Abstract
Introduction
Cyanide is a major chemical threat, and cyanide ingestion carries a higher risk for a supra-lethal dose exposure compared to inhalation but provides an opportunity for effective treatment due to a longer treatment window and a gastrointestinal cyanide reservoir that could be neutralized prior to systemic absorption. We hypothesized that orally administered cobinamide may function as a high-binding affinity scavenger and that gastric alkalinization would reduce cyanide absorption and concurrently increase cobinamide binding, further enhancing antidote effectiveness.
Methods
Thirty New Zealand white rabbits were divided into five groups and were given a lethal dose of oral cyanide poisoning (50 mg). The survival time of animals was monitored with oral cyanide alone, oral cyanide with gastric alkalinization with oral sodium bicarbonate buffer (500 mg), and in combination with either aquohydroxocobinamide or dinitrocobinamide (250 mM). Red blood cell cyanide concentration, plasma cobinamide, and thiocyanate concentrations were measured from blood samples.
Results
In cyanide ingested animals, oral sodium bicarbonate alone significantly prolonged survival time to 20.3 ± 8.6 min compared to 10.5 ± 4.3 min in saline-treated controls, but did not lead to overall survival. Aquohydroxocobinamide and dinitrocobinamide increased survival time to 64 ± 41 (p < 0.05) and 75 ± 16.4 min (p < 0.001), respectively. Compared to aquohydroxocobinamide, dinitrocobinamide showed greater systemic absorption and reduced blood pressure. Dinitrocobinamide also markedly increased the red blood cell cyanide concentration. Under all conditions, the plasma thiocyanate concentration gradually increased with time.
Conclusion
This study demonstrates a promising new approach to treat high-dose cyanide ingestion, with gastric alkalinization alone and in combination with oral cobinamide for treating a supra-lethal dose of orally administered cyanide in rabbits.
Keywords: Oral cyanide poisoning, Cobinamide, Gastric alkalinization, Diffuse optical spectroscopy
Introduction
Cyanide poisoning can occur in a variety of settings; for example, accidental exposure from industrial events, inhalation of combustion products, acts of terrorism, or chemical warfare [1–4]. Doses as little as 50 mg may be fatal to humans. Cyanide is inexpensive and readily accessible, with more than 5.2 billion pounds produced annually worldwide [5]. The cyanide threat from terrorism and industrial accidents is a major concern of the US civilian and military chemical defense programs. Oral ingestion or inhalation of cyanide can cause irreversible injury or death within minutes of exposure [6]. While effective antidotes are available for treating individual victims, no antidote presently exists for a mass casualty scenario or for very large dose of oral ingestions, since current antidotes must be given intravenously in large volumes of fluid.
It would be relatively simple for terrorists to poison water supplies [7–9], and prior threats have been uncovered [7, 8]. Despite very rapid cyanide absorption from the gastrointestinal tract, oral cyanide exposure is more amenable to treatment than inhalation exposure, due to a longer time from exposure to poisoning: death following oral cyanide ingestion can provide therapeutic windows of 30 min to hours, compared to less than 10 min for inhaled cyanide [10]. Moreover, a large reservoir of gastrointestinal cyanide can potentially be prevented from being absorbed following oral ingestion. In this study, we investigated a treatment approach that could be directed to casualties from oral cyanide ingestion.
Treatment of cyanide poisoning includes three general classes of agents: methemoglobin generators and nitric oxide (NO) donors (sodium nitrite, amyl nitrite, and dimethyl aminophenol), sulfur donors (sodium thiosulfate and glutathione) [1, 11], and direct binding agents (hydroxocobalamin and dicobalt edetate). We have been developing cobinamide as a cyanide antidote; it can be administered rapidly in small volumes by intramuscular injection [12–18]. Cobinamide is a promising cyanide antidote, capable of reversing LD80–100 level exposures with an intramuscular (or intravenous) injection of ~1 mL in rabbit models, and equivalently adjusted volumes in other animal models [12–20]. However, for oral ingestions, effects are delayed enough that much larger ingestions can occur [10]. Consequently, much larger antidote doses will be necessary. Intramuscular injection volumes are limited to a maximum of ~4 mL, and systemically administered doses of antidote will be limited by the drug’s side effects.
In addition to a lack of drugs for treating a large number of cyanide-poisoned victims, no effective methods exist for neutralizing large gastrointestinal cyanide reservoirs following oral ingestion.
Cobinamide is the penultimate precursor in hydroxocobalamin biosynthesis, lacking the dimethylbenzimidazole ribonucleotide group coordinated to the lower axial position of the cobalt atom [16]. This leads to several major chemical differences between cobinamide and hydroxocobalamin: (i) each cobinamide molecule can bind two ligands compared to one for hydroxocobalamin, (ii) cobinamide has a much higher affinity for ligands than hydroxocobalamin due to release of a negative trans effect of the bulky dimethylbenzimidiazole group, and (iii) cobinamide is considerably more water soluble than hydroxocobalamin [16]. The extraordinarily high-binding constant of cobinamide for cyanide [KA overall of ≈1022 M−1 [21] allows efficient cyanide neutralization [14–16, 22, 23] and is the basis for cobinamide’s potential use to neutralize a large dose of gastro-intestinal cyanide. However, cobinamide binding of cyanide is pH dependent, with pH > 4 required for effective binding (binding is complete at pH 6–7). This consideration must be addressed for gastric administration.
