Abstract
Objective
To assess which characteristics are associated with failure to receive fertility counseling among a cohort of young women diagnosed with cancer.
Design
A population-based cohort study.
Setting
Not applicable.
Patients
A total of 1,282 cancer survivors, of whom 1,116 met the inclusion criteria for the analysis.
Interventions
None.
Main Outcome Measure(s)
The main outcome in this study was whether or not women reported receiving any information on how cancer treatment might affect their ability to become pregnant at the time of their cancer diagnosis.
Results
Forty percent of cancer survivors reported that they did not receive fertility counseling at the time of cancer diagnosis. Women were more likely to fail to receive counseling if they had a high school education or less (OR=1.90, 95% CI: 0.97, 3.70) or if they had a prior birth (OR=1.92, 95% CI: 1.31, 2.81). Cancer related variables that were associated with a lack of counseling included not receiving chemotherapy as part of treatment (OR=4.39, 95% CI: 2.96, 6.51) and diagnosis with certain cancer types.
Conclusions
Counseling about the risk of infertility and available fertility preservation options is important to cancer patients. Additionally, counseling can make women aware other adverse reproductive outcomes such as early menopause and its associated symptoms. Less educated women and parous women are at particular risk of not getting fertility-related information. Programs that focus on training not just the oncologist, but also other healthcare providers involved with cancer care, to provide fertility counseling may help expand access.
Keywords: cancer survivors, fertility counseling, female fertility
INTRODUCTION
According to Surveillance, Epidemiology, and End Results (SEER) statistics there are approximately 35,000 women between the ages of 15 and 39 who are diagnosed with cancer annually (1). Some cancer treatments alter the normal reproductive anatomy, have the potential to be gonadotoxic, and can lead to secondary amenorrhea. While many women will resume menses after treatment, they may have unrecognized diminished ovarian reserve, which can contribute to a higher risk of premature ovarian insufficiency and infertility (2–6). Furthermore, since adjuvant treatment may continue several years after diagnosis, women may age out of their reproductive window while pursuing these treatments. Registry-based studies have found decreased rates of childbearing and increased probabilities of childlessness among female cancer survivors (7–9).
In 2005 and 2006, the American Society for Reproductive Medicine (ASRM) and the American Society of Clinical Oncology (ASCO) recommended that oncologists provide information to all reproductive-aged cancer patients about how cancer treatment may affect fertility and about fertility preservation options available (10, 11). These guidelines were updated in 2013 to include a broader range of healthcare providers; recognize that oocyte preservation was no longer considered an experimental treatment; and emphasize that referrals to a fertility specialist should not only include women who expressed an interest in fertility preservation, but also those who were ambivalent towards these procedures (12, 13). Fertility counseling is important because the loss of fertility has been reported to be almost as important to reproductive-aged women diagnosed with cancer as concerns about survival (14, 15). Women reported a better quality of life and less distress if they received fertility counseling, regardless of whether they pursued fertility treatment (16–18). Even among women for whom fertility preservation is not an option, the counseling provides an opportunity to process the potential loss of fertility after cancer treatment and discuss alternative reproductive options (e.g., egg donation and surrogacy) (19).
Although cancer treatments have the potential to adversely affect the reproductive system and future fertility is important to many women, some women report that they do not receive information about how cancer treatment could affect their ability to become pregnant. Differences in who receives fertility counseling have been reported by age, race, education, and parity (20–23). Disparities have also been found in which women receive referrals to a fertility specialist to discuss fertility preservation (24, 25).
The objective of this study was to comprehensively assess which characteristics of young women diagnosed with cancer were associated with not receiving fertility counseling, to identify women with potentially unmet need in a population-based study. We examined 3 domains of factors that might influence this discussion, sociodemographic, physician influencing, and patient influencing to take into consideration that the conversation could be initiated by a variety of healthcare providers or the patients themselves.
MATERIALS AND METHODS
The Furthering Understanding of Cancer Health and Survivorship in Adult (FUCHSIA) Women’s Study is a population-based cohort study designed to examine how cancer treatment during the reproductive years affects future fertility. Women were eligible to participate if they were 20 to 35 years old at first diagnosis with a reportable invasive cancer or ductal carcinoma in situ and were diagnosed during 1990–2009 (26). Women had to be reproductive aged (22–45 years) at recruitment, be at least 2 years post diagnosis, have a working telephone, and speak English. Additionally, for this analysis, women who had a hysterectomy or bilateral salpingo-oophorectomy before cancer diagnosis were excluded because they would not have been able to become pregnant at the time of the cancer diagnosis.
Eligible cancer survivors were recruited in collaboration with the Georgia Cancer Registry (GCR), a statewide, population-based registry that collects information on all reportable cancers in Georgia residents. The GCR started collecting information on all incident cancer cases in metropolitan Atlanta before the rest of Georgia and data were available through 2009 at the time of study recruitment. To maximize our sample and minimize differences in treatment over time, data were requested from 1990 through 2009 for metropolitan Atlanta and from 1999 through 2009 for the rest of Georgia. Women who agreed that the study could contact them were invited to complete a telephone interview with a trained interviewer. The interview lasted approximately one hour and collected information about cancer diagnoses and treatments, medical conditions, experience with infertility, pregnancy history, desire for children, and demographic and lifestyle factors. Participants were asked to choose from a pre-specified list of responses to each question, which were pilot tested to ensure they would capture a wide range of experiences (27). In addition, we obtained data on age at diagnosis and cancer type from the GCR. Women consented to participate in the study orally at the time of the interview. The Emory University and the Georgia Department of Public Health Institutional Review Boards approved this study.
