Abstract
Homogeneous nucleation and subsequent cluster growth leads to the formation of new aerosol particles in the atmosphere1. Nucleation of sulphuric acid and organic vapours is thought to be responsible for new particle formation over continents1,2 while iodine oxide vapours have been implicated in particle formation over coastal regions3–7. Molecular clustering pathways involved in atmospheric particle formation have been elucidated in controlled laboratory studies of chemically simple systems2,8–10. But no direct molecular-level observations of nucleation in atmospheric field conditions involving either sulphuric acid, organic or iodine oxide vapours have been reported to date11. Here we report field data from Mace Head, Ireland and supporting data from northern Greenland and Queen Maud Land, Antarctica that allow for the identification of the molecular steps involved in new particle formation in an iodine-rich, coastal atmospheric environment. We find that the formation and initial growth process is almost exclusively driven by iodine oxoacids and iodine oxide vapours with average resulting cluster O:I ratios of 2.4. Based on the high O:I ratio, together with observed high concentrations of iodic acid, HIO3, we suggest that cluster formation primarily proceeds by sequential addition of iodic acid HIO3, followed by intra-cluster restructuring to I2O5 and recycling of water in the atmosphere or upon drying. Overall, our study provides ambient atmospheric molecular-level observations of nucleation, supporting the previously suggested role of iodine containing species in new particle formation3–7, 12–18, and identifies the key nucleating compound.
Elucidation of the key question “how do new clusters and particles form in the atmosphere?” has failed to date due to the lack of a measurement technique sufficiently sensitive to detect or identify the chemical composition of nucleating clusters, given that most new atmospheric particle formation events occur at modest intensities. An exception, in terms of nucleation burst intensity, is coastal new particle formation where exceptionally high formation and growth rates, relative to any other environment, have been observed3,7. The observed rapid new particle formation and growth in coastal air requires extraordinarily high production rates of particle precursors. Biogenic emissions of iodine vapors, mainly I2[14–15,19], from marine algae have been implicated in the production of I atoms by photolysis reactions. I atoms would then rapidly react in a chain of reactions initiated by ozone to form IO radicals3,12,20–21, OIO3,21,22, HIO3,12, HIO3 (Iodic acid)23, I2O2-4[3] and I2O5[16] – all potential precursors of new particles. Particle production from yet unidentified iodine containing vapours has also been experimentally demonstrated3,12,13. Laboratory experiments starting from I2 and ozone suggested that particles formed in dry conditions are composed of I2O5[16, 17].
To pursue identification of the molecular steps involved in new particle formation in an iodine-rich environment, a field campaign was performed at Mace Head coastal station on Irish west coast in August – October 2013. A suite of novel instrumentation, and in particular, a nitrate ion based CI-APi-TOF mass spectrometer capable of resolving the chemical composition of freshly formed electrically neutral clusters9, was applied (Supplementary Information).
