Abstract
Objective
To investigate the direct actions of active 1,25-dihydroxy vitamin D3 (VD3) upon primate follicular development at specific stages of folliculogenesis.
Design
Secondary preantral follicles were isolated from rhesus monkeys ovaries, encapsulated in alginate, and cultured for 40 days. Follicles were randomly assigned to experimental groups of control, low-dose VD3 (LVD3; 25 pg/ml), and high-dose VD3 (HVD3; 100 pg/ml).
Setting
National primate research center.
Animals
Adult, female rhesus macaques (Macaca mulatta).
Intervention(s)
None.
Main Outcome Measure(s)
Follicle survival and growth, as well as oocyte size were assessed. Progesterone (P4), androstenedione (A4), estradiol (E2), and anti-Müllerian hormone (AMH) concentrations in culture media were measured.
Results
Compared with the control group, LVD3 increased preantral follicle survival at week 2 by > 66%, while HVD3 increased antral follicle diameters at week 5. Follicles with diameters ≥ 500 µm at week 5 were categorized as fast-growing follicles. Higher percentages of fast-growing follicles were obtained following HVD3 treatment. Although P4, A4, and E2 production by antral follicles were not altered by VD3, AMH concentrations were 36% higher in LVD3 group relative to controls at week 5. Oocytes with larger diameters were retrieved from antral follicles developed in both LVD3 and HVD3 groups compared with controls.
Conclusion
The addition of low-dose VD3 increased preantral follicle survival and maintained AMH production by antral follicles, while high-dose VD3 improved antral follicle growth. VD3 supplement promoted oocyte growth in in vitro-developed follicles. Direct actions of VD3 on the primate follicle appear to be both dose- and stage-dependent.
Keywords: Vitamin D3, anti-Müllerian hormone, follicle culture, primate folliculogenesis, ovary
Capsule
Vitamin D3 improved primate follicle survival and growth, anti-Müllerian hormone production and oocyte growth in vitro. Vitamin D3 direct actions in the primate follicle appear to be dose- and stage-dependent.
Introduction
Vitamin D is a secosteroid best known for regulating calcium absorption during osteogenesis. In addition, vitamin D affects a wide range of cellular processes including cell proliferation and differentiation, apoptosis, and inflammation (1). There is an increasing recognition that vitamin D also plays important roles in female reproduction. It is believed that normal physiological vitamin D levels are important for optimizing normal reproductive potential in women (2). Although studies have yet to be performed to assess a direct effect of vitamin D on promoting fecundity in women without reproductive dysfunction, several clinical studies suggest an association between adequate vitamin D and successful fertility treatments in women with a history of infertility. Most of these studies examined this relationship in the context of in vitro fertilization (IVF) treatment, wherein those individuals with lower circulating levels of vitamin D had reduced pregnancy rates (3, 4). Serum vitamin D level was also considered a predictor for successful pregnancy following ovulation induction in women with polycystic ovary syndrome (PCOS) who were trying to conceive (5). Vitamin D supplementation was also reported to improve menstrual regularity, follicular development and pregnancy rates in PCOS patients (6, 7). However, mechanism(s) by which sufficient vitamin D may result in improved reproductive outcomes remain unclear.
The circulating precursor of vitamin D (e.g., 25-hydroxy vitamin D) is synthesized from cholesterol predominantly in the skin upon ultraviolet light exposure, with an additional 10% being derived from dietary sources (8). Within target tissues, 25-hydroxy vitamin D is converted to 1,25-dihydroxy vitamin D3 (VD3), which then binds to the vitamin D receptor. Vitamin D receptor, a member of the nuclear hormone receptor superfamily, serves to regulate biological activities through its ability to affect gene transcription following activation by VD3 (9). Vitamin D receptor has been identified in the ovary, particularly in granulosa cells of the follicle (10). Thus, one possible mechanism by which vitamin D is associated with higher pregnancy rates may be by directly improving ovarian follicular development through promoting proliferation and differentiation of granulosa cells (2). However, the direct actions and regulation of vitamin D on ovarian preantral follicle growth and antral follicle maturation have yet to be examined in primate species.
We developed a 3-dimensional culture system whereby nonhuman primate (e.g., rhesus macaque) preantral follicles grow to the antral stage and are functional in terms of steroidogenesis, local growth factor production, and oocyte maturation (11). Vitamin D receptor mRNA expression was identified in in vitro-developed rhesus macaque follicles, which was consistent with the observation in follicles developed in vivo (RNA sequencing data are available at http://www.ncbi.nlm.nih.gov/sra; accession number: SRP044327). This well-established technique provides a valuable model to study the role of endocrine/paracrine factors play in regulating follicular growth and function at specific stages of follicular development. Therefore, studies were designed to examine possible direct actions of VD3 upon specific follicular metrics, oocyte morphological changes, and follicular steroid and anti-Müllerian hormone (AMH) production in macaques.
Material and Methods
Animal use and ovary collection
The general care and housing of rhesus macaques (Macaca mulatta) were provided by the Division of Comparative Medicine at the Oregon National Primate Research Center (ONPRC), Oregon Health & Science University. Animals were pair-caged in a temperature-controlled (22°C), light-regulated (12L:12D) room. The diet consisted of Purina monkey chow containing stabilized VD3 (Ralston-Purina, Richmond, IN, USA), provided twice a day, was supplemented with fresh fruit or vegetables once a day. Water was provided ad libitum. Animals were treated according to the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals. Protocols were approved by the ONPRC Institutional Animal Care and Use Committee (11).