The outcome for this study was defined by a response of yes, no, or don’t remember to the question “Did you talk to a doctor or other health professional about how this cancer treatment could affect your ability to become pregnant?” Follow-up questions collected information on who initiated the discussion, when the discussion occurred, and if they were referred to a fertility specialist. We used these questions to identify women not being counseled about how cancer treatment might affect their future fertility.
Three categories of factors that might influence whether or not a woman received fertility counseling at the time of diagnosis were assessed based on women’s responses during the study interview. These factors included sociodemographic characteristics, factors influencing patients, and factors influencing physicians/other healthcare providers. The categories shared factors as depicted in Figure 1. Sociodemographic characteristics included race, education, income, and insurance status, as well as age, relationship status, and place of residence at the time of diagnosis.
Figure 1.
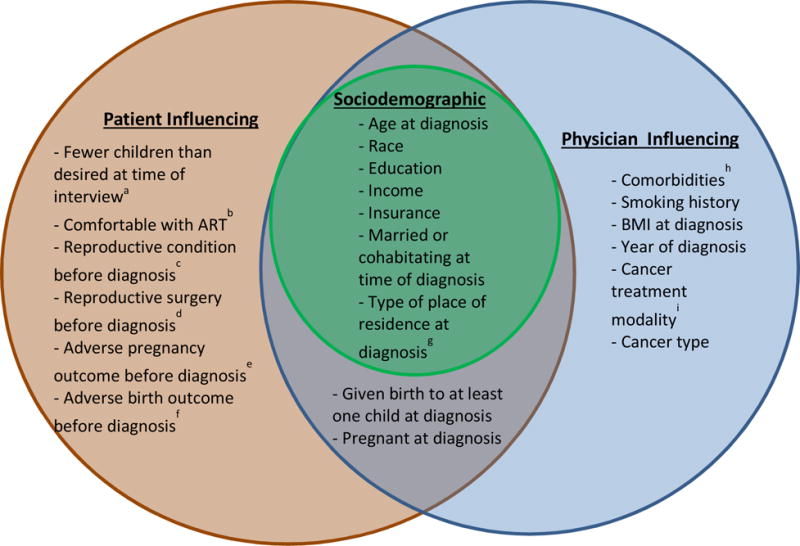
Categories of Factors that May Influence Whether or Not a Woman Receives Fertility Counseling at the Time of Cancer Diagnosis.
aCalculated by subtracting the number of children women gave birth to from the total number they reported they desired
bComfort with assisted reproductive technologies such as in vitro fertilization.
cDiagnosis with polycystic ovary syndrome, endometriosis, or fibroids.
dSurgery on uterus, ovaries, or fallopian tubes.
eMiscarriage or stillbirth.
fPreterm or low birth weight birth
gLevel of urbanization of the county of residence
hChronic hypertension or diabetes.
iChemotherapy and/or radiation.
Factors that might influence a woman’s decision to initiate a discussion with her doctor about how treatment could affect her future fertility at the time of cancer diagnosis included parity, desire for future children, and comfort with assisted reproductive technology. We hypothesized that women with prior obstetric or gynecologic problems would be more likely to initiate a conversation. Reproductive conditions considered included fibroids; endometriosis; polycystic ovary syndrome; history of amenorrhea; and surgery to the uterus, ovaries, or fallopian tubes. Sexually transmitted infections examined included chlamydia, gonorrhea, pelvic inflammatory disease, human papilloma virus, and herpes. Pregnancy at the time of diagnosis and history of miscarriage, stillbirth, elective abortion, ectopic pregnancy, low birth weight or preterm birth were also assessed.
Patient characteristics that may influence physician/other healthcare provider initiated counseling included comorbidities (hypertension, diabetes), smoking status, and obesity, as well as characteristics of the cancer diagnosis (type) and its treatment (chemotherapy, radiation, surgery). Cancers were grouped to assess effects on the reproductive system directly, through radiation to the pelvic region, or by disrupting hormone function. These groups included breast, reproductive (uterine, cervical, and ovarian), Hodgkin’s lymphoma, non-Hodgkin’s lymphoma, brain, thyroid, and other cancer types.
Statistical Analysis
Descriptive statistics were generated for all factors of interest by receipt of counseling. Among those counseled we characterized who initiated the counseling, when it occurred in relation to cancer treatment, and who received a referral. The prevalence of counseling and referral were calculated for each year of diagnosis from 1999–2009 (the years with participants from the whole state). To assess which factors were most strongly associated with not receiving fertility counseling at the time of cancer diagnosis we fit bivariate logistic models for each of the factors hypothesized to influence receipt of counseling followed by fully adjusted multivariable models for each of the three categories of factors.
All of the factors hypothesized were considered important predictors of the outcome conceptually. However, results from the bivariate and fully adjusted multivariable models were used to determine the most influential variables in determining the likelihood of not receiving counseling. Three considerations were taken into account during variable selection: magnitude of effect, precision of the estimate, and statistical significance of the estimate at p<0.10.
