Abstract
The impaired ability to produce or respond to insulin, a hormone synthetized by the pancreatic β-cells, leads to diabetes. There is an excruciating need of finding new approaches to protect or restore these cells once they are lost. Replacement and ex vivo directed reprogramming methods have an undeniable therapeutic potential, yet they exhibit crucial flaws. The in vivo conversion of adult cells to functional insulin-producing cells is a promising alternative for regenerative treatments in diabetes. The stunning natural transdifferentiation potential of the adult endocrine pancreas was recently uncovered. Modulating molecular targets involved in β-cell fate maintenance or in general differentiation mechanisms can further potentiate this intrinsic cell plasticity, which leads to insulin production reconstitution.
Introduction
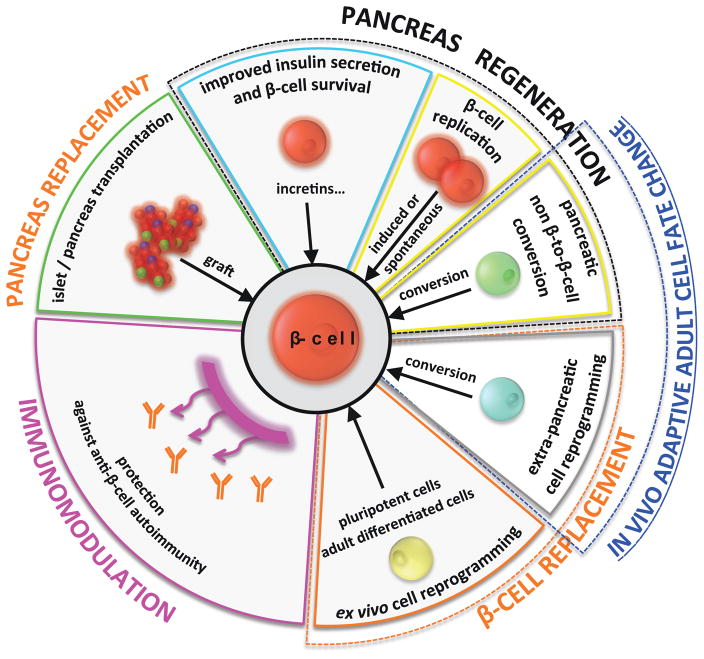
Diabetes, i.e. the excessive production of urine (polyuria) associated with tenacious thirst (polydipsia), is a symptom common to many different diseases characterized by a debilitating persistent excess of glucose in circulating blood (hyperglycemia). The high blood sugar is typical of diabetes mellitus (distinct from diabetes insipidus, a “brain-kidney” disease). Diabetes mellitus results from the impaired ability of either (i) the endocrine pancreas to produce the hormone insulin, or (ii) the target tissues (brain, liver, muscle…) to respond to it, or both. The two prevailing forms of diabetes, named Type 1 and Type 2 (T1D and T2D), are characterized by the loss of insulin-producing islet cells: total or near total in T1D, due to an autoimmune destruction, or variable and partial in T2D1,2. A cure for insulin-dependent diabetes requires the reconstitution of a functional β-cell mass, either through in situ regeneration or by cell-based replacement therapies, i.e. the transplantation of surrogate β-like cells obtained from stem cells3,4 (Figure 1).
Figure 1.
Synopsis of present-day and tentative approaches to treat diabetes. Today, physicians try to maintain and improve insulin secretion and β-cell survival / function; in extreme situations, the only solution is transplantation (of isolated islets or total pancreas). The two prospective broad strategies of the regenerative medicine approach are β-cell replacement and β-cell regeneration. The two largely rely on the exploitation of the recently discovered cell plasticity of the adult. Developing an efficient protective immunomodulation against β-cell autoimmunity will be an additional requirement in T1D conditions.
In recent years, several observations have revealed an astonishing intrinsic plasticity in the pancreatic islets of Langerhans5. These findings allow envisioning new strategies for treating diabetes by exploiting the in vivo transdifferentiation potential of diverse pancreatic cell types (Figure 1). Due to space constrains, in this mini-review we will solely address the main advances towards this goal by focusing exclusively on the experimental settings in which reprogramming into insulin production (either natural or guided, of pancreatic or extra-pancreatic cells) satisfied the following criteria: i) was described in vivo, ii) occurred during postnatal life, and iii) reinstated, transiently or permanently, blood sugar levels (glycemia) in diabetic animals.
Choosing a β-cell reconstitution strategy: replacement vs. regeneration
The last 20 years have seen a rapid development of β-cell replacement strategies, the most common being the allotransplantation of healthy pancreatic islets from cadaveric donors6 (Figure 1). This method is limited by the scarcity of donors and the inherent graft decay, with only 44% of the recipients being insulin-independent 3 years after transplantation7. Consequently, finding a more accessible source of β-cells is mandatory for the development of regenerative therapies to treat diabetes.
An increasingly popular line of research focuses on generating mature insulin-producing cells in vitro starting from human induced pluripotent stem cells (hiPSC), derived from somatic cells of normal donors (such as fibroblasts), as an alternative to islet allotransplantation (Figure 1). Although this approach has the advantage of generating a potentially unlimited number of β-like cells, it still faces some controversy regarding graft rejection complications8,9, thus requiring further research directed at designing optimal delivery methods (encapsulation devices)10, or developing genetically-modified β-like cells from autologous patient-derived iPSC11. Also, most current cell differentiation protocols have limiting flaws linked to heterogeneous yields and tumorigenesis3,12,13.
An alternative approach to the in vitro/ex vivo differentiation of surrogate β-cells is the exploitation of the natural in vivo β-cell regenerative capacity of the pancreas, primarily by stimulating β-cell self-replication14–16 (Figure 1). Nevertheless, this approach is inadequate for treating patients with complete or near-complete absence of β-cells, as reported in many T1D cases, among other limitations.