Ovaries were collected from 3 animals at necropsy (12–13 year old; comparable to 30–35 year old in women). Euthanasia was due to reasons unrelated to reproductive health and necropsy was performed by the Pathology Services Unit, ONPRC. Ovaries were immediately transferred into HEPES-buffered holding media (Cooper Surgical, Inc., Trumbull, CT, USA) and kept at 37 °C for follicle isolation (11).
Follicle isolation, encapsulation, and culture
The process of follicle isolation, encapsulation, and culture was previously reported (11). Briefly, the ovarian cortex was cut into 1 × 1 × 1 mm cubes. Follicles were mechanically isolated using 31-gauge needles. Secondary follicles (diameter 125–225 µm) met criteria for encapsulation if they exhibited an intact basement membrane, 2–4 layers of granulosa cells, and a healthy, centrally located oocyte.
Follicles were individually transferred into 5 µl 0.25% (w/v) sterile sodium alginate (FMC BioPolymers, Philadelphia, PA, USA)-PBS (137 mM NaCL, 10 mM phosphate, 2.7 mM KCl, Invitrogen, Carlsbad, CA, USA). The droplets were cross-linked in 50 mM CaCl2, 140 mM NaCl, 10 mM HEPES solution (pH 7.2). Each encapsulated follicle was placed in individual wells of 48-well plates containing 300 µl alpha minimum essential medium (Invitrogen) containing 6% (v/v) human serum protein supplement (SPS; Cooper Surgical, Inc.), 0.5 mg/ml bovine fetuin, 5 µg/ml insulin, 5µg/ml transferrin, 5 ng/ml sodium selenite (Sigma-Aldrich, St Louis, MO, USA), and 3 ng/ml recombinant human follicle-stimulating hormone (FSH; NV Organon/Merck Sharp & Dohme, Oss, Netherlands) (11).
Follicles from each of the three animals were randomly assigned to 3 experimental groups with 48 follicles/group: (a) CTRL group: control media; (b) LVD3 group: low-dose VD3 (biologically active form of vitamin D3; Sigma-Aldrich), 25 pg/ml; and (c) HVD3 group: high-dose VD3, 100 pg/ml. Doses were determined based on the serum and follicular fluid levels reported in women undergoing ovarian stimulations (12). Follicles were cultured at 37 °C in a 5% O2 environment (in 6% CO2/89% N2) for 40 days. Media (150 µl) was collected and replaced every other day, and stored at −20°C for analyses of steroid hormone and AMH concentrations (11).
Follicle survival and growth
Follicle survival, growth, and antrum formation were assessed weekly using an Olympus CK-40 inverted microscope and an Olympus DP11 digital camera (Olympus Imaging America Inc., Center Valley, PA) as described previously (11). Follicle sizes were determined by measuring the distance from the outer layer of cells at the widest diameter and then the diameter perpendicular to the first measurement by the same individual. The mean of the two values determined the follicle’s overall diameter. The measurements were performed using Image J 1.48 software (National Institutes of Health, Bethesda, MD, USA). Follicles were considered atretic if the oocyte was dark or not surrounded by a layer of granulosa cells, the granulosa cells appeared dark or fragmented, or the follicle diameter decreased. To avoid bias and decrease inter-observer variation, blind analyses were performed by a single investigator (JX).
Ovarian steroid and AMH assays
One media sample collected weekly (the first sample of the week) from each follicle culture was analyzed for progesterone (P4), androstenedione (A4), and estradiol (E2) concentrations by the Endocrine Technology Support Core at ONPRC. P4 and E2 were assayed using a Cobas Elecsys platform (Roche Diagnostics, Indianapolis, IN, USA). A4 concentrations were measured by ELISA using an AA E-1000 kit (Rocky Mountain Diagnostics, Inc., Colorado Springs, CO, USA) according to the manufacturers’ instructions. The sensitivity of the assay was 0.04 ng/ml for 25 µl sample. The standard curve of the assay ranged 0.1–10 ng/ml. Another media sample collected weekly (the second sample of the week) was analyzed for AMH concentrations by ELISA (AL-105 kit, AnshLabs, Webster, TX, USA) based on the manufacturers’ instructions. The sensitivity of the assay was 23 pg/ml for 25 µl sample. The standard curve of the assay ranged 0.08–14.2 ng/ml. Control media was also measured using a Cobas Elecsys kit (catalog number: 06506780160; Roche Diagnostics, Indianapolis, IN, USA) to ensure 25-hydroxy vitamin D3 was not included in the media supplements used for follicle culture.
Oocyte evaluation
Oocyte evaluations were performed on a 37 °C warming plate as previously described (11). Briefly, cumulus cells were removed from cumulus-oocyte complexes in Tyrode’s albumin lactate pyruvate (TALP)-HEPES-BSA (0.3% v/v) medium provided by the Assisted Reproductive Technologies Support Core (ART) at ONPRC to obtain denuded oocytes. Oocytes were then transferred to TALP medium and photographed. Oocyte diameters (excluding the zona pellucida) and conditions were assessed using the same camera and software as described above. Oocytes were considered degenerated if the oocyte was dark or the oocyte cytoplasm became condensed. To avoid bias and decrease inter-observer variation, blind analyses were performed by a single investigator (JX).
Statistical analysis
Statistical significance was determined by SigmaPlot 11 software (SPSS, Inc., Chicago, IL, USA) using a two-way analysis of variance (ANOVA) with repeated measures or one-way ANOVA followed by the Student-Newman-Keuls post hoc test for single time points. Differences were considered significant at P < 0.05 and values are presented as mean ± SEM. Follicle survival represents the percentage of three individual animals in each experimental group. Follicle growth, AMH production and oocyte growth were analyzed for each individual follicle with total follicle numbers indicated in the figure captions, and represent follicles obtained from the three individual animals.