RESULTS
There were 5,137 women initially identified as potentially eligible in the GCR. The registry successfully contacted 60% of these women, of whom 78% agreed to have their name released to the study, and 1% were non-English speaking. Of these, 56% were interviewed (including partial interviews), 17% refused, and the remainder could not be reached for the interview. A total of 1,282 cancer survivors completed the study interview. The distribution of demographic characteristics (age, race, and residence) was similar among participants and those initially identified in the registry. Women were interviewed between May 2012 – February 2013, a median of 7 years after cancer diagnosis (interquartile range [IQR], 5–11). Women who had a hysterectomy or bilateral salpingo-oophorectomy before cancer diagnosis (n=153) or missing information (responded don’t know or refused) for the outcome (n=13) were excluded. Fifty-nine percent of women (n=660) in our study were counseled on how cancer treatment could affect their ability to become pregnant. When we restricted to women diagnosed after the original ASRM counseling guidelines (2005 or later) the proportion of women reporting counseling was similar (62%).
Women were well distributed across the sociodemographic characteristics examined (Table 1). A smaller proportion of women who were not living with a partner compared with those cohabitating (53% vs 62%) and women who were parous compared with women who were nulliparous (54% vs. 64%) reported receiving fertility counseling. Women with lower educational attainment and less insurance were also less likely to report receiving counseling. Half of the study participants were diagnosed between 30–35 years old and most survivors were diagnosed after the year 2000, but counseling was similar across age and year of diagnosis. Breast cancer and Hodgkin lymphoma survivors were the most likely to report receiving counseling. There was a slight but not marked increase in counseling and referral in our study population after the original ASRM and ASCO guidelines in 2005–2006 (Supplemental Figure 1).
Table 1.
Characteristics of Cancer Patients by Whether or Not They Received Fertility Counseling
| Received Fertility Counseling | |||
|---|---|---|---|
| Yes (n=660) |
No (n=456) |
||
| No. (%) | No. (%) | p value | |
| Age at diagnosis (years) | |||
| 20–24 | 96 (58.9) | 67 (41.1) | 0.99 |
| 25–29 | 209 (58.9) | 146 (41.1) | |
| 30–35 | 355 (59.4) | 243 (40.6) | |
| Race | |||
| White | 463 (59.4) | 317 (40.6) | 0.81 |
| Black | 165 (58.1) | 119 (41.9) | |
| Other race | 32 (62.8) | 19 (37.3) | |
| Missing | 1 | ||
| Educationa | |||
| High school or less | 26 (37.1) | 44 (62.9) | <0.01 |
| Some college | 175 (61.0) | 112 (39.0) | |
| College graduate | 247 (59.5) | 168 (40.5) | |
| At least some graduate school | 212 (61.8) | 131 (38.2) | |
| Missing | 1 | ||
| Incomeb | |||
| Less than or equal to $50k | 208 (55.2) | 169 (44.8) | 0.13 |
| $50k – 100k | 242 (61.7) | 150 (38.3) | |
| $100k+ | 199 (61.0) | 127 (39.0) | |
| Missing | 11 | 10 | |
| Insurancec | |||
| Private | 528 (61.5) | 331 (38.5) | 0.01 |
| Self | 35 (61.4) | 22 (38.6) | |
| Public | 47 (50.5) | 46 (49.5) | |
| None | 49 (46.7) | 56 (53.3) | |
| Missing | 1 | 1 | |
| Married or cohabitating at time of diagnosis | |||
| Yes | 463 (62.2) | 282 (37.9) | <0.01 |
| No | 197 (53.1) | 174 (46.9) | |
| Type of place of residence at diagnosisd | |||
| Urbanized | 100 (55.3) | 81 (44.8) | 0.07 |
| Suburban | 367 (57.3) | 273 (42.7) | |
| Small metropolitan | 131 (66.2) | 67 (33.8) | |
| Small town/rural | 62 (63.9) | 35 (36.1) | |
| Given birth to at least one child at diagnosis | |||
| Yes | 286 (53.6) | 248 (46.4) | <0.01 |
| No | 374 (64.3) | 208 (35.7) | |
| Year of diagnosis | |||
| 1990–94 | 10 (66.7) | 5 (33.3) | 0.16 |
| 1995–99 | 67 (51.9) | 62 (48.1) | |
| 2000–04 | 228 (57.4) | 169 (42.6) | |
| 2005–09 | 355 (61.7) | 220 (38.3) | |
| Chemotherapye | |||
| Yes | 466 (72.9) | 173 (27.1) | <0.01 |
| No | 194 (40.7) | 283 (59.3) | |
| Radiatione | |||
| Yes | 345 (67.4) | 167 (32.6) | <0.01 |
| No | 315 (52.2) | 289 (47.9) | |
| Cancer types | |||
| Reproductive (cervix, ovary, uterus) | 51 (81.0) | 12 (19.1) | |
| Breast | 274 (69.7) | 119 (30.3) | |
| Brain | 16 (57.1) | 12 (42.9) | |
| Hodgkin lymphoma | 97 (82.9) | 20 (17.1) | |
| Non-Hodgkin lymphoma | 34 (61.8) | 21 (38.2) | |
| Thyroid | 66 (54.1) | 56 (45.9) | |
| Other cancer type | 122 (36.1) | 216 (63.9) | |
Current education at the time of the interview.
