Abstract
Oncogenic BRAFV600E mutations activate MAP kinase signaling and are associated with treatment resistance and poor prognosis in patients with colorectal cancer (CRC). In BRAFV600E mutant CRCs, treatment failure may be related to BRAFV600E -mediated apoptosis resistance that occurs by an as yet undefined mechanism. We found that BRAFV600E can upregulate anti-apoptotic MCL-1 in a gene dose-dependent manner using CRC cell lines isogenic for BRAF. BRAFV600E -induced MCL-1 upregulation was confirmed by ectopic BRAFV600E expression that activated MEK/ERK signaling to phosphorylate (MCL-1Thr163) and stabilize MCL-1. Upregulation of MCL-1 was mediated by MEK/ERK shown by the ability of ERK siRNA to suppress MCL-1. Stabilization of MCL-1 by phosphorylation was shown by a phosphorylation-mimicking mutant and an unphosphorylated MCL-1 mutant that decreased or increased MCL-1 protein turnover, respectively. MEK/ERK inhibition by cobimetinib suppressed MCL-1 expression/phosphorylation and induced pro-apoptotic BIM to a greater extent than did vemurafenib in BRAFV600E cell lines. MCL-1 knockdown vs control shRNA significantly enhanced cobimetinib-induced apoptosis in vitro and in HT29 colon cancer xenografts. The small molecule MCL-1 inhibitor, A-1210477 also enhanced cobimetinib-induced apoptosis in vitro that was due to disruption of the interaction of MCL-1 with pro-apoptotic BAK and BIM. Knockdown of BIM attenuated BAX, but not BAK, activation by cobimetinib plus A-1210477. In summary, BRAFV600E -mediated MEK/ERK activation can upregulate MCL-1 by phosphorylation/stabilization to confer apoptosis resistance that can be reversed by MCL-1 antagonism combined with cobimetinib, suggesting a novel therapeutic strategy against BRAFV600E mutant CRCs.
Keywords: MCL-1, BRAF, ERK, cobimetinib, A-1210477, apoptosis
INTRODUCTION
Mutational activation of the BRAF oncogene drives tumorigenesis through constitutive activation of the MAPK signaling pathway (1). The most frequent somatic alteration in BRAF is the point mutation T1799A encoding BRAFV600E which results in a several hundred-fold increase in its kinase activity (2). BRAFV600E is detected in approximately 10% of human colorectal cancers (CRCs) where it is associated with poor responsiveness to chemotherapy (3, 4), and poor prognosis (5–9) with frequent peritoneal metastasis (10–12). The subset of CRCs with microsatellite instability (MSI) are enriched with BRAF mutations that are associated with poor outcome in the metastatic setting as is observed in microsatellite stable CRCs (13). In contrast to BRAFV600E melanoma, metastatic CRCs with the same BRAFV600E mutation have shown a lack of sensitivity to BRAF or MEK inhibitor monotherapy in early clinical trials (3). Whereas approximately 50% of patients with metastatic melanoma responded to BRAF inhibition by vemurafenib, the response rate was only 5% among patients with BRAFV600E metastatic CRC (3, 14, 15). The inability of vemurafenib to kill colon cancer cells was shown to be related to rebound EGFR activation, and the addition of inhibitors of EGFR signaling suppressed MEK/ERK activation and induced a synergistic apoptosis (16, 17). These data suggested that apoptosis resistance can be overcome by blockade of reactivated MAPK signaling and in this regard, combined BRAF and MEK inhibition led to further suppression of MAPK signaling and increased therapeutic efficacy (17, 18). Potent inhibition of active, phosphorylated MEK is required for strong inhibition of the MAPK pathway in BRAFV600E tumors, and was achieved using cobimetinib which resulted in superior efficacy in BRAFV600E cancer cells (1). Among patients with BRAFV600E–mutant metastatic melanoma, the addition of cobimetinib to vemurafenib was associated with a significant improvement in progression-free survival in a phase III clinical trial that led to its approval by the U.S. Food and Drug Administration (19). In contrast to results in patients with melanoma, however, treatment of metastatic CRC patients with the combination of the BRAF inhibitor dabrafenib and the MEK inhibitor trametinib showed only a 12% response rate including one complete response (4). These data underscore the need to further elucidate the mechanism of BRAFV600E -mediated drug resistance and, thereby, improve the responsiveness of these cancers using novel therapeutic approaches.
The MAPK pathway links extracellular signals to the machinery that controls cellular processes including apoptosis (20). Existing data indicate a continued dependency on BRAF signaling for cancer cell survival, although the mechanisms by which BRAFV600E or its downstream effectors confer apoptosis resistance are poorly understood. In BRAFV600E melanoma cells, the MAPK pathway is an important regulator of cell survival through the suppression of pro-apoptotic BH3 family proteins and potentially by upregulation of anti-apoptotic proteins (21–23). The importance of anti-apoptotic proteins in CRC has been previously reviewed (24, 25). BIM is a BH3-only protein that induces cell death through antagonizing the anti-apoptotic effects of BCL-2, BCL-XL, and MCL-1 (26). Treatment with inhibitors of MEK and BRAF were shown to prevent the proteasomal degradation of BIM that led to apoptosis (27, 28). Further support for apoptosis as a contributor to mutant BRAF-mediated resistance is suggested by the observation that inhibition of BRAFV600E combined with a BCL-2/BCL-xL antagonist can significantly enhance the cytotoxic effect of vemurafenib in melanoma cell lines(29) which may be relevant to CRC.
We tested the hypothesis that BRAFV600E can confer apoptosis resistance by upregulating the expression of an anti-apoptotic BCL-2 family protein(s). In this report, we demonstrate that BRAFV600E can upregulate anti-apoptotic MCL-1 (30) via its phosphorylation/stabilization to confer apoptosis resistance in human CRC cells. This resistance mechanism was reversed by concurrent MCL-1 antagonism and MEK/ERK inhibition that increased anti-tumor efficacy in vitro and in vivo.
MATERIALS AND METHODS
Cell culture and drugs
The BRAF mutant RKO, HT29 and WiDr cell lines were obtained from the ATCC (Manassas, VA). Isogenic RKO [A19 (BRAFV600E/−/−), T29 (BRAFWT/−/−)], VACO432 [parental (BRAFV600E/WT), VT1 (BRAFWT/−)] and HCT116 #152 (KRASWT/−) human CRC cell lines were obtained (2015) from Dr. B. Vogelstein (GRCF Biorepository & Cell Center, Johns Hopkins University). Parental RKO (BRAFV600E/V600E/WT) cells are triploid, harboring two mutant BRAFV600E alleles and one wild type (WT) BRAF allele. RKO A19 (BRAFV600E/−/−) cells carry a mutant BRAFV600E allele and have knockout of the WT allele. RKO T29 (BRAFWT/−/−) cells contain knockout of two mutant BRAFV600E alleles and carry one WT allele. In contrast, VACO432 cells and HCT116#152 cells are diploid: VACO432 VT1 (BRAFWT/−) cells and HCT116#152 (KRASWT/−) cells contain knockout of one BRAFV600E allele or one KRAS mutant allele, respectively. Authentication of RKO, HT29 and WiDr cell lines was not performed within the previous 6 months. However, cell lines are routinely tested for Mycoplasma contamination every 3 months with a MycoAlert mycoplasma detection set (Lonza, Allendale, NJ). For isogenic BRAF cells, GRCF utilizes a short tandem repeat profiling for authentication. All cell lines, except HEK293T cells, were grown as monolayers in RPMI medium (Invitrogen, Cat # 11875) supplemented with 10% (v/v) FBS and 1% antibiotic-antimycotic (Invitrogen, Cat # 15240). HEK293T cells were utilized for pseudovirus production and were grown in DMEM (Sigma, Cat # D5796) with supplementation as above. Cells were treated with cobimetinib (GDC-0973/XL-518; ActiveBiochem, Cat # A-1180), A-1210477 (Selleckchem, Cat # S7790) or their combination. Both cobimetinib and A-1210477 were prepared as 50 mmol/liter and 10 mmol/liter stock solutions in DMSO (Sigma, Cat # D2650), respectively, and stored at −20 °C.