Adaptive transdifferentiation is a conserved regeneration mechanism
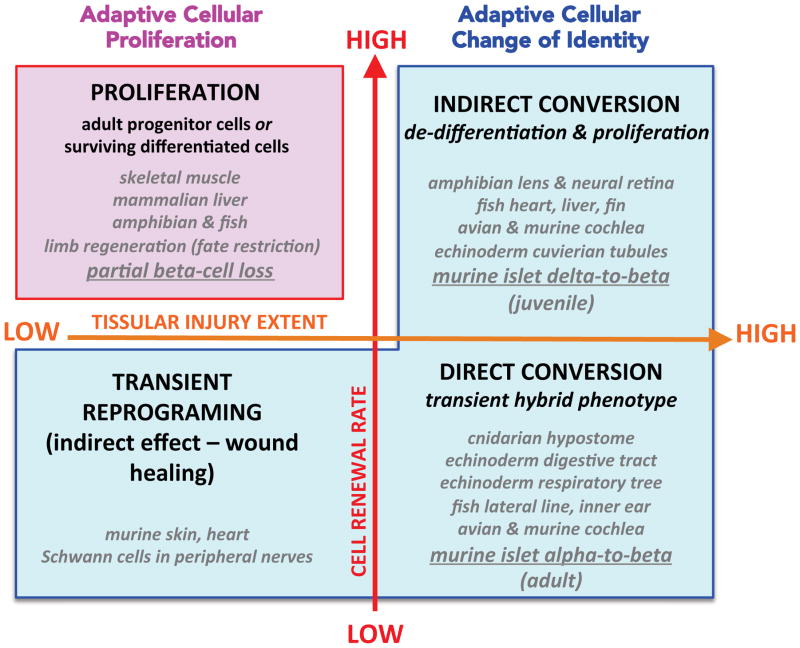
The body has developed two main natural strategies to replenish lost cell populations, which are different depending upon the capacity of the cells to enter the cell cycle (summarized in Figure 2).
Figure 2.
The natural strategies to replenish lost cell populations in vivo rely upon adaptive increased cell proliferation, in tissues with high renewal rates, or on adaptive changes of cell identity (conversion), in tissues with low proliferation capacity. At the tissular injury level, limb amputation does not imply the loss of a given specific cell type, since in the remaining member all cell types are present, in contrast with selective cell ablation situations; therefore, limb regeneration after partial amputation appears as “low tissular injury” condition. The examples listed are referenced in Table 1.
Cell transdifferentiation, conversion, reprogramming or fate change, is a stable switch in cell identity, where a terminally differentiated cell converts into a different mature cell-type, with or without experiencing a transitional proliferative stage (reviewed in17–22). It occurs naturally in response to various stressors (reviewed in23) and represents an ancient and widespread regenerative strategy among metazoans, being described from cnidarians to vertebrates (reviewed in19,24–26; Table 1). However, the transdifferentiation nature of the regenerative process remains controversial in some cases, because it may occur alongside other regenerative mechanisms22,27,28. Two examples are fin regeneration in fish and limb regeneration in amphibians, where cell lineage tracing experiments have revealed that most cell types are lineage-restricted29: upon injury, differentiated cells in the proximity of the wound form the blastema, i.e. they de-differentiate before rebuilding the original tissue by giving rise to the original cell type without a change in fate30–32. Interestingly though, fin regeneration still occurs after osteoblast ablation, thus proving that bone can regenerate from alternative sources33.
Table 1.
Documented examples of regeneration through adaptive cell plasticity in kingdom Animalia.
| Organism | Organ / Tissue | Cell Conversion Type | References |
|---|---|---|---|
| Cnidarians (Hydra vulgaris AEP) | Hypostome (Granular mucous cells ) | Direct | Siebert et al. 200873 |
| Echinoderms (Eupentacta fraudatrix) | Digestive tract (anterior rudiment) | Direct | Mashanov et al. 200574 |
| Echinoderms (Holothuria forskali) | Cuvierian tubules | Indirect | VandenSpiegel et al. 200075 |
| Echinoderms (Apostichopus japonicus) | Respiratory trees | Direct & Indirect | Dolmatov and Ginanova 200976 |
| Fish (Zebrafish) (Danio rerio) | Liver | Indirect | He et al. 201477 |
| Fish (Zebrafish) (Danio rerio) | Heart | Indirect | Zhang et al. 201378 |
| Teleosts (Zebrafish) (Danio rerio) | Fin rays (osteoblasts-depleted fins) | Indirect | Singh et al. 201233 |
| Teleosts (Zebrafish) (Danio rerio) | Lateral line and inner ears (sensory hair cells) | Direct | Reviewed in: Monroe et al. 201579 |
| Amphibians: Anures (Frog) (Xenopus laevis) | Lens regeneration & Neural retina regeneration | Indirect | Yoshii et al. 200780 |
| Amphibians: Urodeles (Newt) (Notophthalmus viridescens) (Triturus viridescens) (Cynops pyrrhogaster) | Wolffian lens regeneration & Neural retina regeneration | Indirect | Reviewed in: Barbosa-Sabanero, et al. 201281; Tsonis et al. 200482 |
| Avians (Chick) (Gallus gallus) | Cochlea (sensory hair cells) | Direct & Indirect | Reviewed in: Stone and Cotanche 200783 |
| Mammals (Mouse) (Mus musculus) JUVENILES |
Pancreas (β-cells) | Indirect | Chera et al. 201450 |
| Mammals (Mouse) (Mus musculus) ADULTS |
Pancreas (β-cells) | Direct | Thorel, Népote et al. 201048 |
| Mammals (Mouse) (Mus musculus) | Skin and heart | Direct | Davis, Burr et al. 201284 Reviewed in: Hinz 200785 |
| Mammals (Mouse) (Mus musculus) | Peripheral nerves (Wallerian degeneration) | Indirect | Arthur-Farraj et al. 201286 Reviewed in: Jessen et al. 201587 Jessen et al. 201522 |
| Mammals (Mouse) (Mus musculus) NEONATAL |
Cochlea (sensory hair cells) | Direct & Indirect | Bramhall et al. 201488 Cox et al. 201489 Reviewed in: Richardson and Atkinson 201590 |
| Mammals (Mouse) (Mus musculus) | Liver (biliary epithelial cells) | unknown | Yanger et al. 201391 |
Two different mechanisms of cell transdifferentiation were described already a century ago: (1) direct conversion, whereby cell type A trans-fates directly into cell type B, in absence of cell division and displaying a transient hybrid phenotype, and (2) indirect conversion, where cell type A de-differentiates, proliferates and re-differentiates acquiring the B cell type fate. The first process is characteristic for morphallaxis, while the second is a type of epimorphosis34 (Figure 2).
