SUMMARY
Although all young children nap, the neurophysiological features and associated developmental trajectories of daytime sleep remain largely unknown. Longitudinal studies of napping physiology are fundamental to understanding sleep regulation during early childhood, a sensitive period in brain and behaviour development and a time when children transition from a biphasic to a monophasic sleep–wakefulness pattern. We investigated daytime sleep in eight healthy children with sleep electroencephalography (EEG) assessments at three longitudinal points: 2 years (2.5–3.0 years), 3 years (3.5–4.0 years) and 5 years (5.5–6.0 years). At each age, we measured nap EEG during three randomized conditions: after 4 h (morning nap), 7 h (afternoon nap) and 10 h (evening nap) duration of prior wakefulness. Developmental changes in sleep were most prevalent in the afternoon nap (e.g. decrease in sleep duration by 30 min from 2 to 3 years and by 20 min from 3 to 5 years). In contrast, nap sleep architecture (% of sleep stages) remained unchanged across age. Maturational changes in non-rapid eye movement sleep EEG power were pronounced in the slow wave activity (SWA, 0.75–4.5 Hz), theta (4.75–7.75 Hz) and sigma (10–15 Hz) frequency ranges. These findings indicate that the primary marker of sleep depth, SWA, is less apparent in daytime naps as children mature. Moreover, our fundamental data provide insight into associations between sleep regulation and functional modifications in the central nervous system during early childhood.
Keywords: brain development, EEG power spectra, napping, sleep electroencephalography, sleep homeostasis, slow wave activity
INTRODUCTION
Regardless of cultural context, young children fulfil part of their 24-h sleep need by napping during the day (Crosby et al., 2005). A major developmental milestone is the consolidation from a biphasic to a monophasic sleep–wakefulness pattern: at age 2 years nearly all children nap, between 3 and 4 years of age napping declines and by age 5 years daytime naps are scarce (Weissbluth, 1995). In addition, naps become shorter in duration and occur later in the day with increasing age (Dales, 1941). This decrease in napping frequency and duration is the main factor accounting for the reported decline in total 24-h sleep duration from toddlerhood throughout the kindergarten years (Crosby et al., 2005; Iglowstein et al., 2003; Kahn et al., 1973). Although observational research has led to a richer understanding of napping behaviour, the neurophysiological features of naps and how they change across early development are largely unknown.
Examining trajectories of napping brain physiology is fundamental for understanding sleep regulation across early childhood (Jenni and Lebourgeois, 2006). In adolescents and adults, extending the duration of wakefulness systematically increases sleep need (Dijk et al., 1987; Jenni et al., 2005), as measured by non-rapid eye movement (NREM) sleep slow wave activity (SWA, 0.75–4.5 Hz) (Achermann and Borbely, 2003). Relatedly, napping dissipates SWA in subsequent night-time sleep (Werth et al., 1996). Furthermore, observational data suggest that afternoon nap sleep onset latency increases with age (Dales, 1941). Only a handful of studies have examined daytime and night-time sleep electroencephalography (EEG) in early life (Kahn et al., 1973; Louis et al., 1997), none of which varied the duration of prior wakefulness systematically by assessing EEG sleep physiology at different times of the day.
In this study, we investigated experimentally the sleep EEG during morning, afternoon and evening naps in a longitudinal cohort of children at ages 2, 3 and 5 years. It has been proposed that the homeostatic build-up of sleep need across the day attenuates across early childhood (Jenni and LeBourgeois, 2006). We thus hypothesized a decline in the primary markers of sleep need (i.e. SWA) with increasing age. Relatedly, we expected changes in secondary markers of sleep need that are related to lighter sleep and lower sleep pressure, including a decrease in nap time in bed and sleep duration and an increase in sleep onset latency and slow wave sleep (SWS) latency across ages 2–5 years.
MATERIALS AND METHODS
Participants
Eight healthy children (five females) were assessed at three longitudinal time-points: ages 2.5–3.0 years (2 Y, n = 8), 3.5–4.0 years (3 Y, n = 8) and 5.5–6.0 years (5 Y, n = 7, one subject did not participate). Inclusion required that children followed a biphasic sleep schedule (night-time sleep opportunity of ≥10.5 h at night and nap opportunity of ≥45 min) for which they fell asleep at least 3 days per week during their nap opportunity at age 2 Y. Generally, children were good sleepers in excellent health; exclusion criteria are described in detail in our previous publications (Berger et al., 2012; Kurth et al., 2013). Families signed a consent form approved by the Brown University institutional review board, and the study was performed according to the Declaration of Helsinki.