Current income at the time of the interview.
Private insurance includes employer, school, military or VA insurance; self includes COBRA, public includes Medicare and Medicaid.
Type of place of residence based on a modified version of the NCHS urban rural categories.
Ever use (yes/no).
Table 2 presents adjusted estimates from the final models after dropping variables that were not associated with counseling (p< 0.1) or did not improve precision. Model 1 was the final model including all variables identified as important when considering all three domains. Factors that remained associated with failure to receive fertility counseling were having less than some college education (OR=1.90, 95% CI: 0.97, 3.70) and being unmarried and not cohabitating at the time of diagnosis (OR=1.90, 95% CI: 1.34, 2.69). Giving birth to at least one child (OR=1.92, 95% CI: 1.31, 2.81) and prior fallopian tube surgery (OR=2.19, 95% CI: 1.29, 3.72) also remained associated with not receiving fertility counseling, as was having met reproductive goals at the time of the interview (OR=1.61, 95% CI: 1.19, 2.17). Women not treated with chemotherapy (OR=4.39, 95% CI: 2.96, 6.51) were less likely to receive information on how their cancer treatment could affect their future fertility in adjusted models. Model 2 was the final model when we only considered factors that might influence whether the physician/other healthcare provider initiated counseling. In this model, education, relationship status, parity, and chemotherapy remained strongly associated with failure to receive counseling.
Table 2.
Adjusted Odds Ratios for Characteristics of Cancer Patients and Not Receiving Fertility Counseling
| Model 1a | Model 2a | |||
|---|---|---|---|---|
| aOR | 95% CI | aOR | 95% CI | |
| Age at diagnosis (years) | ||||
| 20–24 | 1.09 | 0.67, 1.77 | 1.06 | 0.67, 1.67 |
| 25–29 | Reference | Reference | ||
| 30–35 | 1.19 | 0.83, 1.70 | 1.14 | 0.82, 1.59 |
| Race | ||||
| White | Reference | Reference | ||
| Black | 0.97 | 0.67, 1.39 | 1.02 | 0.72, 1.43 |
| Other race | 1.01 | 0.49, 2.08 | 0.86 | 0.44, 1.68 |
| Educationb | ||||
| High school or less | 1.90 | 0.97, 3.70 | 2.43 | 1.29, 4.59 |
| Some college | 1.05 | 0.70, 1.56 | 1.05 | 0.73, 1.52 |
| College graduate | Reference | Reference | ||
| At least some graduate school | 1.23 | 0.87, 1.76 | 1.09 | 0.78, 1.53 |
| Insurancec | ||||
| Private | Reference | Reference | ||
| Self | 1.14 | 0.59, 2.21 | 1.06 | 0.57, 1.99 |
| Public | 1.22 | 0.69, 2.16 | 1.18 | 0.69, 2.01 |
| None | 1.56 | 0.91, 2.67 | 1.43 | 0.88, 2.34 |
| Married or cohabitating at time of diagnosis | ||||
| Yes | Reference | Reference | ||
| No | 1.90 | 1.34, 2.69 | 1.90 | 1.37, 2.62 |
| Place of residence at diagnosisd | ||||
| Large central | 1.11 | 0.74, 1.68 | 1.21 | 0.83, 1.78 |
| Large fringe | Reference | Reference | ||
| Small metropolitan | 0.78 | 0.51, 1.18 | 0.71 | 0.48, 1.05 |
| Non-metropolitan | 0.82 | 0.47, 1.43 | 0.77 | 0.46, 1.30 |
| Given birth to at least one child at diagnosis | ||||
| Yes | 1.92 | 1.31, 2.81 | 2.51 | 1.80, 3.51 |
| No | Reference | Reference | ||
| Fewer children than desired at time of interviewe | ||||
| Yes | Reference | |||
| No | 1.61 | 1.19, 2.17 | ||
| Human Papilloma Virus before diagnosis | ||||
| Yes | Reference | |||
| No | 1.45 | 0.86, 2.46 | ||
| Surgery on fallopian tubes before diagnosis | ||||
| Yes | 2.19 | 1.29, 3.72 | ||
| No | Reference | |||
| Low birth weight or preterm birth before diagnosis | ||||
| Yes | 1.17 | 0.76, 1.80 | ||
| No | Reference | |||
| Chemotherapyf | ||||
| Yes | Reference | Reference | ||
| No | 4.39 | 2.96, 6.51 | 4.16 | 2.89, 6.01 |
| Radiationf | ||||
| Yes | Reference | Reference | ||
| No | 1.12 | 0.80, 1.57 | 1.20 | 0.88, 1.65 |
| Cancer types | ||||
| Reproductive (cervix, ovary, uterus) | 0.12 | 0.06, 0.25 | 0.12 | 0.06, 0.24 |
| Breast | 0.38 | 0.25, 0.57 | 0.40 | 0.27, 0.59 |
| Brain | 0.69 | 0.27, 1.78 | 0.50 | 0.21, 1.21 |
| Hodgkin lymphoma | 0.27 | 0.14, 0.52 | 0.29 | 0.16, 0.52 |
| Non-Hodgkin lymphoma | 0.62 | 0.31, 1.27 | 0.71 | 0.37, 1.37 |
| Thyroid | 0.26 | 0.15, 0.45 | 0.29 | 0.17, 0.47 |
| Other cancer type | Reference | Reference | ||
Estimates are adjusted for all other variables in the model.