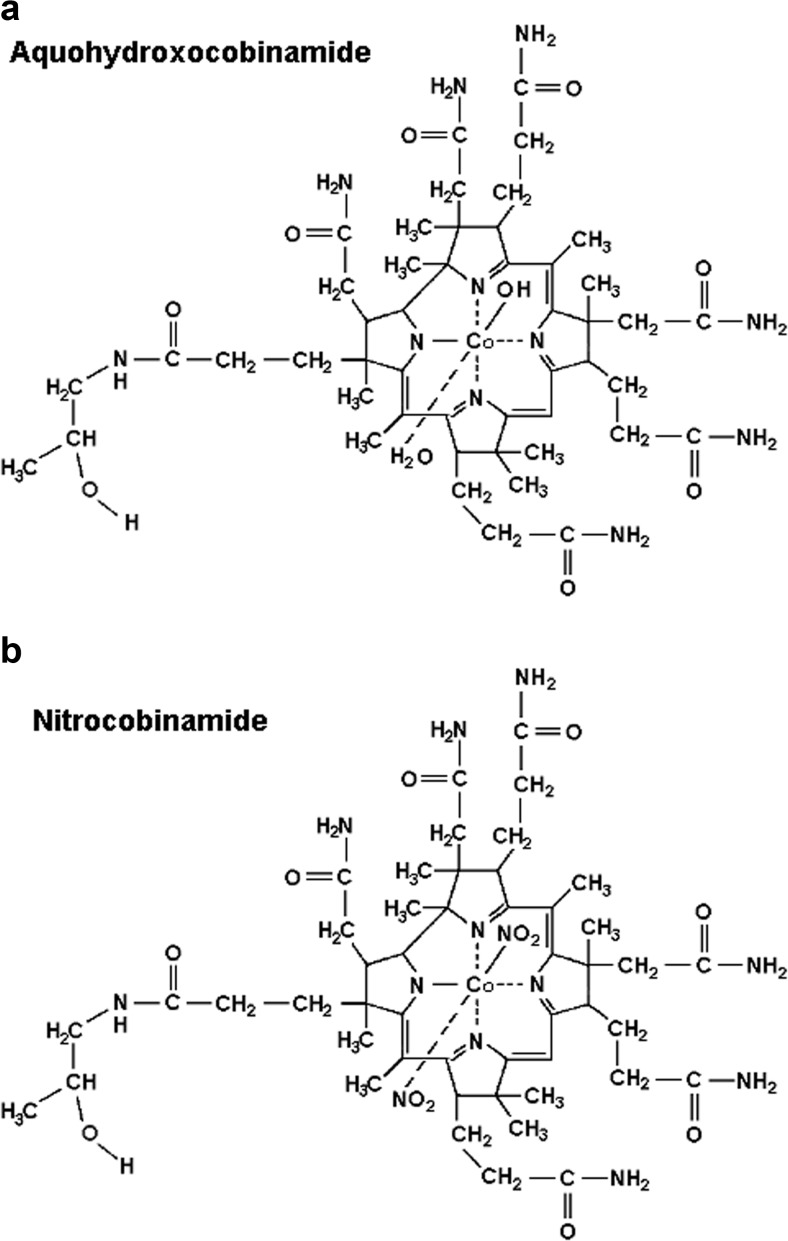
We have shown that both aquohydroxocobinamide (water and hydroxyl molecules bound to the cobalt atom) and dinitrocobinamide (two nitrite groups bound to the cobalt atom) are effective cyanide antidotes [12, 15, 17, 20] (Fig. 1). While dinitrocobinamide is better absorbed after intramuscular injection than aquohydroxocobinamide [20], the two species may not show differential absorption from the gastrointestinal tract. We investigated the effectiveness of both cobinamide species in this study.
Fig. 1.
The structures of aquohydroxocobinamide (a) and dinitrocobinamide (b) are shown. Cobinamide is used generically, without specifying the ligand(s) bound to the cobalt atom. Aquohydroxocobinamide refers to cobinamide with a water and hydroxyl group coordinated to the cobalt atom, without designating which group is in the lower (α) or upper (β) axial position. Nitrite binds to cobalamin via the nitrogen atom and not via one of the two oxygen atoms. Cobinamide with two bound nitrite groups are dinitrocobinamide
In addition, we hypothesized that gastric pH would influence the rate of cyanide absorption. In the stomach, cyanide ion will convert to hydrogen cyanide gas, since the pK a and the boiling point of HCN are 9.3 and 26.3 °C, respectively. While no prior published data directly compare oral cyanide absorption at various gastric pH levels, cyanide is reportedly absorbed as the highly diffusible hydrogen cyanide molecule [24]. Thus, raising gastric pH should delay cyanide absorption, and this should be safe, because exposure of the esophagus to pH up to 11.5 has been shown to be well tolerated and cause no damage [25]. We hypothesize that raising gastric pH should delay the rate of cyanide absorption. This in turn could influence the available time-window for effective antidote administration and potentially affect survival.
Methods
Thirty New Zealand white male pathogen-free rabbits (Western Oregon Rabbit Supply, Philomath, Oregon) weighing 3.5–4.5 kg were used in this study. All procedures were reviewed and approved by the Institutional Animal Care Committee of the University California, Irvine.
Procedures
At 17–20 h prior to initiating the study, food and water were withheld from the rabbits, and an Elizabethan collar was placed to prevent coprophagy. At the start of the study, the rabbits were anesthetized with an intramuscular (IM) injection of a 2:1 ketamine HCL (Ketaject, Phoenix Pharmaceutical Inc., St. Joseph, MI)/xylazine (Anased, Lloyed Laboratories, Shenandoah, IA) mixture. A 23-gauge catheter was placed in the marginal ear vein to allow intravenous access. The animals were intubated using a 3.5-mm cuffed endotracheal tube, which was immediately connected to a Bickford rebreathing anesthesia device; this allowed the animals to breath a source of 1.5–2.5 % isoflurane mixed with room air provided through an Ohmeda V.M.C. anesthesia machine. A 5-F premature infant feeding tube (NG Tube, Bard, Covington, GA) was inserted through the mouth into the stomach to administer cyanide and antidote. Once the feeding tube was positioned in the stomach, air was injected through the feeding tube and the bubbling sound was verified. The animals were connected to a pulse oximeter (Biox 3700 Pulse Oximeter, Ohmeda, Boulder, CO) by placing the ear probe (Datex-Ohmeda TS-E4-H) on the animal’s cheek. Heart rate and oxygen saturation (SPO2) were monitored, as were respiratory rate, end tidal CO2, and end tidal O2 via a Datex Ohmeda, General Electric, S/5 Patient Monitor connected to the endotracheal tube. Femoral arterial and venous cut-downs were performed in the left groin for central line placement to collect blood samples and measure systemic pressure. A 12-in, 18-g catheter (C-PMA-400-FA, Cook Inc., Bloomington, IN) was inserted into both the artery and vein, and a three-way stopcock was connected. A calibrated pressure transducer (TSD104A Transducer and MP100 WSW System, Biopac Systems, Inc., Santa Barbara, CA) was connected to the end of the arterial line to provide continuous blood pressure measurement.
Data Collection
Blood samples for cyanide analysis, blood gases, SPO2, and metabolic data were taken at baseline (before cyanide and antidote administration) at the time of administration, and at 2.5, 5, 10, 15, 30, 45, 60, and 90 min post administration. Systemic blood pressure readings were recorded every minute for the first 10 min after cyanide injection.
Animals that survived until a 90-min post cyanide administration were considered “survivors” and were euthanized with 1 mL of Euthasol (390 mg pentobarbital sodium/50 mg phenytoin sodium; Euthasol, Virbac AH, Inc., Fort Worth, Texas) administered through the marginal ear vein. Animals that died before 90 min were considered “non-survivors.”