Results
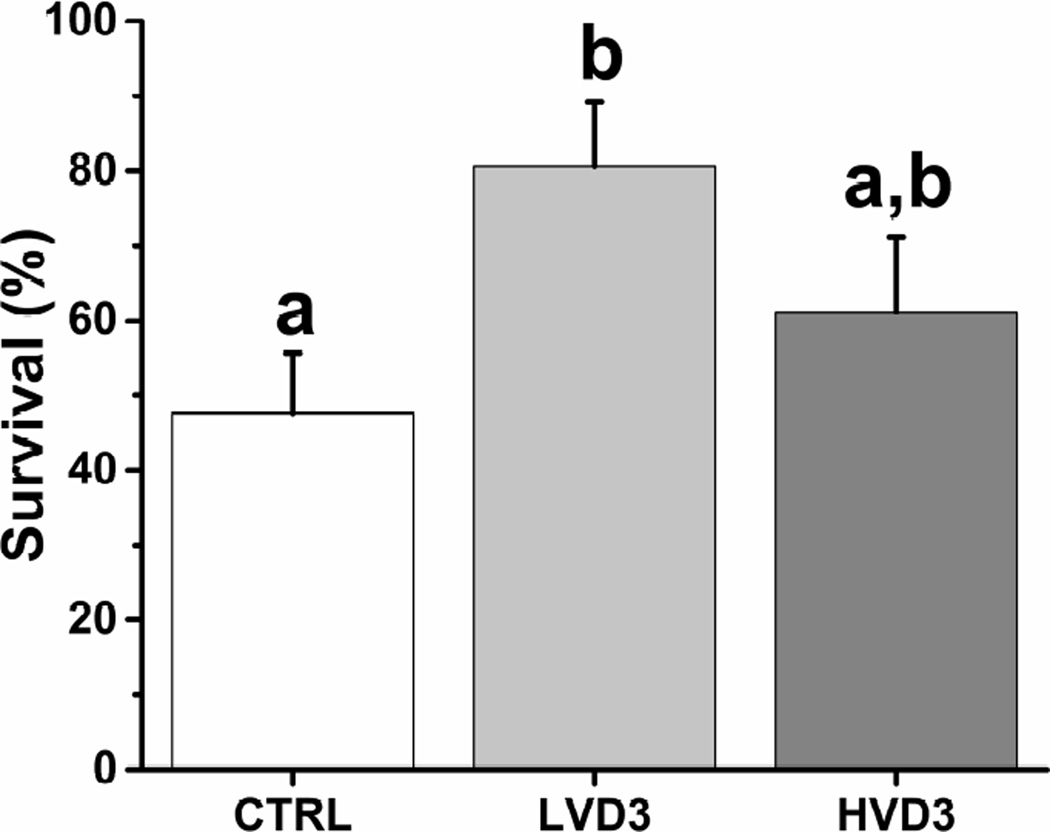
The percentages of rhesus macaque follicles that survived after 2 weeks, when follicles were still at the preantral stage, relative to the total number cultured was 66% greater (P < 0.05) in the presence of LVD3 than in the no-VD3 control group (Figure 1). HVD3 had no effect on follicle survival at 2 weeks of culture relative to controls (Figure 1). Follicle survival rates remained unchanged in all experimental groups after week 2 until the end of culture at week 5.
Figure 1.
The effects of vitamin D3 on rhesus macaque preantral follicle survival after 2 weeks of culture in an alginate matrix. Follicle survival was calculated as the percentage of survived follicles relative to the total number cultured. CTRL, control; LVD3, low-dose (25 pg/ml) vitamin D3 addition; HVD3, high-dose (100 pg/ml) vitamin D3 addition. Significant differences between treatment groups are indicated by different letters (P < 0.05). Data are presented as the mean ± SEM with 3 animals per experimental group.
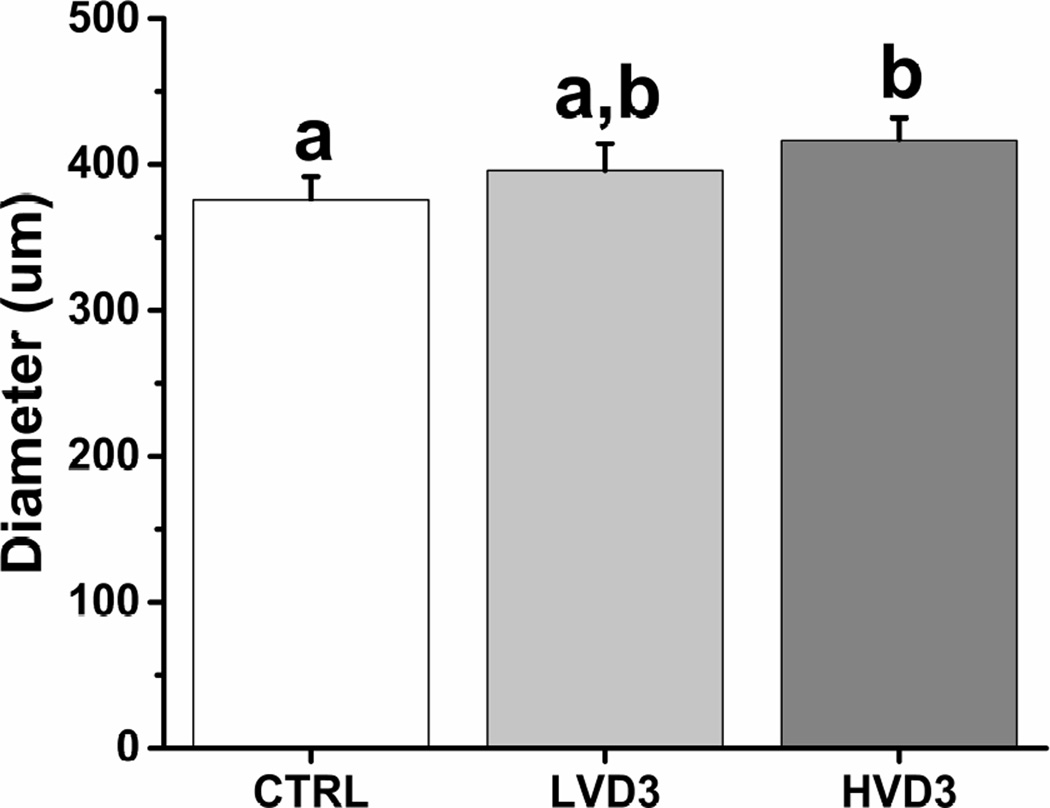
Rhesus macaque follicles that survive in vitro can be divided into distinct cohorts based on their growth rates by week 5, as previously described (13). While non-growing follicles remained at the preantral stage throughout 5 weeks of culture, growing follicles formed an antrum at week 3. The percentages of growing versus total surviving follicles were comparable among all three groups (control = 93.3 ± 0.7%, LVD3 = 95.3 ± 0.5%, HVD3 = 93.3 ± 0.7%). However, though starting at equivalent sizes at the beginning of culture, growing follicles attained a larger diameter (P < 0.05) at week 5 of culture in the HVD3 group compared with those cultured in the absence of VD3 (Figure 2). In contrast, the final sizes of rhesus macaque antral follicles cultured in LVD3 were not significantly different from either the control or HVD3-treated follicles (Figure 2). Growing follicles with diameters ≥ 500 µm at week 5 were termed as fast-growing follicles, as previously described (11). Similar percentages of fast-growing versus total growing follicles were noted in the control and LVD3 groups at week 5 (Supplemental Figure 1), whereas the percentage of fast-growing follicles was significantly greater (P < 0.05) in the HVD3 group (25%) relative to controls (4%).