Lentiviral and retroviral expression of shRNA or cDNA
Virus production in HEK293T cells and transduction of target cells with lentivirus were performed utilizing a standard procedure described previously (31). The non-targeting shRNA expression vector was obtained from Addgene (Cat #1864). Lentiviral BIM and MCL-1 shRNA constructs were previously described (31). Lentiviral BRAFV600E was generated by subcloning its cDNA (Addgene, Cat # 15269) into the lentiviral vector pCDH1-puro-2HA. For transduction of lentiviral constructs (packaged as pseudotyped viral particles) into target cells, the growth medium of recipient cells was replaced with OPTI-MEM (Invitrogen, Cat # 31985) containing 8 μg/ml Polybrene (Sigma, Cat # 107689) and lentivirus. The cells were incubated overnight at 37 °C, and the medium was replaced the following day. Puromycin (2– 4 μg/ml, Sigma; Cat # P8833) was added 48 h post-transduction, and the puromycin-resistant cells were used for subsequent experiments.
Wild-type MCL-1 cDNA was purchased from Origene (Cat # RC200521). MCL-1 AA or DD mutants at T92/T163 were generated using a mutagenesis kit (Agilent, Cat # 200523) and synthesized primers containing the desired mutations, which were then cloned into retroviral pBape-puro-2HA vector.
Transfection of siRNA
Cells were seeded 1 day before transfection at 30 –50% confluence in growth medium without antibiotics. ERK siRNA (Cell Signaling Technology, Cat # 6560) and Lipofectamine RNAiMax (Invitrogen, Cat # 13778150) were diluted in OPTI-MEM medium, mixed gently, and incubated to allow complex formation. The cells were then transfected by adding the RNAi-Lipofectamine complex dropwise to medium to achieve an siRNA concentration of 100 nmol/liter. Cells were then incubated at 37 °C, and knockdown efficiency was determined 48 h post-transfection.
Competitive RT-PCR
Total RNA was extracted from cells using the RNA Easy mini kit (Qiagen, Cat # 74104), and RNA integrity was evaluated using an Agilent Bioanalyzer 2000. Competitive RT-PCR was performed with a one-step RT-PCR kit (Qiagen, Cat # 210210) using the following primer sets containing an equimolar ratio of MCL-1 (forward, 5′-GGGCAGGATTGTGACTCTCATT- 3′; reverse, 5′-GATGCAGCTTTCTTGGTTTATGG-3′) against β-actin (forward, 5′-TCACCCACACTGTGCCCATCTACGA- 3′; reverse, 5′-CAGCGGAACCGCTCATTGCCAATGG-3′). Reverse transcription was coupled with PCR (25 cycles) on a thermocycler (Eppendorf, Mastercycler® GX2). PCR products were quantified on the Agilent Bioanalyzer 2000 using the DNA 12,000 kit (Cat # 5067-1508). In brief, samples were loaded onto DNA microchips, and the DNA fragments were then separated by capillary electrophoresis. The target DNA sizes and relative quantities were calculated on the basis of DNA ladders and an internal marker, respectively. The associated software then generates agarose gel-like images.
Analysis of tumor cell apoptosis in cell culture
Apoptosis was analyzed by annexin V+ staining and quantified by flow cytometry as described previously (32). Briefly, cells were incubated with the study drugs at prespecified time points. Trypsin was added to detach adherent cells that were then combined with floating cells. Cells were pelleted by centrifugation, and the pellet was washed three times in cold PBS. Cells were incubated with annexin V conjugated with FITC (BD Biosciences, Cat # 556419). The labeled cell populations were then quantitated by flow cytometry. For analysis of drug synergy, we calculated a combination index (CI) in cells treated with combination of cobimetinib and A-1210477 at a fixed dose ratio. The means of triplicate experiments were used to compute the CI using CompuSyn software (ComboSyn, Inc.). CI <1 indicated drug synergy.
Immunoprecipitation and immunoblotting
Protein samples were prepared in lysis buffer (5 mmol/liter MgCl2, 137 mmol/liter KCL, 1 mmol/liter EDTA, 1 mmol/liter EGTA, 1% CHAPS, and 10 mmol/liter HEPES (pH 7.5)) supplemented with a protease inhibitor cocktail (Sigma, Cat # P8340). The protein concentration of the samples was measured using the nanodrop method (Thermo Scientific). Cell lysates were incubated with primary antibodies for 3 h at 4 °C. Immunocomplexes were captured using magnetic beads conjugated with protein A/G (Pierce, Waltham, MA) and then washed three times in lysis buffer. Immunoprecipitated proteins were eluted with 2x LDS sample buffer (Invitrogen, Cat # NP0008) and loaded onto a 14% SDS-PAGE gel for separation, followed by an electrical transfer onto PVDF membranes (Bio-Rad, Cat # 1620177). Immunoblotting was then performed as described above. For immunoprecipitation, MCL-1 antibody (BD Biosciences, Cat # 554103) and conformation-specific antibodies for BAK (Millipore, Cat # 06-536) or BAX (Sigma, Cat # B8429) was used as primary antibody. For immunoblotting, primary antibodies included those against MCL-1 (Santa Cruz Biotechnology, Cat # sc-819) and tubulin (Sigma, T4026). All other antibodies were purchased from Cell Signaling.
Mouse xenograft studies
HT29 cells containing stable expression of control (#293) or MCL-1 (#50) shRNA were injected (2 × 106 cells per injection) into the right flank of male immunodeficient nu/nu, SCID mice (Charles River Laboratories) at 6–8 weeks of age. When flank tumors reached approximately 100 mm3, cobimetinib (Genentech) at a dose of 15 mg/kg or vehicle (5% DMSO/30% PEG 300/5% Tween 80/ddH2O) was administered once every 3 days by oral gavage for 14 consecutive days. A replicate of 8 mice for vehicle and 6 mice for cobimetinib treatment per experimental condition were utilized. Two mice from vehicle groups were sacrificed before treatment to examine the baseline efficiency of MCL-1 knockdown. Tumor volume was calculated using the following formula: length × width2 × 0.5. All animals were sacrificed at 72 hrs post last treatment dose and tumor tissue was snap frozen at −80°C for immunoblotting experiments. All the animal experiments were performed under an animal protocol approved by the Mayo Clinic Institutional Animal Care and Use Committee.
Statistical analysis
Statistical analysis was performed on data generated for the extent of apoptosis using the annexin V assay. Experimental data represent the mean ± S.D. for triplicate experiments. Statistical significance was determined using Student’s t test in R programming language. P values < 0.05 were considered statistically significant.
