Abstract
Little is known about associations between viral suppression, adherence, and duration of prior viral suppression in sub-Saharan Africa. Study participants were from the UARTO study in Mbarara, Uganda. We fit regression models to characterize relationships between average adherence, treatment interruptions, and rebound viremia (>400 copies/mL) following a previously undetectable result. Our goal was to understand the impact of prior viral suppression on these relationships. 396 participants contributed 2864 quarterly visits. Restricted to periods with average adherence <50 %, each 10 % increase in adherence reduced the odds of rebound viremia by 74 % [adjusted odds ratio (AOR) = 0.26, P = 0.002] and 29 % (AOR = 0.71, P = 0.057) during the first 12 months of suppression and beyond 12 months respectively, interaction term P = 0.018. Among periods with adherence ≥50 %, the risk of rebound viremia decreased with increasing adherence during the first 12 months of viral suppression (AOR = 0.73 for each 10 % increase, P = 0.001), but not thereafter (AOR = 1.09, P = 0.67), interaction term P = 0.027. In contrast, 72-h interruptions, were associated with increased rebound viremia during the first 12 months (AOR = 1.30, P = 0.009) and after (AOR = 1.39, P = 0.005), interaction term P = 0.69. Completing 12 months of viral suppression decreases the impact of average adherence, but not prolonged treatment interruptions, on risk of rebound viremia.
Keywords: Adherence, Viremia, Suppression, ART
Background
As of 2012, approximately 10 million people living with HIV (PLWH) in sub-Saharan Africa had gained access to antiretroviral therapy (ART) [1]. The long-term success of HIV care programs, including their impact on the health of those infected and the incidence of new infections, will largely depend on maintenance of viral suppression for those in care [2, 3].
Among the most important determinants of viral suppression for PLWH is ART adherence [4–6]. Relationships between adherence and virologic control can vary by duration of suppression [7, 8] and regimen [9, 10]. Moreover, specific patterns of adherence can affect these relationships; with recent data demonstrating that treatment interruptions are independently associated with virologic failure independently of the number of doses taken [11–13]. However, relatively few data are available to describe relationships between adherence and viremia in sub-Saharan Africa, where regimen options differ [14]. Precise understanding of adherence patterns that predict viral suppression are critical to inform patients, providers, and public health programmers about the optimal levels of adherence required to maintain the direct health and preventative benefits of ART.
We assessed relationships between objectively measured ART adherence and rebound viremia among people with HIV in southwestern Uganda. Our primary aim was to assess the relationship between adherence and rebound viremia, and particularly how this relationship varies with the length of prior viral suppression.
Methods
Study Setting and Participants
ART-naïve participants attending the HIV clinic at the Mbarara Regional Referral Hospital were enrolled at ART initiation into the Uganda AIDS Rural Treatment Outcomes (UARTO) study, an observational, prospective cohort beginning in 2005 (NCT01596322) [15]. At baseline and quarterly intervals thereafter, blood was collected for HIV RNA measurements. At each interval, data was collected onto clinical research forms and entered into a study database. Adherence was measured from 2005 through 2011 with Medication Event Monitoring System monitors (MEMS, Aardex, Sweden), electronic pill bottles that record the date and time of each bottle opening. MEMS data was downloaded onto a study computer at each study visit.
Statistical Analyses
Our primary outcome of interest was rebound HIV RNA viremia, defined by a detectable HIV RNA (>400 copies/mL) following a previously undetectable HIV RNA.
Our primary predictor was ART adherence, measured in two ways:
Average adherence, computed for each participant as the number of MEMS cap openings divided by the prescribed number of doses per day averaged across each quarterly interval. Daily adherence was capped at 100 %;
The total number of 72-h treatment interruptions per quarterly interval for each participant, which has been demonstrated to be independently associated with risk of viremia [11–13].
We censored the following observation periods: (1) those in which the prior HIV RNA measurement was detectable, since these events represent a failure of suppression, a distinct clinical phenomenon; (2) those with >150 days between quarterly HIV RNA measurements (to maintain homogeneity of observation periods); (3) periods when 30+ days of medication interruption detected by MEMS was immediately followed by a suppressed HIV RNA (suggesting device non-use [16]); and (4) periods without a corresponding HIV RNA result. We also censored days of known MEMS non-usage, such as when participants were prescribed medications in sealed packages that prevented insertion of ART into the MEMS devices.
