]. Additionally, such genetic modification strategies appear to be a promising new immunotherapeutic approach for the treatment of more types of cancer. Below we discuss the most recent advancements in T cell-based immunotherapy and the current limitations and challenges of using Immune checkpoint blockade (ICB) and adoptive T cell therapy (ACT) approaches.
Immune Checkpoint Blockade Therapy
Many clinical advances in cancer immunotherapy have been based on the finding that blockade of immune IR signaling on the pool of exhausted T cells can reestablish effector function and promote antitumor activity. The emergence of ICB therapy as a strategy to treat cancer was founded on the ability of mAbs to block the interaction between IRs -- such as CTLA-4 and PD-1-- on exhausted T cells, as well as their corresponding ligands on antigen-presenting cells [20]. Blocking such inhibitory interactions promotes expansion and recovery of effector function in exhausted T cells, leading to viral control or tumor regression in preclinical models and cancer patients [11, 14, 21]. Indeed, the success of ICB therapy has been evident for treating a growing list of cancer types, including melanoma, non-small cell lung carcinoma, Hodgkin’s lymphoma, head and neck cancer, mismatch repair-deficient tumors, renal cell carcinoma, ovarian, and bladder cancers, highlighting the fact that such therapeutic strategies are a promising frontier in cancer therapy [20]. The first FDA approved IR-based therapy, ipilimumab, is a mAb targeting CTLA-4. Its use was shown to improve the clinical outcome of patients with advanced melanoma, with a survival rate of at least 3 years in approximately 20% of patients receiving the drug [20]. The clinical success of ipilimumab paved the way for extending ICB strategies to additional IRs (e.g., PD-1) and their corresponding ligands. Recently, the FDA approved mAbs to block the interaction between PD-1 and its ligand, programmed death ligand-1 (PD-L1). Anti-PD-1 mAb drugs (nivolumab and pembrolizumab), and anti-PD-L1 mAb drug (atezolizumab) have improved objective response rates and decreased toxicities in several types of cancer, including advanced melanoma, non-small-cell lung carcinoma, and renal cell carcinoma [22]. Although some patients exhibit partial or complete responses to ICB therapy, many patients remain unresponsive [23]. Additionally, a recent phase I clinical trial (NCT01295827) reported that while pembrolizumab was initially efficacious in patients with advanced melanoma, approximately 30% developed resistance and subsequent tumor relapse within 2 years [24]. Because the efficacy of ICB therapy is currently limited, it is important to understand the molecular barriers preventing treatment success.
Challenges of Immune Checkpoint Blockade Therapy
Despite unprecedented clinical success, a large proportion of patients with cancer fail to present an objective response following ICB therapy [20]. In addition, some patients initially responsive to ICB treatment, experience relapses [24]. These failures in ICB therapy highlight the need to better dissect the potential mechanisms of resistance, and/or the lack of durable responses in some patients. Among the potential challenges to achieving successful ICB responses are (1) the absence of host tumor-specific T cells due to negative selection or physical deletion by cell death of severely exhausted cells; (2) an inability to reactivate the exhausted T cells that are present within the immunosuppressive tumor microenvironment (TME); and/or (3) a failure of re-activated cells to expand and sustain their ability to express effector cytokines and exert tumor-killing activity. These limitations have prompted investigators to question which subset of exhausted T cells might be responding to ICB. Furthermore, several studies are now moving towards addressing whether ICB-responding T cells acquire a specific gene expression program that leads to the generation of long-lived memory T cells, or, whether they revert back to an exhausted state.
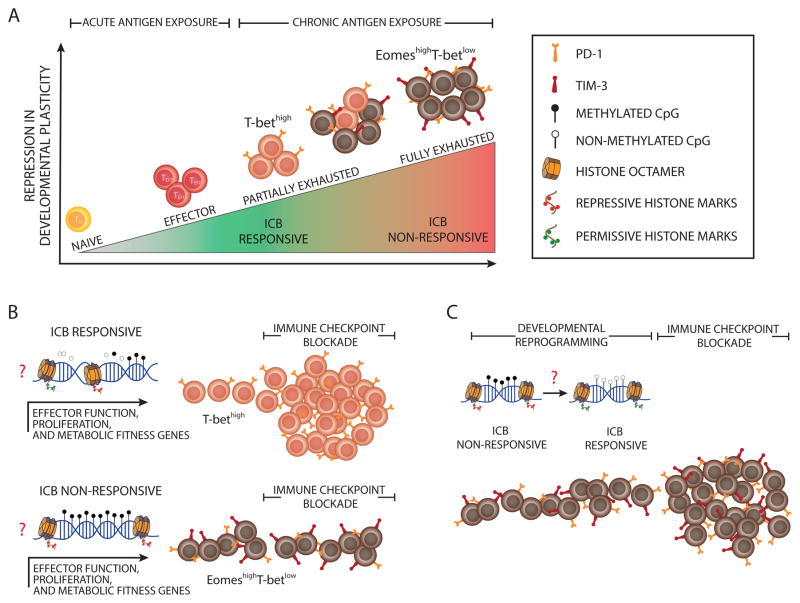
Building upon the initial insight into the role IRs play in T-cell exhaustion, further phenotypic and transcriptional characterization of exhausted T cells have resulted in the discovery of a functional heterogeneity among the pool of exhausted T cells. For instance, the cumulative coexpression of multiple IRs, including PD-1 and Tim-3, has been found to be associated with an increase in the degree of T-cell exhaustion, as indicated by the progressive inability of the more exhausted cells to produce effector cytokines (IL-2, TNFα, and eventually IFNγ), and the gradual loss of their proliferative capacity (Key Figure, Figure 1A) [16, 25, 26]. Key experiments defining the subsets of exhausted T cells and their lineages in a murine model of chronic LCMV infection and in individuals with chronic or resolved Hepatitis C Virus (HCV) infections have revealed the differential expression of T-box transcription factors T-bet and Eomesodermin (Eomes) in these cells [27]. These studies showed that a progenitor subset of exhausted cells, phenotypically characterized as T-bethigh–Eomeslow, retained the potential for ICB-mediated rejuvenation [27]. By contrast, terminally differentiated exhausted T cells (defined as expressing low T-bet and high Eomes) exhibited poor ICB responsiveness (Key Figure, Figure 1) [27]. Thus, severely exhausted T cells (terminally differentiated) have been deemed as refractory to ICB-mediated rejuvenation (Key Figure, Figure 1B) [28]. In an effort to further define which subset(s) of exhausted T cells respond to ICB, two recent studies using the mouse model of chronic LCMV infection reported that the transcription factor Tcf1 (Tcf7 gene) was critical in maintaining the ICB-responsive subset of exhausted T cells expanding during PD-1 blockade [26, 29]. Another group reported a similar finding but ascribed the proliferative burst to a less differentiated CD8+ T cell subset [26], presumably deriving more recently from the thymus. Broadly, each of these studies conclude that a subset of CD8+ T cells, not fully committed to the exhausted fate, provide the proliferative T-cell burst following ICB [30]. However, it remains unclear whether such lack of responsiveness to ICB is due to an inability to rescue the functional response from severely exhausted T cells or whether it is due to the decreased number of antigen-specific T cells resulting from cell death. These data raise new questions about both the current success and failure associated with the clinical use of ICB, as well as whether new therapeutic approaches can be designed to reactivate and enlist fully exhausted T cells into fighting tumors.
