Abstract
Phase synchronization of neuronal oscillations is a fundamental mechanism underlying cognitive processing and behavior, including context-dependent response production and inhibition. Abnormalities in neural synchrony can lead to abnormal information processing and contribute to cognitive and behavioral deficits in neuropsychiatric disorders. However, little is known about genetic and environmental contributions to individual differences in cortical oscillatory dynamics underlying response inhibition. This study examined heritability of event-related phase synchronization of brain oscillations in 302 young female twins including 94 MZ and 57 DZ pairs performing a cued Go/No-Go version of the Continuous Performance Test (CPT). We used the Phase Locking Index (PLI) to assess inter-trial phase clustering (synchrony) in several frequency bands in two time intervals after stimulus onset (0–300 and 301–600 ms). Response inhibition (i.e., successful response suppression in No-Go trials) was characterized by a transient increase in phase synchronization of delta- and theta-band oscillations in the fronto-central midline region. Genetic analysis showed significant heritability of the phase locking measures related to response inhibition, with 30 to 49% of inter-individual variability being accounted for by genetic factors. This is the first study providing evidence for heritability of task-related neural synchrony. The present results suggest that PLI can serve as an indicator of genetically transmitted individual differences in neural substrates of response inhibition.
1. Introduction
Inter-individual variation in human cognition and behavior, both normal and abnormal, is strongly influenced by genetic factors (e.g., Beam and Turkheimer, 2013). However, neurobiological pathways and mechanisms mediating these genetic influences remain poorly understood. Investigating the genetic contribution to individual differences in neural mechanisms supporting specific cognitive function helps to establish meaningful links between genes, brain, and behavior. In particular, identification of heritable neurophenotypic markers for complex, higher order cognitive functions can facilitate finding genes for higher cognition characteristics by focusing analysis on well-characterized neurophysiological processes.
Neural mechanisms of cognitive control are important targets for genetic research because deficits in cognitive control and associated impairments in self-regulation of behavior have been observed in a broad range of psychopathologies including but not limited to schizophrenia, addictive disorders, and attention-deficit hyperactivity disorder (Barch, 2005). Genetic research on the etiology of these disorders has been hindered by the complexity and heterogeneity of diagnostic phenotypes. An alternative strategy is to focus on the identification of intermediate neural phenotypes, or “endophenotypes”, that is, genetically transmitted variability of brain function mediating the association between the genotype and complex behavioral phenotype (Gottesman and Gould, 2003). A recent initiative of the National Institute of Mental Health focuses on the identification of “Research Domain Criteria (RDoC)”, defined as novel brain-based dimensions that cut across diagnostic categories, better represent the underlying neurobiology (Insel et al., 2010), and are amenable to computational modeling (Stephan and Mathys, 2014). Response inhibition is one of such fundamental cross-diagnostic neurocognitive, with most notable deficits observed in the “externalizing spectrum” disorders characterized by impulsive and under-controlled behaviors such as oppositional-defiant and conduct disorders (children), antisocial personality disorder (adults), substance use disorders, and attention-deficit/hyperactivity disorder (ADHD).
It has long been proposed that synchronization binds oscillatory neuronal assemblies into coherent functional networks that provide the basis for perception and action (Livanov, 1934, 1977). Empirical studies and computational modeling indicate that interactions between interconnected neurons comprising a functional network are state-dependent (e.g., Friston et al., 2012). In particular, the dynamic modulation of neuronal responsiveness determines the probability and timing of the generation of action potentials in functionally connected neurons (reviewed in Haider and McCormick, 2009). Animal models and research with human participants have demonstrated the central role of neural oscillations in the dynamic organization of functional networks. Precise temporal coordination of neuronal activity states is achieved through transient phase synchronization of oscillations in neuronal assemblies (Buzsaki and Draguhn, 2004; Klimesch et al., 2007; Livanov, 1977; Llinas et al., 2005; Sauseng and Klimesch, 2008; Uhlhaas and Singer, 2010, 2012). The development of methods for quantifying temporal neural dynamics has allowed researchers to demonstrate the functional significance of neuronal synchronization across a broad range of tasks, conditions, and cognitive processes including sensation, perception, attention, and memory (Benchenane et al., 2011; Klimesch et al., 2007; Livanov, 1977; Palva et al., 2010; Sauseng and Klimesch, 2008; Uhlhaas and Singer, 2010, 2012; Womelsdorf et al., 2007).
Recent studies underscore the role of neural synchrony in higher order, super-ordinate integrative functions such as cognitive control (Cohen, 2011; Nigbur et al., 2012; Nigbur et al., 2011). Nigbur et al. (2011) reported increased power of theta-band (4–8 Hz) oscillations related to conflict processing across different types of conflicts including response inhibition, perceptual conflict (stimulus incongruency), and response conflict. The largest effect was observed in the No-Go condition of the response inhibition task. Importantly, theta enhancement was localized in medio-frontal cortex (MFC) areas within anterior cingulate cortex and (pre-) supplementary motor areas (Nigbur et al., 2012; Nigbur et al., 2011). Other authors have proposed that theta oscillations generated in the medial prefrontal cortex represent a common neurophysiological substrate for the processing the signals of novelty, conflict, error, and punishment (Cavanagh et al., 2012), decision making, as well as action selection in goal-directed behavior (Womelsdorf et al., 2010b). Finally, individual differences in theta-band synchronization predict individual differences in cognitive functioning: lower intertrial phase synchrony in the theta band predicted reduced stability of performance as indicated by reaction time variability (Papenberg et al., 2013), and long-range theta synchrony during cognitive tasks has been found to correlate with general intelligence (Anokhin et al., 1999).
These results are in good agreement with animal evidence. When rats had to choose between two action alternatives, cell assembly phase synchronization peaked at the decision point (Jones and Wilson, 2005). In a genetic mouse model of schizophrenia, hippocampal-prefrontal theta coherence in a spatial working memory task was drastically reduced, and lower theta coherence before training predicted the time it took the animals to learn the task (Benchenane et al., 2011; Sigurdsson et al., 2010). Synchronization of lower frequency (delta-band) oscillations also play an important role in higher cognitive processes such as decision making in monkeys (Nacher et al., 2013), and reduced event-related delta oscillations have been associated with mild cognitive impairment at the prodromal stage of Alzheimer's disease (Yener et al., 2013). Taken together, the available evidence indicates that low-frequency oscillations (delta and theta) play a key role in integrative brain activity supporting higher cognition, and that abnormal event-related oscillations are associated with cognitive deficits.
