Abstract
Several recently developed biomarkers of Alzheimer disease (AD) are invasive, expensive, and difficult to obtain in most clinical settings. Olfactory identification test performance represents a noninvasive, inexpensive biomarker of AD that may have predictive accuracy comparable with neuroimaging measures and biomarkers assessed in cerebrospinal fluid. Neurofibrillary tangles in the olfactory bulb are among the earliest pathologic features of AD and are also seen in the projection pathways from the olfactory bulb to secondary olfactory brain regions, including the piriform and medial temporal cortex, orbitofrontal cortex, and other limbic regions. Odor identification impairment characterizes AD and predicts the clinical transition from mild cognitive impairment to AD in both clinical and community samples. Epidemiologic data indicate that in cognitively intact older adults, impairment in odor identification predicts cognitive decline but that episodic verbal memory impairment does not predict cognitive decline. Odor identification impairment has also been shown to predict mortality in older subjects with mortality risk increasing with greater severity of impairment in odor identification. The exact cause of this association is not known, but olfactory deficits may lead to an increase in accidents in the home, because of the inability to smell and taste food that is unsafe or not smelling a gas leak or fire, and this may increase mortality risk. Standardized tests of odor identification ability are widely available and may provide a useful tool to improve diagnostic and predictive accuracy for cognitive decline, AD, and mortality in older adults.
Keywords: Alzheimer disease, olfaction, odor identification deficits, cognitive decline, Mortality
EARLY MARKERS OF ALZHEIMER DISEASE
The aging of the population has been associated with increased prevalence of diseases associated with age, including Alzheimer disease (AD). Cholinesterase inhibitors and the N-methyl-D-aspartate (NMDA) receptor partial antagonist memantine are approved for the treatment of AD but are associated with limited clinically measurable improvement in cognition. Antiamyloid therapies and other types of experimental therapies have been unsuccessful to date.1,2
Identification of predictors of the clinical transition to a diagnosis of AD is important to estimate prognosis that can be helpful to patients and family members and to target early treatment in patients who are at high risk. Age is a risk factor for most dementia subtypes and for AD more specifically.3 The apolipoprotein E e4 genotype is a well-established risk factor for AD, and although other genes have been identified, none has as strong an association with AD as the apolipoprotein E e4 genotype.4 Measures that clearly distinguish patients with AD from cognitively intact control subjects and are associated with the transition from mild cognitive impairment (MCI) to AD include neuropsychological impairment in tests of memory and executive function, hippocampal and entorhinal cortex atrophy and reduced cortical thickness on magnetic resonance imaging scan of brain, parietotemporal blood flow and metabolism deficits with single-photon emission computed tomography and 18F-fluorodeoxyglucose positron emission tomography (PET), increased uptake/retention with amyloid imaging radiotracers using PET, and decreased β42-amyloid levels and increased tau and phospho-tau levels in cerebrospinal fluid.3
Several of these neurobiologic markers are expensive and often invasive, and each of them typically achieves sensitivity and specificity in the range of 60%–80% in most studies that have examined the prediction of the transition from MCI to a clinical diagnosis of AD.5,6 In clinical practice, noninvasive markers are more practical and feasible, and impairment in odor identification has emerged as an important biomarker with diagnostic group discrimination and predictive utility that are comparable with the more invasive biomarkers.
