Abstract
Injection of human Chorionic Gonadotropin (hCG) directly into the dorsal lymph sac of Xenopus is a commonly used protocol for induction of ovulation, but recent shortages in the stocks of commercially available hCG as well as lack of a well tested alternative have resulted in frustrating experimental delays in laboratories that predominantly use Xenopus in their research. Mammalian Luteinizing Hormones (LH) share structural similarity, functional equivalency, and bind the same receptor as hCG; this suggests that LH may serve as a good alternative to hCG for promoting ovulation in Xenopus. LH has been found to induce maturation of Xenopus oocytes in vitro, but whether it can be used to induce ovulation in vivo has not been examined. Here we compared the ability of four mammalian LH proteins, bovine (bLH), human (hLH), ovine (oLH), porcine (pLH), to induce ovulation in Xenopus when injected into the dorsal lymph sac of sexually mature females. We find that both ovine and human LH, but not bovine or porcine, are good substitutes for hCG for induction of ovulation in WT and J strain X. laevis and X. tropicalis.
Keywords: Xenopus laevis, J strain, luteinizing hormone, ovulation, chorionic gonadotropin
Introduction
Induction of ovulation in Xenopus by injection of human Chorionic Gonadotropin (hCG) directly into the dorsal lymph sac is a well established protocol (Sive et al., 2000). This protocol has its roots in some of the earliest experimental work done in Xenopus more than eight decades ago. In the early 1930s, while studying the role of pituitary hormones in X. laevis pigmentation, Lancelot T. Hogben described that injection of extracts from the anterior pituitary of an ox could induce ovulation in X. laevis at any time of the year and that hypophysectomy resulted in striking ovarian regression (Hogben et al., 1931). This suggested that X. laevis may serve as a good system for detection of gonadotropic activity and directly led to the development of the Xenopus test for early pregnancy in which induction of ovulation was scored following injection of urine collected from human females (Elkan, 1938; Elkan, 1946). Further work in biochemistry led to the purification and identification of hCG as the active component in pregnant urine responsible for induction of ovulation in the frog and eventually lead to commercial availability of purified hCG (Practice Committee of American Society for Reproductive Medicine, Birmingham, Alabama, 2008). The increased access to the purified hormone together with development of protocols for maintenance and breeding of captive X. laevis allowed for its establishment as an experimental model system capable of providing large quantities of equivalent material all year round for use in biochemistry, embryology, and development biology (Gurdon and Hopwood, 2000).
Although Chorionic Gonadotropin (CG) is only found in primate and equine genomes, it is structurally similar to Luteinizing Hormone (LH), which is found in all vertebrates (Choi and Smitz, 2014). Both CG and LH are heterodimer glycoproteins composed of two subunits, α and β (Choi and Smitz, 2014); the α subunit is identical for both, while the β subunits are encoded by distinct genes (Boorstein et al., 1982; Naylor et al., 1983). In vivo, each hormone exists as a cocktail of distinct isoforms resulting from extensive post-translational glycosylation, sialylation and sulphonation (Choi and Smitz, 2014). The differences in the extent of post-translation modification between the two hormones are thought to account for the variability in their perdurance and for the fact that despite both activating the same Luteinizing hormone/choriogonadotropin receptor (LHCGR), each promotes a distinct downstream signaling response (Choi and Smitz, 2014).
In recent years, the intermittent shortages of commercially available hCG have led to sporadic and frustrating interference with experimental work relying on induction of Xenopus ovulation. In spite of the described differences between the two hormones, previous data using Xenopus oocytes and other anuran species suggest that LH derived from mammalian sources is a good candidate as an alternative to hCG in induction of Xenopus ovulation. In amphibians, the final steps of oocyte maturation occur shortly before ovulation and are characterized by meiotic division and the resulting breakdown of nuclear envelope and extrusion of the first polar body (Thornton, 1971). Ovine LH (oLH) was demonstrated as highly specific in promoting oocyte meiotic maturation in isolated X. laevis ovaries (Licht et al., 1976). Furthermore, oLH effectively induced ovulation in ovaries isolated from another anuran, Rana pipiens and also stimulated high levels of progesterone production in ovarian fragments from Rana catesbeiana (Bergers and Li, 1960; Licht et al., 1976; Ogawa et al., 2011).
We decided to investigate whether injection of mammalian LH proteins into the dorsal lymph sac of X. laevis and X. tropicalis would be sufficient to promote ovulation and therefore could be used as an alternative to hCG. We tested the efficiency of LH proteins derived from four distinct mammalian sources: bovine (bLH), human (hLH), ovine (oLH) and porcine (pLH) to induce egg laying in Xenopus by measuring the number of eggs laid. With the recent completion of the X. laevis genome using the inbred J strain X. laevis we compared the responses in both outcrossed wild type (WT) and inbred J strain X. laevis. We found that oLH and hLH are as efficient at inducing spawning as hCG, whereas bLH and pLH were less effective at inducing ovulation. In X. tropicalis, we found that oLH is as efficient as hCG in inducing ovulation, but that the batch size produced by oLH injected females is always smaller. Nonetheless, the oLH X. tropicalis females still produce eggs in numbers sufficient for experimental work. Our results demonstrate that oLH and hLH can be used as a substitute for hCG in Xenopus to promote ovulation.