Antidotes and Reagents
NaCN (Sigma Aldrich) was dissolved in 0.9 % NaCl (50 mg in 10 mL) immediately prior to administration. NaHCO3 was dissolved in distilled H2O (250 mg per 5 mL) and the solution was adjusted to pH 9.0 using 10 N NaOH immediately prior to administration.
Dinitrocobinamide was prepared from hydroxocobalamin as previously described [20]. Aquohydroxocobinamide was prepared from dinitrocobinamide by reducing the cobalt atom to the +1 valency state using sodium borohydride; this simultaneously removes nitrite from cobinamide and reduces nitrite to nitric oxide. The nitric oxide was removed by bubbling nitrogen through the solution, and the sodium borohydride was removed over a C18 reversed-phase column. Air was bubbled through the eluate to oxidize the cobinamide to the +3 valency state, and the resulting aquohydroxocobinamide product was concentrated to a solid under reduced pressure. Both the dinitrocobinamide and aquohydroxocobinamide were >98 % pure as assessed by high-performance liquid chromatography; they were administered to the rabbits through the nasogastric tube as a 250-mM solution (6.5 mL).
Study Design/Treatment Groups
The rabbits were divided into five groups of six animals each and received the following agents via the nasogastric tube:
Cyanide control; received 50 mg of NaCN in 10 mL saline
Cyanide with a single-dose sodium bicarbonate; received 500 mg of NaHCO3 in a 10-mL distilled H2O, followed immediately by 50 mg NaCN in a 10-mL saline
Cyanide with double-dose sodium bicarbonate; received 500 mg of NaHCO3, followed by 50 mg NaCN, and then a second dose of 250 mg NaHCO3 in a 5-mL distilled H2O administered at apnea or 8 min post cyanide injection, whichever came first
Cyanide with dinitrocobinamide and sodium bicarbonate; received 500 mg NaHCO3 followed by 50 mg NaCN, and then 6.5 mL 250 mM dinitrocobinamide
-
Cyanide with aquohydroxocobinamide and sodium bicarbonate; received 500 mg NaHCO3, followed by 50 mg NaCN, and then 6.5 mL 250 mM aquohydroxocobinamide
The total fluid volumes that animals received in each group are 10, 20, 25, 26.5, and 26.5 mL, respectively.
Diffuse Optical Spectroscopy
Diffuse optical spectroscopy (DOS) measurements were obtained through a fiber optic probe with a diode light emitter and the detector at a fixed distance (10 mm) from the source fiber. The probe was placed on the shaved surface of the right inner thigh of the animal for muscle measurements. The broadband DOS system combines multi-frequency domain photon migration with time-independent near-infrared spectroscopy to accurately measure bulk tissue absorption and scattering spectra [26–32]. Tissue concentrations of oxy- and deoxyhemoglobin, water, and CytcOx redox state changes (changes from baseline of the differential between oxidized and reduced CytcOx concentrations) were calculated by a linear least squares fit of the wavelength-dependent extinction coefficient spectra of each chromophore as previously reported. We used oxyhemoglobin and reduced (deoxy) hemoglobin absorption spectra reported by Zijlstra et al. [33] and oxidized CytcOx and reduced CytcOx absorption spectra reported by Moody et al. [34] for subsequent fitting and analysis.
Measurement of Red Blood Cell Cyanide Concentration
Cyanide in blood is bound almost exclusively to ferric(met) hemoglobin in red blood cells (RBCs); thus, the blood cyanide concentration can be measured by separating RBCs from plasma and acidifying the RBCs to release cyanide as HCN gas [35]. Whole blood collected from the animals was immediately cooled to 4 °C and centrifuged, and the plasma and RBC fractions separated. Samples were kept at 4 °C and analyzed within 48 h. The RBCs were lysed in ice-cold water, and the lysates were placed into the outer compartment of a Conway microdiffusion cell. A volume of 10 % trichloroacetic acid equal to the lysate was also added to the outer compartment, and an alkalinized cobinamide solution was added to the center compartment. The cell was capped, and the lysate was mixed with the trichloroacetic acid by gently tilting the chamber. The trichloroacetic acid denatures the hemoglobin and releases HCN gas, which is trapped in the cobinamide solution. The resulting dicyanocobinamide is measured spectrophotometrically as described previously [36]. The cyanide concentration was determined from a standard curve using freshly prepared KCN dissolved in 1 mM NaOH. Duplicate samples showed <15 % variation.
Measurement of Plasma Thiocyanate Concentration
Thiocyanate in the plasma was reduced to cyanide using potassium permanganate as described previously [19]. The resulting cyanide was measured as described above.
Measurement of Plasma Cobinamide Concentration
The plasma cobinamide concentration was measured by converting cobinamide in the samples to dinitrocobinamide by adding >100-fold excess of sodium nitrite over the estimated cobinamide concentration. Sample absorbance at 510 nm was measured, and the cobinamide concentration was calculated by comparison to known standards. This method yielded values within 95–105 % of those obtained by high-performance liquid chromatography.
Statistical Analysis
Data is expressed as mean ± SD. Survival times were compared by Mantel–Cox modeling versus controls. Blood pressure was compared by ANOVA.
Results
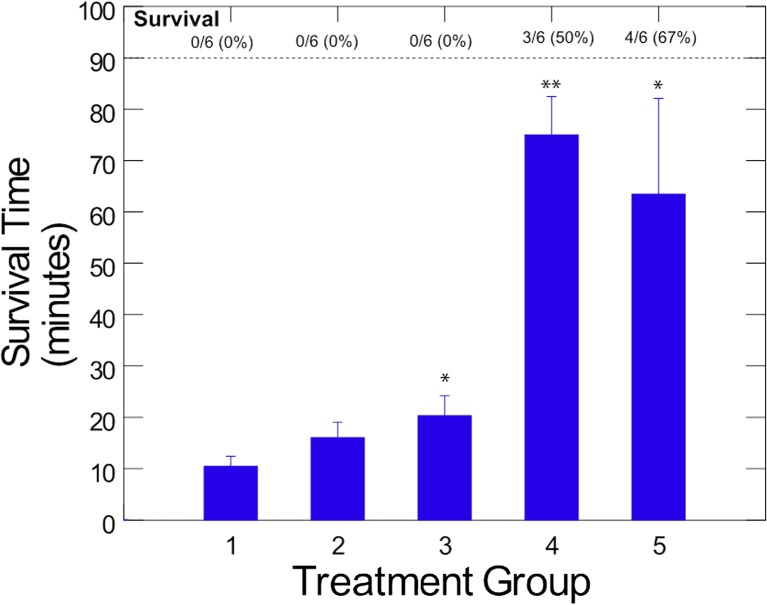
Animals in group 1, cyanide only, developed apnea at 2.6 ± 0.7 min (range 2 to 3.5 min) following cyanide administration and died at 10.5 ± 4.3 min (range 3 to 14 min; Fig. 2). Thus, this is a highly lethal model with rapid onset of apnea and subsequent death.
