Abstract
Purpose
We investigated the visual tracer distribution pattern and serial changes in uptake ratio in different anatomical zones during the natural postoperative course in order to establish a reference for evaluation of patients with complications.
Methods
A total of 36 patients without symptoms after hip or knee arthroplasty were grouped according to the interval between surgery and the scan. The serial changes in SUVmean in each periprosthetic zone were quantified using the volume of interest isocontour method. Images were classified according to the uptake distribution pattern. The uptake ratios in the postoperative period groups were then compared using the Kruskal-Wallis test. The correlation between uptake ratio and postoperative period was then determined.
Results
Tracer distribution patterns in hip prostheses were classified into three types and the patterns in knee prostheses into five types. In hip prostheses, intense osteoblastic activity was observed during 3–6 months and then declined in most patients, but showed a slight increase over 15–25 months in 5–10 % of patients. The correlation coefficients varied among the zones. Significant differences in uptake ratios among the period groups was found for all zones, except zone 8. Porous coated areas showed higher uptake than uncoated areas only for the period the 3–6 months. In knee prostheses, uptake ratios showed a curvilinear pattern, increasing from 3–6 to 8–15 months and declining later. The uptake ratios were different among the period groups. Every zone showed a positive correlation from 3–6 to 8–15 months, and negative correlations from 8–15 to 22–25 months.
Conclusions
This is the first 18F-sodium fluoride PET/CT study investigating the stability of implants and sets a reference for evaluation of patients with complications.
Keywords: Arthroplasty, Positron emission tomography, Postoperative, Prosthesis, Sodium fluoride
Introduction
Artificial joint replacement surgery (AJRS) is the orthopedic procedure of choice for the treatment of the degenerative, arthritic, or injured joints [1]. However, many patients complain of recurring pain after AJRS due to complications, such as infections or loosening [2]. Bone scintigraphy has been used for the diagnosis of complications after AJRS. However, all abnormal areas of uptake around the prosthesis should not be interpreted as complications. As the porous coating of the prosthesis allows ingrowth of bone and fibrous tissue into the prosthesis itself, normal postoperative changes can result in an altered scintigraphic appearance unrelated to a complication. In order to discriminate normal postoperative activity from complications, the natural scintigraphic pattern related to time after AJRS in asymptomatic patients should be investigated.
Several previous studies have investigated normal postoperative patterns on 99mTc-methylene diphosphonate (MDP) bone scans [3–6]. Kim et al. evaluated the degree of uptake within the five periprosthetic zones of a hip joint on sequential bone scans using a visual grading system. In that study, the radioactivity tended to regress according to the time since implantation, but in 31 % of patients, persistent elevations of radioactivity within the greater trochanter, the tip of the prosthesis, and the lesser trochanter were observed for up to 12 months after AJRS, indicating that the regression rate varied depending on the periprosthetic zone [3]. However, conventional planar bone scans are of limited value for assessing postoperative changes in three-dimensional volumetric structures, and the measurement of quantitative parameters, such as the standardized uptake value (SUV), is difficult.
The recently developed 18F-sodium fluoride (NaF) bone PET/CT is a more accurate diagnostic tool for specific bone disorders with the potential to replace conventional 99mTc-MDP studies. Most of the NaF transported to the bone is retained only after a single pass of blood, making it a suitable radiopharmaceutical for the assessment of subtle changes in bone turnover in the bone–prosthesis interface. However, by using a quantification parameter, such as SUV, more objective assessments can be performed with this method than with a conventional bone scan. In addition, 18F-NaF PET/CT studies are more convenient for patients because the scanning time is less than with conventional 99mTc-MDP studies.
Since the first report of the use of AJRS, there has been no prospective study of the use of 18F-NaF PET/CT to demonstrate the natural post-operative findings in bone. To our knowledge, this study is the first that has investigated the utility of 18F-NaF PET/CT bone studies in the evaluation of the natural postoperative course in asymptomatic patients following implantation of a prosthesis.
We used 18F-NaF PET/CT to investigate the serial physiological changes with time after AJRS in asymptomatic patients in order to establish a reference for evaluation of patients with complications.
Materials and Methods
Patient Population
A total of 36 patients (44 prostheses) without symptoms suggesting complications after AJRS of the hip (15 patients, 20 prostheses) or knee (21 patients, 24 prostheses) were enrolled in this study. Patients received an uncemented porous coated hip arthroplasty prosthesis or a cemented knee arthroplasty prosthesis between March 2013 and April 2015. The procedures were performed by two experienced orthopedic surgeons. The patients were grouped according to the interval between surgery and the scan: for the hip procedures, the groups were 3–6 months (seven prostheses), 7–22 months (nine prostheses), and more than 24 months (four prostheses; Table 1), and for the knee procedures, the groups were (3–6 months (four prostheses), 8–15 months (14 prostheses), and 22–25 months (six prostheses; Table 2). During the postoperative period, patients were evaluated during regular follow-ups and regarded as having no clinical evidence of complications according to the following criteria: (1) negative tip culture in the 3-day postoperative study, (2) no subjective symptoms reported by the patient, (3) no radiolucent line on the 3-month postoperative plain radiograph, and (4) erythrocyte sedimentation rate and C-reactive protein within the normal ranges in the 3-month postoperative blood tests. The clinical design of this retrospective study was approved by the ethics committee of the Dong-A Medical Center (DAUHIRB-16-061), with waiver of the need for informed consent.
Table 1.
Characteristics of patients and prostheses in the hip prosthesis group
| Number | Sex/age (years) | Time (months) | Type of prosthesis | Use of cement |
|---|---|---|---|---|
| 1 | F/70 | 3.60 | VerSys taper hip stem; Zimmer, USA | No |
| 2 | F/78 | 3.60 | Bencox hip system; Corentec, Korea | No |
| 3 | M/76 | 4.77 | Bencox hip system; Corentec, Korea | No |
| 4 | M/43 | 6.07 | Bencox hip system; Corentec, Korea | No |
| 5 | M/48 | 6.30 | Bencox hip system; Corentec, Korea | No |
| 6 | M/50 | 6.30 | Bencox hip system; Corentec, Korea | No |
| 7 | F/70 | 7.33 | Bencox hip system; Corentec, Korea | No |
| 8 | F/54 | 11.07 | Bencox hip system; Corentec, Korea | No |
| 9 | F/78 | 12.23 | EcoFit stem; Implantcast, Germany | No |
| 10 | F/52 | 16.10 | Bencox hip system; Corentec, Korea | No |
| 11 | F/70 | 21.43 | Bencox hip system; Corentec, Korea | No |
| 12 | F/30 | 22.00 | Bencox hip system; Corentec, Korea | No |
| 13 | M/62 | 24.97 | Bencox hip system; Corentec, Korea | No |
| 14 | M/31 | 25.57 | Metha short hip stem system; B. Braun-Aesculap, Germany | No |
| 15 | M/57 | 28.77 | Bencox hip system; Corentec, Korea | No |
Table 2.