Materials and Methods
Husbandry
All animals were housed and handled in the National Xenopus Resource in accordance with animal care protocol 15-02B approved by the Marine Biology Laboratory IACUC.
Spawning
Spawning was induced in sexually mature X. laevis wild-type and J strain females by first priming with an injection of 50 IU (WT) or 35 IU (J strain) PMSG, respectively, followed 2–4 days later by the injection of hCG, recombinant hCG, bovine LH, human LH, ovine LH, or porcine LH. Two females per tank were kept at room temperature and were allowed to spawn for 22 hours. The next day each female was squeezed 2–3 times to help induce spawning. After 22 hours, all eggs were collected and the volume of eggs with jelly coats still on was measured and used to estimate the total number of eggs. Eggs were collected into falcon tubes and allowed to settle for at least 5 minutes before volume measurements were made.
A similar approach was used for X. tropicalis where females were primed with 10 IU of hCG or 15 IU of PMSG, then boosted 1–2 days later with hCG, rhCG, or oLH. Following boosting females were kept at 3 per tank at 25–27°C and allowed to lay eggs for 8 hours, after which the eggs were collected, their volumes measured and the average number of eggs laid per female was calculated. While laying, each female was squeezed 1–2 times.
Females that did not produce any eggs during the squeezing nor during frequent observations throughout the experiment as well as the ones that did not show a pronounced engorgement of the labia and as such were not responding to the hormone were considered as not having laid any eggs throughout the experiment.
Egg counting
To convert volume of eggs laid to total number of eggs we first established the average number of eggs per mL laid by J strain and WT X. laevis and X. tropicalis as follows. 5 mL of eggs were collected in a 50 mL falcon tube from three individual females and, after dejellying, the total number of eggs was counted and used to calculate the average number of eggs/mL. This gave 150 eggs/mL for WT X. laevis, 200 eggs/mL for J strain, and 344 eggs/mL for X. tropicalis.
Hormones
Highly purified bovine, human, ovine and porcine Luteinizing Hormones as well as rhCG and hCG were ordered from the National Hormone and Peptide Program (www.humc.edu/hormones). PMSG was procured from Fisher Scientific (Catalog # 50893505).
Statistical analysis
Statistical analysis was performed in MATLAB. The following code was used.
Results
Efficient induction of ovulation in Xenopus with ovine Luteinizing Hormone
To determine if oLH can be used as a substitute for hCG, we compared the ability of each hormone to induce ovulation in vivo in X. laevis in both inbred J strain and outcrossed female X. laevis. Initially, we used oocyte positive females (i.e. those with a demonstrated prior history of oocyte production) that were at least 18 months old. As J strain frogs are smaller than traditional outcrossed frogs we tested various doses of each hormone. Female frogs were initially primed with PMSG 2–4 days prior to injection of hCG or LH and were not fed during this time. The frogs were then injected with different doses of hormone and allowed to lay eggs for 22 hours, after which the eggs were collected and the volume measured; during egg laying, the frogs were manually squeezed three times before collection. At 350 IU, 400 IU, or 450 IU of hCG female J strain frogs produced approximately 2400 eggs (Figure 1A). At the highest dose of 500 IU of hCG J strain female frogs produced an average of 3294 eggs (Figure 1A). In comparison, J strain females also responded well to oLH. At the lowest dose (100 μg) the females laid fewer eggs than hCG, averaging 1880 eggs (Figure 1A). The number of eggs laid increased with higher doses of oLH; at 150 μg they produced 2888 eggs, and females boosted with 200 μg oLH produced 3738 eggs (Figure 1A). Although all three doses were sufficient to induce egg laying, the highest dose of oLH was the most efficient and consistent. All eggs laid by J strain females were of good quality and fertilized efficiently in vitro with J strain male sperm and developed normally (data not shown). We have been using oLH in place of hCG for over six months now and have not seen a large difference in quality of eggs produced and fertilized, nor has this affected the maturation of resulting tadpoles to froglets and adults.
Figure 1. Ovine Luteinizing Hormone efficiently induces spawning in both J strain and WT X. laevis, independent of egg laying history.
