Abstract
Introduction
Synthetic Cannabinoid Receptor Agonists (SCRAs) are the largest group of new psychoactive substances reported to the European Warning System and the United Nations Office on Drugs and Crime to date. The heterogeneous nature and speed of diversification of these compounds make it challenging to accurately characterise and predict harms of these compounds in pre-clinical studies, ahead of their appearance.
Case Report
We report the case of a 19-year-old female who purchased three products from a headshop: two new psychoactive substances (sachets of “cannabis tea” and “mushroom tea”) as well as two LSD blotters. After the “cannabis tea” was smoked and the two LSD blotters and “mushroom tea” were ingested, the patient became tachycardic (HR 128), developed seizures, agitation, visual hallucinations as well as suspected serotonergic toxicity (sustained ankle clonus 20–30 beats) 1–2 hours after use. She was treated with 1 mg of intravenous midazolam. Symptoms/signs resolved within 13 hours. No further supportive care was required. Plasma, blood, and urine samples confirmed the presence of two SCRAs: 5FAKB-48 and 5F-PB-22. The patient also reported therapeutic use of both fluoxetine and citalopram for depression.
Discussion
To the best of our knowledge, this is the first case report of non-fatal intoxication with 5F-AKB-48 with analytical confirmation and exposure times. It also highlights the difficulties in understanding the pattern of toxicity of certain SCRAs in the context of psychotropic medications/co-morbid mental illness.
Keywords: Synthetic cannabinoid receptor agonist, 5F-AKB-48, 5F-PB-22, Novel psychoactive substances, Recreational drug toxicity
Introduction
There has been increasing availability of a wide range of different synthetic cannabinoid receptor agonists (SCRAs) as new psychoactive substances over the last decade [1]. The initial elucidation of the main active constituent of cannabis, D9-tetrahydrocannabinol (THC) in 1965 led to the manufacture of, and experimentation with, early synthetic cannabinoid analogues, which modelled THC [2]. In the late 1980s/early 1990s new synthetic cannabinoids and related naturally occurring endocannabinoids such as anandamide and 2-arachidonylglycerol were described after isolation of the G-protein coupled receptors CB1 and CB2. Human studies have demonstrated pleotropic effects related to CB1 and or CB2 activation including alteration of central nervous system (CNS) functions (reward, memory, cognition and pain perception), modulation of immune/inflammatory responses, cardiovascular effects (vasodilatation), respiratory effects (regulating intrinsic control of airway tone) as well as evidence of involvement in regulatory control of reproduction and reduction of intraocular pressure [3–8]. There has been exploration of these cannabinoid receptors as potential drug targets for anticonvulsants, appetite regulators in obesity, and treatments for drug dependence [9–10]; however adverse effects (gastrointestinal, renal and psychiatric) hindered progress of these compounds through clinical trials as therapeutic agents [11–14].
Published and presented scientific findings from the above studies have been exploited for the development and sale of SCRAs as potential recreational drugs [15]. The Synthetic Cannabinoid Receptor Agonists (SCRAs) were first reported as new psychoactive substances in Europe in 2008 and the first case report of acute SCRA toxicity was from forensic investigators in Austria and Germany in 2008, although ‘legal high’ products involving SCRAs have been sold from at least 2006 [16]. There has been a steady rise in the availability of a wide range of different SCRAs since this time and have been the most common new psychoactive substances reported to the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) with 142 SCRAs reported from 2008 to the end of 2014. Despite this, there are relatively few reports of acute SCRA toxicity; early reports noted a similar pattern of toxicity to cannabis. These include typical undesired neuropsychiatric and cardiovascular effects—drowsiness, paranoia, delusion, hallucination, impaired cognition/memory as well as tachycardia and hypotension [17–18]. More recently reports have suggested that the SCRAs have additional toxicity including acute kidney injury, stimulant (sympathomimetic) toxicity and seizures [19–20]. We present here a case of significant acute toxicity related to analytically confirmed use of two SCRAs: N-(adamantan-1-yl)-1-(5-fluoropentyl)-1H-indazole-3-carboxamide (5F-AKB-48) and 1-(5-Fluoropentyl)-1H-indole-3-carboxylic acid 8-quinolinyl ester (5F-PB-22). Written informed consent was obtained from the patient for publication of this case report.
