Abstract
Individuals in a group may obtain information from other group members about the environment, including the location of a food source or the presence of a predator. Here, we model how information spreads in a group using a susceptible-infected-removed epidemic model. We apply this model to a simulated shoal of fish using the motion dynamics of a coupled oscillator model, in order to test the biological hypothesis that polarized or aligned shoaling leads to faster and more accurate escape responses. The contributions of this study are the (i) application of a probabilistic model of epidemics to the study of collective animal behavior; (ii) testing the biological hypothesis that group cohesion improves predator escape; (iii) quantification of the effect of social cues on startle propagation; and (iv) investigation of the variation in response based on network connectivity. We find that when perfectly aligned individuals in a group are startled, there is a rapid escape by individuals that directly detect the threat, as well as by individuals responding to their neighbors. However, individuals that are not startled do not head away from the threat. In startled groups that are randomly oriented, there is a rapid, accurate response by individuals that directly detect the threat, followed by less accurate responses by individuals responding to neighbor cues. Over the simulation duration, however, even unstartled individuals head away from the threat. This study illustrates a potential speed-accuracy trade-off in the startle response of animal groups, in agreement with several previous experimental studies. Additionally, the model can be applied to a variety of group decision-making processes, including those involving higher-dimensional motion.
There are a variety of benefits to group living, many of which are conferred through information sharing between individuals. One striking example of this is the escape of a shoal of fish away from a potential threat. Fish who are unable to detect a threat directly may still respond rapidly by observing the movements of their neighbors. While differences in shoaling behavior have been observed in fishes, it is unclear how the degree of alignment between individuals affects the response to predation. In this study, we model information spread and the resulting motion of the escape response in order to test the hypothesis that increased alignment results in faster escape. We find that the alignment of the group does affect the escape response, and our results predict behavioral observations of previous studies. While inspired by fish shoals, the model may be of general use in studying the spread of information in other groups of animals as well, including humans. For example, another application of the model may be in emergency planning, where panic spreads through a group. The spread of panic as well as the resulting motion may be modeled based on simple behavioral rules, as was performed in this study for fish shoals.
I. INTRODUCTION
For animals that live in social groups, information about the environment may come directly from individual sensory modalities or through social cues from other members in the group.1–5 In shoaling fish, social cues have been shown to play an important role in anti-predator benefits. For example, the rapid wave of evasion of a shoal of fish away from a predator may be faster than the speed of the approaching predator.6–9 This observation suggests individuals in the group not only receive information from the predator directly but also by observing the behavior of surrounding individuals. Additionally, early predator detection has been observed in groups of fish,10–13 theoretically due to expanded sensory fields from the combined sensory capabilities of individuals in the group.
Behavioral studies suggest that social cues from neighboring fish not only spread information regarding the presence of a threat but can also affect the timing and dynamics of the response itself. Domenici and Batty14 quantified escape behavior of solitary herring Clupea harengus compared with schooling individuals and found that although the escape responses of schooling fish had longer latency, they exhibited higher probability of being startled away from the threat, thereby increasing the accuracy of the response. Godin and Morgan15 also found an increase in escape latency in schooling killifish as compared to solitary individuals startled by a model predator. Further evidence from behavioral, electrophysiological, and molecular studies indicate that the startle response in teleost fish is composed of a decision-making network of neurons16,17 that may be modulated by both social and ecological factors, including the presence of neighboring fish.14,15,18–20
In addition to these experimental studies, there have been several prior modeling studies investigating predator attack on a group.21–24 Many of these models used individual-based approaches to observe patterns of behavior and predict the prey's response to predator movements. Some have included evolutionary dynamics.25 However, there are few modeling approaches that have explicitly studied the influence of neighboring individuals on the startle response. A notable exception is Kolpas et al.,26 in which the authors modeled perturbations of a group of fish by changing the heading of one individual (simulating a rapid turn) in order to examine how changing the spatial position of an individual affects other individuals responding to the heading change.
Groups of fish vary greatly from loose aggregations to shoals and schools, based on the degrees of cohesion and alignment between group members. Many species of fish also exhibit dynamic behaviors that change over time.27,28 Breder29 defined obligate shoaling fishes as those that are polarized or exhibit alignment in heading constantly, and facultative shoaling fishes as those that are polarized occasionally. This terminology is generally used to specify shoaling behaviors as a result of various environmental contexts (e.g., reduced nearest-neighbor distance, and increased alignment in the presence of a threat).30 In this study, we use the term obligate to refer to shoals that are polarized before the presence of a threat, and facultative to refer to shoals that seek to polarize once a threat is detected by at least one member of the group.
While differences in shoaling behavior have been observed in fishes, it is unclear how the degree of polarization affects the response to predation. Aligned individuals are able to respond more quickly to movement changes made by their neighbors31 and, therefore, respond more rapidly to the location of prey or unknown changes in their environment.32 Domenici and Batty14 measured the trajectory of solitary and shoaling fish startling from a threat, and found that solitary fish escape towards or away from the stimulus, whereas shoaling fish tend to escape away from the threat. They hypothesized that when herring are shoaling, the ability of each fish to correct its trajectory is enhanced by the additional information obtained from startled neighbors.
This study uses numerical simulation and modeling methods to test the hypothesis that strong alignment improves a shoal's response to a threat. To do so, we apply a simple model of epidemic contagion to model the probability of response to a threat. Specifically, we use a susceptible-infected-removed (SIR) model33,34 based on three main probabilities: (i) the probability of an individual being susceptible to infection, (ii) the probability of being infected, and (iii) the probability of being cured.33,35,36 Here, we change the terminology slightly to refer to (i) the probability of a fish being susceptible to startle response, (ii) the probability of a fish being startled by either a directly perceived threat or cues from its neighbors, and (iii) the probability of remaining in a startled state, if previously startled. Using this modeling approach, we examine conditions under which a wave of information propagates through a group. (Previously, a similar model has been used to fit to experimental data.19)
While SIR models are an appropriate tool to examine contagion in a graph, these models do not include dynamics. In order to model simple dynamics of the startle response, we use a mathematical model of coupled phase oscillators based on the Kuramoto model.37 The Kuramoto model has been applied to the study of collective behavior and to biological groups, in particular,38–42 in which the phase angles correspond to the direction of motion of each agent. Phase-oscillator models have motivated studies on stability analysis43,44 and stabilization of planar collective motion.45,46 With the SIR-Kuramoto joint model, we are able to investigate the spread of information through a network and to study the orientation dynamics resulting from interactions with other agents.