Key Figure, Figure 1. Epigenetic Barriers to Immune Checkpoint Blockade-Mediated Rejuvenation of T Cells.
(A) The schematic depicts the repression in developmental plasticity acquired by antigen-specific T cells during clonal expansion in response to acute or chronic antigen exposure. Upon acute antigen exposure, naive CD8+ T cells clonally expand and differentiate into effector cells. During chronic high-level antigen exposure, persistent T cell receptor (TCR) stimulation can cause T cell exhaustion. Effector function is progressively repressed during the development of T cell exhaustion, thereby leading to a heterogeneous population of T cells at various levels of exhaustion. Partially exhausted T cells are phenotypically defined by sustained expression of a minimal number of immune inhibitory receptors (IRs) and characterized by differential expression of T-bet and Eomes (T-bethigh Eomeslow T cells), whereas fully exhausted T cells are marked by co-expression of multiple IRs. The immune checkpoint blockade (ICB) obstructs inhibitory signals from immune IRs, resulting in rejuvenation of partially exhausted T cells. (B) The schematic depicts the effect of progressive epigenetic programming that reinforces silencing of genes involved in effector function, proliferation, and metabolic fitness of exhausted T cells. Partially exhausted T cells may retain a degree of epigenetic plasticity at exhaustion-specific silenced genes manifested by partially methylated DNA and deposition of fewer repressive, more permissive histone marks. Terminally differentiated exhausted T cells may be more epigenetically restrained through fully methylated DNA, the deposition of more repressive histone marks, and the loss of permissive histone marks. (C) The diagram models the potential reprogramming of exhaustion-associated epigenetic programs that could be done to remove cell-intrinsic barriers to achieving a better, possibly durable rejuvenation response of fully exhausted T cells following ICB therapy.
Gene expression programs associated with the progressive development of T-cell exhaustion can become reinforced, meaning that multiple exhaustion-associated transcriptional features, such as upregulated PD-1 expression and poor ICB-responsiveness, are stably maintained even after antigen withdrawal. Indeed, this has been demonstrated by adoptive transfer of exhausted T cells into an antigen-free environment, or by showing viral load control ,as in the case of HIV-1 elite controllers or patients exhibiting successful viral control following antiretroviral treatment [31–36]. These findings suggest that a stable cell-intrinsic mechanism is acquired in exhausted T cells (Key Figure, Figure 1). Stabilization of T-cell exhaustion programs may not only limit the rejuvenation capacity of exhausted T cells during ICB treatment but also restrict the ability of rejuvenated cells to generate long-lived antitumor immunity after tumor clearance, and this may be linked to the observed lack of protection against tumor relapses in some treated patients. Although several clinical trials have suggested that the successful objective response following ICB therapy is durable [20, 24, 37, 38], tumor relapses occur in approximately one-third of patients responding to PD-1 blockade, as stated earlier [24]. Such findings highlight the crucial need to understand the difference between acquiring a certain degree of longevity of tumor control after ICB therapy and the requirements for generating tumor-specific T-cell memory [39–41]. If indeed there are heritable “cell division–transmissible” programs repressing the expression of genes regulating effector function, expansion potential, and metabolic fitness of exhausted T cells, in addition to sustaining upregulated expression of IRs, it is plausible to hypothesize that these programs may ultimately terminate the function of the expanded pool of T cells following ICB treatment. Thus, it is possible that ICB-responsive T cells would then be incompetent to generate durable host immunity against the same tumor antigens. This potential contribution of cell-intrinsic barriers to ICB failures makes it necessary for future studies to dissect the molecular mechanisms regulating the maintenance of an exhaustion-associated transcriptional program.
Does Epigenetic Programming Stabilize T-Cell Exhaustion?
Transcriptional programming during cellular differentiation is regulated through coordinated recruitment and/or eviction of transcription factors [42]. The long-term maintenance of acquired transcriptional states is often mediated by stable covalent changes, known as “epigenetic modifications,” to DNA and histone proteins [43]. Epigenetic changes modulate chromatin structure, which regulates the accessibility of transcription factors to specific DNA sequences. In general, the acquisition of DNA methylation or “repressive” histone marks at gene-regulatory regions is coupled with transcriptional repression. By contrast, DNA demethylation or the acquisition of “permissive” histone marks is coupled with an active or poised state of gene expression (Key Figure, Figure 1B) [44]. Acquired epigenetic modifications can be faithfully propagated during cell division after the absence of the initial triggers of epigenetic programming. Such stability of epigenetic programs is fundamental to establishing and maintaining “transcriptional memory” of the environmental stimuli and differentiation cues that a cell transiently experiences [45]. Mounting evidence indicates that key developmental and functional aspects of effector and memory CD8+ T cells are coupled to subset-specific epigenetic modifications, including DNA-methylation programs and histone modifications [46–54] (Box 2).
BOX 2. Epigenetic Regulation of CD8+ T-Cell Development and Differentiation.
Growing evidence suggests that dynamic epigenetic reprogramming is coupled to T-cell development and differentiation. A recent study reported that a stable DNA-methylation program at the proximal enhancer of the cd4 locus (CD4) is essential to enforce cd4 gene silencing in CD8+ T cells, whereas CD4+ T cells lack this program, suggesting an important role of epigenetic programming in securing cell-identity during T-cell development [49]. Genome-wide DNA-methylation profiling of naïve and effector virus-specific CD8+ T cells during acute LCMV infection have revealed that many promoters and putative regulatory cis-elements of effector genes become demethylated during effector T-cell differentiation, along with differential methylation programming at naïve/effector-specific transcription factor binding sites [47]. In another study using a mouse model of influenza infection, mapping histone modifications in antigen-specific CD8+ T-cells showed that the loci of effector genes acquired permissive histone marks during effector and memory T-cell differentiation [52]. Collectively, these data provide evidence that changes in epigenetic programs are coupled with transcriptional reprogramming linked to CD8+ T-cell effector function and fate decision.
