Abstract
Purpose
18F-fluorodeoxyglucose (FDG) PET/CT is useful for staging and evaluating treatment response in patients with diffuse large B-cell lymphoma (DLBCL). A five-point scale model using the mediastinal blood pool (MBP) and liver as references is a recommended method for interpreting treatment response. We evaluated the variability in standardized uptake values (SUVs) of the MBP, liver, and myocardium during chemotherapy in patients with DLBCL.
Methods
We analyzed 60 patients with DLBCL who received rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP) treatment and underwent baseline, interim, and final FDG PET/CT scans. The FDG uptakes of lymphoma lesions, MBP, liver, and myocardium were assessed, and changes in the MBP and liver SUV and possible associated factors were evaluated.
Results
The SUV of the liver did not change significantly during the chemotherapy. However, the SUVmean of MBP showed a significant change though the difference was small (p = 0.019). SUVmean of MBP and liver at baseline and interim scans was significantly lower in patients with advanced Ann Arbor stage on diagnosis. The SUVmean of the MBP and liver was negatively correlated with the volumetric index of lymphoma lesions in baseline scans (r = -0.547, p < 0.001; r = -0.502, p < 0.001). Positive myocardial FDG uptake was more frequently observed in interim and final scans than in the baseline scan, but there was no significant association between the MBP and liver uptake and myocardial uptake.
Conclusions
The SUV of the liver was not significantly changed during R-CHOP chemotherapy in patients with DLBCL, whereas the MBP SUV of the interim scan decreased slightly. However, the SUV of the reference organs may be affected by tumor burden, and this should be considered when assessing follow-up scans. Although myocardial FDG uptake was more frequently observed after R-CHOP chemotherapy, it did not affect the SUV of the MBP and liver.
Electronic supplementary material
The online version of this article (doi:10.1007/s13139-016-0432-y) contains supplementary material, which is available to authorized users.
Keywords: Diffuse large B-cell lymphoma, FDG, PET, Mediastinum, Liver, Myocardium
Introduction
18F-fluorodeoxyglucose (FDG) positron emission tomography-computed tomography (PET/CT) is known to be a useful modality for staging and evaluating treatment response in patients with diffuse large B-cell lymphoma (DLBCL), the most common lymphoid malignancy in adults [1, 2]. A five-point scale model, the Deauville criteria, was introduced to standardize visual interpretation of the treatment response and has become a recommended method for routine clinical use and clinical trials using interim and post-treatment FDG-PET/CT in Hodgkin lymphoma (HL) and some non-Hodgkin lymphomas (NHL) such as DLBCL [2]. In this scale model, FDG uptake in the mediastinum and liver is used as reference [2]. Therefore, it is important to know whether FDG uptake of the mediastinum and liver changes during chemotherapy, as variation in FDG uptake in the reference organs can affect the visual interpretation, causing inaccurate results of score reporting.
Previous studies investigating changes in FDG uptake in the liver and mediastinum during chemotherapy in patients with lymphoma have yielded conflicting results [3–5]. Some studies have reported that FDG uptake in the liver showed significant intra-patient variability during chemotherapy that should be considered when assessing interim PET/CT scan [3, 4], whereas another study demonstrated stable FDG uptake in the liver and mediastinum during chemotherapy [5]. In addition, factors affecting the FDG uptake of the liver and mediastinum are still not clear, and the effect of chemotherapy on FDG uptake of these reference organs is uncertain.
Myocardial FDG uptake is known to have various patterns because of the switch in energy substrate from free fatty acids to glucose mediated by insulin [6]. However, despite the wide variation in physiologic myocardial FDG uptake, the cardiotoxicity of chemotherapeutic agents was one of the factors associated with increased cardiac FDG uptake in an observational study [7]. Doxorubicin, one of the chemotherapy agents in the R-CHOP regimen that is currently the standard first-line chemotherapy for DLBCL, has dose-limiting cardiotoxicity and thus has the potential to affect FDG uptake of the myocardium [8]. However, possible changes in myocardial FDG uptake induced by doxorubicin might be suppressed by dexrazoxane, an iron chelator that is used in some situations for prevention of anthracycline-induced heart injury [9]. Variation in the distribution of FDG uptake in the body possibly leads to alteration in the SUV of the mediastinum and liver. Uncontrolled FDG uptake in the myocardium as well as the change of lymphoma lesion FDG uptake may influence the variation.
We conducted this study to evaluate the variability in FDG uptake in the mediastinum and liver during chemotherapy in patients with DLBCL. We also assessed the FDG uptake of lymphoma lesions and myocardium as possible associated factors of that variation.