Experimental design
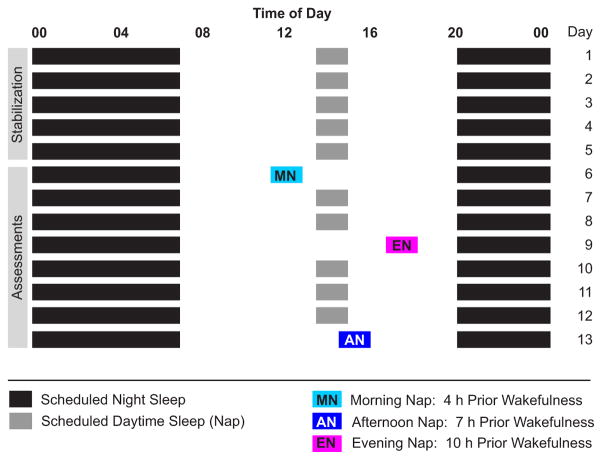
At all ages, children’s nap and night-time sleep schedules were stabilized ≥5 days before each sleep EEG assessment (Fig. 1). This strict bedtime schedule included a minimum 24-h sleep opportunity of 12.5 h, including a daily 45-min nap opportunity (2 and 3 Y) or a minimum 24-h sleep opportunity of 12 h without a nap opportunity (5 Y). Falling asleep during daytime naps throughout the stabilization period was not a requirement for performing subsequent EEG assessments. During the entire study, children slept in their typical environment (home, daycare, family care). Except for the 3 days of nap assessments children adhered to the sleep schedule, which was verified with actigraphy, sleep diaries and daily contact with parents (telephone or e-mail). No caffeine or a deviation of >15 min from the sleep schedule was allowed during the 5 days preceding EEG assessments. At 2 and 3 Y, daytime sleep EEG recordings were performed after 4 h (morning nap; MN), 7 h (afternoon nap; AN) and 10 h (evening nap; EN) of wakefulness (Fig. 1). We also performed AN and EN assessments at age 5 Y, when participants were no longer napping habitually. Two children gave up regular naps by age 3 Y, and all children were not napping at age 5 Y. All EEG recordings occurred in the families’ homes (children did not attend daycare or school on assessment days), where researchers attached electrodes and monitored the subjects continuously with infrared cameras. Parents performed habitual naptime settling routines before each assessment. At least two nights (3.6 nights on average) of regularly scheduled daytime and night-time sleep elapsed between the randomly ordered nap recordings. Children slept until they awakened spontaneously, except for the EN; in this case, children who were still asleep after 60 min or who completed the first full sleep cycle (NREM/rapid eye movement, REM sleep) were awakened due to parental concerns that long daytime sleep would disturb subsequent night-time sleep (two recordings at 2 Y; 1 at 3 Y; 2 at 5 Y).
Figure 1.
Experimental design: an example protocol for a child with a 20:00–07:00 nighttime sleep schedule and a habitual 13:00 daytime nap opportunity (≥12.5 h total time in bed). Experimental nap assessments with varying prior wakefulness time [morning nap (MN) at 11:00, afternoon nap (AN) at 14:00 evening nap (EN) at 17:00] were ordered randomly and occurred on non-consecutive days.
At least three in-home training sessions were performed to introduce parents and children gradually to the study procedures. These visits included explanation of actigraphy use, completion of sleep diaries and acclimatization to electrodes. After each training session children were rewarded with ‘play time’ with researchers and small gifts. For further electrode acclimatization, parents placed two electrodes at different head locations each day prior to naptime during the week before the first EEG recording.
Sleep EEG
Standard sleep EEG recordings were obtained (C3, C4, O1, O2, A1, A2) using a portable Vitaport 3 EEG recorder (Temec Instruments, Kerkrade, the Netherlands). EEG was scored visually in 30-s epochs (C3A2), in agreement with standard criteria (Rechtschaffen and Kales, 1968). Power density spectra of consecutive 30-s epochs [fast-Fourier transform (FFT), Tukey window (r = 0.50, average of 10 4-s epochs overlapping by 1 s; VitaScore, Temec Instruments] were computed for the derivation C3A2. Spectra up to 20 Hz were analysed with a frequency resolution of 0.25 Hz. EEG artefacts were removed semi-automatically: epochs were excluded when power in the 20–40 Hz and SWA band exceeded a threshold based on a moving average determined over 20 30-s epochs. We excluded frequency bins up to 1.25 Hz in all assessments of one child due to sweating artefacts. Because of sensitivity to low-frequency artefacts, 0.25–0.5 Hz were excluded for the comparison of power density spectra.
Data analysis
Analyses were performed with MATLAB (R2011a; MathWorks, Natick, MA, USA). Developmental changes were examined with two separate approaches: first, all children fell asleep during nap opportunities at 2 Y and 3 Y, thus, we compared sleep variables between the first two time-points with paired t-tests) for the MN, AN (n = 8) and EN (n = 7; one recording lost at 2 Y due to technical failure). Secondly, repeated-measures analyses of variance (ANOVA) for the AN and EN were performed including data at ages 2 Y, 3 Y and 5 Y (n = 5; only five of seven children fell asleep during the AN and the EN opportunity at 5 Y). We also utilized repeated-measures ANOVA or paired t-tests to examine prior wakefulness (nap condition: MN, AN, EN) effects on sleep variables. The alpha level was 0.05. Finally, we investigated developmental changes and nap condition differences in EEG power density spectra using the maximal common duration of artefact-free NREM sleep across children, nap conditions and ages (i.e. first 27 min after sleep onset). Bootstrap methods were used to assess the statistical significance in NREM sleep EEG power density spectra across development and nap conditions. This conservative statistic uses a random sample (reshuffling) from the original data pool and controls the false alarm rate (Maris and Oostenveld, 2007). At each 0.25-Hz frequency bin, tests were performed (two-tailed; P < 0.05, dftotal = 14–23) by first generating a random sample 5000 times from the original data pool, and then comparing the resulting distributions in each nap condition and at each age (for details see Maris and Oostenveld, 2007). The same approach was used to compare SWA (EEG power in the 0.75–4.5 Hz range) across nap conditions. Sleep variables are presented as means and standard deviations (mean ± SD), and effect sizes are reported as Cohen’s d or eta-squared, η2.
RESULTS
Descriptive statistics of sleep variables for all naps at each age are presented in Table 1.
Table 1.