The development of T-cell exhaustion may also be regulated by stable epigenetic programming. It was recently reported that the Pdcd1 locus (PD-1 gene) in antigen-specific CD8+ T cells undergoes DNA-demethylation programming during the effector stage of the immune response after viral infection, as in the case of LCMV infection in mice [33]. While functional memory T cells, generated after acute antigen exposure, were able to remethylate the regulatory regions in the Pdcd1 locus, the demethylation epigenetic program was preserved after chronic antigen exposure and coupled to the sustained expression of PD-1 on exhausted T cells [33, 34]. Even after antigen levels were diminished -- as observed in HIV-1 elite controllers or in patients that have successfully controlled HIV-1 infection following antiretroviral treatment --the demethylation status of the Pdcd1 locus was maintained in HIV-1-specific CD8+ T cells. These data suggest that the stability of exhaustion-associated transcriptional programming is regulated by cell-intrinsic mechanisms, possibly through fixation of acquired epigenetic programs [34]. In a recent set of experiments, it was shown that antigen-specific CD8+ T cells isolated from mice during the effector stage of chronic LCMV infection, and adoptively transferred into an antigen-free environment, retained a stable exhaustion-associated demethylation program in the promoter of the Pdcd1locus, even after the T cells were rested from TCR stimulation for 1 month in vivo. Importantly, these cells were still partially resistant to therapeutic PD-1 blockade during a recall LCMV response in the infected mice [35]. These findings further support the idea that exhaustion-associated transcriptional programs are fixed, even after antigen clearance. However, given that epigenetic modifications may become reinforced, it is unclear how these programs are stabilized, and whether they can provide a cell-intrinsic resistance mechanism against ICB-mediated rejuvenation of T cells (Key Figure, Figure 1). The answer to these questions would translate into identifying novel epigenetic targets in exhausted T cells, aiming to extend the dynamic trajectory of the success in cancer immunotherapy (Box 3).
BOX 3. Combinatorial Immunotherapies and Epigenetic Reprogramming Approaches.
Despite the significant benefit of ICB monotherapy in some patients with cancer, many patients fail to show successful and/or durable clinical response. Therefore, various combinations of treatment modalities with ICB are now under clinical investigation for several cancer types. Growing evidence from animal tumor models suggest that combined blockade of multiple IRs (e.g., PD-1, CTLA-4, Tim-3, LAG-3) enhances the rejuvenation of exhausted antitumor T cells [15, 74, 75]. These promising preclinical studies have prompted investigating whether clinical combination of immunotherapies that target non-redundant inhibitory signaling pathways could enhance the efficacy of ICB and/or successfully treat a larger proportion of patients. Recent clinical trials in patients with metastatic melanoma showed that treatment with nivolumab plus ipilimumab resulted in a greater objective response rate and longer progression-free survival compared to monotherapy [76, 77]. This has now fueled more clinical trials to test this combinatorial strategy in other types of cancer.
Epigenetic therapy holds a great potential for combinatorial approaches with ICB therapy. Although the rationale for epigenetic therapy has been widely founded on extensive studies of epigenetic abnormalities in tumor cells driving their transformation -- such as epigenetic silencing of tumor-suppressor genes, emerging evidence suggests a mechanistic immune evasion role of epigenetic reprogramming in tumor cells by silencing immune-related genes and/or tumor-specific antigens. Studies using murine models of ovarian cancer showed that production of Th1-type chemokines (e.g., CXCL9, CXCL10) in tumors is epigenetically repressed and associated with poor T-cell infiltration in the tumor. Treatment with epigenetic modulators, such as DNA- or EZH2-methyltransferase inhibitors, enhanced CXCL9 and CXCL10 chemokine production and T-cell trafficking into the tumor and potentiated tumor regression following ICB therapy [78, 79]. Another study using a melanoma mouse model showed that treatment with a DNA demethylating agent induced upregulation of a type I IFN-gene signature and augmented tumor responses to anti-CTLA-4 mAb treatment [73]. Additionally, several studies suggested that primary sensitization of tumor cells with DNA demethylating agents would enhance the clinical benefit of ICB by improving tumor-antigen presentation and/or upregulating PD-L1 expression on tumor cells (reviewed in [72]).
Given the plausible rationale for combining epigenetic and immune therapies, the concept of epigenetic stabilization of a T-cell exhaustion program -- and its potential contribution to restraining ICB efficacy -- may establish another platform for identifying novel epigenetic targets in exhausted T cells. It may also be possible to better identify the impaired ability of rejuvenated T cells to generate long-lived memory, providing a rationale for developing new combinatorial epigenetic modulator strategies to treat cancer.
The progressive development of T-cell exhaustion described above is largely associated with corresponding changes in gene expression [26, 30]. Such progressive changes in transcriptional programming suggest that the switch from ICB-responsive to unresponsive may be associated with a stable epigenetic state (Key Figure, Figure 1A). Although the potential regulatory role of epigenetic programming in reinforcing terminal differentiation of exhausted T cells remains to be fully explored, one explanation for the restricted rejuvenation capacity of fully exhausted T cells might be that progressively acquired repressive epigenetic modifications -- through methylation of CpG sites in gene regulatory regions and/or deposition of repressive histone marks -- reinforce silencing of genes involved in effector function, proliferation, and metabolic fitness (Key Figure, Figure 1B). As such, fully exhausted T cells could be epigenetically restrained and, consequently, committed to an ICB-refractory state of differentiation (Key Figure, Figure 1B). Although speculative, such ideas do merit further investigation. For example, characterization of histone modifications and DNA methylation changes during the development of T-cell exhaustion and following ICB may be informative in identifying stable exhaustion-associated programs. These proposed studies and others may help design novel approaches to manipulate the epigenome of exhausted T cells.
Irrefutably, ICB therapy can enhance antitumor immune responses by circumventing some of the extrinsic (employed by the tumor) and intrinsic (implicit to T cells) mechanisms that generally facilitate T-cell exhaustion. However, this treatment modality may be less efficacious for patients whose pool of antitumor T cells contains a disproportionate frequency of terminally differentiated exhausted T cells. Alternatively, it may also be largely ineffective in patients failing to establish immunological antitumor memory following ICB therapy, which may be accompanied by higher rates of tumor relapse. In order to broaden the scope of treatment options for patients who relapse or fail to exhibit an objective response following ICB therapy, a viable alternative may be to expand the pool of tumor-specific T cells by genetically engineering human T cells. In the following section, we describe how the use of chimeric antigen receptors (CARs) shows promise in overcoming some of the shortcomings of ICB therapy.