Materials and Methods
Study Subjects
Study subjects were selected from 239 adult patients who were newly diagnosed with DLBCL and underwent more than two FDG PET/CT scans between January 2014 and May 2015. We included 105 patients who received six cycles of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP) treatment with the standard dose and underwent baseline, interim (after 3 cycles), and final (after 6 cycles) FDG PET/CT scans. Among these patients, we excluded 27 who had previous history of chemotherapy and showed abnormal echocardiographic findings in the baseline study, seven patients with a blood glucose level greater than 200 mg/dl or scan delay more than 10 % of 60 min for the FDG PET/CT scan, and three patients who had intra-subject discordance of the PET scanner. An additional eight patients were excluded because the interim and final FDG PET/CT scans were performed more than 1 month after the last cycle of chemotherapy. Consequently, 60 patients were finally enrolled in this study. None of the study subjects had a history of radiation therapy including the heart, mediastinum, and liver in the irradiated field.
This retrospective observational study was approved by our institutional review board with exemption of written consent from the study subjects.
FDG PET/CT Imaging
All patients fasted for at least 6 h before PET/CT studies. After measurement of the blood glucose level, subjects were injected with 5 MBq/kg of FDG. Imaging was performed 60 min later on a Discovery LS (GE Healthcare; n = 3 patients) or a Discovery STe PET/CT scanner (GE Healthcare; n = 57 patients) without intravenous or oral contrast. Whole-body CT was performed with a continuous spiral technique with an eight-slice helical CT (140 keV; 40–120 mA; section width, 5 mm; Discovery LS) or a continuous spiral technique with 16-slice helical CT (140 keV; 30–170 mA; section width, 3.75 mm; Discovery STe). After the CT scan, an emission scan was obtained from head to thigh for 4 min per frame in 2D mode with reconstruction of attenuation-corrected PET images (4.3 × 4.3 × 3.9 mm) using an ordered-subset expectation maximization algorithm (28 subsets, 2 iterations; Discovery LS) or for 2.5 min per frame in 3D mode with reconstruction of attenuation-corrected PET images (3.9 × 3.9 × 3.3 mm) using a 3D ordered-subset expectation maximization algorithm (20 subsets, 2 iterations; Discovery STe).
Image Analysis
Image analysis and data review were done by two experienced nuclear medicine clinicians. The maximum standardized uptake values (SUVmax) and mean standardized uptake values (SUVmean) of the mediastinal blood pool (MBP) and liver were assessed by drawing a volume of interest (VOI). A VOI of 1.5 × 1.5 × 1.5 cm was drawn above the aortic root for MBP, and a VOI of 5 × 5 × 5 cm was placed in the right lobe of the liver, ensuring that VOIs were restricted to areas of physiologic uptake. None of the subjects had high FDG uptake of atherosclerotic plaque in VOIs for the mediastinal blood pool. The procedures were uniformly repeated in basal, interim, and final PET.
Metabolic tumor volume (MTV) and total lesion glycolysis (TLG) of lymphoma lesions were measured by a semiautomatic method to delineate the volume of interest. We measured them for all lymphoma lesions with discernible FDG uptake and drew the boundaries using SUV-based contouring software (volume viewer software on GE Advantage Workstation 4.4). The SUV threshold was set to 2.5, the value suggested as the cutoff value for malignancy by Freudenberg et al. [10]. Volumetric parameters of myocardium and bone marrow were also measured. SUV thresholds for myocardium and bone marrow were MBP SUVmean and SUV of 2.5, respectively.
Statistical Analysis
Data are expressed as mean and standard deviation (SD). Differences in SUVmax and SUVmean of the MBP and liver across the basal, interim, and final PET were assessed by repeated measures ANOVA test. Independent samples t-test was used to compare the MBP and liver SUVmean of subjects according to Ann Arbor stage. We assumed that tumor burden has a close association with the SUVmean of MBP and liver and investigated this further by evaluating the correlation of the MTV and TLG of lymphoma lesions and the SUVmean of the MBP and liver. For the presentation of discrete patterns in the change of the SUVmean of the MBP and liver along the course of chemotherapy according to the initial tumor burden, subjects with MTV of more than 1 SD above the median in the basal scan were considered the high tumor burden group, and the rest of the subjects were grouped as low tumor burden. SUVmean of the MBP and liver of these two groups was evaluated with repeated measures ANOVA test. Association between the SUVmean of the reference organs and volumetric parameters of lymphoma lesions was evaluated using Pearson’s correlation test. Associations with myocardial FDG uptake and bone marrow uptake were also assessed in the same manner. Variations in the MBP and liver SUV and their possible associated factors were analyzed by one-way ANOVA test. Chi-square test was used to compare the frequencies of positive myocardial uptake.