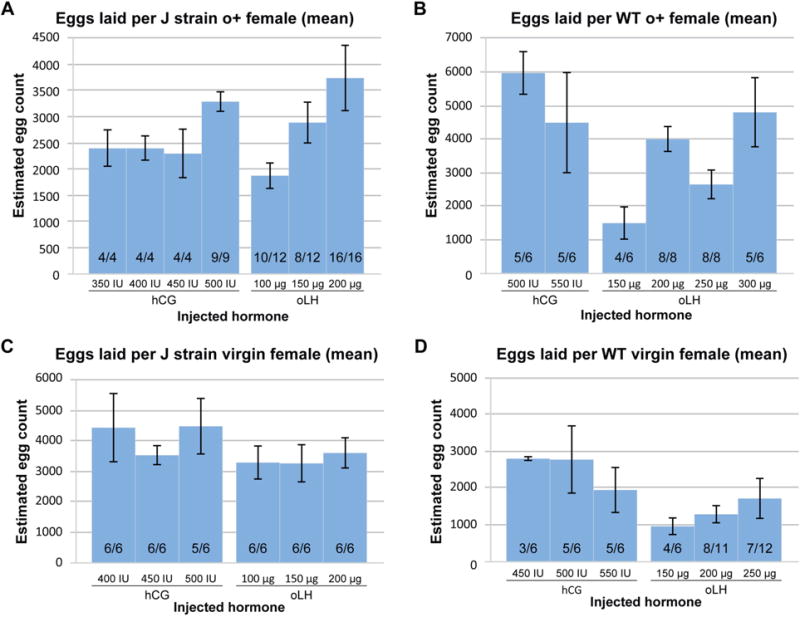
(A) J strain o+, (B) WT o+, (C) J strain virgin, and (D) WT virgin X. laevis females were boosted with either hCG or oLH. The height of each bar indicates the average number of eggs laid per female with the error bars showing the standard error of the mean. The ratios within each bar represent the corresponding number of females that laid eggs per number of females boosted. Only females that laid were included in the calculations of average number of eggs laid and the standard error of the mean. The amount and type of hormone used are indicated on the bottom of each graph.
The inbred J strain is of particular importance due to its use for the sequencing of X. laevis genome; since many laboratories working with X. laevis predominantly use outcrossed wild type (WT) frogs purchased from commercial vendors we examined whether oLH was as effective at inducing ovulation in WT X. laevis. At 500 IU and 550 IU of hCG the females produced an average of 5940 and 4470 eggs (Figure 1B); lower doses of hCG were not effective at inducing egg laying in WT X. laevis. We observed a comparable spawning efficiency at the highest doses of oLH used. At 150 μg oLH the boosted animals produced an average of only 1500 eggs, with only 66% of injected frog laying eggs (Figure 1B). At higher doses we found more consistent numbers of WT frogs laying eggs; at 200 μg they produced 3994 eggs, at 250 μg they produced 2644 eggs, and at the highest dose of 300 μg they produced 4700 eggs (Figure 1B). Thus, oLH is able to induce spawning in oocyte positive outcrossed females at efficiencies comparable to those obtained with hCG, but required slightly higher doses as compared to J strain females. This is consistent with the fact that WT o+ frogs are approximately 60% larger than J strain frogs (Table 1).
Table 1.
Weight and hormone dose of Xenopus laevis and Xenopus tropicalis
| Figure panel | Strain | Breeding history | Experimental condition | individual mass of females used (g) | Mean mass (g) | Amount of hormone/1g of mean mass |
|---|---|---|---|---|---|---|
| 1A | J | 0+ | 350 IU hCG | 58.2, 63.9, 49.3, 57.0 | 57.10 | 6.130 IU |
| 400 IU hCG | 76.9, 66.1, 79.7, 83.7 | 76.60 | 5.222 IU | |||
| 450 IU hCG | 89.7, 72.3, 53.2, 52.9 | 67.03 | 6.714 IU | |||
| 500 IU hCG | 59.0, 80.0, 68.0, 70.0, 60.0, 80.0, 55.7, 91.6, 78.3 | 71.40 | 7.003 IU | |||
| 100 μg oLH | 58.9, 74.6, 72.2, 66.8, 42.5, 52.2, 42.4, 74.0, 101.0, 84.5, 105.0, 96.0 | 72.53 | 1.379 μg | |||
| 150 μg oLH | 66.0, 55.2, 56.2, 69.5, 90.0, 113.5, 94.4, 107.0, 86.0, 95.0, 79.3, 99.2 | 84.28 | 1.780 μg | |||
| 200 μg oLH | 87.0, 72.0, 85.0, 69.0, 92.1, 75.8, 57.8, 74.3, 95.0, 93.5, 97.0, 106.5, 80.1, 70.2, 74.0, 89.0 | 82.39 | 2.427 μg | |||
| 1B | WT | o+ | 500 IU hCG | 79.4, 98.3, 88.5, 75.8, 82.5, 152.4 | 96.15 | 5.200 IU |
| 550 IU hCG | 88.2, 77.6, 63.3, 174.5, 171.2, 112.9 | 114.62 | 4.799 IU | |||
| 150 μg oLH | 97.3, 135.8, 130.4, 93.9, 109.7, 154.3 | 120.23 | 1.248 μg | |||
| 200 μg oLH | 110.4, 139.4, 99.3, 140.4, 171.8, 203.1, 164.5, 259.3 | 161.03 | 1.242 μg | |||
| 250 μg oLH | 126.0, 85.0, 88.7, 87.9, 129.2, 144.4, 145.0, 170.4 | 122.08 | 2.048 μg | |||
| 300 μg oLH | 121.9, 87.8, 137.6, 138.5, 124.1, 195.5 | 134.23 | 2.235 μg | |||
| 1C | J | Virgin | 400 IU hCG | 52.2, 43.3, 62.0, 64.4, 55.5, 34.2 | 51.93 | 7.702 IU |
| 450 IU hCG | 49.7, 52.2, 48.7, 53.4, 60.9, 34.1 | 49.83 | 9.030 IU | |||
| 500 IU hCG | 61.4, 72.1, 64.4, 53.4, 49.7, 41.1 | 57.07 | 8.762 IU | |||
| 100 μg oLH | 75.3, 59.5, 49.6, 60.1, 39.6, 39.8 | 53.98 | 1.852 μg | |||