Chimeric Antigen Receptor T Cells to Overcome Exhaustion
The field of genetically engineering T cells is one of the newest and fastest growing branches of cancer immunotherapy. Interest in gene-transfer approaches focus on the ability of these therapies to bypass negative selection and other mechanisms of immune tolerance resulting in an increased repertoire of tumor-specific T cells [55]. Recent advances in genetic engineering enable T-cell transduction with CARs so that they can be redirected to and activated by tumor antigens. CAR T cells combine single-chain variable fragments (antibody-derived extracellular domain) with T cell–signaling domains (intracellular domains) to induce T-cell activation upon antigen binding. Although the field of adoptive T-cell immunotherapy using transduced T cells with antitumor receptors is still in its infancy, clinical trials are underway and have shown clinically significant anti-tumor responses in chronic lymphocytic leukemia, B-cell lymphoma, melanoma, and neuroblastoma [56]. The benefits of CAR-based therapies stem from four key abilities of CAR T cells: (1) targeting tumor antigens with specificity, (2) targeting surface antigens in a major histocompatibility complex (MHC)-unrestricted manner, (3) harnessing the cytolytic potential of cytotoxic T lymphocytes, and (4) inducing the expansion and persistence of CAR T cells for long-term responses. Historically, the field of adoptive T cell therapy was pioneered by Steven Rosenberg and colleagues, who harnessed the tumor killing activity of autologous lymphocytes from patients’ tumors (referred to as tumor-infiltrating lymphocytes; TILs). They developed methods for the isolation and culture of TILs that could be reinfused into patients following preconditioning with lymphodepleting regimens [17]. As has been shown in clinical trials evaluating the efficacy of adoptive transfer of TILs and the use of mAbs to block the inhibitory signals from co-IRs (e.g., ipilumimab for CTLA-4, and nivolumab or pembrolizumab for PD-1) on T cells, emerging studies suggest that, as a single modality treatment, CAR T cells are efficacious in many settings, all but for a minor subset of patients, [A1] This may be partially due to the redundant regulatory mechanisms that are inherent to all T cells, in addition to our limitations in understanding which T-cell characteristics are essential and predictive of antitumor activity.
Chimeric Antigen Receptor Limitations and Strategies for Improvement
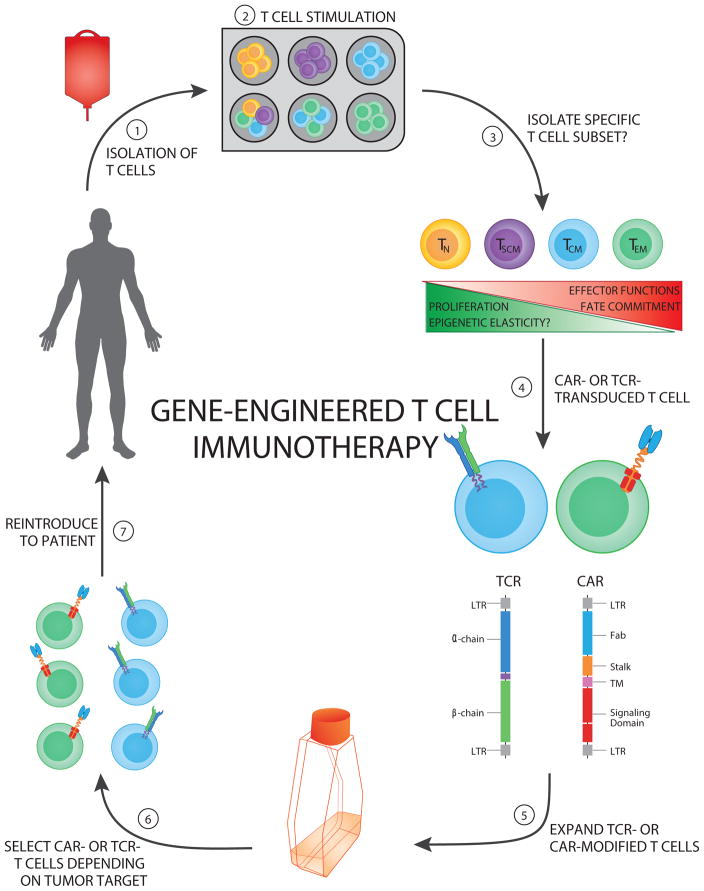
Current clinical protocols use polyclonal T-cell populations as a starting point for the transduction of CAR-modified T cells, but few studies have addressed whether an optimal T-cell subtype and/or manufacturing system should be followed when generating CAR T cells. Recent evidence from clinical reports suggests that the starting cell population, method of expansion, tumor type being treated, and design/engineering approach can all greatly affect the persistence and function of CAR T cells [19, 57]. CAR T-cell persistence most likely plays a major role in determining the efficacy of the anti-tumor response, but due to the lack of consensus on the design of clinical trials among U.S. research institutions, it is difficult to pinpoint which of these factors will contribute to long-term memory against tumor-specific antigens. CAR T cells designed with the 4-1BB–costimulatory domain (as opposed to CD28 signaling components) have been shown to display superior antitumor activity, characterized by reduced tumor burden, decreased exhaustion during ex vivo expansion, and increased persistence in vivo in xenograft mouse models [58, 59]. Furthermore, in a recent clinical trial utilizing CD19 CAR T cells containing the 4-1BB signaling domain to treat patients with refractory CD19+ leukemia and lymphoma, prolonged persistence of CAR T cells was observed (out to 2 years); this exceeded the persistence observed in a similar study utilizing CD19+ CAR T cells containing the CD28 signaling domain [60, 61]. However, the differentiation status of transduced T cells and the conditioning regimen a patient undergoes prior to reintroducing the engineered T cells (e.g., addition or exclusion of alkylating agents, fludarabine, total body irradiation) can also profoundly affect the persistence and cytotoxic potential of CAR T cells [62] (Figure 2). In fact, several studies have shown that lymphodepletion, either by total-body irradiation or cytotoxic drug administration, can create a niche for transferred T-cell expansion and induce homeostatic proliferation due to the accumulation of homeostatic cytokines (e.g., IL-7 and IL-15) [56, 63–65] . Moreover, in vitro culture conditions that stimulate (via anti-CD3/CD28 or stimulator cells) and expand (via cytokines, such as IL-2) T-cell populations, can alter the differentiation status and effector function of such engineered T cells (Figure 2). Although it is unclear whether specific frequencies of infused CAR T cell subsets (e.g., naïve, TN; stem cell memory, TSCM; effector, TEFF; central memory, TCM; or effector memory TEM) have greater clinical efficacy in the context of adoptive T cell therapy, the frequency of CAR-modified T cells with a TSCM-like phenotype positively correlates with their in vivo expansion in patients with B-cell malignancies [66]. Thus, since the differentiation status and effector function of such engineered T cells markedly affect antitumor efficacy, engraftment, and T-cell expansion, efforts are increasing to preserve a more primitive (i.e., less differentiated) T-cell pool and increase the benefits afforded by adoptive T cell therapy (Figure 2) [67, 68].
Figure 2. Genetically-Engineered T cell Immunotherapeutic Approaches.
The schematic depicts the multiuse process of generating genetically engineered human T cells targeted against specific tumor antigens.
(Step 1) Autologous T cells can be isolated from a patient’s peripheral blood mononuclear cells or an excised tumor.
(Step 2) T cell pools or specific subsets can be stimulated with (1) activating CD3 antibody (OKT3), (2) anti-CD3/CD28–coated beads, or (3) allogeneic feeder cell lines.
(Step 3) Specific T cell subsets can be sorted or enriched according to their differentiation status to exploit proliferative and effector functions.