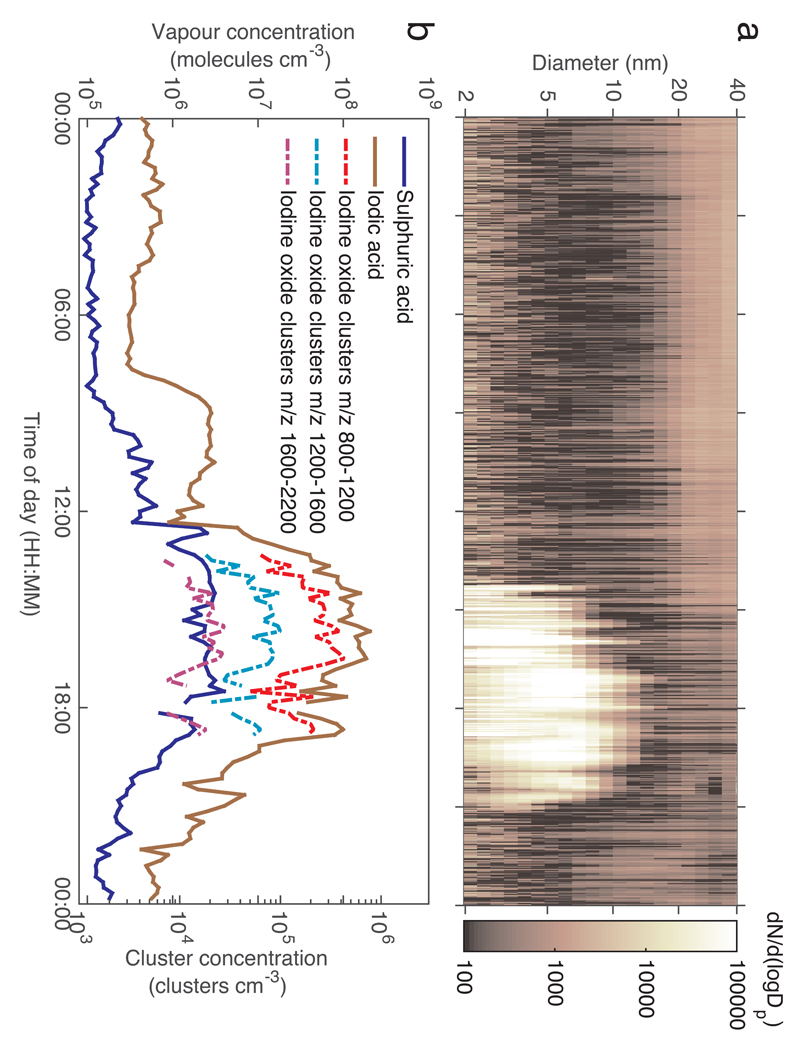
Throughout the campaign, new particle formation associated with low tide and subsequent exposure of seabed macro-algae to ambient air was observed almost every day (Supplementary Information Figure S1). An example of a new particle formation event is shown in Figure 1. Soon after noon when the low tide occurred, a strong burst (a) of new particles was detected with total concentration of >1.5 nm particles exceeding 106 cm-3 and 1.5 – 3 nm clusters reaching concentrations of the order of 105 cm-3 (Supplementary Information Figure S2). Because of the short time between the emission source and the measurement site particles rarely grew up above 10-20 nm. However, previous studies have demonstrated that these particles reach cloud condensation nuclei (CCN) sizes in a matter of few hours24.
Figure 1. Typical particle formation event recorded at Mace Head.
a, The event is initiated slightly after noon at low tide resulting in iodine emissions from marine algae. Particles grow rapidly to 2-10 nm sizes. When the tide gets high again after ~6 hours the particle production stops. b, The event is associated with a slight increase of sulphuric acid, thought typically to be the key player in atmospheric nucleation, but much more predominant increase in iodic acid with peak concentration above 108 cm-3 is observed. Together with iodic acid, iodine oxide clusters with masses exceeding 2000 Da are observed.
Preceding the new particle production event, a strong increase in iodic acid, HIO3, signal was seen (b) with concentrations reaching 108 molecules cm-3 during the course of the event. The observation of iodic acid was unexpected as there have, hitherto, been no reported observations of its presence in ambient air. At the same time, neutral iodine oxide clusters up to over 2000 atomic mass units were detected, confirming that iodine oxides (including HIO3) were almost solely responsible for new particle formation.
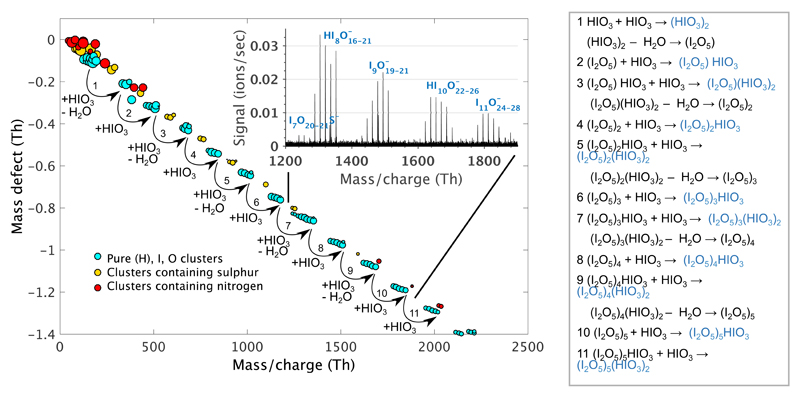
The chemically ionized cluster distribution, representing originally neutral clusters that have charged upon chemical ionization (Supplementary Information), is shown in Figure 2. The depicted mass defects (i.e. total deviation of the molecule/cluster mass from the integer mass defined as the sum of neutrons and protons in the atom nuclei of the molecules) emphasize the high iodine and oxygen content of the measured clusters because oxygen has a moderate and iodine a strong negative mass defect. Even though sulphuric acid, methyl sulphonic acid and highly oxidized multifunctional organic vapours25 were detected, neutral clusters were composed almost exclusively of iodine and oxygen and, to a very small extent, of hydrogen. Chemically ionized clusters contained either none (an odd number of I) or one hydrogen atom (an even number of I), suggesting one (odd I) or two (even I) hydrogen atoms in their original neutral form. Minute quantities of sulphuric acid, as well as nitrogen, in form of ammonia or nitric acid, were detected in clusters, but pure iodine oxide clusters clearly dominated over the other compounds.