NEUROBIOLOGY OF OLFACTION
The experience of smelling an odor is the result of small molecules that enter the nasal cavity, dissolve in the mucosa of the olfactory epithelium, and then interact with olfactory receptor neurons via transmembrane G-protein coupled olfactory receptor proteins.7 Each of these proteins expresses one olfactory receptor, and similar receptors project to the same glomeruli in the olfactory bulb. In these round glomeruli, the axons of olfactory receptor synapse onto apical dendrites of olfactory bulb neurons, which are mitral and tufted cells.8
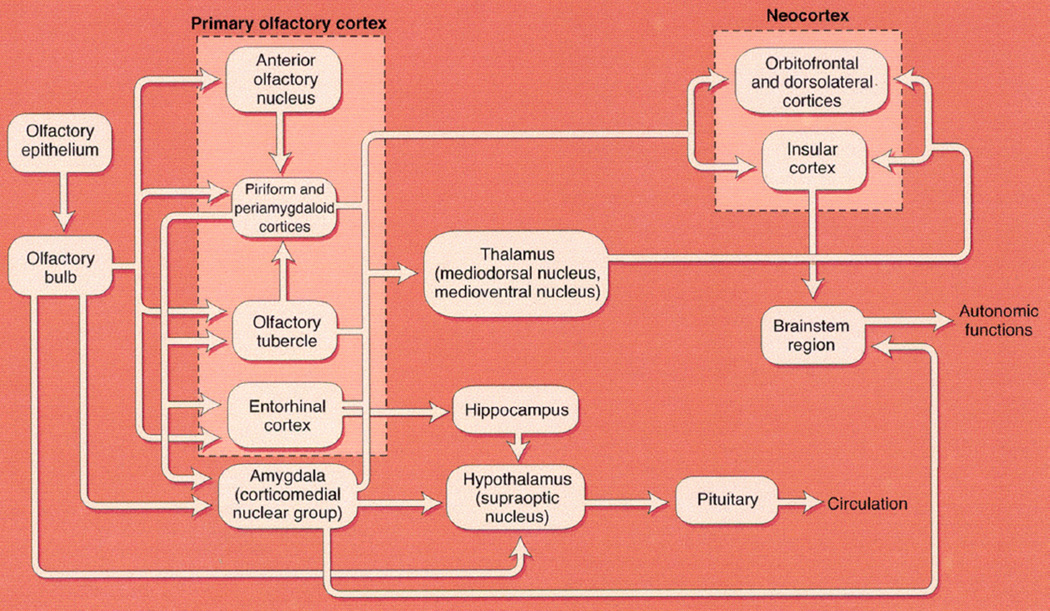
Neurons from the olfactory bulb project to several structures including the amygdala, piriform cortex, and entorhinal cortex, which then project to the orbital and orbitofrontal cortex via the thalamus (Figure 1). The entorhinal cortex provides the main input to the hippocampus. Neuronal connections also occur in reverse, including back to the olfactory bulb. Neurotransmitter input from noradrenergic and serotonergic neurons and cholinergic neurons from the nucleus basalis is modulatory throughout the olfactory pathways.7,9 During the aging process, the number of olfactory receptor neurons declines as olfactory epithelium is gradually replaced with respiratory epithelium.10 Late components of olfactory event-related potentials show increased latency with age, confirming that central structures are involved in the olfactory pathways.11
FIGURE 1.
Central olfactory projections of the olfactory system. Direct connections between the olfactory bulb and hypothalamus may not be present in humans and some other mammals. (Copyright © 2010 RL Doty.).
NEUROPATHOLOGY
Neurofibrillary tangles, a key pathologic feature of AD, are found in olfactory neurons in the olfactory bulb in patients with mild AD, and odor identification deficits during life correlate with tangles in the olfactory bulb and its projection areas at autopsy.12,13 In AD, the olfactory bulb shows minimal mature amyloid plaques, and the neurofibrillary tangle burden with abnormally phosphorylated tau protein is typically higher.14–17 Basic findings from animal models are consistent with these associations: Odor discrimination deficits are not observed in the Tg2576 AD mouse model that is an amyloidogenic mouse model,18 but increased investigation of novel odors is diminished in transgenic mice expressing human tau protein.19
OLFACTION IN NORMAL AGING
There is a general decline in odor identification ability with aging, which accelerates markedly above 70 years of age.20 In older adults without cognitive impairment, age correlates inversely with odor identification test scores.21,22 Practically, this means that absolute scores on olfaction tests cannot be used to define abnormality, and age adjustment needs to be used. Women score slightly better than men on odor identification tests, but this difference is not large and is not detectable in disorders like AD that are associated with severe olfactory pathology.