| 150 μg oLH | 61.5, 74.2, 50.9, 71.1, 33.3, 69.8 | 60.13 | 2.494 μg | |||
| 200 μg oLH | 56.6, 74.5, 55.5, 72.6, 65.4, 48.3 | 62.15 | 3.218 μg | |||
| 1D | WT | Virgin | 450 IU hCG | 73.0, 86.1, 88.3, 98.6, 96.4, 97.7 | 90.02 | 4.999 IU |
| 500 IU hCG | 112.8, 131.5, 104.1, 137.8, 73.4, 123.9 | 113.92 | 4.389 IU | |||
| 550 IU hCG | 108.2, 85.4, 97.4, 114.5, 52.1, 87.8 | 90.90 | 6.051 IU | |||
| 150 μg oLH | 105.9, 105.4, 108.1, 115.6, 96.4, 126.4 | 109.63 | 1.368 μg | |||
| 200 μg oLH | 143.9, 99.8, 110.5, 87.2, 82.4, 81.3, 146.8, 118.5, 82.8, 93.0, 104.3 | 104.58 | 1.912 μg | |||
| 250 μg oLH | 108.5, 135.1, 102.5, 113.8, 104.0, 120.0, 76.5, 128.8, 61.6, 92.5, 87.0, 91.6 | 101.81 | 2.455 μg | |||
| 2A | J | o+ | 100 μg bLH | 55.2, 76.5, 63.4, 82.1, 87.5, 32.9 | 66.27 | 1.509 μg |
| 150 μg bLH | 61.3, 41.3, 82.5, 29.9, 47.4, 88.1 | 58.43 | 2.567 μg | |||
| 200 μg bLH | 95.9, 87.7, 47.5, 49.6, 53.0, 77.6 | 68.54 | 2.918 μg | |||
| 100 μg hLH | 67.4, 55.1, 57.0, 60.1, 99.2, 77.3 | 69.35 | 1.442 μg | |||
| 150 μg hLH | 58.4, 69.7, 93.6, 98.5, 69.1, 53.4 | 73.78 | 2.033 μg | |||
| 200 μg hLH | 49.6, 100.5, 97.0, 67.0, 47.9, 48.2 | 68.37 | 2.925 μg | |||
| 100 μg pLH | 44.4, 68.7, 65.2, 92.5 | 67.70 | 1.477 μg | |||
| 150 μg pLH | 48.2, 91.6, 57.4, 74.2, 70.8, 61.9 | 67.35 | 2.227 μg | |||
| 200 μg pLH | 62.6, 107.9, 64.8, 87.7, 61.0, 85.6 | 78.28 | 2.555 μg | |||
| 2B | WT | 0+ | 200 μg bLH | 164.3, 124.4, 154.1, 107.0, 164.4, 170.8 | 147.50 | 1.356 μg |
| 250 μg bLH | 124.0, 73.8, 85.2, 157.0, 93.6, 200.3 | 122.32 | 2.044 μg | |||
| 200 μg hLH | 129.8, 127.3, 85.0, 132.0, 153.3, 215.2 | 140.43 | 1.424 μg | |||
| 250 μg hLH | 157.6, 125.3, 80.3, 168.7, 106.6, 151.3 | 131.63 | 1.899 μg | |||
| 200 μg pLH | 139.4, 192.7, 165.9, 205.8, 86.2, 112.4 | 150.40 | 1.330 μg | |||
| 250 μg pLH | 171.6, 184.5, 116.5, 166.1, 170.4, 178.9 | 164.67 | 1.518 μg | |||
| 3 | TROP | 0+ | 10 IU hCG/100 IU hCG | 18.1, 23.5, 16.9, 19.9, 17.0, 18.9 | 19.05 | 5.249 IU |
| 10 IU hCG/150 IU hCG | 15.1, 23.2, 17.4, 23.2, 17.2, 13.6 | 18.28 | 8.204 IU | |||
| 10 IU hCG/100 IU rhCG | 16.8, 23.2, 17.9, 14.5, 16.5, 12.6 | 17.00 | 5.882 IU | |||
| 10 IU hCG/150 IU rhCG | 15.6, 20.8, 23.4, 19.7, 13.2, 14.2 | 17.82 | 8.419 IU | |||
| 10 IU hCG/25 μg oLH | 16.0, 14.4, 17.7, 17.1, 11.4, 16.3 | 15.48 | 1.615 μg | |||
| 10 IU hCG/50 μg oLH | 20.3, 16.7, 17.1, 17.4, 16.0, 18.1, 15.9, 20.7, 15.9, 19.2 | 17.73 | 2.820 μg | |||
| 10 IU hCG/75 μg oLH | 14.3, 14.5, 13.6, 14.7, 17.7, 19.8, 15.8, 12.1 | 15.31 | 4.898 μg | |||
| 10 IU hCG/100 μg oLH | 17.9, 13.6, 14.5, 18.7, 11.6, 16.4, 15.9 | 15.51 | 6.446 μg | |||
| 15 IU PMSG/100 IU hCG | 19.0, 16.6, 21.6, 17.9, 13.0, 15.4 | 17.25 | 6.797 IU | |||
| 15 IU PMSG/150 IU hCG | 14.0, 21.1, 21.5, 17.4, 16.8, 15.5 | 17.72 | 8.467 IU | |||
| 15 IU PMSG/100 IU rhCG | 17.8, 24.6, 20.0, 16.3, 16.7, 18.5, 18.6 | 18.93 | 5.283 IU | |||
| 15 IU PMSG/150 IU rhCG | 13.9, 18.8, 16.5, 16.1, 15.2, 14.2, 16.3 | 15.86 | 9.459 IU | |||
| 15 IU PMSG/25 μg oLH | 15.7, 18.0, 11.6, 17.9, 14.3, 16.9, 18.1, 15.5 | 16.00 | 1.563 μg | |||
| 15 IU PMSG/50 μg oLH | 19.2, 15.5, 16.0, 17.1, 15.7, 10.0, 13.8, 19.6, 16.7, 17.2 | 16.08 | 3.109 μg | |||
| 15 IU PMSG/75 μg oLH | 13.2, 17.3, 14.2, 15.7, 19.4, 15.1, 14.9, 11.1, 12.7 | 14.84 | 5.052 μg | |||
| 15 IU PMSG/100 μg oLH | 15.4, 20.2, 15.4, 17.7, 13.3, 17.3, 17.7, 19.3, 20.6 | 17.43 | 5.736 μg |