(Step 4) Engineered T cells can be generated by cloning either T cell receptors (TCRs) or chimeric antigen receptors (CARs) and transducing a patient’s T cells with retro- or lenti-viruses, thus redirecting recognition toward tumor-associated antigens.
(Step 5) Engineered T cells can then be expanded in vitro in the presence of conditioning cytokines (e.g., IL-2 or IL-15) to increase the frequency of tumor-specific T cells generated.
(Step 6) Engineered T cells specific for an antigen (target) of interest are selected.
(Step 7) Engineered T cells can then be reinfused into the patient (usually post-lymphodepletion).
In addition to cell-intrinsic factors, several extrinsic factors limit the clinical efficacy afforded by CAR T cells. For instance, some extrinsic challenges are attributed on the one hand to suppressive components of the TME, such as inhibitory cytokines (e.g., TGF-β and IL-10), inhibitory cell types (e.g., regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs)), as well as inhibitory surface proteins (e.g., PD-L1 and PD-L2 signaling pathways [69, 70]. On the other hand, other challenges are inherent to the nature and tissue-specificity of different solid tumors, which can greatly limit the quantity and quality of tumor-specific antigens being targeted, due to an increased likelihood of off-target toxicities in these types of tumors [69, 70]. One of the greatest obstacles with CAR T cell therapy to be overcome is the identification of suitable targets. This is problematic because of the lack of technological resources to identify novel tumor-specific antigens, as well as the fact that several candidate antigens are also expressed in vital organs. Importantly, several approaches to overcome these limitations are actively being explored by various research institutions. Indeed, attempts are being made to modulate the epigenetic profile of exhausted T cells exposed to chronic levels of antigen (Key Figure, Figure 1). For instance, a recent study reported that exhausted CD8+ T cells isolated from chronic LCMV-infected mice presented reduced levels of the permissive diacetylated histone H3 epigenetic mark compared to effector or memory CD8+ T cells [71]. In vitro treatment of exhausted T cells with an HDAC inhibitor restored diacetylated histone H3 levels, which were associated with improved CD8+ T cell potential to express effector cytokines upon antigen-stimulation. Interestingly, treated T cells could develop into functional memory cells upon adoptive transfer into an antigen-free environment [71]. Of note, studies are currently ongoing to modulate the intracellular signaling domains within a same CAR; these involve CAR T cells harboring third generation CARs that combine CD3ζ, CD28, and 4-1BB signaling domains, yielding promising results. Thus, it will be interesting to follow how these exciting investigations unfold.
Concluding Remarks
There are various instances of ICB success to treat different forms of cancer. In addition, several promising preclinical results and emerging models of immunotherapeutic approaches such as CAR therapy have been reported. Consequently, there is a sound rationale for combining treatment modalities to induce broader antitumor responses. Although the number of potential immunotherapeutic combinations is almost endless, there is growing evidence of the critical role of epigenetic programming in cell transformation and malignancy; this area has attracted special attention in the design of strategies that combine epigenetic modulators and ICB therapy [72]. The exact mechanisms by which epigenetic sensitization improves immunotherapy are still unknown, but several hypotheses are being tested, including that epigenetic therapy (1) increases the expression of tumor-specific antigens (such as cancer testis antigens), especially important in cancers that alter antigen processing to evade recognition by the immune system; (2) inhibits the suppressive function of the TME, by altering epigenetic programs involved in immune evasion, either in the tumor (Box 3), or in immunosuppressive cell types (e.g., Tregs and MDSCs); and/or (3) positively modulates directly and/or indirectly the development and effector function of cytotoxic immune cells, ultimately increasing the killing of transformed cells. Interestingly, treatment with DNA demethylating agents was recently shown to enhance the response to anti-CTLA-4 treatment in a melanoma mouse model; this was achieved by inducing re-expression of endogenous retrovirus dsRNA that triggers type I interferon immune responses in cancer cells [73].
While highly compelling, the specific role of exhaustion-associated epigenetic programs on the TILs’ responsiveness to ICB, and their ability to generate antitumor memory, remains largely unexplored. A better understanding of the fundamental mechanisms involved in the development of T-cell exhaustion is urgently needed to enhance the therapeutic efficacy of ICB and combination therapies to treat malignancies. This information will also very likely contribute to the optimization of existing and newly emerging CAR T cell strategies. Undoubtedly, the recent success of T cell-based immunotherapies is the result of a brilliant marriage between clinical and basic research. While extremely exciting, it is likely that we have just scratched the surface of the potential that T-cell immunotherapy has to offer (see Outstanding Questions and Box 1). It will be of great interest to see how resolution of the questions outlined above furthers the development of this exploding field.
Outstanding Questions.
What cell-intrinsic mechanisms reinforce the stability of transcriptional repression during the development of T cell exhaustion?
Are specific epigenetic programs progressively acquired during the terminal differentiation of exhausted T cells?
Does this exhaustion-associated epigenetic programming restrict the therapeutic efficacy of immune checkpoint blockade?
How are combination therapies independently and mutually influenced by epigenetic changes in a cell? Conversely, how do combination therapies independently and mutually influence epigenetic changes in a cell? How might these changes affect treatment outcomes?
Does the fixation of exhaustion-associated epigenetic programs restrain the ability of rejuvenated T cells to generate long-lived immunity after ICB therapy?
Can developmental reprogramming of terminally differentiated exhausted T cells restore their responsiveness following ICB treatment?
Which is the best human T cell subset for adoptive T cell therapy, taking into account balanced attributes for optimum activation, in vitro expansion, killing capacity, and in vivo persistence?
Can epigenetic reprogramming of CAR T cells during in vitro expansion modulate their long-term potential for in vivo persistence and killing activity?
BOX 1. The Clinician’s Corner.
Exhaustion of persistently antigen-stimulated CD8+ T cells, as in the case of cancer or chronic infections, is associated with a unique transcriptional program characterized by upregulated expression of surface IRs (e.g., PD-1, CTLA-4) and impaired effector functions.
Rejuvenation of exhausted anti-tumor T cells by blocking their surface IRs (immune checkpoint blockade; ICB) has revolutionized the field of cancer immunotherapy with unprecedented success in treating multiple types of cancer.
Many patients with cancer fail to show clinical benefit following ICB and some treated patients experience tumor relapses, raising questions on the molecular mechanisms regulating T cell responsiveness to ICB therapy.
Growing evidence indicates that partially exhausted T cells are the main responders to ICB, whereas severely exhausted cells exhibit poor ICB-responsiveness and stably retain many of the exhaustion-associated transcriptional features even after antigen clearance, suggesting cell-intrinsic barriers in exhausted T cells to ICB therapy.
Epigenetic stabilization of exhaustion-associated transcriptional programs in CD8+ T cells may remain as a potential cell-intrinsic barrier that restrains their ICB-responsiveness and possibly enforces reversion of rejuvenated T cells into the exhausted state. Identifying these epigenetic programs will facilitate development of novel therapeutic strategies to manipulate these epigenetic targets. Preclinical and ongoing clinical studies show promising synergistic potential of standard epigenetic therapies in combination with T cell-based immunotherapy.