Characteristics of patients and prostheses in the knee prosthesis group
| Number | Sex/age (years) | Time (months) | Type of prosthesis | Use of cement |
|---|---|---|---|---|
| 1 | F/68 | 3.17 | Scorpio NRG knee system; Stryker, USA | Yes |
| 2 | F/61 | 3.17 | Attune knee system; DePuy, USA | Yes |
| 3 | F/80 | 3.87 | Scorpio NRG knee system; Stryker, USA | Yes |
| 4 | F/69 | 4.33 | Genesis II; Smith and Nephew, Switzerland | Yes |
| 5 | F/75 | 6.67 | e.motion total knee system, B. Braun-Aesculap, Germany | Yes |
| 6 | F/78 | 7.13 | Scorpio NRG knee system; Stryker, USA | Yes |
| 7 | F/64 | 8.30 | Scorpio NRG knee system; Stryker, USA | Yes |
| 8 | F/82 | 8.43 | Scorpio NRG knee system; Stryker, USA | Yes |
| 9 | M/55 | 10.63 | Scorpio NRG knee system; Stryker, USA | Yes |
| 10 | F/74 | 11.93 | Scorpio NRG knee system; Stryker, USA | Yes |
| 11 | F/61 | 12.27 | Scorpio NRG knee system; Stryker, USA | Yes |
| 12 | F/75 | 12.60 | Scorpio NRG knee system; Stryker, USA | Yes |
| 13 | F/70 | 12.73 | Scorpio NRG knee system; Stryker, USA | Yes |
| 14 | F/76 | 13.90 | Genesis II; Smith and Nephew, Switzerland | Yes |
| 15 | M/73 | 14.30 | e.motion total knee system; B. Braun-Aesculap, Germany | Yes |
| 16 | F/67 | 14.37 | Scorpio NRG knee system; Stryker, USA | Yes |
| 17 | M/46 | 14.60 | e.motion total knee system; B. Braun-Aesculap, Germany | Yes |
| 18 | F/71 | 22.30 | e.motion total knee system; B. Braun-Aesculap, Germany | Yes |
| 19 | M/74 | 24.17 | Scorpio NRG knee system; Stryker, USA | Yes |
| 20 | F/72 | 24.63 | Scorpio NRG knee system; Stryker, USA | Yes |
| 21 | F/70 | 24.77 | e.motion total knee system; B. Braun-Aesculap, Germany | Yes |
18F-Sodium Fluoride PET Imaging
18F-NaF PET/CT was performed using a Biograph mCT flow PET/CT camera (Siemens), which provided an in-plane spatial resolution of 2.0 mm full-width at half-maximum at the center of the field of view. Patients were given an intravenous injection of 0.2 mCi 18F-NaF per kg. Static whole-body bone phase scanning was performed for 30 min at around 60 min after injection of radiotracer in flow mode (0.7 mm/s for the prosthesis area, 1.5 mm/s for other areas) in three-dimensional mode after a CT scan with a slice thickness of 5 mm, a pitch of 1.2, a rotation time of 0.5 s and at 120 kVp and 80 mA. The PET images were reconstructed using point spread of function (True X) and time of flight (200 × 200 matrix, two iterations, and 21 subsets).
Image Interpretation
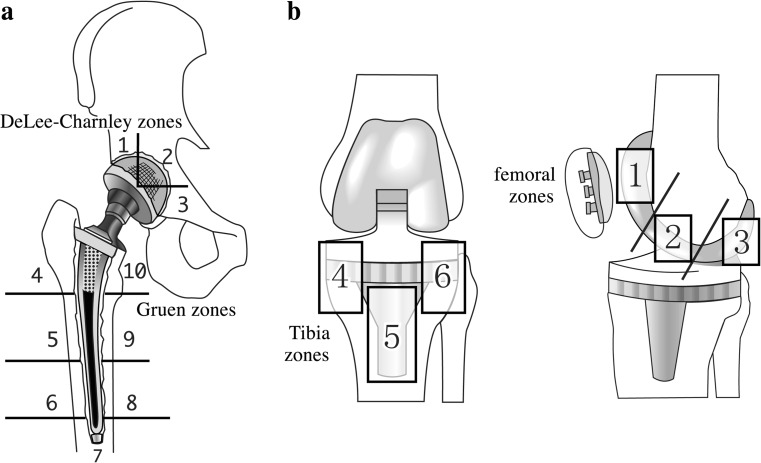
First, the PET images were evaluated visually by experienced nuclear medicine physicians and the uptake patterns classified. Second, the serial changes in SUVmean were analyzed quantitatively based on the volume of interest (VOI) automatic isocontour method. All image processing and measurements were performed using PMOD version 3.7.0. Ten regions of interest were demarcated on the hip prostheses: three acetabular zones, called DeLee and Charnley zones [7], and seven femoral prosthetic zones, called Gruen zones [8] (Fig. 1). The Gruen zones were divided into hydroxyapatite-coated areas (zones 4 and 10) and noncoated areas (zones 5–9) [5]. Six regions of interest were demarcated on the knee prostheses: three femoral zones (zones 1–3) on the sagittal view and three tibial zones (zones 4–6) on the coronal view.
Fig. 1.
a Hip prosthesis. Zones 1–3 in the acetabular component correspond to DeLee-Charnley zones. Zones 4–10 in the femoral component correspond to Gruen zones. b Knee prosthesis. Zones 1–3 are located in the femoral component (on the sagittal view) and zones 4–6 are located in the tibial component (on the coronal view)
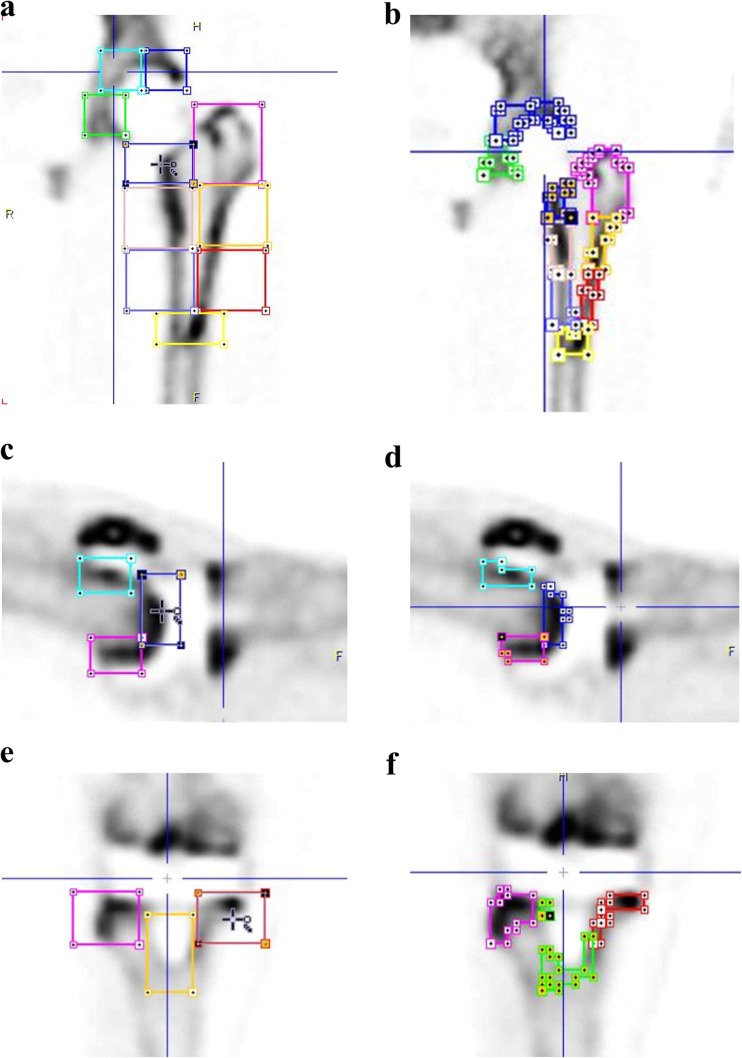
All imaging data were processed with PMOD software (version 7.0; PMOD Technologies Ltd, Zurich, Switzerland). For each image, the CT scans were used for gross localization of organs and placement of the VOI for any given region. A three-dimensional cuboid was systematically generated for each zone using PMOD by specifying a length with x, y, and z axis directions (Fig. 2a).