In order to compare shoaling behaviors, we categorize agents as non-shoaling, obligate shoaling, or facultative shoaling. Additionally, we control for the individual effects of startling due to an external threat, or via neighbor cues, by examining the responses of each shoaling type in the presence or absence of social cues. When there are social cues from neighboring agents, we refer to these groups as attentive. When there is no use of social cues, we refer to these groups as inattentive. We then measure the proportion of fish startling over time, the directionality of the response, and the cohesion of the shoal after the startle response, in order to assess differences in response between the various types of shoaling behavior.
The contributions of this study are (i) application of a probabilistic model of epidemics to the study of animal behavior; (ii) testing the biological hypothesis that shoal cohesion improves predator escape; (iii) quantification of the effect of social cues on startle propagation; and (iv) investigation of the variation in response based on network connectivity. This work has application to the study of collective behavior in fish shoals and other animal groups.
II. METHODS
Consider a network36 of N phase angles indexed by and coupled via a random, undirected communication graph with nodes and edges such that the graph satisfies with probability c, unless otherwise specified. Thus, the edges between the nodes in the graph are built independently and with equal probability. In the absence of noise, these angles synchronize with one another under the shoaling method described next.
Let t represent the discrete time index, and the orientation of individual k at time t be . When not startled, agents are steered according to the following stochastic difference equation:
| (1) |
where ω1 represents the first of two forms of sensory noise. (The second noise source is described later.) Here, the noise is drawn randomly from a Gaussian distribution, N(0, σ1). The term κ is the coupling gain between pairs of agents and is of importance for the synchronization behavior of the model.37 Namely, κ > 0 yields synchronization and κ < 0 yields incoherent behavior.37 The notation denotes the neighbor set of k, which is the set of agents that generate information received by agent k.
A. Probabilistic model of information transmission
In order to study startle propagation in groups, we use a model of information transmission known in computer science and epidemiology literature as a susceptible-infected-remove (SIR) model. Once out of the startled state (i.e., after completing the startle response), agents cannot be re-startled; hence, it is removed. (Theoretically and biologically, if left for a sufficiently long period of time, startles could occur again. However, our goal here is to examine the spread of a single, isolated startle event.)
An important aspect of this model is the inclusion of time t. Let Pext be the probability that agent detects and responds to an external signal, such as a predation threat, at time t = 0. , the probability of agent k being in a startled state at t > 0, includes the probability of indirect detection of threatening stimuli through the interactions with other agents. Let Pint be the probability that an agent perceives (and responds to) a cue from another agent and Psus be the probability that an agent sustains the startle state from one time step to the next. Time t = 0 is the instant at which agents can perceive and respond directly to the threat. , for t > 0, is the probability at time t that agent k sustains a response from time step t − 1 or detects and responds to a neighboring agent in the startle state at time t − 1. (There are only two model states: startled and not startled, where both susceptible and removed agents are non-startled.)
At time step t > 0, each agent k has the following probability of being startled:
| (2) |
where is the probability that agent k was not startled in the previous time step.
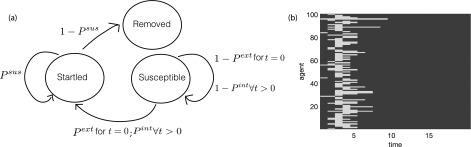
SIR models are a discrete-time Markov chain, i.e., a dynamical system composed of S discrete states. These states and the transitions between them are based on the probabilities in the model (Figure 1(a)). The state of each agent over time can also be represented as cellular automata (Figure 1(b)). In the cellular automata, each cell represents an agent k at a time step t. The color of the cell represents whether the agent is startled or not.
FIG. 1.
(a) The qualitative transition diagram illustrating how agents change state; (b) image depicting change in agent state over time as cellular automata. Blue indicates a non-startled state, and yellow indicates a startled state. On the first time step, seven agents startle due to the external threat via Pext. On subsequent time steps, the startle propagates throughout the shoal.
B. Behavioral rules when startled
Agents execute different behavioral rules depending on what signal initiated the startled state (i.e., an external threat via Pext or an internal cue via Pint), and whether or not the startle has just been initiated.
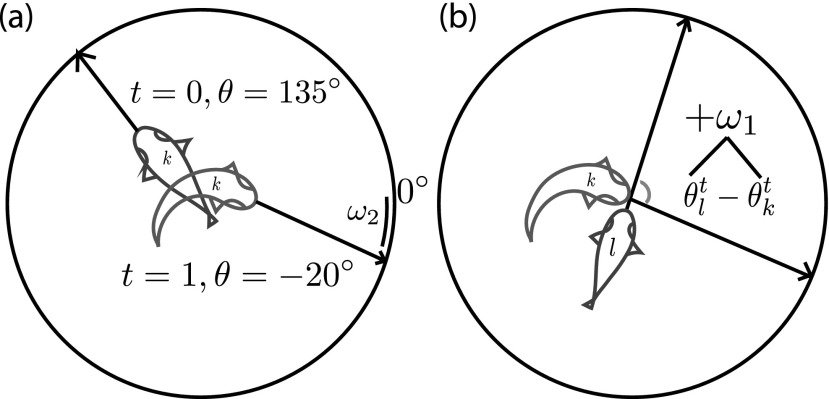
When there is no disturbance or threat to the shoal, agents synchronize at a rate dependent on the gain of the synchronization model, κ. At time t = 0, agents have probability Pext of responding to an external threat. When startled directly from a threat via Pext, agents react by instantaneously reorienting—with some variation due to noise—towards a reference direction, θs (Figure 2(a)). Without loss of generality, the reference direction is considered to be the direction away from the threat and is set to θs = 0. The startle impulse is thus
| (3) |
where ω2 is the variability of directionality in the startle response drawn randomly from a Gaussian distribution, . This behavioral rule simulates the rapid turning behavior of fish during an escape response.47
FIG. 2.