CLINICAL FINDINGS IN MCI AND AD
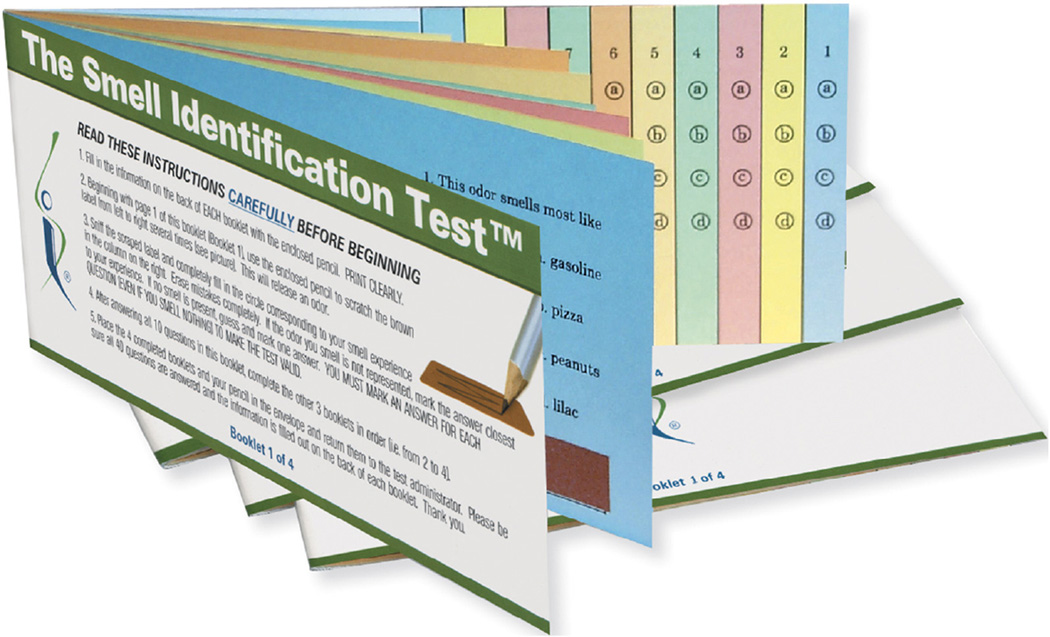
After initial clinical studies established the presence of marked olfactory deficits in patients with AD compared with cognitively intact control subjects,23,24 subsequent research has focused on the utility of impairment in odor identification in predicting the transition from MCI to AD and its association with cognitive decline in older adults without cognitive impairment.5,25 The most prominent olfactory deficit is in odor identification, which involves identifying a specific odor when presented to the nostrils, typically in a multiple-choice test format. Odor identification testing is useful in humans because they possess markedly inferior olfactory abilities when compared with other mammalian species (e.g., canines and rodents), and therefore multiple-choice items with very distinct odors are needed for reliable odor identification testing. For example, in the most widely used odor identification test, the scratch-and-sniff University of Pennsylvania Smell Identification Test (UPSIT), one of the items has four possible choices that are clearly distinct from each other: paint thinner, cherry, coconut, and cheddar cheese (Figure 2).26 There are minor cross-cultural differences in the ability to identify common odors,27 and versions in different languages are available for the UPSIT. Although an odor identification test can be self-administered, it is advisable for a staff member to administer the test to ensure that all test procedures are conducted correctly, including the completion of all items. Odor identification impairment is believed to reflect damage in the brain regions that receive neuronal projections directly or indirectly from the olfactory bulb, including the piriform cortex, amygdala, hippocampal and entorhinal cortex, and orbitofrontal cortex. When the smell is perceived, odor memory and odor naming, which are modulated by several limbic regions, together contribute to the eventual integrated process of odor identification that likely involves the orbitofrontal cortex and other frontal lobe regions.7
FIGURE 2.