In order to determine the occurrence of a cell fate conversion event in response to injury, the resulting differentiated cell type must be i) lineage-traced to its original progenitor, and ii) characterized at the morphological and molecular levels35. These changes can be reflected by wide differences in the transcriptional landscape, including, for instance, the expression of specific genes such as those encoding peptidic hormones and the enzymes involved in their conversion and secretion21.
Cells and their progeny can be irreversibly (genetically) tagged in vivo, in transgenic animals36,37 (reviewed in38–40). The first in vivo cell lineage tracing analysis during mouse embryonic development using the Cre/loxP system was performed by one of us in a study of pancreatic cell fate allocation41. A few examples of in vivo lineage-tracing studies in development and regeneration in other organs are indicated here5,42–47. This cell labeling method eliminates any ambiguity regarding the identity of the precursors of converted cells48–50. Moreover, the same transgenic setup may be used for the simultaneous constitutive or inducible, transient or irreversible, modulation of key genes in the cell type of interest.
The lack of a cell tracing system is a major limitation in any study involving human diabetic patients51. It is also a limiting factor in analyses of experimental diabetes: for instance, in a murine model of severe injury in which pancreatic duct ligation was combined with alloxan-induced β-cell destruction, the authors claimed that α-cells convert into β-like cells based only on marker co-expression, which is never conclusive52.
Guided pancreatic cell transdifferentiation: the artificially induced cell plasticity
In homeostatic conditions, β-cells are located in endocrine units termed islets of Langerhans, intermingled between the acini and ducts of the exocrine pancreas. Besides the preponderant β-cells, pancreatic islets contain other endocrine cell types, each secreting a different hormone: glucagon (α-cells), somatostatin (δ-cells) and pancreatic polypeptide (PP-cells). The regenerative potential of the pancreas was first described almost 20 years ago, yet without adequate lineage tracing tools, when several studies reported the emergence of new endocrine cells, including insulin-producing cells, following tissue injury (reviewed in26). The authors suggested an exocrine cell transdifferentiation process, rather than the recruitment of “dormant” stem cells.
Recent years have witnessed attempts at generating insulin-producing cells in vivo by ectopically inducing β-cell-specific programs in extra-pancreatic and pancreatic cells, either by gene therapy or pharmacologically. The objective of any guided cell-fate switching strategy is to asymptotically reach a “perfect” conversion while minimally interfering with the original target cell population, so as to avoid paradoxical side effects. One example is the study of Melton and colleagues53, who reprogrammed exocrine cells into insulin production in mice by injecting into the pancreas different combinations of adenoviruses encoding 9, 6 or 3 key β-cell transcription factors; the highest conversion rate inversely correlated with adenoviral cocktail complexity. Only 3 key transcription factors were sufficient for β-cell conversion induction: Pdx1 (required during early pancreas development as well as for β-cell maintenance), Ngn3 (required for islet endocrine progenitor fate allocation) and MafA (required for β-cell maturation). In a long-term study, Zhou and colleagues reported the persistence of induced β-like cells for up to 13 months and, interestingly, their aggregation into islet-like structures54. These studies and others55 showed that these 3 factors are absolutely necessary for an efficient acinar-to-β-like transdifferentiation.
In another study56, Heimberg and colleagues reported that the acinar cell transdifferentiation can be pharmacologically induced with cytokines (epidermal growth factor and ciliary neurotrophic factor treatment). The regenerated β-like cells were able, like in the Zhou study, to rescue diabetes and maintain euglycemia for up to 8 months in mice where diabetes was induced by streptozotocin (STZ) administration.
The endocrine pancreas is a natural choice as source of new β-like cells. Due to the difference in ratio between non-β- and β-cells in islets (β-cells represent almost 80% of the islet cell mass in rodents), one concern of the in vivo islet cell conversion towards the β-cell fate is the risk of decreasing or even losing another islet cell type. In this regard, Collombat et al. reported that the transgenic misexpression of the β-cell-specific transcription factor Pax4 in α-cells (Pax4OE) leads to a loss of 77% of them, together with a progressive increase in insulin+ cells57. Indeed, most Pax4OE α-cells became β-like cells. The authors hypothesized that the constant α-to-β-cell conversion process depleted the α-cell compartment, i.e. the pancreatic glucagon content, hence triggering a compensatory α-cell neogenesis through the mobilization of ductal precursors. The newly formed α-cells adopted a β-cell fate, generating a positive feedback loop, which ultimately resulted in islet hyperplasia49 (reviewed in58). Nevertheless, the authors’ interpretation is refuted by the lack of any compensatory α-cell neodifferentiation in a genetic model of almost complete α-cell ablation59, thus raising the possibility that an alternative explanation accounts for the observed series of events. In fact, by using a genetic model allowing the specific and inducible ablation of 98% of adult α-cells, we observed that the remaining 2% α-cell mass maintains basal glucagon levels (glucagonemia)59. This is incidentally encouraging, since an induced massive α-cell conversion could be envisioned in guided α-cell conversion protocols (see below).
The important concept, however, is that the ectopic expression of just one single β-cell-specific transcription factor in α-cells is sufficient to steadily transdifferentiate them towards a stable β-cell phenotype. Moreover, the Pax4OE-induced β-like converted α-cells were able to restore the glycemic control in mice made diabetic with STZ57.
Extra-pancreatic tissues: a new source for guided β-cell generation
Likely, the number and type of factors required for acquiring a β-like cell phenotype vary depending on the targeted source cell type. Most transdifferentiation studies concern the pancreas, but there is also interest in organs with a related developmental origin, such as the liver60,61 and gut62.
In one such study, Slack and colleagues were able to generate glucose sensing insulin-secreting cells in diabetic mice by converting Sox9+ cells, which are located in the small bile ducts of the liver63. Interestingly, transdifferentiation was induced by a single polycistronic adenovirus encoding the same 3 factors Pdx1, Ngn3 and MafA, indicating that this trio is also highly efficient at inducing a β-like cell fate in extra-pancreatic tissues.