Figure 2. Mass defect vs. cluster mass plot depicting the abundance and atomic composition of nucleating neutral clusters during the event.
Cluster distribution is dominated by iodine oxides. Mechanism explaining the cluster formation starting from iodic acid, HIO3, and proceeding through further addition of iodic acid is highlighted in the figure. It should be noted that neutral cluster loses one hydrogen upon chemical ionization and that part of restructuring reactions can take place only upon detection; thus in the atmosphere, depicted clusters may be more hydrated, i.e., part of I2O5 can be in form of two iodic acid molecules.
The average oxygen to iodine ratio (O:I) was generally 2.4, but varied between 2.2 and 2.6 for a fixed number of iodine atoms. This shows that simple condensation of OIO[12], I2O3[17] or I2O4[17] alone, or any combination of compounds with O:I of two or below, is not sufficient to explain the observed cluster formation. However, compounds such as I2O5[16,17,26] or HIO3[23] should have a major contribution to the cluster production. Signals attributable to I2O5 were low, suggesting that I2O5 condensation alone unlikely explains the observed cluster growth. However, the relatively high concentrations of iodic acid, HIO3, point toward its major role in the cluster buildup.
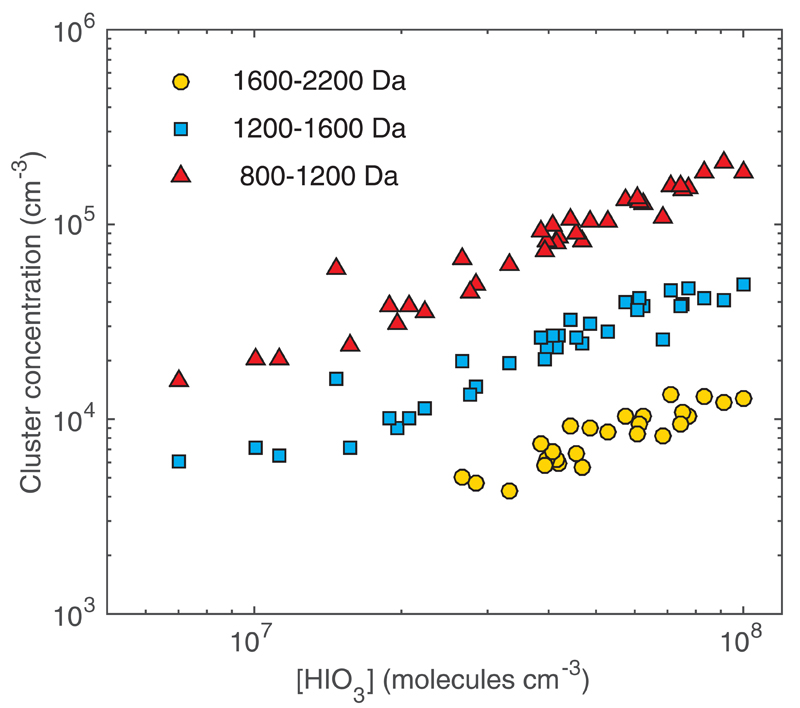
The concentration of HIO3 during the events reached 108 cm-3 and should have been considerably higher in the immediate vicinity of algae beds. Such detected concentration would be sufficient to explain the observed cluster growth rates (Supplementary Information, Table S1). Further evidence on the role of HIO3 comes from the observed cluster concentrations (Figure 3) which depend close to linearly on the HIO3 concentration. Near linear dependencies of cluster and vapour concentrations can in general be caused by arbitrary combinations of clustering mechanisms and sinks. It this particular case, the formation rates are so high that the sinks are likely to play a negligible role. Thus, the linear dependency indicates that the clusters grow by sequential HIO3 additions, which are much faster than the competing evaporation or fragmentation processes. The critical role of HIO3 was found also in a series of supplementary laboratory experiments (Supplementary Information, Figures S4-S11). A chemical mechanism producing iodic acid from primary molecular iodine emissions remains unclear. However, the formation of clusters can, to a large extent, be explained by the uptake of iodic acid and subsequent reaction of two HIO3 molecules in the cluster, resulting in formation of I2O5 + H2O with water evaporating from the cluster (Figure 2). Such a mechanism perfectly explains also the feature that the hydrogen content in the detected (chemically ionized) clusters was either one (even I) or zero (odd I). It should be noted that sampled clusters are exposed to decreased water vapour concentration after entering the vacuum of the mass spectrometer, so further restructuring of HIO3 to I2O5 can take place upon dehydration. Thus, it may be that corresponding clusters in the atmosphere are more hydrated and contain more iodic acid than could be directly inferred from the mass spectra. The general mechanism of the sequential HIO3 addition outlined above, however, remains unaffected even when further dehydration takes place during the sampling.