RESULTS
BRAF V600E mutation upregulates MCL-1 in human CRC cell lines
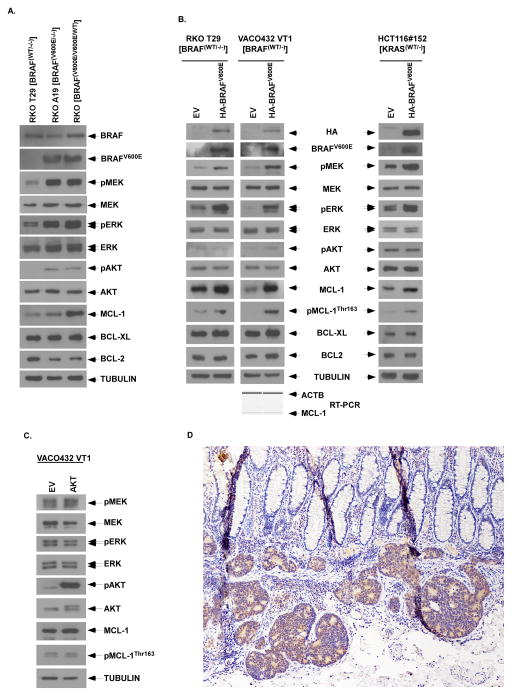
Evidence suggests that BRAFV600E confers apoptosis resistance by an as yet undefined mechanism. To determine whether BRAFV600E is associated with an increase in the expression of anti-apoptotic BCL-2 family proteins, we utilized the isogenic colorectal cancer (CRC) cell lines, RKO parental (BRAFV600E/V600E/WT), RKO A19 (BRAFV600E/−/−) and RKO T29 (BRAFwt/−/−). In cells with BRAFV600E, MEK and ERK were activated and an increase in the number of mutant BRAF alleles was associated with upregulation of MCL-1 in a gene dose-dependent manner (Fig. 1A). In contrast, the gain of BRAFV600E alleles was not associated with an increase in either BCL-XL or BCL-2 expression (Fig. 1A). To confirm the ability of BRAFV600E to upregulate MCL-1, we ectopically expressed BRAFV600E in RKO T29 (BRAFwt/−/−) and VACO432 VT1 (BRAFwt/−) cell lines that was shown to activate MEK/ ERK and to upregulate MCL-1, but not BCL-XL or BCL-2, and to increase MCL-1 phosphorylation at Thr163 (pMCL-1Thr163) (Fig. 1B). Since there was no significant change in MCL-1 transcription by ectopic BRAFV600E, MCL-1 upregulation is likely to occur by a posttranscriptional mechanism (Fig. 1B). We also ectopically expressed BRAFV600E in HCT116#152 (KRASwt/−) derived from a KRAS mutant background which showed similar upregulation of MCL-1 and pMCL-1Thr163 (Fig. 1B). In contrast to BRAFV600E, ectopic expression of constitutively active AKT did not increase pMCL-1Thr163 (Fig. 1C). In a human colon cancer with mutant BRAFV600E, MCL-1 protein expression was shown to be upregulated in the cytoplasm of tumor cells compared to normal colonic epithelial cells indicating the relevance of MCL-1 as a therapeutic target (Fig. 1D).
Figure 1. Mutant BRAF upregulates anti-apoptotic MCL-1 expression.
A, Analysis of protein expression by immunoblotting in parental (BRAFV600E/V600E/WT) and isogenic BRAF cell lines including mutant A19 (BRAFV600E/−/−) and wild-type (WT) T29 (BRAF−/−/WT) RKO cells. Tubulin was utilized as control for protein loading. B,C,D, Ectopic expression of lentiviral mutant BRAF (B) or retroviral AKT (C) was performed in isogenic RKO T29 (BRAF−/−/WT) and VACO432 VT1 (BRAFWT/−) cell lines or in isogenic KRAS WT HCT116#152 (KRASWT/−) cells. A competitive RT-PCR assay was performed to quantitate MCL-1 transcripts using β-actin (ACTB) as an internal control (B, bottom). D, Immunohistochemical expression of MCL-1 in a BRAF mutant human colon cancer cells compared to overlying normal colonic epithelium (100X).
MEK/ERK-mediates BRAFV600E-induced MCL-1 phosphorylation and upregulation
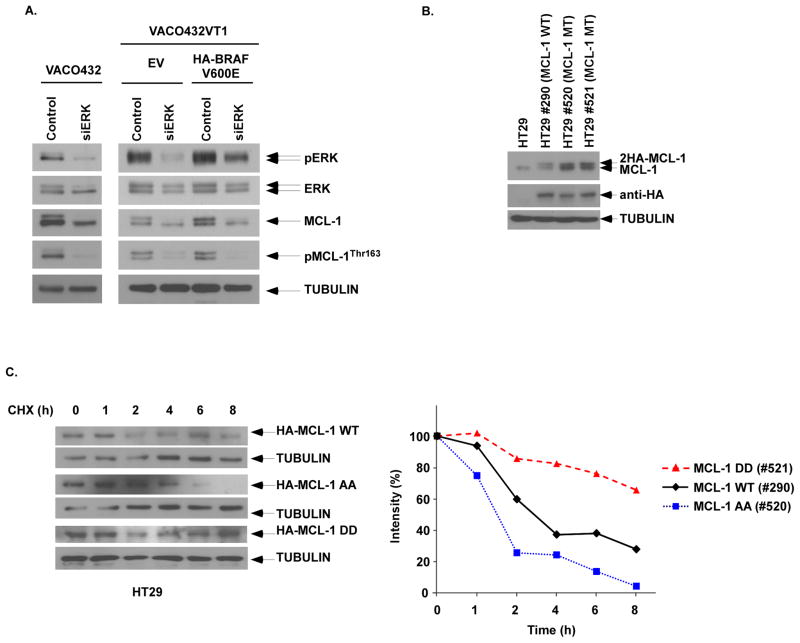
Mutant BRAFV600E is known to cause sustained activation of the MEK-ERK mitogen-activated kinase (MAPK) signaling cascade that controls tumor cell proliferation, apoptosis, and invasion(2). To determine whether mutant BRAFV600E –induced MEK/ERK signaling mediates MCL-1 expression, we silenced ERK by RNA interference in the mutant BRAFV600E VACO432 CRC cell line. In addition to suppression of ERK signaling, knockdown of ERK was shown to inhibit MCL-1 expression and phosphorylation (Fig. 2A). In VACO432 VT1 cells with ectopic BRAFV600E expression, knockdown of ERK was also shown to attenuate MCL-1 expression and to inhibit pMCL-1Thr163 (Fig. 2A).
Figure 2. MEK/ERK signaling phosphorylates MCL-1 resulting in protein stability.
A, ERK knockdown by siRNA was performed in VACO432 (BRAFV600E/WT), as well as isogenic VACO432 VT1 (BRAFWT/−) cells with ectopic BRAFV600E vs empty vector (EV). Protein expression was determined by immunoblotting. B, Ectopic expression of HA-tagged wild type (WT) MCL-1, and phosphorylation-mimicking [T92D/T163D (DD)] or unphosphorylated [T92A/T163A (AA)] MCL-1 mutants was performed in HT29 cells. C, Cell lines were treated with cycloheximide (5 mmol/L) for the indicated times and protein expression against HA-tagged MCL-1 was analyzed by immunoblotting. The level of MCL-1 expression was then quantified by densitometry and normalized using tubulin expression.
To demonstrate the ability of MCL-1 phosphorylation to confer protein stability, we ectopically expressed an HA-tagged, phosphorylation-mimicking double MCL-1 mutant [T92D/T163D (DD)], a nonphosphorylated mutant [T92A/T163A], or wild-type MCL-1 (Fig. 2B). These two residues (T92/T163) have been reported to mediate MCL-1 protein stability by ERK (33). Analysis of protein turnover in cycloheximide chase experiments revealed that Mcl-1-92/163DD, a phosphorylation-mimicking mutant, was more stabilized than was WT Mcl-1 or Mcl-1-92/163AA, a nonphosphorylation mutant (Fig. 2C). While the nonphosphorylation mutant Mcl-1-92/163AA was rapidly degraded, the phosphorylation-mimicking mutant Mcl-1-92/163DD exhibited greater protein stability compared with WT Mcl-1 (Fig. 2C). Taken together, these findings indicate that MEK-ERK signaling phosphorylates and stabilizes MCL-1 to prevent its degradation and thereby, increase its expression.
