Abstract
Introduction
In the developing world, occupation has been identified as a risk factor for snake bite. Such an association has not been described in the USA. The objective of this study was to describe the epidemiology and clinical manifestations of occupational snake bite in patients reported to the ToxIC North American Snakebite Registry (NASBR).
Methods
This was a prospective case series of patients reported to the ToxIC NASBR between January 1, 2014 and November 5, 2015. Variables collected included snake species, patient demographics, date and location of exposure, occupation, bite location, clinical manifestations, and management.
Results
Of 180 adult snake bites reported, 25 (13.9 %; 95 % CI 9.2–19.8 %) were occupational in nature. Rattlesnake envenomations were common (80 %). Most snake bites (96 %) occurred in men. Occupations most associated with snake bite were landscaping (28 %) and working directly with snakes (24 %). Fifty-six percent of bites occurred in an outdoor work environment. Seventy-six percent of envenomations were to the upper extremities. Intentional interaction occurred in 40 % of cases, all of which sustained finger envenomations. No cases presented with apparent acute ethanol intoxication.
Conclusions
The majority of occupational snake bites occurred in men working outdoors and were unintentional injuries. Bites involving the upper extremity tended to result from intentional interactions. Acute ethanol intoxication did not appear to be involved with occupational envenomations.
Keywords: Snake bite, Occupation, Envenomation, Risk factor
Introduction
In the USA, nearly 10,000 snake bites are treated in the Emergency Department (ED) each year resulting in significant morbidity and rare mortality [1]. Both native and non-native snakes are implicated in these bites. In the USA, the Viperidae family (rattlesnakes, cottonmouths, and copperheads), and the Elapidae family (coral snakes) are responsible for native snake envenomations. Viper envenomations typically cause tissue and hematologic toxicity, although neurologic symptoms can occur with some species. Anaphylaxis and shock also rarely occur [2]. Elapid envenomations, conversely, are characterized by neurologic toxicity [3]. Serious envenomations also occur after exposure to captive non-native snake species [4].
Characteristics of individuals sustaining snake envenomations in the USA have been previously described [1, 5––8]. Men are over-represented, frequently comprising approximately 70–80 % of patients [1, 6, 8]. Extremity bites are common, and intentional interaction with snakes is often associated with upper extremity bites [6]. Rattlesnakes are responsible for the majority of venomous bites [1]. Young adults are more frequently affected than children and the elderly [7]. Ethanol intoxication has often been associated with snake bites, particularly when interaction is intentional [6]. Envenomations are more common between March and October, peaking in the summer and fall when human outdoor and snake activities are highest [6].
Worldwide, occupational exposure has been identified as a significant risk factor for snake bite. Although the epidemiology and clinical manifestations of such exposures has been described in other parts of the world [4], data for this population in the USA is lacking. Despite the paucity of data, occupational snake envenomation in the USA does appear to play a significant role. In a recent review, 30 % of venomous snake bites occurred in the workplace [1]. Another review of non-native snake bites reported 11 % occurring in the workplace setting [4]. This study aims to describe the epidemiology and clinical manifestations of occupational snake bites in the USA using data reported to the Toxicology Investigators Consortium (ToxIC) North American Snakebite Registry (NASBR).
Methods
This was a prospective case series. Data reported to the ToxIC NASBR between January 1, 2014 and November 5, 2015 were reviewed.
The ToxIC Registry was established in 2010 by the American College of Medical Toxicology (ACMT) as a novel, prospective toxico-surveillance and research tool. It records all cases cared for at the bedside by medical toxicologists at each of more than fifty sites across the USA that actively contribute cases to the Registry. The Registry allows for pooling of detailed, de-identified clinical information from across all Registry centers.
The Registry is Health Insurance Portability and Accountability Act (HIPAA) compliant and no patient identifiers are available on the database. Participation in the Registry is done in accordance with local institutional and Western Internal Review Board (IRB) policies and procedures.
ACMT’s ToxIC NASBR Sub-Registry is a database that gathers de-identified, detailed prospective information regarding snake bite, clinical manifestations of envenomation, and response to treatment for patients who receive bedside care from medical toxicologists across the USA. Occupational nature of snake bite was determined by the treating clinician for each case and was a mandatory field in the Sub-Registry.
Inclusion criteria were: age ≥ 18 years and occupational exposure leading to snake bite. Data collected included patient demographics, date and location of exposure, occupation, bite location, snake species, clinical manifestations, outcomes, and management. Method of identification of snake was not specified. Data for late bleeding events were obtained from direct patient contact or telephone interview.