Both hCG and oLH efficiently induce ovulation at the highest dose tried. Combining the results from J strain and WT oocyte positive females, 14 females of the 15 injected with hCG and 21 of the 22 injected with oLH ovulated. In both conditions, a single WT female did not spawn. High rates of ovulation do not necessarily mean that both hormones are able to induce production of equally high numbers of eggs. To test the equivalency of the two hormones in relation to the number of eggs produced we performed a two-sample nonparametic Kolmogorov-Smirnov test (Massey, 1951). In our experimental design two females were included per tank and the number of eggs laid was calculated as an average and thus to provide sufficient power for this statistical analysis we combined the J strain and WT data. The tanks that included the WT females that did not lay were also included in the calculation. We were not able to reject the null hypothesis that both hormones would produce equivalent egg number distributions and the asymptotic P-value (considered accurate for our sample sizes) is 0.9183. This supports the conclusion that at the highest doses tested not only are hCG and oLH essentially identical in their efficiency at inducing ovulation but they also produce equivalent numbers of eggs.
To asses whether prior spawning history had any influence on the ability of X. laevis females to respond to oLH, we compared the efficiencies of hCG and oLH to promote egg laying in virgin J strain and WT females. In virgin J strain females boosted with hCG we found similar results with all three doses tested. At 400 and 450 IU all six female frogs laid an average of 4433 and 3533 eggs, respectively, while five out of six females injected with 500 IU produced an average of 4480 eggs (Figure 1C). Boosting virgin J strain females with oLH was also effective with all virgin frogs tested laying eggs; 3300 eggs with 100 μg, 3267 with 150 μg, and 3600 with 200 μg (Figure 1C). Conversely, in WT virgin females we found that oLH was less effective than hCG at inducing egg laying; most hCG injected virgin females laid eggs, whereas the results were more variable with oLH. hCG injected virgin females laid an average of 2800 eggs when injected with 450 IU (3/6), 2775 with 500 IU (5/6), and 1950 with 550 IU (5/6) (Figure 1D). oLH injected virgin WT frogs laid fewer eggs than with hCG, even at the highest dose of oLH. At 150 μg four of six females laid 975 eggs, 1294 eggs were laid by eight of eleven females injected with 200 μg, and at 250 μg the females produced 1714 eggs (Figure 1D). Thus, in virgin female frogs hCG was more effective at inducing egg laying and produced a greater number of eggs than oLH.
Based on our results we found that oLH is an effective substitute for hCG, and the most effective dose correlates with the size of the female frog. Overall, we found J strain frogs weigh approximately 60% less than WT frogs, with J strain virgin frogs averaging 55.8g and J strain o+ frogs weighing 76.2g, whereas WT virgin frogs were on average 102.1g and WT o+ frogs weighing 126.5g (Table 1). To determine the most effective dose we calculated the amount of oLH used (μg) per gram body weight that resulted in the largest number of eggs and highest percentage of injected females laying. In the oLH injections the hormone concentrations tested ranged from 1.38 μg to 3.22 μg per gram of average body mass in J strain frogs, and from 1.25 μg to 2.46 μg per gram of body mass in WT frogs (Table 1). Based on these results we estimate that 2.5 μg/g of oLH is the most effective dose; on average this amounts to 150–200 μg for J strain frogs and 250–300 μg for WT frogs.