MEK/ERK inhibitor cobimetinib downregulates MCL-1 and induces BIM expression
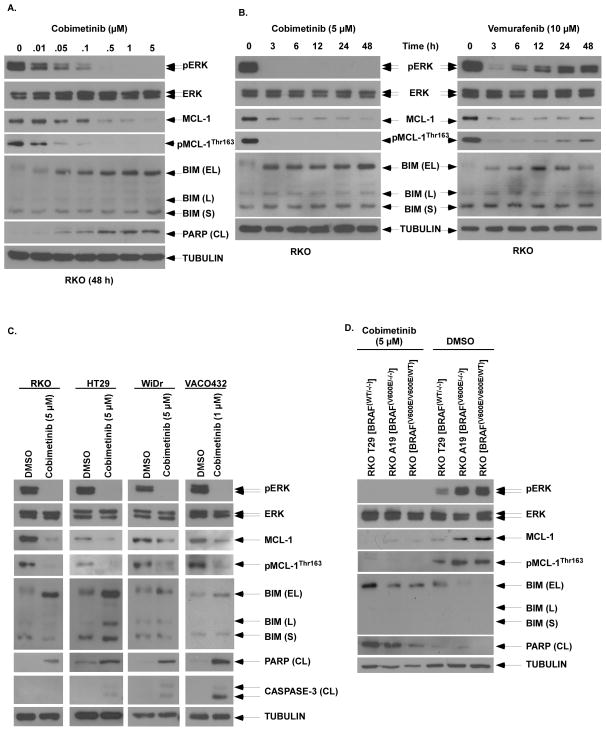
Given the ability of MEK/ERK to regulate MCL-1 expression downstream of BRAFV600E, we determined whether the MEK/ERK inhibitor cobimetinib can suppress MCL-1 and thereby, sensitize mutant BRAF CRC cell lines to apoptosis. Cobimetinib was shown to potently inhibit the phosphorylation of ERK and MCL-1, and to attenuate MCL-1 expression in a dose- and time-dependent manner (Fig. 3A,B) that occurred in association with BIM induction and PARP cleavage (Fig. 3A). In contrast to the sustained effects of cobimetinib, the BRAF inhibitor vemurafenib produced only an early suppression of ERK and MCL-1 expression and phosphorylation (Fig. 3B) whose rebound may be related to EGFR-mediated rebound activation of MAPK signaling (16). To determine whether the observed effects are generalizable, cobimetinib was shown to suppress ERK activation and to downregulate MCL-1 in multiple BRAFV600E CRC cell lines. Cobimetinib was shown to consistently induce pro-apoptotic BIM and to downregulate MCL-1 expression in BRAF mutant CRC cell lines that was associated with cleavage of PRAP and to a lesser extent, caspase-3 (Fig. 3C). Compared to isogenic WT BRAF cell lines, the presence of one or two mutant BRAFV600E alleles was associated attenuated cobimetinib-induced apoptosis that may be related to their MCL-1 upreglation (Fig. 3D).
Figure 3. Cobimetinib treatment inhibits MCL-1 phosphorylation to downregulate expression and induces BIM.
A, RKO cells were treated with increasing doses of cobimetinib for 48 h and protein expression, including BIM isoforms [extra long (EL), long (L) and short (S)] and PARP cleavage (CL), were analyzed by immunboblotting. B, RKO cells were treated with cobimetinib or vemurafenib for the indicated times and expression of pERK/ERK and pMCL-1Thr163/MCL-1 were analyzed by immunoblotting. C, Multiple BRAF mutant CRC cell lines (RKO, HT29, WiDr, VACO432) were treated with cobimetinib at indicated doses for 48 h and protein expression including markers of apoptosis (PARP, caspase-3) were analyzed by immunoblotting. D, RKO BRAF isogenic cell lines with none to two mutant BRAF alleles were treated with cobimetinib vs DMSO and the effect on PARP cleavage was determined.
MCL-1 knockdown enhances cobimetinib-induced apoptosis in vitro and in vivo
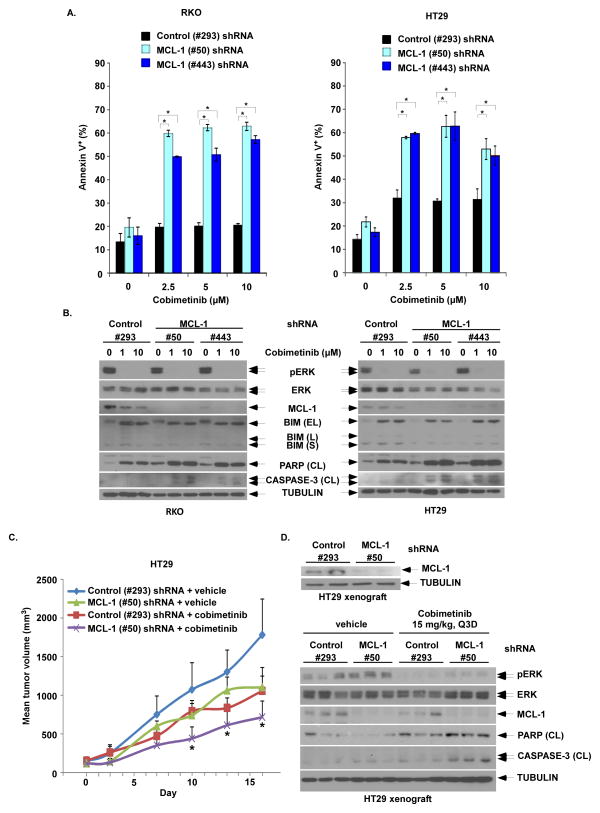
To demonstrate the role of MCL-1 in conferring resistance to cobimetinib, we suppressed MCL-1 using shRNA in RKO and HT29 cell lines and examined drug-induced apoptosis. Knockdown of MCL-1 was shown to increase cobimetinib-induced apoptosis compared to control shRNA cells, as shown by annexin V labeling and cleaved PARP and caspase-3 (Fig. 4, A, B), and this effect was confirmed with a second shRNA with a different MCL-1 targeting sequence (Fig. 4, A, B). These data indicate that antagonism of MCL-1 in mutant BRAFV600E cells treated with cobimetinib can elicit a robust apoptotic response.
Figure 4. MCL-1 knockdown by shRNA enhances cobimetinib-induced apoptosis and anti-tumor efficacy in BRAF mutant CRC cells and tumor xenografts.
A, RKO and HT29 cells were transduced with lentiviral MCL-1 (#50 or #443) vs control shRNA (#293). Cells with stable expression were then incubated with cobimetinib for 48h at the indicated doses. Apoptosis was analyzed by annexin V+ staining that was quantified using flow cytometry. Mean values were derived from triplicate experiments and bars represent S.D. *p<0.05. B, Apoptosis was also analyzed by expression of cleavage (CL) of PARP and CASPASE-3 by immunoblotting in both cell lines. C, HT29 cells containing stable expression of control (#293) or MCL-1 (#50) shRNA were grown as tumor xenografts in SCID mice. Xenograft-bearing mice with dosed with either vehicle or cobimetinib (15 mg/kg every 3 days by oral gavage) for 14 consecutive days. Xenograft mean tumor volumes are plotted against days of treatment for vehicle- and cobimetinib-treated mice. Error bars represent SEM. Statistical significance(*, P < 0.05) is shown for comparison of MCL-1 (#50) shRNA + cobimetinib vs control (#293) shRNA + cobimetinib. D, Pre-treatment expression of MCL-1 in tumors from xenograft-bearing mice was evaluated by immunoblotting (upper panel). Post-treatment expression of pERK/ERK, MCL-1, cleaved PARP and cleaved caspase-3 in tumor xenografts from the 4 groups of tumor-bearing mice were evaluated by immunoblotting (lower panel).
We next determined whether suppression of MCL-1 can enhance cobimetinib-induced tumor regression in vivo. HT29 cells stably expressing control (#293) or MCL-1 (#50) shRNA were grown and then transplanted subcutaneously into SCID mice to generate tumor xenografts that were subsequently treated with vehicle or cobimetinib (15mg/kg Q3D). Prior to treatment, potent suppression of MCL-1 was demonstrated in HT29 tumor xenograft-bearing mice by immunoblotting (Fig. 4D, upper panel). Compared to control, MCL-1 knockdown was shown to suppress tumor growth in untreated mice (Fig 4C). Treatment with cobimetinib significantly suppressed tumor growth in xenograft-bearing mice and was shown to significantly enhance tumor growth inhibition in MCL-1 shRNA tumor xenografts (Fig. 4C). In post-treatment tissue samples from xenograft-bearing mice, we analyzed and compared MEK/ERK activation, MCL-1 expression, and apoptotic markers (cleaved PARP and cleaved caspase-3) among the 4 treatment groups. Suppression of MCL-1 was maintained in knockdown cells (Fig. 4D, lower panel). Consistent with in vitro results, inhibition of ERK phosphorylation by cobimetinib led to downregulation of MCL-1 expression in control shRNA tumor xenografts (Figs 3 and 4D, lower panel). Furthermore, cobimetinib treatment increased cleavage of both PARP and caspase-3 which was further enhanced in MCL-1 knockdown tumor xenografts (Fig. 4D, lower panel).