Descriptive statistics [mean ± standard deviation (SD)] of sleep electroencephalograph (EEG) variables during morning (MN), afternoon (AN) and evening naps (EN) at ages 2 years (2 Y), 3 years (3 Y) and 5 years (5 Y; no evening nap opportunity provided)
| Morning nap
|
Afternoon nap
|
Evening nap
|
||||||
|---|---|---|---|---|---|---|---|---|
| 2 Y | 3 Y | 2 Y | 3 Y | 5 Y | 2 Y | 3 Y | 5 Y | |
| Children falling asleep (%) | 8/8 (100%) | 8/8 (100%) | 7/8 (88%) | 8/8 (100%) | 5/7 (71%) | 8/8 (100%) | 8/8 (100%) | 5/7 (71%) |
| Time in bed (min) | 116.9 ± 34.2 | 104.3 ± 17.9 | 120.6 ± 30.9 | 90.3 ± 18.4 | 76.0 ± 29.8 | 88.5 ± 21.3 | 75.6 ± 11.0 | 79.2 ± 13.1 |
| Sleep duration (min) | 90.0 ± 37.7 | 68.8 ± 20.0 | 105.2 ± 33.6 | 73.6 ± 21.2 | 63.9 ± 30.9 | 73.9 ± 18.2 | 57.3 ± 8.5 | 64.6 ± 9.6 |
| Sleep latency (min) | 24.3 ± 11.3 | 33.0 ± 14.4 | 13.7 ± 10.2 | 15.1 ± 8.0 | 11.0 ± 4.0 | 13.3 ± 11.5 | 17.0 ± 13.2 | 14.1 ± 8.6 |
| SWS latency (min) | 13.1 ± 3.4 | 14.1 ± 3.7 | 11.6 ± 3.3 | 11.6 ± 5.8 | 13.6 ± 4.1 | 9.2 ± 4.1 | 8.1 ± 2.8 | 7.7 ± 1.4 |
| Stage 2 (min) | 42.7 ± 22.2 | 30.8 ± 16.3 | 40.3 ± 18.8 | 25.9 ± 14.0 | 24.1 ± 9.3 | 24.2 ± 11.7 | 14.0 ± 7.2 | 17.1 ± 9.8 |
| Stage 2 (%) | 45.9 ± 8.9 | 43.4 ± 14.9 | 38.2 ± 12.6 | 34.6 ± 17.8 | 41.8 ± 16.7 | 32.4 ± 13.2 | 25.6 ± 14.9 | 25.4 ± 10.1 |
| SWS (min) | 31.9 ± 9.1 | 25.9 ± 11.6 | 47.1 ± 17.7 | 36.9 ± 14.5 | 25.0 ± 17.3 | 41.5 ± 9.4 | 36.9 ± 11.8 | 42.6 ± 9.5 |
| SWS (%) | 39.7 ± 15.0 | 40.2 ± 21.4 | 47.0 ± 18.0 | 50.0 ± 13.3 | 37.6 ± 19.5 | 57.9 ± 16.4 | 63.6 ± 14.2 | 67.0 ± 17.2 |
SWS: slow wave sleep; percentage values were calculated relative to total sleep time.
Nap duration and architecture: developmental aspects
Table 2 shows developmental changes (2 Y, 3 Y) in sleep variables for each nap. For the MN, we found no changes in any sleep measures from 2 Y to 3 Y. Moderate-to-large changes were observed in the AN: children spent less time in bed (30 min) and had shorter sleep durations (32 min) as they matured. Additionally, the time children spent in Stage 2 and SWS decreased between 2 and 3 Y (16 min for Stage 2; 10 min for SWS); however, when taking into account nap duration by analysing relative (%) instead of absolute (min) values, no developmental changes in NREM sleep architecture remained. In the EN, sleep duration decreased (by 17 min), but this finding may depend upon the fact that three individual children did not awaken spontaneously from their naps.
Table 2.
Developmental changes in sleep variables [age 2 years (2 Y) versus 3 years (3 Y); n = 8] for morning (MN), afternoon (AN) and evening naps (EN); significant differences are indicated in bold type
| Nap | Developmental changes (2 Y, 3 Y) Paired t-tests (n = 8) |
|||
|---|---|---|---|---|
| t | d | P | ||
| Time in bed (min) | MN | 0.94 | 0.46 | 0.190 |
| AN | 3.64 | 1.19 | 0.004 | |
| EN | 1.66 | 0.76 | 0.074 | |
| Sleep duration (min) | MN | 1.88 | 0.70 | 0.051 |
| AN | 3.60 | 1.13 | 0.004 | |
| EN† | 3.07 | 1.17 | 0.011 | |
| Sleep latency (min) | MN | −1.39 | −0.68 | 0.103 |
| AN | −0.48 | −0.16 | 0.321 | |
| EN | −0.66 | −0.30 | 0.268 | |
| SWS latency (min) | MN | −1.58 | −0.28 | 0.079 |
| AN | 0.00 | 0.00 | 0.500 | |
| EN | 0.99 | 0.33 | 0.179 | |
| Stage 2 (min) | MN | 1.19 | 0.61 | 0.136 |
| AN | 1.96 | 0.87 | 0.045 | |
| EN | 1.81 | 1.06 | 0.060 | |
| Stage 2 (%)* | MN | 0.36 | 0.20 | 0.726 |
| AN | 0.66 | 0.24 | 0.528 | |
| EN | 0.78 | 0.48 | 0.467 | |
| SWS (min) | MN | 1.20 | 0.57 | 0.134 |
| AN | 2.08 | 0.63 | 0.038 | |
| EN | 0.81 | 0.43 | 0.225 | |
| SWS (%)* | MN | −0.06 | −0.03 | 0.955 |
| AN | −0.43 | −0.19 | 0.679 | |
| EN | −0.66 | −0.37 | 0.535 | |
SWS: slow wave sleep; percentage values were calculated relative to total sleep time.
two-tailed t-test.
consider result with caution because some children were awakened from their evening nap (see Methods).