Fig. 2.
a, c, e Three-dimensional cuboid VOI placement for each zone. In the hip prosthesis, the acetabular VOI in zones 1–3 is 25 × 30 × 25 mm, the femoral VOI in zones 5, 6, 8 and 9 is 37 × 37 × 37 mm; in zone 4 is 37 × 37 × 55 mm; and in zones 7 and 10 is 37 × 37 × 20 mm. In the knee prosthesis, the tibial VOI in zones 4 and 6 is 30 × 30 × 30 mm, and in zone 5 is 27 × 27 × 40 mm. b, d, f. The isocontours to place the VOIs around the structures are automatically detected by setting a threshold of 30 % of the difference between the maximum SUV and minimum SUV
In the hip prostheses, the acetabular VOI in zones 1–3 was 25 × 30 × 25 mm, the femoral VOI in zones 5, 6, 8 and 9 was 37 × 37 × 37 mm, in zone 4 was 37 × 37 × 55 mm, and in zones 7 and 10 was 37 × 37 × 20 mm. In the knee prostheses, the tibial VOI in zones 4 and 6 was 30 × 30 × 30 mm, and in zone 5 was 27 × 27 × 40 mm. Areas affected by metal artifact were carefully avoided. Automatic isocontour detection was then used to refit the VOI around the structures by setting a threshold of a 30 % difference between the maximum SUV and minimum SUV (Fig. 2b). For each contour, the SUVmean was measured.
All of the image processing and quantitative analyses were done by one investigator who was unaware of the interval between surgery and the scan. For reference, the contralateral femur was used in patients receiving hip surgery, or the tibial shaft in patients receiving knee cases, because of their low interindividual variation. The uptake ratio was then calculated by dividing the SUVmean of the periprosthetic zone by the SUV mean of the contralateral distal femur or tibia for hip and knee prostheses, respectively.
Statistics
Numerical data are presented using descriptive statistics expressing values as means ± standard deviation. First, the uptake ratios were compared among the time period groups using the Kruskal-Wallis test with post hoc analysis with the null hypothesis being no difference among the groups. Second, the correlation between uptake ratio and time period was analyzed. A p value less than 0.05 was considered significant.
Results
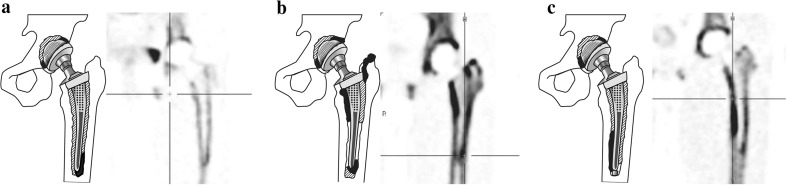
First, the qualitative visual evaluation of tracer distribution was analyzed. Increased 18F-NaF uptake was observed on both attenuation-corrected and uncorrected images. The uptake patterns on the PET images were classified into various types. The classification of the uptake patterns in hip prostheses is shown in Fig. 3 and the frequencies are shown in Table 3.
Fig. 3.
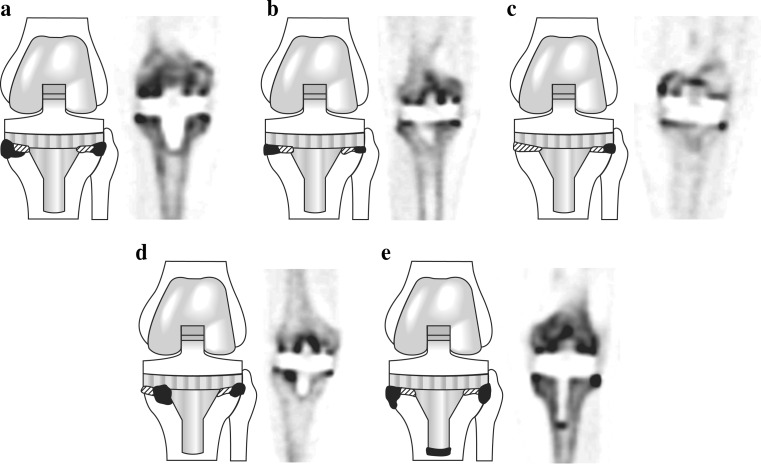
Diagrams and representative PET images showing the three types of uptake pattern in hip prostheses (a type 1, b type 2,. c type 3). Black areas are areas of severely increased uptake and shaded areas are areas of mildly increased uptake
Table 3.
Frequency of each visual classification pattern
| Type 1 | Type 2 | Type 3 | Type 4 | Type 5 | |
|---|---|---|---|---|---|
| Hip | 15 % | 65 % | 20 % | – | – |
| Knee (femoral) | 20.8 % | 37.5 % | 12.5 % | 12.5 % | 20.8 % |
| Knee (tibia) | 37.5 % | 25 % | 25 % | 4.1 % | 8.3 % |
In hip prostheses, a type 1 pattern was mild, homogeneous uptake in all femoral areas (zones 4–10), and focal intense uptake in the femoral tip (zone 7; Fig. 3a). A type 2 pattern was mild, homogeneous uptake in all femoral areas (zones 4–10), but especially intense, localized uptake in the proximal, medial and femoral areas (zones 9 and 10), and focal, intense uptake in the proximal lateral femoral and tip areas (zones 4 and 7; Fig. 3b). A type 3 pattern was mild, homogeneous uptake in all femoral areas (zones 4–10), but intense, localized uptake in the distal, medial femoral areas (zone 8; Fig. 3c). Of the 20 hip prostheses, 3 (15 %) showed a type 1 pattern, 13 (65 %) showed a type 2 pattern, and 4 (20 %) showed a type 3 pattern. Acetabular areas showed mild diffuse uptake in zones 1–3, but focal uptake in zone 1 or 3 in all patients.
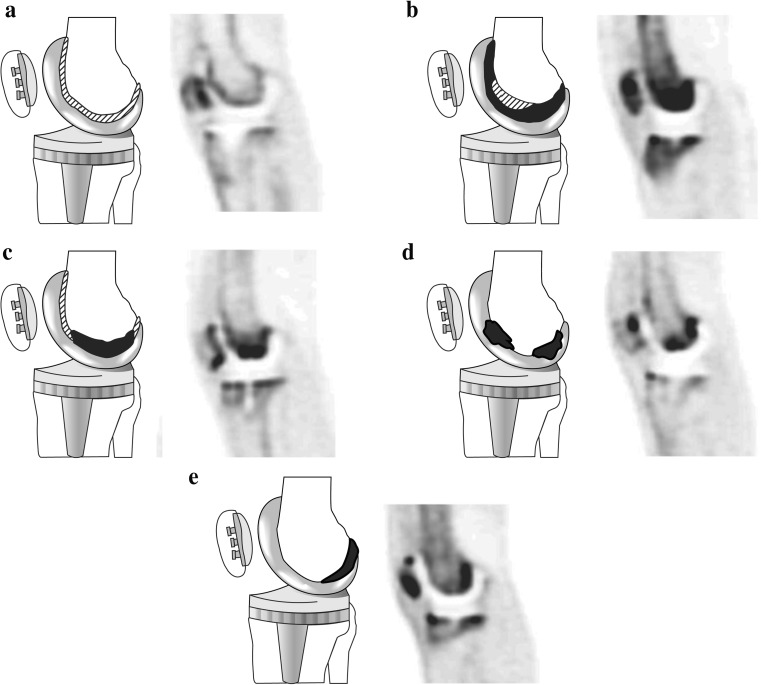
The classification of the uptake patterns in knee prostheses is shown in Fig. 4. In the femoral component of the knee prosthesis, a type 1 pattern was mild, homogeneous uptake in all femoral areas (zones 1–3; Fig. 4a). A type 2 pattern was intense, homogeneous uptake in all femoral areas (zones 1–3; Fig. 4b). A type 3 pattern was mild, homogeneous uptake in all femoral areas (zones 1–3), but more intense, localized uptake in zone 2; Fig. 4c). A type 4 pattern was mild, homogeneous uptake in all femoral areas (zones 1–3), but more intense, localized uptake in zones 2 and 3 (Fig. 4d). A type 5 pattern was mild, homogeneous uptake in all femoral areas (zones 1–3), but more intense, localized uptake in zone 3 (Fig. 4e). In the femoral component, of a total of 24 knee prostheses, five (20.8 %) showed a type 1 pattern, 9 (37.5 %) showed a type 2 pattern, 3 (12.5 %) showed a type 3 pattern, 3 (12.5 %) showed a type 4 pattern, and 4 (20.8 %) showed a type 5 pattern.