The UPSIT. Each page has an odor microencapsulated in a dark rectangle that is scratched with a pencil, sniffed, and then one of four choices of named odors on that page is marked. There are 40 separate pages, each response that correctly identifies the odor receives a score of 1 and an incorrect response a score of 0, and the total score ranges from 0 to 40. (Copyright Sensonics International © 2016.).
Impairment in odor discrimination, which is the ability to differentiate between two or more odors, also occurs in patients with MCI and AD but is slightly less robust than odor identification in distinguishing patient groups.26,28 Odor sensitivity, which is the minimum concentration of an odor for reliable detection, typically is tested by the same odor presented sequentially in increasing concentrations. In AD, odor sensitivity can be impaired and becomes more prominent as the disease progresses beyond the mild stages of dementia.
OLFACTORY IMPAIRMENT AND COGNITIVE DECLINE IN COGNITIVELY INTACT OLDER ADULTS
There is growing evidence that odor identification deficits are associated with cognitive decline, including the transition from normal cognition to MCI.21,25 In a study of a multiethnic community cohort of 1,037 older adults in North Manhattan with an average age of 80 years, impairment in odor identification was superior to deficits in verbal episodic memory in predicting cognitive decline in cognitively intact participants followed for 2–7 years.25 This raises the interesting question of whether an odor identification test may be superior to memory testing in screening older adults at risk of cognitive decline, either for prognostic or potential therapeutic purposes.
Other epidemiologic data also show that odor identification deficits are associated with future cognitive decline.29,30 Therefore, early olfactory impairment may signify early AD pathology and may be useful as part of a preclinical detection strategy, although the magnitude of the impairment in cognitively intact individuals (odds ratios range from 1.4 to 2.0 across studies) is insufficient to be used as the sole biomarker to predict longer term outcome.
There has been little evaluation of odor identification in middle-aged and young adults and a paucity of systematic follow-up studies to assess the risk of cognitive decline and dementia. This gap needs to be filled in future research because of growing evidence that AD neuropathology develops in the brain several decades before clinical symptoms are manifest.3
OLFACTION, MCI, AND AD
The odor identification deficits demonstrated in AD are likely because of lesions in the primary olfactory cortex that comprises the olfactory bulb and its neuronal projections to the piriform, amygdala, and entorhinal cortices and secondary olfactory cortex (hippocampus, orbitofrontal cortex) that receives neuronal projections from the primary olfactory cortex. These brain regions are postulated to be where odors are identified based on integration of odor information received from the olfactory pathway and the projection areas that are involved in the naming and recall of odors.7
In both clinical and epidemiologic samples, there is a clear increase in odor identification deficits from cognitively intact individuals to MCI to AD.25,29,30 In a longitudinal study of 148 outpatients with MCI, broadly defined, baseline odor identification deficits were associated with a fourfold increased risk of conversion from MCI to AD and contributed unique variance in the prediction of conversion from MCI to AD.5 In addition to UPSIT scores, measures of episodic verbal memory, informant report of functional decline, and magnetic resonance imaging hippocampal and entorhinal cortex atrophy comprised a five-variable model that showed predictive accuracy of 85% for the transition from MCI to AD. The correlations among these five measures were moderate, reinforcing the view that multiple clinical markers and biomarkers like odor identification deficits may need to be assessed to improve diagnostic and predictive accuracy in individual patients.