Statistical Analysis
Descriptive statistics were used to report results. The Clopper Pearson method [9] was used to determine 95 % confidence intervals (CI).
Results
Cases
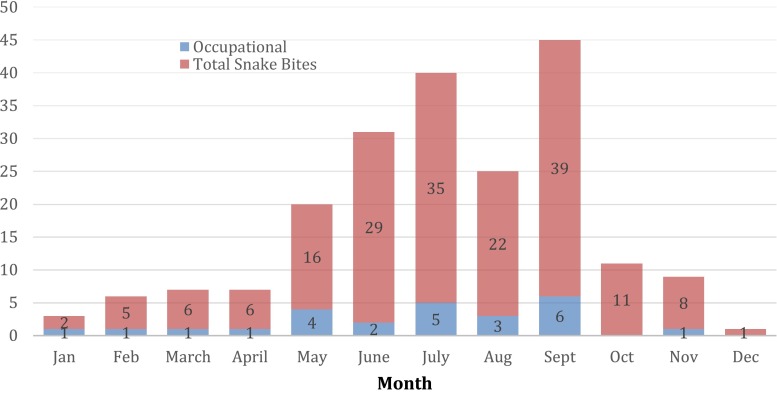
Between January 1, 2014 and November 5, 2015, 180 snake bites in adult patients were reported to the ToxIC Registry. Nine US states were represented. Twenty-five (13.9 %; 95 % CI 9.2–19.8 %) cases occurred during an occupational exposure to the snake and all occupational exposures identified were included in analysis. All occupational envenomations took place in seven US states (Table 1). The majority (52 %) of occupational snake bites occurred in Arizona. The greatest number of cases were reported between May and September, a pattern similar to that of the larger NASBR population. See Fig. 1.
Table 1.
Occupational and total snake bites by state
| US state | Total cases | Occupational cases (% total) |
|---|---|---|
| Arizona | 79 | 13 (17) |
| Texas | 37 | 4 (11) |
| California | 21 | 3 (14) |
| North Carolina | 15 | 1 [7] |
| Missouri | 9 | 1 (11) |
| Colorado | 7 | 0 |
| New Mexico | 6 | 2 (33) |
| Utah | 4 | 1 (25) |
| Pennsylvania | 2 | 0 |
Fig. 1.
Occupational and total snake bites by month
Types of Snakes
There were 20 native rattlesnake, three copperhead, and two non-native pit viper (Crotalus durissus terrificus and Trimeresurus albolabris) snake bites reported. Rattlesnakes were not consistently identified by species. Four (16 %) occupational snake bites occurred after exposure to captive snakes, and the remainder (84 %) were secondary to wild native snakes. Captive snakes included the two non-native snakes, the South American rattlesnake (Crotalus durissus terrificus) and the green pit viper (Trimeresurus albolabris), as well as two native snakes, the Arizona black rattlesnake (Crotalus cerberus) and the western diamondback rattlesnake (Crotalus atrox).
Demographics and Occupation
Twenty-four (96 %) patients were men and one was a woman. The average age was 40.3 years (range 18–66 years). One patient was aged over 65. Two (8 %) patients had sustained previous snake bites. This was the fourth envenomation for one patient, employed as a venomous animal educator.
Six (24 %) patients were employed working directly with snakes and seven (28 %) were employed in landscaping. Fourteen patients (56 %) worked outdoors. See Table 2 for a full list of occupations. Acute ethanol intoxication was not present in any case.
Table 2.