To assess the relationship between adherence, rebound viremia and duration of viral suppression, we first graphically depicted relationships between adherence and rebound viremia over suppression time using lowess plots and noted qualitative changes after approximately 12 months of viral suppression. We then estimated the probability of viral rebound in average adherence strata (≤50, >50–70, >70–90, and >90 %), and by the presence of any treatment interruptions [8, 9, 13]. We used the Z-test of proportions to estimate differences in each adherence stratum by duration of viral suppression less than or greater than 12 months.
Finally, we fitted regression models with each period as a unit of observation, using generalized estimating equations (with robust standard errors) to account for within participant correlation. In the first model, we selected each 10 % stratum in average adherence as the main predictor of interest. In the second and third models, we repeated this analysis but restricted it to periods with an average adherence ≤50 and >50 % respectively, because previous research has shown substantive differences in the risk of rebound viremia at this threshold [8], In the fourth model, the number of 72-h treatment interruptions was the primary predictor. The 72-h threshold was chosen based on previous data, which showed that each day beyond two missed days increases the odds of virologic breakthrough [13, 17]. For each model, we also tested the significance of an interaction term between the primary predictor and duration of viral suppression. We included hypothesized confounders in the model, including age, gender, nadir CD4 T+ lymphocyte count, baseline HIV RNA and ART regimen. Regimens were categorized as one of the following: AZT/3TC/NVP, D4T/3TC/NVP, and AZT/3TC/EFV, which accounted for approximately 98 % of the regimens in use. Statistical analyses were performed using Stata version 13.0 (Statacorp, College Station, TX, USA).
Results
Three hundred and ninety-six participants were observed for a median of 2.8 years [interquartile range (IQR) 1.6–3.7]. Participants were 70 % female, with a median age at enrollment of 35 years (IQR 29–39), median nadir CD4+ T-lymphocyte count of 135 cells/mm3 (IQR 78–198), and median baseline log10 pre-treatment HIV RNA of 5.1 copies/mL (IQR 4.6–5.6). At enrolment, 311 (79 %) were literate, 301 (76 %) had some form of employment while 163 (41 %) were married. The most common ART regimens at initiation were AZT/3TC/NVP, D4T/3TC/NVP, and TDF/3TC/EFV, used by 62, 26 and 10 % of participants, respectively.
Of 4063 observation quarters during follow-up, 1199 (30 %) were censored because: 608 (15 %) had a prior detectable HIV RNA, 303 (8 %) quarters were >150 days, 248 (6 %) had an undetectable HIV RNA immediately following a MEMS interruption of 30+ days, and 40 (1 %) had a missing HIV RNA, leaving a total of 2864 periods included in the analysis. Most quarters (n = 1867, 65 %) were classified by >90 % average adherence, whereas 613 (21 %) had >70–90 % average adherence, 210 (7 %) had >50–70 %, and 174 (6 %) had ≤50 % adherence. Six hundred and fifty-six quarters (23 %) had one or more 72-h treatment interruptions. Of these, 234 (36 %) had 2 or more interruptions. The mean (SD) number of interruptions per quarterly interval was 0.38 (0.91). The median (IQR) gap length before and after the first 12 months of suppression was 135 h (94–135), and 105 h (81–122), respectively, P = 0.12. One hundred and one quarters (4 %) concluded with a detectable HIV RNA > 400 copies/mL.
Having ≥12 months of prior suppression was associated with significantly lower odds of rebound viremia than <12 months of suppression [crude OR = 0.51, 95 % CI (0.34, 0.77), P = 0.001]. In adjusted models, each 10 % increase in adherence was associated with a 26 % reduction in the odds of rebound viremia during the first 12 months of suppression [adjusted odds ratio (AOR) = 0.74, 95 % CI (0.66–0.84), P < 0.001] and 17 % reduction thereafter [AOR = 0.83,95 % CI (0.74–0.92), P < 0.001], interaction term P = 0.17 (Table 1). Restricted to periods with average adherence ≤50 % (174 of 2684 [6 %]), each 10 % increase in adherence reduced the odds of rebound viremia by 74 % [AOR = 0.26, 95 % CI (0.11, 0.62), P = 0.002] and 29 % [AOR = 0.71, 95 % CI (0.50, 1.01), P = 0.057] during the first 12 months of suppression and beyond 12 months respectively, interaction term P = 0.018. However, when we restricted to periods with average adherence >50 % [2690 of 2864 (94 %) quarters], each 10 % increase in adherence yielded a 27 % reduction in the odds of rebound viremia during the first 12 months of viral suppression [AOR = 0.73, 95 % CI (0.61, 0.88), P = 0.001], but there was no evidence of an association with rebound viremia beyond 12 months [AOR = 1.09, 95 % CI (0.78, 1.53), P = 0.67], interaction term P = 0.027.