(a) Illustration of an agent (depicted here as a fish) at a random orientation at the first time step (t = 0, blue), and transitioning to startled state (t = 1, red). When startled directly from a threat, agents react by instantaneously reorienting towards a reference direction, with some noise (ω2). Without loss of generality, the reference direction is set to θs = 0. (b) Illustration of two agents. When startled indirectly by social cues, agents move in the average direction of their startled neighbors. In this illustration, only the difference in orientation between two agents is shown, along with noise (ω1) that is added to the difference in orientation, which represents the noise in the agent's ability to sense their neighbor's orientation.
At the following time steps, t > 0, agents that are currently in a susceptible state (not yet startled) respond to startled neighbors with the probability Pint. Let be the set of startled neighbors of agent k. If startled from neighbor cues, agents react to an impulse directing them in the average orientation of their startled neighbors (Figure 2(b)), i.e.,
| (4) |
Subsequent to the initial transition to the startled state, if agents remain in the startled state via Psus, they follow the behavioral rules of the synchronization model as they would in the susceptible state. While sustaining a startled state, agents may still continue to propagate the response. Table I details the model parameters and the parameter values explored in this study. Simulations were run for 100 time steps. The startle model typically lasted no more than the first 10 time steps for the value of Psus used.
TABLE I.
Parameter space of probabilistic startle model.
| Parameter | Symbols | Values |
|---|---|---|
| Probability of startling to threat | Pext | 0.05, 0.1, 0.5 |
| Probability of startling to a neighbor cue | Pint | 0, 0.05, 0.1, 0.5 |
| Probability of sustaining a response | Psus | 0.5 |
| Coupling gain between agents | κ | 0, 0.2 |
| Initial orientation (obligate) | θ0 | 180° |
| Initial orientation (facultative, non-shoal) | θ0 | Random |
| Standard deviation of noise in synchrony model | σ1 | 2° |
| Standard deviation noise in startle response | σ2 | 20∘ |
| Number of agents | N | 100 |
| Connectivity of random graph | c | 1, 0.5, 0.2 |
C. Shoaling types
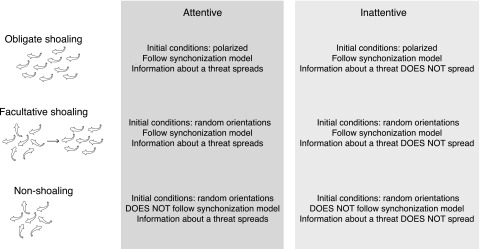
Three shoaling behaviors are investigated: obligate, facultative, and non-shoaling. Obligate shoaling agents are characterized by being initially polarized, and then continuing to follow the synchronization model. Facultative shoaling agents start in random orientations, but in the presence of a threat, they follow the rules of the synchronization model. Non-shoaling agents do not follow the synchrony model.
In addition to these shoaling types, we also distinguish between attentive and inattentive shoals, based on the space they occupy in the probabilistic startle model. Namely, if Pint > 0, the shoal is considered attentive, and the startle will spread, whereas if Pint = 0, then the shoal is considered inattentive and the reaction to the threat does not spread throughout the group. The inattentive group also serves as a control to compare the effects of social information transmission within a group. Table II shows the parameter values for each shoaling type, and Figure 3 details the behavior of each shoal type.
TABLE II.
Shoaling categories.
| Behavior | Initial conditions | Gain | Attentive, inattentive |
|---|---|---|---|
| Obligate | Polarized | κ > 0 | Pint > 0, Pint = 0 |
| Facultative | Random | κ > 0 | Pint > 0, Pint = 0 |
| Non-shoaling | Random | κ = 0 | Pint > 0, Pint = 0 |
FIG. 3.
Categorization of shoaling behavior in the model.
D. Analysis metrics
The proportion of the shoal startled is measured at each time step, which gives the number of fish startled, as well as how rapidly the information spreads. The proportion of the shoal startling will be the same for all shoaling types examined here, because there is no coupling between the motion dynamics of the synchronization model and the startle probability model. This outcome would not be the case if the dynamics of the synchronization model were to change the connectivity between neighbors (the interaction topology), since social information based on neighbor connectivity is a major aspect of the startle probability model.
The orientation dynamics of the responses are measured over time as well and compared between the shoaling groups. We characterize a successful escape from the threat as orienting within ±20° of the reference direction, and investigate two critical time points. The first time point is when all startles have just completed. The second time point is at the end of the simulation.
III. RESULTS
We combined a model of orientation synchronization based on coupled oscillators with a model of information propagation to predict how information would be transferred through a group and how shoaling behavior would affect the dynamics of the startle response.