Figure 2.
The effects of vitamin D3 on rhesus macaque antral follicle growth after 5 weeks of culture in an alginate matrix. Follicle growth was determined by measuring follicle diameters. CTRL, control; LVD3, low-dose (25 pg/ml) vitamin D3 addition; HVD3, high-dose (100 pg/ml) vitamin D3 addition. Significant differences between treatment groups are indicated by different letters (P < 0.05). Data are presented as the mean ± SEM with 20–34 follicles per experimental group.
In vitro-developed macaque antral follicles produce appreciable amounts of ovarian steroids, including P4, A4, and E2, into the culture media as described previously (11). Neither low-dose nor high-dose VD3 addition altered media P4, A4, or E2 concentrations produced by growing follicles compared with controls at week 5 (P4: control = 35.5 ± 11.6 ng/ml, LVD3 = 51.4 ± 13.7 ng/ml, HVD3 = 47.5 ± 11.5 ng/ml; A4: control = 7.7 ± 3.9 pg/ml, LVD3 = 4.7 ± 2.0 pg/ml, HVD3 = 9.0 ± 4.9 pg/ml; E2: control = 466.6 ± 192.1 pg/ml, LVD3 = 360.2 ± 75.2 pg/ml, HVD3 = 390.5 ± 108.3 pg/ml). P4, A4, and E2 production by non-growing follicles was undetectable in the media over 5 weeks of culture regardless of VD3 addition (data not shown).
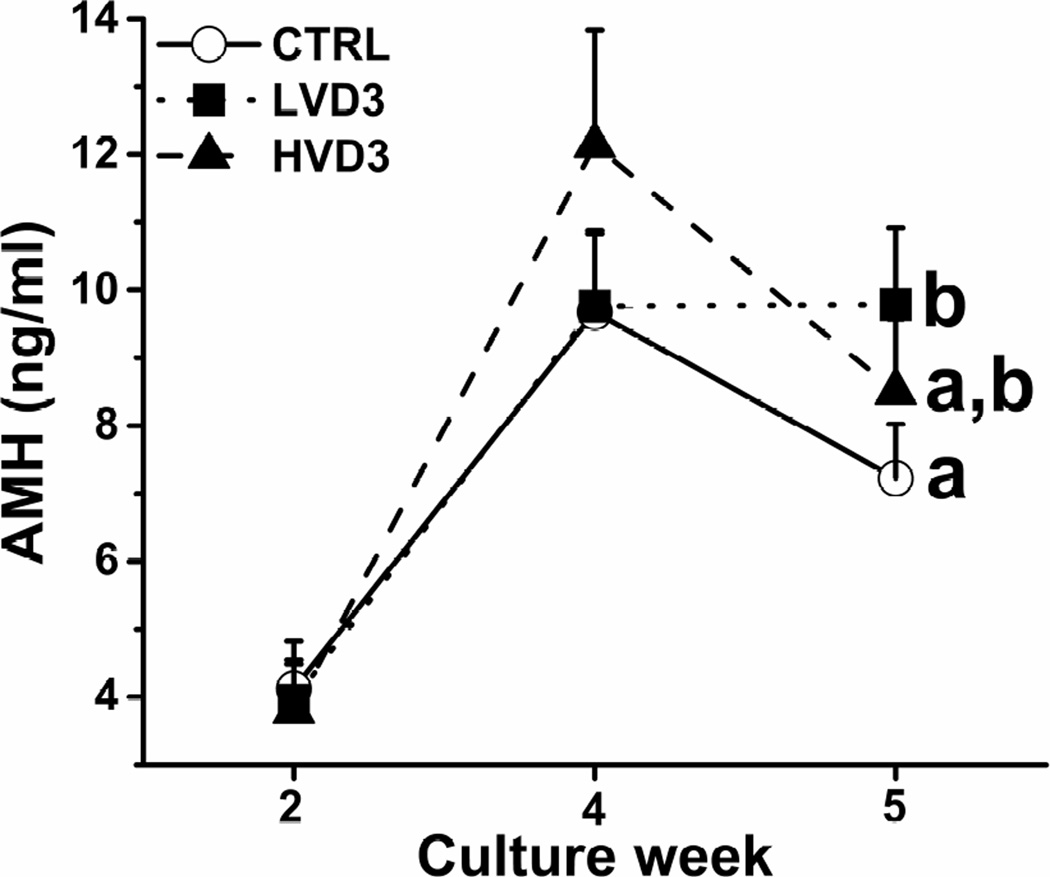
Media AMH concentrations produced by cultured macaque follicles demonstrated similar patterns to those reported previously (11). AMH secreted by growing follicles was detectable at week 2 (preantral stage) and peaked at week 4 (antral stage) (Figure 3). While comparable among all three experimental groups at weeks 2 and 4, AMH levels produced by in vitro-developed antral follicles were higher (P < 0.05) in the LVD3 group than those of the control group at week 5 (Figure 3). The week 5 media AMH levels were not different between the control and HVD3 groups (Figure 3). AMH production by non-growing follicles was unchanged in the culture media over 5 weeks of culture regardless of VD3 addition (data not shown).