Results from one-way repeated-measures ANOVAs testing developmental changes (2 Y, 3 Y, 5 Y) in sleep variables for all naps are presented in Table 3. For the AN, time in bed and sleep duration decreased by ~43 min across ages 2, 3 and 5 Y. Moreover, children spent fewer minutes in SWS (22 min) during the AN as they matured; however, no changes occurred in relative (%) data. In the EN, Stage 2 (% as well as min) increased as children aged. The number of children exhibiting REM sleep at 2 and 3 Y ranged from 4 to 7 (Table 4). By 5 Y, four children showed REM sleep in the AN; only one child showed REM sleep in the EN. Overall, although naps shortened across development, sleep architecture (% of sleep stages) remained largely preserved.
Table 3.
Developmental changes and nap timing differences in sleep variables. One-way repeated-measures (RM) analyses of variance (ANOVAs) (significance indicated in bold type) performed for the afternoon (AN) and evening nap (EN) at ages 2 years (2 Y), 3 years (3 Y) and 5 years (5 Y) with only subjects with complete data (i.e. fell asleep during all nap conditions at all ages; n = 5). Nap timing differences were examined between all naps at 2 Y (n = 7) and 3Y (n = 8)
| Developmental changes (2 Y, 3 Y, 5 Y)
|
Nap condition differences (MN, AN, EN)
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Afternoon nap (n = 5)
|
Evening nap (n = 5)
|
2 Y (n = 7)
|
3 Y (n = 8)
|
|||||||||||||
| F | η2 | P | PH | F | η2 | P | PH | F | η2 | P | PH | F | η2 | P | PH | |
| Time in bed (min) | 8.1 | 0.30 | 0.012 | 2–3 Y 3–5 Y 2–5 Y |
1.0 | 0.20 | 0.423 | – | 5.0 | 0.26 | 0.027 | AN–EN | 5.8 | 0.41 | 0.018 | MN–EN |
| Sleep duration (min) | 7.1 | 0.30 | 0.017 | 2–3 Y 3–5 Y |
1.8 | 0.30 | 0.247 | – | 5.2 | 0.23 | 0.024 | AN–EN | 2.4 | 0.18 | 0.131 | – |
| Sleep latency (min) | 0.7 | 0.04 | 0.532 | – | 0.1 | 0.02 | 0.940 | – | 5.2 | 0.19 | 0.024 | MN–AN AN–EN |
8.9 | 0.31 | 0.004 | MN–AN MN–EN |
| SWS latency (min) | 0.5 | 0.04 | 0.631 | – | 1.6 | 0.20 | 0.273 | – | 3.4 | 0.16 | 0.066 | – | 7.1 | 0.24 | 0.009 | MN–EN |
| Stage 2 (min) | 3.8 | 0.40 | 0.069 | 3–5 Y | 5.5 | 0.48 | 0.044 | NS | 5.2 | 0.18 | 0.023 | AN–EN | 4.9 | 0.30 | 0.028 | AN–EN MN–EN |
| Stage 2 (%) | 0.1 | 0.01 | 0.913 | – | 7.5 | 0.30 | 0.023 | 2–5 Y | 3.1 | 0.19 | 0.080 | – | 7.6 | 0.27 | 0.007 | MN–EN |
| SWS (min) | 9.9 | 0.17 | 0.007 | 3–5 Y 2–5 Y |
0.4 | 0.05 | 0.705 | – | 3.6 | 0.27 | 0.060 | – | 5.1 | 0.26 | 0.025 | NS |
| SWS (%) | 0.3 | 0.05 | 0.776 | – | 1.1 | 0.19 | 0.405 | – | 4.0 | 0.17 | 0.048 | AN–EN | 7.8 | 0.39 | 0.007 | MN–EN AN–EN |
SWS, slow wave sleep; NS, not significant.
Significant post-hoc (PH) paired t-tests denoted by condition.
Table 4.
Proportion of children with rapid eye-movement (REM) sleep in daytime naps and REM sleep variables [mean ± standard deviation (SD)] during morning, afternoon and evening naps at ages 2 years (2 Y), 3 years (3 Y) and 5 years (5 Y).
| Morning nap
|
Afternoon nap
|
Evening nap
|
||||||
|---|---|---|---|---|---|---|---|---|
| 2 Y | 3 Y | 2 Y | 3 Y | 5 Y | 2 Y | 3 Y | 5 Y | |
| Children with REM sleep (%) | 6/8 (75%) | 7/8 (88%) | 6/8 (75%) | 6/8 (75%) | 4/5 (80%) | 4/7 (57%) | 5/8 (63%) | 1/5 (20%) |
| REM sleep latency (min) | 39.3 ± 4.8 | 40.4 ± 11.7 | 49.7 ± 22.6 | 49.9 ± 9.9 | 39.4 ± 17.3 | 53.5 ± 6.2 | 45.5 ± 16.5 | 29.0 |
| REM sleep duration (min) | 19.2 ± 8.9 | 11.9 ± 7.9 | 21.4 ± 11.6 | 11.8 ± 5.5 | 14.5 ± 14.4 | 13.6 ± 11.3 | 7.9 ± 5.8 | 19.0 |
| REM sleep (%) | 17.9 ± 7.0 | 16.3 ± 8.0 | 17.4 ± 8.1 | 17.7 ± 9.5 | 19.5 ± 12.5 | 16.1 ± 9.3 | 13.1 ± 9.0 | 29.9 |
Nap condition differences in sleep variables
At both 2 and 3 Y, the timing of daytime naps (MN, AN, EN) produced differences in a number of sleep variables (Table 3). For example, sleep onset latency was ~15 min longer for the MN than the AN or EN. Also, children exhibited greater SWS% in the EN than the MN or AN. At 5 Y, we observed no nap timing effects (AN versus EN) on sleep variables (all Ps > 0.05).