Case Report
A 19-year-old female was brought to a tertiary-level hospital at Emergency Department by ambulance. She had a past medical history of depression, treated by her primary care physician with fluoxetine and citalopram. Pre-hospital, she was suspected of having tonic seizures having had three witnessed episodes of “rigidity/back arching” associated with urinary incontinence followed by periods of significant drowsiness. On arrival of the ambulance, she had a Glasgow Coma Score of 9/15 (E3, V1, M5) and it was noted by passers by that prior to her “seizures” she had been behaving strangely and appeared to be having visual hallucinations.
On arrival in the Emergency Department (ED) 65 min later, GCS was 10/15 (E3, V1, M6) and she appeared to continue to experience visual hallucinations causing her to reach out at things. She had a heart rate of 128 beats per minute, a blood pressure of 129/97 mmHg, oxygen saturations of 100 % on room air, a respiratory rate of 18 per minute and a temperature of 36.5 °C. She had symmetrical hypertonia with 20–30 beats of ankle clonus; no ocular clonus was demonstrated and the remainder of her neurological examination was normal. The ECG on arrival in the ED demonstrated a sinus tachycardia with a normal QTc and QRS interval. A venous blood gas performed 4.5 h after reported use showed a mild respiratory acidosis (pH 7.25, pCO2 7.83 kPa, HCO3 −25.7 mmol/L and lactate 1.2 mmol/L). Full blood count, blood glucose concentration, renal profile and creatine kinase were normal. In the ED, she was treated with 1 mg of midazolam intravenously (this treatment was given 5 h post exposure, immediately after first blood samples were sent) and admitted for observation. The neurological and neuropsychiatric features had resolved by approximately 13 h from exposure. She required no further treatment.
Prior to discharge the following morning, additional history was taken through an interpreter as the patient was a non- native English speaker. She had bought two new psychoactive substances from a head-shop including a sachet of “cannabis tea” (1.5 g) and a sachet of “mushroom tea” (1.5 g) and reported purchasing two LSD blotters (unknown weight). These were all taken 1–2 h prior to the onset of her symptoms and the subsequent bystander call for an ambulance. The “cannabis tea” was smoked in one roll-up, two LSD blotters that were taken sublingually followed by half of the “mushroom tea” sachet dissolved in some water (unknown amount) and approximately 300 mls were ingested. As the patient observed no effects within 30 min, the remaining contents of the “mushroom tea” sachet (described as “candy pieces”) were chewed. The patient had used methylphenidate every few weeks for the last three months with infrequent cocaine and ‟ecstasy” use. She had lastly used two ecstasy tablets 10 days ago and had not used methylphenidate and cocaine for over 14 days. She was a non-smoker and did not drink alcohol. Apart from the history of depression and use of both citalopram and fluoxetine, the patient denied taking any other medications.
Toxicological Screening
Biological samples were collected from the patient at 5 (plasma) and 17 (blood and urine)-hours post exposure and underwent comprehensive toxicological screening. In relation to SCRA screening, blood, plasma and enzymatically hydrolysed urine samples were prepared for analysis using a liquid/liquid extraction technique with a deuterated analogue of a JWH-18 metabolite as an internal marker. Prepared samples were analysed on a Thermo Q Exactive Focus high resolution accurate mass (HRAM) spectrometer interfaced to a liquid chromatography system. Acquired data were processed against an in-house database containing all synthetic cannabinoids reported to date through forensic intelligence networks. The levels of analytes reported are based on a single point calculation against the internal marker.