Figure 3.
The effects of vitamin D3 on anti-Müllerian hormone (AMH) production by rhesus macaque antral follicles during 5 weeks of culture in an alginate matrix. AMH production was determined by measuring AMH concentrations in the culture media. CTRL, control; LVD3, low-dose (25 pg/ml) vitamin D3 addition; HVD3, high-dose (100 pg/ml) vitamin D3 addition. Significant differences between treatment groups at week 5 are indicated by different letters (P < 0.05). Data are presented as the mean ± SEM with 20–34 follicles per experimental group.
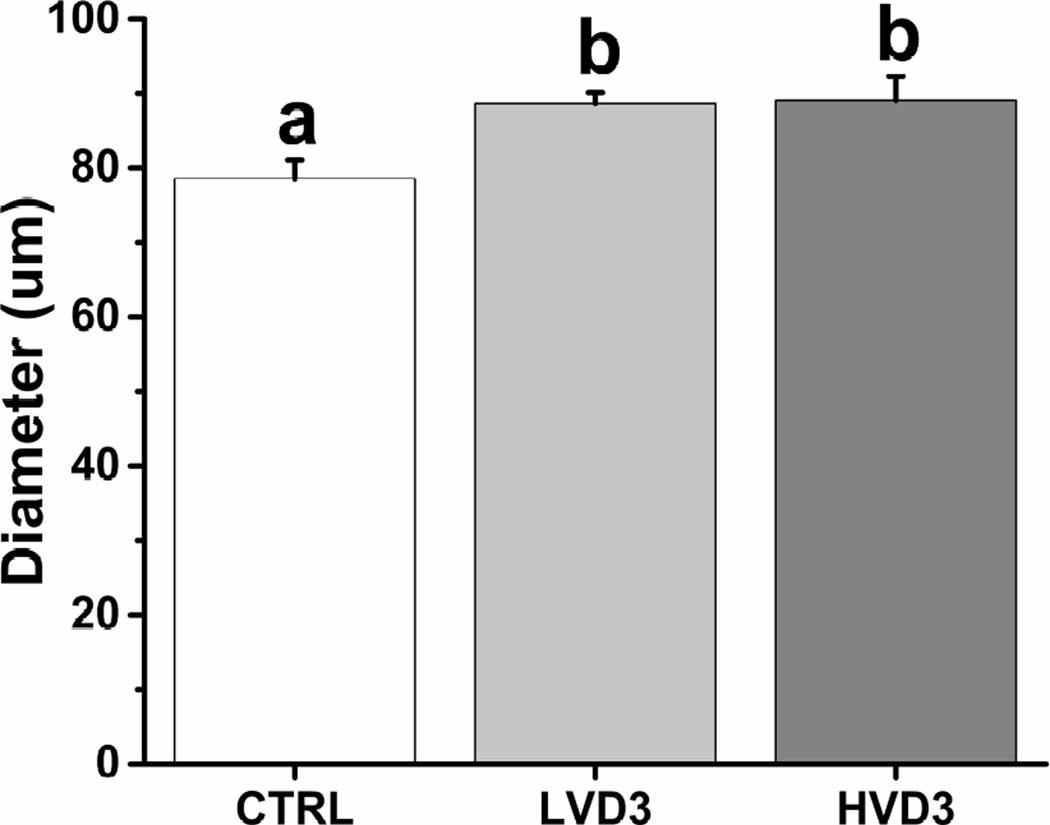
Healthy, germinal vesicle stage oocytes were obtained from in vitro-developed macaque antral follicles after 5 weeks of culture in all three treatment groups. Compared with controls, VD3 treatment did not alter the percentages of healthy versus total oocytes harvested (control = 88.9 ± 1.1%, LVD3 = 93.0 ± 0.7%, HVD3 = 89.6 ± 1.0%). However, both LVD3 and HVD3 treatment increased oocyte diameters at week 5 relative to the control group (P < 0.05) (Figure 4). Oocytes with a diameter greater than 100 µm were only obtained from the VD3-treated follicles (LVD3 = 7.1% and HVD3 = 22.2% of total healthy oocytes harvested).
Figure 4.