In a recent study, we demonstrated significant increase in phase synchronization in delta and theta bands in a Go/No-Go task, as well as both spatial and temporal dissociation between No-Go and Go conditions, such that No-Go stimuli produced stronger phase synchronization in the anterior scalp regions in a time window between 300 and 600 ms after stimulus-onset (Müller & Anokhin, 2012). Other studies also reported phase synchronization in No-Go trials at anterior sites (Beste et al., 2011; Papenberg et al., 2013). Response inhibition tasks produce two major ERP components that discriminate between the Go and No-Go trials. The first is the midline frontal N2. This component is restricted to No-Go trials, and has been observed consistently in visual and auditory tasks (Nieuwenhuis et al., 2004; Nieuwenhuis et al., 2003). The second is the frontal P300, a component that is substantially increased on No-Go trial; this phenomenon is labeled as “No-Go anteriorization” of P300 (Fallgatter and Strik, 1999; Roberts et al., 1994). The No-Go anteriorization has been proposed as a robust topographical marker of the activation of frontal circuitry related to response inhibition (Fallgatter et al., 1997). In experiments designed to separately manipulate conflict and inhibition, N2 and P3 components showed functionally dissociable effects, suggesting that N2 reflects a conflict between two competing response representations (e.g., execute or withhold a response), whereas P3 is increased only when planned movements need to be inhibited (Randall and Smith, 2011). Studies involving both fMRI and ERP measurement in the same Go/No-Go tasks have consistently associated the N2 component with the anterior cingulate cortex activation (Garavan et al., 2002; Mathalon et al., 2003; Swainson et al., 2003; van Veen and Carter, 2002a, b). In contrast, the anteriorized P3 observed in response inhibition condition has been linked to pre-supplementary motor areas presumably involved in motor-inhibitory mechanisms, rather than to conflict processing per se (Huster et al., 2011). The amplitudes of both N2 and P3 No-Go components are highly heritable, with 60 and 58% of inter-individual variability being attributable to genetic factors (Anokhin et al., 2004).
Our recent study suggests that the No-Go N2 component emerges as a result of phase locking of theta-band oscillations, whereas the P3 component is primarily reflecting synchronization in the delta band (Müller and Anokhin, 2012). There is substantial evidence for the functional significance of individual differences in the strength of neural synchrony including associations with cognitive and behavioral performance in both humans (Anokhin et al., 1999; Papenberg et al., 2013) and animals (Benchenane et al., 2011), as well as for the liability of neural synchrony to neuropsychiatric disorders such as schizophrenia, epilepsy, autism, Alzheimer's disease, and Parkinson's disease (reviewed in Uhlhaas and Singer, 2006, 2012). Converging evidence suggests that deficient temporal coordination of neuronal activity leads disrupts a variety of brain functions. In particular, abnormalities in stimulus-evoked neuronal synchronization have been described in schizophrenics and their first-degree relatives, suggesting that neural synchrony may serve as an endophenotype for schizophrenia (Uhlhaas and Singer, 2006, 2010). Neural synchrony is an important determinant of individual differences in normal brain function and behavior, as well as in pathophysiology of brain disorders.
However, surprisingly little is known about the genetic and environmental etiology of individual differences in phase synchrony during cognitive processing. Demonstrating heritability of task-relevant neural synchrony is a first step toward characterization of genetic factors influencing brain synchrony and, hence, core neurophysiological mechanisms of cognition. Here, we investigated heritability of neural synchrony related to response inhibition using a Go/No-Go task in a large sample of monozygotic and dizygotic twins. Neural synchrony was assessed using the phase locking index (PLI), a measure of inter-trial phase clustering of brain oscillations (Cohen and Gulbinaite, 2014).
2. Methods
2.1. Participants
The study sample consisted of 302 young adult female twins aged 18 to 28 years, including 94 MZ and 57 DZ pairs selected from the Missouri Family Registry. Zygosity was determined using a standard set of questions asked to both twins as is typically done in twin genetic research, lab technicians' rating of twins similarity, and, for about 70% of the sample, using genotyping data. Participants were excluded if they had a history of serious head trauma or were using psychoactive medication at the time of testing. All experiments on human research participants were conducted in accordance with the declaration of Helsinki. The study was approved by Washington University Institutional Review Board, and written informed consent was obtained from all participants.
2.2. Materials and procedure
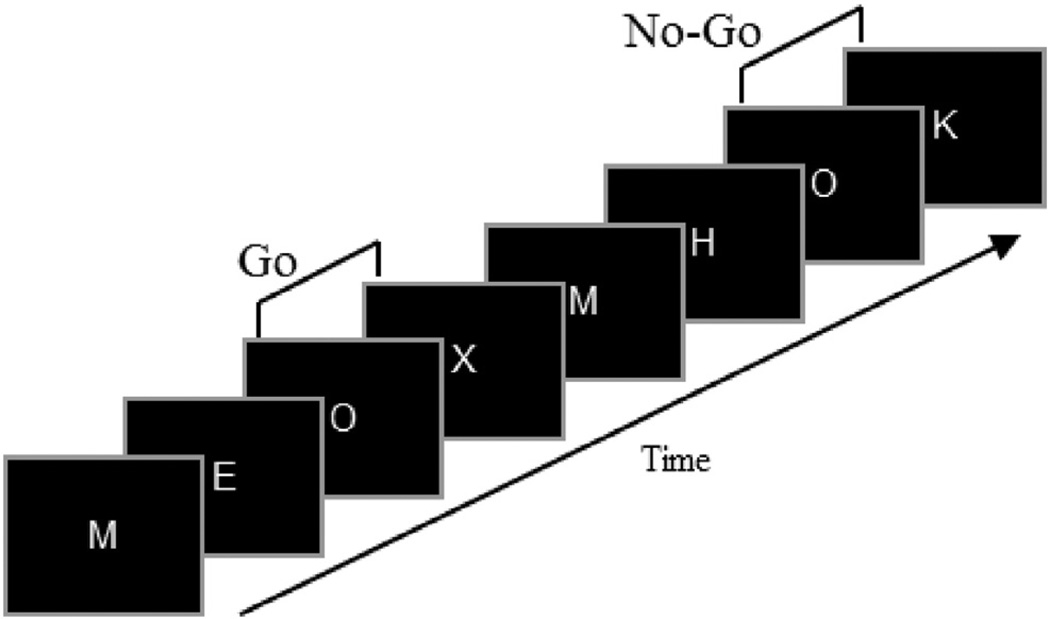
Participants performed a Go/No-Go version of the Continuous Performance Test (CPT) described in previous studies (Anokhin et al., 2004; Fallgatter and Strik, 1999; Lee et al., 2011; Müller and Anokhin, 2012). This task consisted of a series of letters presented sequentially, one at a time, for 0.2 s with a 2 s stimulus onset asynchrony (Fig. 1). Participants were instructed to respond as quickly as possible to the letter X preceded by the letter O by pressing a button on a response pad using the right index finger and to withhold the response, if O was followed by any other letter. Response speed and accuracy were emphasized equally. A total of 400 letters were presented, including 40 O–X (Go) and 40 O–not-X (No-Go) combinations occurring in a pseudo-random order. Eight letters (A, E, H, I, K, L, M, Y) were used as neutral (distracter) stimuli. It is important to note that neutral stimuli were frequent (80% of all stimuli), whereas Go and No-Go stimuli (X and not-X, respectively) were rare and equiprobable (10% each). The response prepotency and, hence, the degree or processing conflict was increased in this task by the relative rareness of the Go and No-Go stimuli and by the fact that the letter O served as a warning cue informing participants that the next letter is likely to be a Go target requiring a speeded response. All Xs were preceded by O, and the O–X contingency was explicitly emphasized in the instruction. Speed and accuracy were equally emphasized by the instruction. The probability and the context of Go and No-Go stimuli was equalized in order to rule out the confounding of Go versus No-Go difference by the well-known oddball effect. For further justification for using Go/No-Go tasks with matched Go and No-Go frequency, see (Lavric et al., 2004). Task performance was quantified using the following measures: speed (mean reaction time in Go trials), accuracy (percent correct responses including correct Go responses and correct No-Go trials), and reaction time variability (standard deviation of reaction time in Go-trials).