This initial plasma sample (5 h post exposure) contained 5F-PB-22 at a concentration of 200 pg/ml together with two metabolites of 5F-AKB-48 [5F-AKB-48 adamantyl hydroxy (900 pg/ml) and 5F-AKB-48 desfluoro hydroxypentyl (5000 pg/ml)], citalopram (10-20 ng/ml), diazepam 10-20 ng/ml and fluoxetine metabolites. Seventeen-hours post-exposure of 5F-PB-22 (40 pg/ml), the two 5F-AKB-48 metabolites [5F-AKB-48 adamantyl hydroxy (150 pg/ml) and 5F-AKB-48 desfluoro hydroxypentyl (800 pg/ml)], citalopram (10-20 ng/ml), fluoxetine (20-30 ng/ml) and diazepam (40-50 ng/ml) was done. The urine sample (17 h post-exposure) contained metabolites of 5F-AKB-48 (1-5 ng/ml range), citalopram (approx. 250 ng/ml), fluoxetine 40 ng/ml and diazepam metabolites (oxazepam 200 ng/ml, temazepam 100 ng/ml) and zolpidem metabolites (10–50 ng/ml range). No other recreational drugs including lysergic acid diethylamide and/or related metabolites, other new psychoactive substances and/or prescription drugs were found in the three biological samples.
Discussion
This case report demonstrates neurotoxicity in an individual associated with the detection of SCRAs 5F-AKB-48 and 5F-PB-22. Features of serotonergic toxicity were also examined in this case. SCRAs may have contributed to this; however, the presence of two serotonergic co-ingestants–fluoxetine and citalopram must be taken into account and are a confounding factor in the pattern of toxicity observed. Interestingly, the presence of diazepam and metabolites as well as zolpidem may represent drug adulterants, as they were not given during the admission and the patient did not report their use. The absence of analytical detection of midazolam and its metabolites 12 h post use, may be associated with its relatively fast metabolism compared with other benzodiazepines. Overall, the patient recovered quickly from this episode, required little clinical intervention and was discharged without complication. To the best of our knowledge, this is the first case report of 5F-AKB-48 use associated with analytical confirmation and exposure times; it also contributes to nascent literature around documented cases and toxicity with 5F-PB-22 use. A total of 134 synthetic cannabinoids have been notified to the EMCDDA as of December 2014, constituting the largest group of new psychoactive substances monitored by the European Warning System [21]. Additionally, 28 % of the 348 new psychoactive substances reported to the United Nations Office on Drugs and Crime from 2008 to 2013 were SCRAs, the largest proportion of new psychoactive substances detected worldwide [22].
5F-AKB-48
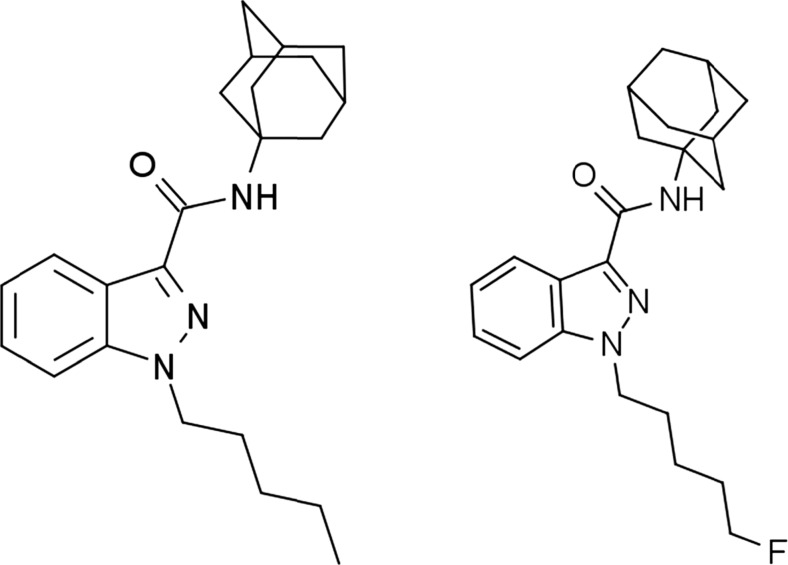
AKB-48 (originally named after a Japanese girl band), also known as APINACA, is a SCRA that was identified in Japanese herbal products in 2012. Structurally, it features an adamantyl group linked to a 1-pentyl-1H-indazole-3-carboxamide (PINACA) base through the amide. 5F-AKB-48 involves a 5-flouropentyl tail linking chain as shown in Fig. 1.