A. Startle propagation
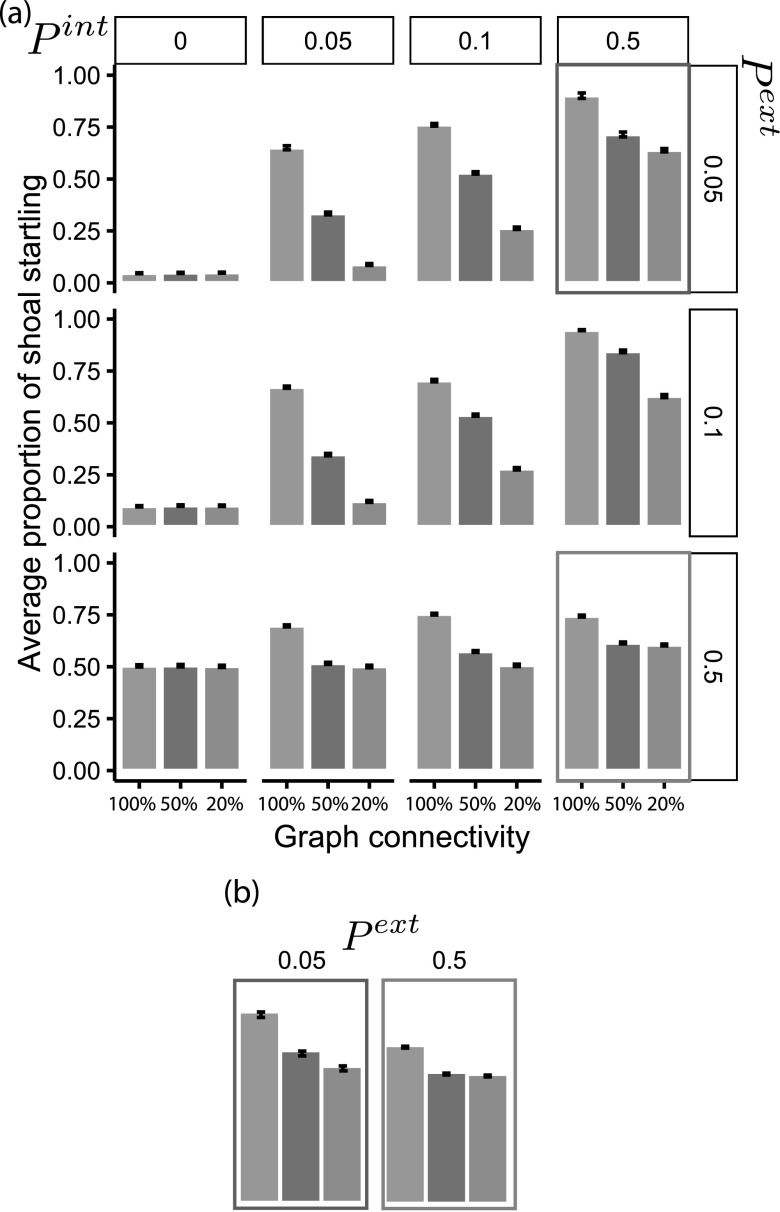
First, we examine the roles of network topology, Pint, and Pext on the spread of information through the group. Figure 4 shows the average proportion of the shoal startling for given Pint and Pext values, and three different network topologies, specified by percent connectivity.
FIG. 4.
(a) Bar plots illustrating the max proportion of the shoal startled (averaged over 300 Monte Carlo runs) for sample values of Pext and Pint. Error bars are 95% confidence intervals. (b) Inset with highlights red and blue boxed subgraphs side-by-side for easier comparison.
When Pint = 0, Figure 4(a) (first column) shows the isolated effect of Pext. Without any social information transmission, a proportion of the shoal startles according to the probability of detecting a threat. Additionally, the proportion of the shoal startling tends to increase with increasing values of Pint and Pext.
A non-intuitive exception to this result is that at higher values of Pint (i.e., 0.5), there are more agents responding when Pext is lower, as opposed to higher (Figure 4(b)). This result can be attributed to the removal aspect of the SIR model, and the value of Psus. If fish startle due to Pext, and the startle responses complete before it can spread to neighboring individuals, it would result in an overall reduced propagation of the response. This result indicates an interaction between the three probabilities in the model and the proportion of agents startling.
As might be expected, more highly connected topologies have a higher proportion of agents responding to the threat. The rate of information spreading happens rapidly in all topology cases, with peak responses occurring in the first or second time step. The difference in peak number of fish responding between topologies can be seen in Figure 4. The greatest difference in the number of fish responding between interaction topologies occurs at low values of Pint and Pext. At higher values of Pint and Pext, all fish startle in response to the threat.
B. Orientation dynamics
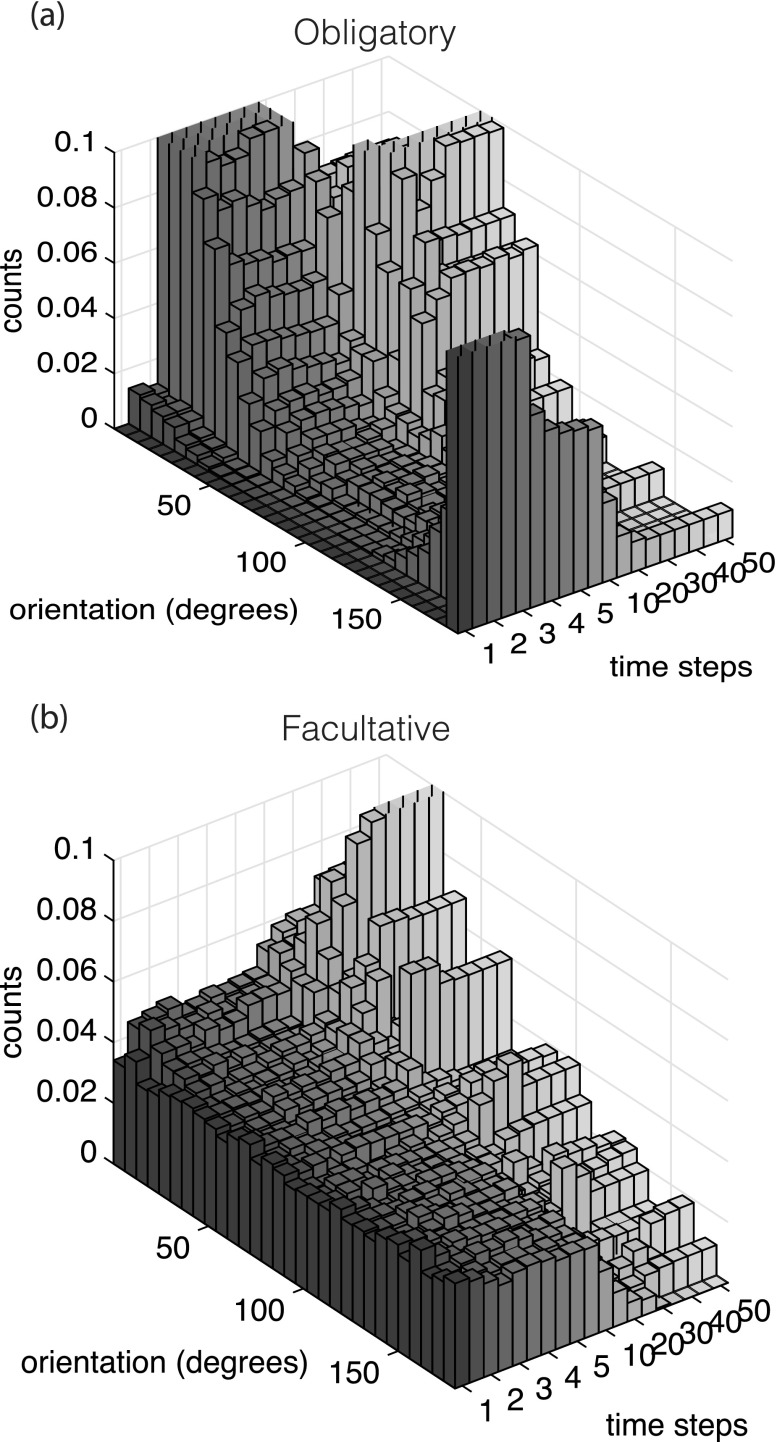
Figure 5 illustrates the orientation histograms over all simulations of obligate and facultative attentive shoals (for inattentive and non-shoaling simulation histograms at the same Pint and Pext). From these histograms, it can be observed that the behaviors in the model occur at two time scales: an initial, rapid response from the startle model, and a slower response driven by the synchronization model. In obligate shoals, there is a higher proportion of the shoal startling away from the threat. However, by the end of the simulation, many of these agents have re-oriented approximately 25°–30° away from the reference direction. In contrast, for the facultative shoals, although fewer individuals respond directly to the threat, by the end of the simulation, a majority of the agents are oriented away from the threat.
FIG. 5.
Waterfall plots displaying orientation histograms over time for attentive obligate and facultative shoaling types, Pint = 0.05 and Pext = 0.05. Note that the time scale plots the first five time steps when most startles occur, then plots once every 10 time steps up until 50, by which time the startles have ended. To see similar images for all cases investigated in this study, see supplementary material.
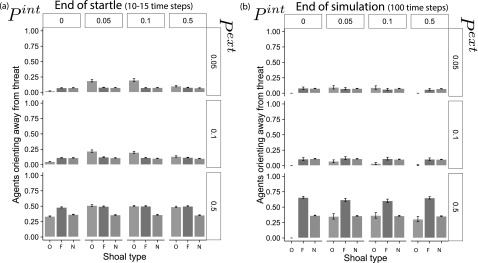