Fig. 1.
The Go/No-Go Continuous Performance Task. Each letter appears on the computer screen for 0.2 s with stimulus onset asynchrony (the interval between the onsets of two consecutive stimuli) of 2.0 s. The subject is instructed to respond as quickly as possible to the letter X which is always preceded by O (effectively serving as a warning cue) but to withhold a response when O is followed by a letter other than X. Thus, O–X and O–non-X combinations constitute Go and No-Go stimuli, respectively (adapted from Fallgatter et al., 1997).
2.3. EEG recording and analysis
The EEG was recorded from 19 scalp locations according to the 10–20 system using an elastic cap with silver/silver-chloride electrodes and a ground electrode on the forehead, with sampling rate of 1000 Hz, and high and low-pass filters set at 0.05 and 70 Hz, respectively. The left mastoid served as reference, and an averaged mastoid reference was digitally computed off-line using the right mastoid recording as a separate channel. Vertical electro-oculogram recording was used for eye-blink artifact correction using a regression-based procedure. After screening for artifacts, EEG signals were subjected to 50 Hz low-pass filtering and segmented related to stimulus (Warning, Go, No-Go, and Neutral) into the 2-s segments with a 0.5 s pre-stimulus baseline and 1.5 s post-stimulus interval.
To characterize the degree of phase consistency across trials independently of amplitude correlation, we computed the Phase Locking Index (PLI) within each condition according to the following algorithm. Using a complex Gabor expansion function, EEG time series of single trials were transformed into a complex time-frequency signal y(fn,t) for frequencies up to 20 Hz with a frequency resolution of 0.5 Hz. Phase Locking Index (PLI) was defined by
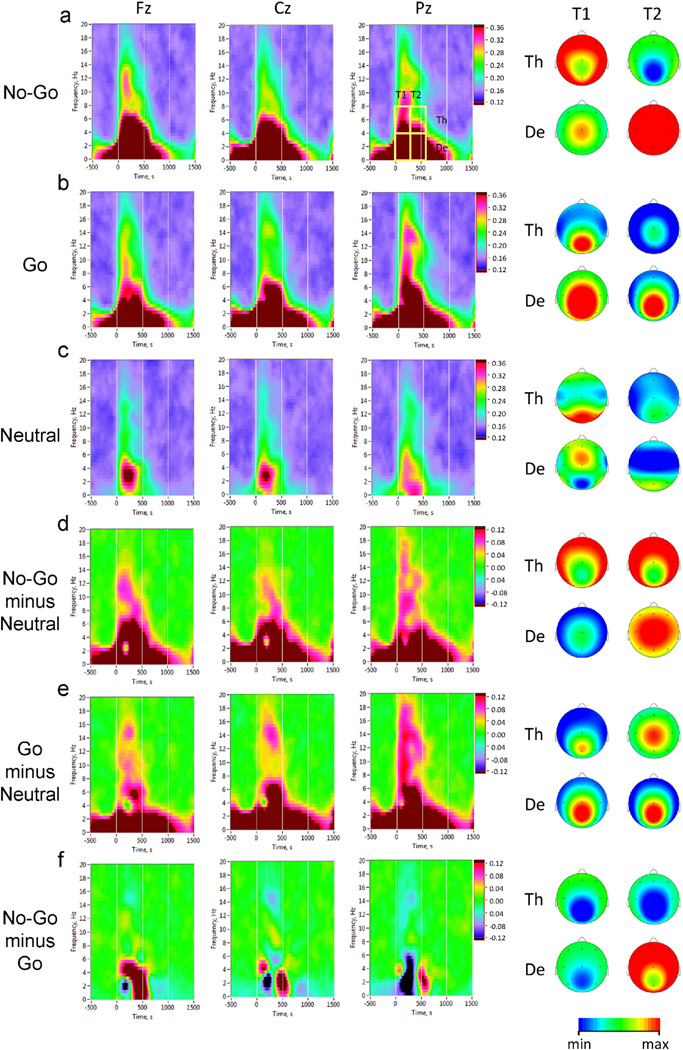
where Φk(fn, t) = arg [ϕ(fn, t)] is instantaneous phase of the signal and 〈·〉 denotes averaging across k trials. PLI varies in the range of 0 to 1, with 0 corresponding to entirely random phase distribution, and 1 indicating a perfect phase coincidence. Because of the fact that the main differences between task conditions were located in delta and theta frequency and within 600 ms after the stimulus onset (Fig. 3), all the measures were subdivided into the two frequency bands (delta: 0.5–4 Hz and theta: 4–8 Hz) and averaged for two consecutive 300-ms post-stimulus time intervals (the first post-stimulus interval: 0–300 ms, and the second post-stimulus interval: 300–600 ms), showing also strongest differences between task conditions. PLI was computed within rectangular regions of interest in the time-frequency space defined by time intervals and frequency bands described above (delta and theta, Fig. 3a). For subsequent statistical analyses, we extracted mean PLI values in each of these rectangular regions in frontal, central and parietal midline scalp regions (electrodes Fz, Cz, and Pz, respectively). In addition, we determined a peak PLI value in the post-stimulus interval (0–1200 ms). These analyses were informed by our previous study in which most significant differences between No-Go and Go conditions with respect to PLI measures were observed at midline electrodes (Müller and Anokhin, 2012).
Fig. 3.
Inter-trial phase clustering in No-Go, Go, and Neutral task conditions. Averaged time-frequency distribution plots of Phase Locking Index (PLI) are shown for representative electrode locations: Fz (midline frontal), Cz (midline central), and Pz (midline parietal). Horizontal axis: time (ms), with zero value corresponding to stimulus onset; vertical axis: EEG oscillations frequency (Hz); phase locking is color coded, with warmer colors indicating higher degree of phase consistency across trials. For statistical analyses, average PLI-values were computed within four time-frequency regions of interest defined by two consecutive 300-ms time windows after the stimulus onset (T1 and T2) and two frequency bands (De = delta and Th = theta). Scalp topography maps of average PLI values within these regions are shown on the right. a: No-Go condition (brain maps are scaled: De, min = 0.40, max = 0.55; Th, min = 0.30, max = 0.36); b: Go condition (brain maps are scaled alike in a); c: Neutral condition (brain maps scaling: De, T1: min = 0.30, max = 0.32; De, T2: min = 0.26, max = 0.28; Th, T1: min = 0.24, max = 0.26; Th, T2: min= 0.20, max = 0.22); d: No-Go–Neutral difference (brain maps scaling: De, T1: min = 0.15, max = 0.30; De, T2: min = 0.25, max = 0.35; Th: min = 0.06, max = 0.12); e: Go–Neutral difference (scaling of brain maps the same as in d); f: No-Go–Go difference (brain maps scaling: De: min= −0.12, max= 0.12; Th: min= 0.0, max = 0.12).