Fig. 1.
AKB-48 and 5F-AKB-48
In vitro studies in rat and mouse models have shown that 5F-AKB-48 (1 μM) has a Ki of 0.87 ± 0.14 nM to CB1 receptors, EC50 31.0 ± 7.5 nM and Emax 190 ± 11 %; and it is a more potent CB1 agonist than JWH-018 (Ki, 3.38 ± 0.63 nM; EC50, 20.2 ± 1.3 nM and Emax, 163 ± 3 %). Further, 5F-AKB-48 (0.1 mg/kg i.v.) as well as 5F-PB-22 (0.01 mg/kg i.v.) were shown to increase in vivo dialysate dopamine concentrations in the shell of the nucleus accumbens of rats [23]. Increasing dopamine transmission as a result of drug use raises the question of abuse liability of these drugs; compulsion to re-dose has been reported in user forums [24].
Users on drug user forums have reported various effects. 5F-AKB-48 used as an SCRA is usually smoked; however, other modes of use have included vapourisation within e-cigarettes, insufflation, formulation as a tea, eyeballing and being used as an ingredient in baked goods [24]. Dose ranges have varied from 1 to 5 mg up to 300 mg over 3 days in experienced users [25]. The onset of effect is reported between 2 and 15 min when smoked and can last 1–2 h. Positive effects include euphoria and an intense state of relaxation felt both mentally and physically (‘body-load’). Negative effects include palpitations, paranoia, intense anxiety and an unpalatable taste in the mouth like ‘burned plastic’ No visual/auditory hallucinations have been reported [26].
5F-PB-22
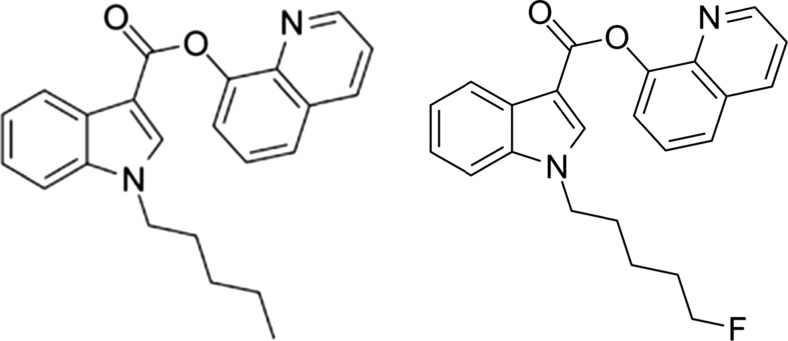
PB-22, 5F-PB-22, JWH-018 and AM2201 belong to a structural class of substances sharing a core indole structure which is substituted at 1 and 3-positions with various functional groups to give rise to these substances. PB-22 (1-pentyl-8-quinolinyl ester-1H-indole-3-carboxylic acid) is a relatively new synthetic cannabinoid, 5F PB-22 shares PB-22 as a base with a 5-fluoropentyl group tail linking chain as shown in Fig. 2.
Fig. 2.
PB-22 and 5F- PB-22
The halogenation, especially fluorination of the established SCRAs for example, the 5-fluoropentyl group to PB- 22 (and also AKB-48), is thought to represent attempts by manufacturers to increase their potency [27]. This was seen in a recent in vitro study in mouse AtT-20 neuroblastoma cells stably transfected with human CB1 or human CB2; PB-22, CB1 EC50 = 5.1 nM; CB2 EC50 = 37 nM compared with twice the relative potency shown with 5F-PB-22 (CB1 EC50 = 2.8 nM; CB2 EC50 = 11 nM). In an in vivo study male Wistar rats PB-22/5F-PB-22 (3 mg/kg i.p.) both decreased body temperature (mean > 1.5 °C) and heart rate measured up to 6 hrs post injection in a dose-dependent fashion [28].