Fig. 2.
Average survival time in oral cyanide exposed rabbits (N = 6/group). New Zealand white rabbits received a supra-lethal dose of oral cyanide and simultaneously received sodium bicarbonate with or without dinitrocobinamide or aquohydroxocobinamide. All animals that survived up to 90 min were euthanized. Groups 1 cyanide alone, 2 cyanide followed by single-dose bicarbonate, 3 cyanide followed by two oral doses of bicarbonate, 4 cyanide followed by single-dose of bicarbonate plus dinitrocobinamide, and 5 cyanide followed by single-dose of bicarbonate plus aquohydroxocobinamide. *p < 0.05 (Mantel–Cox), **p < 0.0001 (Mantel-Cox)
Effect of Bicarbonate
Animals in group 2, single bicarbonate dose, had a mean time to death of 16.1 ± 6.6 min (range 10 to 28 min), and animals in group 3, double bicarbonate dose, had a mean time of death of 20.3 ± 8.6 min (range 10 to 30 min; Fig. 2). Group 3 was significantly different from group 1 (p < 0.04). Thus, gastric alkalinization prolonged survival, but did not prevent death.
Effect of Cobinamide
Animals in group 4, single bicarbonate dose plus dinitrocobinamide, had a mean survival time of 75 ± 16.4 min, with 3/6 animals surviving the full 90-min experimental period (Fig. 2). The survival time was significantly different from that of group 1 animals (p = 0.0001).
Animals in group 5, single bicarbonate dose plus aquohydroxocobinamide, had a mean survival time of 64 ± 41 min, with 4/6 animals surviving the full 90-min experimental period (Fig. 2). Again, this increase in survival time was significantly different from that of group 1 animals (p < 0.05).
The two animals in group 5 that died became apneic within 5 min of cyanide administration and expired in less than 10 min. The rapid onset of apnea and death was faster than even in group 2 animals, suggesting a lack of effective gastric alkalinization in these animals. The three animals in group 4 that expired lived at least 60 min, showing gradual deterioration.
Effect of Cobinamide Formulation on Blood Pressure
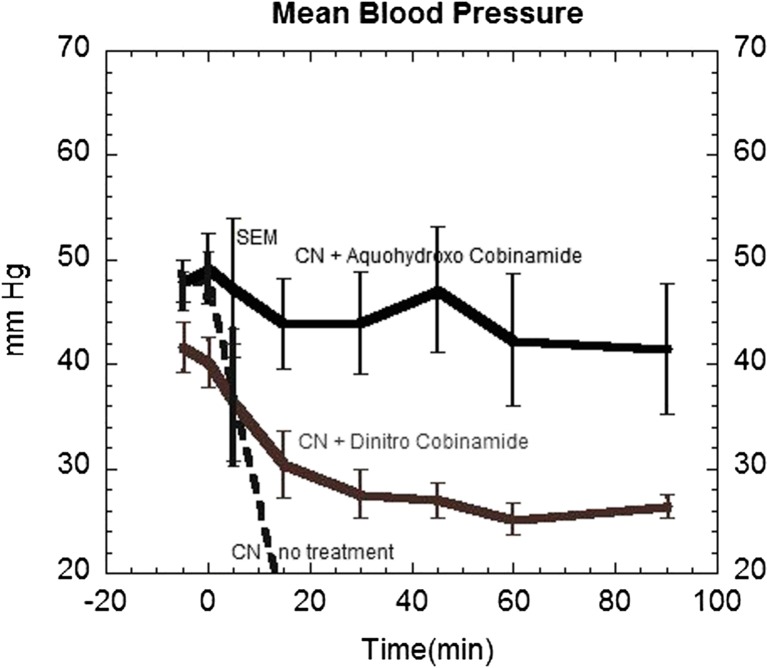
Group 1 animals exhibited rapid cardiovascular collapse with profound hypotension within 1–2 min of receiving the cyanide (Fig. 3). Groups 2 and 3 (single and double bicarbonate doses, respectively) animals also showed rapid fall in blood pressure until expiration, though time until expiration was longer in group 3 than that in controls (group 1).
Fig. 3.
Mean arterial blood pressure in oral cyanide-exposed rabbits. Rabbits received a supra-lethal dose of oral cyanide at time zero as described in the legend of Fig. 1, and their mean arterial blood pressure was measured prior to the cyanide and every 15 min thereafter. Blood pressure in group 1 animals receiving cyanide alone fell rapidly (dotted line) as all animals died within 15 min. Animals in group 5 that received aquohydroxocobinamide maintained their blood pressure throughout the experimental period. Animals in group 4 that received dinitrocobinamide showed a gradual decrease in blood pressure which stabilized at 60 min, but did not return to baseline values (p < 0.05 for group 4 versus group 5)
Group 4 animals (dinitrocobinamide) showed a gradual decrease in blood pressure throughout the study period (Fig. 3). The hypotension was not associated with clinical or diffuse optical spectroscopic evidence of cardiovascular collapse that was seen in group 1 animals. The total nitrite dose received by these animals was 150 mg and likely accounts for the reduced blood pressure. In contrast, animals in group 5 (aquohydroxocobinamide) showed a small, but non-significant decrease in blood pressure throughout the study period (Fig. 3). The blood pressure difference between groups 4 and 5 animals was significantly different (p < 0.05).