2.4. Statistical analysis and assessment of heritability
For genetic analysis, we selected PLI variables that were most sensitive to response inhibition, i.e., showed the best discrimination between the No-Go and other conditions, Go and Neutral (distracter stimuli). First, we examined the significance of No-Go–Go and No-Go–Neutral differences for all PLI variables using a paired t-test. We then ranked the obtained t-values and selected “No-Go-specific” PLI variables with the highest t-values for subsequent genetic analysis using the following stringent criterion: both No-Go N Neutral and No-Go N Go difference had to be significant at p < 0.001 after Bonferroni correction for multiple tests (n = 18).
Next, for each of the six selected PLI measures (Tables 1 and 2) we computed intrapair twin correlations and variance-covariance matrices separately for MZ and DZ twins. To estimate the relative contribution of genetic (heritability) and environmental sources to the total phenotypic variance, we used the model-fitting approach to genetic analysis of twin data (Neale and Cardon, 1992; Posthuma et al., 2003; Rijsdijk and Sham, 2002). Linear structural equation models were fitted to variance–covariance matrices computed separately for MZ and DZ twins using the Mx program (Neale et al., 2002). These models assume that phenotypic variance arises from the following factors: additive genetic influences (A), non-additive genetic influences (D) or environmental influences shared by family members (C), and individually unique (unshared) environmental influences (E). Path coefficients corresponding to these factors were estimated using a maximum likelihood method, and a χ2 statistic was used to assess the goodness-of-fit of each model, where low χ2 values indicate a good fit. For individual variables, we fit ADE or ACE model depending on which model was suggested by the relative size of MZ and DZ correlation (ADE model if rMZ > 2rDZ and ACE model if rMZ < 2rDZ). The significance of C or D paths was tested by comparing the goodness of fit of the reduced (AE) model to the ACE or ADE model, respectively. If dropping a path significantly reduced the goodness of fit (the χ2 difference was significant), the path was retained in the model, otherwise the more parsimonious model was chosen (i.e., the one that accounted for the variance equally well, but with fewer parameters). In addition, different models were compared using Akaike's information criterion (AIC, computed as χ2 − 2df), which provides a combined measure of goodness-of-fit and parsimony for a given model. The model with the lower AIC is considered as better fitting.
Table 1.
Correlations between neural synchrony (Phase Locking Index, PLI) and task performance.
| Measure | % correct | Mean RT | RT variability |
|---|---|---|---|
| Mean PLI, 0–300 ms, theta (Fz) | 0.13* | −0.29** | −0.24** |
| Mean PLI, 300–600 ms, delta (Fz) | 0.35** | −0.30** | −0.38** |
| Mean PLI, 300–600 ms, theta (Fz) | 0.27** | −0.26** | −0.36** |
| Mean PLI, 300–600 ms, delta (Cz) | 0.39** | −0.33** | −0.43** |
| Peak PLI, delta (Fz) | 0.38** | −0.34** | −0.44** |
| Peak PLI, theta (Fz) | 0.24* | −0.27** | −0.30** |
| Neural synchrony factor | 0.39** | −0.38** | −0.46** |
Mean RT is mean reaction time to target (Go) stimuli; RT variability is a standard deviation of RT; Neural synchrony factor is the score on the first principal component extracted from PLI variables; significance of correlation coefficients:
p < 0.05;
p < 0.01.
Table 2.
Twin correlations and heritability estimates for Phase Locking Index (PLI) associated with response inhibition.
| Measure | rMZ (n = 69) | rDZ (n = 40) | a2 or c2, (95% CI) | e2 (95% CI) | χ2, df = 4 | p | AIC |
|---|---|---|---|---|---|---|---|
| Mean PLI, 0–300 ms, theta (Fz) | 0.41** | 0.05 | 0.37 (0.20–0.53) | 0.63 (0.47–0.80) | 3.76 | 0.44 | −4.24 |
| Mean PLI, 300–600 ms, delta (Fz) | 0.33** | 0.07 | 0.30 (0.11–0.47) | 0.70 (0.53–0.89) | 3.74 | 0.44 | −4.27 |
| Mean PLI, 300–600 ms, Theta (Fz) | 0.38** | −0.10 | 0.30 (0.12–0.47) | 0.70 (0.53–0.88) | 5.61 | 0.23 | −2.39 |
| Mean PLI, 300–600 ms, delta (Cz) | 0.48** | 0.21 | 0.49 (0.32–0.62) | 0.51 (0.38–0.68) | 3.88 | 0.42 | −4.12 |
| Peak PLI, Delta (Fz) | 0.39** | 0.09 | 0.40 (0.21–0.56) | 0.60 (0.44–0.79) | 6.59 | 0.16 | −1.41 |
| Peak PLI, Theta (Fz) | 0.36* | −0.01 | 0.32 (0.14–0.49) | 0.68 (0.51–0.77) | 5.59 | 0.23 | −2.41 |
| Neural synchrony factor | 0.44** | 0.07 | 0.43 (0.25–0.58) | 0.57 (0.42–0.75) | 5.54 | 0.24 | −2.46 |
Notes: rMZ and rDZ are intrapair correlations for MZ and DZ twins, respectively. Variance component estimates are based on the best-fitting “AE” or “CE” models: a2 is the proportion of total phenotypic variance explained by genetic factors (heritability) in AE model; c2 is the proportion of variance explained by shared environmental (familial, etc.) environmental factors; e2 is the proportion of variance due to environmental factors including measurement error (95% confidence intervals of the maximum likelihood estimates of the variance components are shown in brackets). Chi-square shows the goodness of fit, with lower χ2 values and higher associated p-values indicating a better fit; AIC is Akaike's Information Criterion (χ2-2df). Significance levels:
p < 0.05;
p < 0.01.