Overexpression of β-cell-specific factors is not the only alternative for making β-like cells. For instance, although the transcription factor FoxO1 is expressed in β-cells, it is not a β-cell-specific marker, and its inactivation does affect neither the formation nor the cell architecture of the endocrine pancreas. By inactivating FoxO1 in gut Ngn3+ enteroendocrine precursor cells of mice64, Accili and co-workers directed their differentiation towards a β-cell fate. The resulting cells expressed β-cell maturity markers and were able to cure diabetes.
This group also showed that, intriguingly, the constitutive selective inactivation of FoxO1 in β-cells leads to their de-differentiation in situations of metabolic stress65. This observation has led the authors to postulate that, instead of dying, perhaps some β-cells dedifferentiate in T2D.
Spontaneous reprogramming of pancreatic islet non-β-cells: the innate plasticity
To accurately determine if the adult pancreas can regenerate new β-cells once they are lost, like in T1D, and to characterize the cellular and molecular programs activated in the pancreas in response to β-cell loss, we used specific cell tracing tools in a transgenic model of inducible acute, rapid, selective and total β-cell removal48,50.
We found that, unexpectedly, β-cell ablation triggers the natural and spontaneous reprogramming of a small fraction (~2%) of the adult glucagon-producing α-cells into insulin production. This α-to-β conversion includes an intermediary hybrid transitional stage of insulin+/glucagon+ bihormonal cells, which was confirmed by genetic lineage tracing analyses48. Of note, this is one of the very few reported cases of naturally occurring direct transdifferentiation in the animal kingdom, as it occurs in the absence of cell proliferation. Probably as a consequence of the proliferation-independent character of the change of identity process, α-cells retain the capacity of engaging into insulin production in aged mice as well, demonstrating a persistence of plasticity throughout life, which could have clinically relevant implications50.
Further studies on the influence of age on β-cell reconstitution following total β-cell ablation revealed that juvenile prepubescent mice always recover from diabetes after near-total β-cell ablation50. Moreover, their recovery was faster, being able to regain a glycemic control by 5 months of regeneration, correlated with a rapid replenishment of the β-cell pool. Surprisingly, genetic lineage-tracing studies revealed a completely novel regeneration mechanism, involving the massive recruitment of another hormonal cell type, δ-cells, which de-differentiate to a progenitor stage, reenter the cell cycle, and recapitulate embryonic development to become insulin producers. Interestingly, this early timeline of the juvenile regeneration mechanism resembles closely the one described for lens regeneration in urodeles (reviewed in5,21,25). The δ-to-β reprogramming is confined to a very restrictive window at the beginning of regeneration and is required for generating a “minimal pool” of insulin producing β-like cells. These regenerated β-like cells secreted insulin and expressed typical β-cell-specific markers, yet they displayed striking differences in the expression of key cell cycle regulators. For this reason, these cells were able to undergo several rounds of proliferation and finally reconstituted up to 30–70% of the age-matched control β-cell mass. Notably, even after 2 years of regeneration the recovered β-cell mass never exceeded the one of age-matched controls suggesting a stringent regulation of the regeneration event, consistent with the observations in classical regenerative structures and systems.
The efficient juvenile regeneration mechanism raises the interest for developing “rejuvenation” strategies aimed at fostering or maintaining the juvenile regenerative mechanism later in life50. In this respect, the initial, yet limited, comparative transcriptional analysis following total β-cell loss revealed that FoxO1 and its downstream effectors display a divergent behaviour in juvenile and adult δ-cells upon injury. FoxO1 is a transcription factor involved in cell cycle and senescence regulation. Its decrease in juvenile δ-cells and the detected low levels of its direct targets, such as senescence markers Cdkn1a (p21) and Cdkn1b (p15Ink4b), was consistent with an increased proliferative capacity. In contrast, following injury, FoxO1 and cyclin-dependent kinase inhibitors were upregulated in adult δ-cells, thus potentially explaining their incapacity to engage into an efficient regenerative program during adulthood. Interestingly, the juvenile mechanism could be somewhat mimicked with a pharmacological inhibition of FoxO1 after injury, which promoted the δ-to-β conversion in adulthood50.
Prospective
The production of experimental models for selective cell ablation in vivo will continue to lead to discoveries about tissue homeostasis and adaptive cell plasticity during embryonic development and during regeneration in pathological conditions or after injury, as has been the case in the pancreas48,66.
In vivo generation of surrogate β-cells from alternative non-β-cell sources represents a promising approach to treat diabetes, especially when β-cell loss is total. It appears that there may be efficient regeneration of β-cells in children with T1D or having undergone subtotal pancreatectomy5,67,68. Also, insulin+/glucagon+ bihormonal cells have been described in human diabetic patients69–71 and upon ex vivo epigenetic manipulation72, suggesting that human α-cells might also display the plasticity allowing insulin production. Yet, whether these are reprogrammed α-cells remains mysterious; since it is not possible to lineage-trace them to their origin, these could on the contrary be de-differentiated β-cells that start expressing glucagon, as claimed by some51.
The natural regeneration potential of the pancreas is age-dependent, with two distinct reprogramming mechanisms being employed according to the age at which the regenerative stressor occurs. The efficient juvenile regeneration is proliferation-dependent and hence restricted to a narrow window during early post-natal life. Current evidence suggests that unknown factors promote the accumulation of senescence markers in adult pancreatic islet cells, thus triggering a steep proliferation potential decrease and blocking the efficient juvenile regenerative program. These factors could be linked to sexual maturation, ageing, dietary change or other. In contrast, the adult regeneration mechanism is proliferation-independent and consequently the cell senescence status does not affect the 1:1 direct transdifferentiation event (age-independent). A consequence of these observations is that, besides cell sources and target molecules, one should also consider the age of a tissue when designing replacement strategies.