Table 1. Multivariable logistic regression models for correlates of rebound HIV-1 RNA viremia (>400 copies/mL) showing effect of adherence by duration of viral suppression.
| <12 months of viral suppression | ≥12 months of viral suppression | Interaction term between average adherence and duration of viral suppression P value |
|||
|---|---|---|---|---|---|
|
|
|
||||
| AOR (95 % CI) | P value | AOR (95 % CI) | P value | ||
| Average adherence models (each 10 % of adherence) | |||||
| Total cohort | 0.74 (0.66, 0.84) | <0.001 | 0.83 (0.74, 0.92) | <0.001 | 0.17 |
| Restricted to average adherence ≤50 | 0.26 (0.11, 0.62) | 0.002 | 0.71 (0.50, 1.01) | 0.057 | 0.018 |
| Restricted to average adherence >50 % | 0.73 (0.61, 0.88) | 0.001 | 1.09 (0.78, 1.53) | 0.67 | 0.027 |
| 72-h treatment interruption model | |||||
| Number of 72+ h treatment gaps | 1.30 (1.07, 1.59) | 0.009 | 1.39 (1.09, 1.70) | 0.005 | 0.69 |
Multivariable models adjusted for age, gender, nadir CD+ T-lymphocyte count, pre-treatment HIV RNA, and ART regimen
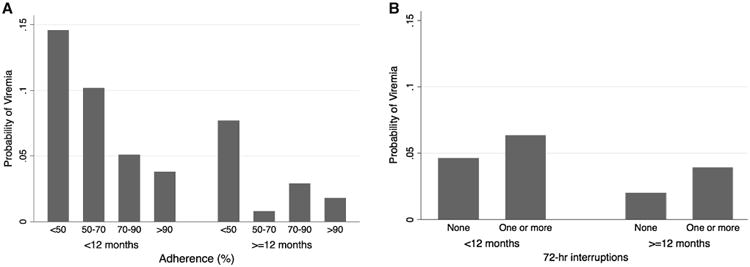
In contrast to average adherence, the association between treatment interruptions and rebound viremia did not depend on duration of prior viral suppression. The presence of any 72-h treatment interruptions increased the odds of rebound viremia during the first 12 months of viral suppression [AOR = 1.30, 95 % CI (1.07, 1.59), P = 0.009] and after [AOR = 1.39, 95 % CI (1.09, 1.70), P = 0.005], interaction term P = 0.69. Although statistically significant in adjusted models, the absolute difference in risk of rebound viremia between those who had 72-h treatment interruptions and those who did not in the first 12 months (6.3 vs 3.9 %) and after 12 months (4.6 vs 2.0 %), was relatively small (Fig. 1).
Fig. 1. Crude risk of rebound viremia, stratified by duration of viral suppression by average adherence (a) or number of 72-h treatment interruptions (b).
Discussion
In a cohort of PLWH in southwestern Uganda taking NNRTI-based ART, the risk of rebound viremia decreased appreciably with increasing duration of viral suppression. This was most pronounced among study participants with greater than 12 months of prior viral suppression, among whom we found no evidence of an impact on rebound viremia, from increasing average adherence over 50 %. Of note, whereas risk of rebound viremia decreased with duration of viral suppression, the risk remained significant even after 12 months of suppression for those with ≤50 % adherence and those with one or more 72-h treatment interruption.
These data have three important implications for HIV practitioners and PLWHs in sub-Saharan Africa. First, the first 12 months of sustained viral suppression is the least forgiving of poor adherence. Similar patterns have been demonstrated in the United States and Canada in populations taking primarily unboosted or boosted protease inhibitor-based regimens [7, 8]. Our data add to this literature by showing similar relationships for those taking almost exclusively NNRTI-based regimens, which are commonly used in sub-Saharan Africa. These data reinforce the value of adherence interventions, particularly early after treatment initiation, when drug adherence has the greatest impact on suppression. Second, whereas prior data on NNRTIs have demonstrated appreciable risk for rebound viremia with diminishing adherence <90 % [10], our data suggest that this effect diminishes with increasing duration of suppression. While perfect adherence should be the goal for every patient, average adherence as low as 50 % might be sufficient to maintain suppression for NNRTI-based regimens after achieving at least 12 months of durable viral suppression [18]. Lastly, our data contribute to a growing literature demonstrating that treatment interruptions are an important risk factor for rebound viremia regardless of the duration of prior viral suppression [10]. In our cohort, although risk of viremia decreased over time, an increasing number of treatment interruptions were independently associated with rebound viremia even after 12 months of suppression. Interventions to decrease risk of prolonged treatment interruptions might protect against this risk.