3. Results
3.1. ERPs in the Go/No-Go task: morphology and scalp topography
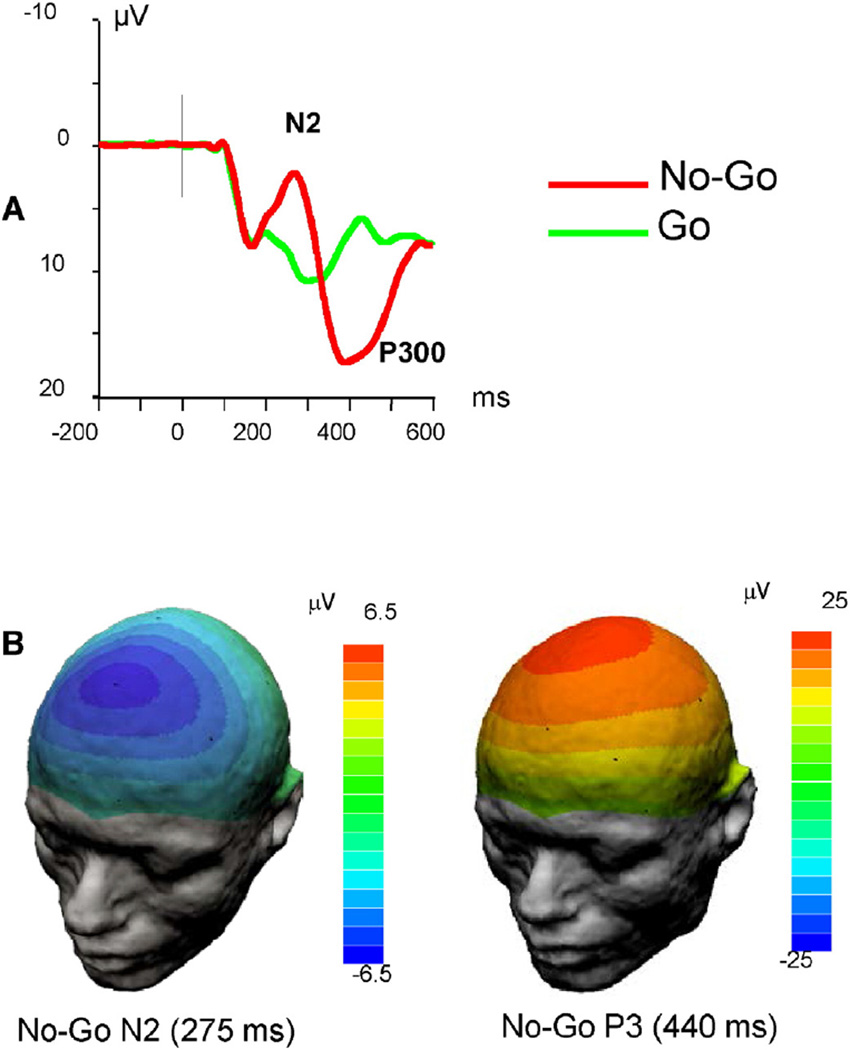
Averaged ERP waveforms revealed a striking difference between responses to Go and No-Go stimuli (Fig. 2). First, in the No-Go condition, there was a prominent frontal N2 component that was virtually absent in the Go condition. Second, in the No-Go condition, the P3 peak was shifted toward anterior (fronto-central) areas relative to the Go condition in which P3 component peaked in the midline parietal region similar to a classical oddball paradigm. Finally, P3 latency was increased in the No-Go condition relative to the Go condition. This “anteriorization” and slowing of P3 has been described in previous studies with this paradigm (Fallgatter and Strik, 1999; Lee et al., 2011).
Fig. 2.
ERPs elicited in the Go/No-Go task. A. Averaged ERP waveforms recorded at the frontal midline (Fz) scalp location. Note dramatic differences in the response waveform between Go and No-Go conditions. B. Scalp potential topography maps for No-Go N2 and P3 components (peak values).
3.2. Phase synchrony of inhibition-related neural oscillations
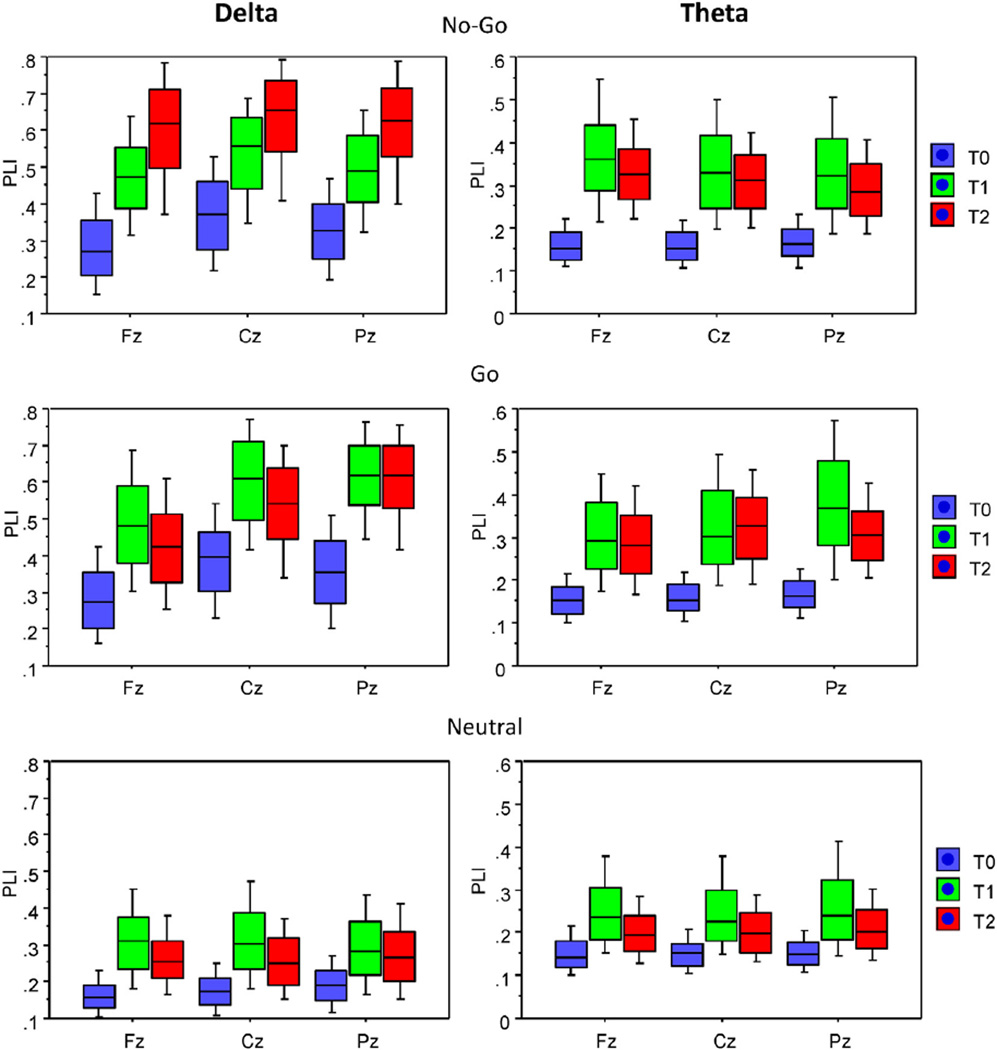
Analysis of phase relations showed a highly significant increase in inter-trial phase synchronization as indicated by PLI in both Go and No-Go conditions relative to the neutral condition (Fig. 3). Furthermore, PLI measures reliably discriminated between No-Go and Go conditions, with phase synchronization in No-Go trials being stronger than in Go trials in the time window of 300–600 ms post-stimulus at frontal and central midline sites (cf. also Fig. 4). These findings informed the selection of No-Go specific variables for genetic analyses (see Section 2.4 of Methods above). A total of 18 variables were tested for No-Go N Go and No-Go N Neutral differences. Six variables met out cut-off criteria, with t-values for No-Go N Go ranging from 5.9 to 18.4, and t-values for No-Go N Neutral ranging from 19.5 to 37.8 (df = 253, p < 0.001 after Bonferroni correction for multiple t-tests). These variables included the mean PLI value in the theta band in the early (0–300 ms) window at Fz electrode, mean PLI values in both theta and delta bands in the late (300–600 ms) window at Fz, the mean delta-PLI in the late window at Cz, and peak PLI values in both theta and delta bands at Fz. Furthermore, since the PLI variables showed significant positive intercorrelations ranging from 0.27 to 0.93, we conducted a principal component analysis and extracted individual scores on the first component that accounted for 64.4% of the total variance in the six PLI measures. These scores were considered as a general Neural Synchrony Factor and included in the genetic analysis along with individual PLI variables.
Fig. 4.