Current education at the time of the interview.
Private insurance includes employer, school, military or VA insurance; self includes COBRA, public includes Medicare and Medicaid.
Type of place of residence based on a modified version of the NCHS urban rural categories.
Calculated by subtracting the number of children women gave birth to from the total number they reported they desired.
Ever use (yes/no).
Among women who were counseled, the discussion was initiated most often by the oncologist (44%) or the woman or her partner (40%) and was initiated 16% of the time by another healthcare provider. Most of these discussions occurred before cancer treatment (78.4%); however, 12.6% occurred during treatment and 9.0% after treatment. The timing of the discussion differed by who initiated the conversation (Table 3). Only 2.2% of conversations started by the oncologist were after treatment, while 13.4% of fertility discussions initiated by the patient or her partner and 16.8% initiated by another healthcare provider occurred after treatment. Of those counseled, 44 breast cancer survivors, 15 Hodgkin lymphoma survivors, and 26 survivors of all the other cancer types received a referral to a reproductive specialist.
Table 3.
Timing and Initiator of Fertility Counseling Among Cancer Patients
| Person Initiating Fertility Counseling | |||
|---|---|---|---|
| Patient or Patient’s Partner (n=254) |
Oncologist (n=276) |
Another healthcare provider (n=101) |
|
| Timing of Discussion | % | % | % |
| Before Treatment | 70.5 | 88.0 | 71.3 |
| During Treatment | 16.1 | 9.8 | 11.9 |
| After Treatment | 13.4 | 2.2 | 16.8 |
DISCUSSION
While life-saving cancer treatment is the priority at the time of diagnosis, survivorship issues should not be ignored. In our study, women with less education, who were unmarried, or already had a child were less likely to receive counseling suggesting that these characteristics make the healthcare provider, cancer patient, or both less likely to initiate a discussion about how cancer treatment can affect the reproductive system. An encouraging result from our study was that women with fewer children than desired were more likely to receive counseling than those who did not desire future children, and many of the other factors we examined showed no disparities in the receipt of counseling. Yet, the overall proportion of women who reported receiving fertility counseling at the time of diagnosis was only 60%, which is similar to other studies (20, 24). Further, only 13% of women who received counseling were referred to a fertility specialist to discuss fertility preservation.
Although many women who received fertility counseling in our study initiated the conversation themselves, the importance of a healthcare provider initiated conversation should not be overlooked. Depending on women to ask for fertility counseling puts the burden on the patient to know that cancer treatment could affect future fertility and the availability of fertility preservation. The 2006 ASCO guidelines note that patients may not mention fertility preservation, so physicians may not be aware of how important it is to them unless they ask (10). Further, women were most likely to receive counseling earlier if the conversation was initiated by their oncologist compared with a self-initiated discussion or one started by another healthcare provider. Early counseling provides women with information on the possible effects cancer treatment can have on reproductive function before exposure to gonadotoxic treatments and provides a greater opportunity to integrate fertility preservation into their cancer treatment plan.
In all analyses, having no college education was associated with an increased likelihood of failing to receive fertility counseling. This is consistent with a study that found that women with less education were less likely to be counseled about the fertility-related issues that could arise due to cancer treatment (21). Less educated women may not report being counseled because they never received information about the potential for cancer treatment to compromise future fertility. Alternatively, the information may have been provided at a level beyond their health literacy. Income, which is related to education, was not associated with receipt of counseling in our study. Income at the time of the interview may have been influenced by life stage with women reporting some college education having lower income because they were still in school or in entry level jobs. Additionally, education level may have had a greater individual influence on whether or not women received counseling compared with income, which was collected as a household measure and reflects both the participant’s income and that of her partner if married or cohabitating. Women who were not in a relationship were less likely to be counseled compared with women with a partner. However, it is equally important to counsel single women on the potential for damage to their reproductive system, especially if they want the opportunity to have a child in the future. This is particularly pertinent now that oocyte freezing is no longer considered an experimental treatment (28). These findings indicate a need to tailor counseling to meet women’s specific situations, especially because women with some identifiable characteristics may be less inclined to initiate a discussion about fertility.
Survivors of reproductive cancers were the most likely to have been counseled, which may be because of the direct connection between these cancers and fertility. However, although counseling in this group of cancer survivors is high, close to 20% of women diagnosed with a reproductive cancer reported that they did not receive fertility counseling. Our results correspond with those in another study that found cancer type influenced a related outcome, receipt of a referral to a fertility specialist, with breast cancer and lymphoma survivors most likely to be referred (29).
Fertility preservation and treatment are costly medical procedures (30, 31). Our results suggest that women who have no insurance or are publicly insured are less likely to receive fertility counseling. Concern about the financial burden of cancer treatment and the perception that these options are cost prohibitive may be contributing to this lack of counseling. Increased awareness of advocacy organizations that provide funding for fertility preservation for cancer patients could increase the number of women who bring up a discussion about fertility preservation and serve as a resource for healthcare providers to direct patients to when they are providing counseling (12).