Fig. 4.
Diagrams and representative PET images showing the five types of uptake pattern in the femoral component of knee prostheses (a type 1, b type 2,. c type 3, d type 4, e type 5) Black areas represent areas of severely increased uptake and shaded areas represent areas of mildly increased uptake
In the tibial component of the knee prostheses, a type 1 pattern was focal, equal uptake in the medial and lateral border of the tibial areas (zones 4 and 6; Fig. 5a). A type 2 pattern was focal uptake in both the medial and lateral border of the tibial areas (zones 4 and 6), but more intense uptake in the medial compartment (zone 4; Fig. 5b). A type 3 pattern was focal uptake in both the medial and lateral border of the tibial areas (zones 4 and 6), but more intense uptake in the medial areas are areas of mildly increased uptake compartment (zone 6; Fig. 5c). A type 4 pattern was mild, homogeneous uptake in both the medial and lateral portion of the tibia, but more intense, localized uptake in the medial border of the bone–prosthesis interface in zone 4 (Fig. 5d). A type 5 pattern was focal uptake in both the medial and lateral border of the tibial areas (zones 4 and 6), as well as in the central compartment (zone 5; Fig. 5e). In the tibial component, of a total of 24 knee prostheses, 9 (37.5 %) showed a type 1 pattern, 6 (25 %) showed a type 2 pattern, 6 (25 %) showed a type 3 pattern, 1 (4.1 %) showed a type 4 pattern, and 2 (8.3 %) showed a type 5 pattern.
Fig. 5.
Diagrams and representative PET images showing the five types of uptake pattern in the tibial component of knee prostheses (a type 1, b type 2,. c type 3, d type 4, e type 5) Black areas represent areas of severely increased uptake and shaded areas represent areas of mildly increased uptake
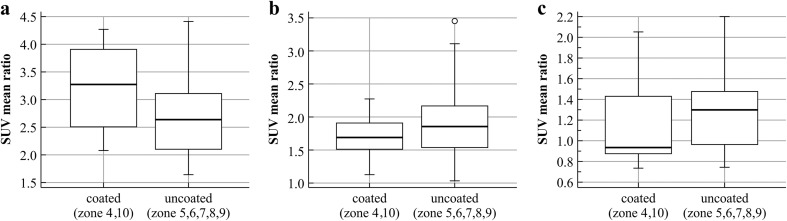
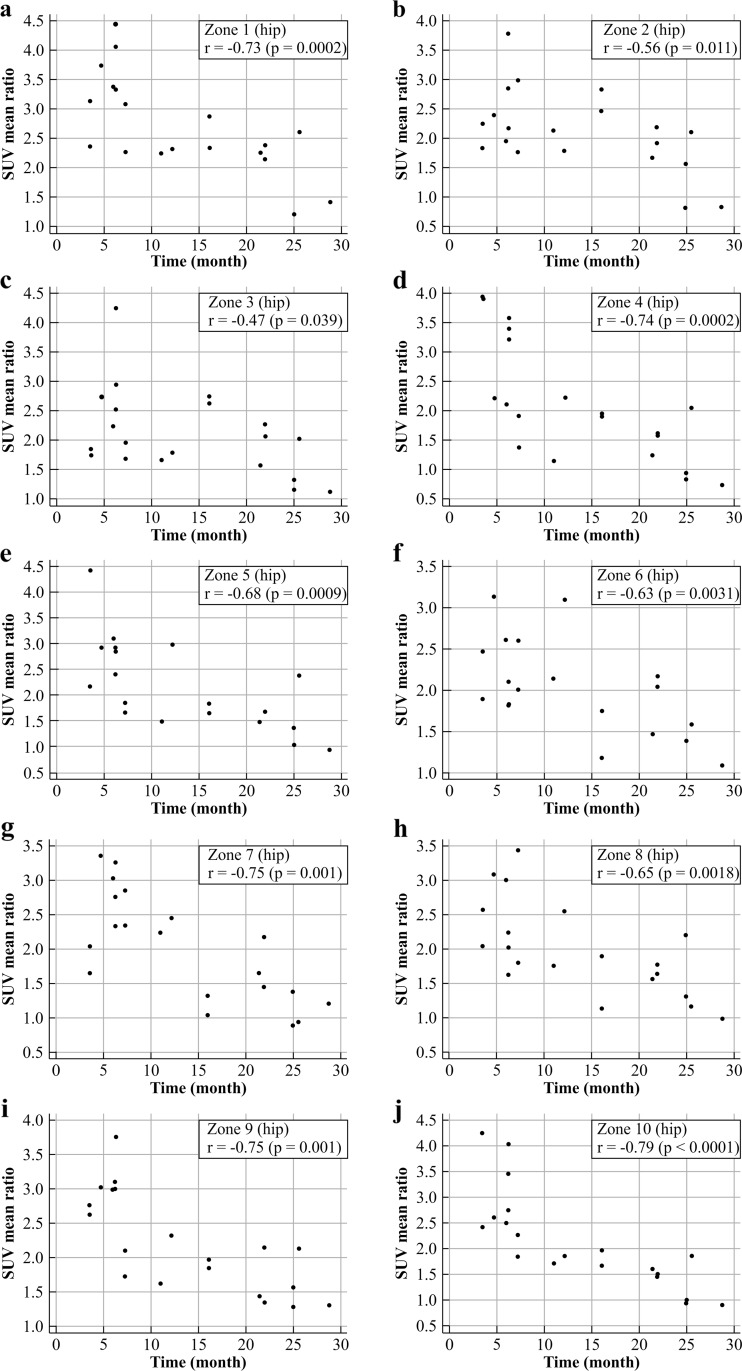
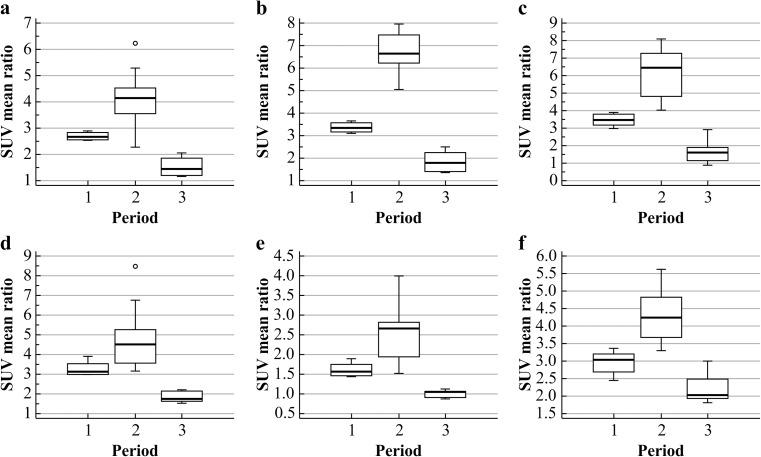
From the quantitative analysis of the hip prostheses, the mean uptake ratios for the periods 3–6, 7–22 months, and more than 24 months are shown in Table 4 and Fig. 6. Comparing the uptake ratios among the time periods, all zones except zone 8 showed significant differences in SUVmean ratio among the three period groups (Kruskal-Wallis test, p < 0.05). As shown in Table 5 and Fig. 7, in the femoral component of the hip prostheses, the uptake ratio in the porous coated area (zones 4 and 10) was significantly higher (Mann–Whitney test) than in the uncoated area (zones 5–9) only during the first 3–6 months after surgery. In the correlation analysis, although the uptake ratio in all zones tended to decrease, the correlation coefficient tended to vary among the different zones (Fig. 8). From the quantitative analysis of the knee prostheses, the mean uptake ratios for the periods 3–6, 8–15 and 22–25 months are presented in Table 6.