MCL-1 inhibitor, A-1210477, synergistically enhances cobimetinib-induced apoptosis
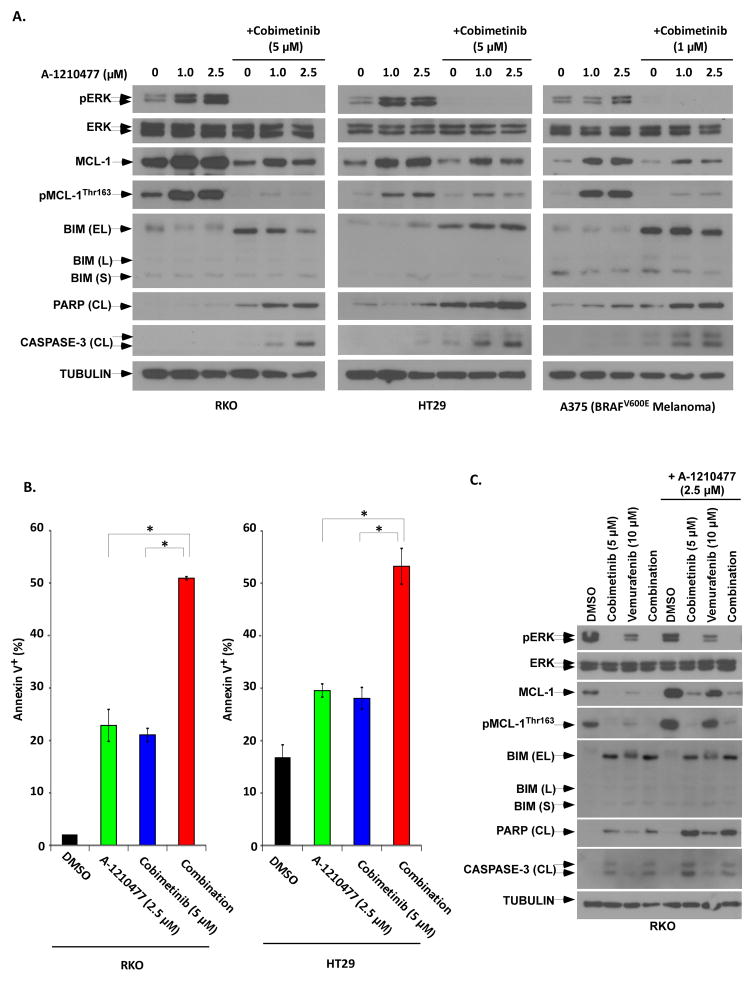
To gain further mechanistic insight, we evaluated the small molecule MCL-1 inhibitor A-1210477 (34) that has been shown to demonstrate selectivity for MCL-1 in cancer cells (34). As previously reported in other tumor cell types(34–36), we found that treatment with A-1210477 upregulates MCL-1 expression in BRAF mutant CRC cells and in the melanoma cell line A375 in a dose-depending manner (Fig. 5A). Treatment with A-1210477 was shown to upregulate MCL-1 together with phosphorylation of ERK and MCL-1, all of which were abrogated by the addition of cobimetinib (Fig. 5A). The combination of cobimetinib and A-1210477 resulted in MCL-1 downregulation, and was associated with increased cleavage of PARP and caspase-3 in CRC and melanoma cell lines (Fig. 5A). Furthemore, the drug combination triggered a 2-fold increase in apoptosis, shown by annexin-V labeling, compared to either drug alone in BRAF mutant CRC cell lines (Fig. 5B). A synergistic interaction was seen between cobimetinib and A-1210477 as determined by calculation of the combination index (CI) [CI < 1] (data not shown). Compared to cobimetinib, the combination of vemurafenib with A-1210477 triggered less apoptosis and the triple drug combination failed to further enhance apoptosis. These data indicate that cobimetinib shows greater efficacy combined with an MCL-1 antagonist than it does with the BRAF inhibitor, vemurafenib (Fig. 5C).
Figure 5. The combination of a small molecule MCL-1 antagonist, A-1210477, with cobimetinib potently induce apoptosis.
A, CRC (RKO, HT29) and melanoma (A375) cell lines were treated with A-1210477 alone or combined with cobimetinib for 48 at the indicated doses. ERK activation, BIM isoform expression, and cleavage of PARP and CASPASE3 were analyzed by immunoblotting. B, RKO and HT29 cell lines were treated with A-1210477 alone or combined with cobimetinib for 48 h, and apoptosis was quantified by annexin V+ staining using flow cytometry. Mean values of triplicate experiments are shown; bars represent S.D; *p<0.05. A synergistic interaction between A-1210477 and cobimetinib (data not shown) was found by calculation of a combination index (CI). C, RKO cells were treated with combimetib, vemurafenib, or their combination in the presence or absence of A-1210477 for 48 h. Protein expression including apoptotic markers was then determined by immunoblotting.
Cobimetinib plus A-1210477 induces apoptosis via pro-apoptotic BIM and BAX, and release of BAK from MCL-1
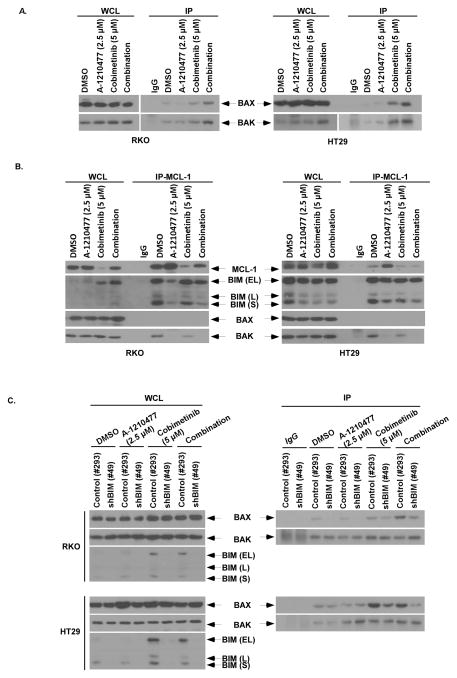
We further examined the mechanism underlying the observed synergistic interaction between cobimetinib and A-1210477. We found that the drug combination activated BAX, as shown by its conformational change, and BAK to a greater extent than did either drug alone (Fig. 6A). Analysis of protein-protein interactions using immunoprecipitation revealed that A-1210477 disrupted the association between MCL-1 and BAK as well as MCL-1 and BIM in RKO and HT29 cell lines (Fig. 6B), confirming the ability of A-1210477 to antagonize MCL-1. The ability of BIM to promote apoptosis induced by the drug combination was demonstrated using BIM shRNA that attenuated BAX, but not BAK, activation.
Figure 6. A-1210477 releases BAK from MCL-1 and cobimetinb induces BIM that is required for BAX activation.
A, RKO and HT29 cell lines were treated with A-1210477, cobimetinib, or in combination for 16 h. Immunoprecipitation (IP) was performed in whole cell lysates (WCL) using conformation-specific antibodies against BAK or BAX (6A7). Normal rabbit (for BAK) and mouse (for BAX) IgG served as antibody controls. B, Cells were treated with A-1210477, cobimetinib, or their combination for 3 h and IP was then performed in WCL using an anti-MCL-1 antibody. Co-precipitated protein complexes were probed for BIM, BAK or BAX by immunoblotting. Normal rabbit IgG served as an antibody control. C, RKO and HT29 cell lines with stable expression of control (#293) or BIM (#49) shRNA were incubated with A-1210477, cobimetinib. or their combination for 16 h. IP was then performed in WCLs using conformation-specific antibodies against BAK or BAX in these cell lines. The effect of BIM shRNA on BIM protein expression was confirmed by immunoblotting.