Results
Patient Characteristics
Patient characteristics are presented in Table 1. Among 60 study subjects, 31 (51.7 %) were male, and the mean age was 53.0 years. According to the Ann Arbor staging system, 25 patients were stage I, 14 were stage II, 8 were stage III, and 13 were stage IV. Mean aspartate aminotransferase (AST)/alanine aminotransferase (ALT) activities before the baseline, interim, and final PET studies were 23.0/22.5, 21.6/26.0, and 25.4/28.9 U/l, respectively. Eight patients (13.3 %) had diabetes mellitus, and seven patients received dexrazoxane treatment during R-CHOP chemotherapy. The mean cumulative dose of doxorubicin was 299.7 mg/m2 after six cycles of R-CHOP. The mean time interval from chemotherapy to the PET study was 16.8 days and 16.3 days for interim and final scans, respectively.
Table 1.
Characteristics of study patients (n = 60)
| Number | ||
|---|---|---|
| Mean age, years (range) | 53.0 (24.2–78.7) | |
| Sex | Male | 31 (51.7 %) |
| Female | 29 (48.3 %) | |
| Diabetes mellitus | 8 (13.3 %) | |
| Blood glucose level, mg/dl, mean (range) | Baseline | 100.9 (73–152) |
| Interim | 101.5 (80–190) | |
| Final | 104.7 (74–185) | |
| Serum AST/ALT, U/l, mean (range) | Before baseline | 23.0 (11–57)/22.5 (7–65) |
| Before interim PET | 21.6 (8–53)/26.0 (6–109) | |
| Before final PET | 25.4 (12–56)/28.9 (5–92) | |
| Mean initial LVEF % (range) | 64.6 (55–75) | |
| Use of dexrazoxane | Prior to interim scan | 5 (8.3 %) |
| Prior to final scan | 7 (11.7 %) | |
| Cumulative dose of doxorubicin, mg/m2, mean (range) | Prior to interim scan | 149.8 (138.1–158.4) |
| Prior to final scan | 299.7 (289.1–310.6) | |
| Time interval from chemotherapy, days, mean (range) | Prior to interim scan | 16.3 (6.6–28.4) |
| Prior to final scan | 16.8 (7.8–26.8) |
AST aspartate aminotransferase, ALT alanine aminotransferase, LVEF left ventricle ejection fraction
SUV of Reference Organs and Correlation with Lymphoma Lesion Uptake
Mean SUVmax of the MBP in baseline, interim, and final PET was 1.76, 1.66, and 1.75, respectively. Mean SUVmean of the MBP was 1.42, 1.32, and 1.40 for baseline, interim, and final PET. Mean SUVmax of the liver in baseline, interim, and final PET was 3.00, 2.98, and 3.08, respectively, and mean SUVmean of the liver was 1.98, 1.96, and 2.04. The SUVmax and SUVmean of the liver did not change significantly during chemotherapy (Table 2). However, the mean SUVmean of the MBP showed a significant change in the baseline, interim, and final FDG PET/CT scans, although the difference was small (1.42 vs. 1.32 vs. 1.40; repeated measures ANOVA test, p = 0.019; Table 2). The difference in the mean SUVmax of the MBP was not significant (p = 0.074).