Occupation of snake bite victims
| Occupation | Cases |
|---|---|
| Landscaper | 7 |
| Snake handler/caretaker | 3 |
| Construction worker | 2 |
| Oil field worker | 2 |
| Snake remover | 1 |
| Venomous animal educator | 1 |
| Camp counselor | 1 |
| Mechanic | 1 |
| Engineer | 1 |
| Truck driver | 1 |
| Medicine man | 1 |
| Animal care facility manager | 1 |
| Biologist | 1 |
| Corrections officer | 1 |
| Professional mountain biker | 1 |
Envenomation Details
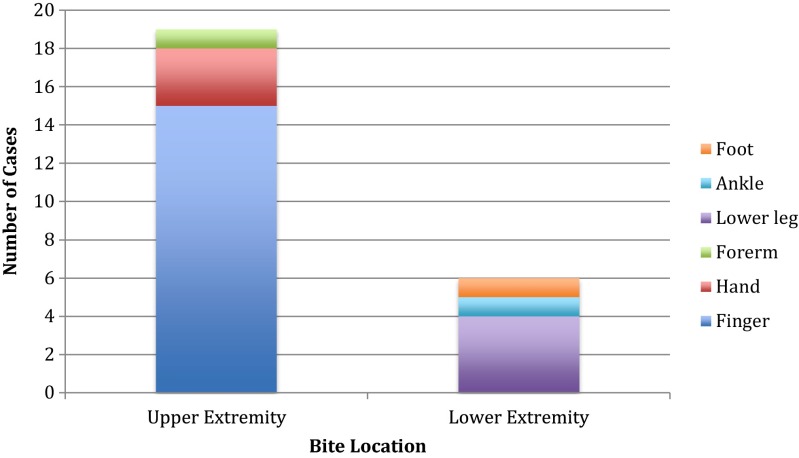
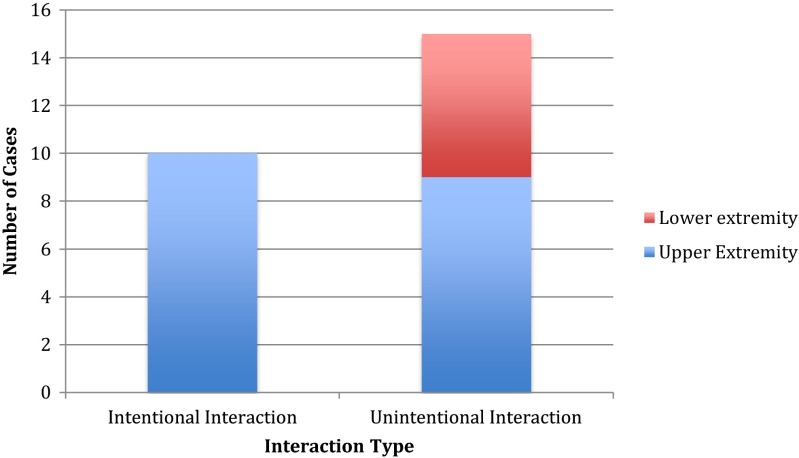
Nineteen (76 %) snake bites occurred to the upper extremities, predominantly to the finger, and six (24 %) occurred to the lower extremities, predominantly to the lower leg. See Fig. 2. Fifteen (60 %) cases were “legitimate bites,” meaning the interaction with the snake was unintentional. Ten (40 %) cases involved intentional interaction with the snake, and all of these individuals sustained upper extremity finger bites (Fig. 3). All patients working directly with snakes also sustained finger bites. None of the lower extremity bites involved intentional interaction with the snake. The single woman was bitten on the finger after intentional interaction with a snake that had incidentally entered her animal care facility.
Fig. 2.
Anatomic location of occupational snake bites
Fig. 3.
Patterns of intentional and unintentional occupational snake bites
Outcomes and Management
Field therapy in the form of ice application was performed in one case. Time to presentation to health care was 3 h or less in all but one case in which there was a 48 h delay (average 3.4 h, range 20 min to 48 h). Antivenom (Crofab™, BTG International in 22 cases, Green Pit Viper Thai Red Cross Antivenom in 1 case) was given in 23 (92 %) cases, with an average total dose of 10 vials (range 2–24 vials) per case. Mean time to antivenom administration after snake bite was 5.6 h (range 1–48 h). Clinical manifestations and laboratory results are described in Table 3 and Table 4, respectively. One patient was administered prophylactic antibiotics, and one underwent wound debridement. There were no fasciotomies. No patient was treated for late bleeding and there were no deaths.
Table 3.
Incidence of clinical manifestations in occupational snake bites
| Rattlesnakes (% total) | Copperheads (% total) | Non-natives (% total) | |
|---|---|---|---|
| Swelling | 19 (100) | 3 (100) | 2 (100) |
| Ecchymosis | 7 (37) | 1 (33) | 1 (50) |
| Erythema | 7 (37) | 2 (67) | 0 |
| Emesis | 2 (11) | 0 | 1 (50) |
| Neurotoxicity | 2 (11) | 0 | 1 (50) |
| Hypotension | 0 | 0 | 0 |
| Minor Bleeding | 3 (16) | 0 | 0 |
| Necrosis | 1 [5] | 0 | 0 |
| Angioedema | 1 [5] | 0 | 0 |
Table 4.