That average adherence and treatment interruptions impacted rebound viremia differently may be attributed to disparities in therapeutic coverage arising from the differences in the patterns of missed doses. Unlike treatment interruptions, average adherence assumes a consistent drug exposure; that is, that the missed doses are spaced uniformly over the adherence period. As has been previously shown [13], different patterns of missing the same number of doses can impact a drug's therapeutic coverage, and thereby rebound viremia differently. A single 7-day interruption in 1 month will result in lower therapeutic coverage compared to 7 single-day interruptions spread out across the month. Therefore, adequate therapeutic drug levels are likely to be less affected by interspaced interruptions as with average adherence than by a lone, prolonged interruption.
The physiologic basis of our primary finding, that prolonged duration of viral suppression decreases the risk of viral rebound, might be explained by properties of virologic decay after initiation of effective ART. Rapid decreases in HIV RNA are observed during the first 6–9 months of treatment through activity against plasma virions and productively infected cells [19]. After approximately 1 year of effective therapy, virologic decay slows to the rate of latently infected cell death. Similarly, risk of rebound viremia with poor ART adherence is highest relatively early after suppression, when activated infected lymphocytes are more likely to be present, enabling ongoing viral replication during periods with low drug levels. Once activated cells are eliminated of HIV, latently infected cells must be newly activated to cause viral rebound, which might explain persistent viral suppression with relatively low levels of active drug. Extreme examples of this phenomenon include the re-emergence of detectable viremia after months or years off of therapy in patients with very low numbers of latently infected cells after bone marrow transplantation [20].
This study has a number of limitations. MEMS is an imperfect adherence measure and is subject to misclassification (e.g., device non-usage and openings without pill removal). We attempted to mitigate these effects by censoring periods of known non-MEMS usage, and those suggestive of non-usage in which long interruptions did not predict viremia. Second, our data are largely restricted to common first-line treatment regimens in resource-limited settings and have limited generalizability either to commonly used first-line regimens in resource-rich areas or second line regimens in sub-Saharan Africa. Third, findings from the post hoc stratified analyses may be an artifact of the data due to a cohort effect, and should be interpreted with caution. Lastly, as with all observational studies, there is risk of residual or unmeasured confounders, which could alternately explain the relationships we detected.
In summary, in a long-term study of objective ART adherence among people on NNRTI-based regimens in rural Uganda, increasing duration of viral suppression significantly decreases risk of rebound viremia for those with >50 % adherence. Importantly, the risk of rebound viremia persists for those with average adherence ≤50 % and for 72-h treatment interruptions, independent of prior suppression time. To optimize the benefits of ART in the region, adherence interventions should focus on early periods of treatment and resuppression and measures to limit prolonged treatment interruptions.
Acknowledgments
This work was supported by U.S. National Institutes of Health (NIH) R01MH054907 and P30AI27763. Additional study funding was provided by the Mark and Lisa Schwartz Family Foundation, the Sullivan Family Foundation, and the Bacca Foundation. The authors also acknowledge the following additional sources of support: K23MH087228 (JEH) K24MH087227 (DRB); and K23MH099916 (MJS).
Footnotes
Prior Presentation This manuscript was presented at ID Week 2014 in Philadelphia, PA, September 2014.
Conflict of Interest All authors have no conflict of interest related to this work. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Compliance with Ethical Standards: Ethics Statement Study procedures were reviewed and approved by institutional review committees at Partners Healthcare, the University of California at San Francisco, and the Mbarara University of Science and Technology.
Ethical Approval All procedures performed involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent All participants gave written informed consent.
References
- 1.UNAIDS. UNAIDS Report on the Global AIDS Epidemic. 2013 http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf.
- 2.Skarbinski J, Rosenberg E, Paz-Bailey G, et al. Human immunodeficiency virus transmission at each step of the care continuum in the United States. JAMA Intern Med. 2015;175(4):588–96. doi: 10.1001/jamainternmed.2014.8180. [DOI] [PubMed] [Google Scholar]
- 3.HIV Care Saves Lives. 2014 CDC.gov.