Male ND4 Swiss-Webster mice (n = 8, 320-350 g) were studied for the presence of discriminative stimulus effects. Delta9–THC and six cannabinoid compounds UR-144, XLR-11, AKB-48, PB-22, and AB-FUBINACA and 5F-PB-22 were used. Injections of 1 ml/kg i.p. of vehicle (ethanol/Cremophor EL/0.9 % saline 1:1:18) versus 5F-PB-22 (0.5 mg/kg; ED50 0.039 ± 0.21 mg/kg) fully substituted for the discriminative stimulus effects of Δ9-THC (3 mg/kg) between 30 to 60 min after administration. However, rates of responding were decreased at 5, 15, 30, and 60 min after 5F-PB-22 [F(5,25)=3.87 P < 0.01], with marked suppression at 15 min after administration, such that four of six rats did not earn a food pellet making the results overall difficult to interpret [29]. Delta9-THC ED50 values on mice drug discrimination were comparatively higher at 0.85 ± 0.12 mg/kg and locomotor activity at 11.14 ± 0.10 mg/kg. Locomotor studies determined the average horizontal activity counts in 10-minute bins as a function of time and dose of each compound; 5F-PB-22 produced depressant effects at 0.5 and 1 mg/kg within 10 minutes after injection and effects lasted 110 minutes (ED50 0.25 ± 0.05 mg/kg). However, a two-way analysis of variance conducted on horizontal activity counts/10-minute did not indicate a significant effect of treatment [F (4,35) = 1.79, P = 0.153] [29].
There are few detailed experiences on drug user forums with 5F-PB-22. Positive effects included a heady euphoria. Negative effects reported are nausea, vomiting, confusion, poor coordination, anxiety, as well as, accounts of seizures. There were no reports of auditory/visual hallucinations. Users opted from 0.5–4 mg doses usually by smoking. Interestingly, some users reported strong compulsions to re-dose and withdrawal symptoms (headache, nausea, vomiting) with persistent cravings to re-use persisting a week after cessation [26].
Trecki et al. reported on clusters of SCRAs resulting in severe toxicity and death in the United States. There were two documented cases of death associated with 5F-PB-22 in July (Iowa) and October 2013 (Nebraska). No further details of these cases were discussed [30]. Delayed onset-seizures (4 h after the product was last smoked) were reported in a 23-year-old male. 5F-PB-22 concentrations were detected on separate plasma samples: 85 pg/ml at 5.5 h and 91 pg/ml at 8.3 h [31]. However, other SCRAs were detected including PB-22 which has been associated with seizures [32], as well as AM2233 [33], BB-22 and JWH-122 [34].