Table 4.
Uptake ratios and their percentage decreases compared with the initial ratios for the periods 3–6, 7–22 months, and more than 24 months in the ten zones of hip prostheses
| Zone | 3–6 months | 7–22 months | More than 24 months | p value (Kruskal-Wallis) | Post hoc analysis | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Decrease (%) | Mean | SD | Decrease (%) | |||
| 1 | 3.491 | 0.670 | 2.439 | 0.317 | −30.142 | 1.616 | 0.673 | −53.709 | 0.0023 | 1 > 2,3 |
| 2 | 2.454 | 0.663 | 2.184 | 0.473 | −11.020 | 1.329 | 0.625 | −45.853 | 0.0337 | 1,2 > 3 |
| 3 | 2.615 | 0.841 | 2.046 | 0.422 | −21.793 | 1.422 | 0.420 | −45.642 | 0.0227 | 1 > 3 |
| 4 | 3.170 | 0.744 | 1.660 | 0.362 | −47.612 | 1.143 | 0.606 | −63.954 | 0.0015 | 1 > 2,3 |
| 5 | 2.965 | 0.712 | 1.817 | 0.454 | −38.851 | 1.437 | 0.660 | −51.452 | 0.0019 | 1 > 2,3 |
| 6 | 2.260 | 0.495 | 2.048 | 0.572 | −9.363 | 1.358 | 0.203 | −39.915 | 0.0236 | 1,2 > 3 |
| 7 | 2.626 | 0.643 | 1.939 | 0.604 | −26.152 | 1.099 | 0.231 | −58.133 | 0.0074 | 1,2 > 3 |
| 8 | 2.362 | 0.535 | 1.944 | 0.667 | −17.688 | 1.413 | 0.540 | −40.171 | 0.058 | |
| 9 | 3.038 | 0.362 | 1.834 | 0.331 | −39.623 | 1.571 | 0.393 | −48.286 | 0.0010 | 1 > 2,3 |
| 10 | 3.145 | 0.762 | 1.767 | 0.250 | −43.809 | 1.174 | 0.462 | −62.668 | 0.0008 | 1 > 2 > 3 |
Fig. 6.
Uptake ratios during the periods 3–6 months (group 1), 7–22 months (group 2), and more than 24 months (group 3) in the ten periprosthetic zones of hip prostheses
Table 5.
Uptake ratios and differences in the ratios in coated and uncoated areas for the periods 3–6, 7–22 months, and more than 24 months in the femoral components of hip prostheses
| Coated areas | Uncoated areas | Difference | p value | |
|---|---|---|---|---|
| 3–6 months | 3.16 (0.72) | 2.66 (0.65) | −0.501 | 0.028 |
| 7–22 months | 1.71 (0.307) | 1.89 (0.52) | 0.1712 | 0.270 |
| >24 months | 1.158 (0.499) | 1.316 (0.419) | 0.1584 | 0.169 |
The values presented are means (standard deviations)
Fig. 7.
Differences in mean uptake ratios between coated and uncoated areas in the femoral components of hip prostheses (a 3–6 months, b 7–22 months, c more than 24 months)
Fig. 8.
Changes in uptake ratios during the natural postoperative course in the ten periprosthetic zones of hip prostheses. Correlation coefficients and p values are also shown. a Zone 1, b Zone 2, c Zone 3, d Zone 4, e Zone 5, f Zone 6, g Zone 7, h Zone 8, i Zone 9, j Zone 10
Table 6.
Uptake ratios and their percentage decreases compared with their initial ratios for the periods 3–6, 8–15, 22–25 months in the six zones of knee prostheses
| Zone | 3–6 months | 8–15 months | 22–25 months | p value (Kruskal-Wallis) | Post hoc analysis | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Decrease (%) | Mean | SD | Decrease (%) | |||
| 1 | 2.683 | 0.145 | 4.143 | 0.952 | 54.421 | 1.505 | 0.357 | −43.911 | 0.000241 | 2 > 1 > 3 |
| 2 | 3.370 | 0.228 | 6.673 | 0.956 | 98.047 | 1.838 | 0.478 | −45.451 | 0.000123 | 2 > 1 > 3 |
| 3 | 3.471 | 0.433 | 6.128 | 1.373 | 76.565 | 1.685 | 0.693 | −51.453 | 0.000123 | 2 > 1 > 3 |
| 4 | 3.239 | 0.446 | 4.843 | 1.483 | 49.542 | 1.828 | 0.267 | −43.570 | 0.000241 | 2 > 1 > 3 |
| 5 | 1.620 | 0.199 | 2.585 | 0.748 | 59.574 | 1.014 | 0.092 | −37.419 | 0.000324 | 2 > 1 > 3 |
| 6 | 2.951 | 0.385 | 4.291 | 0.774 | 45.424 | 2.223 | 0.448 | −24.655 | 0.000282 | 2 > 1 > 3 |
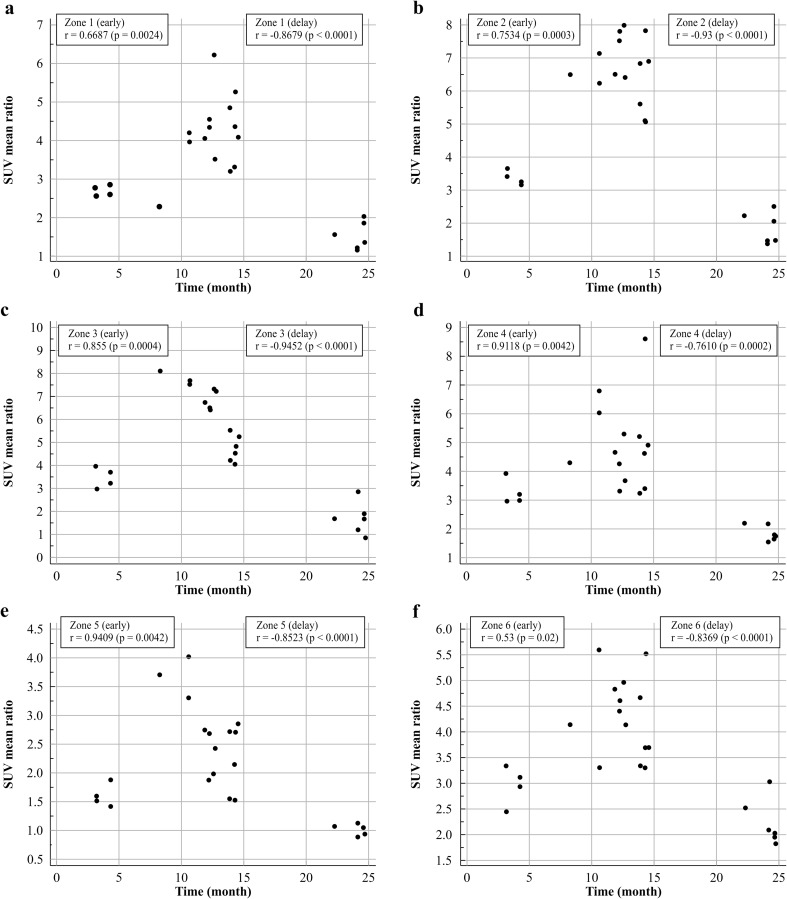
The uptake ratios in the knee prostheses showed a different pattern of change from that in the hip prostheses. There were differences in the uptake ratios among the three periods (3–6, 8–15 and 22–25 months): there were significant increases from 3–6 to 8–15 months, and significant decreases from 8–15 to 22–25 months in all six periprosthetic zones, as shown in Table 4 and Fig. 9. As shown in Fig. 10, every zone showed a positive correlation from 3–6 to 8–15 months (early period), and a negative correlation from 8–15 to 22–25 months (delayed period), although each zone had characteristic correlation coefficients.