Table 2.
Change in standardized uptake values (SUVs) in the MBP, liver, and lesions during R-CHOP therapy in study patients (n = 60)
| Baseline PET | Interim PET | Final PET | p value a | |
|---|---|---|---|---|
| MBP SUVmax (mean ± SD) | 1.76 ± 0.33 | 1.66 ± 0.30 | 1.75 ± 0.25 | 0.074 |
| MBP SUVmean (mean ± SD) | 1.42 ± 0.27 | 1.32 ± 0.21 | 1.40 ± 0.20 | 0.019 |
| Liver SUVmax (mean ± SD) | 3.00 ± 0.57 | 2.98 ± 0.42 | 3.08 ± 0.45 | 0.340 |
| Liver SUVmean (mean ± SD) | 1.98 ± 0.42 | 1.96 ± 0.30 | 2.04 ± 0.29 | 0.092 |
| Lymphoma lesion FDG uptake | ||||
| MTV, cm3 (mean ± SD) | 278.15 ± 410.92 | 1.16 ± 4.53 | 0.51 ± 2.88 | <0.001 |
| TLG (mean ± SD) | 1934.88 ± 2814.10 | 4.78 ± 20.92 | 2.37 ± 13.85 | <0.001 |
| Bone marrow FDG uptake | ||||
| MTV, cm3 (mean ± SD) | 12.85 ± 30.23 | 63.27 ± 121.45 | 59.05 ± 99.84 | 0.003 |
| TLG (mean ± SD) | 36.45 ± 86.81 | 197.12 ± 422.51 | 177.03 ± 316.27 | 0.002 |
aRepeated measures ANOVA
MTV metabolic tumor volume, TLG total lesion glycolysis
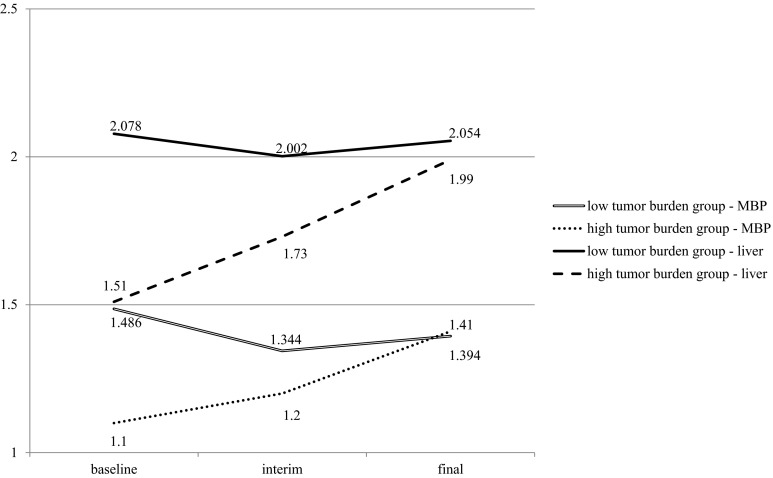
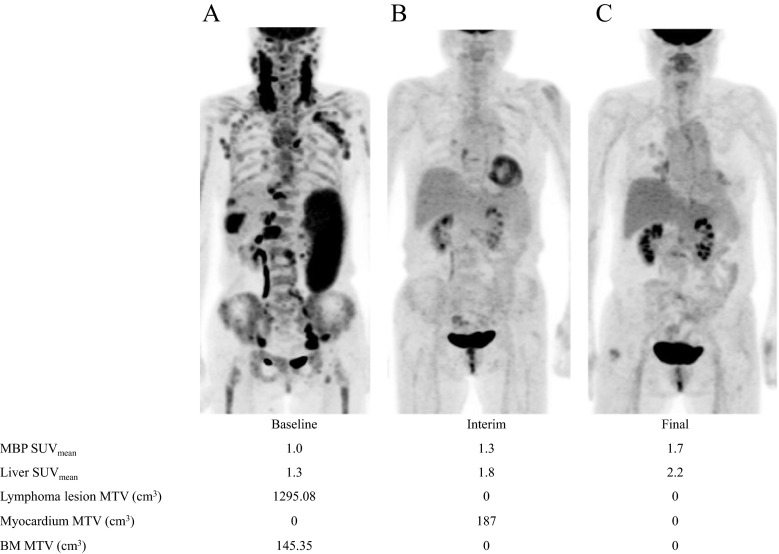
We observed that the SUVmean values of the MBP and liver were significantly lower at baseline and interim PET scans in patients with Ann Arbor stage III or IV disease on diagnosis than in patients with stage I or II disease. However, the difference between these two groups was not significant in the final scans (Supplemental Table 1). MTV and TLG were negatively correlated with the SUVmean of the MBP and liver in baseline scans (Table 3). Pearson’s correlation coefficients between the MTV and SUVmean in the baseline scan were -0.547 for the MBP (p < 0.001) and -0.502 for the liver (p < 0.001). Correlation coefficients for the TLG and SUVmean of the MBP and liver were -0.511 (p < 0.001) and -0.517 (p < 0.001), respectively. However, there was no significant correlation demonstrated in interim and final PET scans. The high tumor burden group was defined as subjects who have an MTV of the lymphoma lesion greater than 1 SD (410.92 cm3) above the median (99.82 cm3) in the baseline scan. Ten patients were included in the high tumor burden group, and the remaining 50 patients were included in the low tumor burden group. SUVmean of the MBP and liver at baseline, interim, and final scans was significantly different between the two groups (Fig. 1, repeated measures ANOVA test, between-subject effects p = 0.001 and 0.001, respectively). A representative case of the high tumor burden group is presented in Fig. 2.
Table 3.