Diffuse Optical Spectroscopy
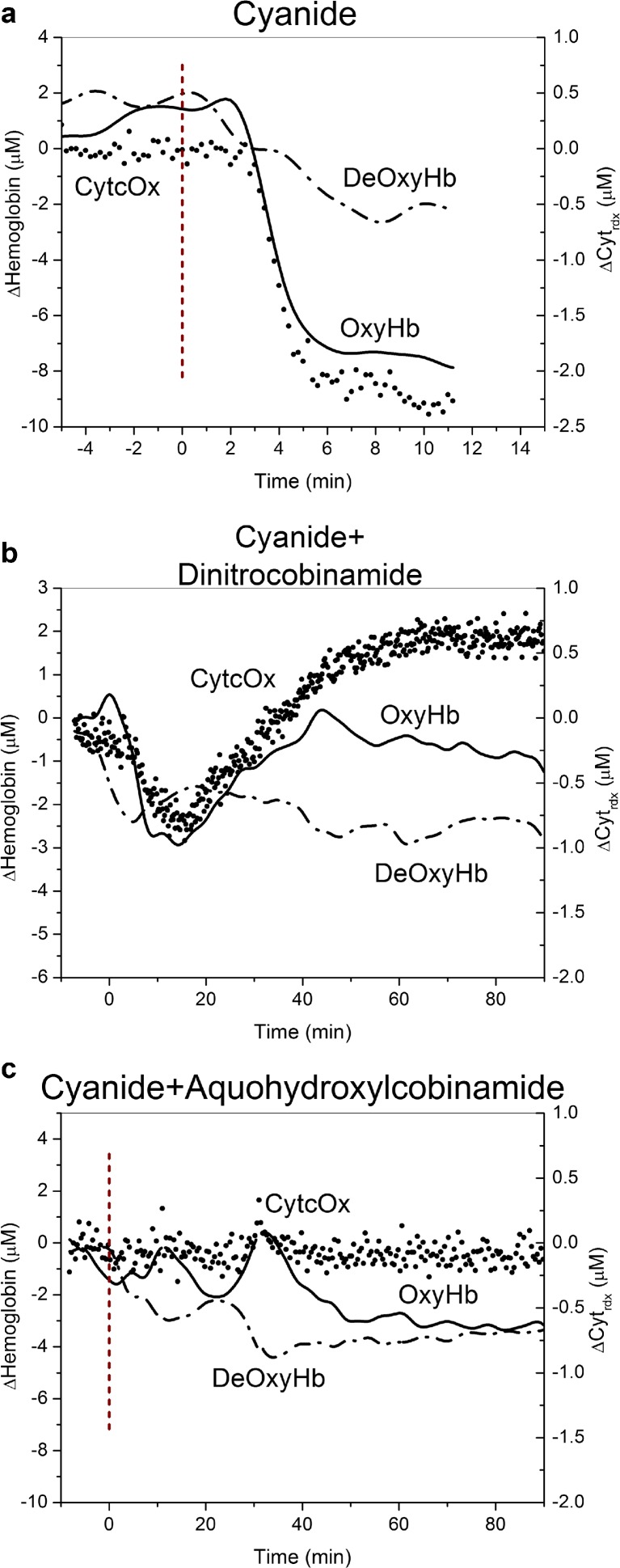
Group 1 animals showed an initial increase in oxyhemoglobin and decrease in deoxyhemoglobin concentrations following cyanide administration (Fig. 4a). This was followed by a very rapid onset of apnea and cardiovascular collapse with an accompanied precipitous fall in oxyhemoglobin and rise in deoxyhemoglobin prior to death (Fig. 4a).
Fig. 4.
Oxy- and deoxyhemoglobin concentrations and cytochrome c oxidase redox state in oral cyanide-exposed rabbits. Animals received a supra-lethal dose of oral cyanide at time zero as described in the legend to Fig. 2; oxyhemoglobin (red lines) and deoxyhemoglobin (blue lines) concentrations (left y-axis) and cytochrome c oxidase (CytcOx) redox state (black circles, right y-axis) were monitored continuously by diffuse optical spectroscopy (DOS). a Group 1 animals, cyanide alone—the oxyhemoglobin concentration rose initially and then fell precipitously as the animals experienced cardiac decompensation and died. CytcOx redox state fell continuously. b Group 4 animals, dinitrocobinamide treatment—the oxyhemoglobin concentration rose initially, then decreased, but gradually recovered towards baseline values. CytcOx redox state decreased initially, but then recovered. c Group 5 animals, aquohydroxocobinamide treatment—the oxyhemoglobin concentration rose initially, then showed a small decrease, but recovered fully. The CytcOx state showed no significant change
In animals in groups 2 and 3, the initial rise in oxyhemoglobin and fall in deoxyhemoglobin concentrations were somewhat more gradual, consistent with a reduced rate of cyanide absorption. This was subsequently followed by apnea and a precipitous fall in the oxyhemoglobin concentration as a terminal event.
In animals in groups 4 and 5, the oxyhemoglobin concentration rose initially, then declined to varying degrees, and stabilized in the surviving animals at concentrations close to baseline by 90 min (Fig. 4b, c).
The cytochrome C oxidase redox state fell in group 1 animals, consistent with cyanide inhibition of the enzyme. Group 4 animals showed an initial fall in the CytcOx redox state that subsequently recovered (Fig. 4b). This was different from group 5 animals where the CytcOx redox state remained stable throughout the study (Fig. 4c; the basis for this difference between groups 4 and 5 animals is addressed in the “Discussion” section).
Red Blood Cell Cyanide Concentration
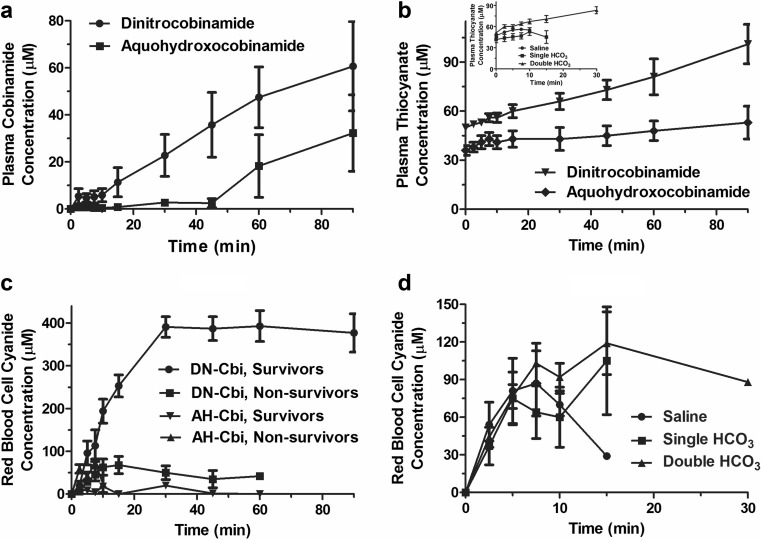
The red blood cell cyanide concentration increased linearly during the first 7.5 min post cyanide administration in groups 1 (cyanide alone), 2, and 3 (cyanide plus bicarbonate; Fig. 5a). The subsequent flattening in the red blood cell cyanide concentration in these animals was possibly from decreased splanchnic perfusion with decreased cyanide absorption, since the blood pressure was falling at this time (Fig. 3). The red blood cell cyanide concentration increased similarly in group 4 (dinitrocobinamide) and group 5 (aquohydroxocobinamide) animals during the first 7.5 min post cyanide administration and then fell gradually in group 5 animals (Fig. 5b). This was a marked contrast to the group 4 dinitrocobinamide animals where the red blood cell cyanide concentration continued to increase until 30 min in the surviving animals, reaching much higher values than in any of the other groups (Fig. 5b). The marked increase in blood cyanide concentration in the group 4 animals suggested that nitrite was absorbed and induced methemoglobinemia; the methemoglobin could then bind cyanide, raising the red blood cell cyanide concentration.