Fig. 9.
Uptake ratios for the periods 3–6 months (period 1), 8–15 months (period 2), 22–25 months (period 3) in the six zones of knee prostheses. a Zone 1, b Zone 2, c Zone 3, d Zone 4, e Zone 5, f Zone 6
Fig. 10.
Changes in uptake ratios during the natural postoperative course in the six periprosthetic zones of knee prostheses. The total period is divided into an early period and a delayed period. Correlation coefficients and p values are also shown. a Zone 1, b Zone 2, c Zone 3, d Zone 4, e Zone 5, f Zone 6
Discussion
In this study, we performed both visual and quantitative analyses to evaluate in detail the distribution of new bone formation and serial changes in uptake intensity (SUVmean ratio) longitudinally for the different zones during the natural postoperative course using 18F-NaF PET/CT.
In the visual analysis of the hip prostheses, tracer distribution was classified into three subtypes. The majority of patients (65 %) showed a type 2 pattern (localized uptake in the bone–prosthesis interface of the proximal medial femoral areas). The uptake patterns found in our study have some points of difference in relation to those found in previous studies using other tracers. In a study using FDG PET to evaluate persistent nonspecific uptake after hip arthroplasty, none of the patients evaluated prospectively (nine prostheses) or those evaluated retrospectively (21 prostheses) had any enhanced uptake along the bone–prosthesis interface corresponding to zones 5, 6, 8 and 9 in our study [9]. Because no enhanced uptake along the bone–prosthesis interface was observed in asymptomatic patients on FDG PET, any increased uptake along the bone–prosthesis interface corresponding to zones 5, 6, 8 and 9 could be regarded as infection. However, in our study, increased uptake, suggestive of continued bone remodeling, was observed along the bone–prosthesis interface corresponding to zones 5, 6, 8 and 9 during the natural postoperative course. Furthermore, a study using 99mTc-MDP to evaluate asymptomatic patients after AJRS showed increased uptake in the medial, distal and lateral segments [10]. However, a comparison between the medial and lateral segments revealed that activity in the medial segment was always less than or equal to that in the lateral segment for any given scanning time: the intensity of uptake in the medial segment was less than, equal to, and greater than that in the lateral segment in 30, 69, and 1 % of the scans, respectively. These findings conflict with our results showing that in the majority of scans (65 %) the uptake showed a medial dominant pattern (type 2). In the visual analysis of the knee prostheses, tracer distribution was classified into five subtypes in the femoral and tibial components, respectively. There have been few previous studies investigating the visual tracer distribution pattern in asymptomatic patients.
In the hip prosthesis group, quantitative analysis of the uptake ratio during the natural postoperative course showed that the uptake ratio generally tended to decrease in proportion to the time since implantation, but the pattern of decrease varied among the different prosthetic zones. In hip prostheses, during the first 3–6 months after surgery, there was intense bone-forming activity, and a significant decrease in bone uptake was observed during the period 5–10 months after surgery. By then, uptake had declined in most patients, but 5–10 % of patients showed a slight increase in uptake during the period 15–20 months in zones 1–3, during the period 10–15 months in zones 5 and 6, and during the period 20–25 months in zones 4–8. Uptake then declined at a similar rate (zones 5 and 7) or a slightly greater rate (zones 1–4, 6, and 8–10) compared with that in the contralateral normal bone. In the correlation analysis, although the uptake ratio in all zones tended to decrease, the correlation coefficient tended to vary among the different zones: zones 1, 4, 7, 9 and 10 showed a strong correlation, zones 5, 6 and 8 showed a moderate correlation, and zones 2 and 3 showed a fair negative correlations.
Comparing the uptake ratios among the time periods, there were significant differences in the SUVmean ratio among the three periods (3–6, 7–22, and more than 24 months), except in zone 8. Comparing the uptake ratio of the porous coated area (zones 4 and 10) with that of the uncoated area (zones 5–9) of the femoral part, the uptake ratio in the coated area was significantly higher than that in the uncoated area only in the first 3–6 months following surgery. However, after 6 months, there was no difference in the uptake ratio between the areas. These findings correlate well with previous results from a 99mTc-MDP bone scan study [5]. The uptake in the hydroxyapatite-coated zone was significantly higher than in the noncoated zones (p < 0.05) in the first 3 months. The uptake ratio decreased much faster in the hydroxyapatite-coated metaphyseal zone, but was higher in all time periods in the non-coated diaphyseal zone of the femoral stem areas.
Several studies have addressed the changing pattern in uptake intensity during the natural postoperative course. 99mTc-MDP bone scintigraphy was performed in 62 asymptomatic patients (80 joints) after cementless hydroxyapatite-coated total hip arthroplasty, and ten healthy controls who had not undergone surgery, from 1 to 48 months [5]. In the control group, the metaphyseal area in the proximal femur showed a physiologically higher uptake ratio than the diaphyseal area. The acetabulum also showed a higher uptake ratio than the proximal femur. In the patient group, the uptake ratio in the femoral stem and the acetabular cup decreased between 1 and 3 months after surgery and stabilized after that time [5]. In another study using 99mTc-HEDP, the uptake ratios showed a changing pattern during the postoperative period from 3 to 12 months in asymptomatic patients [11].
The uptake ratios decreased rapidly during 3–6 months, and reached a nadir at 6–9 months after surgery, then gradually decreased during 9–12 months. The initial uptake ratio of the acetabular area declined from 3.6 at 3 to 2.7 at 6 months (p < 0.01). After 6 months, the differences in the uptake ratios were not significant. The ratio in the femoral areas declined rapidly from 4.0 to 1.6 after 6 months (p < 0.01). Three months later, the ratio was 1.2. The uptake ratio in four patients increased to 3.15 during the later follow-up, without any signs of infection or loosening. The uptake ratio declined more rapidly in the femoral component than in the acetabular component.
The uptake ratios in the knee prostheses showed a different pattern of change than in the hip prostheses. Previous bone scan studies have shown that the remodeling time for bone to return to normal after implantation of a total hip prosthesis is very different from that after implantation of a knee prosthesis [1]. The uptake ratio in every zone showed a curvilinear pattern with a significant increase from 3–6 to 8–15 months. After a peak during 10–15 months, the uptake ratio rapidly decreased to a similar level (zone 5) or slightly higher level (zones 1–4 and 6) during 22–25 months compared with the levels in contralateral normal bone. There was a significant difference in the uptake ratios among the three periods (3–6, 8–15, and 22–25 months) in all periprosthetic zones (zones 1–6). Every zone showed a positive correlation from 3–6 to 8–15 months (early period), and a negative correlations from 8–15 to 22–25 months (delayed period), although each zone had a characteristic correlation coefficient. During the early period, strong positive correlations were observed in zones 2–5 and moderate or fair positive correlations were observed in zones 1 and 6. During the late period, strong negative correlations were observed in all zones. In a study to establish the natural course of radionuclide levels following bone scan imaging after total knee replacement surgery, 41 consecutive patients were evaluated for 24 months after surgery [6]. A substantial increase in uptake was still found 12 months after surgery in 20 % of the knees, and a definite increase in uptake was found 24 months after surgery in 12.5 % of the knees.