We quantify these results focusing on two time points: (i) when startles end and (ii) when the simulation ends. Figure 6 shows the average proportion of successful escapes at the time step when the last startle state ended (Figure 6(a)) and the last time step in the simulation (Figure 6(b)). At the completion of the startle responses in the group, obligate shoaling agents show a higher proportion of the shoal escaping the threat as compared to facultative and non-shoaling groups. The exceptions to this result are when the shoal is inattentive (no neighbor information), or when Pext is high. When there is no social information, facultative and non-shoaling groups show a higher proportion of agents oriented away from the threat. However, this result is due to the fact that these groups are initialized in a random orientation. So, by chance, more agents are likely to be oriented away from the threat. Given that the average number of agents startling in all shoal groups is the same, the agents that startle in the obligate shoal group exhibit a more accurate response according to this criterion (Figure 6(a)).
FIG. 6.
Bar plots illustrating the average proportion (over 300 Monte Carlo runs) of the shoal oriented ±20° from the reference direction. Error bars are 95% confidence intervals. (a) Orientation at the end of startles, and (b) orientation at the end of the simulation.
Figure 6(b) illustrates the proportion of agents oriented to the reference direction at the end of the simulation. For the cases where Pext is 0.1 or 0.5, there are noticeably more facultative shoaling agents oriented towards the reference direction than obligate shoaling agents. For the highest value of Pext, where there would be more first responders, there are approximately 10% more than immediately following the startle responses, suggesting that non-startled agents were recruited to the direction away from the threat, but at a much slower rate. In comparison, there are significantly fewer obligate shoaling agents oriented towards the reference direction at the end of the simulation than immediately following the startle responses, suggesting a strong influence of the role of non-startled agents in determining the orientation of the synchrony model.
Thus, obligate shoaling agents respond much more rapidly than facultative shoaling agents to a threat (0%–20% more of the shoal escaping at the end of the last startle response). Facultative shoaling fish, however, are able to exert stronger influence on their shoal-mates orientation through the synchronization model. The supplementary figure depicts waterfall plots of histograms of agent orientation over time, normalized by 30 000 (100 agents × 300 Monte Carlo runs), bounded between 0 and π.
IV. DISCUSSION
This study uses an epidemic model paired with a model of coupled oscillators to model information transmission in groups of fish. We examine potential differences in startle response behavior based on shoaling properties and investigate the spread of information transmission through a group using a model of contagion.
To the best of our knowledge, this study is the first to apply an epidemic model to the study of fish startle response behavior and the first to numerically analyze potential effects of polarization on startle response behavior. This model specifies probabilities for responding to an external threat, being startled by neighbors, and remaining in a startled state. The probability model alone yields results that resemble a cellular automaton, with two explicit states: startled and non-startled. When paired with the coupled oscillator system, behavioral dynamics can be evaluated. Importantly, the parameter space of the oscillator model was categorized into non-shoaling, facultative shoaling, and obligate shoaling types, based on the level of alignment of group members. The model predicts faster spreading of the startle response in previously polarized groups (obligatory shoalers), and slower, but more accurate, responses in facultative shoalers.