Box plots of average PLI values under the three task conditions (No-Go, Go, and Neutral) for three midline electrodes (Fz, Cz, and Pz) within the three time intervals before and after the stimulus onset. Box plots show10th, 25th, 50th (median), 75th and 90th percentiles of PLI values. Time intervals: T0 = −300–0 ms (baseline), T1 = 0–300 ms, and T2 = 300–600 ms.
All of phase locking measures correlated significantly with task performance (Table 1). For example, the peak delta-band phase locking values at frontal midline showed correlations of 0.38, −0.34, and −0.44 with accuracy, speed, and reaction time variability, respectively. Other measures showed similar patterns of correlations, suggesting that greater phase consistency of the neural response predicts faster, more accurate, and more stable performance.
3.3. Twin correlations and heritability of neural synchrony
Twin correlations as well as the results of genetic model fitting for the selected PLI measures are presented in Table 2. All MZ twin correlations were statistically reliable, indicating familial effects. DZ correlations were generally lower than MZ correlations. Genetic model fitting showed significant heritability for most of the variables, with just one exception in which there was a significant familial effect but no reliable separation between genetic and common environmental transmission. The Neural Synchrony Factor showed a modest but highly significant heritability, indicating that 34% of observed inter-individual variability in neural synchrony related to response inhibition is attributable to genetic factors. Overall, these results demonstrate that individual differences in task-related oscillatory brain dynamics associated with response inhibition have a strong heritable component.
4. Discussion
In this study, we identified measures of transient phase synchronization (phase locking) that were highly specific to response inhibition (successful response suppression in No-Go trials of a Go/No-Go task). Analysis of these measures in monozygotic and dizygotic twins revealed significant genetic influences on inter-individual variability in the degree of phase coordination of neuronal oscillations. To our knowledge, this is the first evidence for genetically transmitted differences in neural synchrony underlying neurophysiological mechanisms of cognitive control.
The present analyses indicate that response inhibition is characterized by a transient increase in phase consistency of delta- and theta-band oscillations in anterior midline regions of the scalp. This result is consistent with marked differences in the averaged ERP response showing the No-Go specific frontal N2 component and an enhancement of the slower P3 response in the fronto-central region (see Fig. 2). The relative timing of inter-trial phase synchronization and averaged ERP components suggests that theta-band synchronization contributes more to the N2 component than to the P3 component, whereas delta-band synchronization contributes mainly to the P3 component. This interpretation is in line with previous studies, and supports the proposal that frontal midline theta generated in the medial prefrontal cortex is a neurophysiological substrate for processing novelty, conflict, error, and punishment (Cavanagh et al., 2012), which also contributes to decision making and action selection in goal-directed behavior (Womelsdorf et al., 2010b). We have previously shown that the degree of long-range theta-band synchronization during the performance of cognitive tasks predicts general intelligence, suggesting that neural synchrony in the theta band plays important role in the organization of higher-order cognitive processes (Anokhin et al., 1999). Converging evidence from both animal and human studies suggests that anterior midline theta reflects the activity of the anterior cingulate cortex (ACC) as a key node in the cognitive control network (Womelsdorf et al., 2010b), with dynamic links to other components of the network (Cavanagh et al., 2012). In particular, studies using electrophysiological recordings from macaque anterior cingulate strongly suggest that theta rhythmic synchronization serves as a neural representation of task-relevant stimulus-response mappings (Womelsdorf et al., 2010a). Studies in humans using intracranial recordings during tasks known to recruit cognitive control indicate that the theta rhythm originates predominantly in superficial layers of the ACC, with phase locking of synaptic activity in the theta range starting before the behavioral response and lasting for several hundred milliseconds, presumably reflecting ACC-neocortical interactions (Wang et al., 2005).
It is important to note that the present study also provides evidence for the functional significance of the inter-trial phase locking. Individual differences in both theta- and delta-band PLI showed significant associations with task performance, including accuracy, reaction time, and, most notably, inter-trial reaction time variability (RTV), consistent with other relevant studies (Bender et al., 2015; Papenberg et al., 2013). Specifically, weaker inter-trial phase clustering as indicated by lower PLI values predicted greater RTV (r = −0.46, p < 0.001). There is a growing literature linking heightened RTV to cognitive and behavioral deficits in psychopathology such as ADHD, autism spectrum disorders (reviewed in Karalunas et al., 2014; Saville et al., 2015) and schizophrenia (Karantinos et al., 2014). This evidence is consistent with the general notion that trial-to-trial variability of a neuronal response could potentially interfere with the organism's ability to utilize sensory information to guide behavior (Ledberg et al., 2012).
Taken together, the present results suggest that neural synchrony can potentially serve as a useful intermediate neurophenotype for the understanding of genetic determination of normal and abnormal behavior. In the normal range, individual differences in neural synchronization have been associated with individual differences in task (Papenberg et al., 2013) and cognitive abilities (Anokhin et al., 1999). In addition, mechanisms of neural synchronization have been linked to neuropsychiatric disorders (Uhlhaas and Singer, 2012). Many of these disorders are also characterized by deficits of cognitive control functions, in particular, response inhibition. Thus, delineating genetically transmitted differences in neural mechanisms of cognitive control may help to discern the neurogenetic etiology of these disorders.
The present finding of significant heritability of inter-trial phase synchrony raises the question of specific genes contribute to the observed genetic influences. The emerging evidence about the cellular and molecular mechanisms underlying the synchronization of neuronal responses can be used to inform the search for candidate genes. GABA-ergic interneurons play an important role in the synchronization of neuronal activity, which is modulated by muscarinic acetylcholinic receptors and common neuromodulators such as dopamine and 5HT (reviewed in Uhlhaas and Singer, 2012). Dopamine injections lead to an increase of hippocampal-prefrontal coherence and reshaping of phase preferences of pyramidal cells (reviewed in Benchenane et al., 2011). Furthermore, dopamine, as well as norepinephrine and acetylcholine, may modulate the resonant properties of prefrontal pyramidal neurons (Benchenane et al., 2011). Investigations of functional genetic variants contributing to neural synchrony may help to provide more mechanistic accounts of the association between genetic variance and variance in behavior in the normal and pathological range.
Certain limitations of the present study need to be acknowledged. First, we used sensor-level analysis due to the modest number of EEG sensors available, which precluded a reliable reconstruction of source-level dynamics. Note, however, that the present analysis focused on temporal network-level dynamics, rather than anatomical localization, and that N2 and P3 components elicited in Go/No-Go tasks have been localized to anterior and mid-cingulate regions in previous studies (Huster et al., 2010). Ongoing studies in our laboratories using denser electrode arrays will provide data for a better spatial localization of task-related phase synchrony effects. Second, given that the present analyses are restricted to a single cognitive task, the generalizability of the present findings to related tasks and contexts is unclear, and needs to be established by further research. Stimulus-locked phase synchronization of cortical oscillations has been described in a variety of cognitive tasks, and the genetic contribution to individual differences to performance may vary across tasks. Hence, genetic studies of neural synchrony should be extended to other tasks recruiting cognitive control to determine commonality versus specificity of genetic influences.