Human but not bovine nor porcine Luteinizing Hormones promote efficient egg laying in X. laevis
The amino acid sequences of Luteinizing Hormone in various species are highly similar, but contain different posttranslational modifications. We decided to test whether using Luteinizing Hormone derived from three alternate mammalian sources, bovine (bLH), human (hLH), and porcine (pLH), would demonstrate a similar ability to promote egg laying in X. laevis as oLH and hCG did. For consistency, we tested the effectiveness of other LH preparations only in o+ J strain and WT frogs and found comparable results in both strains. In J strain females, bLH was an inconsistent inducer of egg laying, with only 300 eggs produced by two of six female frogs injected with 100 μg, 1950 eggs laid by four of six injected with 150 μg, and 3500 eggs laid by only two of six injected with 250 μg (Figure 2A). hLH was considerably more effective at inducing spawning with 100 μg producing an average of 3200 eggs, 150 μg producing 4500 eggs, and 200 μg producing 3456 eggs (Figure 2A). Boosting with pLH resulted in poor and inconsistent spawning with none of the four females injected with 100 μg producing any eggs, only two of six injected with 150 μg producing an average of 1400 eggs, and two of six injected with 200 μg producing an average of 500 (Figure 2A).
Figure 2. Efficiency of spawning in J strain and WT o+ females boosted with either bLH, hLH, or pLH.
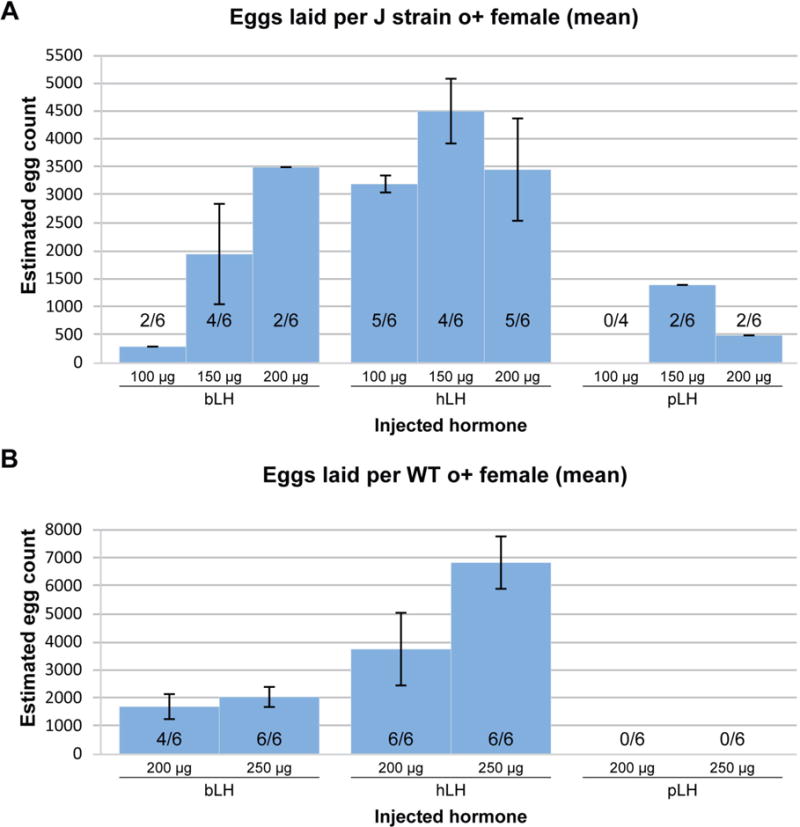
(A) J strain o+, and (B) WT o+ X. laevis females were boosted with either bLH, hLH, or pLH. The height of each bar indicates the average number of eggs laid per female with the error bars showing the standard error of the mean. The ratios within or above each bar represent the corresponding number of females that laid eggs per number of females boosted. Only females that laid were included in the calculations of average number of eggs laid and the standard error of the mean. The amount and type of hormone used are indicated beneath the bars.
WT females showed somewhat similar responses to these three mammalian hormones. They responded more consistently to bLH but produced small egg batches. Four of six injected with 200 μg of bLH produced an average of 1706 eggs, and all six injected with 250 μg produced 2050 eggs on average (Figure 2B). hLH was more efficient at inducing spawning. All six females injected with 200μg laid an average of 3756 eggs, and all six frogs injected with 250 μg laying 6825 eggs on average (Figure 2B). None of the six females injected with 200 μg of pLH nor the six injected with 250 μg of it laid any eggs (Figure 2B). When accounting for the body mass of the females injected, on average all the three hormones were tested at concentrations spanning a comparable range to oLH (Table 1). In conclusion, only human LH, and not bLH nor pLH, was as effective as ovine LH at inducing egg laying in X. laevis.
Boosting X. tropicalis females with oLH promotes egg laying
X. tropicalis is the other species within the Xenopus genus that is commonly used as a developmental biology model system. As a final test, we decided to investigate whether, just as in X. laevis, boosting with oLH could be used to promote egg laying in X. tropicalis. Furthermore, we also investigated whether egg laying reponse to the boosting hormone tested, was differentially affected by prior priming of the females with either 10 IU of hCG or 15 IU of PMSG.