Correlation between the SUVmean of the reference organs and tumor burden and bone marrow uptake at each scan
| Baseline PET | Interim PET | Final PET | ||||||
|---|---|---|---|---|---|---|---|---|
| MBP | Liver | MBP | Liver | MBP | Liver | |||
| Lymphoma lesion | MTV | r a | −0.547 | −0.502 | 0.050 | 0.037 | 0.111 | 0.084 |
| p value a | <0.001 | <0.001 | 0.705 | 0.781 | 0.399 | 0.522 | ||
| TLG | r a | −0.511 | −0.517 | 0.025 | −0.011 | 0.106 | 0.075 | |
| p value a | <0.001 | <0.001 | 0.848 | 0.932 | 0.419 | 0.568 | ||
| Bone marrow | MTV | r a | −0.280 | −0.272 | −0.312 | −0.172 | 0.029 | 0.100 |
| p value a | 0.030 | 0.035 | 0.015 | 0.188 | 0.824 | 0.448 | ||
| TLG | r a | −0.288 | −0.278 | −0.320 | −0.173 | 0.018 | 0.093 | |
| p value a | 0.025 | 0.032 | 0.013 | 0.186 | 0.892 | 0.480 | ||
aPearson’s correlation
MTV metabolic tumor volume, TLG total lesion glycolysis
Fig. 1.
Estimated marginal means of the mediastinal blood pool (MBP) and liver SUVmean in patients with high tumor burden (greater than 1 SD above the median MTV) and low tumor burden at baseline. Each group showed a significantly different pattern of change in the SUVmean of the MBP and liver along the course of chemotherapy (repeated measures ANOVA test, between-subject effects p = 0.001, 0.001 respectively)
Fig. 2.
Baseline, interim, and final PET scans of a 74-year-old female with DLBCL. SUVmean of the mediastinal blood pool (MBP), liver, and metabolic tumor volume (MTV) of the lymphoma lesion, myocardium, and bone marrow (BM) is demonstrated
Bone marrow uptake was additionally measured in the baseline, interim, and final scans, and significantly higher MTV and TLG in the interim and final scan than baseline scan were demonstrated (repeated measures ANOVA test, p = 0.003 and 0.002; Table 2). Bone marrow MTV and TLG were negatively correlated with the SUVmean of the MBP and liver in baseline scans (Table 3). The MTV and TLG of bone marrow were negatively correlated with the MBP SUVmean in interim scans, too. Otherwise, the bone marrow FDG uptake and SUVmean of the MBP and liver showed no significant correlation.
Intra-Patient Variability in the SUV of the MBP, Liver, and Possible Associated Factors
We evaluated the association of intra-patient variability in the SUVmean of the MBP, liver, and other factors (Table 4). When comparing the interim scan to the baseline scan, the SUVmean of the MBP decreased by more than 10 % in 23 patients (38.3 %) and increased by more than 10% in 11 patients (18.3 %). The SUVmean of the liver decreased by more than 10 % in 20 patients (33.3 %) and increased in 18 patients (30 %). When we compared the final scan to the baseline scan, the SUVmean of the MBP decreased in 18 patients (30 %) and increased in 17 patients (28.3 %), and the SUVmean of the liver decreased in 16 patients (26.7 %) and increased in 24 patients (40 %). Patients with an increased SUVmean of the MBP in the final scan compared to baseline scan showed a more advanced Ann Arbor stage at diagnosis (p = 0.001). In other circumstances, scans with an increased SUVmean relative to the baseline scan tended to be associated with a more advanced Ann Arbor stage, although without statistical significance. The change of the mean serum AST/ALT level of the interim or final PET scans that showed an increase in the SUVmean of the liver of more than 10 % from the baseline scans was not significantly different from that of the interim or final scans showing a SUVmean of the liver that was 90-110 % of the baseline scans. Assessment of the correlation between the SUVmean of the liver and mean AST/ALT level at each time point also revealed no significant association (Pearson correlation test, r = 0.047, p = 0.719 for baseline scan; r = -0.003, p = 0.984 for interim scan; r = 0.180, p = 0.170 for final scan). There was no significant difference in body weight change among the subgroups according to the variability of the MBP SUVmean. However, in the subgroups according to variability of the liver SUVmean, a significant difference in body weight change was demonstrated (Table 5, p = 0.006).
Table 4.
Comparison of the initial stage and change in body weight according to change in the MBP SUVmean
| MBP SUVmean change (Interim scan from baseline scan) |
MBP SUVmean change (Final scan from baseline scan) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Decrease ≥ 10 % | Within 90–110 % of baseline | Increase ≥ 10 % | p value a | Decrease ≥10 % |
Within 90–110 % of baseline | Increase ≥ 10 % | p value a | |
| 23 | 26 | 11 | 18 | 25 | 17 | |||
| Ann Arbor stage at diagnosis | 1.74 | 2.38 | 2.45 | 0.106 | 1.78 | 1.84 | 3.00 | 0.001 |
| Body weight change (% of baseline) | −1.5 | 0.3 | −2.1 | 0.280 | −2.3 | 0.3 | 2.6 | 0.131 |
a One-way analysis of variances among groups
Table 5.