EEG power density spectra and SWA
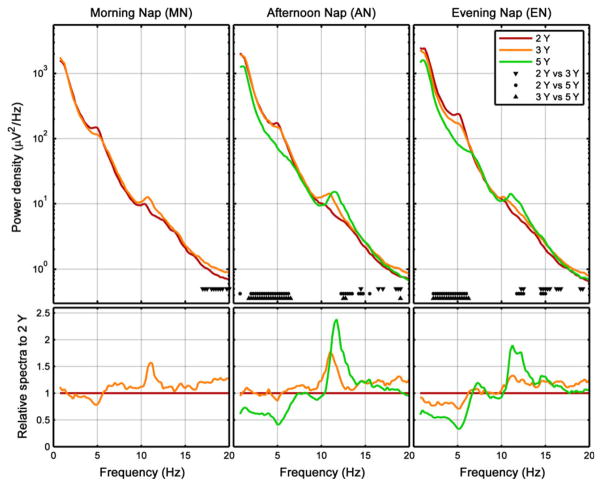
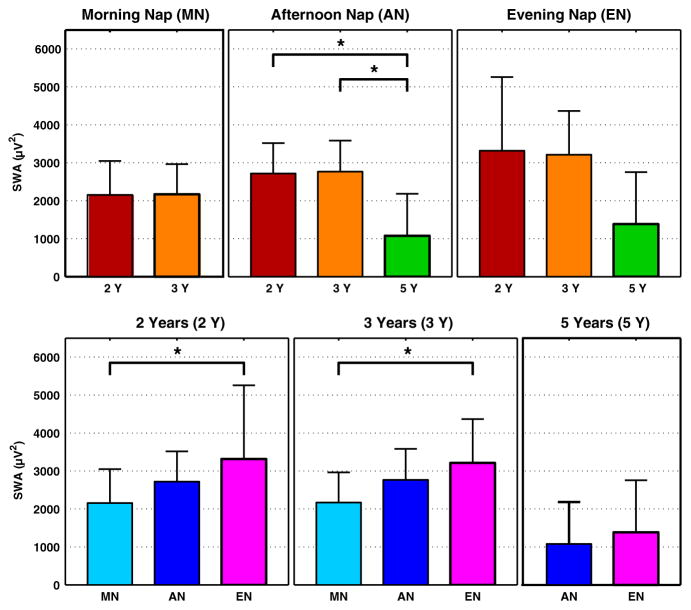
In the MN, EEG power density was increased in the beta frequency range (>16 Hz) at 3 Y compared to 2 Y (Fig. 2, left). In the AN, SWA (0.75–4.5 Hz) and theta power (4.75–7.75 Hz) were higher at 2 Y versus 5 Y and at 3 Y versus 5 Y, but did not differ between 2 and 3 Y (Fig. 2, middle; Fig. 4, top row). Furthermore, power in the sigma frequency band (10–15 Hz) was significantly higher at 5 Y compared to both 2 and 3 Y. Such developmental changes in EEG power density and SWA in the EN were comparable to those in the AN (similar frequency ranges; Fig. 2, right; Fig. 4, top row).
Figure 2.
Developmental conditions: average power density spectra (first 27 min of artefact-free epochs of non-rapid eye movement (NREM) sleep, which is the common maximal duration across recordings and subjects) for absolute (top row) and relative data (bottom row, calculated relative to 2 years). Markers at bottom of subplots refer to significant differences between age groups (2, 3, 5 years).
Figure 4.
Average slow wave activity [SWA; mean ± standard deviation (SD) first 27 min of artefact-free epochs of non-rapid eye movement (NREM) sleep] for nap conditions (top row) and developmental stage (bottom row). *indicates significant differences between age groups (2, 3, 5 years; top row) and nap conditions [morning nap (MN), afternoon nap (AN), evening nap (EN); bottom row].
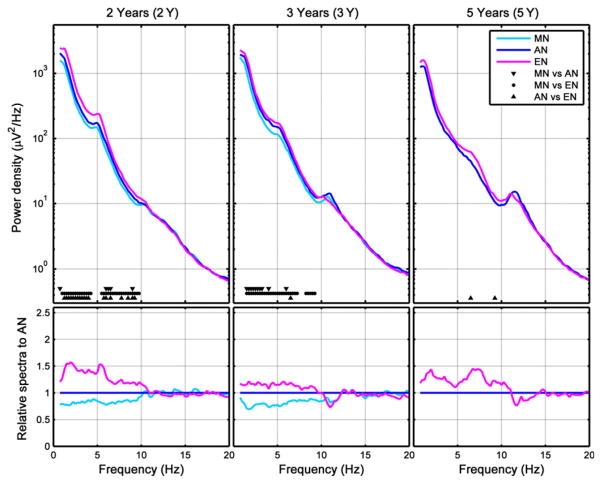
As a final step, we compared EEG power density between nap conditions at each age. At 2 Y, SWA and theta power increased across the day (Fig. 3, left; Fig. 4, bottom row), corresponding with the gradual increase in the duration of prior wakefulness. Because relative data were expressed proportionate to AN, SWA and theta power were below 1 for MN (Fig. 3, left and middle). A similar pattern was observed at 3 Y, except that the differences between the AN and EN dissipated (Fig. 3, middle). At 5 Y, EEG power and SWA differences disappeared entirely (Fig. 3, right; Fig. 4, bottom row). In sum, as children aged, daytime naps contained less SWA and theta activity. Most differences between nap EEG conditions were found at the age of 2 Y.