Fig. 5.
Red blood cell cyanide, plasma thiocyanate, and plasma cobinamide concentrations in oral cyanide-exposed rabbits. Rabbits received oral cyanide with or without sodium bicarbonate and dinitrocobinamide or aquohydroxocobinamide at time zero, and then plasma cobinamide (a), plasma thiocyanate (b), and red blood cell cyanide (c and d) concentrations were measured at the indicated times. In a and the inset of c, data are shown for group 1 animals (cyanide alone with saline), group 2 animals (cyanide plus single bicarbonate administration), and group 3 animals (cyanide with double bicarbonate administration); the x-axis ends at 30 min because these animals had all died by that time. The other panels show data for group 4 (cyanide plus single bicarbonate administration and dinitrocobinamide) and group 5 animals (cyanide plus single bicarbonate administration and aquohydroxocobinamide) and show the full 90-min time scale on the x-axis
Plasma Thiocyanate Concentration
We found that the plasma thiocyanate concentration increased gradually in all five groups of animals (Fig. 5c). This gradual increase was likely from slow conversion of cyanide to thiocyanate, consistent with a limited pool of available sulfane sulfur.
Plasma Cobinamide Concentration
Consistent with our findings that dinitrocobinamide binds less to the extracellular matrix and is absorbed better after intramuscular injection than aquohydroxocobinamide, we found that dinitrocobinamide was absorbed faster and more efficiently than aquohydroxocobinamide after oral ingestion, yielding higher plasma concentrations (Fig. 5d).
Discussion
Administration of high-dose oral cyanide in the rabbit model developed in this study resulted in acute, rapidly lethal cyanide poisoning, with a relatively narrow time window for effective antidote treatment. The cyanide dose was extremely high, 50 mg in a 3-kg rabbit (lethal human exposure can occur with as little as 50–70 mg in a 70-kg adult). This dose is more than twice the LD80 dose for the rabbits and leads to 100 % lethality in less than 15 min. Diffuse optical spectroscopy and hemodynamic changes were seen within seconds to minutes following this amount of oral cyanide. Apnea developed as early as 2 min following ingestion in animals that did not receive antidote or bicarbonate treatment, and all animals were apneic within 4 min without antidote administration. In human beings, if they are alive at the time of potential rescue, then they still have a gastric reserve of cyanide that could be maintained in the ionic form by gastric alkalinization. So the model reflects giving antidote and gastric alkalinization prior to the lethal effects of the gastric cyanide bolus.
Gastric alkalinization with sodium bicarbonate significantly prolonged survival time, without yielding full recovery. Several factors could contribute to the prolonged survival time. Gastric alkalinization would be expected to shift gastric cyanide from predominantly HCN, which should be highly membrane diffusible, to ionic CN−, which is likely less membrane diffusible. Alkalinization of the stomach contents was confirmed by gastric sampling following bicarbonate administration. No prior studies have clarified whether ionic CN− or nonionic HCN (or both) are absorbed through the gastrointestinal tract. However, it has been presumed that because HCN is more diffusible, it is more rapidly absorbed. The findings of our study lend support to this hypothesis because we found a significantly delayed time to death with gastric bicarbonate, with evidence of a dose response. One would not expect gastric neutralization alone to be curative of lethal cyanide ingestion, since continued gastric acid production would lead to eventual cyanide absorption and CN− ion may be absorbed, albeit at a slower rate. These findings suggest that gastric pH alkalinization may be valuable for extending the time window for antidotes to be effective with oral cyanide ingestion. Another possible mechanism of action of the bicarbonate is that it could have been absorbed systemically and decreased cyanide-induced metabolic acidosis. However, it seems unlikely that significant amounts of bicarbonate were absorbed within the first 5 min following cyanide ingestion, which would be required to reverse the rapid toxicity and apnea development from cyanide. Furthermore, diffuse optical spectroscopy measures of systemic CytcOx redox state and oxy- and deoxyhemoglobin saturations suggested delayed systemic cyanide effects, rather than neutralization of cyanide-induced acidosis. Further studies with frequent systemic lactate and anion-gap metabolic acidosis measurements could more definitively answer this question.
Oral cobinamide administration following cyanide ingestion led to a significant improvement in overall survival. The most likely site of cobinamide’s action was in the stomach or proximal small bowel where it could bind cyanide, preventing it from being absorbed. For aquohydroxocobinamide, plasma cobinamide concentrations did not show any substantial increase until 45 min after administration; all control and single bicarbonate-treated animals were dead long before that, and thus, the cobinamide almost certainly had to be acting within the gastrointestinal tract. The dose of cobinamide was well tolerated acutely. It is likely that even greater survival benefits could be achieved with higher doses of oral cobinamide. In addition, one could consider a non-absorbable cobinamide formulation that could allow very high doses of antidote to be administered orally, reducing risk of systemically absorbed cobinamide-induced side effects.
Two formulations of cobinamide were investigated in this study, dinitrocobinamide and aquohydroxocobinamide. Dinitrocobinamide is currently being developed for intramuscular administration. It would be convenient to have one single formulation for oral as well as intramuscular use, and dinitrocobinamide is chemically more stable than aquohydroxocobinamide. Dinitrocobinamide is more rapidly and completely absorbed than aquohydroxocobinamide following intramuscular injection. However, blood pressure was substantially lower in dinitrocobinamide-treated animals than in aquohydroxocobinamide-treated animals. The clinical features of the hypotension were distinct from the acute terminal decompensation from cyanide, which causes a precipitous drop in blood pressure. We reasoned that the hypotension in dinitrocobinamide-treated animals was most likely secondary to the large amount of nitrite (3.25 mmol = 150 mg), some of which was likely systemically absorbed. Therefore, we tested aquohydroxocobinamide. It did not result in hypotension and was equally or more effective in improving survival than dinitrocobinamide. Two animals that did not survive in the aquohydroxocobinamide treatment group died quickly, i.e., within 10 min of receiving cyanide. This was even faster than the animals treated with a single dose of bicarbonate, suggesting poor gastric alkalinization in these two animals. Perhaps in these two animals, a technical problem occurred such that the bicarbonate and/or the cobinamide were not delivered well to the stomach or a large gastric bubble that occurs when bicarbonate is converted to carbon dioxide in the stomach could serve as a protected site for the gas phase HCN. Future gastric buffering studies may help answer these questions.