A limitation of this study is that our outcome is based on patient recall of an event that took place a median of 7 years ago. Additionally, being diagnosed with cancer is a stressful time in a woman’s life and information provided by the oncologist or other healthcare provider at and around the time of diagnosis may not be remembered completely by survivors. However, for the subset of women in our study who had fertility counseling noted in their medical record, over 80% reported receiving counseling in their interview, indicating high recall of this event.
The quality of fertility counseling was unmeasured in our study. Among women who received counseling, some may not have gotten all the information they needed to make informed decisions about fertility preservation. This may have contributed to the higher prevalence of counseling in our cohort compared with other studies that use a stricter definition of counseling which includes a discussion about fertility preservation or receipt of a referral to a fertility specialist (24, 32). However, many of our women were diagnosed before the original ASCO and ASRM guidelines about fertility counseling in cancer survivors were released, so we focused on the broader question of whether or not women received any information about how cancer treatment could affect their ability to become pregnant. Cancer stage may have influenced whether or not a healthcare provider offered fertility counseling to their patient, however stage was missing for many of our participants. Among breast cancer survivors for whom we had the most complete data on stage, participants with stage 0 breast cancer were least likely to be counseled (56%) and those with stage 4 most likely (92%), but the prevalence of counseling was similar across stages 1–3 (70–71%). Lastly, some of our demographic variables including education, income, and insurance were measured at the time of the interview. We used these variables as proxies for status at the time of diagnosis, but there could be misclassification, especially for women diagnosed many years before the interview.
A strength of this study is that it included all cancers. This enabled us to see differences in receipt of counseling across cancer types grouped by how they might affect fertility. In our study, women with certain cancers were more likely to report receiving counseling than others. Because our study included women diagnosed over several decades, we could compare counseling and referral before and after the guidelines for universal counseling by ASRM in 2005 and ASCO in 2006. When we looked at counseling and referral however, we did not see a meaningful increase in more recent years although it is possible that if we were able to include women diagnosed more recently, we may have seen an increase in fertility counseling after the updated 2013 guidelines. Often the perceived need for fertility counseling is based on whether or not women already had children at the time of cancer diagnosis. In studies of referral to a specialist for a fertility preservation consultation, authors found that nulliparous women were more likely to receive a referral (32, 33). In our study we were able to look at the unique outcome of having fewer children than desired which captures women who have not yet reached their desired family size, regardless of parity. Lastly, our study was population-based and reflected the demographic make-up of Georgia allowing us to examine black-white racial differences in counseling adding to the literature on disparities in receipt of counseling (21).
Concerns about delay in treatment and risk of recurrence result in some women not receiving fertility counseling, but many of these concerns are not evidence-based. Early referral and accelerated ovarian stimulation protocols allow certain women to take advantage of a window of opportunity between early treatment that does not affect fertility (e.g., some surgeries) and the start of potentially gonadotoxic treatment regimens (34–36). However, women need to be counseled and referred to a specialist at or close to the time of diagnosis to take advantage of this opportunity. Women with hormone responsive cancers are sometimes advised not to become pregnant because of concern of increased risk of cancer recurrence (37). This advice continues despite several recent studies that show no increased risk of recurrence in women who use in vitro fertilization to become pregnant after cancer (38–41). Regardless of women’s specific characteristics, available options, and pursuit of fertility preservation, fertility counseling can improve the quality of life of cancer survivors (16–18).
For some women a well-coordinated cancer treatment and fertility preservation strategy at the time of diagnosis may allow them to have the children they want in the future without compromising their treatment plan. In our study population, early counseling was most likely to occur if the oncologist initiated the discussion; however, oncologists are already conveying large quantities of information to patients regarding their cancer diagnosis and treatment plan. Training other healthcare providers that are involved with the initial care of cancer patients to offer fertility counseling is part of the most recent recommendations by the ASRM and ASCO regarding fertility counseling and can help reach women early in the treatment process who do not receive or do not recall receiving information from their oncologist (12, 13). More broadly, this type of counseling can prepare women for what to expect with regard to changes in their reproductive system after cancer treatment which includes the ability to have a child, but extends to an increased risk of early menopause and the symptoms associated with this transition.
Supplementary Material
Acknowledgments
The Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant 1R01HD066059 and Reproductive, Perinatal, & Pediatric Training Grant T32HD052460, and the Health Resources and Service Administration Training Grant T03MC07651-06 provided funding for this research. This research was supported in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences. The authors would like to thank Amy Fothergill for all data inquiries and support on the paper. The authors have no conflicts of interest to report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental Figure 1. Prevalence of Counseling and Referral from 1999–2009 in the FUCHSIA Women’s Study
Counseling: Received fertility counseling at the time of cancer diagnosis.
Referral: Received a referral to a fertility specialist at the time of cancer diagnosis.
ASRM: Year the original ASRM guidelines on fertility counseling in cancer survivors.
ASCO: Year the original ASCO guidelines in fertility counseling in cancer survivors.