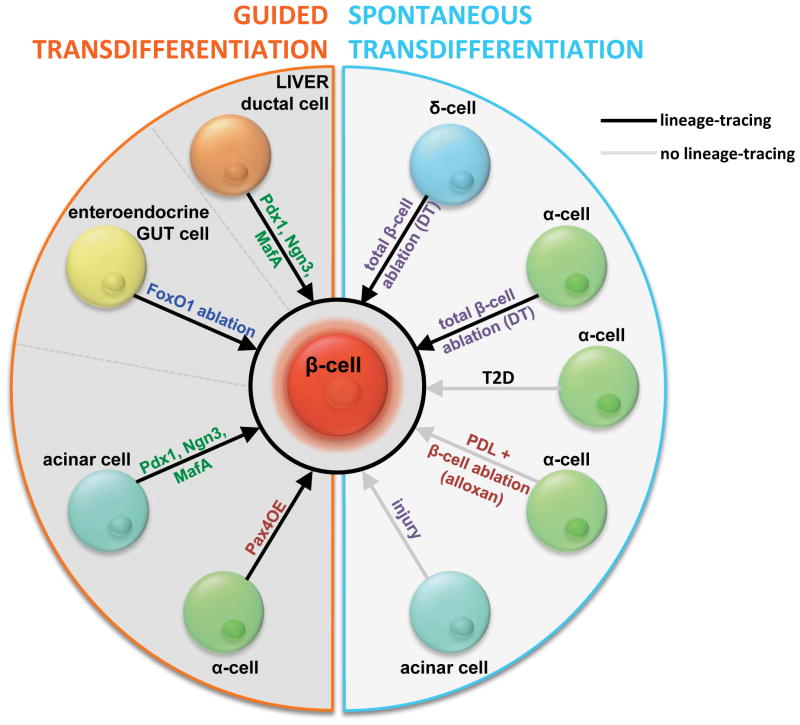
Understanding the cellular and molecular basis of innate adaptive islet plasticity and pancreas development is a requirement for designing efficient guided transdifferentiation strategies. In recent years we have seen a diversification of methods for the generation of replacement β-cells from non-β-cells (Figure 3), including the up-regulation of β-cell-specific factors, as well as the modulation of general regulators of cell differentiation and the cell cycle, both in pancreatic and extra-pancreatic tissues. Given the functional complexity of β-cells, it is unlikely that the artificial modulation of one single factor will suffice to produce a bona-fide β-cell equivalent; rather, a design involving the combinatorial regulation of key β-cell factors and the niche influence will be required.
Figure 3.
Integrative view of the innate and guided cell conversion approaches aimed at reconstituting lost β-cells, classified according to the cell type of origin and the nature of the reprogramming stimuli. DT, diphtheria toxin-mediated β-cell ablation (as used in references #49 and #51); PDL, pancreatic duct ligation (surgical method to trigger pancreatitis in rodent models, and therefore study pancreatic tissue remodeling).
Future studies will help identifying the critical molecular targets and signals, which foster the plasticity leading to the generation of replacement β-like cells.
Acknowledgments
We are most grateful to Luiza Ghila, for carefully reading and editing the manuscript. We also thank Kenichiro Furuyama, Fabrizio Thorel and Daniel Ortega for insightful comments and suggestions. S. C. is supported by grants from the Research Council of Norway (NFR) and the Novo Nordisk Foundation. P.L.H. is supported by grants from the NIH/NIDDK, the Swiss National Science Foundation, the Juvenile Diabetes Research Foundation and the European Union. We apologize for the papers not cited here because of space or scope limitations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gillespie KM. Type 1 diabetes: pathogenesis and prevention. CMAJ. 2006;175:165–170. doi: 10.1503/cmaj.060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alejandro EU, Gregg B, Blandino-Rosano M, Cras-Meneur C, Bernal-Mizrachi E. Natural history of beta-cell adaptation and failure in type 2 diabetes. Mol Aspects Med. 2015;42:19–41. doi: 10.1016/j.mam.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *3.Pagliuca FW, et al. Generation of functional human pancreatic beta cells in vitro. Cell. 2014;159:428–439. doi: 10.1016/j.cell.2014.09.040. With an scalable suspension-based culture system, the authors generated glucose-responsive insulin-producing cells using sequential modulation of multiple signaling pathways in a three-dimensional cell culture system, without any genetic modification. These are the first surrogate differentiated glucose-responsive insulin-producing cells obtained in vitro, which function like adult human β-cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Russ HA, et al. Controlled induction of human pancreatic progenitors produces functional beta-like cells in vitro. EMBO J. 2015;34:1759–1772. doi: 10.15252/embj.201591058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desgraz R, Bonal C, Herrera PL. β-cell regeneration: the pancreatic intrinsic faculty. Trends Endocrinol Metab. 2011;22:34–43. doi: 10.1016/j.tem.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Call MK, Grogg MW, Tsonis PA. Eye on regeneration. Anat Rec B New Anat. 2005;287:42–48. doi: 10.1002/ar.b.20084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruni A, Gala-Lopez B, Pepper AR, Abualhassan NS, Shapiro AJ. Islet cell transplantation for the treatment of type 1 diabetes: recent advances and future challenges. Diabetes Metab Syndr Obes. 2014;7:211–223. doi: 10.2147/dmso.s50789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao T, Zhang ZN, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212–215. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
- 9.Araki R, et al. Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells. Nature. 2013;494:100–104. doi: 10.1038/nature11807. [DOI] [PubMed] [Google Scholar]
- *10.Vegas AJ, et al. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat Med. 2016 doi: 10.1038/nm.4030. Using triazole–thiomorpholine dioxide alginate capsules, the authors were able to transplant glucose-responsive mature β-cells, which were derived from human embryonic stem cells. This is the first study showing glycemic correction in diabetic mice without any immunosuppression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **11.Hua H, et al. iPSC-derived β cells model diabetes due to glucokinase deficiency. J Clin Invest. 2013;123:3146–3153. doi: 10.1172/JCI67638. Using skin biopsies from two MODY2 subjects, the authors generated induced glucose-responsive mature β-cells and showed that higher glucose levels are needed to stimulate insulin secretion in GCK mutant β-cells. This is the first study showing mutation-specific phenotype reversion upon gene sequence correction by homologous recombination. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Rezania A, et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol. 2014;32:1121–1133. doi: 10.1038/nbt.3033. [DOI] [PubMed] [Google Scholar]
- 13.Robinton DA, Daley GQ. The promise of induced pluripotent stem cells in research and therapy. Nature. 2012;481:295–305. doi: 10.1038/nature10761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 15.Li W, et al. mTORC1 pathway mediates beta cell compensatory proliferation in 60 % partial-pancreatectomy mice. Endocrine. 2016 doi: 10.1007/s12020-016-0861-5. [DOI] [PubMed] [Google Scholar]