Of the individuals primed with hCG and then boosted with 100 IU of hCG four in six produced an average of 731 eggs, and five in six boosted with 150 IU produced an average of 1651 (Figure 3). Surprisingly, the females gave a mixed response to recombinant hCG (rhCG). Five of the six females boosted with 100 IU rhCG produced an average of 1174 eggs but only three of the six boosted with 150 IU laid 573 eggs (Figure 3). The females responded more consistently to oLH than they did to rhCG, though the average clutch size did not reach the amount resulting from boosting with 150 IU of hCG. Five of six females boosted with 25 μg of oLH produced an average of 860 eggs, nine of ten boosted with 50 μg produced 898, seven of eight boosted with 75 μg produced 1057, and six of seven boosted with 100 μg produced 803 (Figure 3).
Figure 3. Spawning efficiency in X. tropicalis females first primed with either hCG or PMSG and then boosted with hCG, rhCG, or oLH.
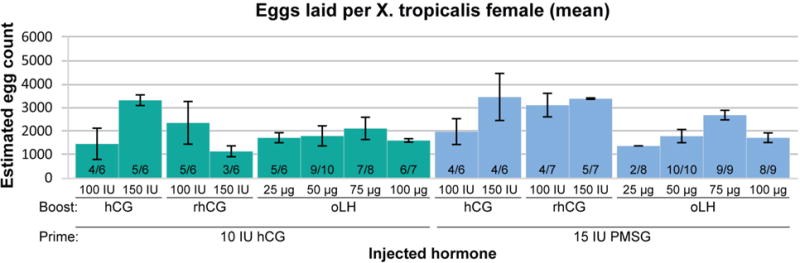
X. tropicalis females were primed with either 10IU of hCG (green bars) or 15IU of PMSG (blue bars). The height of each bar indicates the average number of eggs laid per female with the error bars showing the standard error of the mean. The ratios within each bar represent the number of females that laid eggs per number of females boosted. Only females that laid were included in the calculations of average number of eggs laid and the standard error of the mean. The amount and type of hormone used are indicated on the bottom of the graph.
The main observable difference between priming with 10 IU of hCG and 15 IU of PMSG was the much better response to boosting with rhCG in the PMSG primed females. Among those, four of six boosted with 100 IU of hCG produced an average of 989 eggs, and four of six boosted with 150 IU laid an average of 1720 eggs (Figure 3). Among the females boosted with rhCG four of seven boosted with 100 IU produced an average of 1548 eggs and 5 of seven boosted with 150 IU produced an average of 1686 eggs (Figure 3). PMSG priming didn’t seem to have as much effect on increasing the clutch sizes of animals boosted with oLH; nonetheless, these females still laid eggs in numbers sufficient for experimental work. Two of eight individuals boosted with 25 μg produced an average of 688 eggs, all ten boosted with 50 μg produced 894, all nine boosted with 75 μg produced 1338, and eight of the nine boosted with 100 μg produced 860 (Figure 3). In the X. tropicalis oLH trials we tested a range of concentrations from 1.56μg to 6.45μg/g body mass, with approximately 5 μg per gram providing the largest batch size (Table 1). In conclusion, as in X. laevis, boosting with oLH promotes efficient egg laying in X. tropicalis at clutch sizes sufficiently large for experimental work. Furthermore, priming with 15 IU of PMSG produces essentially similar response to the boosting hormone as priming with 10 IU of hCG.
Discussion
In this paper, we describe a convenient and efficient method for induction of ovulation in Xenopus that can be used in lieu of the established protocol relying on injection of hCG into the dorsal lymph sac (Sive et al., 2000). The only difference is substitution of hCG with oLH, with the overall technique still based on dorsal lymph sac injection and thus not requiring any additional training. Our results clearly demonstrate that in both WT and J strain X. laevis females, oLH is capable of inducing ovulation as efficiently as hCG independent of prior breeding history. The numbers of eggs laid are generally comparable between the two hormones with the only potential exception being that WT virgin females do not appear to lay as many eggs when injected with oLH. It is possible that a higher amount if oLH may increase the number of eggs laid, but in general we found that approximately 2.5 μg/g of oLH was sufficient to replicate results produced by hCG.
oLH is also effective at inducing ovulation in X. tropicalis. We tested a range of concentrations from approximatelly 1.5 μg to 6.5 μg per gram of body mass. We observed that the females respond best to 5 μg of oLH per gram of body mass. At this concentration they spawned at frequencies surpassing those of females injected with hCG (94% vs 75%), however oLH boosted females never produced as many eggs as those boosted with hCG. In this case, increasing the amount of oLH may not be a viable approach for increasing the number of eggs laid, as in our experiments higher concentrations actually decreased the average number of eggs laid. Finally, we observed that X. tropicalis primed with either hCG or PMSG responded equally well to boosting with oLH.
In X. laevis we also tested responses to three additional luteinizing hormones, bovine, human and porcine LH. Only hLH consistently produced results, which suggests it may also serve as a good substitute for boosting with hCG. Although we were disappointed that not all of the mammalian hormones tested produced a similarly high ovulation respone, this observation is not at all surprising. A previous comparison of LH preparations from nine species of eutherian mammals demonstrated a broad range in their ability to promote X. laevis oocyte maturation in vitro, with the ovine derived LH being the most effective (Licht and Papkoff, 1976).