There are a number of limitations of this study. We relied on the clinical evidence for the placement of the feeding tube for the study instead of employing imaging methods such as X-ray of the verification. While it is unlikely that the feeding tube position had changed since animals were immobile under the anesthesia, we cannot rule out the possibility. Also, there is a possibility that rapidly infused fluid volume may force contents beyond the gastric lumen and it may alter the outcome. Also, the dilution of stomach NaCN could possibly have affected the apnea/death time difference between the control, untreated groups (group 1), and the single and double bicarbonate groups (groups 2 and 3), respectively.
All animals were anesthetized as required by our animal review committee; therefore, the effects of anesthetic cannot be determined. However, anesthetic agents would not likely have a substantial cyanide protective effect, given the extremely large doses of cyanide administered. Another limitation is that our animal review committee requires that the experiments be relatively short and that the animals are euthanized at the end. This limitation leads to an underestimation of the effectiveness of cobinamide, since all animals were sacrificed at 90 min post cyanide exposure, and many of the cobinamide-treated animals would likely have survived much longer. A short experimental period also did not allow us to evaluate long-term outcomes, side effects, and neurological recovery; these clearly need to be investigated in terms of long-term impact of an oral cyanide antidote. And finally, in a mass casualty exposure, only conscious victims could receive an oral antidote. Thus, unconscious victims would either not be treated in such a scenario, or require other modes of antidote administration, probably limiting their total tolerable antidote dose.
In this study, the antidote was administered very shortly after cyanide ingestion. Further investigations also will be required to determine the maximum time window for antidote administration to be effective. This will likely be dependent on the rate of oral intake, total ingested dose, individual tolerance, and presence or absence of gastric food. Further studies are needed to define the optimal antidote formulation, timing of antidote, and whether the antidote will prevent long-term effects of cyanide exposure. In addition, there is the need for optimization of the bicarbonate dose or the investigation of other gastric pH alkalinization approaches since it is not known whether the improved survival in the animals receiving two doses of oral bicarbonate was due to the timing of the second dose or the greater total bicarbonate dose received. The maximum tolerable oral dose of cobinamide and the maximum cyanide ingestion, which can be neutralized, need to be studied, too.
In conclusion, this study demonstrates a promising new approach to treat high-dose oral cyanide ingestion and could provide a model for FDA approval for treating oral cyanide poisoning with cobinamide and gastric alkalinization.
Abbreviations
- DOS
Diffuse optical spectroscopy
- FD
Frequency domain
- SS
Steady state
- OxyHb
Oxyhemoglobin
- DeoxyHb
Deoxyhemoglobin
- CytcOx
Cytochrome C oxidase
Compliance with Ethical Standards
Conflicts of Interest
None
Sources of Funding
This study is supported by CounterACT NIH # 1U54 NS079201, CounterACT NIH # U01 NS058030, LAMMP # 445474-30136, and AMRMC W81XWH-12-2-0098.
References
- 1.SI B, TG B. Medical aspects of chemical and biological warfare. Chapter 10, cyanide poisoning. In: FR S, ET T, DR F, Borden Institute (U.S.), editors. Textbook of military medicine. Part I, warfare, weaponry, and the casualty. Washington, D.C.: Borden Institute, Walter Reed Army Medical Center; Office of the Surgeon General, U.S. Army; U.S. Army Medical Dept. Center and School; U.S. Army Medical Research and Material Command; Uniformed Services University of the Health Sciences; 1997. pp. 272–286. [Google Scholar]
- 2.Eckstein M. Cyanide as a chemical terrorism weapon. JEMS. 2004;29(8):suppl 22–suppl 31. [PubMed] [Google Scholar]
- 3.Gracia R, Shepherd G. Cyanide poisoning and its treatment. Pharmacotherapy. 2004;24(10):1358–1365. doi: 10.1592/phco.24.14.1358.43149. [DOI] [PubMed] [Google Scholar]
- 4.Martin CO, Adams HP., Jr Neurological aspects of biological and chemical terrorism: a review for neurologists. Arch Neurol. 2003;60(1):21–25. doi: 10.1001/archneur.60.1.21. [DOI] [PubMed] [Google Scholar]
- 5.Dzombak DA, Ghosh RS, Wong-Chong GM. Cyanide in water and soil: chemistry, risk, and management. Boca Raton, FL: CRC Press; 2006. [Google Scholar]
- 6.Suskind R, editor. The one percent doctrine: deep inside America’s pursuit of its enemies since 9/11. New York, NY: Simon & Schuster; 2006. [Google Scholar]
- 7.Beering P. Threats on tap: understanding the terrorist threat to water. J Water Resour Plan Manag. 2002;128(3):163–167. doi: 10.1061/(ASCE)0733-9496(2002)128:3(163). [DOI] [Google Scholar]
- 8.Keim ME. Terrorism involving cyanide: the prospect of improving preparedness in the prehospital setting. Prehosp Disaster Med. 2006;21(SupplementS2):s56–s60. doi: 10.1017/S1049023X00015910. [DOI] [PubMed] [Google Scholar]
- 9.Khan AS, Swerdlow DL, Juranek DD. Precautions against biological and chemical terrorism directed at food and water supplies. Public Health Rep. 2001;116(1):3–14. doi: 10.1016/S0033-3549(04)50017-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhattacharya R. Antidotes to cyanide poisoning: present status. Indian J Pharm. 2000;32(2):94–101. [Google Scholar]
- 11.Cummings TF. The treatment of cyanide poisoning. Occup Med (Lond) 2004;54(2):82–85. doi: 10.1093/occmed/kqh020. [DOI] [PubMed] [Google Scholar]