Mean laboratory results in occupational snake bites
| Rattlesnakes (range) | Copperheads (range) | Non-natives (range) | |
|---|---|---|---|
| Platelet nadir (K/mm3) | 216 (51–310) n = 20 | 168 (104–214) n = 3 | 177 (169–184) n = 2 |
| Fibrinogen nadir (mg/dL) | 272 (153–415) n = 20 | 251 (227–274) n = 2 | 97 (<30–164)a n = 2 |
| Prothrombin Time peak (sec) | 21 (11- > 120)b n = 19 | 12 (11–12) n = 3 | 20 (16–24) n = 2 |
aFor fibrinogen < 30, a value of 30 was used to calculate the mean
bFor prothrombin time > 120, a value of 120 was used to calculate the mean
Discussion
Occupation as a risk factor for snake bite has been described in other countries. In South East Asia, farming is the occupation most associated with snake bite and farmers represent approximately 80 % of snake bite victims in India [10]. In such an environment, bites to the lower extremities predominate. This contrasts significantly from the results seen in this study, in which there was not a single reported case of occupational snake bite in a farmer. In this study, the majority of bites were to landscapers, followed by snake handlers and others whose employment places them in direct contact with snakes.
Also in contrast to data from South East Asia, the majority of bites in this study were to upper extremities, frequently accompanied by intentional interaction with the snake. This data is in agreement with other modern studies of snake bites in the USA which also demonstrate an association between intentional interaction with snakes and upper extremity bites. The majority (60 %) of bites in this study involved unintentional interaction with the snake. These data, in contrast, differ from most modern snake bite studies in the USA in which the majority of bites result from intentional interaction with snakes [4, 6, 7]. Factors that may have contributed to an increased proportion of unintentional envenomations in this population are speculative but may include absence of ethanol intoxication and occupations exposing victims to snake habitats.
Similar to other snake bite series, male victims predominated. Perhaps the preponderance of men, rather than women, employed in landscaping contributed to the persistence of this trend in this series. Additionally, bites were most likely to occur in the summer and fall months in this group, months when snakes are more active. Overall, individuals are more frequently out of doors both recreationally and for employment purposes during these months, however this may not hold true for more southern states such as Arizona.
Uniquely, ethanol intoxication was not present in any case of occupational snake bite, a finding that differs significantly from other studies of snake bite victims. This is somewhat expected, as consuming ethanol or other intoxicants while working is typically discouraged. Although it would be expected that clinicians at the bedside would be able to detect ethanol intoxication in these patients, victims of occupational envenomations may have ulterior motivations to deny ethanol intoxication under such circumstances. Method of determination of ethanol intoxication (i.e., clinician judgment, patient report or laboratory confirmation) was not reported.
Limitations
This review of data reported to the NASBR Sub-Registry presents limitations inherent to voluntary reporting of data to a registry. Although the NASBR undergoes quality assurance review to identify and correct errors or omissions in data entry, it is possible that all errors were not identified. Notably, the majority of cases took place in Arizona. Data reported for occupations and snake species are thus skewed towards those typical of this geographical region. Limited availability of follow up data in a large number of cases prevented accurate reporting of such outcomes. Lastly, the small number of occupational envenomations resulted in a small sample size. This limited conclusions that can be drawn from the data and limited generalizability of findings.
Conclusions
Occupational exposure represented 13.9 % of snake bites in adults reported to the ToxIC Registry. The majority of occupational snake bites occurred in men working outdoors and were unintentional injuries. Bites involving the upper extremity tended to result from intentional interactions. Acute ethanol intoxication did not appear to be involved with occupational envenomations. These findings need to be validated in a population with a large sample size.