Another important direction for future research is to determine the relationship between event-related phase synchronization of brain oscillations and the blood oxygenation level dependent (BOLD) response that unfolds at a larger temporal scale. In particular, it is important to establish whether these two neuroimaging modalities capture common or distinct neurophysiological mechanisms of information processing. As has been argued before, in some cases rapid temporal dynamics of neural activity can be significantly related to task events while the overall amount of activity averaged over time is not (Cohen, 2011). Given that phase synchronization of ongoing neural oscillations can occur in the absence of an overall increase in the amount of activity in a given cortical volume, neural synchrony and overall level of neural activity (reflected in the BOLD signal) may represent separable and complementary aspects of task-related “activation”.
In conclusion, the results of the present study suggests that individual differences in event-related neuronal phase synchronization are a promising intermediate phenotype for the study of genetic influences on normal and abnormal human behavior. Future studies should examine behavioral correlates of genetically transmitted individual differences in task-related neural synchrony, including cognitive performance, temperament, and liability to psychiatric disease as indicated by family history.
Acknowledgments
This work was supported by grants DA018899 and DA027096 from the National Institutes of Health (NIH) to A.A., and by the Max Planck Society. The authors also acknowledge the generous giving of time and effort by the study participants.
References
- Anokhin AP, Lutzenberger W, Birbaumer N. Spatiotemporal organization of brain dynamics and intelligence: an EEG study in adolescents. Int. J. Psychophysiol. 1999;33:259–273. doi: 10.1016/s0167-8760(99)00064-1. [DOI] [PubMed] [Google Scholar]
- Anokhin AP, Heath AC, Myers E. Genetics, prefrontal cortex, and cognitive control: a twin study of event-related potentials in a response inhibition task. Neurosci. Lett. 2004;368:314–318. doi: 10.1016/j.neulet.2004.07.036. [DOI] [PubMed] [Google Scholar]
- Barch DM. The cognitive neuroscience of schizophrenia. Annu. Rev. Clin. Psychol. 2005;1:321–353. doi: 10.1146/annurev.clinpsy.1.102803.143959. [DOI] [PubMed] [Google Scholar]
- Beam CR, Turkheimer E. Phenotype-environment correlations in longitudinal twin models. Dev. Psychopathol. 2013;25:7–16. doi: 10.1017/S0954579412000867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benchenane K, Tiesinga PH, Battaglia FP. Oscillations in the prefrontal cortex: a gateway to memory and attention. Curr. Opin. Neurobiol. 2011;21:475–485. doi: 10.1016/j.conb.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Bender S, Banaschewski T, Roessner V, Klein C, Rietschel M, Feige B, Brandeis D, Laucht M. Variability of single trial brain activation predicts fluctuations in reaction time. Biol. Psychol. 2015;106:50–60. doi: 10.1016/j.biopsycho.2015.01.013. [DOI] [PubMed] [Google Scholar]
- Beste C, Ness V, Falkenstein M, Saft C. On the role of fronto-striatal neural synchronization processes for response inhibition—evidence from ERP phase-synchronization analyses in pre-manifest Huntington's disease gene mutation carriers. Neuropsychologia. 2011;49:3484–3493. doi: 10.1016/j.neuropsychologia.2011.08.024. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Cavanagh JF, Zambrano-Vazquez L, Allen JJ. Theta lingua franca: a common midfrontal substrate for action monitoring processes. Psychophysiology. 2012;49:220–238. doi: 10.1111/j.1469-8986.2011.01293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX. It's about time. Front. Hum. Neurosci. 2011;5:2. doi: 10.3389/fnhum.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Gulbinaite R. Five methodological challenges in cognitive electrophysiology. NeuroImage. 2014;85(Pt 2):702–710. doi: 10.1016/j.neuroimage.2013.08.010. [DOI] [PubMed] [Google Scholar]
- Fallgatter AJ, Strik WK. The NoGo-anteriorization as a neurophysiological standard-index for cognitive response control. Int. J. Psychophysiol. 1999;32:233–238. doi: 10.1016/s0167-8760(99)00018-5. [DOI] [PubMed] [Google Scholar]
- Fallgatter AJ, Brandeis D, Strik WK. A robust assessment of the NoGo-anteriorisation of P300 microstates in a cued Continuous Performance Test. Brain Topogr. 1997;9:295–302. doi: 10.1007/BF01464484. [DOI] [PubMed] [Google Scholar]
- Friston K, Breakspear M, Deco G. Perception and self-organized instability. Front. Comput. Neurosci. 2012;6:44. doi: 10.3389/fncom.2012.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Murphy K, Roche RA, Stein EA. Dissociable executive functions in the dynamic control of behavior: inhibition, error detection, and correction. NeuroImage. 2002;17:1820–1829. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am. J. Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Haider B, McCormick DA. Rapid neocortical dynamics: cellular and network mechanisms. Neuron. 2009;62:171–189. doi: 10.1016/j.neuron.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huster RJ, Westerhausen R, Pantev C, Konrad C. The role of the cingulate cortex as neural generator of the N200 and P300 in a tactile response inhibition task. Hum. Brain Mapp. 2010;31:1260–1271. doi: 10.1002/hbm.20933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huster RJ, Eichele T, Enriquez-Geppert S, Wollbrink A, Kugel H, Konrad C, Pantev C. Multimodal imaging of functional networks and event-related potentials in performance monitoring. NeuroImage. 2011;56:1588–1597. doi: 10.1016/j.neuroimage.2011.03.039. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am. J. Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Jones MW, Wilson MA. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biol. 2005;3:e402. doi: 10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalunas SL, Geurts HM, Konrad K, Bender S, Nigg JT. Annual research review: Reaction time variability in ADHD and autism spectrum disorders: measurement and mechanisms of a proposed trans-diagnostic phenotype. J. Child Psychol. Psychiatry. 2014;55:685–710. doi: 10.1111/jcpp.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karantinos T, Tsoukas E, Mantas A, Kattoulas E, Stefanis NC, Evdokimidis I, Smyrnis N. Increased intra-subject reaction time variability in the volitional control of movement in schizophrenia. Psychiatry Res. 2014;215:26–32. doi: 10.1016/j.psychres.2013.10.031. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S, Gruber W, Freunberger R. Event-related phase reorganization may explain evoked neural dynamics. Neurosci. Biobehav. Rev. 2007;31:1003–1016. doi: 10.1016/j.neubiorev.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Lavric A, Pizzagalli DA, Forstmeier S. When 'go' and 'nogo' are equally frequent: ERP components and cortical tomography. Eur. J. Neurosci. 2004;20:2483–2488. doi: 10.1111/j.1460-9568.2004.03683.x. [DOI] [PubMed] [Google Scholar]