Several studies have shown that impairment in odor identification strongly distinguishes AD from control subjects,23,24 and to our knowledge, every published study has shown this group difference. There is also consistent evidence that impairment in odor identification predicts the transition from MCI to AD with predictive utility that is comparable with other more invasive and expensive biomarkers. Head-to-head comparisons of odor identification deficits with cerebrospinal fluid and PET imaging markers in large samples are limited, but ongoing research should help to clarify their relative diagnostic and predictive utility.31
INCREASED MORTALITY RISK
An intriguing, relatively new, finding is that impaired odor identification is associated with increased mortality risk in older adults. In a multiethnic community cohort, 349 of 1,169 individuals (29.9%) died during an average follow-up period of 4 years.32 The association between lower UPSIT scores and increased mortality remained even after controlling for age, gender, education, depression, dementia diagnosis, alcohol abuse, head injury, medical comorbidity, smoking, body mass index, and vision and hearing impairment. The hazard ratios for mortality risk increased with decrease in UPSIT scores from 1.58 for the second quartile to 3.81 for the lowest quartile for UPSIT scores. To date, three of four published studies have found this association to be significant.32–35 The one study that did not find a significant association also reported the association of odor identification impairment with increased mortality, but it lost significance after controlling for serum cholesterol and cognitive performance.35 Possible explanations for the association between impaired odor identification and mortality include an increase in the risk of eating spoiled food, cooking-related accidents, and inability to smell gas fumes or fire. Empirical research is lacking on these possibilities in individuals with odor identification impairment. Published studies have focused on older adults, and prospective follow-up studies of young to middle-aged adults with odor identification impairment are needed to shed further light on the association between odor identification impairment and increased mortality risk.
LIMITATIONS TO OLFACTORY TESTING
The UPSIT, which on average takes 20 minutes to administer, is highly reliable and well validated. Shorter versions of the test (e.g., the 12-item Brief Smell Identification Test [B-SIT], which takes 7 minutes on average) are almost as useful as the long version in distinguishing patient groups and predicting the future clinical diagnosis of AD.24 Other commercially available odor identification tests are comparable with the UPSIT in distinguishing groups of patients (e.g., Sniffin‘ Sticks, Sense Trading, The Netherlands).
Odor identification test performance is lower in active smokers and individuals with current upper respiratory tract infections and in a small number of individuals who have congenital anosmia and cannot perform above minimal levels on such tests. Past smoking affects olfactory function, and return of function is related to the amount of previous smoking.36
Odor identification tests are not highly specific and can be abnormal in persons with schizophrenia, Parkinson disease, Lewy body dementia, and possibly vascular dementia.37,38 Therefore, if an odor identification test is used for screening or to estimate prognosis in individuals with cognitive decline, MCI, or AD, it is important to exclude these neurologic and psychiatric disorders before administering the test.
Odor identification impairment increases with age, and the cutoffs for normality can range from the mid-to-high 30s for the 40-item UPSIT (scoring range: 0–40) in young adults to the high 20s to low 30s for individuals in their eighties. Similar age-adjusted scores are needed for all other currently available odor identification tests, and absolute cutoff scores cannot be used uniformly for adults across the lifespan. Therefore, odor identification testing is best used as information that provides added utility to a thorough clinical diagnostic workup, supplemented by other investigations as needed.
CONCLUSIONS
In AD, the evidence clearly demonstrates that olfactory dysfunction, typically assessed by an odor identification test, occurs early in the disease process, even at a preclinical stage where such a test may be superior to testing for verbal episodic memory in predicting longer term cognitive outcome. Odor identification impairment shows moderate predictive utility for cognitive decline in cognitively intact older adults and for the transition from normal cognition to MCI and strong predictive utility for the transition from MCI to AD. Practically, to improve predictive accuracy, olfactory testing may need to be combined with other measures for potential use as a screening tool to identify individuals at risk of cognitive decline in the general population. Relative to cognitive testing and assessment of other biomarkers, the largely unique variance contributed by impairment in odor identification to the prediction of MCI and AD suggests that it may be particularly useful when combined with other tests for such purposes. Age-related changes occur with olfaction, but they also occur with virtually all markers of MCI and AD: decline in cognitive test performance, measures of magnetic resonance imaging atrophy, 18F-fluorodeoxyglucose PET and amyloid PET abnormalities, and decreased β42-amyloid with increased tau and phosphor-tau levels in cerebrospinal fluid.39–42
In addition to the advantages of being easy to administer and cost-effective because of its relatively low price, an odor identification test may be a useful measure to select/stratify patients in treatment trials of cognitively impaired patients or prevention trials in cognitively intact individuals, because olfactory deficits can predict cognitive decline in cognitively intact individuals and are an early biomarker of AD neuropathology. Odor identification tests remain primarily a research tool, but some clinical practitioners do use them. The current clinical recommendation is that odor identification tests can be administered to provide potentially useful information only when combined with a thorough clinical, neuropsychological, and, if necessary, neuroimaging evaluation for patients who present with cognitive decline and an uncertain diagnosis, taking into account the specific conditions that need to be excluded before administering an odor identification test. The observation that odor identification impairment predicts increased mortality has important public health implications that go beyond its potential diagnostic and predictive utility in cognitively impaired older adults and requires further investigation.