Acknowledgments
The authors express gratitude to the staff at the American College of Medical Toxicology (ACMT) for support of the North American Snakebite Registry (NASBR) within the ToxIC Registry project. We would also like to thank the members of the 2015 ToxIC Snakebite Study (TICSS) group: Anna Arroyo-Plascencia, Vikhyat S. Bebarta, Michael C. Beuhler, William Boroughf, Jeffrey Brent, Daniel Brooks, E. Martin Caravati, James D. Cao, Nathan Charlton, Steven Curry, Michael Darracq, William Dribben, Kimberlie Graeme, Spencer Greene, Benjamin Hatten, Kennon Heard, C William Heise, Janetta Iwanicki, Aaron Min Kang, William P Kerns II, Thomas Kibby, Joshua King, Ronald Kirschner, Kurt Kleinschmidt, Ken Kulig, Michael Levine, Rachel Levitan, Elizabeth Moore, Philip Moore, Michael Mullins, Eleanor Oakley, Ayrn O’Connor, Nancy Onisko, Angie Padilla-Jones, Tammy Phan, Frank LoVecchio, Anne-Michelle Ruha, Steven A. Seifert, Daniel J Sessions, Aaron Skolnik, Eric Smith, Meghan Spyres, An Tran, S. Eliza Halcomb, Evan S. Schwarz, Shawn M. Varney, Rais Vohra, Brandon J. Warrick, Sam G. Wang, Paul Wax, and Brian J. Wolk.
Compliance with Ethical Standards
Conflicts of Interest
Authors Meghan Spyres MD, Anne-Michelle Ruha MD, Steven Seifert MD, Nancy Onisko DO, Angela Padilla-Jones RN, and Eric Smith MSIS have no conflicts of interest to declare.
Funding
There was no direct funding for this project.
BTG International sponsored an unrestricted grant to ACMT for the NASBR registry.
References
- 1.O’Neil ME, Mack KA, Gilchrist J, Wozniak, EJ: Snakebite injuries treated in United States emergency departments, 2001–2004. Wilderness Environ Med, 2007 cited 2016 Mar 26;18:281–287. Available from Pubmed: http://www.ncbi.nlm.nih.gov/pubmed/18076294. [DOI] [PubMed]
- 2.Lavonas EJ, Ruha AM, Banner W et al. Unified treatment algorithm for the management of crotaline snakebite in the United States: results from an evidence-informed consensus workgroup. BMC Emerg Med, 2011[cited 2016 Mar 26];11:1–15. Available from Pubmed: http://www.ncbi.nlm.nih.gov/pubmed/21291549 [DOI] [PMC free article] [PubMed]
- 3.Ruha A, Pizon AF. Native (US) venomous snakes and lizards. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, editors. Goldfrank’s toxicologic emergencies, 10e. New York: McGraw-Hill; 2015. pp. 1537–1546. [Google Scholar]
- 4.Warrick BJ, Boyer LV, Seifert SA. Non-native (exotic) snake envenomations in the U.S.; 2005–2011. Toxins, 2014 [cited 2016 Mar 26];6:2899–2911. Available from Pubmed: http://www.ncbi.nlm.nih.gov/pubmed/25268980. [DOI] [PMC free article] [PubMed]
- 5.Downey DJ, Omer GE, Moneim, MS. New Mexico rattlesnake bites. Demographic review and guidelines for treatment. J Trauma,1991 [cited 2016 Mar 26];31:1380–1386. Available from Pubmed: http://www.ncbi.nlm.nih.gov/pubmed/1942147. [PubMed]
- 6.Curry SC, Horning D, Brady P et al. The legitimacy of rattlesnake bites in Central Arizona. Ann Emerg Med, 1989 [cited 2016 Mar 26];18:658–663. Available from Pubmed: http://www.ncbi.nlm.nih.gov/pubmed/2729691 [DOI] [PubMed]
- 7.Wingert WA, Chan, L. Rattlesnake bites in southern California and rationale for recommended treatment. West J Med, 1988 [cited 2016 Mar 26];148:37–44. Available from Pubmed. [PMC free article] [PubMed]
- 8.Tanen DA, Ruha AM, Graeme KA, Curry, SC. Epidemiology and hospital course of rattlesnake envenomations cared for at a tertiary care referral center in Central Arizona. Acad Emerg Med, 2001[cited 2016 Mar 26];8:177–182. Available from Pubmed: http://www.ncbi.nlm.nih.gov/pubmed/11157295 [DOI] [PubMed]
- 9.Pezzullo, JC. Interactive Statistics Pages [Internet]. Washington DC: [Publisher unknown]; [Year unknown] [updated 2009 May 25; cited 2016 April 16]. Available from: http://statpages.info/confint.html
- 10.Bhalla G, Mhaskar D, Agrawal A. A study of clinical profile of snake bite at a tertiary care center. Toxicol Int, 2014:21:203–208. Available from Pubmed: http://www.ncbi.nlm.nih.gov/pubmed/25253932 [DOI] [PMC free article] [PubMed]