We find differences in startle response behavior between shoaling types, even for attentive obligatory and facultative shoals, where the only difference between them is the initial orientation of the shoal when the threat is introduced. The results of the model reinforce the idea that there are benefits of polarized shoaling.31 The model also predicts that randomly oriented shoals may be better at transferring information to uninformed individuals, leading to a slower, but more accurate response. A speed-accuracy trade-off may be a strategy used by fish shoals in response to a predator.47
We investigated different values of Pext and Pint, which varies the number of informed individuals and the rate of information transmission among group members, respectively. Rosenthal et al.48 found differences in the fish who startled first in the group, where first responders were more likely to be found closer to the group boundary (front and sides) than non-responders and, even for shoaling behavior, fish at the front of the shoal have been shown to exert more influence over the heading of their neighbors.49 The value of Pext in our model may be similarly varied based on spatial information. Additionally, the parameter Psus was kept fixed for this study. However, the value of Psus has been shown to play an important role in the timing of epidemic spreading33 and can be varied as well.
Our model could also be used to assess differences in group size and number of responders necessary to propagate information. A small number of knowledgeable individuals has been previously shown to elicit a response from both small and large groups.50–53 However, in smaller groups, a greater proportion of knowledgeable individuals is needed to elicit a response,52,54 and it is more likely that inaccurate decisions will be made.55 Unlike simple contagion processes such as a disease epidemic, where multiple ties dampen the spread of disease,56 multiple ties in social networks provide a reinforcement effect, while connected neighbors not responding inhibit a response.48
Here, we assume undirected coupling between individuals in the group. Fish shoals and other animal groups are likely to have weighted, directed connections48 since the sensory (visual, lateral line, and acoustic) cues fish receive from their neighbors tend not to be symmetrical. In this model, spatial cues were not considered, but even with the simplest version of the model, our results aligned with previous experimental studies.19,31,47 Additionally, the startle probability model captures the reinforcement effect found in the social contagion and information propagation within fish shoals.
If directed coupling were to be included in this model, we might expect agents with more connections to other individuals to have a greater probability of responding via Pint than those with fewer connections. Additionally, individuals with connections to first responders (those startled by Pext) would be more likely to respond to the threat than those not connected to first responders.
In this study, low probabilities of Pext and Pint were used. The greater the number of connections between agents, the lower this number needs to be to avoid saturation of the response (due to reinforcement). It may also be possible to scale this parameter with distance or number of neighbors, such that an agent may have a high probability of interacting with close neighbors, but reduced probability of being startled by more distant neighbors, thereby weighting the connections between individuals.
The model is quite generalizable. It includes two behaviors (synchronizing and startling) that occur on different time scales and may involve separate network interactions. This model may be used to test other hypotheses regarding startle response behaviors in fish, including information transmission based on location within a shoal, different network interactions, and heterogenous groups. It is important to note that the synchronization model and the probabilistic startle model operate independently in this study, but may interact depending on how the interaction topology between agents is specified. Additionally, this model may be used to investigate different information-transmission or decision-making behaviors in animal groups.
SUPPLEMENTARY MATERIAL
See supplementary material for the waterfall plots of all orientation histograms.
ACKNOWLEDGMENTS
The authors acknowledge the University of Maryland supercomputing resources (http://www.it.umd.edu/hpcc) made available in conducting the research reported in this paper. This work was funded in part by the National Science Foundation, Grant No. CMMI 0954361 to D. A. Paley. This work was also funded in part by training Grant No. DC-00046 from the National Institute of Deafness and Communicative Disorders of the National Institutes of Health to A. Chicoli, and a Wylie Dissertation Fellowship from the University of Maryland to A. Chicoli.
References
- 1. Pulliam H. R., “ On the advantages of flocking,” J. Theor. Biol. , 419–422 (1973). 10.1016/0022-5193(73)90184-7 [DOI] [PubMed] [Google Scholar]
- 2. Lazarus J., “ The early warning function of flocking in birds: An experimental study with captive Quelea,” Anim. Behav. , 855–865 (1979). 10.1016/0003-3472(79)90023-X [DOI] [Google Scholar]
- 3. Giraldeau L., Valone T. J., and Templeton J. J., “ Potential disadvantages of using socially acquired information,” Philos. Trans. R. Soc. London, Ser. B , 1559–1566 (2002). 10.1098/rstb.2002.1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boland C. R. J., “ An experimental test of predator detection rates using groups of free-living emus,” Ethology , 209–222 (2003). 10.1046/j.1439-0310.2003.00860.x [DOI] [Google Scholar]