Figure 3. Cluster concentrations, grouped into three mass classes, depend almost linearly on the HIO3-concentration suggesting that the main clustering mechanism is addition of HIO3.
The average O:I ratio of 2.4 suggests that besides the major role of HIO3, other iodine oxoacids – iodous acid (HIO2) or hypoiodous acid (HIO) – could act in the same way as HIO3. That would readily explain the variation of oxygenation in the observed clusters. Both acids were detected during the event, but with much lower intensities than that of iodic acid with lower limit concentration estimates of 2·106 molecules cm-3 and 1·106 molecules cm-3 for iodous and hypoiodous acid, respectively, during the peak concentration period. Rather than these small iodine oxoacids, it is likely that non-hydrogen containing compounds with two iodine atoms (I2O2-5) have been condensed on clusters to some extent. Condensation of IO or OIO should have been less pronounced because hydrogen atom was detected only in every second group of the clusters separated by I2O5. Addition of IO or OIO to a cluster, formed according to above proposed scheme, would result in hydrogen containing peaks in the cluster spectrum also for odd number of I. The overall mechanism should therefore be a mixture of straightforward addition of HIO3 accompanied by restructuring and water recycling and less pronounced addition of HIO, HIO2 and/or I2O2-5 compounds. Figure 2 depicts the process starting from HIO3 molecule and proceeding purely via addition of HIO3 accompanied with the loss of water. However, a parallel process, involving only non-acidic iodine oxides, I2O2-5, cannot be fully excluded. In the mass range of 1000–2000 Da, 65% to 73% of the total cluster mass can be readily explained by HIO3 and I2O5, while the minimum of 27% (in case only I2O3 is co-condensing) or the maximum of 35% (only I2O4 is co-condensing) of this mass is explained by less-oxygenated compounds.
In addition to Mace Head, we employed a CI-APi-TOF mass spectrometer at two field campaigns carried out at high-latitude sites exposed periodically to marine air masses: Station Nord in northern Greenland during February – August 2015 and Aboa research station in Queen Maud Land, Antarctica, during November 2014 – February 2015 (Supplementary information). In Greenland, we started to observe elevated concentrations of iodic acid after the sunrise in late February, often associated with new particle formation events. During such events, the HIO3 concentrations tended to be much higher than that of sulphuric acid (Fig. S12), and it seems that the cluster formation could be explained virtually purely with the HIO3 clustering mechanism (Fig. S13). In Antarctica, we measured gas-phase iodic acid well above the instrumental limit-of-detection despite the distance of more than 100 km to the Antarctic coast (Fig. S15). This observation suggests that the oceanic areas surrounding the Antarctic continent may be strong sources of molecular iodine that is converted to iodic acid in gas phase reactions either at the emission area or during the transportation to our measurement site.