Acknowledgments
This study was supported by U.S. National Institute on Aging grant R01AG041795. Dr. Devanand has received research support from Avanir, serves on the Scientific Advisory Boards of AbbVie and Astellas, and has been a consultant to Intercellular Therapies.
References
- 1.Doody RS, Raman R, Farlow M, et al. A phase 3 trial of semagacestat for treatment of Alzheimer’s disease. N Engl J Med. 2013;369:341–350. doi: 10.1056/NEJMoa1210951. [DOI] [PubMed] [Google Scholar]
- 2.Salloway S, Sperling R, Fox NC, et al. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N Engl J Med. 2014;370:322–333. doi: 10.1056/NEJMoa1304839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nordberg A. Dementia in 2014. Towards early diagnosis in Alzheimer disease. Nat Rev Neurol. 2015;11:69–70. doi: 10.1038/nrneurol.2014.257. [DOI] [PubMed] [Google Scholar]
- 4.Van Cauwenberghe C, Van Broeckhoven C, Sleegers K. The genetic landscape of Alzheimer disease: clinical implications and perspectives. Genet Med. 2016;18:421–430. doi: 10.1038/gim.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devanand DP, Liu X, Tabert MH, et al. Combining early markers strongly predicts conversion from mild cognitive impairment to Alzheimer’s disease. Biol Psychiatry. 2008;64:871–879. doi: 10.1016/j.biopsych.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teipel S, Drzezga A, Grothe MJ, et al. Multimodal imaging in Alzheimer’s disease: validity and usefulness for early detection. Lancet Neurol. 2015;14:1037–1053. doi: 10.1016/S1474-4422(15)00093-9. [DOI] [PubMed] [Google Scholar]
- 7.Masurkar AV, Devanand DP. Olfactory dysfunction in the elderly: basic circuitry and alterations with normal aging and Alzheimer’s disease, in neurology of aging. Curr Geriatr Rep. 2014;3:91–100. doi: 10.1007/s13670-014-0080-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meisami E, Mikhail L, Baim D, et al. Human olfactory bulb: aging of glomeruli and mitral cells and a search for the accessory olfactory bulb. Ann N Y Acad Sci. 1998;855:708–715. doi: 10.1111/j.1749-6632.1998.tb10649.x. [DOI] [PubMed] [Google Scholar]
- 9.Guerin D, Sacquet J, Mandairon N, et al. Early locus coeruleus degeneration and olfactory dysfunctions in Tg2576 mice. Neurobiol Aging. 2009;30:272–283. doi: 10.1016/j.neurobiolaging.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 10.Paik SI, Lehman MN, Seiden AM, et al. Human olfactory biopsy. The influence of age and receptor distribution. Arch Otolaryngol Head Neck Surg. 1992;118:731–738. doi: 10.1001/archotol.1992.01880070061012. [DOI] [PubMed] [Google Scholar]
- 11.Morgan CD, Geisler MW, Covington JW, et al. Olfactory P3 in young and older adults. Psychophysiology. 1999;36:281–287. doi: 10.1017/s0048577299980265. [DOI] [PubMed] [Google Scholar]
- 12.Hyman BT, Arriagada PV, Van Hoesen GW. Pathologic changes in the olfactory system in aging and Alzheimer’s disease. Ann N Y Acad Sci. 1991;640:14–19. doi: 10.1111/j.1749-6632.1991.tb00184.x. [DOI] [PubMed] [Google Scholar]
- 13.Wilson RS, Arnold SE, Schneider JA, et al. The relationship between cerebral Alzheimer’s disease pathology and odour identification in old age. J Neurol Neurosurg Psychiatry. 2007;78:30–35. doi: 10.1136/jnnp.2006.099721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mann DM, Tucker CM, Yates PO. Alzheimer’s disease: an olfactory connection? Mech Ageing Dev. 1988;42:1–15. doi: 10.1016/0047-6374(88)90058-9. [DOI] [PubMed] [Google Scholar]