References
- 1.Surveillance, Epidemiology, and End Results (SEER) Program. Based on the November 2012 SEER data submission. National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch; 2013. Age-Adjusted SEER Incidence Rates By Age At Diagnosis/Death All Sites, All Races, Female 2000–2010 (SEER 18) www.seer.cancer.gov. [Google Scholar]
- 2.Gracia CR, Sammel MD, Freeman E, Prewitt M, Carlson C, Ray A, et al. Impact of cancer therapies on ovarian reserve. Fertility and sterility. 2012;97:134–40 e1. doi: 10.1016/j.fertnstert.2011.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Letourneau JM, Ebbel EE, Katz PP, Oktay KH, McCulloch CE, Ai WZ, et al. Acute ovarian failure underestimates age-specific reproductive impairment for young women undergoing chemotherapy for cancer. Cancer. 2012;118:1933–9. doi: 10.1002/cncr.26403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larsen EC, Muller J, Schmiegelow K, Rechnitzer C, Andersen AN. Reduced ovarian function in long-term survivors of radiation- and chemotherapy-treated childhood cancer. The Journal of clinical endocrinology and metabolism. 2003;88:5307–14. doi: 10.1210/jc.2003-030352. [DOI] [PubMed] [Google Scholar]
- 5.Levine JM, Kelvin JF, Quinn GP, Gracia CR. Infertility in reproductive-age female cancer survivors. Cancer. 2015 doi: 10.1002/cncr.29181. [DOI] [PubMed] [Google Scholar]
- 6.Jacobson MH, Mertens AC, Spencer JB, Manatunga AK, Howards PP. Menses resumption after cancer treatment-induced amenorrhea occurs early or not at all. Fertility and sterility. 2015 doi: 10.1016/j.fertnstert.2015.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Syse A, Kravdal O, Tretli S. Parenthood after cancer - a population-based study. Psycho-oncology. 2007;16:920–7. doi: 10.1002/pon.1154. [DOI] [PubMed] [Google Scholar]
- 8.Madanat LM, Malila N, Dyba T, Hakulinen T, Sankila R, Boice JD, Jr, et al. Probability of parenthood after early onset cancer: a population-based study. International journal of cancer Journal international du cancer. 2008;123:2891–8. doi: 10.1002/ijc.23842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cvancarova M, Samuelsen SO, Magelssen H, Fossa SD. Reproduction rates after cancer treatment: experience from the Norwegian radium hospital. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27:334–43. doi: 10.1200/JCO.2007.15.3130. [DOI] [PubMed] [Google Scholar]
- 10.Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2006;24:2917–31. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 11.Fertility preservation and reproduction in cancer patients. Fertility and sterility. 2005;83:1622–8. doi: 10.1016/j.fertnstert.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 12.Loren AW, Mangu PB, Beck LN, Brennan L, Magdalinski AJ, Partridge AH, et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2013;31:2500–10. doi: 10.1200/JCO.2013.49.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fertility preservation and reproduction in patients facing gonadotoxic therapies: a committee opinion. Fertility and sterility. 2013;100:1224–31. doi: 10.1016/j.fertnstert.2013.08.041. [DOI] [PubMed] [Google Scholar]
- 14.Partridge AH, Gelber S, Peppercorn J, Sampson E, Knudsen K, Laufer M, et al. Web-based survey of fertility issues in young women with breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2004;22:4174–83. doi: 10.1200/JCO.2004.01.159. [DOI] [PubMed] [Google Scholar]
- 15.Schover LR. Motivation for parenthood after cancer: a review. Journal of the National Cancer Institute Monographs. 2005:2–5. doi: 10.1093/jncimonographs/lgi010. [DOI] [PubMed] [Google Scholar]
- 16.Wilkes S, Coulson S, Crosland A, Rubin G, Stewart J. Experience of fertility preservation among younger people diagnosed with cancer. Human fertility (Cambridge, England) 2010;13:151–8. doi: 10.3109/14647273.2010.503359. [DOI] [PubMed] [Google Scholar]
- 17.Jeruss JS, Woodruff TK. Preservation of fertility in patients with cancer. The New England journal of medicine. 2009;360:902–11. doi: 10.1056/NEJMra0801454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bastings L, Baysal O, Beerendonk CC, IntHout J, Traas MA, Verhaak CM, et al. Deciding about fertility preservation after specialist counselling. Human reproduction (Oxford, England) 2014;29:1721–9. doi: 10.1093/humrep/deu136. [DOI] [PubMed] [Google Scholar]
- 19.Tschudin S, Bitzer J. Psychological aspects of fertility preservation in men and women affected by cancer and other life-threatening diseases. Human reproduction update. 2009;15:587–97. doi: 10.1093/humupd/dmp015. [DOI] [PubMed] [Google Scholar]
- 20.Letourneau JM, Ebbel EE, Katz PP, Katz A, Ai WZ, Chien AJ, et al. Pretreatment fertility counseling and fertility preservation improve quality of life in reproductive age women with cancer. Cancer. 2012;118:1710–7. doi: 10.1002/cncr.26459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Letourneau JM, Smith JF, Ebbel EE, Craig A, Katz PP, Cedars MI, et al. Racial, socioeconomic, and demographic disparities in access to fertility preservation in young women diagnosed with cancer. Cancer. 2012;118:4579–88. doi: 10.1002/cncr.26649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duffy CM, Allen SM, Clark MA. Discussions regarding reproductive health for young women with breast cancer undergoing chemotherapy. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2005;23:766–73. doi: 10.1200/JCO.2005.01.134. [DOI] [PubMed] [Google Scholar]