Our measurements point toward prominent gas-phase iodic acid production associated with iodine emissions from different coastal areas, and show that this compound forms very actively growing molecular clusters. However, iodine is not only emitted in coastal areas but also in the open ocean environment27,28 as well as from Arctic and Antarctic sea ice29–33. At the present stage, it remains uncertain if the described particle formation mechanism plays a role in the open ocean environment and if it can make an important contribution to climate-relevant processes.
Supplementary Material
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Acknowledgements
This work was partly funded by Academy of Finland (center of excellence project 1118615 and project 251427), the PEGASOS project (funded by the European Commission under the Framework Program 7 (FP7-ENV-2010-265148)); ACTRIS, the EPA-Ireland, Nordic Centre of Excellence (CRAICC), Finnish Antarctic Research Program, and the European Research Council (ATMNUCLE, grant 227463, MOCAPAF, grant 257360 and COALA, grant 638703).
Footnotes
Author Contibutions
MS, NS, TJ, SR, JKa, AF, OP performed the field measurements, MS, NS, TJ, JKo and HJ, analysed the data, HH suggested the chemical mechanism and performed quantum chemical calculations, SR, TB and MS performed the laboratory experiments, MS, VMK and COD wrote the manuscript. MS, MK, HJ and COD designed the measurements, DC and COD organised the Field Study at Mace Head and contributed to the data analysis. All authors contributed to data interpretation and contributed to the final manuscript development.
Author information
Reprints and permissions information is available at www.nature.com/reprints. Readers are welcome to comment on the online version of the paper.
The authors declare no competing financial interests.
Computer code TofTools for Matlab, used for mass spectrometer data processing, is available upon request.
References
- 1.Kulmala M, et al. Chemistry of atmospheric nucleation: On the recent advances on precursor characterization and atmospheric cluster composition in connection with atmospheric new particle formation. Annu Rev Phys Chem. 2014;65:21–37. doi: 10.1146/annurev-physchem-040412-110014. [DOI] [PubMed] [Google Scholar]
- 2.Riccobono F, et al. Oxidation products of biogenic emissions contribute to nucleation of atmospheric particles. Science. 2014;344:717–721. doi: 10.1126/science.1243527. [DOI] [PubMed] [Google Scholar]
- 3.O’Dowd CD, et al. Marine aerosol formation from biogenic iodine emissions. Nature. 2002;417:632–636. doi: 10.1038/nature00775. [DOI] [PubMed] [Google Scholar]
- 4.Yoon YJ, O’Dowd CD, Jennings SG, Lee SH. Statistical characteristics and predictability of particle formation events in Mace Head. J Geophys Res. 2006;111:D13204. [Google Scholar]
- 5.O’Dowd CD, de Leeuw G. Marine aerosol production: A review of the current knowledge. Phil Trans R Soc A. 2007;365:1753–1774. doi: 10.1098/rsta.2007.2043. [DOI] [PubMed] [Google Scholar]
- 6.McFiggans G, et al. Iodine-mediated coastal particle formation: an overview of the Reactive Halogens in the Marine Boundary Layer (RHaMBLe) Roscoff coastal study. Atmos Chem Phys. 2010;10:2975–2999. [Google Scholar]
- 7.Mahajan AS, et al. Concurrent observations of atomic iodine, molecular iodine and ultrafine particles in a coastal environment. Atmos Chem Phys. 2011;11:2545–2555. [Google Scholar]
- 8.Kirkby J, et al. Role of sulphuric acid, ammonia and galactic cosmic rays in atmospheric aerosol nucleation. Nature. 2011;476:429–433. doi: 10.1038/nature10343. [DOI] [PubMed] [Google Scholar]