- 15.Reyes PF, Deems DA, Suarez MG. Olfactory-related changes in Alzheimer’s disease: a quantitative neuropathologic study. Brain Res Bull. 1993;32:1–5. doi: 10.1016/0361-9230(93)90310-8. [DOI] [PubMed] [Google Scholar]
- 16.Kovacs T, Cairns NJ, Lantos PL. beta-amyloid deposition and neurofibrillary tangle formation in the olfactory bulb in ageing and Alzheimer’s disease. Neuropathol Appl Neurobiol. 1999;25:481–491. doi: 10.1046/j.1365-2990.1999.00208.x. [DOI] [PubMed] [Google Scholar]
- 17.Mundinano IC, Caballero MC, Ordonez C, et al. Increased dopaminergic cells and protein aggregates in the olfactory bulb of patients with neurodegenerative disorders. Acta Neuropathol. 2011;122:61–74. doi: 10.1007/s00401-011-0830-2. [DOI] [PubMed] [Google Scholar]
- 18.Xu W, Lopez-Guzman M, Schoen C, et al. Spared piriform cortical single-unit odor processing and odor discrimination in the Tg2576 mouse model of Alzheimer’s disease. PLoS ONE. 2014;9:e106431. doi: 10.1371/journal.pone.0106431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Macknin JB, Higuchi M, Lee VM, et al. Olfactory dysfunction occurs in transgenic mice overexpressing human tau protein. Brain Res. 2004;1000:174–178. doi: 10.1016/j.brainres.2004.01.047. [DOI] [PubMed] [Google Scholar]
- 20.Doty RL, Shaman P, Applebaum SL, et al. Smell identification ability: changes with age. Science. 1984;226:1441–1443. doi: 10.1126/science.6505700. [DOI] [PubMed] [Google Scholar]
- 21.Wilson RS, Schneider JA, Arnold SE, et al. Olfactory identification and incidence of mild cognitive impairment in older age. Arch Gen Psychiatry. 2007;64:802–808. doi: 10.1001/archpsyc.64.7.802. [DOI] [PubMed] [Google Scholar]
- 22.Devanand DP, Tabert MH, Cuasay K, et al. Olfactory identification deficits and MCI in a multi-ethnic elderly community sample. Neurobiol Aging. 2010;31:1593–1600. doi: 10.1016/j.neurobiolaging.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doty RL, Reyes PF, Gregor T. Presence of both odor identification and detection deficits in Alzheimer’s disease. Brain Res Bull. 1987;18:597–600. doi: 10.1016/0361-9230(87)90129-8. [DOI] [PubMed] [Google Scholar]
- 24.Tabert MH, Liu X, Doty RL, et al. A 10-item smell identification scale related to risk for Alzheimer’s disease. Ann Neurol. 2005;58:155–160. doi: 10.1002/ana.20533. [DOI] [PubMed] [Google Scholar]
- 25.Devanand DP, Lee S, Manly JJ, et al. Olfactory deficits predict cognitive decline and Alzheimer’s dementia in an urban community. Neurology. 2015;84:182–189. doi: 10.1212/WNL.0000000000001132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doty RL, Frye RE, Agrawal U. Internal consistency reliability of the fractionated and whole University of Pennsylvania Smell Identification Test. Percept Psychophys. 1989;45:381–384. doi: 10.3758/bf03210709. [DOI] [PubMed] [Google Scholar]
- 27.Conti MZ, Vicini-Chilovi B, Riva M, et al. Odor identification deficit predicts clinical conversion from mild cognitive impairment to dementia due to Alzheimer’s disease. Arch Clin Neuropsychol. 2013;28:391–399. doi: 10.1093/arclin/act032. [DOI] [PubMed] [Google Scholar]