- 12.Bebarta VS, Tanen DA, Boudreau S, Castaneda M, Zarzabal LA, Vargas T, et al. Intravenous cobinamide versus hydroxocobalamin for acute treatment of severe cyanide poisoning in a swine (Sus scrofa) model. Ann Emerg Med. 2014 doi: 10.1016/j.annemergmed.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brenner M, Kim JG, Lee J, Mahon SB, Lemor D, Ahdout R, et al. Sulfanegen sodium treatment in a rabbit model of sub-lethal cyanide toxicity. Toxicol Appl Pharmacol. 2010;248(3):269–276. doi: 10.1016/j.taap.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brenner M, Kim JG, Mahon SB, Lee J, Kreuter KA, Blackledge W, et al. Intramuscular cobinamide sulfite in a rabbit model of sublethal cyanide toxicity. Ann Emerg Med. 2010;55(4):352–363. doi: 10.1016/j.annemergmed.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brenner M, Mahon SB, Lee J, Kim J, Mukai D, Goodman S, et al. Comparison of cobinamide to hydroxocobalamin in reversing cyanide physiologic effects in rabbits using diffuse optical spectroscopy monitoring. J Biomed Opt. 2010;15(1):017001. doi: 10.1117/1.3290816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Broderick KE, Potluri P, Zhuang S, Scheffler IE, Sharma VS, Pilz RB, et al. Cyanide detoxification by the cobalamin precursor cobinamide. Exp Biol Med. 2006;231(5):641–649. doi: 10.1177/153537020623100519. [DOI] [PubMed] [Google Scholar]
- 17.Chan A, Crankshaw DL, Monteil A, Patterson SE, Nagasawa HT, Briggs JE, et al. The combination of cobinamide and sulfanegen is highly effective in mouse models of cyanide poisoning. Clin Toxicol. 2011;49(5):366–373. doi: 10.3109/15563650.2011.584879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim JG, Lee J, Mahon SB, Mukai D, Patterson SE, Boss GR, et al. Noninvasive monitoring of treatment response in a rabbit cyanide toxicity model reveals differences in brain and muscle metabolism. J Biomed Opt. 2012;17(10):105005. doi: 10.1117/1.JBO.17.10.105005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan A, Balasubramanian M, Blackledge W, Mohammad OM, Alvarez L, Boss GR, et al. Cobinamide is superior to other treatments in a mouse model of cyanide poisoning. Clin Toxicol (Phila) 2010;48(7):709–717. doi: 10.3109/15563650.2010.505197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan A, Jiang J, Fridman A, Guo LT, Shelton GD, Liu M-T, et al. Nitrocobinamide, a new cyanide antidote that can be administered by intramuscular injection. J Med Chem. 2015;58(4):1750–1759. doi: 10.1021/jm501565k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayward GC, Hill HA, Pratt JM, Vanston NJ, Williams RJ. The chemistry of vitamin B 12. IV. The thermodynamic trans-effect. J Chem Soc Perkin 1. 1965:6485–93. [PubMed]
- 22.Bebarta VS, Pitotti RL, Boudreau S, Tanen DA. Intraosseous versus intravenous infusion of hydroxocobalamin for the treatment of acute severe cyanide toxicity in a swine model. Acad Emerg Med Off J Soc Acad Emerg Med. 2014;21(11):1203–11. doi: 10.1111/acem.12518. [DOI] [PubMed] [Google Scholar]
- 23.Broderick KE, Balasubramanian M, Chan A, Potluri P, Feala J, Belke DD, et al. The cobalamin precursor cobinamide detoxifies nitroprusside-generated cyanide. Exp Biol Med. 2007;232(6):789–798. [PubMed] [Google Scholar]
- 24.Newhouse K, Chiu N. Toxicological review of hydrogen cyanide and cyanide salts. In: Agency USEP, editor. Washington, DC: EPA http://www.epa.gov/iris/toxreviews/0060tr.pdf; Accessed on 20 March 2016.
- 25.Atug O, Dobrucali A, Orlando R. Critical pH level of lye (NaOH) for esophageal injury. Dig Dis Sci. 2009;54(5):980–987. doi: 10.1007/s10620-009-0767-7. [DOI] [PubMed] [Google Scholar]
- 26.Bevilacqua F, Berger AJ, Cerussi AE, Jakubowski D, Tromberg BJ. Broadband absorption spectroscopy in turbid media by combined frequency-domain and steady-state methods. Appl Opt (USA) 2000;39(34):6498–6507. doi: 10.1364/AO.39.006498. [DOI] [PubMed] [Google Scholar]
- 27.Lee J, Armstrong J, Kreuter K, Tromberg BJ, Brenner M. Non-invasive in vivo diffuse optical spectroscopy monitoring of cyanide poisoning in a rabbit model. Physiol Meas. 2007;28(9):1057–1066. doi: 10.1088/0967-3334/28/9/007. [DOI] [PubMed] [Google Scholar]
- 28.Lee J, El-Abaddi N, Duke A, Cerussi AE, Brenner M, Tromberg BJ. Noninvasive in vivo monitoring of methemoglobin formation and reduction with broadband diffuse optical spectroscopy. J Appl Physiol. 2006;100(2):615–622. doi: 10.1152/japplphysiol.00424.2004. [DOI] [PubMed] [Google Scholar]
- 29.Lee J, Keuter KA, Kim J, Tran A, Uppal A, Mukai D, et al. Noninvasive in vivo monitoring of cyanide toxicity and treatment using diffuse optical spectroscopy in a rabbit model. Mil Med. 2009;174(6):615–621. doi: 10.7205/MILMED-D-02-7408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee J, Kim JG, Mahon SB, Mukai D, Yoon D, Boss GR, et al. Noninvasive optical cytochrome c oxidase redox state measurements using diffuse optical spectroscopy. J Biomed Opt. 2014;19(5):055001. doi: 10.1117/1.JBO.19.5.055001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merritt S, Gulsen G, Chiou G, Chu Y, Deng C, Cerussi AE, et al. Comparison of water and lipid content measurements using diffuse optical spectroscopy and MRI in emulsion phantoms. Technol Cancer Res Treat. 2003;2(6):563–569. doi: 10.1177/153303460300200608. [DOI] [PubMed] [Google Scholar]
- 32.Pham TH, Coquoz O, Fishikin JB, Anderson E, Tromberg BJ. Broad bandwidth frequency domain instrument for quantitative tissue optical spectroscopy. Rev Sci Instrum. 2000;71:2500–2513. doi: 10.1063/1.1150665. [DOI] [Google Scholar]
- 33.Zijlstra WG, Buursma A, Assendelft OW. Visible and near-infrared absorption spectra of human and animal haemoglobin determination and application. Zeist, The Netherlands: CRC Press; 2000. [Google Scholar]
- 34.Rich PR, Moody AJ. Chapter 10 cytochrome c oxidase. In: Milazzo G, Graber P, editors. Treatise on bioelectrochemistry, vol. 3 Bioenergetics. Basel: Birkhauser Verlag; 1996. [Google Scholar]
- 35.Lundquist P, Rosling H, Sorbo B. Determination of cyanide in whole blood, erythrocytes, and plasma. Clin Chem. 1985;31(4):591–595. [PubMed] [Google Scholar]
- 36.Blackledge WC, Blackledge CW, Griesel A, Mahon SB, Brenner M, Pilz RB, et al. New facile method to measure cyanide in blood. Anal Chem. 2010;82(10):4216–4221. doi: 10.1021/ac100519z. [DOI] [PMC free article] [PubMed] [Google Scholar]