The effects of vitamin D3 on oocyte growth in rhesus macaque antral follicles after 5 weeks of culture in an alginate matrix. Oocyte growth was determined by measuring oocyte diameters. CTRL, control; LVD3, low-dose (25 pg/ml) vitamin D3 addition; HVD3, high-dose (100 pg/ml) vitamin D3 addition. Significant differences between treatment groups are indicated by different letters (P < 0.05). Data are presented as the mean ± SEM with 14–28 oocytes per experimental group.
Discussion
There are numerous studies supporting the salutary effects of vitamin D upon female reproduction in both animal models and in humans (2, 14). However, data regarding the role of vitamin D in reproductive physiology thus far are almost all correlative. The mechanisms of action by which the beneficial effects of vitamin D may occur remain unclear. The current study, using a non-human primate model, demonstrates for the first time that vitamin D has a direct positive effect upon ovarian folliculogenesis. These data support a role for local actions of vitamin D on primate follicular development/function, which are dose- and stage-dependent. The mechanism of this effect is likely to be via vitamin D receptors expressed in the growing follicles (http://www.ncbi.nlm.nih.gov/sra; accession number: SRP044327).
Survival during the initial two weeks of culture, when macaque follicles are at the preantral stage, is critical for subsequent follicular development in vitro. Follicles that survive the first two weeks adapt to the in vitro environment and are viable for the remaining 3 weeks of culture, as demonstrated in the current study. Low-dose VD3 appeared to be more effective than high-dose VD3 in promoting early folliculogenesis by increasing preantral follicle survival. Previous studies suggested that FSH was a survival factor for preantral follicles developed in vitro in both nonhuman primates and humans (15, 16). Although not demonstrated in primate species, FSH receptor mRNA expression was up-regulated in non-luteinized granulosa cells in hens following VD3 treatment in culture (17). Therefore, exogenous VD3 may increase FSH sensitivity in macaque follicles, and thereby promote follicle survival in vitro. However, with limited numbers of granulosa cells in the preantral follicle, high VD3 levels may have limited effects on follicle survival by causing exaggerated gene expression via the activation of vitamin D receptor (18). It is noteworthy that vitamin D deficiency is associated with primary ovarian insufficiency in women probably due to increased follicular atresia (19), though the mechanism undertaken by vitamin D on improving follicle survival in vivo may be different from that of in vitro.
Following antrum formation at week 3 of culture, growing macaque follicles progressed to the small antral stage by increasing their diameters through an additional two weeks of in vitro development. High-dose VD3 appeared to have a greater impact than low-dose VD3 on promoting later folliculogenesis by improving small antral follicle growth. The data were consistent with findings in hens in which a significant enhancement of cell proliferation was observed for non-luteinized granulosa cells incubated with VD3 (17). A previous study indicated that a relative high-dose VD3 not only down-regulated AMH receptor II gene expression, but also significantly reduced the phosphorylation and nuclear localization of SMAD 1/5/8, which are critical for AMH signaling, in cultured granulosa cells collected from IVF patients (20). Growing ovarian follicles isolated from rhesus macaques produce AMH, and AMH directly inhibits the growth and maturation of antral follicles (13). Therefore, one potential mechanism whereby exogenous VD3 may counteract the repressive effect of AMH on antral follicle development would be by limiting AMH receptor expression and signaling, and in turn improving differentiation of granulosa cells in the antral follicle. Vitamin D treatment may also directly promote growth of the follicle by increasing granulosa cell numbers based upon its ability to induce proliferation in other cell types. For example, vitamin D was shown to induce endometrial cell proliferation in women with polycystic ovarian syndrome (PCOS) during intrauterine insemination cycles (21).
An effect of VD3 on steroid production was not noted in the current study, possibly due to variability between follicles. Sample sizes need to be increased in future studies to discern VD3 actions on follicular steroidogenesis. However, low-dose VD3 increased AMH production by in vitro-derived macaque antral follicles. The data are consistent with observations from studies using a human prostate cancer cell line, in which a relative low-dose VD3 analog treatment in vitro upregulated AMH mRNA expression (22). A functional vitamin D receptor response element located in the promoter region of vitamin D-regulated genes was later identified in the promoter region of the AMH gene in human prostate cancer cells, accounting for the direct effect of vitamin D on AMH mRNA expression (23). In clinical studies, positive correlations were observed between circulating vitamin D and serum AMH levels in late-reproductive-aged women (24). A rise in AMH production following vitamin D supplementation was reported in patients without a history of infertility who were vitamin D deficient due to seasonal variation (25). The relationship between vitamin D and AMH may reflect vitamin D-regulated follicular AMH production, a systematic biomarker of follicular activities, by the ovary.
In the current study, vitamin D exhibited a positive impact upon oocyte growth in in vitro-developed macaque antral follicles wherein they reached a final size that was comparable to that of in vivo-matured oocytes, which are greater than 100 µm in diameter in macaques (26). It is well-known that communication via gap junctions within the cumulus-oocyte complex is critical for oocyte metabolism and maturation. Gap junctions, formed of connexin proteins, permit the exchange of regulatory molecules between oocyte and its surrounding cumulus cells (27). Previous studies indicated that VD3 treatment enhanced connexin expression and assembly into functional gap junctions while preventing androgen-induced connexin degradation in cultured human prostate cancer cells (28). The VD3-induced gap junction-mediated communication was also observed in human skin fibroblasts through increasing connexin protein and mRNA levels (29). Thus, it is speculated that one direct mechanism by which vitamin D may promote oocyte growth and cumulus cell proliferation and differentiation is by facilitating gap junction formation and/or function.