The late bone-forming peak activity observed in our study has also been found in previous studies using bone scan tracers. Kim et al. evaluated the natural course of radioactivity following sequential 99mTc bone scans after cementless total hip replacement [3]. In the distal one third of the stem (zones 6 and 8 in our study), the radioactivity regressed to the same levels as in a normal distal femur within 12 months. However, in areas around the greater and lesser trochanter, radioactivity in the proximal one third of the stem, the tip (zones 4, 5, 7, 9, and 10 in our study), tended to decrease initially, but persisted for up to 12 months in 31–37 % of patients. In another prospective 99mTc-MDP bone scan study evaluating 97 patients who underwent hip arthroplasty and were not experiencing any symptoms, 10 % of patients showed persistent activity in the acetabulum, greater trochanter, and prosthesis tip at 2 years after surgery, representing delayed bone remodeling [1].
This late, osteoblastic reaction for biological fixation is caused by the remodeling activity between the implant and ingrowing bone [12–14]. The physiological cellular events occur in a stable, uncomplicated prosthesis and consist of a reparative, noninfectious inflammation, and subsequently a proliferation of connective tissue, restoring the tissue integrity. The first event following the inflammatory stimulus is focal vasodilatation and increased vessel permeability. After the migration of macrophages and monocytes, fibroblasts migrate toward the inflammatory focus and form collagen. The maturation and consolidation of granulation tissue terminates the inflammatory process. However, early protein reactions with the implant activate the immune system by producing hapten complexes. At the prosthesis–bone interface, multinucleated giant cells, plasma cells, macrophages, and lymphocytes may remain in the perivascular sleeve in the vicinity of the implant.
There were several factors affecting our study results regarding the tracer distribution pattern and serial changes in the uptake ratio during the natural postoperative course. First, different results among various studies can be attributed to the well-known differences in the molecular uptake mechanisms of 99mTc-MDP, 18F-FDG, and 18F-NaF. The uptake mechanism for 18F-FDG is arthroplasty induces sterile inflammation in the periprosthetic areas, caused by activation of macrophages and fibroblasts by polyethylene wear-induced particles, and the subsequent outgrowth of aggressive granulomatous tissue that has an increased energy requirement, which is reflected by an increased uptake of FDG [12, 13]. Polyethylene particle-generated chronic inflammatory reactions around the neck of the prosthesis are common findings several months or even years after surgery in patients without evidence of infection or loosening [14].
Although both FDG and NaF detect similar inflammatory areas, they reflect different immunological reactions [15]. 18F-NaF has different uptake mechanisms. In 18F-NaF PET, 18F is directly assimilated into the bone matrix, as fluoride ions exchange with hydroxyl groups in the hydroxyapatite crystals of bone to produce fluoroapatite [16]. 18F localization in the bone structure depends on regional blood flow, as well as on osteoblastic new bone formation [17]. Detection of inflammation using 18F-NaF PET is based on the assumption that it accumulates in minerals created by both osteoblasts and osteoclasts, activated by the immune response to bone infection [18]. Direct comparison of the uptake patterns and SUVmax of 18F-FDG and 18F-NaF PET for detection of an inflammatory focus in periprosthetic hip joints has shown that the tracer accumulation patterns are similar, although the capsule regions were excluded from NaF PET calculations because 18F-NaF does not detect inflammation in the soft tissue. However, FDG PET uptake was significantly associated with positive tissue examination, but on the other hand, there was only a slight association between NaF PET uptake and regions positive on tissue examination [15].
The binding mechanism of 99mTc-MDP involves chemisorption to the hydroxyapatite structure of the bone tissue, and the deposition of 99mTc-MDP occurs at the site of mineralization of osteoid and at the osteocytic lacunae, not osteoclasts [19]. The net transport of fluoride into the bone better reflects bone metabolism than that of 99mTc-MDP, because fluoride is directly incorporated into the bone matrix. Due to low levels of protein binding, high capillary permeability, and a high first-pass extraction ratio, uptake of 18F-NaF into the bone mineralization surface is twofold higher than that of 99mTc-MDP [20].
Second, the difference in serial uptake patterns between hip and knee prostheses can be attributed to the differences in the types of prosthesis, especially whether or not cement is used. In our study, cement was not used in hip arthroplasties and was used in all knee arthroplasties. As the design of the prosthesis and the stress shielding patterns were not the same in each study, uptake patterns and stabilization times may differ depending on the type of prosthesis.
In the cementless, porous coated prosthesis, as used in our study, there is direct contact between the prosthetic material and the bone tissue that grows in the pores. The bone tissue occupies the majority of pores with a diameter more than 100 μm [21]. Abundant osteoblastic growth can occur at the interface with the cortical walls or healthy cancellous bone tissue [21]. When interacting with the cortical bone, a cancellization process with the adjacent interface can occur [21].
The porous coating of an implant surface has been used to facilitate long-term secondary stability, and to enhance the growth of bone into the prosthetic surface [22].
Increased uptake in the different zones is affected by different biological mechanisms. In areas around the greater and lesser tro-chanter and in the proximal one third of the stem high uptake can be caused by bony ingrowth and delayed remodeling, secondary to the healing process of osteotomy. In the tip areas, high uptake is caused by secondary reactive hyperostosis induced by micromotion around the prosthesis [10, 12, 23, 24]. In contrast, in a cement prosthesis, a fibrous membrane may be created at the bone–cement interface in a stable prosthesis. In this membrane, rare, small cement debris particles are present, surrounded by macrophages, giant cells, and a few areas of fibrocartilaginous metaplasia [25, 26]. The membrane is composed of three layers: macrophages and giant cells in the layer bordering the cement; fibrous tissue in the middle layer; and granulation tissue rich in fibroblasts, macrophages, round cells, and blood vessels in the layer facing the bone [27].
Third, different quantification methods may have been used for evaluation in different studies. Here, we used the automatic isocontour method to refit the VOIs along the structures by setting a threshold of a 30 % difference between the maximum and minimum SUV in the given VOI. Different thresholds and VOI sizes can give different results. Moreover, compared with previous planer gamma camera imaging, 18F-NaF PET/CT allows absolute quantification of the radioactivity of three-dimensional structures.
Longitudinal studies of a larger population with longer follow-up periods are recommended for better assessment of the natural changes in the uptake patterns. Furthermore, although we used contralateral bone as the normal reference, a more accurate study comparing an age-matched, normal population should be conducted in the future. Although we evaluated only the delayed bone phase of 18F-NaF PET, evaluating uptake in the early, dynamic, perfusion phase would give more integrated information about asymptomatic patients after AJRS.
Conclusion
Using the most promising tools with excellent sensitivity and specificity in the evaluation of joint replacements, we presented a detailed picture of the visual tracer distribution and serial changes in the quantitative uptake ratio for different anatomical zones during the natural postoperative period. The clinical significance of our work is that this is the first 18F-NaF PET/CT study that gives information concerning the stability of the implants and has established a reference for the evaluation of patients with complications.
Acknowledgments
This work was supported by the Busan Metropolitan City research fund.