- 23.Vadaparampil ST, Christie J, Quinn GP, Fleming P, Stowe C, Bower B, et al. A pilot study to examine patient awareness and provider discussion of the impact of cancer treatment on fertility in a registry-based sample of African American women with breast cancer. Supportive care in cancer: official journal of the Multinational Association of Supportive Care in Cancer. 2012;20:2559–64. doi: 10.1007/s00520-012-1380-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shnorhavorian M, Harlan LC, Smith AW, Keegan TH, Lynch CF, Prasad PK, et al. Fertility preservation knowledge, counseling, and actions among adolescent and young adult patients with cancer: A population-based study. Cancer. 2015;121:3499–506. doi: 10.1002/cncr.29328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quinn GP, Block RG, Clayman ML, Kelvin J, Arvey SR, Lee JH, et al. If you did not document it, it did not happen: rates of documentation of discussion of infertility risk in adolescent and young adult oncology patients’ medical records. J Oncol Pract. 2015;11:137–44. doi: 10.1200/JOP.2014.000786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adamo M, Johnson C, Ruhl J, Dickie L. SEER program coding and staging manual. Bethesda, MD: National Cancer Institute; 2010. [Google Scholar]
- 27.Chin HB, Johnson CY, Kim KH, Knight JH, Mertens AC, Mink PJ, et al. Piloting a computer assisted telephone interview: the FUCHSIA Women’s Study. BMC Womens Health. 2014;14:149. doi: 10.1186/s12905-014-0149-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mature oocyte cryopreservation: a guideline. Fertility and sterility. 2013;99:37–43. doi: 10.1016/j.fertnstert.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 29.Quinn GP, Vadaparampil ST, King L, Miree CA, Wilson C, Raj O, et al. Impact of physicians’ personal discomfort and patient prognosis on discussion of fertility preservation with young cancer patients. Patient education and counseling. 2009;77:338–43. doi: 10.1016/j.pec.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Collins J. Cost-effectiveness of in vitro fertilization. Seminars in reproductive medicine. 2001;19:279–89. doi: 10.1055/s-2001-18047. [DOI] [PubMed] [Google Scholar]
- 31.Bann CM, Treiman K, Squiers L, Tzeng J, Nutt S, Arvey S, et al. Cancer Survivors’ Use of Fertility Preservation. Journal of women’s health (2002) 2015;24:1030–7. doi: 10.1089/jwh.2014.5160. [DOI] [PubMed] [Google Scholar]
- 32.Bastings L, Baysal O, Beerendonk CC, Braat DD, Nelen WL. Referral for fertility preservation counselling in female cancer patients. Human reproduction (Oxford, England) 2014;29:2228–37. doi: 10.1093/humrep/deu186. [DOI] [PubMed] [Google Scholar]
- 33.Goodman LR, Balthazar U, Kim J, Mersereau JE. Trends of socioeconomic disparities in referral patterns for fertility preservation consultation. Human reproduction (Oxford, England) 2012;27:2076–81. doi: 10.1093/humrep/des133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von Wolff M, Montag M, Dittrich R, Denschlag D, Nawroth F, Lawrenz B. Fertility preservation in women–a practical guide to preservation techniques and therapeutic strategies in breast cancer, Hodgkin’s lymphoma and borderline ovarian tumours by the fertility preservation network FertiPROTEKT. Archives of gynecology and obstetrics. 2011;284:427–35. doi: 10.1007/s00404-011-1874-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.von Wolff M, Thaler CJ, Frambach T, Zeeb C, Lawrenz B, Popovici RM, et al. Ovarian stimulation to cryopreserve fertilized oocytes in cancer patients can be started in the luteal phase. Fertility and sterility. 2009;92:1360–5. doi: 10.1016/j.fertnstert.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 36.Lee S, Ozkavukcu S, Heytens E, Moy F, Oktay K. Value of early referral to fertility preservation in young women with breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28:4683–6. doi: 10.1200/JCO.2010.30.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Surbone A, Petrek JA. Childbearing issues in breast carcinoma survivors. Cancer. 1997;79:1271–8. [PubMed] [Google Scholar]
- 38.Kroman N, Jensen MB, Wohlfahrt J, Ejlertsen B. Pregnancy after treatment of breast cancer–a population-based study on behalf of Danish Breast Cancer Cooperative Group. Acta oncologica (Stockholm, Sweden) 2008;47:545–9. doi: 10.1080/02841860801935491. [DOI] [PubMed] [Google Scholar]
- 39.Kroman N, Jensen MB, Melbye M, Wohlfahrt J, Mouridsen HT. Should women be advised against pregnancy after breast-cancer treatment? Lancet. 1997;350:319–22. doi: 10.1016/S0140-6736(97)03052-3. [DOI] [PubMed] [Google Scholar]
- 40.Hemminki K, Forsti A, Sundquist J, Ji J. Risk of familial breast cancer is not increased after pregnancy. Breast cancer research and treatment. 2008;108:417–20. doi: 10.1007/s10549-007-9611-y. [DOI] [PubMed] [Google Scholar]
- 41.Azim AA, Costantini-Ferrando M, Oktay K. Safety of fertility preservation by ovarian stimulation with letrozole and gonadotropins in patients with breast cancer: a prospective controlled study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2008;26:2630–5. doi: 10.1200/JCO.2007.14.8700. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