Deficiency of vitamin D is common and ranges from 20–90% of reproductive-age women in North America (30). Increased rates of vitamin D deficiency were also reported in women with PCOS (31, 32). Thus, the current findings that vitamin D directly acts on primate follicular development may provide insights in understanding vitamin D deficiency-related reproductive dysfunction in women and in improving ovarian function by vitamin D supplementation. This work may also have promising implications for facilitating in vitro maturation (IVM) of mature and immature oocytes in assisted reproductive technologies (ART). Further investigation will likely delineate more precise dosing and timing of vitamin D exposure to promote stage-specific maturation and morphological development of the primate follicle. Further study will also assess if oocyte morphological changes have an impact upon the downstream development of embryo quality and ultimately their implantation rates.
Supplementary Material
Acknowledgments
We are grateful for the assistance provided by members of the Division of Comparative Medicine, the Pathology Services Unit, the Endocrine Technology Support Core, and the Assisted Reproductive Technologies Support Core at ONPRC. The valuable assistance of Ms. Maralee Lawson, ONPRC, with follicle culture is appreciated.
This work was supported by the National Institutes of Health (NIH) Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) R01HD082208 (JX), NIH Office of Research on Women's Health/NICHD K12HD043488 (Building Interdisciplinary Research Careers in Women’s Health, BIRCWH) (JX), NIH Office of the Director P51OD011092 (Oregon National Primate Research Center) (JX, JDH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jing Xu, Email: xujin@ohsu.edu.
Jon D. Hennebold, Email: henneboj@ohsu.edu.
David B. Seifer, Email: David.B.Seifer@hitchcock.org, David.B.Seifer@Dartmouth.edu.
References
- 1.Verstuyf A, Carmeliet G, Bouillon R, Mathieu C. Vitamin D: a pleiotropic hormone. Kidney Int. 2010;78:140–145. doi: 10.1038/ki.2010.17. [DOI] [PubMed] [Google Scholar]
- 2.Irani M, Merhi Z. Role of vitamin D in ovarian physiology and its implication in reproduction: a systematic review. Fertil Steril. 2014;102:460–468. doi: 10.1016/j.fertnstert.2014.04.046. [DOI] [PubMed] [Google Scholar]
- 3.Ozkan S, Jindal S, Greenseid K, Shu J, Zeitlian G, Hickmon C, et al. Replete vitamin D stores predict reproductive success following in vitro fertilization. Fertil Steril. 2010;94:1314–1319. doi: 10.1016/j.fertnstert.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paffoni A, Ferrari S, Viganò P, Pagliardini L, Papaleo E, Candiani M, et al. Vitamin D deficiency and infertility: insights from in vitro fertilization cycles. J Clin Endocrinol Metab. 2014;99:E2372–E2376. doi: 10.1210/jc.2014-1802. [DOI] [PubMed] [Google Scholar]
- 5.Pal L, Zhang H, Williams J, Santoro NF, Diamond MP, Schlaff WD, et al. Vitamin D Status Relates to Reproductive Outcome in Women with Polycystic Ovary Syndrome: Secondary Analysis of a Multicenter Randomized Controlled Trial. J Clin Endocrinol Metab. 2016;101:3027–3035. doi: 10.1210/jc.2015-4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thys-Jacobs S, Donovan D, Papadopoulos A, Sarrel P, Bilezikian JP. Vitamin D and calcium dysregulation in the polycystic ovarian syndrome. Steroids. 1999;64:430–435. doi: 10.1016/s0039-128x(99)00012-4. [DOI] [PubMed] [Google Scholar]
- 7.Firouzabadi Rd, Aflatoonian A, Modarresi S, Sekhavat L, MohammadTaheri S. Therapeutic effects of calcium & vitamin D supplementation in women with PCOS. Complement Ther Clin Pract. 2012;18:85–88. doi: 10.1016/j.ctcp.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Wagner CL, Taylor SN, Dawodu A, Johnson DD, Hollis BW. Vitamin D and its role during pregnancy in attaining optimal health of mother and fetus. Nutrients. 2012;4:208–230. doi: 10.3390/nu4030208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Norman AW. Minireview: vitamin D receptor: new assignments for an already busy receptor. Endocrinology. 2006;147:5542–5548. doi: 10.1210/en.2006-0946. [DOI] [PubMed] [Google Scholar]
- 10.Stumpf WE. Vitamin D sites and mechanisms of action: a histochemical perspective. Reflections on the utility of autoradiography and cytopharmacology for drug targeting. Histochem Cell Biol. 1995;104:417–427. doi: 10.1007/BF01464331. [DOI] [PubMed] [Google Scholar]
- 11.Xu J, Lawson MS, Yeoman RR, Molskness TA, Ting AY, Stouffer RL, et al. Fibrin promotes development and function of macaque primary follicles during encapsulated three-dimensional culture. Hum Reprod. 2013;28:2187–2200. doi: 10.1093/humrep/det093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Potashnik G, Lunenfeld E, Levitas E, Itskovitz J, Albutiano S, Yankowitz N, et al. The relationship between endogenous oestradiol and vitamin D3 metabolites in serum and follicular fluid during ovarian stimulation for in-vitro fertilization and embryo transfer. Hum Reprod. 1992;7:1357–1360. doi: 10.1093/oxfordjournals.humrep.a137573. [DOI] [PubMed] [Google Scholar]