Compliance with Ethical Standards
Conflicts of Interest
The authors declare that they don’t have conflicts of interest.
Ethical Statement
The study was approved by an institutional review board or equivalent and has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All subjects in the study gave written informed consent or the institutional review board waived the need to obtain informed consent.
Footnotes
The manuscript has not been published previously, is not under consideration for publication elsewhere, and has been approved by all of the authors.
References
- 1.Utz JA, Lull RJ, Galvin EG. Asymptomatic total hip prosthesis: natural history determined using Tc-99m MDP bone scans. Radiology. 1986;161:509–12. doi: 10.1148/radiology.161.2.3763923. [DOI] [PubMed] [Google Scholar]
- 2.Li D, Miles K, Wraight E. Bone scintigraphy of hip prostheses: can analysis of patterns of abnormality improve accuracy? Clin Nucl Med. 1994;19:112–5. doi: 10.1097/00003072-199402000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Kim HS, Suh J, Han CD, Kim Y, Lee JD. Sequential Tc-99m MDP bone scans after cementless total hip arthroplasty in asymptomatic patients. Clin Nucl Med. 1997;22:6–12. doi: 10.1097/00003072-199701000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Meidan Z, Weisman S, Baron J, Binderman I. Technetium 99m-MDP scintigraphy of patients undergoing implant prosthetic proce-dures: a follow-up study. J Periodontol. 1994;65:330–5. doi: 10.1902/jop.1994.65.4.330. [DOI] [PubMed] [Google Scholar]
- 5.Suh KT, Lee CB, Kim IJ. Natural progress of a bone scan after cementless hydroxyapatite-coated total hip arthroplasty. Clin Orthop Relat Res. 2001;389:134–42. doi: 10.1097/00003086-200108000-00019. [DOI] [PubMed] [Google Scholar]
- 6.Duus B, Boeckstyns M, Stadeager C. The natural course of radionuclide bone scanning in the evaluation of total knee replacement—a 2 year prospective study. Clin Radiol. 1990;41:341–3. doi: 10.1016/S0009-9260(05)81699-X. [DOI] [PubMed] [Google Scholar]
- 7.DeLee JG, Charnley J. Radiological demarcation of cemented sockets in total hip replacement. Clin Orthop Relat Res. 1976;121:20–32. [PubMed] [Google Scholar]
- 8.Gruen TA, McNeice GM, Amstutz HC. “Modes of failure” of cemented stem-type femoral components: a radiographic analysis of loosening. Clin Orthop Relat Res. 1979;141:17–27. [PubMed] [Google Scholar]
- 9.Zhuang H, Chacko TK, Hickeson M, Stevenson K, Feng Q, Ponzo F, et al. Persistent non-specific FDG uptake on PET imaging fol-lowing hip arthroplasty. Eur J Nucl Med Mol Imaging. 2002;29:1328–33. doi: 10.1007/s00259-002-0886-2. [DOI] [PubMed] [Google Scholar]
- 10.Oswald SG, Van Nostrand D, Savory CG, Callaghan JJ. Three phase bone scan and indium white blood cell scintigraphy following porous coated hip arthroplasty: a prospective study of the prosthetic tip. J Nucl Med. 1989;30:1321–31. [PubMed] [Google Scholar]
- 11.Creutzig H. Bone imaging after total replacement arthroplasty of the hip joint. A follow-up with different radiopharmaceuticals. Eur J Nucl Med. 1976;1:177–80. doi: 10.1007/BF00257968. [DOI] [PubMed] [Google Scholar]
- 12.Engh CA, Bobyn JD, Glassman AH. Porous-coated hip replacement. The factors governing bone ingrowth, stress shielding, and clinical results. J Bone Joint Surg Br. 1987;69:45–55. doi: 10.1302/0301-620X.69B1.3818732. [DOI] [PubMed] [Google Scholar]
- 13.Pilliar RM, Cameron HU, Macnab I. Porous surface layered prosthetic devices. Biomed Eng. 1975;10:126–31. [PubMed] [Google Scholar]
- 14.Andriacchi TP, Stanwyck TS, Galante JO. Knee biomechanics and total knee replacement. J Arthroplast. 1986;1:211–9. doi: 10.1016/S0883-5403(86)80033-X. [DOI] [PubMed] [Google Scholar]
- 15.Choe H, Inaba Y, Kobayashi N, Miyamae Y, Ike H, Yukizawa Y, et al. (18)F-fluorodeoxy glucose and (18)F fluoride PET for detection of inflammation focus in periprosthetic hip joint infection cases. Mod Rheumatol. 2015;25:322–4. doi: 10.3109/14397595.2014.931505. [DOI] [PubMed] [Google Scholar]
- 16.Grynpas MD. Fluoride effects on bone crystals. J Bone Miner Res. 1990;5:S169–75. doi: 10.1002/jbmr.5650051362. [DOI] [PubMed] [Google Scholar]
- 17.Segall G, Delbeke D, Stabin MG, Even-Sapir E, Fair J, Sajdak R, et al. SNM practice guideline for sodium 18F-fluoride PET/CT bone scans 1.0. J Nucl Med. 2010;51:1813–20. doi: 10.2967/jnumed.110.082263. [DOI] [PubMed] [Google Scholar]
- 18.Shi S, Zhang X. Interaction of Staphylococcus aureus with osteoblasts (Review) Exp Ther Med. 2012;3:367–70. doi: 10.3892/etm.2011.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Francis MD, Ferguson DL, Tofe AJ, Bevan JA, Michaels SE. Comparative evaluation of three diphosphonates: in vitro adsorption (C- 14 labeled) and in vivo osteogenic uptake (Tc-99m complexed) J Nucl Med. 1980;21:1185–9. [PubMed] [Google Scholar]
- 20.Grant FD, Fahey FH, Packard AB, Davis RT, Alavi A, Treves ST. Skeletal PET with 18F-fluoride: applying new technology to an old tracer. J Nucl Med. 2008;49:68–78. doi: 10.2967/jnumed.106.037200. [DOI] [PubMed] [Google Scholar]
- 21.Ong KL, Lovald S, Black J. Orthopaedic biomaterials in research and practice. New York: CRC Press; 2014. [Google Scholar]
- 22.Nourbash PS, Paprosky WG. Cementless femoral design concerns: rationale for extensive porous coating. Clin Orthop Relat Res. 1998;355:189–99. doi: 10.1097/00003086-199810000-00020. [DOI] [PubMed] [Google Scholar]
- 23.Weiss PE, Mall JC, Hoffer PB, Murray WR, Rodrigo JJ, Genant HK. 99mTc-methylene diphosphonate bone imaging in the evaluation of total hip prostheses. Radiology. 1979;133:727–9. doi: 10.1148/133.3.727. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan PA, Montesi SA, Jardon OM, Gregory PR. Bone-ingrowth hip prostheses in asymptomatic patients: radiographic features. Radiology. 1988;169:221–7. doi: 10.1148/radiology.169.1.3420262. [DOI] [PubMed] [Google Scholar]
- 25.Pizzoferrato A, Ciapetti G, Stea S, Toni A. Cellular events in the mechanisms of prosthesis loosening. Clin Mater. 1991;7:51–81. doi: 10.1016/0267-6605(91)90057-M. [DOI] [PubMed] [Google Scholar]
- 26.Jones LC, Hungerford DS. Cement disease. Clin Orthop Relat Res. 1987;225:192–206. [PubMed] [Google Scholar]
- 27.Linder L, Carlsson AS. The bone-cement interface in hip arthroplasty: a histologic and enzyme study of stable components. Acta Orthop Scand. 1986;57:495–500. doi: 10.3109/17453678609014777. [DOI] [PubMed] [Google Scholar]