In conclusion, we found that boosting Xenopus with oLH can be used in place of hCG to induce ovulation, at a dose of approximately 2.5 μg/g oLH in X. laevis and twice that concentration in X. tropicalis. Furthermore, as a practical comparison, the oLH available from the National Hormone and Peptide Program is priced at $150.00 per 10mg, which ends up costing $3.00 per boosting injection per WT X. laevis female. The hCG available from Sigma-Aldrich (Catalog # CG10) when bought in bulk of 10 vials each containing 10000 IU is priced at $956.00 which brings the cost of each boosting injection to $4.78. Thus, the use of oLH will not only aid in avoiding any disruption in experimental work resulting from shortages in commercially available hCG but might also aid in lowering the overall experimental costs.
Highlights.
Identification of Luteinizing Hormone as an effective replacement for hCG in both X. laevis and X. tropicalis.
Comparison of functional efficacy of different species LH demonstrates that human and ovine work best, with porcine and bovine not as effective.
J strain X. laevis frogs are slightly smaller than traditional outcrossed WT frogs and require slightly less hormone to induce ovulation.
Inbred J strain X. laevis eggs are slightly smaller than WT frogs.
Acknowledgments
We wish to thank Sean McNamara, Brian Suh, Lynn Ware and Alyssa Parker for their care of the frogs and to Esther Pearl for her help throughout this project. This work was supported by a grant from the NIH P40OD010997.
References
- Bergers AC, Li CH. Amphibian ovulation in vitro induced by mammalian pituitary hormones and progesterone. Endocrinology. 1960;66:255–259. doi: 10.1210/endo-66-2-255. [DOI] [PubMed] [Google Scholar]
- Boorstein WR, Vamvakopoulos NC, Fiddes JC. Human chorionic gonadotropin β-subunit is encoded by at least eight genes arranged in tandem and inverted pairs. Nature. 1982;300:419–422. doi: 10.1038/300419a0. [DOI] [PubMed] [Google Scholar]
- Choi J, Smitz J. Luteinizing hormone and human chorionic gonadotropin: Origins of difference. Mol Cell Endocrinol. 2014;383:203–213. doi: 10.1016/j.mce.2013.12.009. [DOI] [PubMed] [Google Scholar]
- Elkan ER. The Xenopus Pregnancy Test. Br Med J. 1938;2:1253–1274.2. doi: 10.1136/bmj.2.4067.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkan RE. A New Test for Pregnancy. Postgraduate Medical Journal. 1946;22:87–93. doi: 10.1136/pgmj.22.245.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdon JB, Hopwood N. The introduction of Xenopus laevis into developmental biology: of empire, pregnancy testing and ribosomal genes. Int J Dev Biol. 2000;44:43–50. [PubMed] [Google Scholar]
- Hogben L, Charles E, Slome D. Studies On The Pituitary. Journal of Experimental Biology. 1931;8:345–354. [Google Scholar]
- Licht P, Licht P, Papkoff H, Papkoff H, Farmer SW, Farmer SW, Muller CH, Muller CH, Tsui HW, Tsui HW, Crews D, Crews D. Evolution of gonadotropin structure and function. Recent Prog Horm Res. 1976;33:169–248. doi: 10.1016/b978-0-12-571133-3.50012-x. [DOI] [PubMed] [Google Scholar]
- Licht P, Papkoff H. Species specificity in the response of an in vitro amphibian (Xenopus laevis) ovulation assay to mammalian luteinizing hormones. Gen Comp Endocrinol. 1976;29:552–555. doi: 10.1016/0016-6480(76)90039-3. [DOI] [PubMed] [Google Scholar]
- Massey FJ., Jr The Kolmogorov-Smirnov test for goodness of fit. Journal of the American statistical. 1951;46:68–78. doi: 10.1080/01621459.1951.10500769. [DOI] [Google Scholar]
- Naylor SL, Chin WW, Goodman HM, Lalley PA, Grzeschik KH, Sakaguchi AY. Chromosome assignment of genes encoding the alpha and beta subunits of glycoprotein hormones in man and mouse. Somat Cell Mol Genet. 1983;9:757–770. doi: 10.1007/BF01539478. [DOI] [PubMed] [Google Scholar]
- Ogawa A, Dake J, Iwashina YK, Tokumoto T. Induction of ovulation in Xenopus without hCG injection: the effect of adding steroids into the aquatic environment. Reprod Biol Endocrinol. 2011;9:11. doi: 10.1186/1477-7827-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Practice Committee of American Society for Reproductive Medicine, Birmingham, Alabama. Gonadotropin preparations: past, present, and future perspectives. Fertility and Sterility. 2008;90:S13–20. doi: 10.1016/j.fertnstert.2008.08.031. [DOI] [PubMed] [Google Scholar]
- Sive HL, Grainger RM, Harland RM. Early development of Xenopus laevis: a laboratory manual 2000 [Google Scholar]
- Thornton VF. A bioassay for progesterone and gonadotropins based on the meiotic division of Xenopus oocytes in vitro. Gen Comp Endocrinol. 1971;16:599–605. doi: 10.1016/0016-6480(71)90125-0. [DOI] [PubMed] [Google Scholar]