Comparison of the initial stage, change in the AST/ALT ratio, and change in body weight according to change in the liver SUVmean
| Liver SUVmean change (Interim scan from baseline scan) |
Liver SUVmean change (Final scan from baseline scan) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Decrease ≥10 % | Within 90–110 % of baseline | Increase ≥10 % | p value a | Decrease ≥10 % |
Within 90–110 % of baseline | Increase ≥10 % | p value a | |
| 20 | 22 | 18 | 16 | 20 | 24 | |||
| Ann Arbor stage | 1.90 | 2.14 | 2.44 | 0.377 | 1.88 | 2.00 | 2.46 | 0.253 |
| Mean AST/ALTb
(% compared to baseline) |
121 | 112 | 102 | 0.482 | 124 | 130 | 131 | 0.948 |
| Body weight change (% of baseline) | −1.2 | −0.3 | −1.0 | 0.840 | −4.4 | 1.0 | 2.6 | 0.006 |
aOne-way analysis of variances among groups
b(Follow-up AST + follow-up ALT)/(baseline AST + baseline AST) × 100
Change in Myocardial FDG Uptake During Chemotherapy
Myocardial FDG uptake was positive in 26 (43.3 %), 38 (63.3 %), and 39 (65.0 %) patients on baseline, interim, and final PET scans, respectively. Positive myocardial FDG uptake was demonstrated more frequently in PET scans performed after the start of R-CHOP than in baseline PET (Table 6; 64.2 % vs. 43.3 %, p = 0.008). However, the MTV or TLG of the myocardium was not significantly changed during chemotherapy. In addition, there was no significant difference in the frequency of positive myocardial FDG uptake between interim and final PET scans. The frequency of positive myocardial FDG uptake in the final scans was not significantly different between patients with or without dexrazoxane (42.9 % vs. 67.3 %, p = 0.226). MBP and liver SUVmean were not significantly correlated with myocardial MTV or TLG (Supplemental Table 2).
Table 6.
Change in myocardial FDG uptake during chemotherapy
| Baseline PET | Follow-up PET | p value | ||
|---|---|---|---|---|
| Interim | Final | |||
| Positive myocardial uptake (n) | 26 (43.3 %) | 38 (63.3 %) | 39 (65.0 %) | 0.008a |
| SUVmax (range) | 2.8–17.8 | 2.8–16.9 | 2.5–11.4 | |
| Total myocardium MTV, cm3 (mean ± SD) | 107.49 ± 173.91 | 147.51 ± 169.51 | 118.95 ± 133.49 | 0.289b |
| Total myocardium TLG, cm3 (mean ± SD) | 382.73 ± 699.67 | 474.18 ± 662.61 | 360.24 ± 441.48 | 0.337b |
| LV MTV, cm3 (mean ± SD) | 98.02 ± 149.22 | 138.35 ± 151.44 | 116.06 ± 129.38 | 0.183b |
| LV TLG, cm3 (mean ± SD) | 366.72 ± 661.52 | 456.85 ± 618.28 | 355.35 ± 434.60 | 0.378b |
aChi-square test, brepeated measures ANOVA
MTV metabolic tumor volume, TLG total lesion glycolysis, LV left ventricle
Discussion
This study demonstrated that liver FDG uptake did not change significantly throughout the course of R-CHOP chemotherapy in patients with DLBCL. The SUVmean of the MBP was changed along the course of chemotherapy though the difference was small. Patients with advanced stage at diagnosis showed significantly lower MBP and liver FDG uptake in basal and interim scans than patients with early stage. MTV and TLG of lymphoma lesions demonstrated significant negative correlation with the SUVmean of the MBP and liver in the baseline scan. These findings suggest that the tumor burden may be a significant factor affecting the SUV of the reference organs. Although positive myocardial uptake was more frequently observed in interim or final scans than baseline scans, the volumetric index did not change significantly after chemotherapy. The mean SUV of the MBP and liver were not significantly correlated with myocardial FDG uptake.
The five-point scale system using FDG uptake of the MBP and liver as a reference is a well-established reporting method for PET in patients with HL and some types of NHL, including DLBCL [11]. This scale has been validated for use in interim and final FDG PET scans and is recommended for reporting results of PET scans [11]. However, although this method is based on the assumption that FDG uptake in these reference organs does not show significant intra-patient variability, previous studies have shown conflicting results regarding this issue [3, 5]. Several studies have reported changes in the SUV of the MBP and liver after chemotherapy in patients with lymphoma. Ceriani et al. reported that interim liver FDG uptake was significantly increased compared to baseline or final scans in patients with DLBCL [3]. They suggested that reversible liver metabolic changes during the course of chemotherapy might induce such differences. However, FDG uptake in the liver was not affected by R-CHOP chemotherapy in the study of Kaya et al. [5], concordant with the results of the present study. Compared with previous studies [3, 5], we found that the MBP SUV of the interim scan was lower than that of the baseline or final scans. However, as the difference in mean SUV was only 0.1 or less, this might have a minimal clinical impact.