- 16.Wang G, et al. First quantitative high-throughput screen in zebrafish identifies novel pathways for increasing pancreatic beta-cell mass. Elife. 2015;4 doi: 10.7554/eLife.08261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsonis PA. Regeneration via transdifferentiation: the lens and hair cells. Hear Res. 2007;227:28–31. doi: 10.1016/j.heares.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 18.Tsonis PA. Regenerative biology: the emerging field of tissue repair and restoration. Differentiation. 2002;70:397–409. doi: 10.1046/j.1432-0436.2002.700802.x. [DOI] [PubMed] [Google Scholar]
- 19.Tsonis PA. Regeneration in vertebrates. Dev Biol. 2000;221:273–284. doi: 10.1006/dbio.2000.9667. [DOI] [PubMed] [Google Scholar]
- 20.Sisakhtnezhad S, Matin MM. Transdifferentiation: a cell and molecular reprogramming process. Cell Tissue Res. 2012;348:379–396. doi: 10.1007/s00441-012-1403-y. [DOI] [PubMed] [Google Scholar]
- 21.Slack JM. Metaplasia and transdifferentiation: from pure biology to the clinic. Nat Rev Mol Cell Biol. 2007;8:369–378. doi: 10.1038/nrm2146. [DOI] [PubMed] [Google Scholar]
- 22.Jessen KR, Mirsky R, Arthur-Farraj P. The Role of Cell Plasticity in Tissue Repair: Adaptive Cellular Reprogramming. Dev Cell. 2015;34:613–620. doi: 10.1016/j.devcel.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Thowfeequ S, Myatt EJ, Tosh D. Transdifferentiation in developmental biology, disease, and in therapy. Dev Dyn. 2007;236:3208–3217. doi: 10.1002/dvdy.21336. [DOI] [PubMed] [Google Scholar]
- 24.Poss KD. Advances in understanding tissue regenerative capacity and mechanisms in animals. Nat Rev Genet. 2010;11:710–722. doi: 10.1038/nrg2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knapp D, Tanaka EM. Regeneration and reprogramming. Curr Opin Genet Dev. 2012;22:485–493. doi: 10.1016/j.gde.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 26.Jopling C, Boue S, Izpisua Belmonte JC. Dedifferentiation, transdifferentiation and reprogramming: three routes to regeneration. Nat Rev Mol Cell Biol. 2011;12:79–89. doi: 10.1038/nrm3043. [DOI] [PubMed] [Google Scholar]
- 27.Gemberling M, Bailey TJ, Hyde DR, Poss KD. The zebrafish as a model for complex tissue regeneration. Trends Genet. 2013;29:611–620. doi: 10.1016/j.tig.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kragl M, et al. Novel insights into the flexibility of cell and positional identity during urodele limb regeneration. Cold Spring Harb Symp Quant Biol. 2008;73:583–592. doi: 10.1101/sqb.2008.73.034. [DOI] [PubMed] [Google Scholar]
- 29.Tu S, Johnson SL. Fate restriction in the growing and regenerating zebrafish fin. Dev Cell. 2011;20:725–732. doi: 10.1016/j.devcel.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knopf F, et al. Bone regenerates via dedifferentiation of osteoblasts in the zebrafish fin. Dev Cell. 2011;20:713–724. doi: 10.1016/j.devcel.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 31.Sousa S, et al. Differentiated skeletal cells contribute to blastema formation during zebrafish fin regeneration. Development. 2011;138:3897–3905. doi: 10.1242/dev.064717. [DOI] [PubMed] [Google Scholar]
- 32.Kragl M, et al. Cells keep a memory of their tissue origin during axolotl limb regeneration. Nature. 2009;460:60–65. doi: 10.1038/nature08152. [DOI] [PubMed] [Google Scholar]
- 33.Singh SP, Holdway JE, Poss KD. Regeneration of amputated zebrafish fin rays from de novo osteoblasts. Dev Cell. 2012;22:879–886. doi: 10.1016/j.devcel.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morgan TH. REGENERATION AND LIABILITY TO INJURY. Science. 1901;14:235–248. doi: 10.1126/science.14.346.235. [DOI] [PubMed] [Google Scholar]
- 35.Eguchi G, Kodama R. Transdifferentiation. Curr Opin Cell Biol. 1993;5:1023–1028. doi: 10.1016/0955-0674(93)90087-7. [DOI] [PubMed] [Google Scholar]
- 36.Herrera PL. Defining the cell lineages of the islets of Langerhans using transgenic mice. Int J Dev Biol. 2002;46:97–103. [PubMed] [Google Scholar]
- 37.Herrera PL, Nepote V, Delacour A. Pancreatic cell lineage analyses in mice. Endocrine. 2002;19:267–278. doi: 10.1385/ENDO:19:3:267. [DOI] [PubMed] [Google Scholar]
- 38.Weissman TA, Pan YA. Brainbow: new resources and emerging biological applications for multicolor genetic labeling and analysis. Genetics. 2015;199:293–306. doi: 10.1534/genetics.114.172510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Keymeulen A, Blanpain C. Tracing epithelial stem cells during development, homeostasis, and repair. J Cell Biol. 2012;197:575–584. doi: 10.1083/jcb.201201041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thirumangalathu S, Barlow LA. In vivo fate tracing studies of mammalian taste cell progenitors. Ann N Y Acad Sci. 2009;1170:34–38. doi: 10.1111/j.1749-6632.2009.04371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herrera PL. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development. 2000;127:2317–2322. doi: 10.1242/dev.127.11.2317. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki A. Evidence of cell-fate conversion from hepatocytes to cholangiocytes in the injured liver: in-vivo genetic lineage-tracing approaches. Curr Opin Gastroenterol. 2015;31:247–251. doi: 10.1097/mog.0000000000000172. [DOI] [PubMed] [Google Scholar]
- 43.Romagnani P, Rinkevich Y, Dekel B. The use of lineage tracing to study kidney injury and regeneration. Nat Rev Nephrol. 2015;11:420–431. doi: 10.1038/nrneph.2015.67. [DOI] [PubMed] [Google Scholar]
- 44.Joseph C, et al. Deciphering hematopoietic stem cells in their niches: a critical appraisal of genetic models, lineage tracing, and imaging strategies. Cell Stem Cell. 2013;13:520–533. doi: 10.1016/j.stem.2013.10.010. [DOI] [PubMed] [Google Scholar]
- **45.Desgraz R, Herrera PL. Pancreatic neurogenin 3-expressing cells are unipotent islet precursors. Development. 2009;136:3567–3574. doi: 10.1242/dev.039214. Using MADM, a genetic system in which a Cre-dependent chromosomal translocation labels rare cells, at extremely low mosaic efficiency, the authors performed in vivo clonal analyses in mice to study the proliferation and differentiation of very large numbers of single Ngn3(+). This study shows that at the single cell level, Ngn3(+) cells are not pluripotent, but unipotent islet precursors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell. 2013;154:274–284. doi: 10.1016/j.cell.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 47.Blanpain C, Simons BD. Unravelling stem cell dynamics by lineage tracing. Nat Rev Mol Cell Biol. 2013;14:489–502. doi: 10.1038/nrm3625. [DOI] [PubMed] [Google Scholar]