Behonick et al. described four fatalities associated with the analytical detection of 5F PB-22 [35]. The first case was a 17-year-old male with a past history of poly-recreational drug use who had acute dyspnoea progressing to collapse and died despite resuscitative efforts. 5F-PB-22 (1.1 ng/mL), ethanol (0.033 g/dL), amiodarone (administered during resuscitative efforts) and caffeine (concentration not provided) were detected on a post-mortem femoral blood sample. Death was attributed to 5F-PB-22 intoxication. The second case was a 27-year-old male who presented to an ED with a several day history of worsening anorexia, fever, vomiting and diffuse abdominal pain and went on to develop multi-organ failure and an eventual PEA arrest. He had been using paracetamol, two tablets, twice daily for 2–3 days prior to admission and smoking marijuana. The paracetamol concentration was 6.8 mcg/mL and he was treated with n-acetylcysteine. A pair of hospital serum specimens obtained a day before death (9.5 h apart) indicated the presence of Carboxy-THC at concentrations of 246 and 176 ng/mL. A third serum specimen (7 h before death) detected 5F-PB-22 (1.3 ng/mL). The autopsy revealed 80–90 % hepatocellular necrosis, acute tubular necrosis and acute respiratory distress syndrome. The cause of death was listed as “fulminant liver failure in the setting of THC (marijuana) and 5F-PB-22 (synthetic cannabinoid) exposure”. Other drugs detected included piperacillin, levofloxacin and lorazepam which were presumably related to treatment efforts. The third case was an 18-year-old male declared dead pre-hospital. He was noted to have numerous mixed alcoholic beverages and to be smoking synthetic marijuana (K2/Spice) the night before. At autopsy, bilateral pulmonary and abdominal organ (liver, spleen and kidneys) vasocongestion were reported. An iliac post-mortem blood sample detected 5F-PB-22 (1.5 ng/mL); no other drugs were detected. The final case was a 19-year -old male who was discovered 2 days after a party. The main autopsy findings were bilateral pulmonary oedema, necrotizing granulomatous inflammation with histoplasma microorganisms and congestion of viscera; 5F-PB-22 (1.5 ng/mL) was detected in a superior vena cava blood sample. The stated cause of death was suspected acute drug intoxication using the synthetic cannabinoid 5F-PB-22. No other drugs were detected.
Conclusion
We describe a case of significant toxicity associated with analytically confirmed 5F-AKB-48 and 5F-PB-22 use. Associated symptoms/signs resolved within 13 hours and the patient did not suffer any long-term sequelae. Pre-clinical studies available indicate potent CB1 agonism and potential dopaminergic effects as a likely mechanism for these effects. It is important that clinical toxicologists characterise the pattern of toxicity associated with these SCRA to inform legislative decisions as well as harm minimisation efforts.
Compliance with Ethical Standards
Conflict of Interest
Authors Rachelle Abouchedid, James H. Ho, Simon Hudson, Alison Dines, John RH. Archer, David M. Wood and Paul I. Dargan declare that they have no conflict of interest.
Professor Dargan reports that he is a member of the UK Advisory Council on the Misuse of Drugs and the Scientific Committee of the European Monitoring Centre for Drugs and Drug Addiction.
Sources of Funding
None
Informed Consent
Consent for publication of this case was obtained and provided to the journal in accordance with JMT policy.
References
- 1.EMCDDA. European Drug Report (Trends and Developments). 2015. http://www.emcdda.europa.eu/edr2015. Accessed: 15 Nov 2015.
- 2.Mechoulam R, Gaoni Y. A total synthesis of D1-delta-1-tetrahydrocannabinol, the active constituent of hashish. J Am Chem Soc. 1965;87:3273–3275. doi: 10.1021/ja01092a065. [DOI] [PubMed] [Google Scholar]
- 3.Wagner JA, Jarai Z, Batkai S, Kunos G. Hemodynamic effects of cannabinoids: coronary and cerebral vasodilation mediated by cannabinoid CB1 receptors. Eur J Pharmacol. 2001;423:203–210. doi: 10.1016/S0014-2999(01)01112-8. [DOI] [PubMed] [Google Scholar]