- 5. Kurvers R. H. J. M., Wolf M., and Krause J., “ Humans use social information to adjust their quorum thresholds adaptively in a simulated predator detection experiment,” Behav. Ecol. Sociobiol. , 449–456 (2014). 10.1007/s00265-013-1659-6 [DOI] [Google Scholar]
- 6. Treherne J. E. and Foster W. A., “ Group transmission of predator avoidance-behavior in a marine insect – The Trafalgar effect,” Anim. Behav. , 911–917 (1981). 10.1016/S0003-3472(81)80028-0 [DOI] [Google Scholar]
- 7. Gerlotto F., Bertrand S., Bez N., and Gutierrez M., “ Waves of agitation inside anchovy schools observed with multibeam sonar: A way to transmit information in response to predation,” ICES J. Mar. Sci. , 1405–1417 (2006). 10.1016/j.icesjms.2006.04.023 [DOI] [Google Scholar]
- 8. Marras S., Batty R. S., and Domenici P., “ Information transfer and anti-predator maneuvers in schooling herring,” Adapt. Behav. , 44–56 (2012). 10.1177/1059712311426799 [DOI] [Google Scholar]
- 9. Herbert-Read J. E., Buhl J., Hu F., Ward A. J. W., and Sumpter D. J. T., “ Initiation and spread of escape waves within animal groups,” R. Soc. Open Sci. , 140355 (2015); e-print arXiv:1409.6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Magurran A. E., Oulton W. J., and Pitcher T. J., “ Vigilant behaviour and shoal size in minnows,” Z. Tierpsychol. , 167–178 (1985). 10.1111/j.1439-0310.1985.tb01386.x [DOI] [Google Scholar]
- 11. Webb P. W., “ Does schooling reduce fast-start response latencies in teleosts?,” Comp. Biochem. Physiol. , 231–234 (1980). 10.1016/0300-9629(80)90230-3 [DOI] [Google Scholar]
- 12. Tegeder R. W. and Krause J., “ Density dependence and numerosity in fright stimulated aggregation behavior in shoaling fish,” Philos. Trans. R. Soc. B , 381–390 (1995). 10.1098/rstb.1995.0172 [DOI] [Google Scholar]
- 13. Brown C. and Warburton K., “ Social mechanisms enhance escape responses in shoals of rainbowfish, Melanotaenia duboulayi,” Environ. Biol. Fishes , 455–459 (1999). 10.1023/A:1007518710790 [DOI] [Google Scholar]
- 14. Domenici P. and Batty R. S., “ Escape behaviour of solitary herring (Clupea harengus) and comparisons with schooling individuals,” Mar. Biol. , 29–38 (1997). 10.1007/s002270050065 [DOI] [Google Scholar]
- 15. Godin J. G. J. and Morgan M. J., “ Predator avoidance and school size in a cyprinodontid fish, the banded killifish (Fundulus diaphanus Lesueur),” Behav. Ecol. , 105–110 (1985). 10.1007/BF00295142 [DOI] [Google Scholar]
- 16. Zottoli S. J. and Faber D. S., “ The Mauthner cell: What has it taught us?,” Neuroscientist , 26–38 (2000). 10.1177/107385840000600111 [DOI] [Google Scholar]
- 17. Korn H. and Faber D. S., “ The Mauthner cell half a century later: A neurobiological model for decision-making?,” Neuron , 13–28 (2005). 10.1016/j.neuron.2005.05.019 [DOI] [PubMed] [Google Scholar]
- 18. Medan V. and Preuss T., “ The Mauthner-cell circuit of fish as a model system for startle plasticity,” J. Physiol. Paris , 129–140 (2014). 10.1016/j.jphysparis.2014.07.006 [DOI] [PubMed] [Google Scholar]
- 19. Chicoli A., Butail S., Lun Y., Bak-Coleman J., Coombs S., and Paley D. A., “ The effects of flow on schooling Devario aequipinnatus: School structure, startle response and information transmission,” J. Fish Biol. , 1401–1421 (2014). 10.1111/jfb.12365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Diamond K., “ Environmental effects on fish escape responses: Impact of flow on the escape performance of the Hawaiian stream goby Sicyopterus stimpsoni,” Ph.D. thesis, Clemson University, 2015. [Google Scholar]
- 21. Vabø R. and Nøttestad L., “ An individual based model of fish school reactions: predicting anti-predator behaviour as observed in nature,” Fish. Oceanogr. , 155–171 (1997). 10.1046/j.1365-2419.1997.00037.x [DOI] [Google Scholar]
- 22. Inada Y. and Kawachi K., “ Order and flexibility in the motion of fish schools,” J. Theor. Biol. , 371–387 (2002). 10.1006/jtbi.2001.2449 [DOI] [PubMed] [Google Scholar]
- 23. Zheng M., Kashimori Y., Hoshino O., Fujita K., and Kambara T., “ Behavior pattern (innate action) of individuals in fish schools generating efficient collective evasion from predation,” J. Theor. Biol. , 153–167 (2005). 10.1016/j.jtbi.2004.12.025 [DOI] [PubMed] [Google Scholar]
- 24. Lee S. H., Pak H. K., and Chon T. S., “ Dynamics of prey-flock escaping behavior in response to predator's attack,” J. Theor. Biol. , 250–259 (2006). 10.1016/j.jtbi.2005.09.009 [DOI] [PubMed] [Google Scholar]
- 25. Wood A. J. and Ackland G. J., “ Evolving the selfish herd: emergence of distinct aggregating strategies in an individual-based model,” Proc. R. Soc. B: Biol. Sci. , 1637–1642 (2007). 10.1098/rspb.2007.0306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kolpas A., Busch M., Li H., Couzin I. D., Petzold L., and Moehlis J., “ How the spatial position of individuals affects their influence on swarms: A numerical comparison of two popular swarm dynamics models,” PloS One , e58525 (2013). 10.1371/journal.pone.0058525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Miller N. Y. and Gerlai R., “ Oscillations in shoal cohesion in zebrafish (Danio rerio),” Behav. Brain Res. , 148–151 (2008). 10.1016/j.bbr.2008.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Viscido S. V., Parrish J. K., and Grünbaum D., “ Individual behavior and emergent properties of fish schools: A comparison of observation and theory,” Mar. Ecol.: Prog. Ser. , 239–249 (2004). 10.3354/meps273239 [DOI] [Google Scholar]
- 29. Breder C., “ Studies on the social groupings of fishes,” Bull. Mus. Am. Nat. Hist. , 393–482 (1959); available at http://digitallibrary.amnh.org/handle/2246/1187. [Google Scholar]
- 30. Delcourt J. and Poncin P., “ Shoals and schools: Back to the heuristic definitions and quantitative references,” Rev. Fish Biol. Fish. , 595–619 (2012). 10.1007/s11160-012-9260-z [DOI] [Google Scholar]