DISCUSSION
To date, the treatment of human CRCs with mutant BRAF has been largely unsuccessful due to intrinsic/acquired resistance that includes reactivation of signaling through the MAPK pathway (37). Studies indicate that the MAPK pathway is reactivated in 70–79% of melanomas with acquired vemurafenib or dabrafenib resistance (37, 38). The recovery of MAPK signaling can occur by rebound EGFR activation and/or the acquisition of mutations in NRAS, MEK1/2, and BRAF-splice form mutants (39–41). These data indicate the need for potent inhibition of activated MEK and support a strategy of combined BRAF and MEK inhibition in CRC which has been shown to be effective in BRAF mutant melanoma (19). However, this strategy was recently shown to be largely ineffective against human CRCs (4). We, therefore, tested the hypothesis that apoptosis resistance is a major contributor to therapeutic failure in mutant BRAF CRCs. We found that activation of MEK/ERK by mutant BRAF can upregulate anti-apoptotic MCL-1 by its phosphorylation and stabilization to prevent protein turnover. Mcl-1 is known to play a critical role in tumor cell survival and is overexpressed in many human cancers. We demonstrated that phosphorylation can stabilize MCL-1 using a MCL-1 phosphorylation-mimicking mutant and an unphosphorylated MCL-1 mutant. Phoshosphorylation is known to influence the ubiquitination and thus degradation of the modified protein, with examples including c-Myc (42, 43), androgen receptor (44) or the yeast transcriptional factor, Rpn4 (45). In a BRAF mutant human colon cancer, we observed that MCL-1 proteins were overexpressed in tumor cells compared to normal colonic epithelia. Upregulation of MCL-1 protein was increased in a BRAFV600E gene-dose-dependent manner in CRC cell lines isogenic for BRAF (that differ in the number of mutant BRAF alleles), and MCL-1 upregulation/phosphorylation was confirmed in cells with ectopic BRAFV600E expression. In human melanoma cells, mutant BRAF was reported to increase MCL-1 expression by the STAT3 transcription factor (46). However, MCL-1 was not transcriptionally regulated in our CRC cell lines indicating that its posttranscriptional regulation results in enhanced protein stability.
Since mutant BRAF activates downstream MAPK signaling, we determined if ERK activation is an important contributor to MCL-1 upregulation. Knockdown of ERK was shown to attenuate MCL-1 expression and phosphorylation. Furthermore, we made the novel observation that the selective MEK/ERK inhibitor cobimetinib can suppress MCL-1 expression and phosphorylation in CRC cells. In melanoma cell lines, conflicting data exist for the effect of MEK inhibitors on MCL-1 expression in that MEK inhibition by U0126 (47) was shown to downregulate MCL-1 whereas trametinib (48) increased MCL-1 expression. Cobimetinib-induced apoptosis was attenuated by mutant BRAF in isogenic CRC cells where MCL-1 was shown to be upregulated. Suppression of MCL-1 by shRNA or using the selective small molecule MCL-1 inhibitor A-1210477 was shown to potently increase cobimetinib-induced apoptosis, indicating that MCL-1 can mediate apoptosis resistance by mutant BRAF. We confirmed this observation in colon cancer xenograft-bearing mice whereby either MCL-1 antagonism or cobimetinib treatment suppressed tumor growth compared to their respective controls, and cobimetinib significantly enhanced tumor growth inhibition in MCL-1 knockdown vs control xenograft-bearing mice. Treatment with cobimetinib enhanced cleavage of both PARP and caspase-3 in tumor xenografts derived from MCL-1 knockdown compared to control shRNA cells.
To gain mechanistic insight into the ability of MCL-1 antagonism to enhance cobimetinib-induced apoptosis, we utilized the small molecule MCL-1 inhibitor, A-1210477, which has shown ‘on-target’ cancer cell cytotoxicity as monotherapy (34). By inhibiting MCL-1, A-1210477 was shown to interact synergistically with the BCL-2/BCL-xL inhibitor ABT-263 (navitoclax) to kill a variety of cancer cell types (34–36). We found that the combination of A-1210477 and cobimetinib was synergistic as shown by a mechanism that involved disruption of the interaction of MCL-1 with the pro-apoptotic BH3-only proteins BIM and BAK by A-1210477, and by the ability of cobimetinib to suppress MCL-1 and induce BIM expression. MCL-1 is known to bind to and neutralize BIM and BAK (49), and release of BIM from MCL-1 contributes to apoptosis induction. Studies have shown that BIM induction is an important mechanism by which MEK/ERK inhibitors, including cobimetinib, can cooperatively kill tumor cells (50–52). Whereas ERK phosphorylation of BIM targets the protein for polyubiquitination and proteosomal degradation (53), we previously reported that the MEK/ERK inhibitor, GDC-0623, inhibits BIM phosphorylation at Ser69 to increase protein stability(50). Depending on the specific motif that is modified, phosphorylation can either inhibit ubiquitination-triggered protein degradation (e.g., BIM) or promote proteins stability (e.g., MCL-1) (54). The importance of BIM induction in triggering apoptosis was shown in BIM knockdown cells where BAX activation was attenuated by drug combination, consistent with evidence that BIM preferentially activates BAX whereas BID preferentially activates BAK (55). These data support dual targeting of MCL-1 by reducing its expression, antagonizing its function, and promoting BIM which cooperatively enhance apoptosis.
We found that cobimetinib inhibited ERK activation and suppressed MCL-1 to a greater extent than did vemurafenib in vitro. Compared to its combination with cobimetinib, the addition of A-1210477 to vemurafenib triggered less apoptosis, and the triple drug combination failed to further enhance apoptosis that was likely due to the observed weaker ability of vemurafenib to induce BIM and inhibit MCL-1 expression. These data, albeit limited, suggest that the combination of a MEK/ERK inhibitor with an MCL-1 antagonist may be a more potent inducer of apoptosis compared to its combination with a BRAF inhibitor in CRC cell lines. Furthermore, the ability of MCL-1 to confer resistance to MEK/ERK and BRAF inhibition may explain, in part, the disappointing response rate for the combination of dabrafenib and trametinib in patients with metastatic CRC (4). Cobimetinib is being studied in clinical trials in patients with metastatic CRC and was found to be well tolerated at the same dose (60 mg daily) (56) which is approved by FDA for treatment of metastatic melanoma in combination with vemurafenib (19). To date, neither A-1210477 nor other inhibitors of MCL-1 have undergone testing in humans. However, our demonstration that cobimetinib in combination with MCL-1 antagonism can enhance tumor growth inhibition in vivo suggests the clinical relevance of this strategy.
In conclusion, we identify MEK/ERK-mediated MCL-1 upregulation by mutant BRAF to be an important mechanism of apoptosis resistance that contributes to poor therapeutic efficacy in BRAF mutant CRC cells. In addition to potent MEK/ERK inhibition, cobimetinib was shown to attenuate MCL-1 phosphorylation/stabilization to increase apoptotic susceptibility that was enhanced by concurrent MCL-1 antagonism. The combination of cobimetinb with MCL-1 gene knockdown significantly inhibited BRAFV600E mutant CRC tumor growth in xenograft-bearing mice that was associated with enhanced tumor cell apoptosis, suggesting a promising and novel therapeutic strategy for the treatment of mutant BRAF CRCs.
Acknowledgments
Support: Hisato Kawakami was a recipient of a fellowship grant from the Uehara Memorial Foundation, Japan. This work was supported, in part, by grants from the National Cancer Institute/National Institutes of Health (Grant number R01 CA113681 to F. A. S.; the Mayo Clinic Cancer Center [Grant number P30 CA15083; and the Mayo Clinic Center for Cell Signaling in Gastroenterology [Grant number P30 DK084567]).
The authors wish to thank Deborah Frank for her very capable secretarial assistance in the preparation of this manuscript.