- 4.Haberer JE, Kahane J, Kigozi I, et al. Real-time adherence monitoring for HIV antiretroviral therapy. AIDS Behav. 2010;14(6):1340–6. doi: 10.1007/s10461-010-9799-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haberer JE, Robbins GK, Ybarra M, et al. Real-time electronic adherence monitoring is feasible, comparable to unannounced pill counts, and acceptable. AIDS Behav. 2012;16(2):375–82. doi: 10.1007/s10461-011-9933-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnsten JH, Demas PA, Farzadegan H, et al. Antiretroviral therapy adherence and viral suppression in HIV-infected drug users: comparison of self-report and electronic monitoring. Clin Infect Dis. 2001;33(8):1417–23. doi: 10.1086/323201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lima VD, Bangsberg DR, Harrigan PR, et al. Risk of viral failure declines with duration of suppression on highly active antiretroviral therapy irrespective of adherence level. J Acquir Immune Defic Syndr. 2010;55(4):460–5. doi: 10.1097/QAI.0b013e3181f2ac87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenblum M, Deeks SG, van der Laan M, Bangsberg DR. The risk of virologic failure decreases with duration of HIV suppression, at greater than 50% adherence to antiretroviral therapy. PLoS ONE. 2009;4(9):e7196. doi: 10.1371/journal.pone.0007196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133(1):21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 10.Nachega JB, Hislop M, Dowdy DW, Chaisson RE, Regensberg L, Maartens G. Adherence to nonnucleoside reverse transcriptase inhibitor-based HIV therapy and virologic outcomes. Ann Intern Med. 2007;146(8):564–73. doi: 10.7326/0003-4819-146-8-200704170-00007. [DOI] [PubMed] [Google Scholar]
- 11.Parienti JJ, Massari V, Descamps D, et al. Predictors of virologic failure and resistance in HIV-infected patients treated with nevirapine- or efavirenz-based antiretroviral therapy. Clin Infect Dis. 2004;38(9):1311–6. doi: 10.1086/383572. [DOI] [PubMed] [Google Scholar]
- 12.Oyugi JH, Byakika-Tusiime J, Ragland K, et al. Treatment interruptions predict resistance in HIV-positive individuals purchasing fixed-dose combination antiretroviral therapy in Kampala, Uganda. Aids. 2007;21(8):965–71. doi: 10.1097/QAD.0b013e32802e6bfa. [DOI] [PubMed] [Google Scholar]
- 13.Genberg BL, Wilson IB, Bangsberg DR, et al. Patterns of antiretroviral therapy adherence and impact on HIV RNA among patients in North America. Aids. 2012;26(11):1415–23. doi: 10.1097/QAD.0b013e328354bed6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO. On the use of antiretroviral drugs for treating and preventing HIV infection. World Health Organisation; 2014. [Google Scholar]
- 15.Adakun SA, Siedner MJ, Muzoora C, et al. Higher baseline CD4 cell count predicts treatment interruptions and persistent viremia in patients initiating ARVs in rural Uganda. J Acquir Immune Defic Syndr. 2013;62(3):317–21. doi: 10.1097/QAI.0b013e3182800daf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haberer JE, Musinguzi N, Boum Y, II, et al. Duration of antiretroviral therapy adherence interruption is associated with risk of virologic rebound as determined by real-time adherence monitoring in rural Uganda. J Acquir Immune Defic Syndr. 2015;70(4):386–92. doi: 10.1097/QAI.0000000000000737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parienti JJ, Das-Douglas M, Massari V, et al. Not all missed doses are the same: sustained NNRTI treatment interruptions predict HIV rebound at low-to-moderate adherence levels. PLoS ONE. 2008;3(7):e2783. doi: 10.1371/journal.pone.0002783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bangsberg DR. Less than 95% adherence to nonnucleoside reverse-transcriptase inhibitor therapy can lead to viral suppression. Clin Infect Dis. 2006;43(7):939–41. doi: 10.1086/507526. [DOI] [PubMed] [Google Scholar]
- 19.Siliciano JD, Siliciano RF. Latency and viral persistence in HIV-1 infection. J Clin Investig. 2000;106(7):823–5. doi: 10.1172/JCI11246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henrich TJ, Hanhauser E, Marty FM, et al. Antiretroviral-free HIV-1 remission and viral rebound after allogeneic stem cell transplantation: report of 2 cases. Ann Intern Med. 2014;161(5):319–27. doi: 10.7326/M14-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]