- 31. Couzin I. D., Krause J., James R., Ruxton G. D., and Franks N. R., “ Collective memory and spatial sorting in animal groups,” J. Theor. Biol. , 1–11 (2002). 10.1006/jtbi.2002.3065 [DOI] [PubMed] [Google Scholar]
- 32. Laland K. and Williams K., “ Shoaling generates social learning of foraging information in guppies,” Anim. Behav. , 1161–1169 (1997). 10.1006/anbe.1996.0318 [DOI] [PubMed] [Google Scholar]
- 33. Billings L., Spears W. M., and Schwartz I. B., “ A unified prediction of computer virus spread in connected networks,” Phys. Lett. A , 261–266 (2002). 10.1016/S0375-9601(02)00152-4 [DOI] [Google Scholar]
- 34. Kempe D., Kleinberg J., and Tardos E., “ Maximing the spread of influence through a social network,” Theory Comput. , 105–147 (2015). 10.4086/toc.2015.v011a004 [DOI] [Google Scholar]
- 35. Kermack W. O. and McKendrick A. G., “ A contribution to the mathematical theory of epidemics,” Proc. R. Soc. London, Ser. A , 700–721 (1927). 10.1098/rspa.1927.0118 [DOI] [Google Scholar]
- 36. Mesbahi M. and Egerstedt M., Graph Theoretic Methods in Multi-Agent Networks ( Princeton University Press, New Jersey, 2010). [Google Scholar]
- 37. Kuramoto Y., “ Self-entrainment of a population of coupled non-linear oscillators,” in International Symposium on Mathematical Problems in Theoretical Physics ( Springer, 1975), pp. 420–422. [Google Scholar]
- 38. Boi S., Couzin I. D., Buono N. D., Franks N. R., and Britton N. F., “ Coupled oscillators and activity waves in ant colonies,” R. Soc. , 371–378 (1999). 10.1098/rspb.1999.0647 [DOI] [Google Scholar]
- 39. Strogatz S., “ From Kuramoto to Crawford: Exploring the onset of synchronization in populations of coupled oscillators,” Physica D , 1–20 (2000). 10.1016/S0167-2789(00)00094-4 [DOI] [Google Scholar]
- 40. Nabet B., Leonard N. E., Couzin I. D., and Levin S. A., “ Dynamics of decision making in animal group motion,” J. Nonlinear Sci. , 399–435 (2009). 10.1007/s00332-008-9038-6 [DOI] [Google Scholar]
- 41. Leonard N. E., Shen T., Nabet B., Scardovi L., Couzin I. D., and Levin S. A., “ Decision versus compromise for animal groups in motion,” Proc. Natl. Acad. Sci. U.S.A. , 227–232 (2012). 10.1073/pnas.1118318108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chicoli A., Bak-Coleman J., Coombs S., and Paley D. A., “ Rheotaxis performance increases with group size in a coupled phase model with sensory noise,” Eur. Phys. J.: Spec. Top. , 3233–3244 (2015). 10.1140/epjst/e2015-50080-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jadbabaie A., Lin J., and Morse A. S., “ Coordination of groups of mobile autonomous agents using nearest neighbor rules,” IEEE Trans. Autom. Control , 988–1001 (2003). 10.1109/TAC.2003.812781 [DOI] [Google Scholar]
- 44. Moreau L., “ Stability of multiagent systems with time-dependent communication links,” IEEE Trans. Autom. Control , 169–182 (2005). 10.1109/TAC.2004.841888 [DOI] [Google Scholar]
- 45. Sepulchre R., Paley D. A., and Leonard N. E., “ Stabilization of planar collective motion with limited communication,” IEEE Trans. Autom. Control , 706–719 (2008). 10.1109/TAC.2008.919857 [DOI] [Google Scholar]
- 46. Paley D. A., Leonard N. E., Sepulchre R., and Couzin I. D., “ Spatial models of bi-stability in biological collectives,” in Proceedings of the 46th IEEE Conference on Decision and Control New Orleans, LA, USA, Dec. 12–14, 2007 (2007), pp. 4851–4856. [Google Scholar]
- 47. Domenici P. and Blake R. W., “ The kinematics and performance of fish fast-start swimming,” J. Exp. Biol. , 1165–1178 (1997); available at http://jeb.biologists.org/content/200/8/1165. [DOI] [PubMed] [Google Scholar]
- 48. Rosenthal S. B., Twomey C. R., Hartnett A. T., Wu H. S., and Couzin I. D., “ Revealing the hidden networks of interaction in mobile animal groups allows prediction of complex behavioral contagion,” Proc. Natl. Acad. Sci. U.S.A. , 4690–4695 (2015). 10.1073/pnas.1420068112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Krause J., Hoare D., Krause S., Hemelrijk C. K., and Rubenstein D. I., “ Leadership in fish shoals,” Fish Fish. , 82–89 (2000). 10.1111/j.1467-2979.2000.tb00001.x [DOI] [Google Scholar]
- 50. Gueron S., Levin S. A., and Rubenstein D. I., “ The dynamics of herds: From individuals to aggregations,” J. Theor. Biol. , 85–98 (1996). 10.1006/jtbi.1996.01448796191 [DOI] [Google Scholar]
- 51. Reebs S. G., “ Can a minority of informed leaders determine the foraging movements of a fish shoal?,” Anim. Behav. , 403–409 (2000). 10.1006/anbe.1999.1314 [DOI] [PubMed] [Google Scholar]
- 52. Huse G., Railsback S., and Feronö A., “ Modelling changes in migration pattern of herring: collective behaviour and numerical domination,” J. Fish Biol. , 571–582 (2002). 10.1111/j.1095-8649.2002.tb01685.x [DOI] [Google Scholar]
- 53. Mirabet V., Fréon P., and Lett C., “ Factors affecting information transfer from knowledgeable to naive individuals in groups,” Behav. Ecol. Sociobiol. , 159–171 (2008). 10.1007/s00265-008-0647-8 [DOI] [Google Scholar]
- 54. Couzin I. D., Krause J., Franks N. R., and Levin S. A., “ Effective leadership and decision-making in animal groups on the move,” Nature , 513–516 (2005). 10.1038/nature03236 [DOI] [PubMed] [Google Scholar]
- 55. Ward A. J. W., Sumpter D. J. T., Couzin I. D., Hart P. J. B., and Krause J., “ Quorum decision-making facilitates information transfer in fish shoals,” Proc. Natl. Acad. Sci. U.S.A. , 6948–6953 (2008). 10.1073/pnas.0710344105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Centola D. M. and Macy M., “ Complex contagions and the weakness of long ties,” Am. J. Sociol. , 702–734 (2007). 10.1086/521848 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See supplementary material for the waterfall plots of all orientation histograms.