- **48.Thorel F, et al. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature. 2010;464:1149–1154. doi: 10.1038/nature08894. Using a genetic model of β-cell ablation (RIP-DTR), the authors showed that the murine pancreas has a innate ability to regenerate new insulin-producing cells. This is the first study to show intra-islet plasticity and direct transdifferentiation of one endocrine cell into another type of endocrine cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Al-Hasani K, et al. Adult duct-lining cells can reprogram into beta-like cells able to counter repeated cycles of toxin-induced diabetes. Dev Cell. 2013;26:86–100. doi: 10.1016/j.devcel.2013.05.018. [DOI] [PubMed] [Google Scholar]
- **50.Chera S, et al. Diabetes recovery by age-dependent conversion of pancreatic delta-cells into insulin producers. Nature. 2014;514:503–507. doi: 10.1038/nature13633. Total ablation of β-cells in prepubertal mice induces dedifferentiation of somatostating-producing cells and their redifferentiation into insulin-producing cells. This is the first study showing spontaneous diabetes recovery after total β-cell ablation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spijker HS, et al. Loss of β-Cell Identity Occurs in Type 2 Diabetes and Is Associated With Islet Amyloid Deposits. Diabetes. 2015;64:2928–2938. doi: 10.2337/db14-1752. [DOI] [PubMed] [Google Scholar]
- 52.Chung CH, Hao E, Piran R, Keinan E, Levine F. Pancreatic beta-cell neogenesis by direct conversion from mature alpha-cells. Stem Cells. 2010;28:1630–1638. doi: 10.1002/stem.482. [DOI] [PubMed] [Google Scholar]
- **53.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. Using adenoviruses encoding 3 transcription factors (Ngn3, Pdx1, Mafa), the authors were able to reprogram differentiated pancreatic exocrine cells into β-like cells in adult mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li W, et al. Long-term persistence and development of induced pancreatic beta cells generated by lineage conversion of acinar cells. Nat Biotechnol. 2014;32:1223–1230. doi: 10.1038/nbt.3082. [DOI] [PubMed] [Google Scholar]
- 55.Heller RS, et al. Improved glucose tolerance and acinar dysmorphogenesis by targeted expression of transcription factor PDX-1 to the exocrine pancreas. Diabetes. 2001;50:1553–1561. doi: 10.2337/diabetes.50.7.1553. [DOI] [PubMed] [Google Scholar]
- **56.Baeyens L, et al. Transient cytokine treatment induces acinar cell reprogramming and regenerates functional beta cell mass in diabetic mice. Nat Biotechnol. 2014;32:76–83. doi: 10.1038/nbt.2747. Using transient cytokine exposure, the authors were able to promote acinar-to-β-cell reprogramming via Stat3 signaling. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- **57.Collombat P, et al. The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into alpha and subsequently beta cells. Cell. 2009;138:449–462. doi: 10.1016/j.cell.2009.05.035. Using using different cell-type specific promoters, the authors showed that Pax4 forces endocrine precursor cells to adopt a β-cell fate. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ben-Othman N, et al. From pancreatic islet formation to beta-cell regeneration. Diabetes Res Clin Pract. 2013;101:1–9. doi: 10.1016/j.diabres.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 59.Thorel F, et al. Normal glucagon signaling and β-cell function after near-total α-cell ablation in adult mice. Diabetes. 2011;60:2872–2882. doi: 10.2337/db11-0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rodriguez-Seguel E, et al. Mutually exclusive signaling signatures define the hepatic and pancreatic progenitor cell lineage divergence. Genes Dev. 2013;27:1932–1946. doi: 10.1101/gad.220244.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zaret KS, Grompe M. Generation and regeneration of cells of the liver and pancreas. Science. 2008;322:1490–1494. doi: 10.1126/science.1161431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen YJ, et al. De novo formation of insulin-producing “neo-beta cell islets” from intestinal crypts. Cell Rep. 2014;6:1046–1058. doi: 10.1016/j.celrep.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **63.Banga A, Akinci E, Greder LV, Dutton JR, Slack JM. In vivo reprogramming of Sox9+ cells in the liver to insulin-secreting ducts. Proc Natl Acad Sci U S A. 2012;109:15336–15341. doi: 10.1073/pnas.1201701109. Using adenoviral polycistronic constructs (Ngn3, Pdx1, Mafa), the authors were able to reprogram liver cells into insulin-producing cells. This study shows that insulin+ cells do not arise from hepatocytes, but from a SOX9+ population, likely from small bile ducts or peri-biliary glands. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **64.Talchai C, Xuan S, Kitamura T, DePinho RA, Accili D. Generation of functional insulin-producing cells in the gut by Foxo1 ablation. Nat Genet. 2012;44:406–412. S401. doi: 10.1038/ng.2215. Upon FoxO1 inactivation in gut epithelium, the authors showed that the Neurog3(+) enteroendocrine progenitor cells give rise to gut insulin-producingg cells, which expressed markers of mature β cells and secreted insulin in response to glucose and sulfonylureas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **65.Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic beta cell dedifferentiation as a mechanism of diabetic beta cell failure. Cell. 2012;150:1223–1234. doi: 10.1016/j.cell.2012.07.029. Under conditions of physiological stress, adult murine β-cells lacking FoxO1 acquire a progenitor-like state that permits their conversion to other pancreatic endocrine cell types. This is the first study showing the role of FoxO1 in β-cell homeostasis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Herrera PL, et al. Ablation of islet endocrine cells by targeted expression of hormone-promoter-driven toxigenes. Proc Natl Acad Sci U S A. 1994;91:12999–13003. doi: 10.1073/pnas.91.26.12999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Karges B, et al. Complete long-term recovery of beta-cell function in autoimmune type 1 diabetes after insulin treatment. Diabetes Care. 2004;27:1207–1208. doi: 10.2337/diacare.27.5.1207. [DOI] [PubMed] [Google Scholar]