Patients with Ann Arbor stage I or II disease at diagnosis showed higher SUVs of the MBP and liver in baseline and interim scans than patients with Ann Arbor stage III or IV. This difference was not seen in the final scan. We hypothesized that the relatively large burden of malignant FDG-avid lesions of advanced-stage disease induces a lower SUV at other sites of the body; however, as most of the FDG-avid malignant lesions were resolved after chemotherapy, there would be no previous effect on the SUV of the MBP or liver. To evaluate this hypothesis, we measured the MTV and TLG of the lymphoma lesion in each scan and revealed a significant negative correlation among the MBP, liver SUV and MTV, and TLG of lymphoma lesions in the baseline scan. Most lymphoma lesions showed markedly decreased FDG uptake in interim and final scans and therefore no significant correlation between the volumetric index of the lymphoma lesion and the MBP and liver SUV after chemotherapy. These findings support our supposition and, to the best of our knowledge, are the first demonstration of a close association between the intra-patient variability of the SUV in reference organs and tumor burden.
Several previous studies have shown an association between elevated serum liver enzyme levels and the SUV of the liver [12, 13]. However, serum liver enzyme levels were not correlated with the liver SUV in this study; serum AST and ALT were not significantly changed during or after chemotherapy and did not show a consistent trend with or without variability of the liver SUVmean. Subgroups with an increase of more than 10 % of the liver SUVmean in the final scan compared to baseline scan showed increased body weight at the same time, whereas subgroups with a decrease of more than 10% of the liver SUVmean in the final scan demonstrated decreased body weight. There has been a report that the liver SUVmean increased with body weight in patients with breast cancer [14]. This may be related to the altered metabolism but the specific mechanism is unclear.
Myocardial FDG uptake was positive in 26 of 60 (43.3 %) baseline scans and in 77 of 120 (64.2 %) follow-up scans. This is consistent with a previous report of cardiotoxicity chemotherapy as a variable associated with higher cardiac FDG uptake [7]. Another study has suggested that increased myocardial FDG uptake might be a marker of cardiotoxicity associated with adriamycin-based chemotherapy [15]. However, in our study, there was no significant difference in the frequency of positive myocardial FDG uptake between the interim and final scans despite the two-fold higher cumulative dose of doxorubicin for the final scans or in the volumetric index of myocardial FDG uptake between baseline and follow-up scans. Furthermore, use of the cardiac protectant dexrazoxane was not associated with lower myocardial FDG uptake in follow-up scans. We also evaluated the possible association between myocardial FDG uptake and MBP/liver uptake and observed that there was no significant association between MBP and liver uptake and myocardial uptake at each time point. Therefore, myocardial FDG uptake may not affect the assessment of treatment response by FDG PET/CT using the five-point scale in patients with DLBCL.
This retrospective study has several limitations. First, although there are many indicators of hepatic function, this study used only serum AST and ALT levels for evaluation. Second, food intake and drug use before the 6-h fast were not controlled and might influence the level of myocardial FDG uptake. The results of this study will require confirmation by further prospective well-controlled studies.
Conclusion
The present study demonstrated that the SUV of the liver was not significantly changed during R-CHOP chemotherapy in patients with DLBCL, whereas the MBP SUV of the interim scan decreased slightly. However, patients with a large tumor burden at diagnosis showed a lower SUV of the MBP and liver in baseline and interim scans. SUV of the reference organs might be affected by the tumor burden, and this should be considered when assessing interim and follow-up PET/CT scans. Although myocardial FDG uptake was more frequently observed after R-CHOP chemotherapy, myocardial FDG uptake did not affect the SUV of the MBP or liver.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(DOC 50 kb)
Acknowledgment
None.
Compliance with Ethical Standards
Conflict of Interest
Soo Jeong Kim, Hyun Kyung Yi, Chae Hong Lim, Young Seok Cho, Joon Young Choi, Yearn Seong Choe, Kyung-Han Lee, Byung-Tae Kim, and Seung-Hwan Moon declare that they have no conflicts of interest.
Ethical Statement
Our study was performed in compliance with the regulations of our institution and generally accepted guidelines governing such work. Each author participated sufficiently in the work to take responsibility for the content. We confirm that this manuscript is original, is not under simultaneous consideration by another journal, and has not been previously published. There are no financial associations that might cause a conflict of interest.