Figure 3.
Nap conditions: average power density spectra [first 27 min of artefact-free epochs of non-rapid eye movement (NREM) sleep] for absolute (top row) and relative data (bottom row, calculated relative to AN). Markers at bottom of subplots refer to significant differences between nap conditions (morning nap MN; afternoon nap AN; evening nap EN).
DISCUSSION
This study examined developmental changes in napping neurophysiology across ages 2–5 years by utilizing an experimental protocol to gradually increase sleep need across the waking day. Overall, advancing age and nap timing were associated with alterations in sleep duration and EEG physiology, while sleep architecture remained largely preserved. Our results suggest that as children mature, they spend less time in deep sleep and exhibit fewer differences in SWA when naps are taken at different times of the day. We propose that the daytime sleep EEG (i) reflects the maturation of the central nervous system, (ii) mirrors neurophysiological changes associated with the increasing tolerance of long wakefulness bouts and (iii) provides a tool to understand children’s behavioural regulation in the context of sleep duration and timing.
Sleep regulation changes between infancy (Bes et al., 1991; Jenni et al., 2004) and adolescence (Campbell and Feinberg, 2009; Jenni et al., 2005). Extended wakefulness in newborns does not trigger the typical homeostatic response of amplified SWA but instead increases sleep duration and consolidation (Alfoldi et al., 1990; Anders and Roffwarg, 1973). During the first 2 years of life, age-related changes in sleep physiology are more prominent in naps compared to nocturnal sleep (e.g. increases in waking; decreases in sleep duration, NREM sleep and SWS) (Louis et al., 1997). Here, we found not only a developmental decrease in markers of deep sleep (SWS%) and sleep latency, but also a similar SWA response to increased prior wakefulness with advancing age. Interestingly, such nap timing differences in sleep were pronounced at 2 and 3 Y but diminished by 5 Y. These findings imply that the homeostatic build-up of sleep need across the day attenuates across early childhood (Jenni and Lebourgeois, 2006). These results may also provide a physiological explanation for the natural decline in napping as well as the diminished behavioural consequences of extending wakefulness in older compared to younger preschool children.
In adults, power in SWA and theta frequencies is a monotonic function of the duration of prior wakefulness (Dijk et al., 1987). We found no developmental differences in SWA and theta power between 2 and 3 Y in the MN, suggesting that the neural response to low sleep need is similar in 2- and 3-year-olds. This is supported further by the observation of no developmental change in sleep latency in the MN. Because MN and AN power spectra did not differ largely at 2 Y, our data may indicate that napping in the morning or afternoon is important to maintain vigilance and functioning during the toddler years. In line with this, the AN—the most ‘naturalistic’ timing of daytime sleep at the ages considered—may indeed offer the most functional benefits, which are discussed in recent studies of restricted daytime sleep in habitually napping preschoolers (Berger et al., 2012; Kurdziel et al., 2013; Miller et al., 2014). Finally, the effects of increasing time awake on spectral power was most evident at 2 and 3 Y and was limited to slow wave and theta frequencies. Based upon animal data, these findings imply a decrease in the extent of neural recruitment as children mature (Vyazovskiy et al., 2007). When neural circuits are mature, the homeostatic response to systematically extended wakefulness does not elicit a different response, e.g. comparing younger with older adults (Campbell and Feinberg, 2005).
Our objective findings build upon those from parent reports showing a decline in nap duration across childhood (Iglowstein et al., 2003; Weissbluth, 1995). The shortening of naps is ubiquitous and evolutionarily significant, as it is consistent with the increasing consolidation of sleep and wake bouts that exist across a variety of mammalian species (e.g. Meier and Berger, 1965). Our data also extend current knowledge by showing that although naps shorten across the preschool years, daytime sleep architecture remains largely unchanged. These observations provide further evidence of maturation of the homeostatic system, such that extending waking may be moderated through the alteration of sleep depth rather than sleep architecture, i.e. percentage of sleep stages. Contrary to our hypothesis that was based upon an early observational study with preschool children (Dales, 1941), we did not find a developmental increase in napping sleep onset latency, which is consistent with electrophysiological data across infancy and toddlerhood (Louis et al., 1997). Interestingly, two participants did not fall asleep during the 60-min AN and EN nap opportunities at age 5 Y. Thus, in order to include these children in our analysis of a developmental change in sleep onset latency, we utilized a strategy that is a standard in the field for multiple sleep latency test data (Taylor et al., 2005). By setting sleep onset latency to the duration of the nap opportunity (i.e. 60 min), we observed a non-significant trend towards an increase in sleep latency with advancing age in the AN (14.4 ± 10.7 at 2 Y; 15.3 ± 8.7 at 3 Y; 25.0 ± 24.1 at 5 Y, P = 0.08).