Footnotes
Conflict of Interest. The authors report no conflicts related to the content of the manuscript.
References
- 1.Hatzivassiliou G, Haling JR, Chen H, Song K, Price S, Heald R, et al. Mechanism of MEK inhibition determines efficacy in mutant KRAS- versus BRAF-driven cancers. Nature. 2013;501:232–6. doi: 10.1038/nature12441. [DOI] [PubMed] [Google Scholar]
- 2.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 3.Kopetz S, Desai J, Chan E, Hecht JR, O’Dwyer PJ, Maru D, et al. Phase II Pilot Study of Vemurafenib in Patients With Metastatic BRAF-Mutated Colorectal Cancer. J Clin Oncol. 2015;33:4032–8. doi: 10.1200/JCO.2015.63.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corcoran RB, Atreya CE, Falchook GS, Kwak EL, Ryan DP, Bendell JC, et al. Combined BRAF and MEK Inhibition With Dabrafenib and Trametinib in BRAF V600-Mutant Colorectal Cancer. J Clin Oncol. 2015;33:4023–31. doi: 10.1200/JCO.2015.63.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roth AD, Tejpar S, Delorenzi M, Yan P, Fiocca R, Klingbiel D, et al. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J Clin Oncol. 2010;28:466–74. doi: 10.1200/JCO.2009.23.3452. [DOI] [PubMed] [Google Scholar]
- 6.Farina-Sarasqueta A, van Lijnschoten G, Moerland E, Creemers GJ, Lemmens VE, Rutten HJ, et al. The BRAF V600E mutation is an independent prognostic factor for survival in stage II and stage III colon cancer patients. Ann Oncol. 2010;21:2396–402. doi: 10.1093/annonc/mdq258. [DOI] [PubMed] [Google Scholar]
- 7.Ogino S, Shima K, Meyerhardt JA, McCleary NJ, Ng K, Hollis D, et al. Predictive and prognostic roles of BRAF mutation in stage III colon cancer: results from intergroup trial CALGB 89803. Clin Cancer Res. 2012;18:890–900. doi: 10.1158/1078-0432.CCR-11-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sinicrope FA, Shi Q, Smyrk TC, Thibodeau SN, Dienstmann R, Guinney J, et al. Molecular markers identify subtypes of stage III colon cancer associated with patient outcomes. Gastroenterology. 2015;148:88–99. doi: 10.1053/j.gastro.2014.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phipps AI, Limburg PJ, Baron JA, Burnett-Hartman AN, Weisenberger DJ, Laird PW, et al. Association between molecular subtypes of colorectal cancer and patient survival. Gastroenterology. 2015;148:77–87. e2. doi: 10.1053/j.gastro.2014.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tie J, Gibbs P, Lipton L, Christie M, Jorissen RN, Burgess AW, et al. Optimizing targeted therapeutic development: analysis of a colorectal cancer patient population with the BRAF(V600E) mutation. Int J Cancer. 2011;128:2075–84. doi: 10.1002/ijc.25555. [DOI] [PubMed] [Google Scholar]
- 11.Tran B, Kopetz S, Tie J, Gibbs P, Jiang ZQ, Lieu CH, et al. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer. 2011;117:4623–32. doi: 10.1002/cncr.26086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilcox RE, Shi Q, Sinicrope FA, Sargent DJ, Foster NR, Meyers JP, et al. Influence of molecular alterations on site-specific (ss) time to recurrence (TTR) following adjuvant therapy in resected colon cancer (CC) (Alliance Trial N0147) Journal of Clinical Oncology. 2015:33. [Google Scholar]
- 13.Goldstein J, Tran B, Ensor J, Gibbs P, Wong HL, Wong SF, et al. Multicenter retrospective analysis of metastatic colorectal cancer (CRC) with high-level microsatellite instability (MSI-H) Ann Oncol. 2014;25:1032–8. doi: 10.1093/annonc/mdu100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sosman JA, Kim KB, Schuchter L, Gonzalez R, Pavlick AC, Weber JS, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366:707–14. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–16. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prahallad A, Sun C, Huang S, Di Nicolantonio F, Salazar R, Zecchin D, et al. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature. 2012;483:100–3. doi: 10.1038/nature10868. [DOI] [PubMed] [Google Scholar]
- 17.Corcoran RB, Ebi H, Turke AB, Coffee EM, Nishino M, Cogdill AP, et al. EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov. 2012;2:227–35. doi: 10.1158/2159-8290.CD-11-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corcoran RB, Dias-Santagata D, Bergethon K, Iafrate AJ, Settleman J, Engelman JA. BRAF gene amplification can promote acquired resistance to MEK inhibitors in cancer cells harboring the BRAF V600E mutation. Sci Signal. 2010;3:ra84. doi: 10.1126/scisignal.2001148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larkin J, Ascierto PA, Dreno B, Atkinson V, Liszkay G, Maio M, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371:1867–76. doi: 10.1056/NEJMoa1408868. [DOI] [PubMed] [Google Scholar]
- 20.Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–90. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 21.Cartlidge RA, Thomas GR, Cagnol S, Jong KA, Molton SA, Finch AJ, et al. Oncogenic BRAF(V600E) inhibits BIM expression to promote melanoma cell survival. Pigment Cell Melanoma Res. 2008;21:534–44. doi: 10.1111/j.1755-148X.2008.00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang CC, Lucas K, Avery-Kiejda KA, Wade M, deBock CE, Thorne RF, et al. Up-regulation of Mcl-1 is critical for survival of human melanoma cells upon endoplasmic reticulum stress. Cancer Res. 2008;68:6708–17. doi: 10.1158/0008-5472.CAN-08-0349. [DOI] [PubMed] [Google Scholar]
- 23.Boisvert-Adamo K, Aplin AE. Mutant B-RAF mediates resistance to anoikis via Bad and Bim. Oncogene. 2008;27:3301–12. doi: 10.1038/sj.onc.1211003. [DOI] [PubMed] [Google Scholar]
- 24.Zhang L, Yu J. Role of apoptosis in colon cancer biology, therapy, and prevention. Curr Colorectal Cancer Rep. 2013:9. doi: 10.1007/s11888-013-0188-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masuoka HC, Sinicrope FA. Clinical relevance of apoptotic regulatory proteins in colorectal cancers. Curr Colorectal Cancer Rep. 2010;6:111–7. [Google Scholar]
- 26.Ley R, Ewings KE, Hadfield K, Cook SJ. Regulatory phosphorylation of Bim: sorting out the ERK from the JNK. Cell Death Differ. 2005;12:1008–14. doi: 10.1038/sj.cdd.4401688. [DOI] [PubMed] [Google Scholar]
- 27.Lucas KM, Mohana-Kumaran N, Lau D, Zhang XD, Hersey P, Huang DC, et al. Modulation of NOXA and MCL-1 as a strategy for sensitizing melanoma cells to the BH3-mimetic ABT-737. Clin Cancer Res. 2012;18:783–95. doi: 10.1158/1078-0432.CCR-11-1166. [DOI] [PubMed] [Google Scholar]
- 28.Luciano F, Jacquel A, Colosetti P, Herrant M, Cagnol S, Pages G, et al. Phosphorylation of Bim-EL by Erk1/2 on serine 69 promotes its degradation via the proteasome pathway and regulates its proapoptotic function. Oncogene. 2003;22:6785–93. doi: 10.1038/sj.onc.1206792. [DOI] [PubMed] [Google Scholar]