- 9.Kürten A, et al. Neutral molecular cluster formation of sulfuric acid-dimethylamine observed in real time under atmospheric conditions. Proc Nat Acad Sci. 2014;111:15019–15024. doi: 10.1073/pnas.1404853111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirkby J, et al. Ion-induced nucleation of pure biogenic particles. Nature. 2016;533:521–526. doi: 10.1038/nature17953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kulmala M, et al. Direct Observations of Atmospheric Aerosol Nucleation. Science. 2013;339:943–946. doi: 10.1126/science.1227385. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman T, O’Dowd CD, Seinfeld JH. Iodine oxide homogeneous nucleation: An explanation for coastal new particle production. Geophys Res Lett. 2001;28:1949–1952. [Google Scholar]
- 13.Jimenez JL, et al. New particle formation from photooxidation of diiodomethane (CH2I2) J Geophys Res. 2003;108(D10):4318. [Google Scholar]
- 14.McFiggans G, et al. Direct evidence for coastal iodine particles from Laminaria macroalgae—Linkage to emissions of molecular iodine. Atmos Chem Phys. 2004;4:701–713. [Google Scholar]
- 15.Saiz-Lopez A, Plane JMC. Novel iodine chemistry in the marine boundary layer. Geophys Res Lett. 2004;31:L04112. [Google Scholar]
- 16.Saunders RW, Plane JMC. Formation pathways and composition of iodine oxide ultra-fine particles. Environ Chem. 2005;2:299–303. [Google Scholar]
- 17.Saunders RW, et al. Studies of the Formation and Growth of Aerosol from Molecular Iodine Precursor. Zeitschrift für Physikalische Chemie. 2010;224:1095–1117. [Google Scholar]
- 18.Ehn M, et al. Growth rates during coastal and marine new particle formation in western Ireland. J Geophys Res. 2010;115:D18218. [Google Scholar]
- 19.Huang R-J, et al. In situ measurements of molecular iodine in the marine boundary layer: the link to macroalgae and the implications for O3, IO, OIO and NOx. Atmos Chem Phys. 2010;10:4823–4833. [Google Scholar]
- 20.Alicke B, Hebestreit K, Stutz J, Platt U. Iodine oxide in the marine boundary layer. Nature. 1999;397:572–573. [Google Scholar]
- 21.Atkinson R, et al. Evaluated Kinetic and Photochemical Data for Atmospheric Chemistry: Supplement VIII, Halogen Species Evaluation for Atmospheric Chemistry. J Phys Ref Data. 2000;29:167–266. [Google Scholar]
- 22.Bloss WJ, Rowley DM, Cox RA, Jones RL. Kinetics and products of the IO self-reaction. J Phys Chem A. 2001;105:7840–7854. [Google Scholar]
- 23.Sunder S, Vikis AC. Raman spectra of iodine oxyacids produced by the gas-phase reaction of iodine with ozone in the presence of water vapour. Canadian Journal of Spectroscopy. 1987;32:45–48. [Google Scholar]
- 24.O’Dowd CD. On the spatial extent and evolution of coastal aerosol plumes. J Geophys Res. 2002;107 doi: 10.1029/2001JD000422. [DOI] [Google Scholar]
- 25.Ehn M, et al. A large source of low-volatility secondary organic aerosol. Nature. 2014;506:476–479. doi: 10.1038/nature13032. [DOI] [PubMed] [Google Scholar]
- 26.Saiz-Lopez A, et al. Atmospheric chemistry of iodine. Chem Rev. 2011;112:1773–1804. doi: 10.1021/cr200029u. [DOI] [PubMed] [Google Scholar]
- 27.Mahajan AS, et al. Measurement and modelling of tropospheric reactive halogen species over the tropical Atlantic Ocean. Atmos Chem Phys. 2010;10:4611–4624. [Google Scholar]
- 28.Lawler MJ, Mahajan AS, Saiz-Lopez A, Saltzman ES. Observations of I2 at a remote marine site. Atmos Chem Phys. 2014;14:2669–2678. [Google Scholar]
- 29.Saiz-Lopez A, et al. On the vertical distribution of boundary layer halogens over coastal Antarctica: Implications for O3, HOx, NOx and the Hg lifetime. Atmos Chem Phys. 2008;8:887–900. [Google Scholar]
- 30.Mahajan AS, et al. Evidence of reactive iodine chemistry in the Arctic boundary layer. J Geophys Res. 2010;115:D20303. [Google Scholar]
- 31.Atkinson HM, et al. Iodine emissions from the sea ice of the Weddell Sea. Atmos Chem Phys. 2012;12:11229–11244. [Google Scholar]
- 32.Allan J, et al. Iodine observed in new particle formation events in the Arctic atmosphere during ACCACIA. Atmos Chem Phys. 2015;15:5599–5609. [Google Scholar]
- 33.Roscoe HK. Particles and iodine compounds in coastal Antarctica. J Geophys Res. 2015 doi: 10.1002/2015JD023301. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.