- Ledberg A, Montagnini A, Coppola R, Bressler SL. Reduced variability of ongoing and evoked cortical activity leads to improved behavioral performance. PLoS One. 2012;7:e43166. doi: 10.1371/journal.pone.0043166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TW, Yu YW, Wu HC, Chen TJ. Do resting brain dynamics predict oddball evoked-potential? BMC Neurosci. 2011;12:121. doi: 10.1186/1471-2202-12-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livanov MN. Analysis of bioelectrical oscillations in the cerebral cortex of rabbit. Contemp. Neuropathol. Psychiatry Psychohygiene. 1934;3:98–115. [Google Scholar]
- Livanov MN. Spatial Organization of Cerebral Processes. New York Toronto: John Wiley & Sons; 1977. [Google Scholar]
- Llinas R, Urbano FJ, Leznik E, Ramirez RR, van Marle HJ. Rhythmic and dysrhythmic thalamocortical dynamics: GABA systems and the edge effect. Trends Neurosci. 2005;28:325–333. doi: 10.1016/j.tins.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Whitfield SL, Ford JM. Anatomy of an error: ERP and fMRI. Biol. Psychol. 2003;64:119–141. doi: 10.1016/s0301-0511(03)00105-4. [DOI] [PubMed] [Google Scholar]
- Müller V, Anokhin AP. Neural synchrony during response production and inhibition. PLoS One. 2012;7:e38931. doi: 10.1371/journal.pone.0038931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacher V, Ledberg A, Deco G, Romo R. Coherent delta-band oscillations between cortical areas correlate with decision making. Proc. Natl. Acad. Sci. U. S. A. 2013 doi: 10.1073/pnas.1314681110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale MC, Cardon LR. Methodology for Genetic Studies of Twins and Families. Dordrecht: Kluwer Academic Publishers; 1992. [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx:Statistical Modeling. 6th. Richmond, VA: Department of Psychiatry; 2002. [Google Scholar]
- Nieuwenhuis S, Yeung N, van den Wildenberg W, Ridderinkhof KR. Electrophysiological correlates of anterior cingulate function in a go/no-go task: effects of response conflict and trial type frequency. Cogn. Affect. Behav. Neurosci. 2003;3:17–26. doi: 10.3758/cabn.3.1.17. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N, Cohen JD. Stimulus modality, perceptual overlap, and the go/no-go N2. Psychophysiology. 2004;41:157–160. doi: 10.1046/j.1469-8986.2003.00128.x. [DOI] [PubMed] [Google Scholar]
- Nigbur R, Ivanova G, Sturmer B. Theta power as a marker for cognitive interference. Clin. Neurophysiol. 2011;122:2185–2194. doi: 10.1016/j.clinph.2011.03.030. [DOI] [PubMed] [Google Scholar]
- Nigbur R, Cohen MX, Ridderinkhof KR, Sturmer B. Theta dynamics reveal domain-specific control over stimulus and response conflict. J. Cogn. Neurosci. 2012;24:1264–1274. doi: 10.1162/jocn_a_00128. [DOI] [PubMed] [Google Scholar]
- Palva JM, Monto S, Kulashekhar S, Palva S. Neuronal synchrony reveals working memory networks and predicts individual memory capacity. Proc. Natl. Acad. Sci. U. S. A. 2010;107:7580–7585. doi: 10.1073/pnas.0913113107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papenberg G, Hammerer D, Müller V, Lindenberger U, Li SC. Lower theta intertrial phase coherence during performance monitoring is related to higher reaction time variability: a lifespan study. NeuroImage. 2013 doi: 10.1016/j.neuroimage.2013.07.032. [DOI] [PubMed] [Google Scholar]
- Posthuma D, Beem AL, de Geus EJ, van Baal GC, von Hjelmborg JB, Iachine I, Boomsma DI. Theory and practice in quantitative genetics. Twin Res. 2003;6:361–376. doi: 10.1375/136905203770326367. [DOI] [PubMed] [Google Scholar]
- Randall WM, Smith JL. Conflict and inhibition in the cued-Go/NoGo task. Clin. Neurophysiol. 2011;122:2400–2407. doi: 10.1016/j.clinph.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Rijsdijk FV, Sham PC. Analytic approaches to twin data using structural equation models. Brief. Bioinform. 2002;3:119–133. doi: 10.1093/bib/3.2.119. [DOI] [PubMed] [Google Scholar]
- Roberts LE, Rau H, Lutzenberger W, Birbaumer N. Mapping P300 waves onto inhibition: Go/No-Go discrimination. Electroencephalogr. Clin. Neurophysiol. 1994;92:44–55. doi: 10.1016/0168-5597(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W. What does phase information of oscillatory brain activity tell us about cognitive processes? Neurosci. Biobehav. Rev. 2008;32:1001–1013. doi: 10.1016/j.neubiorev.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Saville CW, Feige B, Kluckert C, Bender S, Biscaldi M, Berger A, Fleischhaker C, Henighausen K, Klein C. Increased reaction time variability in attention-deficit hyperactivity disorder as a response-related phenomenon: evidence from single-trial event-related potentials. J. Child Psychol. Psychiatry. 2015;56:801–813. doi: 10.1111/jcpp.12348. [DOI] [PubMed] [Google Scholar]
- Sigurdsson T, Stark KL, Karayiorgou M, Gogos JA, Gordon JA. Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia. Nature. 2010;464:763–767. doi: 10.1038/nature08855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Mathys C. Computational approaches to psychiatry. Curr. Opin. Neurobiol. 2014;25:85–92. doi: 10.1016/j.conb.2013.12.007. [DOI] [PubMed] [Google Scholar]
- Swainson R, Cunnington R, Jackson GM, Rorden C, Peters AM, Morris PG, Jackson SR. Cognitive control mechanisms revealed by ERP and fMRI: evidence from repeated task-switching. J. Cogn. Neurosci. 2003;15:785–799. doi: 10.1162/089892903322370717. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52:155–168. doi: 10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat. Rev. Neurosci. 2010;11:100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Neuronal dynamics and neuropsychiatric disorders: toward a translational paradigm for dysfunctional large-scale networks. Neuron. 2012;75:963–980. doi: 10.1016/j.neuron.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Van Veen V, Carter CS. The timing of action-monitoring processes in the anterior cingulate cortex. J. Cogn. Neurosci. 2002a;14:593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiol. Behav. 2002b;77:477–482. doi: 10.1016/s0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- Wang C, Ulbert I, Schomer DL, Marinkovic K, Halgren E. Responses of human anterior cingulate cortex microdomains to error detection, conflict monitoring, stimulus-response mapping, familiarity, and orienting. J. Neurosci. 2005;25:604–613. doi: 10.1523/JNEUROSCI.4151-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womelsdorf T, Schoffelen JM, Oostenveld R, Singer W, Desimone R, Engel AK, Fries P. Modulation of neuronal interactions through neuronal synchronization. Science. 2007;316:1609–1612. doi: 10.1126/science.1139597. [DOI] [PubMed] [Google Scholar]
- Womelsdorf T, Johnston K, Vinck M, Everling S. Theta-activity in anterior cingulate cortex predicts task rules and their adjustments following errors. Proc. Natl. Acad. Sci. U. S. A. 2010a;107:5248–5253. doi: 10.1073/pnas.0906194107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womelsdorf T, Vinck M, Leung LS, Everling S. Selective theta-synchronization of choice-relevant information subserves goal-directed behavior. Front. Hum. Neurosci. 2010b;4:210. doi: 10.3389/fnhum.2010.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yener GG, Kurt P, Emek-Savas DD, Guntekin B, Basar E. Reduced visual event-related delta oscillatory responses in amnestic mild cognitive impairment. J. Alzheimers Dis. 2013;37:759–767. doi: 10.3233/JAD-130569. [DOI] [PubMed] [Google Scholar]