- 4.Calignano A, Katona I, Desarnaud F, Giuffrida A, La Rana G, Mackie K, Freund TF, Piomelli D. Bidirectional control of airway responsiveness by endogenous cannabinoids. Nature. 2000;408:96–101. doi: 10.1038/35040576. [DOI] [PubMed] [Google Scholar]
- 5.De Petrocellis L, Melck D, Bisogno T, Di Marzo V. Endocannabinoids and fatty acid amides in cancer, inflammation and related disorders. Chem Phys Lipids. 2000;108:191–209. doi: 10.1016/S0009-3084(00)00196-1. [DOI] [PubMed] [Google Scholar]
- 6.Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science. 2002;296:678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- 7.Tomida I, Pertwee RG, Azuara-Blanco A. Cannabinoids and glaucoma. Br J Ophthalmol. 2004;88:708–713. doi: 10.1136/bjo.2003.032250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maccarrone M. CB2 receptors in reproduction. Br J Pharmacol. 2008;153:189–198. doi: 10.1038/sj.bjp.0707444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Foll B, Goldberg SR. Cannabinoid CB1 receptor antagonists as promising new medications for drug dependence. JPET. 2005;312:875–883. doi: 10.1124/jpet.104.077974. [DOI] [PubMed] [Google Scholar]
- 10.Cota D, Marsicano G, Lutz B, Vicennati V, Stalla GK, Pasquali R, Pagotto U. Endogenous cannabinoid system as a modulator of food intake. Int J Obes. 2003;27:289–301. doi: 10.1038/sj.ijo.0802250. [DOI] [PubMed] [Google Scholar]
- 11.Janero DR, Makriyannis A. Cannabinoid receptor antagonists: pharmacological opportunities, clinical experience, and translational prognosis. Expert Opin Emerg Drugs. 2009;14:43–65. doi: 10.1517/14728210902736568. [DOI] [PubMed] [Google Scholar]
- 12.Devane WA, Dysarz FA, 3rd, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34:605–613. [PubMed] [Google Scholar]
- 13.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 14.Everett RM, Descotes G, Rollin M, Greener Y, Bradford JC, Benziger DP, Ward SJ. Nephrotoxicity of pravadoline maleate (WIN 48098-6) in dogs: evidence of maleic acid-induced acute tubular necrosis. Fundam Appl Toxicol. 1993;21:59–65. doi: 10.1006/faat.1993.1072. [DOI] [PubMed] [Google Scholar]
- 15.Wiley JL, Marusich JA, Huffman JW, Balster RL, Thomas BF. Hijacking of basic research: the case of synthetic cannabinoids. Methods Rep RTI Press. 2011;2011:17971. doi: 10.3768/rtipress.2011.op.0007.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.EMCDDA. Perspectives on drugs. Synthetic cannabinoids in Europe. 2015. http://www.emcdda.europa.eu/attachements.cfm/att_212361_EN_Synthetic%20cannabinoids_updated2015.pdf. Accessed 22 Dec 2015.
- 17.Karila L, Roux P, Rolland B, Benyamina A, Reynaud M, Aubin HJ, Lançon C. Acute and long-term effects of cannabis use: a review. Curr Pharm Des. 2014;20:4112–4118. doi: 10.2174/13816128113199990620. [DOI] [PubMed] [Google Scholar]
- 18.Schneir AB, Cullen J, Ly BT. “Spice” girls: synthetic cannabinoid intoxication. J Emerg Med. 2011;40:296–299. doi: 10.1016/j.jemermed.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Tait RJ, Caldicott D, Mountain D, Hill SL, Lenton S. A systematic review of adverse events arising from the use of synthetic cannabinoids and their associated treatment. Clin Toxicol (Phila) 2015;15:1–13. doi: 10.3109/15563650.2015.1110590. [DOI] [PubMed] [Google Scholar]
- 20.Lovett C, Wood DM, Dargan PI. Pharmacology and toxicology of the synthetic cannabinoid receptor agonists. Réanimation. 2015;24:527–541. doi: 10.1007/s13546-015-1104-4. [DOI] [Google Scholar]
- 21.EMCDDA–Europol joint publication. EMCDDA–Europol 2014 Annual Report on the implementation of Council Decision 2005/387/JHA. 2014. http://www.emcdda.europa.eu/attachements.cfm/att_240380_EN_TDAN15001ENN.pdf. Accessed 22 Nov 2015.
- 22.United Nations Office on Drugs and Crime. Global synthetic drugs assessment amphetamine-type stimulants and new psychoactive substances. 2014. https://www.unodc.org/documents/scientific/2014_Global_Synthetic_Drugs_Assessment_web.pdf. Accessed 22 Dec 2015.