Trends.
Immune checkpoint blockade-mediated expansion of partially exhausted CD8+ T cell has resulted in unprecedented clinical responses in multiple types of cancer.
Multiple hallmarks of transcriptional programming during the development of T cell exhaustion are stably inherited, even after the level of specific antigen has diminished.
Stability of a permissive transcriptional program at the PD-1 promoter in exhausted CD8+ T cells is coupled to stable epigenetic modifications.
Exhausted T cells remain partially refractory to immune checkpoint blockade therapy, even after resting in an antigen-free environment.
Current CAR T cell therapeutic approaches remain vulnerable to T cell exhaustion
Acknowledgments
We thank Dr. Nisha Badders for scientific editing (St. Jude Children’s Research Hospital). This work was supported by the National Institutes of Health grant 1R01AI114442 (to B.Y.), R01 AI107625 (to P.T.) and ALSAC (to B.Y. and P.T.).
Glossary
- T-cell exhaustion
Dysfunctional state acquired by T cells experiencing persistent TCR stimulation characterized by upregulated expression of immune inhibitory receptors (e.g., PD-1, CTLA-4, Tim-3), impaired effector function, poor proliferation, and metabolic defects.
- Murine lymphocytic choriomeningitis virus (LCMV) infections
Two strains of LCMV—Armstrong and Clone 13—differing in two amino acids only, have been widely used to study host immune responses during acute vs. chronic virus infection. In mice, the Armstrong strain is cleared within 8–10 days post-infection, whereas the Clone 13 strain causes a more disseminated chronic infection with higher viral titers that persist for a prolonged period of time.
- Inhibitory receptors (IRs)
Various immune checkpoints are inducibly expressed on the surface of activated T lymphocytes to tightly control T cell function, not only during exhaustion, but also during acute T cell activation, differentiation, and autoimmune responses. Immune IRs, such as CTLA-4 and PD-1, negatively regulate T cell function using different regulatory mechanisms, including competition against co-stimulatory receptors for the binding of a common ligand; interfering with activating signals downstream of the TCR; or upregulating inhibitory genes involved in T cell suppression.
- Immune checkpoint blockade (ICB)
Therapeutic strategy to block inhibitory signaling from immune IRs and their ligands. Several mAbs blocking CTLA-4 or PD-1—PD-L1 inhibitory interactions (generally on T cells) have recently gained FDA approval for the treatment of various malignancies, and remain some of the most promising anti-oncogenic treatment strategies.
- Chimeric antigen receptors (CARs)
Genetically engineered receptors designed to combine both antigen-specificity and T cell-activating properties in a single molecule. They can be expressed on human T cells to target specific tumor antigens.
- Ipilimumab
Fully human mAb that blocks the surface inhibitory receptor CTLA-4 (Yervoy, Bristol-Myers Squibb Company). It was approved by the FDA in March 2011 to treat metastatic melanoma, and the approval was extended in 2015 to treat surgically resectable melanoma.
- Nivolumab
Fully human mAb that blocks the surface inhibitory receptor PD-1 (Opdivo, Bristol-Myers Squibb Company). It was approved by the FDA in December 2014 to treat metastatic melanoma. Growing clinical evidence of its efficacy in several types cancer resulted in extending the FDA approval of nivolumab to also treat stage IV non-small cell lung cancer (NSCLC; squamous and adenocarcinoma), renal cell carcinoma, Hodgkin’s lymphoma in 2015 and 2016. A combination of nivolumab with ipilimumab has been approved to treat unresectable or metastatic melanoma.
- Pembrolizumab
Humanized mAb that blocks the PD-1 receptor (Keytruda, Merck Sharp and Dohme Corp.). It was approved by the FDA in September 2014 to treat metastatic melanoma, then extended to treat stage IV NSCLC and recurrent or metastatic head and neck squamous cell carcinoma in 2015 and 2016.
- Atezolizumab
Humanized mAb that block the PD-L1 ligand (Tecentriq, Genentech Inc.). It is the first anti-PD-L1 product to be approved by the FDA in 2016 and has been indicated to treat the most common type of bladder cancer, called urothelial carcinoma.
- Antigen-presenting cells
Heterogeneous populations of immune cells that can display antigen-derived epitopes over their surface after being complexed with the major histocompatibility complex proteins (MHC) class I or II. These epitopes can be recognized by T cells using their antigen-specific T-cell receptor (TCR). Intracellular pathogen-infected or transformed cells can also present pathogen- or tumor-derived epitopes complexed with MHC class I proteins to be recognized by CD8+ T cells.
- T-bet and Eomesodermin
T-bet (T-box expressed in T cells) and Eomes (eomesodermin): T-box transcription factors that play regulatory roles in the development and functions of immune cells including T lymphocytes and NK cells. T-bet was originally identified as a master lineage-defining transcription factor that promotes the differentiation of mouse Th1 CD4+ T cells while inhibiting Th2 and Th17 CD4+ T cell differentiation. Additionally, upregulated expression of T-bet and Eomes is critical for regulating effector and effector memory CD8+ T cell differentiation. Differential expression of T-bet and Eomes in exhausted CD8+ T cells defined the functional heterogeneity in exhausted T cell populations.
- HIV-elite controllers
A rare subset of patients with human immunodeficiency virus (HIV) infection who are able to control the viral burden, maintaining undetectable viremia without any treatment (in conventional assays).
- Epigenetic modifications
Covalent heritable modifications that are acquired on histone tail proteins or DNA without changing DNA sequences. They regulate gene expression programs either through repressive epigenetic changes that induce gene silencing, or permissive changes that promote active or poised states of gene expression. Because such changes are cell division-transmissible, they play critical regulatory roles in cell differentiation and cell identity maintenance.
- Tumor microenvironment (TME)
Tissue environment that surrounds and feeds tumor cells and includes immune cells, other non-transformed cells, secreted molecules, extracellular matrix, and blood vessels. Tumor cells can dynamically change their microenvironment and TME can affect the growth and spread of a tumor.
- Tumor-infiltrating lymphocytes (TILs)
Lymphocytes that are recruited to, and infiltrate the TME to control tumor growth. These cells have been exploited for adoptive T cell therapy by isolating them from patients’ tumors, and expanding them ex vivo before being reinfused back into patients.
- Regulatory T cells (Tregs)
Subset of CD4+ T cells that suppress the effector function of other T cell subsets. One role consists of conferring protection against autoimmune responses by maintaining tolerance to self-antigens. They also control the development of immunopathologies in the host by modulating immune responses to non-self-antigens.
- Myeloid-derived suppressor cells (MDSCs)
Heterogeneous myeloid cell populations found in tumor, infection, or inflammatory sites; they are able to suppress T cell functions.