- 68.Karges B, et al. Immunological mechanisms associated with long-term remission of human type 1 diabetes. Diabetes Metab Res Rev. 2006;22:184–189. doi: 10.1002/dmrr.600. [DOI] [PubMed] [Google Scholar]
- 69.Yoneda S, et al. Predominance of beta-cell neogenesis rather than replication in humans with an impaired glucose tolerance and newly diagnosed diabetes. J Clin Endocrinol Metab. 2013;98:2053–2061. doi: 10.1210/jc.2012-3832. [DOI] [PubMed] [Google Scholar]
- 70.White MG, et al. Expression of mesenchymal and alpha-cell phenotypic markers in islet beta-cells in recently diagnosed diabetes. Diabetes Care. 2013;36:3818–3820. doi: 10.2337/dc13-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Butler AE, et al. Marked expansion of exocrine and endocrine pancreas with incretin therapy in humans with increased exocrine pancreas dysplasia and the potential for glucagon-producing neuroendocrine tumors. Diabetes. 2013;62:2595–2604. doi: 10.2337/db12-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bramswig NC, et al. Epigenomic plasticity enables human pancreatic alpha to beta cell reprogramming. J Clin Invest. 2013;123:1275–1284. doi: 10.1172/jci66514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Siebert S, Anton-Erxleben F, Bosch TC. Cell type complexity in the basal metazoan Hydra is maintained by both stem cell based mechanisms and transdifferentiation. Dev Biol. 2008;313:13–24. doi: 10.1016/j.ydbio.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 74.Mashanov VS, Dolmatov IY, Heinzeller T. Transdifferentiation in holothurian gut regeneration. Biol Bull. 2005;209:184–193. doi: 10.2307/3593108. [DOI] [PubMed] [Google Scholar]
- 75.VandenSpiegel D, Jangoux M, Flammang P. Maintaining the line of defense: regeneration of Cuvierian tubules in the sea cucumber Holothuria forskali (Echinodermata, Holothuroidea) Biol Bull. 2000;198:34–49. doi: 10.2307/1542802. [DOI] [PubMed] [Google Scholar]
- 76.Dolmatov IY, Ginanova TT. Post-autotomy regeneration of respiratory trees in the holothurian Apostichopus japonicus (Holothuroidea, Aspidochirotida) Cell Tissue Res. 2009;336:41–58. doi: 10.1007/s00441-009-0761-6. [DOI] [PubMed] [Google Scholar]
- 77.He J, Lu H, Zou Q, Luo L. Regeneration of liver after extreme hepatocyte loss occurs mainly via biliary transdifferentiation in zebrafish. Gastroenterology. 2014;146:789–800.e788. doi: 10.1053/j.gastro.2013.11.045. [DOI] [PubMed] [Google Scholar]
- 78.Zhang R, et al. In vivo cardiac reprogramming contributes to zebrafish heart regeneration. Nature. 2013;498:497–501. doi: 10.1038/nature12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Monroe JD, Rajadinakaran G, Smith ME. Sensory hair cell death and regeneration in fishes. Front Cell Neurosci. 2015;9:131. doi: 10.3389/fncel.2015.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yoshii C, Ueda Y, Okamoto M, Araki M. Neural retinal regeneration in the anuran amphibian Xenopus laevis post-metamorphosis: transdifferentiation of retinal pigmented epithelium regenerates the neural retina. Dev Biol. 2007;303:45–56. doi: 10.1016/j.ydbio.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 81.Barbosa-Sabanero K, et al. Lens and retina regeneration: new perspectives from model organisms. Biochem J. 2012;447:321–334. doi: 10.1042/BJ20120813. [DOI] [PubMed] [Google Scholar]
- 82.Tsonis PA, Madhavan M, Tancous EE, Del Rio-Tsonis K. A newt’s eye view of lens regeneration. Int J Dev Biol. 2004;48:975–980. doi: 10.1387/ijdb.041867pt. [DOI] [PubMed] [Google Scholar]
- 83.Stone JS, Cotanche DA. Hair cell regeneration in the avian auditory epithelium. Int J Dev Biol. 2007;51:633–647. doi: 10.1387/ijdb.072408js. [DOI] [PubMed] [Google Scholar]
- 84.Davis J, Burr AR, Davis GF, Birnbaumer L, Molkentin JD. A TRPC6-dependent pathway for myofibroblast transdifferentiation and wound healing in vivo. Dev Cell. 2012;23:705–715. doi: 10.1016/j.devcel.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526–537. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]
- 86.Arthur-Farraj PJ, et al. c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron. 2012;75:633–647. doi: 10.1016/j.neuron.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jessen KR, Mirsky R, Lloyd AC. Schwann Cells: Development and Role in Nerve Repair. Cold Spring Harb Perspect Biol. 2015;7:a020487. doi: 10.1101/cshperspect.a020487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bramhall NF, Shi F, Arnold K, Hochedlinger K, Edge AS. Lgr5-positive supporting cells generate new hair cells in the postnatal cochlea. Stem Cell Reports. 2014;2:311–322. doi: 10.1016/j.stemcr.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cox BC, et al. Spontaneous hair cell regeneration in the neonatal mouse cochlea in vivo. Development. 2014;141:816–829. doi: 10.1242/dev.103036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Richardson RT, Atkinson PJ. Atoh1 gene therapy in the cochlea for hair cell regeneration. Expert Opin Biol Ther. 2015;15:417–430. doi: 10.1517/14712598.2015.1009889. [DOI] [PubMed] [Google Scholar]
- 91.Yanger K, et al. Robust cellular reprogramming occurs spontaneously during liver regeneration. Genes Dev. 2013;27:719–724. doi: 10.1101/gad.207803.112. [DOI] [PMC free article] [PubMed] [Google Scholar]