- 23.De Luca MA, Castelli MP, Loi B, Porcu A, Martorelli M, Miliano C, Kellett K, Davidson C, Stair LJ, Schifano F, Di Chiara G. Native CB1 receptor affinity, intrinsic activity and accumbens shell dopamine stimulant properties of third generation SPICE/K2 cannabinoids: BB-22, 5F-PB-22, 5F-AKB-48 and STS-135. Neuropharmacology. 2015;11. [DOI] [PubMed]
- 24.BlueLight. AKB-48-F Experience report. http://www.bluelight.org/vb/threads/647964-AKB-48-F-Experience-report. Accessed 15 Nov 2015.
- 25.BlueLight. 627774-Cannabinoid-AKB-48. http://www.bluelight.org/vb/threads/627774-Cannabinoid-AKB-48. Accessed 15 Nov 2015.
- 26.Drugs-Forum. Experiences - 5F-AKB48, BB-22, 5F-PB22, STS-135 Last Gen Noids comparison from experience. https://drugs-forum.com/forum/showthread.php?t=226101. Accessed 15 Nov 2015.
- 27.Gurney SMR, Scott KS, Kacinko SL, Presley BC, Logan BK. Pharmacology, toxicology, and adverse effects of synthetic cannabinoid drugs. Forensic Sci Rev. 2014;26:53–78. [PubMed] [Google Scholar]
- 28.Banister SD, Stuart J, Kevin RC, Edington A, Longworth M, Wilkinson SM, Beinat C, Buchanan AS, Hibbs DE, Glass M, Connor M, McGregor IS, Kassiou M. Effects of Bioisosteric fluorine in synthetic cannabinoid designer drugs JWH-018, AM-2201, UR-144, XLR-11, PB-22, 5F-PB-22, APICA, and STS-135. ACS Chem Neurosci. 2015;6:1445–1458. doi: 10.1021/acschemneuro.5b00107. [DOI] [PubMed] [Google Scholar]
- 29.Gatch MB, Forster MJ. Δ9-tetrahydrocannabinol-like effects of novel synthetic cannabinoids found on the gray market. Behav Pharmacol. 2015;26:460–468. doi: 10.1097/FBP.0000000000000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trecki J, Gerona RR, Schwartz MD. Synthetic cannabinoid–related illnesses and deaths. (perspective) N Engl J Med. 2015;373:2. doi: 10.1056/NEJMp1505328. [DOI] [PubMed] [Google Scholar]
- 31.Schep LJ, Slaughter RJ, Hudson S, Place R, Watts M. Delayed seizure-like activity following analytically confirmed use of previously unreported synthetic cannabinoid analogues. Hum Exp Toxicol. 2015;34:557–560. doi: 10.1177/0960327114550886. [DOI] [PubMed] [Google Scholar]
- 32.Gugelmann H, Gerona R, Li C, Tsutaoka B, Olson KR, Lung D. ‘Crazy monkey’ poisons man and dog: human and canine seizures due to PB-22, a novel synthetic cannabinoid. Clin Toxicol (Phila) 2014;52:635–638. doi: 10.3109/15563650.2014.925562. [DOI] [PubMed] [Google Scholar]
- 33.Hussain F, Al-musawi H, Al-khateeb E, Abu sayf A. Ischemic cerebrovasular accident, uncontrolled seizuresand acute myocardial infarction associated with syn thetic marijuana abuse. Am J Intern Med. 2014;2:138–114. [Google Scholar]
- 34.Hermanns-Clausen M, Kneisel S, Szabo B, Auwärter V. Acute toxicity due to the confirmed consumption of synthetic cannabinoids: clinical and laboratory findings. Addiction. 2013;108:534–544. doi: 10.1111/j.1360-0443.2012.04078.x. [DOI] [PubMed] [Google Scholar]
- 35.Behonick G, Shanks KG, Firchau DJ, Mathur G, Lynch CF, Nashelsky M, Jaskierny DJ, Meroueh C. Four postmortem case reports with quantitative detection of the synthetic cannabinoid, 5F-PB-22. J Anal Toxicol. 2014;38:559–562. doi: 10.1093/jat/bku048. [DOI] [PMC free article] [PubMed] [Google Scholar]