Our data imply that naps in preschool children consist of approximately half the amount of Stage 2 sleep (%) and almost twice as much SWS (%) compared to the daytime naps of adults (Dijk et al., 1987; Werth et al., 1996). Further, we observed a developmental increase in sigma power. Although sigma power is related to sleep spindle bursts (Dijk, 1995), it does not distinguish between background EEG activity and phasic spindle activity. Spindles originate from the thalamic nuclei and are synchronized by cortico-cortical and thalamo-cortical loops (Kandel and Buzsaki, 1997). Similar to the sigma EEG signature in nocturnal sleep (Doucette et al., 2015), the maturation of sigma power and other spindle characteristics across the preschool years may reflect the increasing integrity of thalamo-cortical networks and development of circuits associated with sleep-dependent memory formation (Diekelmann and Born, 2010) and may provide learning benefits, as observed in midday naps (Kurdziel et al., 2013). However, developmental changes in the functional benefits of daytime sleep and their associated physiological features need further investigation, particularly as children’s nighttime sleep may promote greater functional gains compared to adults (Wilhelm et al., 2013).
Although we achieved medium-to-large effect sizes in this relatively small sample, research on larger community samples and those experiencing inadequate sleep is warranted to understand further the role of sleep in neurodevelopment. The generalizability of our results needs to be considered with caution because we enrolled only healthy, regularly napping children; however, our strict study criteria ensured control of known confounds that affect sleep regulation. All participants were provided with nap opportunities at strictly scheduled times of the day and at ages when children were napping or had given up their regular naps. This kind of challenging protocol is crucial to examine fundamentally the early development of sleep regulation using EEG. Further, although we were not able to make statistical comparisons about REM sleep in our cohort, our descriptive data are important for researchers who study napping and cognitive, emotional and social functioning. Also, due to our small sample size, we did not utilize corrections or a statistical model to test interactions between developmental stage and nap timing on sleep and spectral EEG measures. We believe that this is an important research direction for further understanding of the early development of sleep regulation. Finally, the circadian effect of slow frequency power in daytime naps is negligible in adults (Dijk et al., 1987), but we cannot exclude the possibility that circadian factors contributed to our findings in children, especially when considering the recently reported cross-talk between homeostatic and circadian factors affecting clock-gene expression (Curie et al., 2013) or markers of neuronal plasticity (Lazar et al., 2015). Relatedly, an important future question is whether nap timing is associated with distinct functional benefits, as shown in adults (Lovato and Lack, 2010). Considering that SWA reflects neurodevelopment (Kurth et al., 2010), we posit that the natural dropping of naps in young children arises from a reduction of sleep pressure, which is coordinated through brain network development (Lebourgeois et al., 2012). The current investigation provides rich objective data for understanding children’s neurophysiological trajectories of naps and for gaining insight into sleep regulation development.
Acknowledgments
The authors declare no competing financial interests. This work was supported by the National Institute of Mental Health (K01-MH74643, R01-MH086566 to MKL) and the Swiss National Science Foundation (320030-130766 and 32003B-146643 to PA, PBZHP3-138801 and PBZHP3-147180 to SK). We thank our research staff, laboratory technicians, students and participants.
Footnotes
AUTHOR CONTRIBUTIONS
ML, PA and OJ designed the research; ML performed the research; SK, LP, PA, TR and IM analysed the data; SK, JL, ML and PA wrote the paper.
CONFLICT OF INTEREST
None.
References
- Achermann P, Borbely AA. Mathematical models of sleep regulation. Front Biosci. 2003;8:s683–s693. doi: 10.2741/1064. [DOI] [PubMed] [Google Scholar]
- Alfoldi P, Tobler I, Borbely AA. Sleep regulation in rats during early development. Am J Physiol. 1990;258:R634–R644. doi: 10.1152/ajpregu.1990.258.3.R634. [DOI] [PubMed] [Google Scholar]
- Anders TF, Roffwarg HP. The effects of selective interruption and deprivation of sleep in the human newborn. Dev Psychobiol. 1973;6:77–89. doi: 10.1002/dev.420060110. [DOI] [PubMed] [Google Scholar]
- Berger RH, Miller AL, Seifer R, Cares SR, Lebourgeois MK. Acute sleep restriction effects on emotion responses in 30- to 36-month-old children. J Sleep Res. 2012;21:235–246. doi: 10.1111/j.1365-2869.2011.00962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bes F, Schulz H, Navelet Y, Salzarulo P. The distribution of slow-wave sleep across the night: a comparison for infants, children, and adults. Sleep. 1991;14:5–12. doi: 10.1093/sleep/14.1.5. [DOI] [PubMed] [Google Scholar]
- Campbell IG, Feinberg I. Homeostatic sleep response to naps is similar in normal elderly and young adults. Neurobiol Aging. 2005;26:135–144. doi: 10.1016/j.neurobiolaging.2004.02.021. [DOI] [PubMed] [Google Scholar]
- Campbell IG, Feinberg I. Longitudinal trajectories of non-rapid eye movement delta and theta EEG as indicators of adolescent brain maturation. Proc Natl Acad Sci USA. 2009;106:5177–5180. doi: 10.1073/pnas.0812947106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby B, Lebourgeois MK, Harsh J. Racial differences in reported napping and nocturnal sleep in 2- to 8-year-old children. Pediatrics. 2005;115:225–232. doi: 10.1542/peds.2004-0815D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curie T, Mongrain V, Dorsaz S, Mang GM, Emmenegger Y, Franken P. Homeostatic and circadian contribution to EEG and molecular state variables of sleep regulation. Sleep. 2013;36:311–323. doi: 10.5665/sleep.2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dales RJ. Afternoon sleep in a group of nursery-school children. Pedagog Semin J Gen. 1941;58:161–180. [Google Scholar]
- Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- Dijk DJ. EEG slow waves and sleep spindles: windows on the sleeping brain. Behav Brain Res. 1995;69:109–116. doi: 10.1016/0166-4328(95)00007-g. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Beersma DG, Daan S. EEG power density during nap sleep: reflection of an hourglass measuring the duration of prior wakefulness. J Biolog Rhythms. 1987;2:207–219. doi: 10.1177/074873048700200304. [DOI] [PubMed] [Google Scholar]
- Doucette MR, Kurth S, Chevalier N, Munakata Y, Lebourgeois MK. Topography of slow sigma power during sleep is associated with processing speed in preschool children. Brain Sci. 2015;5:494–508. doi: 10.3390/brainsci5040494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglowstein I, Jenni OG, Molinari L, Largo RH. Sleep duration from infancy to adolescence: reference values and generational trends. Pediatrics. 2003;111:302–307. doi: 10.1542/peds.111.2.302. [DOI] [PubMed] [Google Scholar]
- Jenni OG, Lebourgeois MK. Understanding sleep–wake behavior and sleep disorders in children: the value of a model. Curr Opin Psychiatry. 2006;19:282–287. doi: 10.1097/01.yco.0000218599.32969.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenni OG, Borbely AA, Achermann P. Development of the nocturnal sleep electroencephalogram in human infants. Am J Physiol Regul Integr Comp Physiol. 2004;286:R528–R538. doi: 10.1152/ajpregu.00503.2003. [DOI] [PubMed] [Google Scholar]
- Jenni OG, Achermann P, Carskadon MA. Homeostatic sleep regulation in adolescents. Sleep. 2005;28:1446–1454. doi: 10.1093/sleep/28.11.1446. [DOI] [PubMed] [Google Scholar]
- Kahn E, Fisher C, Edwards A, Davis DM. 24-Hour sleep patterns. A comparison between 2- to 3-year-old and 4- to 6-year-old children. Arch Gen Psychiatry. 1973;29:380–385. doi: 10.1001/archpsyc.1973.04200030068010. [DOI] [PubMed] [Google Scholar]
- Kandel A, Buzsaki G. Cellular-synaptic generation of sleep spindles, spike-and-wave discharges, and evoked thalamocortical responses in the neocortex of the rat. J Neurosci. 1997;17:6783–6797. doi: 10.1523/JNEUROSCI.17-17-06783.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurdziel L, Duclos K, Spencer RM. Sleep spindles in midday naps enhance learning in preschool children. Proc Natl Acad Sci USA. 2013;110:17267–17272. doi: 10.1073/pnas.1306418110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth S, Ringli M, Geiger A, Lebourgeois M, Jenni OG, Huber R. Mapping of cortical activity in the first two decades of life: a high-density sleep electroencephalogram study. J Neurosci. 2010;30:13211–13219. doi: 10.1523/JNEUROSCI.2532-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth S, Achermann P, Rusterholz T, Lebourgeois MK. Development of brain EEG connectivity across early childhood: does sleep play a role? Brain Sci. 2013;3:1445–1460. doi: 10.3390/brainsci3041445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar AS, Lazar ZI, Dijk DJ. Circadian regulation of slow waves in human sleep: topographical aspects. NeuroImage. 2015;116:123–134. doi: 10.1016/j.neuroimage.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBourgeois MK, Rusterholz T, Jenni OG, Carskadon MA, Achermann P. Do the dynamics of sleep homeostasis change across early childhood? Sleep. 2012;35:A21. [Google Scholar]
- Louis J, Cannard C, Bastuji H, Challamel MJ. Sleep ontogenesis revisited: a longitudinal 24-hour home polygraphic study on 15 normal infants during the first two years of life. Sleep. 1997;20:323–233. doi: 10.1093/sleep/20.5.323. [DOI] [PubMed] [Google Scholar]
- Lovato N, Lack L. The effects of napping on cognitive functioning. Prog Brain Res. 2010;185:155–166. doi: 10.1016/B978-0-444-53702-7.00009-9. [DOI] [PubMed] [Google Scholar]
- Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 2007;164:177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Meier GW, Berger RJ. Development of sleep and wakefulness patterns in the infant rhesus monkey. Exp Neurol. 1965;12:257–277. doi: 10.1016/0014-4886(65)90071-3. [DOI] [PubMed] [Google Scholar]
- Miller AL, Seifer R, Crossin R, Lebourgeois MK. Toddler’s self-regulation strategies in a challenge context are nap-dependent. J Sleep Res. 2015;24:279–87. doi: 10.1111/jsr.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. US Public Health Service, US Government Printing Office; Washington DC: 1968. [Google Scholar]
- Taylor DJ, Jenni OG, Acebo C, Carskadon MA. Sleep tendency during extended wakefulness: insights into adolescent sleep regulation and behavior. J Sleep Res. 2005;14:239–244. doi: 10.1111/j.1365-2869.2005.00467.x. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy VV, Riedner BA, Cirelli C, Tononi G. Sleep homeostasis and cortical synchronization: II. A local field potential study of sleep slow waves in the rat. Sleep. 2007;30:1631–1642. doi: 10.1093/sleep/30.12.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissbluth M. Naps in children: 6 months–7 years. Sleep. 1995;18:82–87. doi: 10.1093/sleep/18.2.82. [DOI] [PubMed] [Google Scholar]
- Werth E, Dijk DJ, Achermann P, Borbely AA. Dynamics of the sleep EEG after an early evening nap: experimental data and simulations. Am J Physiol. 1996;271:R501–R510. doi: 10.1152/ajpregu.1996.271.3.R501. [DOI] [PubMed] [Google Scholar]
- Wilhelm I, Rose M, Imhof KI, Rasch B, Buchel C, Born J. The sleeping child outplays the adult’s capacity to convert implicit into explicit knowledge. Nat Neurosci. 2013;16:391–393. doi: 10.1038/nn.3343. [DOI] [PubMed] [Google Scholar]