- 29.Frederick DT, Salas Fragomeni RA, Schalck A, Ferreiro-Neira I, Hoff T, Cooper ZA, et al. Clinical profiling of BCL-2 family members in the setting of BRAF inhibition offers a rationale for targeting de novo resistance using BH3 mimetics. PLoS One. 2014;9:e101286. doi: 10.1371/journal.pone.0101286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akgul C. Mcl-1 is a potential therapeutic target in multiple types of cancer. Cell Mol Life Sci. 2009;66:1326–36. doi: 10.1007/s00018-008-8637-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang S, Sinicrope FA. BH3 mimetic ABT-737 potentiates TRAIL-mediated apoptotic signaling by unsequestering Bim and Bak in human pancreatic cancer cells. Cancer Res. 2008;68:2944–51. doi: 10.1158/0008-5472.CAN-07-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang S, Okumura K, Sinicrope FA. BH3 mimetic obatoclax enhances TRAIL-mediated apoptosis in human pancreatic cancer cells. Clin Cancer Res. 2009;15:150–9. doi: 10.1158/1078-0432.CCR-08-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ding Q, Huo L, Yang JY, Xia W, Wei Y, Liao Y, et al. Down-regulation of myeloid cell leukemia-1 through inhibiting Erk/Pin 1 pathway by sorafenib facilitates chemosensitization in breast cancer. Cancer Res. 2008;68:6109–17. doi: 10.1158/0008-5472.CAN-08-0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leverson JD, Zhang H, Chen J, Tahir SK, Phillips DC, Xue J, et al. Potent and selective small-molecule MCL-1 inhibitors demonstrate on-target cancer cell killing activity as single agents and in combination with ABT-263 (navitoclax) Cell Death Dis. 2015;6:e1590. doi: 10.1038/cddis.2014.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phillips DC, Xiao Y, Lam LT, Litvinovich E, Roberts-Rapp L, Souers AJ, et al. Loss in MCL-1 function sensitizes non-Hodgkin’s lymphoma cell lines to the BCL-2-selective inhibitor venetoclax (ABT-199) Blood Cancer J. 2015;5:e368. doi: 10.1038/bcj.2015.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao Y, Nimmer P, Sheppard GS, Bruncko M, Hessler P, Lu X, et al. MCL-1 Is a Key Determinant of Breast Cancer Cell Survival: Validation of MCL-1 Dependency Utilizing a Highly Selective Small Molecule Inhibitor. Mol Cancer Ther. 2015;14:1837–47. doi: 10.1158/1535-7163.MCT-14-0928. [DOI] [PubMed] [Google Scholar]
- 37.Shi H, Hugo W, Kong X, Hong A, Koya RC, Moriceau G, et al. Acquired resistance and clonal evolution in melanoma during BRAF inhibitor therapy. Cancer Discov. 2014;4:80–93. doi: 10.1158/2159-8290.CD-13-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rizos H, Menzies AM, Pupo GM, Carlino MS, Fung C, Hyman J, et al. BRAF inhibitor resistance mechanisms in metastatic melanoma: spectrum and clinical impact. Clin Cancer Res. 2014;20:1965–77. doi: 10.1158/1078-0432.CCR-13-3122. [DOI] [PubMed] [Google Scholar]
- 39.Van Allen EM, Wagle N, Sucker A, Treacy DJ, Johannessen CM, Goetz EM, et al. The genetic landscape of clinical resistance to RAF inhibition in metastatic melanoma. Cancer Discov. 2014;4:94–109. doi: 10.1158/2159-8290.CD-13-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nazarian R, Shi H, Wang Q, Kong X, Koya RC, Lee H, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468:973–7. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poulikakos PI, Persaud Y, Janakiraman M, Kong X, Ng C, Moriceau G, et al. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E) Nature. 2011;480:387–90. doi: 10.1038/nature10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Welcker M, Orian A, Jin J, Grim JE, Harper JW, Eisenman RN, et al. The Fbw7 tumor suppressor regulates glycogen synthase kinase 3 phosphorylation-dependent c-Myc protein degradation. Proc Natl Acad Sci U S A. 2004;101:9085–90. doi: 10.1073/pnas.0402770101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yada M, Hatakeyama S, Kamura T, Nishiyama M, Tsunematsu R, Imaki H, et al. Phosphorylation-dependent degradation of c-Myc is mediated by the F-box protein Fbw7. Embo J. 2004;23:2116–25. doi: 10.1038/sj.emboj.7600217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin HK, Wang L, Hu YC, Altuwaijri S, Chang C. Phosphorylation-dependent ubiquitylation and degradation of androgen receptor by Akt require Mdm2 E3 ligase. Embo J. 2002;21:4037–48. doi: 10.1093/emboj/cdf406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ju D, Xu H, Wang X, Xie Y. Ubiquitin-mediated degradation of Rpn4 is controlled by a phosphorylation-dependent ubiquitylation signal. Biochim Biophys Acta. 2007;1773:1672–80. doi: 10.1016/j.bbamcr.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 46.Becker TM, Boyd SC, Mijatov B, Gowrishankar K, Snoyman S, Pupo GM, et al. Mutant B-RAF-Mcl-1 survival signaling depends on the STAT3 transcription factor. Oncogene. 2014;33:1158–66. doi: 10.1038/onc.2013.45. [DOI] [PubMed] [Google Scholar]
- 47.Wang YF, Jiang CC, Kiejda KA, Gillespie S, Zhang XD, Hersey P. Apoptosis induction in human melanoma cells by inhibition of MEK is caspase-independent and mediated by the Bcl-2 family members PUMA, Bim, and Mcl-1. Clin Cancer Res. 2007;13:4934–42. doi: 10.1158/1078-0432.CCR-07-0665. [DOI] [PubMed] [Google Scholar]
- 48.Fofaria NM, Frederick DT, Sullivan RJ, Flaherty KT, Srivastava SK. Overexpression of Mcl-1 confers resistance to BRAFV600E inhibitors alone and in combination with MEK1/2 inhibitors in melanoma. Oncotarget. 2015;6:40535–56. doi: 10.18632/oncotarget.5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morales AA, Kurtoglu M, Matulis SM, Liu J, Siefker D, Gutman DM, et al. Distribution of Bim determines Mcl-1 dependence or codependence with Bcl-xL/Bcl-2 in Mcl-1-expressing myeloma cells. Blood. 2011;118:1329–39. doi: 10.1182/blood-2011-01-327197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zaanan A, Okamoto K, Kawakami H, Khazaie K, Huang S, Sinicrope FA. The Mutant KRAS Gene Up-regulates BCL-XL Protein via STAT3 to Confer Apoptosis Resistance That Is Reversed by BIM Protein Induction and BCL-XL Antagonism. J Biol Chem. 2015;290:23838–49. doi: 10.1074/jbc.M115.657833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bhalla S, Evens AM, Dai B, Prachand S, Gordon LI, Gartenhaus RB. The novel anti-MEK small molecule AZD6244 induces BIM-dependent and AKT-independent apoptosis in diffuse large B-cell lymphoma. Blood. 2011;118:1052–61. doi: 10.1182/blood-2011-03-340109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O’Reilly LA, Kruse EA, Puthalakath H, Kelly PN, Kaufmann T, Huang DC, et al. MEK/ERK-mediated phosphorylation of Bim is required to ensure survival of T and B lymphocytes during mitogenic stimulation. J Immunol. 2009;183:261–9. doi: 10.4049/jimmunol.0803853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wiggins CM, Johnson M, Cook SJ. Refining the minimal sequence required for ERK1/2-dependent poly-ubiquitination and proteasome-dependent turnover of BIM. Cell Signal. 2010;22:801–8. doi: 10.1016/j.cellsig.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 54.Nguyen LK, Kolch W, Kholodenko BN. When ubiquitination meets phosphorylation: a systems biology perspective of EGFR/MAPK signalling. Cell Commun Signal. 2013;11:52. doi: 10.1186/1478-811X-11-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sarosiek KA, Chi X, Bachman JA, Sims JJ, Montero J, Patel L, et al. BID preferentially activates BAK while BIM preferentially activates BAX, affecting chemotherapy response. Mol Cell. 2013;51:751–65. doi: 10.1016/j.molcel.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bendell JC, Kim TW, Goh BC, Wallin J, Oh DY, Han SW, et al. Clinical activity and safety of cobimetinib (cobi) and atezolizumab in colorectal cancer (CRC) J Clin Oncol. 2016;34(suppl) abstr 3502. [Google Scholar]