- 13.Xu J, Bishop CV, Lawson MS, Park BS, Xu F. Anti-Müllerian hormone promotes preantral follicle growth, but inhibits antral follicle maturation and dominant follicle selection in primates. Hum Reprod. 2016;31:1522–1530. doi: 10.1093/humrep/dew100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luk J, Torrealday S, Neal Perry G, Pal L. Relevance of vitamin D in reproduction. Hum Reprod. 2012;27:3015–3027. doi: 10.1093/humrep/des248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu J, Bernuci MP, Lawson MS, Yeoman RR, Fisher TE, Zelinski MB, et al. Survival, growth, and maturation of secondary follicles from prepubertal, young, and older adult rhesus monkeys during encapsulated three-dimensional culture: effects of gonadotropins and insulin. Reproduction. 2010;140:685–697. doi: 10.1530/REP-10-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wright CS, Hovatta O, Margara R, Trew G, Winston RM, Franks S, et al. Effects of follicle-stimulating hormone and serum substitution on the in-vitro growth of human ovarian follicles. Hum Reprod. 1999;14:1555–1562. doi: 10.1093/humrep/14.6.1555. [DOI] [PubMed] [Google Scholar]
- 17.Wojtusik J, Johnson PA. Vitamin D regulates anti-Mullerian hormone expression in granulosa cells of the hen. Biol Reprod. 2012;86:91. doi: 10.1095/biolreprod.111.094110. [DOI] [PubMed] [Google Scholar]
- 18.Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr. 2008;88:582S–586S. doi: 10.1093/ajcn/88.2.582S. [DOI] [PubMed] [Google Scholar]
- 19.Kebapcilar AG, Kulaksizoglu M, Kebapcilar L, Gonen MS, Unlü A, Topcu A, et al. Is there a link between premature ovarian failure and serum concentrations of vitamin D, zinc, and copper? Menopause. 2013;20:94–99. doi: 10.1097/gme.0b013e31826015ca. [DOI] [PubMed] [Google Scholar]
- 20.Merhi Z, Doswell A, Krebs K, Cipolla M. Vitamin D alters genes involved in follicular development and steroidogenesis in human cumulus granulosa cells. J Clin Endocrinol Metab. 2014;99:E1137–E1145. doi: 10.1210/jc.2013-4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asadi M, Matin N, Frootan M, Mohamadpour J, Qorbani M, Tanha FD. Vitamin D improves endometrial thickness in PCOS women who need intrauterine insemination: a randomized double-blind placebo-controlled trial. Arch Gynecol Obstet. 2014;289:865–870. doi: 10.1007/s00404-013-3055-x. [DOI] [PubMed] [Google Scholar]
- 22.Krishnan AV, Moreno J, Nonn L, Malloy P, Swami S, Peng L, et al. Novel pathways that contribute to the anti-proliferative and chemopreventive activities of calcitriol in prostate cancer. J Steroid Biochem Mol Biol. 2007;103:694–702. doi: 10.1016/j.jsbmb.2006.12.051. [DOI] [PubMed] [Google Scholar]
- 23.Malloy PJ, Peng L, Wang J, Feldman D. Interaction of the vitamin D receptor with a vitamin D response element in the Mullerian-inhibiting substance (MIS) promoter: regulation of MIS expression by calcitriol in prostate cancer cells. Endocrinology. 2009;150:1580–1587. doi: 10.1210/en.2008-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merhi ZO, Seifer DB, Weedon J, Adeyemi O, Holman S, Anastos K, et al. Circulating vitamin D correlates with serum antimüllerian hormone levels in late-reproductive-aged women: Women's Interagency HIV Study. Fertil Steril. 2012;98:228–234. doi: 10.1016/j.fertnstert.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dennis NA, Houghton LA, Jones GT, van Rij AM, Morgan K, McLennan IS. The level of serum anti-Müllerian hormone correlates with vitamin D status in men and women but not in boys. J Clin Endocrinol Metab. 2012;97:2450–2455. doi: 10.1210/jc.2012-1213. [DOI] [PubMed] [Google Scholar]
- 26.Buse E, Zöller M, Van Esch E. The macaque ovary, with special reference to the cynomolgus macaque (Macaca fascicularis) Toxicol Pathol. 2008;36:24S–66S. [Google Scholar]
- 27.Kimura N, Hoshino Y, Totsukawa K, Sato E. Cellular and molecular events during oocyte maturation in mammals: molecules of cumulus-oocyte complex matrix and signalling pathways regulating meiotic progression. Soc Reprod Fertil Suppl. 2007;63:327–342. [PubMed] [Google Scholar]
- 28.Kelsey L, Katoch P, Ray A, Mitra S, Chakraborty S, Lin MF, et al. Vitamin D3 regulates the formation and degradation of gap junctions in androgen-responsive human prostate cancer cells. PLoS One. 2014;9:e106437. doi: 10.1371/journal.pone.0106437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clairmont A, Tessman D, Stock A, Nicolai S, Stahl W, Sies H. Induction of gap junctional intercellular communication by vitamin D in human skin fibroblasts is dependent on the nuclear Induction of gap junctional intercellular communication by vitamin D in human skin fibroblasts is dependent on the nuclear vitamin D receptor. Carcinogenesis. 1996;17:1389–1391. doi: 10.1093/carcin/17.6.1389. [DOI] [PubMed] [Google Scholar]
- 30.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 31.Li HW, Brereton RE, Anderson RA, Wallace AM, Ho CK. Vitamin D deficiency is common and associated with metabolic risk factors in patients with polycystic ovary syndrome. Metabolism. 2011;60:1475–1481. doi: 10.1016/j.metabol.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Wehr E, Trummer O, Giuliani A, Gruber HJ, Pieber TR, Obermayer-Pietsch B. Vitamin D-associated polymorphisms are related to insulin resistance and vitamin D deficiency in polycystic ovary syndrome. Eur J Endocrinol. 2011;164:741–749. doi: 10.1530/EJE-11-0134. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.