- Epigenetic modulators
Chemical compounds that can modulate disease-related epigenetic abnormalities by targeting regulators of epigenetic processes including epigenetic writers (e.g., DNA methyltransferases—DNMTs, histone methyltransferases—HMTs, histone acetyltransferases—HATs), epigenetic readers (e.g., bromodomain-containing proteins), and epigenetic erasers (e.g., histone deacetylases—HDACs, lysine demethylases—KDMs). For example, 5-azacytidine and 5-aza-2′-deoxycytidine are FDA-approved DNA demethylating agents (inhibitors of DNMTs) to treat some hematologic cancers, such as myelodysplastic syndrome. HDAC inhibitors are also approved for the treatment of cutaneous and peripheral T-cell lymphoma.
- Adoptive T cell therapy
Therapeutic approach that depends on isolating autologous TILs from a patient’s tumor and expanding them ex vivo to achieve greater numbers of TIls. Expanded TILs are reinfused back into the patient after preconditioning with lymphodepleting regimens.
- Negative selection
the process by which lymphocytes bearing receptors that react too strongly with self-antigens are eliminated from the repertoire in order to maintain self-tolerance.
- 4-1BB-co-stimulatory domain
The portion of the CAR’s cytoplasmic tail that includes the addition of the CD137 (also known as 4-1BB) costimulatory protein receptor’s signaling domain in order to enhance cell signaling and antitumor potency.
- Homeostatic proliferation
Proliferative response of lymphocytes induced by a lymphopenic environment to maintain a constant level of lymphocytes.
- Allogeneic feeder cell lines
Cell lines (stimulators) that are used to stimulate the activation and expansion of responder T cells due to their expression of foreign histocompatibility antigens (e.g., MHC class I or MHC class II molecules).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kaech SM, Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nature reviews. Immunology. 2012;12:749–761. doi: 10.1038/nri3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moskophidis D, et al. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 3.Zajac AJ, et al. Viral immune evasion due to persistence of activated T cells without effector function. The Journal of experimental medicine. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallimore A, et al. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. The Journal of experimental medicine. 1998;187:1383–1393. doi: 10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuller MJ, Zajac AJ. Ablation of CD8 and CD4 T cell responses by high viral loads. J Immunol. 2003;170:477–486. doi: 10.4049/jimmunol.170.1.477. [DOI] [PubMed] [Google Scholar]
- 6.Wherry EJ, et al. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. Journal of virology. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goepfert PA, et al. A significant number of human immunodeficiency virus epitope-specific cytotoxic T lymphocytes detected by tetramer binding do not produce gamma interferon. Journal of virology. 2000;74:10249–10255. doi: 10.1128/jvi.74.21.10249-10255.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lechner F, et al. Analysis of successful immune responses in persons infected with hepatitis C virus. The Journal of experimental medicine. 2000;191:1499–1512. doi: 10.1084/jem.191.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee PP, et al. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nature medicine. 1999;5:677–685. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 10.Wherry EJ, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Barber DL, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 12.Wherry EJ. T cell exhaustion. Nature immunology. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 13.Bengsch B, et al. Bioenergetic Insufficiencies Due to Metabolic Alterations Regulated by the Inhibitory Receptor PD-1 Are an Early Driver of CD8+ T Cell Exhaustion. Immunity. 2016 doi: 10.1016/j.immuni.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leach DR, et al. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 15.Sakuishi K, et al. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. The Journal of experimental medicine. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin HT, et al. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14733–14738. doi: 10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348:62–68. doi: 10.1126/science.aaa4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee DW, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davila ML, et al. How do CARs work?: Early insights from recent clinical studies targeting CD19. Oncoimmunology. 2012;1:1577–1583. doi: 10.4161/onci.22524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161:205–214. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohaegbulam KC, et al. Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends in molecular medicine. 2015;21:24–33. doi: 10.1016/j.molmed.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma W, et al. Current status and perspectives in translational biomarker research for PD-1/PD-L1 immune checkpoint blockade therapy. Journal of Hematology & Oncology. 2016;9:1–21. doi: 10.1186/s13045-016-0277-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bu X, et al. Learning from PD-1 Resistance: New Combination Strategies. Trends in molecular medicine. 2016;22:448–451. doi: 10.1016/j.molmed.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ribas A, et al. Association of Pembrolizumab With Tumor Response and Survival Among Patients With Advanced Melanoma. Jama. 2016;315:1600–1609. doi: 10.1001/jama.2016.4059. [DOI] [PubMed] [Google Scholar]
- 25.Blackburn SD, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nature immunology. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Im SJ, et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature. 2016 doi: 10.1038/nature19330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paley MA, et al. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science. 2012;338:1220–1225. doi: 10.1126/science.1229620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blackburn SD, et al. Selective expansion of a subset of exhausted CD8 T cells by alphaPD-L1 blockade. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:15016–15021. doi: 10.1073/pnas.0801497105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Utzschneider DT, et al. T Cell Factor 1-Expressing Memory-like CD8(+) T Cells Sustain the Immune Response to Chronic Viral Infections. Immunity. 2016;45:415–427. doi: 10.1016/j.immuni.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 30.He R, et al. Follicular CXCR5-expressing CD8+ T cells curtail chronic viral infection. Nature. 2016 doi: 10.1038/nature19317. [DOI] [PubMed] [Google Scholar]
- 31.Angelosanto JM, et al. Progressive loss of memory T cell potential and commitment to exhaustion during chronic viral infection. Journal of virology. 2012;86:8161–8170. doi: 10.1128/JVI.00889-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Utzschneider DT, et al. T cells maintain an exhausted phenotype after antigen withdrawal and population reexpansion. Nature immunology. 2013;14:603–610. doi: 10.1038/ni.2606. [DOI] [PubMed] [Google Scholar]
- 33.Youngblood B, et al. Chronic virus infection enforces demethylation of the locus that encodes PD-1 in antigen-specific CD8+ T cells. Immunity. 2011;35:13. doi: 10.1016/j.immuni.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Youngblood B, et al. Cutting Edge: Prolonged Exposure to HIV Reinforces a Poised Epigenetic Program for PD-1 Expression in Virus-Specific CD8 T Cells. J Immunol. 2013;191:540–544. doi: 10.4049/jimmunol.1203161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahn E, et al. Demethylation of the PD-1 promoter is imprinted during the effector phase of CD8 T cell exhaustion. Journal of virology. 2016 doi: 10.1128/JVI.00798-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schietinger A, et al. Tumor-Specific T Cell Dysfunction Is a Dynamic Antigen-Driven Differentiation Program Initiated Early during Tumorigenesis. Immunity. 2016;45:389–401. doi: 10.1016/j.immuni.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Topalian SL, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32:1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schadendorf D, et al. Pooled Analysis of Long-Term Survival Data From Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33:1889–1894. doi: 10.1200/JCO.2014.56.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou J, et al. Persistence of multiple tumor-specific T-cell clones is associated with complete tumor regression in a melanoma patient receiving adoptive cell transfer therapy. J Immunother. 2005;28:53–62. doi: 10.1097/00002371-200501000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosenberg SA, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chandran SS, et al. Tumor-Specific Effector CD8+ T Cells That Can Establish Immunological Memory in Humans after Adoptive Transfer Are Marked by Expression of IL7 Receptor and c-myc. Cancer research. 2015;75:3216–3226. doi: 10.1158/0008-5472.CAN-15-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Voss TC, Hager GL. Dynamic regulation of transcriptional states by chromatin and transcription factors. Nature reviews. Genetics. 2014;15:69–81. doi: 10.1038/nrg3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reik W. Stability and flexibility of epigenetic gene regulation in mammalian development. Nature. 2007;447:425–432. doi: 10.1038/nature05918. [DOI] [PubMed] [Google Scholar]