- 28.Djordjevic J, Jones-Gotman M, De Sousa K, et al. Olfaction in patients with mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging. 2008;29:693–706. doi: 10.1016/j.neurobiolaging.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 29.Graves AB, Bowen JD, Rajaram L, et al. Impaired olfaction as a marker for cognitive decline: interaction with apolipoprotein E epsilon4 status. Neurology. 1999;53:1480–1487. doi: 10.1212/wnl.53.7.1480. [DOI] [PubMed] [Google Scholar]
- 30.Schubert CR, Carmichael LL, Murphy C, et al. Olfaction and the 5-year incidence of cognitive impairment in an epidemiological study of older adults. J Am Geriatr Soc. 2008;56:1517–1521. doi: 10.1111/j.1532-5415.2008.01826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts RO, Christianson TJ, Kremers WK, et al. Association between olfactory dysfunction and amnestic mild cognitive impairment and Alzheimer disease dementia. JAMA Neurol. 2016;73:93–101. doi: 10.1001/jamaneurol.2015.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Devanand DP, Lee S, Manly J, et al. Olfactory identification deficits and increased mortality in the community. Ann Neurol. 2015;78:401–411. doi: 10.1002/ana.24447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson RS, Lei Y, Bennett DA. Odor identification and mortality in old age. Chem Senses. 2011;36:63–67. doi: 10.1093/chemse/bjq098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pinto J, Wroblewski KE, Kern DW, et al. Olfactory dysfunction predicts 5-year mortality in older adults. PLoS ONE. 2014;9:e107541. doi: 10.1371/journal.pone.0107541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gopinath B, Sue CM, Kifley A, et al. The association between olfactory impairment and total mortality in older adults. J Gerontol A Biol Sci Med Sci. 2012;67A:204–209. doi: 10.1093/gerona/glr165. [DOI] [PubMed] [Google Scholar]
- 36.Frye RE, Schwartz BS, Doty RL. Dose-related effects of cigarette smoking on olfactory function. JAMA. 1990;263:1233–1236. [PubMed] [Google Scholar]
- 37.Ross GW, Abbott RD, Petrovitch H, et al. Association of olfactory dysfunction with incidental Lewy bodies. Mov Disord. 2006;21:2062–2067. doi: 10.1002/mds.21076. [DOI] [PubMed] [Google Scholar]
- 38.Doty RL. Olfaction in Parkinson’s disease and related disorders. Neurobiol Dis. 2012;46:527–552. doi: 10.1016/j.nbd.2011.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kantarci K, Senjem ML, Lowe VJ, et al. Effects of age on the glucose metabolic changes in mild cognitive impairment. AJNR Am J Neuroradiol. 2010;31:1247–1253. doi: 10.3174/ajnr.A2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Erten-Lyons D, Dodge HH, Woltjer R, et al. Neuropathologic basis of age-associated brain atrophy. JAMA Neurol. 2013;70:616–622. doi: 10.1001/jamaneurol.2013.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scheinin NM, Wikman K, Jula A, et al. Cortical 11C-PIB uptake is associated with age, APOE genotype, and gender in “healthy aging”. J Alzheimers Dis. 2014;41:193–202. doi: 10.3233/JAD-132783. [DOI] [PubMed] [Google Scholar]
- 42.Mattsson N, Rosén E, Hansson O, et al. Age and diagnostic performance of Alzheimer disease CSF biomarkers. Neurology. 2012;78:468–476. doi: 10.1212/WNL.0b013e3182477eed. [DOI] [PMC free article] [PubMed] [Google Scholar]