References
- 1.Martelli M, Ferreri AJ, Agostinelli C, Di Rocco A, Pfreundschuh M, Pileri SA. Diffuse large B-cell lymphoma. Crit Rev Oncol Hematol. 2013;87:146–71. doi: 10.1016/j.critrevonc.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network. Non-Hodkin’s lymphomas (Version 2.2015). http://www.nccn.org/professionals/physician_gls/pdf/nhl.pdf. Accessed 15 June 2015.
- 3.Ceriani L, Suriano S, Ruberto T, Zucca E, Giovanella L. 18F-FDG uptake changes in liver and mediastinum during chemotherapy in patients with diffuse large B-cell lymphoma. Clin Nucl Med. 2012;37:949–52. doi: 10.1097/RLU.0b013e318263831d. [DOI] [PubMed] [Google Scholar]
- 4.Chiaravalloti A, Danieli R, Abbatiello P, Di Pietro B, Travascio L, Cantonetti M, et al. Factors affecting intrapatient liver and mediastinal blood pool (1)(8)F-FDG standardized uptake value changes during ABVD chemotherapy in Hodgkin’s lymphoma. Eur J Nucl Med Mol Imaging. 2014;41:1123–32. doi: 10.1007/s00259-014-2703-0. [DOI] [PubMed] [Google Scholar]
- 5.Kaya B, Dostbil Z, Ismailoglu M, Tasdemir B. Sahin O. Clin Imaging: Effect of R-CHOP chemotherapy on liver and mediastinal blood pool F-FDG standardized uptake values in patients with non-Hodgkin’s lymphoma; 2015. [DOI] [PubMed] [Google Scholar]
- 6.Lobert P, Brown RK, Dvorak RA, Corbett JR, Kazerooni EA, Wong KK. Spectrum of physiological and pathological cardiac and pericardial uptake of FDG in oncology PET-CT. Clin Radiol. 2013;68:e59–71. doi: 10.1016/j.crad.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Israel O, Weiler-Sagie M, Rispler S, Bar-Shalom R, Frenkel A, Keidar Z, et al. PET/CT quantitation of the effect of patient-related factors on cardiac 18F-FDG uptake. J Nucl Med. 2007;48:234–9. [PubMed] [Google Scholar]
- 8.Sawyer DB, Peng X, Chen B, Pentassuglia L, Lim CC. Mechanisms of anthracycline cardiac injury: can we identify strategies for cardioprotection? Prog Cardiovasc Dis. 2010;53:105–13. doi: 10.1016/j.pcad.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Limat S, Daguindau E, Cahn JY, Nerich V, Brion A, Perrin S, et al. Incidence and risk-factors of CHOP/R-CHOP-related cardiotoxicity in patients with aggressive non-Hodgkin’s lymphoma. J Clin Pharm Ther. 2014;39:168–74. doi: 10.1111/jcpt.12124. [DOI] [PubMed] [Google Scholar]
- 10.Freudenberg LS, Antoch G, Schutt P, Beyer T, Jentzen W, Muller SP, et al. FDG-PET/CT in re-staging of patients with lymphoma. Eur J Nucl Med Mol Imaging. 2004;31:325–9. doi: 10.1007/s00259-003-1375-y. [DOI] [PubMed] [Google Scholar]
- 11.Barrington SF, Mikhaeel NG, Kostakoglu L, Meignan M, Hutchings M, Mueller SP, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol. 2014;32:3048–58. doi: 10.1200/JCO.2013.53.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin CY, Ding HJ, Lin T, Lin CC, Kuo TH, Kao CH. Positive correlation between serum liver enzyme levels and standard uptake values of liver on FDG-PET. Clin Imaging. 2010;34:109–12. doi: 10.1016/j.clinimag.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Pak K, Kim SJ, Kim IJ, Kim K, Kim H, Kim SJ. Hepatic FDG uptake is not associated with hepatic steatosis but with visceral fat volume in cancer screening. Nucl Med Mol Imaging. 2012;46:176–81. doi: 10.1007/s13139-012-0143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Groheux D, Delord M, Rubello D, Colletti PM, Nguyen ML, Hindie E. Variation of liver SUV on (18)FDG-PET/CT studies in women with breast cancer. Clin Nucl Med. 2013;38:422–5. doi: 10.1097/RLU.0b013e3182872f0e. [DOI] [PubMed] [Google Scholar]
- 15.Borde C, Kand P, Basu S. Enhanced myocardial fluorodeoxyglucose uptake following Adriamycin-based therapy: Evidence of early chemotherapeutic cardiotoxicity? World J Radiol. 2012;4:220–3. doi: 10.4329/wjr.v4.i5.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 50 kb)