- 44.Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nature reviews. Genetics. 2012;13:484–492. doi: 10.1038/nrg3230. [DOI] [PubMed] [Google Scholar]
- 45.D'Urso A, Brickner JH. Mechanisms of epigenetic memory. Trends in genetics : TIG. 2014;30:230–236. doi: 10.1016/j.tig.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dogra P, et al. Generating long-lived CD8 T cell memory: insights from epigenetic programs. European journal of immunology. 2016 doi: 10.1002/eji.201545550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scharer CD, et al. Global DNA Methylation Remodeling Accompanies CD8 T Cell Effector Function. J Immunol. 2013 doi: 10.4049/jimmunol.1301395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Denton AE, et al. Differentiation-dependent functional and epigenetic landscapes for cytokine genes in virus-specific CD8+ T cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:15306–15311. doi: 10.1073/pnas.1112520108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sellars M, et al. Regulation of DNA methylation dictates Cd4 expression during the development of helper and cytotoxic T cell lineages. Nature immunology. 2015;16:746–754. doi: 10.1038/ni.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zediak VP, et al. Cutting edge: persistently open chromatin at effector gene loci in resting memory CD8+ T cells independent of transcriptional status. J Immunol. 2011;186:2705–2709. doi: 10.4049/jimmunol.1003741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Araki Y, et al. Histone acetylation facilitates rapid and robust memory CD8 T cell response through differential expression of effector molecules (eomesodermin and its targets: perforin and granzyme B) J Immunol. 2008;180:8102–8108. doi: 10.4049/jimmunol.180.12.8102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Russ BE, et al. Distinct Epigenetic Signatures Delineate Transcriptional Programs during Virus-Specific CD8 T Cell Differentiation. Immunity. 2014;41:853–865. doi: 10.1016/j.immuni.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kersh EN, et al. Rapid demethylation of the IFN-gamma gene occurs in memory but not naive CD8 T cells. J Immunol. 2006;176:4083–4093. doi: 10.4049/jimmunol.176.7.4083. [DOI] [PubMed] [Google Scholar]
- 54.Crompton JG, et al. Lineage relationship of CD8+ T cell subsets is revealed by progressive changes in the epigenetic landscape. Cellular & molecular immunology. 2015 doi: 10.1038/cmi.2015.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.June CH, et al. Adoptive cellular therapy: a race to the finish line. Science translational medicine. 2015;7:280ps287. doi: 10.1126/scitranslmed.aaa3643. [DOI] [PubMed] [Google Scholar]
- 56.Lee DW, et al. The future is now: chimeric antigen receptors as new targeted therapies for childhood cancer. Clin Cancer Res. 2012;18:2780–2790. doi: 10.1158/1078-0432.CCR-11-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Srivastava S, Riddell SR. Engineering CAR-T cells: Design concepts. Trends Immunol. 2015;36:494–502. doi: 10.1016/j.it.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao Z, et al. Structural Design of Engineered Costimulation Determines Tumor Rejection Kinetics and Persistence of CAR T Cells. Cancer cell. 2015;28:415–428. doi: 10.1016/j.ccell.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Long AH, et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nature medicine. 2015;21:581–590. doi: 10.1038/nm.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Davila ML, et al. Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Science translational medicine. 2014;6:224ra225. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maude SL, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. The New England journal of medicine. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khalil DN, et al. The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nature reviews. Clinical oncology. 2016;13:394. doi: 10.1038/nrclinonc.2016.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ernst B, et al. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 1999;11:173–181. doi: 10.1016/s1074-7613(00)80092-8. [DOI] [PubMed] [Google Scholar]
- 64.Hataye J, et al. Naive and memory CD4+ T cell survival controlled by clonal abundance. Science. 2006;312:114–116. doi: 10.1126/science.1124228. [DOI] [PubMed] [Google Scholar]
- 65.Turtle CJ, et al. A distinct subset of self-renewing human memory CD8+ T cells survives cytotoxic chemotherapy. Immunity. 2009;31:834–844. doi: 10.1016/j.immuni.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu Y, et al. Closely related T-memory stem cells correlate with in vivo expansion of CAR.CD19-T cells and are preserved by IL-7 and IL-15. Blood. 2014;123:3750–3759. doi: 10.1182/blood-2014-01-552174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Klebanoff CA, et al. Memory T cell-driven differentiation of naive cells impairs adoptive immunotherapy. The Journal of clinical investigation. 2016;126:318–334. doi: 10.1172/JCI81217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Restifo NP, et al. Adoptive immunotherapy for cancer: harnessing the T cell response. Nature reviews. Immunology. 2012;12:269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gajewski TF, et al. Innate and adaptive immune cells in the tumor microenvironment. Nature immunology. 2013;14:1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shiao SL, et al. Immune microenvironments in solid tumors: new targets for therapy. Genes & development. 2011;25:2559–2572. doi: 10.1101/gad.169029.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang F, et al. Epigenetic manipulation restores functions of defective CD8(+) T cells from chronic viral infection. Molecular therapy : the journal of the American Society of Gene Therapy. 2014;22:1698–1706. doi: 10.1038/mt.2014.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chiappinelli KB, et al. Combining Epigenetic and Immunotherapy to Combat Cancer. Cancer research. 2016;76:1683–1689. doi: 10.1158/0008-5472.CAN-15-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chiappinelli KB, et al. Inhibiting DNA Methylation Causes an Interferon Response in Cancer via dsRNA Including Endogenous Retroviruses. Cell. 2016;164:1073. doi: 10.1016/j.cell.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 74.Curran MA, et al. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Woo SR, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer research. 2012;72:917–927. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Larkin J, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. The New England journal of medicine. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Postow MA, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. The New England journal of medicine. 2015;372:2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Peng D, et al. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature. 2015;527:249–253. doi: 10.1038/nature15520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang L, et al. Decitabine Enhances Lymphocyte Migration and Function and Synergizes with CTLA-4 Blockade in a Murine Ovarian Cancer Model. Cancer immunology research. 2015;3:1030–1041. doi: 10.1158/2326-6066.CIR-15-0073. [DOI] [PubMed] [Google Scholar]